Abstract
Small cell lung cancer (SCLC) is a highly aggressive tumor type for which limited therapeutic progress has been made. Platinum-based chemotherapy with or without thoracic radiotherapy remains the backbone of treatment, but most patients with SCLC acquire therapeutic resistance. Given the need for more effective therapies, better elucidation of the molecular pathogenesis of SCLC is imperative. The phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) pathway is frequently activated in SCLC and strongly associated with resistance to ionizing radiation in many solid tumors. This pathway is an important regulator of cancer cell glucose metabolism, and its activation probably effects radioresistance by influencing bioenergetic processes in SCLC. Glucose metabolism has three main branches—aerobic glycolysis, oxidative phosphorylation, and the pentose phosphate pathway—involved in radioresistance. The interaction between the PI3K/AKT/mTOR pathway and glucose metabolism is largely mediated by hypoxia-inducible factor 1 (HIF-1) signaling. The PI3K/AKT/mTOR pathway also influences glucose metabolism through other mechanisms to participate in radioresistance, including inhibiting the ubiquitination of rate-limiting enzymes of the pentose phosphate pathway. This review summarizes our understanding of links among the PI3K/AKT/mTOR pathway, hypoxia, and glucose metabolism in SCLC radioresistance and highlights promising research directions to promote cancer cell death and improve the clinical outcome of patients with this devastating disease.
Keywords: Small cell lung cancer, PI3K/AKT/mTOR pathway, Glucose metabolism, Radioresistance
Highlights
-
•
The phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) pathway was frequently activated in small cell lung cancer (SCLC) and was closely associated with radioresistance.
-
•
This pathway can be involved in regulating glucose metabolism, which can affect radioresistance by influencing energy metabolism.
-
•
The interaction between the PI3K/AKT/mTOR pathway and glucose metabolism was primarily mediated by hypoxia-inducible factor 1 (HIF-1) signaling, and this pathway may also affect glucose metabolism through other mechanisms, including inhibition of the ubiquitinated degradation of the rate-limiting enzyme of the pentose phosphate pathway (G6PD).
Graphical abstract
Introduction
Lung carcinoma is a serious public health problem globally with high morbidity and mortality, giving rise to high socio-economic pressure.1 Small cell lung cancer (SCLC) accounts for about 15% of lung cancers and has the highest-grade malignancy among all subtypes of lung cancer.2 SCLC can be either limited-stage (LS-SCLC) or extensive-stage (ES-SCLC). Concurrent chemoradiotherapy (CCRT) remains the standard of care for LS-SCLC.2,3 Despite the high initial response rate to chemotherapy and radiotherapy, therapeutic resistance almost always occurs in SCLC, followed by recurrence or disease progression.2,4 The resistance of SCLC to radiotherapy remains an important challenge given the low 5-year survival rate in patients with this devastating disease.5 Given the requirement for more effective therapies, delineation of the molecular pathogenesis of SCLC is imperative.
The phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) signaling is activated following ionizing radiation, which increases cancer cell survival. This pathway was also found to be activated in patients with relapsed SCLC who previously received CCRT compared with that in treatment-naïve patients.6 Furthermore, the PI3K/AKT/mTOR pathway regulates hypoxia-inducible factor 1 (HIF-1)α expression through phosphorylation of two downstream effectors: ribosomal protein S6 kinase (p70S6K) and eukaryotic translation initiation factor 4E (eIF-4E)-binding protein 1 (4E-BP1).7,8 mTOR contains two structurally different multiprotein complexes—mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2)—which promote aerobic glycolysis, glutaminolysis, and the pentose phosphate pathway (PPP) by regulating the expression and activity of transcription factors such as HIF-1α and myelocytomatosis oncogene (MYC) and either activating or inactivating metabolic enzymes (such as glucose transporters, hexokinase, and glutamine synthetase).9, 10, 11
Hypoxia frequently occurs in solid tumors and can prevent fixation of DNA damage by oxygen, believed to be the mechanism underlying radioresistance.12 In addition, the hypoxic microenvironment can activate the HIF-1 pathway, which helps cancer cells avoid death via upregulation of their glucose uptake and utilization.13,14 Hypoxia is also involved in cancer cell proliferation, angiogenesis, metastasis, and pH regulation, which can coordinate to reduce the efficacy of radiation treatment, eventually leading to cancer relapse.14
Cancer cells preferentially utilize glycolysis even in the presence of oxygen, producing large quantities of lactic acid as the metabolic byproduct, known as the Warburg effect. High glycolytic states of cancers significantly correlate with radiation resistance and aggressive biological behavior.15,16 The Warburg effect is involved in resistance to radiotherapy and/or chemotherapy.17 The PPP is another branch of glucose metabolism, and tumor cells upregulate the PPP to produce reductive metabolites and detoxify chemoradiotherapy-induced reactive oxygens species (ROS).18,19 Therefore, targeting the glucose metabolism of tumor cells is a potential therapeutic approach for improving the efficacy of radiation therapy.
In this review, we summarize and discuss how the PI3K/AKT/mTOR and HIF-1 pathways influence SCLC radioresistance by modifying glucose metabolism in cancer cells. We also discuss suppression of the PI3K/AKT/mTOR pathway, hypoxia, and glucose metabolism as a potential therapeutic strategy to overcome this radioresistance.
Radioresistance of SCLC cells
CCRT remains the standard first-line treatment for LS-SCLC, and thoracic radiotherapy can improve the local control rate, reduce recurrence risk, and prolong survival for patients with ES-SCLC.20,21 Direct and indirect effects can be induced by applying ionizing radiation especially in rapidly proliferating tumor cells. The former refers to direct interactions between particles and relevant macromolecules, whereby the damage can usually be repaired. The latter plays a dominant role in biological damage, which leads to interactions between water and oxygen molecules near and inside tumor cells. Subsequently, ROS, including superoxide and hydroxyl radicals, are produced. These accumulate to cause irreversible damage to DNA and other macromolecules in tumor cells and provoke apoptosis by inducing cellular stress response and activating the intrinsic apoptosis cascade.22,23 Although an initial high sensitivity to chemotherapy and radiotherapy is observed, treatment resistance almost always develops in patients with SCLC. Resistance to radiation is a complex phenomenon that requires further elucidation; nonetheless, two potential explanations can be given. First, the surviving cancer cells were forced to adapt and acquire resistance under the multiple exposures of ionizing radiation. Second, cancer cells with high radiosensitivity are killed by radiation, but those with low radiosensitivity survive, proliferate rapidly, and become predominant. Preclinical and clinical studies have revealed some potential mechanisms underlying radioresistance, including alteration of DNA repair capacity, gene expression regulation, cell cycle arrest, autophagy induction, cancer cell glucose metabolism reprogramming, and cancer stem cell activity.24, 25, 26
Role of the PI3K/AKT/mTOR pathway, hypoxia, glucose metabolism, and radiotherapy in SCLC radioresistance
PI3K/AKT/mTOR pathway in SCLC
The PI3K/AKT/mTOR signaling pathway is essential to the cell cycle, cell growth, cell survival, glucose metabolism, and protein synthesis [Figure 1]. This pathway is frequently altered across various malignancies, including SCLC, breast cancer, and ovarian cancer. PI3Ks belong to the intercellular lipid kinase family, which phosphorylates the 3′-OH functional group of phosphatidylinositol. PI3Ks have three different subtypes, subtype 1 being the most important, as it can transform phosphatidylinositol biphosphate (PIP2) into phosphatidylinositol triphosphate (PIP3). As a secondary messenger, PIP3 recruits and activates kinases with the pleckstrin homology domain, such as PI3K-dependent kinase 1 (PDK1). Phosphatase and tensin homology (PTEN) is a tumor-suppressor gene that dephosphorylates PIP3 and is an important negative regulator of the PI3K/AKT/mTOR pathway.27,28 PTEN mutation or deletion frequently occurs in malignancies, and its epigenetic silencing has been reported to induce overactivation of the PI3K/AKT/mTOR pathway in various cancers.28
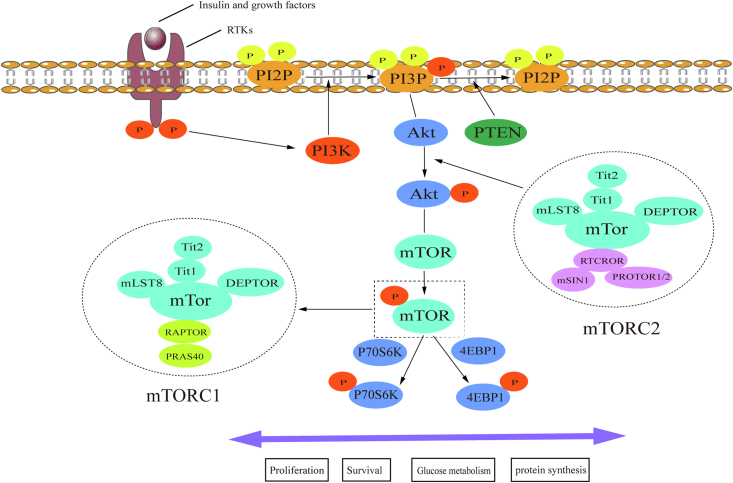
Figure 1.
The PI3K/AKT/mTOR pathway. Insulin or growth factors trigger PI3K activation, which causes PIP2 phosphorylation and PIP3 production. PTEN antagonizes the kinase activity of PI3K by dephosphorylating PIP3. As a phosphate source, PIP3 transfers a phosphate group to AKT and activates it. AKT contains different regulatory sites that can be phosphorylated by mTORC2. As a kinase, AKT phosphorylates downstream targets such as mTORC1, which eventually regulates cell proliferation, survival, glucose metabolism, and protein synthesis through p70S6K and 4EBP1 phosphorylation. PI3K: Phosphoinositide 3-kinase; AKT: Protein kinase B; mTOR: Mammalian target of rapamycin; PIP2: Phosphatidylinositol-4,5-bisphosphate; PIP3: Phosphatidylinositol-3,4,5-trisphosphate; mTORC: mTOR complex.
