Abstract
Stage conversion between bradyzoites and tachyzoites was investigated in C57BL/6 mice chronically infected with the ME-49 strain of Toxoplasma gondii. In order to promote bradyzoite-tachyzoite conversion, mice were treated in vivo with neutralizing doses of anti-gamma interferon (IFN-γ) or anti-tumor necrosis factor alpha (TNF-α) antibodies. Expression of parasite-specific antigens SAG-1, SAG-2, and heat shock protein 70 (Hsp-70) was visualized in the central nervous system by immunocytochemistry and measured by photometric assay. The immunosuppressive effect of anti-IFN-γ or anti-TNF-α treatment was immediate, leading to parasite stage conversion as indicated by the increased expression of tachyzoite-specific antigens (SAG-1 and SAG-2) and by rapid parasite replication. We also observed expression of high levels of Hsp-70 during a short period of conversion of bradyzoites to tachyzoites. Our data suggest that Hsp-70 may have an important role in the process of bradyzoite-tachyzoite conversion during the reactivation of chronic toxoplasmosis.
Toxoplasma gondii is an infectious pathogen that causes toxoplasmosis. During the acute phase of infection, the tachyzoite stage of the parasite undergoes an initial period of rapid multiplication. In immunocompetent individuals, tachyzoite multiplication is inhibited by the immune response. The outcome of this immunologic response to the tachyzoite results in the development of bradyzoites, the hallmark of chronic infection. In selected immunodeficiencies, and in particular AIDS, bradyzoites may escape from the cyst and revert to tachyzoites that multiply unhampered, resulting in the extensive and often fatal tissue damage associated with toxoplasmic encephalitis (23). In vivo studies in experimental models indicate that gamma interferon (IFN-γ) is a major cytokine that mediates resistance against T. gondii infection (35). CD4+ and CD8+ lymphocytes are involved in the prevention of disease reactivation, probably through the production of IFN-γ (10, 11, 19, 20). In vivo and in vitro experiments also suggest a crucial role for both IFN-γ and tumor necrosis factor alpha (TNF-α) in the induction of nitric oxide-mediated microbicidal activity (1, 8, 15, 22, 29, 32).
Reactivation of a latent infection culminates in the conversion of bradyzoites to tachyzoites, an event that has been investigated in vitro (4, 5, 33). In vitro studies demonstrated that differentiation from the tachyzoite to the bradyzoite stage can be induced by external stress factors, such as increased pH of the cell culture medium, a shift of the temperature from 37 to 43°C, or treatment with sodium arsenite (34). During stage differentiation from tachyzoite to bradyzoite, a stage-specific heat shock protein (Hsp)/BAG-1 antigen is expressed. This bradyzoite-specific protein showed similarity to the small Hsp from plants (6). In vitro exposure of tachyzoites of T. gondii ME-49 to pH 8.1 facilitates their conversion to bradyzoites, during which time the parasites may express a 72-kDa protein that is believed to be part of the Hsp-70 family (38).
The molecular events surrounding the conversion of the bradyzoite to the tachyzoite during reactivation of chronic infection with T. gondii have not been explored. In mice, relapsing toxoplasmic encephalitis is associated with an increased expression of SAG-1 and SAG-2 mRNAs in the central nervous system (CNS) (12, 13). In this study, C57BL/6 mice infected with the ME-49 strain of T. gondii were immunosuppressed by treatment with anti-IFN-γ or anti-TNF-α monoclonal antibody (MAb), and the effect on expression of SAG-1 and SAG-2 as well as Hsp was examined.
