Abstract
Preimplantation genetic testing (PGT), which was developed as an alternative to prenatal genetic testing, allows couples to avoid pregnancies with abnormal chromosomes and the subsequent termination of the affected fetus. Originally used for early onset monogenic conditions, PGT is now used to prevent various types of inherited cancer conditions based on the development of PGT technology, assisted reproductive techniques (ARTs), and in vitro fertilization (IVF). This review provides insights into the potential benefits and challenges associated with the application of PGT for hereditary cancer and provides an overview of the existing literature on this test, with a particular focus on the current challenges related to laws, ethics, counseling, and technology. Additionally, this review predicts the future potential applications of this method. Although PGT may be utilized to predict and prevent hereditary cancer, each case should be comprehensively evaluated. The motives of couples must be assessed to prevent the misuse of this technique for eugenic purposes, and non-pathogenic phenotypes must be carefully evaluated. Pathological cases that require this technology should also be carefully considered based on legal and ethical reasoning. PGT may be the preferred treatment for hereditary cancer cases; however, such cases require careful case-by-case evaluations. Therefore, this study concludes that multidisciplinary counseling and support for patients and their families are essential to ensure that PGT is a viable option that meets all legal and ethical concerns.
Keywords: Preimplantation genetic testing, Hereditary cancer, Late-onset diseases, Ethics, Legal restrictions
Graphical abstract
Highlights
-
•
Preimplantation genetic testing (PGT) identifies and prevents hereditary cancer by selecting embryo-free pathogenic mutations.
-
•
Careful consideration and multidisciplinary support and guidelines are necessary to make PGT an acceptable option.
-
•
Guidelines should be developed to assist stakeholders and practitioners in making decisions surrounding PGT.
-
•
Non-disclosure testing raises ethical and moral concerns, thus requiring careful consideration on a case-by-case basis.
-
•
Genetic applications continue to advance and objections are unlikely to endure.
Introduction
In 1989, Handyside et al.1 were among the pioneers of preimplantation genetic testing (PGT) and utilized the technique to examine a single embryo cell at the 6–8 cell stage. This approach aims to prevent the transmission of X-linked recessive diseases by evaluating female embryos prior to transfer.1 Since then, significant advances have been made in genetic screening tools, which has led to the discovery of numerous genes that are responsible for inherited cancers. These discoveries have facilitated the identification of pathogenic variants at early stages, thus allowing for cancer diagnosis, prevention, and treatment and improving survival rates.2
Initially, PGT was extensively applied as an alternative to prenatal amniocentesis testing (PNT) to detect early onset monogenic life-threatening diseases.3 The identification and selection of unaffected embryos through PGT help families avoid going through “invasive procedures like PNT or probably termination of pregnancy of affected embryos.4 Although single-cell genetic testing is challenging and the procedures involve complex steps, PGT has moved from the experimental phase and now represents an alternative to prenatal screening.
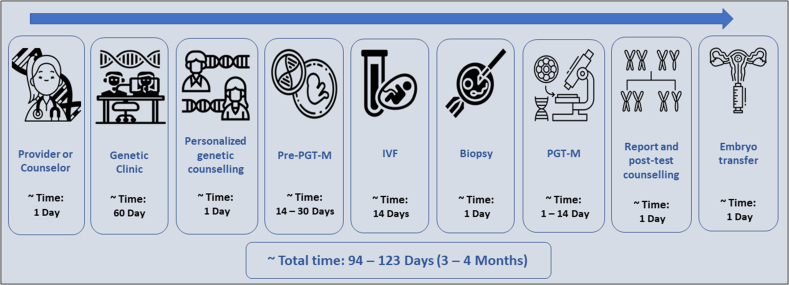
PGT is an assisted reproductive technique (ART) that combines in vitro fertilization (IVF) with embryo culture. Essentially, this testing procedure requires couples to begin IVF so that PGT for monogenic disorders (PGT-M) can be performed to select a non-affected embryo.5 The first step in PGT is the unequivocal identification of the causative gene and pathogenic variant within the relevant affected family members. Subsequently, an appropriate technique is used to examine and identify embryos that are negative for a given disease.6 These steps and the approximate time required until completion are summarized in Figure 1.