As a downstream kinase of PI3K, AKT is completely activated when its T308 and S473 residues are phosphorylated by mTORC2 and PDK1. This then causes phosphorylation of enzymes such as glycogen synthase kinase 3 beta (GSK3β), hexokinase 2 (HK2), murine double minute 2 proto-oncogene (MDM2), tuberous sclerosis 2 (TSC2), B-cell chronic lymphocytic leukemia/lymphoma 2 (BCL2) associated agonist of cell death (BAD), mTOR, and HIF-1.29,30 AKT is involved in a wide range of cellular processes, including glucose metabolism and cell proliferation and survival. mTOR is a downstream kinase of the PI3K/AKT pathway and contains two kinds of multiprotein complexes: mTORC1 and mTORC2 [Figure 1].
mTORC1 is a rapamycin- and nutrient-sensitive complex that plays an important role in cell growth, proliferation, and survival by regulating the expression of many proteins via mTORC1-induced phosphorylation of p70S6K and 4EBP1.30 Phospho-mTOR (a marker of mTOR kinase activity) and phospho-p70S6K (a downstream target of mTORC1) is present in 55% and 91% of SCLC tumors, respectively.31,32 Through immunohistochemical analysis, 66% of SCLC tissues were found to be positive for phospho-4EBP1 (another downstream target of mTORC1), and non-smoking patients and those with metastasis were found to have higher positivity rates for p-4EBP1. In addition, patients with higher levels of p-4EBP1 had less favorable survival (P = 0.016).33
mTORC2 regulates cytoskeletal organization and is insensitive to rapamycin. This complex also indirectly affects glucose metabolism and cell proliferation and survival by phosphorylating and activating AKT.30 Rapamycin-insensitive companion of mTOR (RICTOR), encoding a scaffold protein of mTORC2, was found to be the most frequently amplified gene in 10%–15% of patients with SCLC, using next-generation sequencing or fluorescence in situ hybridization.31,34 Furthermore, the copy number variation of RICTOR was correlated with its protein expression level in SCLC cell lines.35 The positivity rate for RICTOR and phospho-AKT (a downstream kinase of mTORC2) was found to be 37% and 42%, respectively, using immunohistochemical analysis.31 Chemotaxis and scratch wound assays also revealed that SCLC cell lines with RICTOR amplification could migrate more quickly and were more susceptible to mTOR inhibitors. Furthermore, patients with RICTOR amplification had considerably worse survival than those without (P = 0.021).35
Genomic profiling studies have greatly improved our understanding of the molecular characteristics of SCLC.34,36,37 Comprehensive genomic studies have revealed that 7%–36% of SCLC tumors harbor PTEN, PIK3CA, AKT2, AKT3, RICTOR, and MTOR mutations.37,38 In a bioinformatic analysis of 130 blood samples from patients with ES-SCLC, those harboring PI3K/AKT/mTOR alterations were associated with a higher blood tumor mutational burden.39 A recent study found that PI3K/AKT/mTOR pathway activation contributes to phenotypic switching from suspension to adhesion or semi-adhesion growth pattern and confers SCLC cells with resistance to chemotherapy.40 microRNA (miRNA, miR) polymorphisms in the PI3K/AKT/mTOR pathway are important prognostic factors among patients with LS-SCLC, and three single-nucleotide polymorphisms—MTOR: rs2536 (T > C), PIK3R1: rs3756668 (A > G), and PIK3R1: rs12755 (A > C)—are correlated with favorable prognosis.41
Role of the PI3K/AKT/mTOR pathway in radioresistance
Understanding the mechanisms underlying radioresistance is relevant for exploring new therapeutic strategies. With the rapid development of advanced analytical methods, including genomics and proteomics, progress has been made in the exploration of radioresistance-associated signaling pathways. The effects of activating the PI3K/AKT/mTOR pathway on radioresistance have been extensively studied. miR-410 contributes to epithelial-mesenchymal transition (EMT) and resistance to ionizing radiation by activating PI3K/AKT/mTOR signaling in non-SCLC (NSCLC) both in vitro and in vivo.42 Through label-free quantitative liquid-chromatography/tandem-mass spectrometry, this pathway was found to be the most activated pathway correlated with radioresistance in three prostate cancer cell lines resistant to ionizing radiation.43 A recent study that compared paired SCLC samples from 11 patients with LS-SCLC at diagnosis and relapse using genomic analyses demonstrated that genes belonging to the PI3K/AKT signaling pathway were significantly enriched in the relapse samples.6
The PI3K/AKT/mTOR pathway may induce resistance to ionizing radiation in tumor cells via neoangiogenesis, cell cycle, DNA repair, and hypoxia. Ionizing radiation causes reoxygenation and neoangiogenesis in tumor cells, and this is partially due to vascular endothelial growth factor (VEGF) upregulation through HIF-1α activation regulated by the PI3K/AKT/mTOR pathway. Then, VEGF preserves endothelial cells against radiation-induced damage by activating the PI3K/AKT/mTOR pathway, causing upregulation of anti-apoptotic proteins, including Bcl2.44 Antiangiogenesis could cause vasculature normalization, enhancing perfusion and alleviating hypoxia, thus improving the cytotoxic effects of radiation. Some compounds, including PI3K/AKT inhibitors, can disrupt the vasculature through direct or indirect inhibition of VEGF. A combination of low-dose LY294002 (PI3K inhibitor) and cisplatin considerably improved the efficacy of radiotherapy by decreasing neovascularization.45 Ionizing radiation can lead to p53-dependent or p53-independent G1 and G2 arrest of the cell cycle, but the PI3K/AKT/mTOR pathway overrides the p53-independent cell cycle arrest by activating cyclin D and inactivating the cell cycle-dependent kinase inhibitor p27.46 DNA damage repair depends on three important kinases—DNA-dependent protein kinase catalytic subunit (DNA-PKcs), ataxia-telangiectasia mutated (ATM), and ATM- and RAD3-related proteins—and the dual PI3K/mTOR inhibitor NVP-BEZ235 can potently inhibit ATM- and DNA-PKC-mediated DNA double-strand break repair, increase DNA damage in cancer cells, and enhance the cytotoxic effect of radiotherapy.47,48
Hypoxia in SCLC
Hypoxia, involving low oxygenation levels, is one of the most important hallmarks of cancer; oxygen levels in cancer are <2%, whereas normal oxygen concentration is 3.2–12.3%. Hypoxia is mainly caused by the rapid proliferation and dysfunctional vascularization of and consumption of available oxygen in tumor cells.49 The HIF-1 pathway is activated in cancer cells exposed to a hypoxic microenvironment. HIF-1 is an important transcription factor consisting of HIF-1α and HIF-1β. HIF-1β shows constitutive expression, but the expression and function of HIF-1α are largely controlled by the oxygen level. Under low oxygen concentrations, HIF-1α avoids degradation and accumulates to help cancer cells resist the temporary stress. Activation of HIF-1 signaling can upregulate the transcription of many genes in glucose metabolism, such as those encoding glucose transporters (GLUTs), glycolytic enzymes, and carbonic anhydrases. Regulation of these genes in tumor cells leads to metabolic conversion from aerobic respiration to glycolysis and utilization of the PPP. Although not all cancer cells are exposed to hypoxia, the adaptive response to hypoxia confers more aggressive and therapy-resistance properties to cancer cells.50,51
Patients with SCLC frequently experience breathing difficulty due to obstruction by tumors, pleural effusion, chronic obstructive pulmonary disease, and tar from cigarettes, causing insufficient oxygenation in blood and tissues. Additionally, chemotherapy often induces anemia in patients, decreasing their ability to transport oxygen in the blood.52 Histological analysis of biopsy samples has shown hypoxic regions in more than 50% of patients newly diagnosed with SCLC, and this value may be higher when considering the small size of samples analyzed and the failure to reveal full intra-tumor heterogeneity.53 The presence of hypoxic regions in SCLC is strongly associated with tumor progression and unfavorable survival, and HIF-1α is an independent prognostic factor (P < 0.003) among patients with SCLC, even after adjusting for clinical parameters.54 Moreover, HIF-2α is correlated with shorter survival in patients with SCLC,55 so both HIF-1α and HIF-2α are attractive molecular targets in SCLC.
Role of hypoxia in radioresistance
Ionizing radiation kills tumor cells primarily by inducing ROS production under oxygenated conditions. In hypoxic cells, ROS formation is limited, DNA damage can be repaired, and death can be avoided.56 Therefore, tumor cells in a well-oxygenated environment are far more sensitive to ionizing radiation than hypoxic cells. To measure this phenomenon, the oxygen enhancement ratio, which is the ratio of radiation doses delivered under hypoxia to normal oxygen concentration required to achieve the same biological end-points, can be utilized. In fact, radiation doses in hypoxic cells are evidently higher than those in normoxic cells to reach the same mortality rate. Tumor cells in a hypoxic environment are two to three times more resistant to ionizing radiation than those in a normoxic environment.49,57 The concentration of and duration of exposure to oxygen are important factors in this regard. To achieve efficient cytotoxicity, an adequate molecular oxygen level is required during radiation therapy or at least during the lifetime of the ROS induced by ionizing radiation.