Female C57BL/6 mice, 4 to 5 weeks old, were infected with 10 to 20 cysts of the ME-49 strain of T. gondii and received weekly treatment with 3 mg of rat immunoglobulin G1 MAb specific for either IFN-γ (XMG-6), TNF-α (HT-11-22), or control β-galactosidase (GL-113), beginning at 4 weeks postinfection (11, 12). The animals treated with anti-IFN-γ antibody were killed in a CO2 chamber and decapitated at 0, 1, 3, 5, 7, 9, 10, and 12 days after the initiation of the immunosuppressive treatment, and those treated with anti-TNF-α were killed at 12 days. The brains were removed and fixed in Bouin-Hollande fixative for 24 h and transferred to 70% ethanol before processing for paraffin sectioning (12). For immunocytochemistry (14), mouse brain sections, 4 mm thick, were obtained from paraffin blocks. To localize SAG-1, SAG-2, and 70-kDa Hsp, paraffin sections were deparaffinized and antigenic unmasking was done with a microwave oven (31). The sections were incubated for 30 min at 37°C in 2% unlabeled sheep serum to reduce nonspecific binding and then incubated in polyclonal rabbit primary antibody against SAG-1 or SAG-2 antigen or Hsp-70 (1:25) at 4°C overnight. The polyclonal antibody to Hsp was raised against the 3/4 C-terminal region of Hsp-70 from Leishmania (Viannia) braziliensis, the most polymorphic portion of this molecule (2). Secondary biotinylated antibodies were sheep anti-rabbit antibodies. The sensitivity was improved with the avidin-biotin technique (ABC kit, PK-4000; Vector Laboratories, Inc., Burlingame, Calif.). The reaction was visualized by incubating the section with 3,3′-diaminobenzidine tetrahydrochloride (Sigma) for 5 min. The slides were studied with an Olympus light microscope and photographed with Kodak film (100 ASA). Control slides were incubated in the unlabeled rabbit serum. The measurement of the staining intensity was done with the UTHSCSA Image Tool program from the University of Texas Health Science Center, San Antonio.
Morbidity was assessed by histochemical enumeration of cyst numbers and determination of sizes as well as distribution within the CNS with brain sections from chronically infected mice. Periodic acid-Schiff stain (PAS) was used as a specific stain to identify the cyst membrane that contains the bradyzoite stage of T. gondii. Polyclonal antibodies against SAG-1 or SAG-2 were used as specific markers for tachyzoites (16–18, 24). The expression of parasite Hsps during bradyzoite-tachyzoite conversion was evaluated because these proteins may be involved in the stage transformation of parasites (38). The parameters measured to determine T. gondii stage conversion included (i) the frequency of free tachyzoites; (ii) the average number of cysts within the brain; (iii) cyst diameters; and (iv) the intensity of SAG-1, SAG-2, Hsp-70, or PAS staining during bradyzoite-tachyzoite conversion in brains of chronically infected animals, analyzed before and after the treatment with anti-IFN-γ or anti-TNF-α MAb (Table 1 and Fig. 1 and 2).
TABLE 1.
Free tachyzoites, cyst numbers, cyst diameters, and cyst PAS staining in brains of C57BL/6 mice chronically infected with T. gondii and treated with various MAbsa
| MAb treatment and day after initiation | Free-tachyzoite scoreb | No. of cysts per section | Diameter of cysts (μm) | % PAS-positive cysts |
|---|---|---|---|---|
| Anti-β-galactosidase (control) | 1+ | 3.5 ± 2.1c | 45.4 ± 14.4d | 96 |
| Anti-IFN-γ day 0 | 1+ | 6.8 ± 3.8c | 39.8 ± 14.2d | 100 |
| Anti-IFN-γ day 1 | — | 10.1 ± 1.3 | 46.7 ± 23.5 | 100 |
| Anti-IFN-γ day 3 | — | 8.6 ± 4.4 | 43.6 ± 12.0 | 81 |
| Anti-IFN-γ day 5 | 2+ | 11.8 ± 5.8 | 46.8 ± 19.7 | 80 |
| Anti-IFN-γ day 7 | 3+ | 30.0 ± 20.5 | 29.1 ± 17.1 | 60 |
| Anti-IFN-γ day 9 | 4+ | 49.5 ± 0.7 | 25.6 ± 14.2 | 27 |
| Anti-IFN-γ day 10 | 3+ | 21.6 ± 7.4 | 31.1 ± 12.5 | 45 |
| Anti-IFN-γ day 12 | 5+ | 128.5 ± 49.4e | 25.6 ± 14.0 | 50 |
| Anti-TNF-α day 12 | 4+ | 31.6 ± 28.3 | 23.3 ± 12.7 | 80 |
The results are the means and standard deviations of assays performed on three mice/group.