Figure 1.
Workflow of the assisted reproductive technique that includes all stages from disease diagnosis to embryo transfer, with the approximate duration of each of the following stages (days): pre-preimplantation genetic testing for monogenic disorders, in vitro fertilization, and preimplantation genetic testing for monogenic disorders. ART: Assisted reproductive technique; IVF: In vitro fertilization; PGT-M: Preimplantation genetic testing for monogenic disorders; Pre-PGT-M: Pre-preimplantation genetic testing for monogenic disorders.
The PGT technique was initially applied to one cell on the third day after fertilization in the blastomere stage, and deoxyribonucleic acid (DNA) was extracted from a single cell to examine the presence of one specific genetic defect.7 The number of cells in an embryo biopsy varies from one to six depending on the embryo stage. For example, biopsy of one to two cells is achievable at the cleavage stage of an embryo, while biopsy of five to ten cells is possible from the trophectoderm at the blastocyst stage.8,9
Previously, couples mainly underwent PGT-M for monogenic disorders that cause life-limiting childhood-onset diseases; however, PGT has been increasingly used for late-onset disorders, such as Huntington's disease and hereditary cancer.6 Currently, genetic testing is broadly offered for hereditary cancer susceptibility genes; moreover, the criterion for testing is reproductive age. Therefore, the Human Fertilisation and Embryology Authority (HFEA) approved PGT for certain genetic cancer predisposition syndromes.6
People with inherited precancerous syndromes are at risk of developing tumors and may require severe and serious procedures and surgeries to increase their likelihood of survival. Couples who undergo PGT testing are seeking to spare their future children from experiencing these life-threatening diseases.7
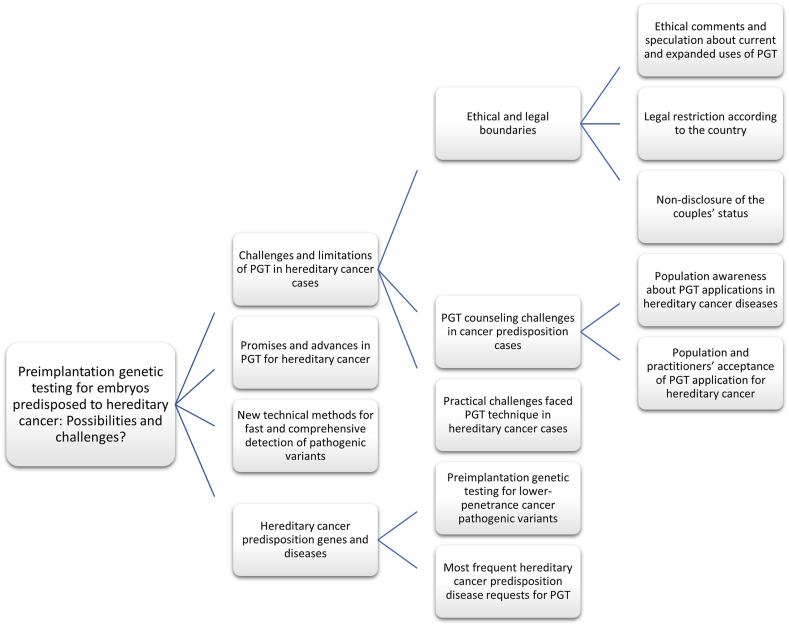
As PGT testing for hereditary cancer susceptibility genes is rapidly developing both scientifically and technically, annual scientific reviews are required that discuss the associated ethical issues and challenges and summarize the latest developments in this field. Such a review must include the efficacy and efficiency of any new technical methods, such as extended PGT (ePGT) and its ethical limitations, and discussions on polygenic PGT and its approval. The review presented here provides an overview of recent advancements in this area and discusses the challenges and expected benefits of testing hereditary cancer genes using PGT as an ART. The schema diagram illustrates the hierarchical and relational nature of each section in this review article, facilitating readers' comprehension of its scope, objectives, and findings in an organized and systematic way [Figure 2].
Figure 2.
Schema diagram for the content. PGT: Preimplantation genetic testing.