Moreover, the cell cycle affects sensitivity to radiation, and hypoxia can influence cell cycle progression, during which the radioresistance of tumor cells is dynamically altered. Cancer cells in the G2-M phase are most sensitive to radiotherapy, those in the G1 phase are less radiosensitive, and those in the S phase are least radiosensitive.58 Under hypoxic conditions, S-phase entry is hindered by HIF-1α through its regulation of genes encoding proteins in cell cycle regulation. p21 and p27, important cyclin-dependent kinase inhibitors, are significantly upregulated under hypoxia, leading to cell cycle arrest and reduced radiotherapy efficacy.59
Role of glucose metabolism in SCLC
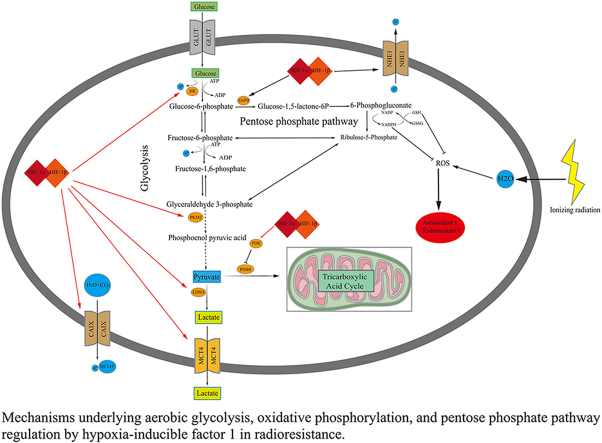
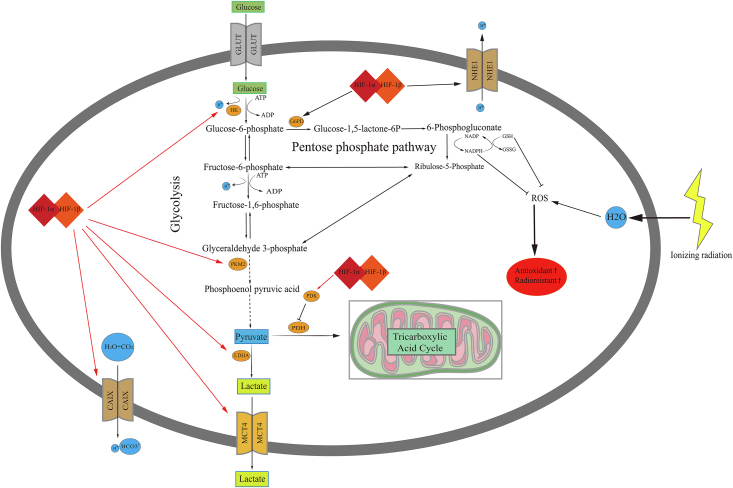
To meet their energy and biosynthetic demands, cancer cells prefer glycolysis over oxidative phosphorylation (OXPHOS), even in the presence of oxygen. Using fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT), the significant increase in glucose uptake by cancer cells has been used to evaluate therapy response and diagnose SCLC, particularly LS-SCLC.60,61 According to FDG-PET/CT results, maximum or integrated standardized values of glucose uptake, metabolic tumor volume, and total lesion glycolysis are negatively associated with the prognosis of patients with SCLC.62 Glucose metabolism promotes cell proliferation and growth by rapidly producing energy, generating intermediate products used to synthesize nucleotides, amino acids, and lipids, and maintaining redox homeostasis.63 The major source of nicotinamide adenine dinucleotide phosphate (NADPH) in the cytoplasm is the PPP, which is required to scavenge cellular ROS and eventually enhance the antioxidant capacity of tumor cells [Figure 2].18,19 Hence, glucose metabolism, mainly comprising glycolysis, the PPP, and the citric acid cycle, shows potential and is attracting growing attention as a therapeutic target in SCLC.
Figure 2.
Mechanisms underlying aerobic glycolysis, OXPHOS, and PPP regulation by HIF-1 in radioresistance. ADP: Adenosine diphosphate; ATP: Adenosine triphosphate; CAIX: Carbonic anhydrase 9; G6PD: Glucose-6-phosphate dehydrogenase; GLUT: Glucose transporters; GSH: Glutathione; GSSG: Glutathione disulfide; HIF-1: Hypoxia-inducible factor-1; HK: Hexokinase; LDHA: Lactate dehydrogenase A; MCT4: Monocarboxylate transporter 4; NHE1: Naþ/Hþ exchanger isoform 1; OXPHOS: Oxidative phosphorylation; PDH: Pyruvate dehydrogenase; PDK: Pyruvate dehydrogenase kinase; PPP: Pentose phosphate pathway; ROS: Reactive oxygen species.
Elevated glucose uptake and lactate production are crucial hallmarks of glucose metabolism in cancer cells. Specific genetic alterations can cause metabolic liabilities in tumors. Overexpression of genes with oncogenic functions, including c-MYC, RAS, PI3K, and AKT, increases glucose uptake and upregulates transporters or enzymes involved in aerobic glycolysis and/or the PPP.64 Conversely, p53 serves as a tumor suppressor by inhibiting glycolysis or the PPP and promoting OXPHOS.65 TP53 is either deleted or mutated across various tumors, including SCLC. According to comprehensive genomic profiling, 90% of SCLC cases lack functional p53,66 so its regulation of glucose metabolism is mostly lacking. Moreover, amplification or overexpression of MYC and other genetic alterations leading to overactivation of the PI3K/AKT/mTOR pathway are frequently observed in patients with SCLC. Thus, a large proportion of SCLC tumors exhibit high states of glycolysis and/or PPP.
A recent study divided SCLC cells into two groups—MYCHigh and MYCLow—and explored the differences between glycolysis and OXPHOS both in vitro and in vivo.67 The MYCHigh group was sensitive to glycolysis inhibition, whereas the MYCLow group remained primarily unresponsive, suggesting that MYC defined the predominant metabolic phenotype. Hence, it was concluded that SCLC tumors with high MYC expression strongly depend on glycolysis, whereas those with either low or no MYC expression mainly exhibit the OXPHOS phenotype. In addition, correlations between metabolites and prognosis in SCLC cases have been reported. A retrospective analysis of 98 patients with SCLC who underwent pretreatment FDG-PET/CT revealed that increased levels of serum lactate dehydrogenase (LDH), which catalyzes the final stage of aerobic glycolysis, were associated with overall survival (OS) (hazard ratio [HR] = 1.8, 95% confidence interval [CI]: 1.16–2.77, P = 0.008) and progression-free survival (HR = 1.71, 95% CI: 1.11–2.64, P = 0.015).68 Recently, a similar study on 234 patients with SCLC also demonstrated the independent prognostic value of LDH and reported patients with pretreatment LDH ≥215.70 U/L to have significantly unfavorable survival (HR = 1.468, 95% CI: 1.069–2.017, P = 0.018).69 In another study, pretreatment LDH level was negatively correlated with relapse-free survival (HR = 2.8, 95% CI: 1.03–7.52, P = 0.043), but LDH level and glucose transporter 1 (GLUT-1) were associated with a higher objective response rate (ORR) among 41 patients with SCLC who underwent CCRT.70
Role of glucose metabolism in radioresistance
Emerging evidence has revealed that tumor cells in which glycolysis activity and/or the PPP are significantly upregulated are less sensitive to radiotherapy and have more aggressive phenotypes. Irradiation-induced ROS not only damages cancer cell DNA but also elevates the glucose uptake by tumor cells during reoxygenation; a kind of antioxidant superoxide dismutase (SOD) mimic was found to evidently reduce the glucose uptake induced by irradiation and suppress the glycolytic switch.71 Tyrosine phosphorylation caused by the SRC proto-oncogene, non-receptor tyrosine kinase (SRC) family kinase Fyn was shown to increase 6-phosphogluconate dehydrogenase activity in glioma by enhancing its binding affinity to NADP+ and activating the PPP to produce NADPH and ribose-5-phosphate, which detoxify intracellular ROS and promote DNA synthesis, finally leading to glioma cell proliferation and radioresistance.18 To explore clinically relevant radioresistance, many radioresistant cell models have been established, and cancer cells are frequently exposed to x-rays 15–30 times (sustained irradiation) to induce radioresistance. Radioresistant head and neck squamous cells presented significantly different metabolic alterations in which glucose uptake and fatty acid biosynthesis were enhanced, the PPP was upregulated, and OXPHOS was downregulated.72 Moreover, radioresistant glioblastoma multiforme (GBM) cells showed a higher glycolytic state and increased stemness through the Spy1-(CAP-Gly Domain Containing Linker Protein 3)CLIP3 axis, and rescuing CLIP3 expression was shown to be a potential approach for reversing GBM radioresistance.26
A recent study using proteomic, metabolomic, and metabolic flux analyses suggested that compared with their parental cells, radioresistant hepatocellular carcinoma cells exhibited elevated glucose flux and higher mRNA levels of genes involved in glycolysis, including HK2 and GLUT-1, causing cardiolipin upregulation, which reduces the efficacy of irradiation by suppressing the release of cytochrome C to activate the apoptosis cascade.13 miR-223-3p was reported to contribute to radioresistance in prostate cancer by increasing glycolysis via targeting Forkhead Box O3 (FOXO3a).73 Patients with oral squamous cell carcinoma with higher GLUT-1 expression were less responsive to preoperative radiation therapy at 36 Gy than those with lower GLUT-1 expression (P = 0.005).74
To the best of our knowledge, no study related to SCLC cells with secondary radioresistance has been published yet. CPH 54A and CPH 54B are distinct SCLC cell subpopulations established from the same patient. Despite their common genomic origin, the cell lines exhibit distinct phenotypes.75 CPH 54B has stronger radioresistance than CPH 54A both in vivo and in vitro. Furthermore, CPH 54B tumors have lower oxygen consumption, steady-state adenosine triphosphate (ATP) levels, cellular ATP concentrations, and higher lactate production than CPH 54A tumors. Interestingly, CPH 54B tumors have higher mRNA and protein levels of GLUT-1 and correspondingly higher FDG uptake both in vitro and in vivo, suggesting the glycolytic predominance of CPH 54B.76
The PI3K/AKT/mTOR pathway and hypoxia
PI3K activation upregulates HIF-1α protein expression and plays an important role in regulating protein synthesis through its target AKT and downstream component mTOR. eIF-4E is the mRNA 5′ cap-binding protein that participates in the translation of many proteins. The family of repressor proteins, 4E-binding proteins (4E-BPs), control eIF-4E activity, and the phosphorylation status of 4E-BPs determine whether they bind to eIF-4E. On the one hand, mTOR mediates its action by phosphorylating 4E-BP1, after which 4EBP1 is released from eIF-4E, leading to the failure of inhibiting cap-dependent mRNA translation and initiation of cap-dependent translation.77,78 On the other hand, mTOR causes the phosphorylation of p70S6K and then phosphorylates its substrate, the ribosomal protein S6, inducing the translation of many proteins [Figure 1].78 Based on the understanding of these mechanisms, it was concluded that HIF-1 translation could be greatly increased in tumor cells by activating the PI3K/AKT/mTOR pathway.