1+, one lesion containing few tachyzoites in three to five histopathologic sections; 2+, one or more lesions containing tachyzoites in two sections; 3+, one or more parasite-containing lesions per section; 4+, one or more parasite-containing lesions per section and at least one extensive lesion containing abundant tachyzoites; 5+, more than one extensive lesion containing abundant tachyzoites. —, not detected.
Significantly different from values obtained for mice treated with IFN-γ and sacrificed on day 1 and days 5 to 12 and mice treated with TNF-α and sacrificed on day 12 (P ≤ 0.0295).
Significantly different from values obtained for mice treated with IFN-γ and sacrificed on days 7 to 12 and mice treated with TNF-α and sacrificed on day 12 (P ≤ 0.0255).
Significantly different from values obtained for mice with all other conditions of immunosuppression (P ≤ 0.0290). For the methodological details, see the text.
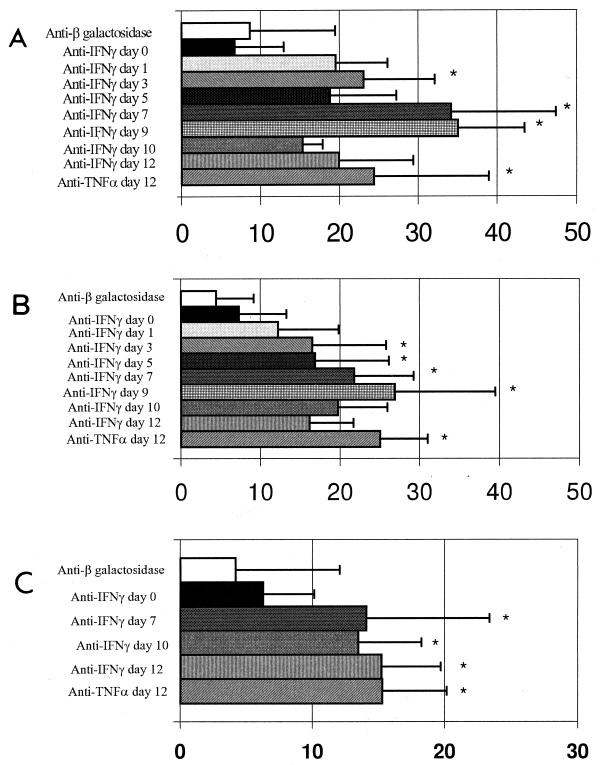
FIG. 1.
Detection of SAG-1 (A), SAG-2 (B), and Hsp-70 (C) antigens by photometric assay in brain cysts from mice chronically infected with T. gondii. The data were obtained from several cysts analyzed from groups of three mice. Intensities of expression under the indicated treatment conditions are shown in absorbance units. Asterisks indicate values significantly different from those obtained with controls (P < 0.05).
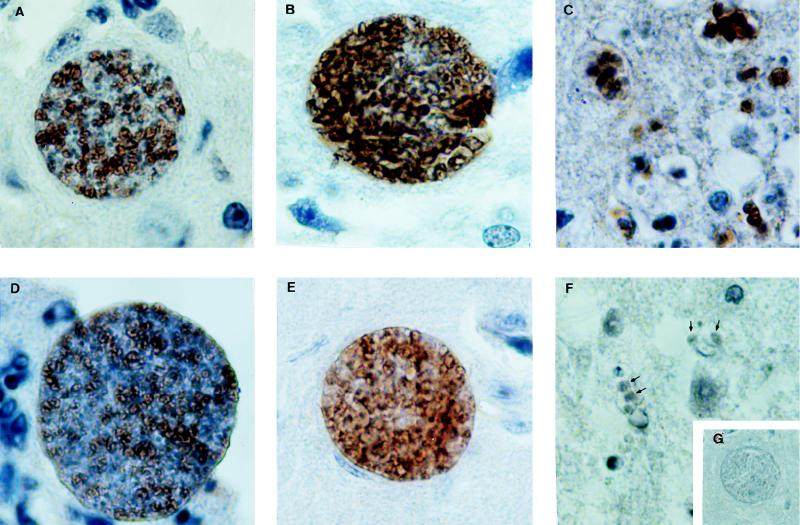
FIG. 2.