Hereditary cancer predisposition genes and diseases
The HFEA approved the use of PGT testing for certain cancer predisposition genes, such as BRCA1 and BRCA2 for breast and ovarian cancer, and disorders, such as Lynch syndrome for bowel, endometrial, and ovarian cancer; familial adenomatous polyposis (FAP) for bowel polyps with malignant potential; Li-Fraumeni syndrome for breast cancer and other soft tissue tumors, and neurofibromatosis type 2 for schwannomas.10 In 2006, the HFEA authorized the PGT procedure for pathogenic variants, such as BRCA1 and BRCA2, and hereditary nonpolyposis colorectal cancer (HNPCC). These conditions are separate from those previously authorized in the United Kingdom (UK) because they include three factors: symptoms that appear at old age, pathogenic variants that have low penetrance, and the possibility of treatment. Previously, PGT was only authorized by the HFEA for cases that included one or two conditions mentioned above, which represented the main criterion for authorizing the PGT procedure.3,10
These variants (BRCA1, BRCA2, and HNPCC) are pathogenic, although most do not have complete penetrance, which means that not all individuals carrying these variants express disease pathogenicity. For instance, the incidence of breast cancer (BC) and ovarian cancer (OC) reaches 80% and 50% for BRCA pathogenic variant carriers, respectively. In detail, a 60–80% risk of developing BC before the age of 70 is observed for carriers of the BRCA1 or BRCA2 pathogenic variants, a 30–60% risk of developing OC is observed for carriers of BRCA1 gene pathogenic variants, and a 5–20% risk of developing OC before age 70 is observed for carriers of BRCA2.3
The likelihood of transmission of the genetic pathogenic variant responsible for cancer from parents to their offspring varies between diseases. For individuals who carry the MEN2A gene pathogenic variant, the probability of transmitting the pathogenic rearranged during transfection (RET) variant to their offspring is 50%,11 and the same probability is observed for individuals who carry germline retinoblastoma 1 (RB1) gene pathogenic variants responsible for the development of hereditary RB.7 In RB, 45–50% of patients who develop the disease by the germline pathogenic variant are capable of transmitting the disease to their offspring,12 although RB is also caused by non-inherited pathogenic variants (somatic carcinomas).7
The prevalence of multiple endocrine neoplasia type 1 (MEN1) disease is 1 in 50,000 individuals and the disease appears between 5 and 80 years of age. Studies have indicated that the clinical manifestations of MEN1 appear in 80% of patients in their 5th decade of life.13,14
Aggressive carcinomas may be caused by pathogenic germline variants.15 These cancers are categorized as adult-onset predisposition syndromes, for which treatments are available that reduce their risks. However, compared to other sporadic malignancies, hereditary cancers usually appear at a young age and involve a high risk of multiple primary tumors. These conflicting characteristics of this disease have led to controversy regarding the relevance of PGT as a reproductive choice for couples with these pathogenic variants.6
The management of these cancers mostly includes invasive screening tests and prophylactic surgery, such as colectomy, mastectomy, or oophorectomy, which have negative psychological effects on patients and their quality of life.
Preimplantation genetic testing for lower-penetrance cancer pathogenic variants
Continuous developments in the field of PGT and ART in recent years have made PGT a clinically accurate, safe, and well-established procedure.16 PGT is an attractive option for couples who carry genetic pathogenic variants that cause severe, highly pathogenic, or fatal cancers because in such cases, the pathogenic variants responsible for causing cancer show complete penetrance. More recently, a trend of applying PGT-M has been observed for such cases, such as late-onset syndromes that include cancer predisposition disorders with low penetrance rates.17
Although the use of PGT for susceptible inherited cancer is still debated,18 PGT represents an option for preventing hereditary cancers, and the selection of cancer predisposition-free embryos can prevent the risk of clinical pregnancy termination. Even with the requirement of undergoing IVF, PGT is still a better option for obtaining unaffected offspring.17
PGT is a safe prediction method and a suitable alternative for individuals suffering from these pathogenic variants who do not wish to undergo PNT. However, a number of countries continue to object to the use of this method for late-onset diseases where the degree of penetration is incomplete, such as hereditary cancers. Nevertheless, the application of PGT for hereditary cancers is increasing in the United States (US),19 and it is a superior option for avoiding the ethical dilemmas associated with PNT.