Wan et al. revealed that HIF-1α promoted proliferation and angiogenesis of residual SCLC (NCI–H446 cell line) and NSCLC (NCI–H1650 cell line) cells following hyperthermia treatment. In NSCLC, HIF-1α expression was shown to be controlled by both the PI3K/AKT and extracellular signal-regulated kinase (ERK) pathways, whereas, in SCLC, HIF-1α levels were reported to be controlled by only the PI3K/AKT pathway.79 The suppressor of cytokine signaling 3 (SOCS3) was reported to target the PI3K/AKT pathway to block HIF-1α expression and then inhibit the proliferation and angiogenesis of SCLC cells.80 Moreover, cytochalasin H was found to reduce HIF-1α protein accumulation and downregulate VEGF through the PI3K/AKT/P70S6K and ERK1/2 pathways to suppress angiogenesis in NSCLC cells.81 Similarly, Huaier downregulated HIF-1α and inhibited glycolysis in lung cancer both in vivo and in vitro, including glucose transport and lactic acid production, by inactivating the PI3K/AKT pathway.82
Hypoxia and reprogramming of glucose metabolism
Hypoxia is strongly associated with many metabolic adaptations in cancer that promote tumor cell growth and angiogenesis, an example of which is the adaptation of glucose metabolism. Glucose metabolism is also closely associated with glutamine and fatty acid metabolism. Hypoxia affects three main branches of glucose metabolism in tumor cells: the PPP, glycolysis, and OXPHOS [Figure 2]. First, HIF-1α is a positive transcriptional regulator of rate-limiting enzymes for the PPP, including glucose-6-phosphate dehydrogenase (G6PD). Glucose-6-phosphate (G6P), the substrate of G6PD, is utilized in the PPP as well as glycolysis to synthesize many key nucleotides (including ribonucleotides and deoxyribonucleotides) and amino acid precursors in cancer cell proliferation and growth. A recent study revealed that HIF-1α could regulate glucose metabolism and sensitivity to imatinib through the PPP in gastrointestinal stromal tumors.83 Upon exposure to low-dose radiation, normal human cells were shown to exhibit HIF-1α-mediated metabolic alterations with reduced mitochondrial gene expression and enhanced expression of genes encoding glucose transporters and glycolysis and PPP enzymes.84 Conversely, glucose metabolism through the PPP, rather than glycolysis, contributed to the stabilization of the HIF-1α protein under hypoxia.85
Second, 14 kinds of glucose transporters play important roles in glucose uptake, the first step of aerobic glycolysis. The most representative example is GLUT-1, an important transcriptional target of HIF-1α. Genetic alterations and hypoxia were found to increase GLUT-1 expression in a HIF-1α-dependent manner, leading to increased glucose uptake and contributing to glycolysis and angiogenesis in SCLC cells.86 Besides GLUTs, other important rate-limiting enzymes of glycolysis, such as pyruvate kinase M2 (PKM2), hexokinases (HKs), and LDHA, are positively regulated by HIF-1α. Hypoxia was shown to induce cisplatin resistance in NSCLC cells via exosomal PKM2, which transmitted the cisplatin resistance to sensitive tumor cells both in vitro and in vivo by promoting glycolysis, more antioxidant metabolite generation, and lethal-ROS (induced by cisplatin) neutralization.87 Moreover, hypoxia increased HKII transcription in A549 NSCLC cells in a HIF-1-dependent manner.88 In aerobic glycolysis, pyruvate generated in the last step is converted to lactate instead of acetyl coenzyme A in a HIF-1α-dependent manner under the action of LDHA, causing cancer acidosis in hypoxic regions. Subsequently, under the action of monocarboxylate transporter 4 (MCT4), which is also regulated by HIF-1α, lactate is removed from the cytoplasm to maintain the acid-base equilibrium of the tumor cells [Figure 2]. The outcome was a relatively alkaline intracellular environment favored by tumor cells and an acidic extracellular environment, which promoted extracellular matrix breakdown and increased the potential for metastasis89 which is characteristic of SCLC.
Third, transient HIF-1α activation was shown to increase the levels of LDHA and the phosphorylated E1α subunit of pyruvate dehydrogenase (p-PDH-E1α) in lung cancer. This indicates that the switch in glucose metabolism from OXPHOS to glycolysis and lactic acid accumulation, and the HIF-1 inhibitor YC-1, suppressed the switch in glucose metabolism, elevated intratumoral ROS levels, and finally inhibited the metastasis of lung cancer.90
The PI3K/AKT/mTOR pathway and glucose metabolism
The PI3K/AKT/mTOR pathway can regulate glucose metabolism through HIF-1α and MYC, as discussed earlier. Other mechanisms of the PI3K/AKT/mTOR pathway in terms of regulating cancer glucose metabolism have also been identified. Cheng et al. reported that PI3K/AKT pathway activation inhibits ubiquitylation and degradation of G6PD, the key regulator of the PPP, by suppressing the E3 ligase TRIM21 and upregulating the PPP.91 These authors also found that metabolites of the PPP enhance AKT activation and further promote the rewiring of glucose metabolism by downregulating the AKT inhibitor PHLDA3.91 A recent study revealed that mTOR inhibition could reduce G6PD expression and NADPH levels and increase ROS levels, inducing ROS-dependent death in T-cell acute lymphoblastic leukemia.92 Furthermore, a combination of baicalein, wogonin, and oroxylin-A was reported to suppress EMT progress through PI3K/AKT-TWIST1-glycolysis signaling in NSCLC cells.93
Targeting the PI3K/AKT/mTOR pathway, hypoxia, and glucose metabolism to improve radiotherapy efficacy
PI3K/AKT/mTOR pathway inhibition
Many small molecules have been developed as inhibitors of the PI3K/AKT/mTOR pathway. These small molecules can be classified into two major types, namely, single inhibitors that only inhibit PI3K, AKT, or mTOR signaling proteins, and dual inhibitors that block PI3K and mTOR signaling. Blocking the PI3K/AKT/mTOR pathway could enhance the sensitivity of cancer cells to radiation therapy. Clinical trials of combined PI3K/AKT/mTOR inhibitors and radiotherapy in solid tumors are summarized in Table 1.
Table 1.
Summary of clinical trials of combined PI3K/AKT/mTOR inhibitors and radiotherapy in solid tumors.
| Name of inhibitors | Targets | Year | Tumor types | Number of participants | ClinicalTrials.gov identifier or EU Clinical Trials Register | Phase | Radiotherapy dose (fraction no. X radiation) | Clinical observations |
|---|---|---|---|---|---|---|---|---|
| Voxtalisib | PI3K/mTOR | 2015 | Stage III-IV NSCLC | 26 | EudraCT No.2007-001698-27 | I | 28–66 Gy in 14–33 fractions | Pulmonary toxicity should be carefully monitored. |
| Voxtalisib | PI3K/mTOR | 2015 | High-grade GBM | 54 | NCT00704080 | I | 1.8–2 Gy/fraction up to 60 Gy, 5 days/week | A favorable safety profile and a moderate level of PI3K/mTOR pathway inhibition were observed. |
| Buparlisib | PI3K | 2020 | Newly diagnosed GBM | 22 | NCT01473901 | I | 30 × 2 Gy | A challenging safety profile of buparlisib in combination with radiotherapy and temozolomide was observed. |
| Alpelisib | PI3K | 2020 | Locoregionally advanced HNSCC | 9 | NCT02537223 | I | 35 × 2 Gy | Alpelisib at 200 mg had a manageable safety profile in combination with cisplatin-based chemoradiation. |
| Alpelisib | PI3K | 2022 | Stage III-IVB HNSCC | 11 | NCT02282371 | I | 33 × 2.12 Gy or 35 × 2 Gy | The recommended dose of alpelisib is 250 mg/d in combination with cetuximab and intensity-modulated radiation therapy. |
| Nelfinavir | PI3K/AKT | 2016 | Advanced rectal cancer | 10 | EudraCT No.2010-020621-40 | NA | 5 × 5 Gy, 7 days | Nelfinavir combined with radiotherapy is well tolerated and is associated with increased blood flow to rectal tumors. |
| Temsirolimus | mTOR | 2014 | NSCLC | 10 | NA | I | 14 × 2.5 Gy, 5 days/week | The combination of temsirolimus 15 mg weekly and thoracic radiation is well tolerated. |
| Temsirolimus | mTOR | 2010 | Newly diagnosed GBM | 12 | NA | I | 30 × 2 Gy | The increased infection rate with temsirolimus combined with chemo-radiotherapy was observed. |
| Rapamycin | mTOR | 2007 | Stage III NSCLC | 7 | NA | I | 30 × 2 Gy | Combination therapy with rapamycin, radiation, and cisplatin was well tolerated. |
| Rapamycin | mTOR | 2015 | Primary resectable rectal cancer | 13 (Phase I), 31 (Phase II) | NCT00409994 | I/II | Short-course hypofractionated radiotherapy (5 × 5 Gy) | Obvious decrease in metabolic activity, but no significant pCR was found. |
| Everolimus | mTOR | 2017 | Locally advanced rectal cancer | 12 | EudraCT No.2010-022087-13 | Ib | (28 × 1.8 Gy), 5 days/week | The manageable safety profile of everolimus combined with standard chemoradiation. |
| Everolimus | mTOR | 2013 | High-risk locally advanced prostate cancer | 14 | NCT00943956 | I | 37 × 2 Gy | Concomitant hormone-radiotherapy and everolimus were well-tolerated, and the recommended MTD of everolimus is 5 mg/day. |
| Everolimus | mTOR | 2017 | Prostate cancer with biochemical recurrence | 18 | NA | I | 37 × 1.8 Gy | Everolimus at a dose of ≤10 mg/d is safe and tolerable in combination with fractionated post-prostatectomy radiotherapy. |
| Everolimus | mTOR | 2016 | Locally advanced cervix cancer | 13 | NCT01217177 | I | 25 × 1.8 Gy for radiotherapy and 4 × 6 Gy for brachytherapy | The MTD of everolimus in combination with cisplatin and radiotherapy was 5 mg/d. |
| Everolimus | mTOR | 2011 | Newly diagnosed GBM | 18 | NA | I | 30 × 2 Gy | Everolimus combined with radiation and temozolomide was reasonably well tolerated. |
| Everolimus | mTOR | 2015 | Newly diagnosed GBM | 100 | NA | II | 30 × 2 Gy | Combining everolimus with conventional chemoradiation had moderate toxicity. |
| Everolimus | mTOR | 2018 | Newly diagnosed GBM | 171 | NCT01062399 | II | 30 × 2 Gy | Combining everolimus with conventional chemoradiation increased treatment-related toxicities and did not improve PFS. |
AKT: Protein kinase B; GBM: Glioblastoma; HNSCC: Head and neck squamous cell carcinoma; MTD: Maximum tolerated dose; mTOR: Mammalian target of rapamycin; NSCLC: Non-small cell lung cancer; pCR: Pathologic complete response; PI3K: Pphosphoinositide 3-kinase.