Illustration of SAG-1 (top panels) and Hsp-70 (bottom panels) immunoperoxidase staining in parasites inside cysts (A, B, D, and E) and free tachyzoites (C and F) of T. gondii present in the CNSs of animals treated with either control MAb (A and D) or anti-IFN-γ (B, C, E, and F) and shown at a magnification of ×244. Background staining with an unrelated control polyclonal antibody is shown in the inset (G) at a magnification of ×101. For the methodological details, see the text.
Rapid parasite replication was observed between days 5 and 7 after treatment with anti-IFN-γ MAb, as indicated by the presence of free tachyzoites and PAS-negative pseudocysts (20%), which represent newly formed cysts (Table 1). Morphologic analysis indicated that the maximum parasite burden occurred between days 7 and 9. Increased numbers of free tachyzoites and pseudocysts (Table 1) were observed in samples of brain tissue, suggesting cyst rupture, bradyzoite-tachyzoite conversion, tachyzoite replication, and active infection of host cells. Of note was an increase in the percentage and absolute number of PAS-positive cysts after day 9 of treatment with anti-IFN-γ MAb, indicating that the transformation of tachyzoites to bradyzoites persisted in spite of immunosuppressive treatment. Although the relative numbers of pseudocysts decreased, there was an increase in the absolute numbers of pseudocysts and free tachyzoites. These observations demonstrate that development of new cysts as well as maximal tachyzoite replication occurred after day 9 of treatment with anti-IFN-γ.
In contrast to a previous study (39), the results of the immunocytochemistry analysis demonstrated the expression of stage-specific tachyzoite antigens on parasites within the cyst membrane (14, 31). Some cysts from infected mice treated with MAb had few parasites expressing SAG-1 and SAG-2 antigens. The discrepancy between our observations and the previous report may be explained in part by our use of T. gondii ME-49. During chronic infection in highly susceptible C57BL/6 mice (7, 36), this strain can maintain a continuous and more dynamic cyst turnover (9, 26). Nevertheless, our data also show that in vivo neutralization of either IFN-γ or TNF-α in mice chronically infected (for 4 weeks) with T. gondii ME-49 resulted in a dramatic enhancement and homogeneous expression of SAG-1 and SAG-2 inside the brain cysts. This suggests that the majority of the parasites inside cysts begin to express tachyzoite-specific antigens after cytokine neutralization. A photometric assay was used to measure the expression of SAG-1 and SAG-2 in brain cysts. As shown in Fig. 1A, 1 day after initiation of anti-IFN-γ treatment, the expression of SAG-1 was enhanced. The highest expression of SAG-1 by parasites in the CNSs of animals was recorded at 7 to 9 days after treatment with anti-IFN-γ MAb (Fig. 1A and 2B). A decrease in the intensity of SAG-1 expression was observed after day 10 of treatment with anti-IFN-γ. Similar kinetics were observed when the expression of SAG-2 by T. gondii during treatment with anti-IFN-γ MAb was evaluated (Fig. 1B). An increase in SAG-1 and SAG-2 expression was also observed in parasites in the CNSs of mice chronically infected with T. gondii and treated with anti-TNF-α MAb (Fig. 1A and B).
In order to study the expression of T. gondii Hsp-70 during stage conversion from bradyzoites to tachyzoites, we used rabbit polyclonal antibodies raised against the last three quarters of the C-terminal region of Hsp-70 from L. (V.) braziliensis (2). Neutralization of endogenous IFN-γ or TNF-α in chronically infected C57BL/6 mice resulted in homogeneous expression of Hsp-70 in some brain cysts (Fig. 1C and 2E). Interestingly, some of the cysts from immunosuppressed animals did not express this protein. By photometric assay, increased expression of Hsp-70 was observed in animals receiving anti-IFN-γ MAb after 7 days of treatment. These mice had an increase in number of free tachyzoites in relation to cyst numbers (Table 1). Fully differentiated free tachyzoites in the brain tissue did not express Hsp-70 (Fig. 2F). Thus, our data indicate that maximal expression of Hsp-70 occurs in encysted parasites during a short period of parasite stage conversion. We were unable to determine whether expression of Hsp-70 occurs during bradyzoite-tachyzoite conversion or tachyzoite-bradyzoite conversion. The fact that the maximal intensity of Hsp expression was observed at late stages of reactivation may indicate that Hsp-70 is primarily expressed during tachyzoite-bradyzoite conversion.