The main and most important argument for using PGT in such cases is to prevent the inheritance of severe diseases. Abstractly, PNT and PGT do not select a child or embryo carrying a specific disease but rather provide a cure for the disease. PGT technology is superior to PNT because it avoids the ethical issues of PNT, which might identify a disease that could lead to the termination of the pregnancy and disposal of the fetus.20 Rechitsky and Kuliev revealed that the largest group of non-traditional indications was detected via PGT-M for 23 cancer predisposition syndromes, including BRCA1 and BRCA2, and they accounted for more than one-third of the observed cases. The application of this procedure resulted in the birth of 271 children free of cancer predisposition genes, making it the world's largest series.21
For diseases that may be preventable or treatable, a request for PNT is uncommon because of the low penetrance rate. Realistically, a mutual unwillingness is observed among professionals and parents to terminate the pregnancy if the child has a predisposition to pathogenic variants. Instead, embryo selection using PGT represents an acceptable alternative to avoid the clinical termination of pregnancy.6 Research by Lee et al. found that only one in three couples agreed to use invasive PNT with the possibility of terminating the pregnancy.22 Similarly, a study by Derks-Smeet et al. showed that the majority of couples with hereditary breast and ovarian cancer (HBOC) pathogenic variants refused to undergo PNT and even refused to follow natural conception.23
The demand for genetic testing for cancer diseases is increasing, and the survival rate for young patients exposed to cancer is also increasing. This situation has led to an increased demand for PGT to prevent such diseases because PNT has many flaws, such as ethical concerns regarding the decision to terminate a pregnancy and moral, religious, and social obstacles that may prevent abortion according to the laws of some countries.3 Moreover, diseases that arise due to a hereditary predisposition with incomplete penetrance of pathogenic variants cannot be considered a reason for prenatal screening because termination of pregnancy in these cases is based on exposure to the disease for genetically based reasons only. In such cases, we cannot be sure that the child tested would develop the disease.17
Most frequent hereditary cancer predisposition disease requests for preimplantation genetic testing
The most requests for PGT-M in terms of late-onset inherited conditions are for cancer predisposition syndrome. Cases of these cancers are shown in Table 1.
Table 1.
Key characteristics detailed in publications on preimplantation genetic testing for monogenic disorders for hereditary predisposition to cancer diseases.
| Name | Inheritance cancer syndrome | Mechanism of cancer | Variant | Name of the disease | Reference |
|---|---|---|---|---|---|
| MEN2 | Autosomal dominant | Germline pathogenic variants of the RET proto-oncogene | C.1858T > C, P.C620R germline variant |
MTC, parathyroid tumors, and pheochromocytoma and Hirschsprung's disease | Würgler et al.11 |
| MEN1 | Autosomal dominant | Associated with germline and somatic inactivating mutations in the MEN1 gene | Chromosome 11q13, encodes the 610 amino acid protein menin | Tumors in multiple endocrine tissues | Lima et al.13 |
| HBOC | Autosomal dominant | BRCA2; BRCA1 gene pathogenic variant | C.7436_7805del [GenBank U43746]); BRCA1 pathogenic variant carrier (5273G[A In exon 19) | Breast cancer, ovarian cancer, pancreatic cancer, and prostate cancer | Lee et al.22 and Ramón et al.27 |
| FAP | Autosomal dominant | Truncating germline pathogenic variants in the APC gene | APC gene, C.532-8G > A (NG_008481:G93262g > A); an AGTT deletion pathogenic variant in exon 15 of the APC gene | FAP is hundreds or even thousands of polyps growing in the gastrointestinal tract, primarily in the colon | Lee et al.22 and Davis et al.28 |
| RB1 | Autosomal dominant | Caused by pathogenic variants in the RB1 gene | Single base pair substitution at the 5′ end of intron 12 (splice site pathogenic variant) of the RB gene (1353 + 1G→A). Normal GT splice consensus sequence following exon 12 is changed by this G→A transition pathogenic variant | Retinoblastoma: malignant tumor of the retina | Xu et al.7 |
| LS; also known as HNPCC | Autosomal dominant | Germline pathogenic variants in DNA MMR genes: MLH1, MSH2, MSH6, EPCAM, and PMS2 genes |