The single PI3K inhibitor LY294002 enhances the cytotoxicity of low concentrations of etoposide by blocking AKT signaling in SCLC cells.94 Similarly, the dual PI3K/mTOR inhibitor GSK2126458 can synergistically inhibit SCLC proliferation when combined with cisplatin and etoposide.40 Furthermore, the dual PI3K and histone deacetylase inhibitor FK228 significantly potentiates the inhibitory effect of ionizing radiation on human radioresistant SCLC cells, mainly by inducing chromatin decondensation and impairing DNA repair competency.95
Hypoxia inhibition
Hypoxia target therapy focuses on applying bioreductive prodrugs and inhibiting molecular targets usually highly expressed in tumor cells. Oxygen itself is regarded as a radiosensitizer, and tumor-bearing mice are more susceptible to ionizing radiation when kept under oxygen pressure three times the normal atmospheric pressure compared with those under normal atmospheric pressure.96 Bioreductive prodrugs selectively target and kill hypoxic cells. These prodrugs are metabolized under the strongly reductive conditions of hypoxic regions and then release the active drugs cytotoxic to the hypoxic cells. Therefore, the potential of these prodrugs to increase radiotherapy and chemotherapy efficiency makes them highly attractive in cancer therapy. Tirapazamine is one of the best characterized clinical bioreductive prodrugs, and several preclinical studies have evaluated its synergistic efficacy with radiation treatment in lung cancer cell lines and mouse models.97 Tirapazamine and radiation have been combined to treat refectory solid tumors in several clinical trials. For example, S0004, a phase I clinical trial, evaluated the addition of tirapazamine to cisplatin/etoposide and once-daily thoracic radiotherapy among patients with LS-SCLC; the ORR was 80%, and the median OS was 22 months.98 Subsequently, the phase II trial SWOG 0222 was initiated but had to be terminated early because a parallel trial reported excessive toxicity.99 The results of SWOG 0222 were similar to those of S0004 in that the ORR was 63%, median progression-free survival was 11 months, and median OS was 21 months, but 46% of patients presented with grade 4 adverse events, mainly from hematologic toxicity. Although tirapazamine was not investigated further, targeting hypoxia for SCLC treatment remains an attractive prospect.
Many drugs have been found to suppress the expression of HIF-1α and its target genes and have entered clinical trials, as summarized in Table 2. A phase Ib/II clinical trial reported acceptable toxicity levels among patients with relapsed or refractory SCLC treated with ganetespib plus doxorubicin, highlighting the potential of ganetespib for SCLC treatment.100 Admittedly, there are some limitations to targeting HIF-1 in cancer treatment. First, owing to the multiple isoforms of HIF-1, a potential functional redundancy can limit the efficacy of targeting HIF-1. Second, HIF-1 is also expressed in non-hypoxic regions and normal tissues, which could lead to possible off-target effects. Third, 10%–20% of patients with SCLC present with brain metastasis at the time of initial diagnosis, and 50%–80% of patients finally develop brain metastasis during the therapeutic process.101 Therefore, the blood-brain barrier permeability of drugs targeting hypoxia should be comprehensively evaluated in preclinical and clinical studies.
Table 2.
Summary of drugs targeted against HIF-1 reaching clinical evaluation.
| Name of drug | Mechanism of action | Tumor types | Number of participants | ClinicalTrials.gov identifier | Phase |
|---|---|---|---|---|---|
| EZN-2968 | Antisense oligonucleotide inhibitor of HIF-1α | Advanced solid tumors with liver metastases | 9 | NCT01120288 | I |
| RO7070179 | HIF-1α mRNA antagonist | Hepatocellular carcinoma | 9 | NCT02564614 | I |
| PX-478 | Decrease HIF-1 accumulation by promoting ubiquitination and decreasing transcription and translation | Advanced solid tumors and lymphoma | 45 | NCT00522652 | I |
| AFP464 | Interfere HIF-1 mRNA | Advanced solid tumors | 60 | NCT00369200 | I |
| Topotecan | Suppress HIF-1 translation via targeting topoisomerase I | Refractory advanced solid neoplasms expressing HIF-1 alpha | 16 | NCT00117013 | I |
| Alvespimycin | Inhibit Hsp90, inducing HIF-1 degradation | Solid tumors and lymphomas | 40 | NCT00088868 | I |
| STA-9090 | Inhibit Hsp90, inducing HIF-1 degradation | Relapsed or refractory small cell lung cancer | 25 | NCT01173523 | II |
| MBM-02 | Inhibit both HIF-1α and HIF-2α | Prostate cancer in biochemical recurrence | 55 | NCT04876755 | II |
| CRLX101 | Inhibit the HIF→(CAIX) →VEGF→VEGFR2 pathway | Recurrent platinum-resistant ovarian, tubal, and peritoneal cancer | 63 | NCT01652079 | II |
| EZN-2208 | Suppress HIF-1 translation via targeting topoisomerase I | Metastatic breast cancer | 160 | NCT01036113 | II |
| Digoxin | Suppress HIF-1 translation | Newly diagnosed operable breast cancer | 6 | NCT01763931 | II |
α: Alpha; HIF-1: Hypoxia-inducible factor.
Glucose metabolism inhibition
Multiple drugs targeting pivotal enzymes or transporters in glucose metabolism have been identified, some of which have entered preclinical and/or clinical trials.63,64 Clinical trials of these drugs in combination with radiotherapy are summarized in Table 3. WZB117 is a selective inhibitor of GLUT-1 shown to inhibit glucose metabolism in breast cancer; it also re-sensitizes radioresistant breast cancer cells to radiation.102 Lonidamine is the first orally administered small-molecule inhibitor of mitochondria-bounded hexokinase to suppress glycolysis. 2DG is structurally similar to glucose, and mechanically, this molecule competitively inhibits HK2, which regulates the first rate-limiting step of glycolysis. In addition, 3-BrPA was found to inhibit glycolysis, disturb redox homeostasis, and decrease nucleotide synthesis in triple-negative breast cancer, finally enhancing the inhibitory effect of ionizing radiation.103
Table 3.
Clinical trials of targeting enzymes or transporters of glucose metabolism in the combination of radiotherapy in solid tumors.
| Name of inhibitors | Targets | Year | Tumor types | Number of participants | Phase | Radiotherapy schedule | Clinical observations |
|---|---|---|---|---|---|---|---|
| Lonidamine | Hexokinase | 1994 | Clinically localized but nonresectable NSCLC | 310 | III | 1.8 Gy/fraction up to 55–60 Gy, 5 days/week | No significant advantages in the lonidamine-treated population in OS, PFS, and the median duration of local control were observed. |
| Lonidamine | Hexokinase | 1994 | Stages II-IV HNSCC | 97 | III | 1.5 Gy/fraction up to 60–65 Gy | The addition of lonidamine to hypofractionated radiotherapy was correlated with longer DFS. |
| Lonidamine | Hexokinase | 1989 | Brain metastases | 58 | II | 3000 cGy of WBRT | No serious organ toxicity or myelosuppression was observed. |
| 2-deoxy-d-glucose | Hexokinase | 1996 | Supratentorial GBM (grade III/IV) | 20 | I/II | 4 × 5 Gy/fraction/week, WBRT | The feasibility of administering the treatment (2-deoxy-d-glucose + 5 Gy) was well tolerated. |
| 2-deoxy-d-glucose | Hexokinase | 2006 | Grade IV GBM | 70 | II | 7 × 5 Gy/fraction/week to residual tumor +3 cm margin | Restlessness in a few patients. No life-threatening changes in vital parameters. |
| Dichloroacetate | Pyruvate dehydrogenase kinase | 2022 | Unresected, locally advanced HNSCC | 45 | II | 35 × 2 Gy | Adding dichloroacetate to cisplatin-based chemoradiotherapy appeared safe with no detrimental effect on survival and expected metabolite changes. |
DFS: Disease-free survival; GBM: Glioblastoma; HNSCC: Head and neck squamous cell carcinoma; NSCLC: Non-small cell lung cancer; OS: Overall survival; PFS: Progression-free survival; WBRT: Whole-brain radiotherapy.
Another important glycolytic enzyme, pyruvate dehydrogenase kinase (PDK), can similarly be targeted using specific small-molecule inhibitors. PDK participates in regulating the rate and amount of pyruvate entering the tricarboxylic acid cycle by inhibiting dehydrogenase activity. Some studies reported that PDK suppression alters glucose metabolism and elevates oxygen consumption in cancer cells, finally increasing ROS production and susceptibility to ionizing radiation.104, 105, 106 Dichloroacetate (DCA) is a kind of small-molecule inhibitor that can reverse the glycolytic phenotype and suppress cell proliferation and angiogenesis.106 In combination with ionizing radiation or anticancer platinum compounds, DCA has shown a synergistically inhibitory effect against several types of cancer cell lines, including those for SCLC and NSCLC.107,108 Similarly, a recent study on NSCLC revealed that DCA combined with EGFR tyrosine kinase inhibitors and/or radiotherapy significantly increased the cytotoxic effects by redirecting glucose metabolism toward OXPHOS and decreasing lactate generation.109
LDHA is a specific inhibitor of FX-11 that can enhance the radiosensitivity of prostate cancer cells with acquired radioresistance by decreasing EMT, hypoxia, and autophagy and increasing DNA double-strand break events and apoptosis.110 Furthermore, AZD3965, in combination with fractioned radiation, was more efficacious at inhibiting monocarboxylate transporter 1 (MCT1) than using either modality alone in SCLC xenografts.111 The carbonic anhydrase inhibitor indisulam can also apparently suppress tumor cell growth and sensitize GBM cells to radiotherapy and chemotherapy.112
Conclusion
SCLC is a fast-growing, highly aggressive cancer with a poor prognosis and limited effective therapy options. The rapid emergence of radiotherapy resistance in SCLC is a key contributor to treatment failure and poor survival. PI3K/AKT/mTOR pathway activation and hypoxia are known mechanisms underlying radioresistance. The PI3K/AKT/mTOR pathway can increase HIF-1α expression at the transcription level, and both the PI3K/AKT/mTOR pathway and HIF-1α play key roles in regulating the expression of key enzymes and/or transporters in glucose metabolism, including glycolysis, OXPHOS, and the PPP. Herein, we summarized promising avenues of research and provided insights into potential treatments to promote cancer cell death and improve the clinical prognosis of patients with this devastating disease. Admittedly, the relationship among the PI3K/AKT/mTOR pathway, hypoxia, and glucose metabolism is complex, and further studies should concentrate on how these signaling pathways interact to influence SCLC radioresistance.