The Hsps have been shown to be highly conserved among a wide variety of organisms. Although Hsp functions are not completely understood, these proteins are essential for survival of the cell (3, 30) and are involved in a variety of biological functions within the cell, including preservation and recovery of various protein complexes and degradation of denatured proteins. Environmental stresses, such as heat shock, starvation, and alkaline pH, can induce cell differentiation, a process associated with induction of Hsp expression (21, 28). In nature, transfer of parasites from one environment to another or parasite stage conversion is frequently associated with expression of Hsp (3, 27, 37). Hsps are also important immunologic targets in response to pathogens (25). In vitro, environmental stresses, such as alkaline pH, can drive the transformation of tachyzoites to bradyzoites. Associated with this transformation is the expression of a T. gondii-specific antigen that has some homology to the small Hsp from plants (6). In our study, neutralization of endogenous IFN-γ or TNF-α or depletion of T-cell subsets (data not shown) enhanced expression of Hsp-70 inside brain cysts from immunosuppressed mice. Interestingly, Hsp-70 was not intensively expressed by bradyzoites from immunocompetent mice or by free tachyzoites in brain lesions from immunosuppressed animals. These findings suggest that Hsp-70 may have an important role in T. gondii adaptation during this differentiation event.
Acknowledgments
We thank Joao Kazuyuki Kajiwara from the Laboratory of Morphology at the Faculdade de Medicina de Ribeirao Preto at the Universidade de Sao Paulo, where the photometric and morphometric assays were performed, for helpful discussions. We also thank Antonio Gomes de Amorim Filho from the Escola Paulista de Medicina for providing us with anti-Hsp antiserum.
This work was supported by grants from Brazilian Research Councils (CNPq, CAPES, and FAPEMIG).
REFERENCES
- 1.Adams L B, Hibbs J B, Jr, Taintor R R, Krahenbuhl J L. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from l-arginine. J Immunol. 1990;144:2725–2729. [PubMed] [Google Scholar]
- 2.Amorim-Filho A G. A proteina de “heat shock” de 70 kDa de Leishmania (V.) braziliensis como alvo da resposta imune humoral na leishmaniose mucocutanea. Ph.D. thesis. Sao Paulo, Brazil: Escola Paulista de Medicina; 1994. [Google Scholar]
- 3.Bensaude O, Babinet C, Morange M, Jacob F. Heat shock protein, first major products of zygotic gene activity in mouse embryo. Nature. 1983;305:331–333. doi: 10.1038/305331a0. [DOI] [PubMed] [Google Scholar]
- 4.Bohne W, Heesemann J, Gross U. Induction of bradyzoite-specific Toxoplasma gondii antigens in gamma interferon-treated mouse macrophages. Infect Immun. 1993;61:1141–1145. doi: 10.1128/iai.61.3.1141-1145.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohne W, Heesemann J, Gross U. Reduced replication of Toxoplasma gondii is necessary for induction of bradyzoite-specific antigens: a possible role for nitric oxide in triggering stage conversion. Infect Immun. 1994;62:1761–1767. doi: 10.1128/iai.62.5.1761-1767.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohne W, Gross U, Ferguson D J P. Cloning and characterization of a bradyzoite-specifically expressed gene (hsp30/bag1) of Toxoplasma gondii, related to genes encoding small heat-shock proteins of plants. Mol Microbiol. 1995;16:1221–1230. doi: 10.1111/j.1365-2958.1995.tb02344.x. [DOI] [PubMed] [Google Scholar]