Hmsh2 (homolog of the prokaryotic DNA MMR gene muts) And Hmlhl, Hpmsl, and Hpms2 (all homologs of the prokaryotic DNA MMR gene mutl). Pathogenic variants in each of the four genes have been found in the germline cells of HNPCC families | CRC | Marra et al.29 and Dewanwala et al.30 |
| LFS | Autosomal dominant | Heterozygous pathogenic variants in the P53 gene | Germline pathogenic variants in the P53 gene on chromosome 17p13.1 | Osteosarcoma, breast cancer, brain neoplasm, leukemia, and adrenal tumors. inheritance | Ilic et al.31 |
| NF2 | Autosomal dominant | Pathogenic variants in the NF2 gene | Single base pair substitution in the NF2 gene. G/C substitution at nucleotide 8240. NF2 gene on chromosome 22q12.2 |
Development of histologically benign tumors in the CNS. These include unilateral vestibular schwannoma | Abou-Sleiman et al.32 |
| NF1 | Autosomal dominant | Nonsense pathogenic variant in the NF1 gene that changes the codon to a STOP codon | NF1 gene is located on chromosome 17q11.2. NF1c.4495C > T (NM_000267) | Young patient with typical neurofibromatosis type 1 diagnosed with a unilateral vestibular schwannoma | Lee et al.22 and Huq et al.33 |
APC: Adenomatous polyposis coli; CNS: Central nervous system; CRC: Colorectal cancer; FAP: Familial adenomatous polyposis; HBOC: Hereditary breast and ovarian cancer; HNPCC: Hereditary nonpolyposis colorectal cancer; LFS: Li-Fraumeni syndrome; LS: Lynch syndrome; MEN1: Multiple endocrine neoplasia type 1; MEN2: Multiple endocrine neoplasia type 2; MMR: Mismatch repair; MTC: Medullary thyroid cancer; NF1: Neurofibromatosis type 1; NF2: Neurofibromatosis type 2; RB1: Retinoblastoma; RET: Rearranged during transfection.
In 1998, Ao et al. led the efforts to use PGT-M for cancer predisposition syndromes by performing the test for individuals with familial polyposis coli, and they performed the first whole-genome amplification (WGA) in addition to PGT-M, where pathogenic variants were detected by direct and indirect methods using linked informative markers to screen pathogenic variants of the APC gene. This strategy increased the accuracy of PGT-M screening for dominant disorders.24
The requests for PGT for cancer predisposition screening by couples with a genetic predisposition to late-onset disorders steadily increased after Verlinsky et al. first described PGT for cancer in 1999.17 Verlinsky et al. applied PGT for inherited predisposition for the first time in late 1999 for couples carrying p53 tumor suppressor gene variants,25 which opened the door to examining a variety of indicators of a genetic predisposition to cancer syndromes through PGT, mainly for common diseases that were never considered suitable for PNT.17
According to Harper et al., the most frequent requests for PGT-M are inherited cancer predisposition syndromes such as RB, Li-Fraumeni syndrome, neurofibromatosis I and II, and familial adenomatous polyposis coli.26
BC caused by BRCA1 and BRCA2 gene pathogenic variants are the most frequent genetic predisposition cancer condition assessed via PGT-M.34, 35, 36, 37 Kuliev et al. reported that 30% of cases (59 of 175) were tested, and the pathogenic variants in BRCA1 and BRCA2 were from the paternal side.17
BC and colon cancer are the most common cancer syndromes.3 The BC pathogenic variants BRCA1 and BRCA2 can either have no effect (non-penetrance) or result in BC or OC with variable risks.38
BRCA1 and BRCA2 were reported as the most frequently screened genes for PGT-M (271 of 792 cycles), and requests are also common for cancers such as neurofibromatosis, FAP 1, Fanconi anemia, HNPCC, and tuberous sclerosis.5
According to Kuliev et al.,17 634 of 966 embryos free of inherited cancer gene pathogenic variants were identified via PGT and transplanted at a rate of 1.52 embryos per transfer case, which resulted in 387 clinical pregnancies (61% success rate). These cases resulted in the birth of healthy children who were primarily free of the hereditary cancer susceptibility pathogenic variant examined. Kuliev et al. stated that with the exception of BC, most abnormalities are considered rather rare and inherited in an autosomal dominant form, as shown in Table 1, with a prevalence ratio of 1 in 5000 for FAP and 1 in 15,000 for RB disease in the US. These ratios increased to 1 in 36,000 for von Hippel-Lindau and were much greater than for other diseases.17