Funding
This study is supported by the National Natural Science Foundation of China (No. 81672972).
Author contributions
Huan Deng takes responsibility for the integrity of the data and the accuracy of the analysis. Drafting of the manuscript: Huan Deng and Ying Jin. Supervision: Ying Jin and Ming Chen. Concept and design: All authors. Acquisition, analysis, and interpretation of data: All authors. Critical revision of the manuscript for important intellectual content: All authors. All authors read and approved the final manuscript.
Ethics statement
None.
Data availability statement
The datasets used in the current study are available from the corresponding author on reasonable request.
Conflicts of interest
None.
Acknowledgment
The authors thank Smart (http://smart.servier.com/) for the images presented in this study.
Contributor Information
Ying Jin, Email: jinying@zjcc.org.cn.
Ming Chen, Email: chenming@zjcc.org.cn.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., et al. Cancer statistics, 2021. CA A Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Yang S., Zhang Z., Wang Q. Emerging therapies for small cell lung cancer. J Hematol Oncol. 2019;12:47. doi: 10.1186/s13045-019-0731-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li D., Deng C., Zheng Q., et al. Impact of adjuvant therapy on survival in surgically resected limited-stage small cell lung cancer. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.704517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zugazagoitia J., Paz-Ares L. Extensive-stage small-cell lung cancer: first-line and second-line treatment options. J Clin Oncol. 2022;40:671–680. doi: 10.1200/jco.21.01881. [DOI] [PubMed] [Google Scholar]
- 5.Yang K., Zhao Y., Du Y., et al. Evaluation of hippo pathway and CD133 in radiation resistance in small-cell lung cancer. J Oncol. 2021;2021 doi: 10.1155/2021/8842554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin Y., Chen Y., Tang H., et al. Activation of PI3K/AKT pathway is a potential mechanism of treatment resistance in small cell lung cancer. Clin Cancer Res. 2022;28:526–539. doi: 10.1158/1078-0432.ccr-21-1943. [DOI] [PubMed] [Google Scholar]
- 7.Chen K., Lin Z.W., He S.M., et al. Metformin inhibits the proliferation of rheumatoid arthritis fibroblast-like synoviocytes through IGF-IR/PI3K/AKT/m-TOR pathway. Biomed Pharmacother. 2019;115 doi: 10.1016/j.biopha.2019.108875. [DOI] [PubMed] [Google Scholar]
- 8.Baturcam E., Vollmer S., Schlüter H., et al. MEK inhibition drives anti-viral defence in RV but not RSV challenged human airway epithelial cells through AKT/p70S6K/4E-BP1 signalling. Cell Commun Signal. 2019;17:78. doi: 10.1186/s12964-019-0378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu B., Huang Z.B., Chen X., et al. Mammalian target of rapamycin 2 (MTOR2) and C-MYC modulate glucosamine-6-phosphate synthesis in glioblastoma (GBM) cells through glutamine: fructose-6-phosphate aminotransferase (GFAT1) Cell Mol Neurobiol. 2019;39:415–434. doi: 10.1007/s10571-019-00659-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ponnusamy L., Natarajan S.R., Manoharan R. MARK2 potentiate aerobic glycolysis-mediated cell growth in breast cancer through regulating mTOR/HIF-1α and p53 pathways. J Cell Biochem. 2022;123:759–771. doi: 10.1002/jcb.30219. [DOI] [PubMed] [Google Scholar]
- 11.Li J., Hu Z.Q., Yu S., et al. CircRPN2 inhibits aerobic glycolysis and metastasis in hepatocellular carcinoma. Cancer Res. 2022;82:1055–1069. doi: 10.1158/0008-5472.can-21-1259. [DOI] [PubMed] [Google Scholar]
- 12.Köthe A., Bizzocchi N., Safai S., et al. Investigating the potential of proton therapy for hypoxia-targeted dose escalation in non-small cell lung cancer. Radiat Oncol. 2021;16:199. doi: 10.1186/s13014-021-01914-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang Y., Zhan Y., Xie Y., et al. Integration of glucose and cardiolipin anabolism confers radiation resistance of HCC. Hepatology. 2021;75:1386–1401. doi: 10.1002/hep.32177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kabakov A.E., Yakimova A.O. Hypoxia-induced cancer cell responses driving radioresistance of hypoxic tumors: approaches to targeting and radiosensitizing. Cancers. 2021;13:1102. doi: 10.3390/cancers13051102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shim B.Y., Jung J.H., Lee K.M., et al. Glucose transporter 1 (GLUT1) of anaerobic glycolysis as predictive and prognostic values in neoadjuvant chemoradiotherapy and laparoscopic surgery for locally advanced rectal cancer. Int J Colorectal Dis. 2013;28:375–383. doi: 10.1007/s00384-012-1542-3. [DOI] [PubMed] [Google Scholar]
- 16.Qin F., Fan Q., Yu P.K.N., et al. Properties and gene expression profiling of acquired radioresistance in mouse breast cancer cells. Ann Transl Med. 2021;9:628. doi: 10.21037/atm-20-4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray M., Turnbull A.K., Ward C., et al. Development and characterisation of acquired radioresistant breast cancer cell lines. Radiat Oncol. 2019;14:64. doi: 10.1186/s13014-019-1268-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu R., Li W., Tao B., et al. Tyrosine phosphorylation activates 6-phosphogluconate dehydrogenase and promotes tumor growth and radiation resistance. Nat Commun. 2019;10:991. doi: 10.1038/s41467-019-08921-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Y., Gao W., Zhang Y., et al. ABT737 reverses cisplatin resistance by targeting glucose metabolism of human ovarian cancer cells. Int J Oncol. 2018;53:1055–1068. doi: 10.3892/ijo.2018.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slotman B.J., van Tinteren H., Praag J.O., et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet. 2015;385:36–42. doi: 10.1016/s0140-6736(14)61085-0. [DOI] [PubMed] [Google Scholar]
- 21.Sun J.M., Ahn Y.C., Choi E.K., et al. Phase III trial of concurrent thoracic radiotherapy with either first- or third-cycle chemotherapy for limited-disease small-cell lung cancer. Ann Oncol. 2013;24:2088–2092. doi: 10.1093/annonc/mdt140. [DOI] [PubMed] [Google Scholar]
- 22.Yang P., Luo X., Li J., et al. Ionizing radiation upregulates glutamine metabolism and induces cell death via accumulation of reactive oxygen species. Oxid Med Cell Longev. 2021;2021 doi: 10.1155/2021/5826932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawamura K., Qi F., Kobayashi J. Potential relationship between the biological effects of low-dose irradiation and mitochondrial ROS production. J Radiat Res. 2018;59(suppl_2):ii91–ii97. doi: 10.1093/jrr/rrx091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdel-Rafei M.K., Thabet N.M., Rashed L.A., et al. Canagliflozin, a SGLT-2 inhibitor, relieves ER stress, modulates autophagy and induces apoptosis in irradiated HepG2 cells: signal transduction between PI3K/AKT/GSK-3β/mTOR and Wnt/β-catenin pathways; in vitro. J Cancer Res Therapeut. 2021;17:1404–1418. doi: 10.4103/jcrt.jcrt_963_19. [DOI] [PubMed] [Google Scholar]
- 25.Tang Z., Dokic I., Knoll M., et al. Radioresistance and transcriptional reprograming of invasive glioblastoma cells. Int J Radiat Oncol Biol Phys. 2022;112:499–513. doi: 10.1016/j.ijrobp.2021.09.017. [DOI] [PubMed] [Google Scholar]
- 26.Kang H., Lee S., Kim K., et al. Downregulated CLIP3 induces radioresistance by enhancing stemness and glycolytic flux in glioblastoma. J Exp Clin Cancer Res. 2021;40:282. doi: 10.1186/s13046-021-02077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu G., Yang S., Liu Y., et al. The adenosine-A2a receptor regulates the radioresistance of gastric cancer via PI3K-AKT-mTOR pathway. Int J Clin Oncol. 2022;27:911–920. doi: 10.1007/s10147-022-02123-x. [DOI] [PubMed] [Google Scholar]
- 28.Xu Y.J., Wei R.S., Li X.H., et al. MiR-421 promotes lipid metabolism by targeting PTEN via activating PI3K/AKT/mTOR pathway in non-small cell lung cancer. Epigenomics. 2022;14:121–138. doi: 10.2217/epi-2021-0229. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y., Miao X., Jiang Y., et al. The synergistic antitumor effect of IL-6 neutralization with NVP-BEZ235 in hepatocellular carcinoma. Cell Death Dis. 2022;13:146. doi: 10.1038/s41419-022-04583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barzegar Behrooz A., Talaie Z., Jusheghani F., et al. Wnt and PI3K/Akt/mTOR survival pathways as therapeutic targets in glioblastoma. Int J Mol Sci. 2022;23:1353. doi: 10.3390/ijms23031353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krencz I., Sebestyen A., Papay J., et al. Correlation between immunohistochemistry and RICTOR fluorescence in situ hybridization amplification in small cell lung carcinoma. Hum Pathol. 2019;93:74–80. doi: 10.1016/j.humpath.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 32.Schmid K., Bago-Horvath Z., Berger W., et al. Dual inhibition of EGFR and mTOR pathways in small cell lung cancer. Br J Cancer. 2010;103:622–628. doi: 10.1038/sj.bjc.6605761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roh M.S., Lee J.H., Kang K.W., et al. Phosphorylated 4E-binding protein 1 expression is associated with poor prognosis in small-cell lung cancer. Virchows Arch. 2015;467:667–673. doi: 10.1007/s00428-015-1860-2. [DOI] [PubMed] [Google Scholar]