- 7.Brown C R, Mcleod R. Class I MHC genes and CD8+ T cells determine cyst number in Toxoplasma gondii infection. J Immunol. 1990;145:3438–3441. [PubMed] [Google Scholar]
- 8.Deckert-Schluter M, Bluethmann H, Rang A, Hof H, Schluter D. Crucial role of TNF receptor type 1 (p55), but not of TNF receptor type 2 (p75), in murine toxoplasmosis. J Immunol. 1998;160:3427–3436. [PubMed] [Google Scholar]
- 9.Ferguson D J P, Huskinson-Mark J, Araujo F G, Remington J S. A morphological study of chronic cerebral toxoplasmosis in mice: comparison of four different strains of Toxoplasma gondii. Parasitol Res. 1994;80:493–501. doi: 10.1007/BF00932696. [DOI] [PubMed] [Google Scholar]
- 10.Gazzinelli R, Yuhui X, Hieny S, Cheever A, Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol. 1992;149:175–180. [PubMed] [Google Scholar]
- 11.Gazzinelli R T, Hakim F T, Hieny S, Shearer G, Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-γ production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol. 1991;146:286–292. [PubMed] [Google Scholar]
- 12.Gazzinelli R T, Eltoum I, Wynn T A, Sher A. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-α and correlates with the down-regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J Immunol. 1993;151:3672–3681. [PubMed] [Google Scholar]
- 13.Gazzinelli R T, Brezin A, Li Q, Nussenblatt R B, Chan C C. Toxoplasma gondii: acquired ocular toxoplasmosis in the murine model, protective role of TNF-α and IFN-γ. Exp Parasitol. 1994;78:217–229. doi: 10.1006/expr.1994.1022. [DOI] [PubMed] [Google Scholar]
- 14.Gosselin E J, Cate C C, Pettengill O S, Sorenson G D. Immunocytochemistry: its evolution and criteria for its application in the study of epon-embedded cells and tissue. Am J Anat. 1986;175:135–160. doi: 10.1002/aja.1001750205. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi S, Chan C C, Gazzinelli R T, Roberge F G. Contribution of nitric oxide to the host parasite equilibrium in toxoplasmosis. J Immunol. 1996;156:1476–1481. [PubMed] [Google Scholar]
- 16.Kasper L H, Crabb J H, Pfefferkorn E R. Purification of a major membrane protein of Toxoplasma gondii by immunoabsorption with a monoclonal antibody. J Immunol. 1983;130:2407–2412. [PubMed] [Google Scholar]
- 17.Kasper L H, Currie K M, Bradley M S. An unexpected response to vaccination with a purified major membrane tachyzoite antigen (P30) of Toxoplasma gondii. J Immunol. 1985;134:3426–3431. [PubMed] [Google Scholar]
- 18.Kasper L H. Identification of stage-specific antigens of Toxoplasma gondii. Infect Immun. 1989;57:668–672. doi: 10.1128/iai.57.3.668-672.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kasper L H, Khan I A, Ely K H, Buelow R, Boothroyd J C. Antigen-specific (p30) mouse CD8+ T cells are cytotoxic against Toxoplasma gondii-infected peritoneal macrophages. J Immunol. 1992;148:1493–1498. [PubMed] [Google Scholar]
- 20.Khan I A, Ely K H, Kasper L H. A purified parasite antigen (p30) mediates CD8+ T cell immunity against fatal Toxoplasma gondii infection in mice. J Immunol. 1991;147:3501–3506. [PubMed] [Google Scholar]
- 21.Kurtz S, Rossi J, Petko L, Lindquist S. An ancient developmental induction: heat-shock proteins induced in sporulation and oogenesis. Science. 1986;231:1154–1157. doi: 10.1126/science.3511530. [DOI] [PubMed] [Google Scholar]
- 22.Langermans J A M, Van Der Hulst M E B, Nibbering P H, Hiemstra P S, Fransen L, Van Furth R. IFN-γ induced l-arginine-dependent toxoplasmastatic activity in murine peritoneal macrophages is mediated by endogenous tumor necrosis factor-α. J Immunol. 1992;148:568–574. [PubMed] [Google Scholar]