A steady rise has been observed in PGT applications for hereditary cancer predisposition syndromes since the first case was described in 1999, for a total of 429 patients, 792 cycles, and 46 genes in 2020.17,24,25
New technical methods for fast and comprehensive detection of pathogenic variants
In 1992, comparative genomic hybridization (CGH) was developed by a group of scientists for the comprehensive analysis of embryos to detect somatic pathogenic variants in cancer cells, and it represented the first technique in the field of molecular cytogenetics.39 The major advances in this area have been attributed to the shift from old and less effective technologies, such as fluorescence in situ hybridization (FISH), to new technical methods, such as next-generation sequencing (NGS), which is used for whole-exome sequencing (WES) or whole-genome sequencing (WGS) and DNA microarray.40
Several advances have been made in PGT-M from a practical perspective, such as the substitution of singleplex polymerase chain reaction (PCR) by multiplex PCR for single cells. This method represents the main option for detecting monogenic disorders; however, genome-wide haplotyping and sequencing technologies have been used to replace regular PCR methods over the past 10 years.41,42
One of the advantages of NGS is its ability to simultaneously perform genotyping and chromosome copy number determination with high accuracy and reliability. This technology facilitates the creation of a global platform for assessing monogenic disorders (including the detection of de novo pathogenic variants). Although NGS technology is promising, more comprehensive evaluations and verification are required to incorporate it into clinical practice for PGT. Because of the continuous improvements that are being made to reduce its costs and increase its productivity and suitability for cancer cases and polygenic diseases, time is required to adapt workflows, computational pipelines, and tools to realize the flexibility of PGT services.43
The remarkable and sharp reduction in the cost of DNA sequencing has made the PGT technique move toward a sequencing-based solution in which multiple assays (for PGT-M, preimplantation genetic testing for aneuploidy [PGT-A], and preimplantation genetic testing for chromosome structural rearrangements [PGT-SR]) can be performed in a single run. The effective development of human genome sequencing analysis tools and the application of machine learning to biobank data on a global level has led to the expansion and evolution of PGT to include the most common genetic diseases of polygenic nature.42,44
Among the other major improvements in this area is the use of WGA based on the multiple displacement amplification (MDA) method. This is the preferred method for PCR-based or single nucleotide polymorphism (SNP)-based applications in monogenic disorder screening because it more effectively covers the genome and has lower error rates. The integration of MDA and PCR is the latest application based on WGA operations. Therefore, many genome-wide platforms are now available to implement PGT, such as array CGH, SNP arrays, and NGS, which require the use of WGA technology. However, the possibility of bias and artifacts during the WGA process must be considered.16
Another fast-growing technology is DNA amplification via quantitative PCR (qPCR) to directly detect pathogenic variants by amplifying one or more closely related polymorphic markers. This method performs direct and indirect genotyping assessments to reduce the risks related to allelic dropout (ADO) and increase the accuracy and ability to detect contamination in examined samples. Studies have demonstrated the accuracy and validity of the results of this method to be more than 98%.45
One of the recent new approaches to PGT technique is the Variant haplophasing around the target by long-read sequencing, using third-generation sequencing (TGS) as part of the overall workup of PGT SR and PGT-M.46 Comprehensive parental SNP profiles around the targets are used to identify useful polymorphic markers that make clinical PGT designs easy and simple, and thus allow discrimination between carrier and non-carrier embryos. This allows rapid selection of closely related informative markers around the region of interest for patient-specific test design and has the ability to set the phase without additional blood samples from the extended family.47 Table 2 lists the advantages and disadvantages of existing technical applications.
Table 2.