- 34.Ross J.S., Wang K., Elkadi O.R., et al. Next-generation sequencing reveals frequent consistent genomic alterations in small cell undifferentiated lung cancer. J Clin Pathol. 2014;67:772–776. doi: 10.1136/jclinpath-2014-202447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakre N., Wildey G., Behtaj M., et al. RICTOR amplification identifies a subgroup in small cell lung cancer and predicts response to drugs targeting mTOR. Oncotarget. 2017;8:5992–6002. doi: 10.18632/oncotarget.13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin M.W., Su K.Y., Su T.J., et al. Clinicopathological and genomic comparisons between different histologic components in combined small cell lung cancer and non-small cell lung cancer. Lung Cancer. 2018;125:282–290. doi: 10.1016/j.lungcan.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Udagawa H., Umemura S., Murakami I., et al. Genetic profiling-based prognostic prediction of patients with advanced small-cell lung cancer in large scale analysis. Lung Cancer. 2018;126:182–188. doi: 10.1016/j.lungcan.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 38.Umemura S., Mimaki S., Makinoshima H., et al. Therapeutic priority of the PI3K/AKT/mTOR pathway in small cell lung cancers as revealed by a comprehensive genomic analysis. J Thorac Oncol. 2014;9:1324–1331. doi: 10.1097/jto.0000000000000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang J., Wang X., Lu J., et al. Genomic profiling of circulating tumor DNA from patients with extensive-stage small cell lung cancer identifies potentially actionable alterations. J Cancer. 2021;12:5099–5105. doi: 10.7150/jca.55134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X., Li C., Guo C., et al. PI3K/Akt/mTOR signaling orchestrates the phenotypic transition and chemo-resistance of small cell lung cancer. J Genet Genom. 2021;48:640–651. doi: 10.1016/j.jgg.2021.04.001. [DOI] [PubMed] [Google Scholar]
- 41.Jiang W., Zhang W., Wu L., et al. MicroRNA- related polymorphisms in PI3K/Akt/mTOR pathway genes are predictive of limited-disease small cell lung cancer treatment outcomes. BioMed Res Int. 2017;2017 doi: 10.1155/2017/6501385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan Y., Liao H., Pu Q., et al. miR-410 induces both epithelial-mesenchymal transition and radioresistance through activation of the PI3K/mTOR pathway in non-small cell lung cancer. Signal Transduct Targeted Ther. 2020;5:85. doi: 10.1038/s41392-020-0182-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang L., Graham P.H., Ni J., et al. Targeting PI3K/Akt/mTOR signaling pathway in the treatment of prostate cancer radioresistance. Crit Rev Oncol Hematol. 2015;96:507–517. doi: 10.1016/j.critrevonc.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 44.Kumar P., Miller A.I., Polverini P.J. p38 MAPK mediates gamma-irradiation-induced endothelial cell apoptosis, and vascular endothelial growth factor protects endothelial cells through the phosphoinositide 3-kinase-Akt-Bcl-2 pathway. J Biol Chem. 2004;279:43352–43360. doi: 10.1074/jbc.m405777200. [DOI] [PubMed] [Google Scholar]
- 45.Kumar P., Benedict R., Urzua F., et al. Combination treatment significantly enhances the efficacy of antitumor therapy by preferentially targeting angiogenesis. Lab Invest. 2005;85:756–767. doi: 10.1038/labinvest.3700272. [DOI] [PubMed] [Google Scholar]
- 46.Iida M., Harari P.M., Wheeler D.L., et al. Targeting AKT/PKB to improve treatment outcomes for solid tumors. Mutat Res. 2020;819–820 doi: 10.1016/j.mrfmmm.2020.111690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gil del Alcazar C.R., Hardebeck M.C., Mukherjee B., et al. Inhibition of DNA double-strand break repair by the dual PI3K/mTOR inhibitor NVP-BEZ235 as a strategy for radiosensitization of glioblastoma. Clin Cancer Res. 2014;20:1235–1248. doi: 10.1158/1078-0432.ccr-13-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mukherjee B., Tomimatsu N., Amancherla K., et al. The dual PI3K/mTOR inhibitor NVP-BEZ235 is a potent inhibitor of ATM- and DNA-PKCs-mediated DNA damage responses. Neoplasia. 2012;14:34–43. doi: 10.1593/neo.111512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zdrowowicz M., Spisz P., Hać A., et al. Influence of hypoxia on radiosensitization of cancer cells by 5-bromo-2'-deoxyuridine. Int J Mol Sci. 2022;23:1429. doi: 10.3390/ijms23031429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang J., Jiang Y., Zhang Y.G., et al. The effects of hypoxia on mitochondrial function and metabolism in gastric cancer cells. Transl Cancer Res. 2021;10:817–826. doi: 10.21037/tcr-20-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dong S., Liang S., Cheng Z., et al. ROS/PI3K/Akt and Wnt/β-catenin signalings activate HIF-1α-induced metabolic reprogramming to impart 5-fluorouracil resistance in colorectal cancer. J Exp Clin Cancer Res. 2022;41:15. doi: 10.1186/s13046-021-02229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng S., Evans W.K., Stys-Norman D., et al. Chemotherapy for relapsed small cell lung cancer: a systematic review and practice guideline. J Thorac Oncol. 2007;2:348–354. doi: 10.1097/01.jto.0000263720.15062.51. [DOI] [PubMed] [Google Scholar]
- 53.Lee G.W., Go S.I., Cho Y.J., et al. Hypoxia-inducible factor-1α and excision repair cross-complementing 1 in patients with small cell lung cancer who received front-line platinum-based chemotherapy: a retrospective study. J Thorac Oncol. 2012;7:528–534. doi: 10.1097/jto.0b013e3182417830. [DOI] [PubMed] [Google Scholar]
- 54.Lin C.S., Liu T.C., Lee M.T., et al. Independent prognostic value of hypoxia-inducible factor 1-alpha expression in small cell lung cancer. Int J Med Sci. 2017;14:785–790. doi: 10.7150/ijms.19512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luan Y., Gao C., Miao Y., et al. Clinicopathological and prognostic significance of HIF-1α and HIF-2α expression in small cell lung cancer. Pathol Res Pract. 2013;209:184–189. doi: 10.1016/j.prp.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 56.Philip B., Ito K., Moreno-Sánchez R., et al. HIF expression and the role of hypoxic microenvironments within primary tumours as protective sites driving cancer stem cell renewal and metastatic progression. Carcinogenesis. 2013;34:1699–1707. doi: 10.1093/carcin/bgt209. [DOI] [PubMed] [Google Scholar]
- 57.Rockwell S., Dobrucki I.T., Kim E.Y., et al. Hypoxia and radiation therapy: past history, ongoing research, and future promise. Curr Mol Med. 2009;9:442–458. doi: 10.2174/156652409788167087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pawlik T.M., Keyomarsi K. Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:928–942. doi: 10.1016/j.ijrobp.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 59.Green S.L., Freiberg R.A., Giaccia A.J. p21(Cip1) and p27(Kip1) regulate cell cycle reentry after hypoxic stress but are not necessary for hypoxia-induced arrest. Mol Cell Biol. 2001;21:1196–1206. doi: 10.1128/mcb.21.4.1196-1206.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gardin I. Methods to delineate tumour for radiotherapy by fluorodeoxyglucose positron emission tomography. Cancer Radiother. 2020;24:418–422. doi: 10.1016/j.canrad.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 61.Oh J.R., Seo J.H., Chong A., et al. Whole-body metabolic tumour volume of 18F-FDG PET/CT improves the prediction of prognosis in small cell lung cancer. Eur J Nucl Med Mol Imag. 2012;39:925–935. doi: 10.1007/s00259-011-2059-7. [DOI] [PubMed] [Google Scholar]
- 62.Lee J.W., Choi J.S., Lyu J., et al. Prognostic significance of (18)F-fluorodeoxyglucose uptake of bone marrow measured on positron emission tomography in patients with small cell lung cancer. Lung Cancer. 2018;118:41–47. doi: 10.1016/j.lungcan.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 63.Luengo A., Gui D.Y., Vander Heiden M.G. Targeting metabolism for cancer therapy. Cell Chem Biol. 2017;24:1161–1180. doi: 10.1016/j.chembiol.2017.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoxhaj G., Manning B.D. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer. 2020;20:74–88. doi: 10.1038/s41568-019-0216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schwartzenberg-Bar-Yoseph F., Armoni M., Karnieli E. The tumor suppressor p53 down-regulates glucose transporters GLUT1 and GLUT4 gene expression. Cancer Res. 2004;64:2627–2633. doi: 10.1158/0008-5472.can-03-0846. [DOI] [PubMed] [Google Scholar]
- 66.Saltos A., Shafique M., Chiappori A. Update on the biology, management, and treatment of small cell lung cancer (SCLC) Front Oncol. 2020;10:1074. doi: 10.3389/fonc.2020.01074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cargill K.R., Stewart C.A., Park E.M., et al. Targeting MYC-enhanced glycolysis for the treatment of small cell lung cancer. Cancer Metabol. 2021;9:33. doi: 10.1186/s40170-021-00270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu D., Ma T., Niu Z., et al. Prognostic significance of metabolic parameters measured by (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with small cell lung cancer. Lung Cancer. 2011;73:332–337. doi: 10.1016/j.lungcan.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 69.He M., Chi X., Shi X., et al. Value of pretreatment serum lactate dehydrogenase as a prognostic and predictive factor for small-cell lung cancer patients treated with first-line platinum-containing chemotherapy. Thorac Cancer. 2021;12:3101–3109. doi: 10.1111/1759-7714.13581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee J., Kim J.O., Jung C.K., et al. Metabolic activity on [18f]-fluorodeoxyglucose-positron emission tomography/computed tomography and glucose transporter-1 expression might predict clinical outcomes in patients with limited disease small-cell lung cancer who receive concurrent chemoradiation. Clin Lung Cancer. 2014;15:e13–e21. doi: 10.1016/j.cllc.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 71.Zhong J., Rajaram N., Brizel D.M., et al. Radiation induces aerobic glycolysis through reactive oxygen species. Radiother Oncol. 2013;106:390–396. doi: 10.1016/j.radonc.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mims J., Bansal N., Bharadwaj M.S., et al. Energy metabolism in a matched model of radiation resistance for head and neck squamous cell cancer. Radiat Res. 2015;183:291–304. doi: 10.1667/rr13828.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou K., Wei Y., Li X., et al. MiR-223-3p targets FOXO3a to inhibit radiosensitivity in prostate cancer by activating glycolysis. Life Sci. 2021;282 doi: 10.1016/j.lfs.2021.119798. [DOI] [PubMed] [Google Scholar]
- 74.Kunkel M., Reichert T.E., Benz P., et al. Overexpression of Glut-1 and increased glucose metabolism in tumors are associated with a poor prognosis in patients with oral squamous cell carcinoma. Cancer. 2003;97:1015–1024. doi: 10.1002/cncr.11159. [DOI] [PubMed] [Google Scholar]