- 23.Luft B J, Brooks R G, Conley F K, McCabe R E, Remington J S. Toxoplasmic encephalitis in patients with acquired immune response deficiency syndrome. JAMA. 1984;252:913–917. [PubMed] [Google Scholar]
- 24.Mcleod R, Mack D, Brown C. Toxoplasma gondii: new advances in cellular and molecular biology. Exp Parasitol. 1991;72:109–121. doi: 10.1016/0014-4894(91)90129-k. [DOI] [PubMed] [Google Scholar]
- 25.Newport G, Culpepper J, Agabian N. Parasite heat-shock proteins. Parasitol Today. 1988;4:306–312. doi: 10.1016/0169-4758(88)90111-1. [DOI] [PubMed] [Google Scholar]
- 26.Pavesio C E N, Chiappino M L, Setzer P Y, Nichols B A. Toxoplasma gondii: differentiation and death of bradyzoites. Parasitol Res. 1992;78:1–9. doi: 10.1007/BF00936173. [DOI] [PubMed] [Google Scholar]
- 27.Polla B S. Heat shock proteins in host-parasite interactions. Immunol Today. 1991;12:A38–A41. doi: 10.1016/S0167-5699(05)80011-8. [DOI] [PubMed] [Google Scholar]
- 28.Reiner D S, Shinnick T M, Ardeshir F, Gillin F D. Encystation of Giardia lamblia leads to expression of antigens recognized by antibodies against conserved heat shock proteins. Infect Immun. 1992;60:5312–5315. doi: 10.1128/iai.60.12.5312-5315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scharton-Kersten T M, Yap G, Sher A. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med. 1997;185:1261–1273. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlesinger M J. Heat shock proteins. J Biol Chem. 1990;256:12111–12114. [PubMed] [Google Scholar]
- 31.Sharma H M, Kauffman M, Mcgaughy V R. Improved immunoperoxidase staining using microwave slide drying. Lab Med. 1990;21:658–660. [Google Scholar]
- 32.Sibley L D, Adams L, Fukutomi Y. Tumor necrosis factor-α triggers antitoxoplasmal activity of IFN-γ primed macrophages. J Immunol. 1991;147:2340–2345. [PubMed] [Google Scholar]
- 33.Soete M, Fortier B, Camus D, Dubremetz J F. Toxoplasma gondii: kinetics of bradyzoite-tachyzoite interconversion in vitro. Exp Parasitol. 1993;76:259–264. doi: 10.1006/expr.1993.1031. [DOI] [PubMed] [Google Scholar]
- 34.Soete M, Camus D, Dubremetz J F. Experimental induction of bradyzoite-specific antigen expression and cyst formation by the RH strain of Toxoplasma gondii in vitro. Exp Parasitol. 1994;78:361–370. doi: 10.1006/expr.1994.1039. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki Y, Orellana M A, Schreiber R D, Remington J S. Interferon-γ: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki Y, Joh K, Orellana M A, Conley F K, Remington J S. A gene(s) within the H-2D region determines the development of toxoplasmic encephalitis in mice. Immunology. 1991;74:732–739. [PMC free article] [PubMed] [Google Scholar]
- 37.Van Der Ploeg L H T, Giannini S H, Cantor C R. Heat shock genes: regulatory role for differentiation in parasitic protozoa. Science. 1985;228:1443–1446. doi: 10.1126/science.4012301. [DOI] [PubMed] [Google Scholar]
- 38.Weiss L M, Laplace D, Takvorian P, Tanowitz H B, Wittner M. The association of the stress responses and Toxoplasma gondii bradyzoite development. J Eukaryot Microbiol. 1996;43:120S. doi: 10.1111/j.1550-7408.1996.tb05036.x. [DOI] [PubMed] [Google Scholar]
- 39.Woodison G, Smith J E. Identification of the dominant cyst antigens of Toxoplasma gondii. Parasitology. 1990;100:389–392. doi: 10.1017/s0031182000078665. [DOI] [PubMed] [Google Scholar]