A comparison between the advantages and disadvantages of existing technical applications.
| Technical principle | Advantages | Disadvantages |
|---|---|---|
| Comparative genomic hybridization (CGH) | First technique in the molecular cytogenetics field, allows for comprehensive analysis | Less effective than newer technologies |
| Fluorescence in situ hybridization (FISH) | Good for detecting specific DNA sequences, allows for visualization of specific genetic regions | Limited in scope, not comprehensive |
| Next generation sequencing (NGS) | Simultaneously perform genotyping and chromosome copy number determination with increased accuracy and reliability, promising for cancer cases and polygenic diseases | Needs more comprehensive evaluation and verification to become part of clinical practice, continuous improvement undergoing |
| Real-time polymerase chain reaction (RT-PCR) | Directly detects mutations with amplification of one or more closely related polymorphic markers, high accuracy, and validity | Limited in scope, not comprehensive |
| DNA microarray | High-throughput screening, can detect multiple mutations simultaneously | Limited in scope, not comprehensive |
| Multiplex conventional PCR | High-throughput screening, allows for the detection of multiple mutations simultaneously | Limited in scope, not comprehensive |
| Next generation capture | Highly sensitive and specific, can detect mutations at a low allelic frequency | Requires pre-selection of target regions, limited in scope |
| Protein microarray | Can detect protein–protein interactions, the potential for identifying new drug targets | Limited in scope, not comprehensive |
| Sanger sequencing | Reliable and accurate, suitable for detecting small mutations and indels | Low throughput, time-consuming and expensive |
| Whole exome sequencing (WES) | Can cover most coding regions of the genome, can detect both known and unknown mutations | Does not cover non-coding regions or structural variations, may miss mutations in poorly covered regions |
| Whole genome sequencing (WGS) | Can cover the entire genome, can detect both known and unknown mutations and structural variations | Expensive and computationally intensive, may generate large amounts of data that require significant storage and processing resources |
| Polymerase chain reaction (PCR) | Highly sensitive and specific, can detect low levels of mutations | Requires prior knowledge of target regions, may generate false positives due to amplification of non-specific products |
| Microsatellite instability analysis (MSI) | Can detect mutations in microsatellite regions, useful for detecting Lynch syndrome | Limited to certain types of cancers, may miss mutations in non-microsatellite regions |
| Methylation-specific PCR (MSP) | Can detect DNA methylation patterns, useful for detecting epigenetic changes associated with cancer | Limited to detecting methylation changes, may miss mutations in non-methylated regions |
Potential benefits and advances in preimplantation genetic testing used for hereditary cancers
Since 1990, the list of genetic conditions referred for PGT has increased due to the remarkable developments in PGT-M, which have also increased the success rates of positive pregnancies and births of genetically unaffected children. Such developments are likely to continue with advancements in the techniques and protocols used in PGT analysis and ART, respectively.17 Li-Fraumeni syndrome (p53 tumor suppressor gene pathogenic variants),25 neurofibromatosis type 2,32 neurofibromatosis type 1,48 familial adenomatous polyposis coli,24 and RB are some of the reported autosomal dominant inherited cancer cases in which PGT was used as an alternative preventive option.7,36,49, 50, 51, 52, 53 The developments in IVF, particularly embryo culture techniques in conjunction with the evolution of the freezing processes of the embryos using ultra-rapid freezing or so-called vitrification, have led to a major shift in utilizing day 5 (D5) biopsies instead of day 3 (D3) biopsies. This development facilitates the screening of biopsied embryos by two tests, such as PGT-M and PGT-A, to select the best embryos, thus increasing the pregnancy rate.5 Shifting to D5 biopsy demonstrated high efficacy and limited harm to embryos, and it also provided sufficient time to perform the necessary analyses compared with D3 biopsies, where the results must be released within 2 days to make decisions on embryo transfer. Therefore, the ability to fully multiply the genome from one cell or from several cells has been realized.40
These developments have been used to identify the nucleotide sequences of large areas of the genome, which has led to more comprehensive and accurate analyses and an all-in-one solution for PGT-A, PGT-SR, and PGT-M using multiple techniques, including NGS and SNP microarray.54 Regardless of the scientific debate on the clinical value of the PGT-A test for preventing miscarriage or its role in obtaining a healthy child,55 this approach will definitely decrease the time, labor, and cost of the test.