- 75.Spang-Thomsen M., Clerici M., Engelholm S.A., et al. Growth kinetics and in vivo radiosensitivity in nude mice of two subpopulations derived from a single human small cell carcinoma of the lung. Eur J Cancer Clin Oncol. 1986;22:549–556. doi: 10.1016/0277-5379(86)90042-8. [DOI] [PubMed] [Google Scholar]
- 76.Pedersen M.W., Holm S., Lund E.L., et al. Coregulation of glucose uptake and vascular endothelial growth factor (VEGF) in two small-cell lung cancer (SCLC) sublines in vivo and in vitro. Neoplasia. 2001;3:80–87. doi: 10.1038/sj.neo.7900133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qin X., Jiang B., Zhang Y. 4E-BP1, a multifactor regulated multifunctional protein. Cell Cycle. 2016;15:781–786. doi: 10.1080/15384101.2016.1151581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thoreen C.C., Chantranupong L., Keys H.R., et al. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature. 2012;485:109–113. doi: 10.1038/nature11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wan J., Wu W. Hyperthermia induced HIF-1a expression of lung cancer through AKT and ERK signaling pathways. J Exp Clin Cancer Res. 2016;35:119. doi: 10.1186/s13046-016-0399-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wan J., Che Y., Kang N., et al. SOCS3 blocks HIF-1α expression to inhibit proliferation and angiogenesis of human small cell lung cancer by downregulating activation of Akt, but not STAT3. Mol Med Rep. 2015;12:83–92. doi: 10.3892/mmr.2015.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ma Y., Xiu Z., Zhou Z., et al. Cytochalasin H inhibits angiogenesis via the suppression of HIF-1α protein accumulation and VEGF expression through PI3K/AKT/P70S6K and ERK1/2 signaling pathways in non-small cell lung cancer cells. J Cancer. 2019;10:1997–2005. doi: 10.7150/jca.29933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu X., Liu L., Chen K., et al. Huaier shows anti-cancer activities by inhibition of cell growth, migration and energy metabolism in lung cancer through PI3K/AKT/HIF-1α pathway. J Cell Mol Med. 2021;25:2228–2237. doi: 10.1111/jcmm.16215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xu K., He Z., Chen M., et al. HIF-1α regulates cellular metabolism, and Imatinib resistance by targeting phosphogluconate dehydrogenase in gastrointestinal stromal tumors. Cell Death Dis. 2020;11:586. doi: 10.1038/s41419-020-02768-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lall R., Ganapathy S., Yang M., et al. Low-dose radiation exposure induces a HIF-1-mediated adaptive and protective metabolic response. Cell Death Differ. 2014;21:836–844. doi: 10.1038/cdd.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Osada-Oka M., Hashiba Y., Akiba S., et al. Glucose is necessary for stabilization of hypoxia-inducible factor-1alpha under hypoxia: contribution of the pentose phosphate pathway to this stabilization. FEBS Lett. 2010;584:3073–3079. doi: 10.1016/j.febslet.2010.05.046. [DOI] [PubMed] [Google Scholar]
- 86.Wan J., Chai H., Yu Z., et al. HIF-1α effects on angiogenic potential in human small cell lung carcinoma. J Exp Clin Cancer Res. 2011;30:77. doi: 10.1186/1756-9966-30-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang D., Zhao C., Xu F., et al. Cisplatin-resistant NSCLC cells induced by hypoxia transmit resistance to sensitive cells through exosomal PKM2. Theranostics. 2021;11:2860–2875. doi: 10.7150/thno.51797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Riddle S.R., Ahmad A., Ahmad S., et al. Hypoxia induces hexokinase II gene expression in human lung cell line A549. Am J Physiol Cell Mol Physiol. 2000;278:L407–L416. doi: 10.1152/ajplung.2000.278.2.l407. [DOI] [PubMed] [Google Scholar]
- 89.Chiche J., Ilc K., Laferrière J., et al. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 2009;69:358–368. doi: 10.1158/0008-5472.can-08-2470. [DOI] [PubMed] [Google Scholar]
- 90.Zhao T., Zhu Y., Morinibu A., et al. HIF-1-mediated metabolic reprogramming reduces ROS levels and facilitates the metastatic colonization of cancers in lungs. Sci Rep. 2014;4:3793. doi: 10.1038/srep03793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cheng J., Huang Y., Zhang X., et al. TRIM21 and PHLDA3 negatively regulate the crosstalk between the PI3K/AKT pathway and PPP metabolism. Nat Commun. 2020;11:1880. doi: 10.1038/s41467-020-15819-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Silic-Benussi M., Sharova E., Ciccarese F., et al. mTOR inhibition downregulates glucose-6-phosphate dehydrogenase and induces ROS-dependent death in T-cell acute lymphoblastic leukemia cells. Redox Biol. 2022;51 doi: 10.1016/j.redox.2022.102268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cao H.J., Zhou W., Xian X.L., et al. A mixture of baicalein, wogonin, and oroxylin-A inhibits EMT in the A549 cell line via the PI3K/AKT-TWIST1-glycolysis pathway. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.821485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Krystal G.W., Sulanke G., Litz J. Inhibition of phosphatidylinositol 3-kinase-Akt signaling blocks growth, promotes apoptosis, and enhances sensitivity of small cell lung cancer cells to chemotherapy. Mol Cancer Therapeut. 2002;1:913–922. [PubMed] [Google Scholar]
- 95.Li H., Ma L., Bian X., et al. FK228 sensitizes radioresistant small cell lung cancer cells to radiation. Clin Epigenet. 2021;13:41. doi: 10.1186/s13148-021-01025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Powers W.E., Tolmach L.J. Demonstration of an anoxic component in a mouse tumor-cell population by in vivo assay of survival following irradiation. Radiology. 1964;83:328–336. doi: 10.1148/83.2.328. [DOI] [PubMed] [Google Scholar]
- 97.Wilson W.R., Hay M.P. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 98.Siim B.G., Pruijn F.B., Sturman J.R., et al. Selective potentiation of the hypoxic cytotoxicity of tirapazamine by its 1-N-oxide metabolite SR 4317. Cancer Res. 2004;64:736–742. doi: 10.1158/0008-5472.can-03-2488. [DOI] [PubMed] [Google Scholar]
- 99.Le Q.T., McCoy J., Williamson S., et al. Phase I study of tirapazamine plus cisplatin/etoposide and concurrent thoracic radiotherapy in limited-stage small cell lung cancer (S0004): a Southwest Oncology Group study. Clin Cancer Res. 2004;10:5418–5424. doi: 10.1158/1078-0432.ccr-04-0436. [DOI] [PubMed] [Google Scholar]
- 100.Subramaniam D.S., Liu S.V., Crawford J., et al. A phase Ib/II study of ganetespib with doxorubicin in advanced solid tumors including relapsed-refractory small cell lung cancer. Front Oncol. 2018;8:64. doi: 10.3389/fonc.2018.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li N., Chu Y., Song Q. Brain metastasis in patients with small cell lung cancer. Int J Gen Med. 2021;14:10131–10139. doi: 10.2147/IJGM.S342009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhao F., Ming J., Zhou Y., et al. Inhibition of Glut1 by WZB117 sensitizes radioresistant breast cancer cells to irradiation. Cancer Chemother Pharmacol. 2016;77:963–972. doi: 10.1007/s00280-016-3007-9. [DOI] [PubMed] [Google Scholar]
- 103.Skaripa-Koukelli I., Hauton D., Walsby-Tickle J., et al. 3-Bromopyruvate-mediated MCT1-dependent metabolic perturbation sensitizes triple negative breast cancer cells to ionizing radiation. Cancer Metabol. 2021;9:37. doi: 10.1186/s40170-021-00273-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Woolbright B.L., Rajendran G., Harris R.A., et al. Metabolic flexibility in cancer: targeting the pyruvate dehydrogenase kinase:pyruvate dehydrogenase axis. Mol Cancer Therapeut. 2019;18:1673–1681. doi: 10.1158/1535-7163.mct-19-0079. [DOI] [PubMed] [Google Scholar]
- 105.Tataranni T., Piccoli C. Dichloroacetate (DCA) and cancer: an overview towards clinical applications. Oxid Med Cell Longev. 2019;2019 doi: 10.1155/2019/8201079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cook K.M., Shen H., McKelvey K.J., et al. Targeting glucose metabolism of cancer cells with dichloroacetate to radiosensitize high-grade gliomas. Int J Mol Sci. 2021;22:7265. doi: 10.3390/ijms22147265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Olszewski U., Poulsen T.T., Ulsperger E., et al. In vitro cytotoxicity of combinations of dichloroacetate with anticancer platinum compounds. Clin Pharmacol. 2010;2:177–183. doi: 10.2147/cpaa.s11795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Allen K.T., Chin-Sinex H., DeLuca T., et al. Dichloroacetate alters Warburg metabolism, inhibits cell growth, and increases the X-ray sensitivity of human A549 and H1299 NSC lung cancer cells. Free Radic Biol Med. 2015;89:263–273. doi: 10.1016/j.freeradbiomed.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 109.Dyrstad S.E., Lotsberg M.L., Tan T.Z., et al. Blocking aerobic glycolysis by targeting pyruvate dehydrogenase kinase in combination with EGFR TKI and ionizing radiation increases therapeutic effect in non-small cell lung cancer cells. Cancers. 2021;13:941. doi: 10.3390/cancers13050941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hao J., Graham P., Chang L., et al. Proteomic identification of the lactate dehydrogenase A in a radioresistant prostate cancer xenograft mouse model for improving radiotherapy. Oncotarget. 2016;7:74269–74285. doi: 10.18632/oncotarget.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bola B.M., Chadwick A.L., Michopoulos F., et al. Inhibition of monocarboxylate transporter-1 (MCT1) by AZD3965 enhances radiosensitivity by reducing lactate transport. Mol Cancer Therapeut. 2014;13:2805–2816. doi: 10.1158/1535-7163.mct-13-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Teixeira S.A., Viapiano M.S., Andrade A.F., et al. The carbonic anhydrase inhibitor E7070 sensitizes glioblastoma cells to radio- and chemotherapy and reduces tumor growth. Mol Neurobiol. 2021;58:4520–4534. doi: 10.1007/s12035-021-02437-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used in the current study are available from the corresponding author on reasonable request.