Consistent with the aforementioned ideas, major developments have been observed in all aspects of ART, especially the work of PGT, which has greatly improved the ability to genetically examine and interrogate gametes and embryos, such as by routine laser techniques, which is used for the collection of cells and aids in hatching, cutting, and removal.17
Such developments have been made in parallel with determining the genetic sequence of the human genome in the early 2000s and precisely identifying the genetic basis for a large number of genetic diseases resulting from a single genetic pathogenic variant. Such findings have led to an expansion in the range of diseases that can be treated through PGT, which includes three main groups of cases: childhood fatal diseases, late-onset diseases, and different types of cancers. Moreover, human leukocyte antigen (HLA) testing has been developed to match organ and tissue transplant recipients with compatible donors to enable the use of cord blood stem cells for transplantation to affected children in the family.5
Owing to significant improvements in our understanding of the molecular basis of cancers and the ability to sequence genes or whole exons, the predisposition for inherited cancer is considered a major emerging indicator for PGT.17
Progress in human genome analysis has directly impacted the application of PGT, making it possible to combine the analysis of the whole chromosome and one pathogenic variant and evaluate mitochondrial DNA in one assay.56 It has also been expanded so that the expected diagnosis may be based on epigenetic and transcriptomic evaluations.56 These potential technical goals include improving disease prognosis, serving patients at an older reproductive age, and improving IVF outcomes.56
PGT can prevent the transmission of pathogenic variants from parents to their offspring, thereby reducing the risk of passing on suffering to future generations. Thus, if PGT is applied periodically and consistently to such variants as a reproductive option, this technique may reduce the prevalence of genetic diseases caused by these variants in the community.11 Therefore, the future of PGT is moving toward ePGT, which includes polygenic and late-onset diseases and epigenetics, transcriptomic, and non-invasive assessments of embryos.56 The future application of ePGT could provide additional information on clinical characteristics or prognostic outlooks.
The use of central online databases to integrate and share data globally is another promising development, as suggested by El-Toukhy and Braude,6 who also mentioned that these databases should be validated using a comprehensive set of strict validation rules to prevent minor errors and emphasized that this method is one of the simplest, most efficient, and least prone to data errors.6 This approach has been implemented in other genetic databases available online; therefore, it can be used to link other international databases that include different areas of healthcare, such as cancer registration, to identify potential associations among IVF, PGT, and cancer. In setting up these databases, the raw data would be accessible to researchers to facilitate further studies.6 New diseases have been added to the list of diseases being examined and treated by PGT, including polygenic diseases (PGT-P), such as hereditary cancers, owing to the machine learning applied to data from population-level biobanks.57
The following represent advances in PGT that could be utilized for hereditary cancer.
-
1.
Non-invasive PGT: The development of non-invasive PGT, which can be performed without the need for embryo biopsy, is another promising area of research. This method involves analysis of the spent culture medium surrounding the embryo, which contains genetic material shed by the developing embryo.
-
2.
Machine learning and population-level biobanks: The application of machine learning to data from population-level biobanks has enabled the identification of potential genetic associations with hereditary cancers. This could lead to the development of new polygenic disease models for PGT-P that could improve disease prognosis and treatment. Machine-learning algorithms are being developed to analyze PGT data and identify patterns that may be difficult for human analysts to detect. This could lead to more accurate and efficient PGT testing as well as the identification of new genetic markers associated with hereditary cancer predispositions.
-
3.
Improved techniques and protocols: The development of improved techniques and protocols in PGT has made it possible to genetically examine and interrogate gametes and embryos more accurately. For example, the routine use of laser techniques to collect cells and aid hatching, cutting, and removal has become more widespread, thus making the process less invasive and more efficient.
-
4.
Multi-omics analysis: Multi-omics analysis is an emerging area of research that combines multiple omics technologies to better understand the complexity of genetic diseases, including hereditary cancer. This approach could lead to a more comprehensive PGT that incorporates information from multiple sources.
-
5.
Single-cell analysis: Single-cell analysis in PGT has become more common and allowed for the examination of genetic material from individual cells. This can provide more accurate information about genetic abnormalities as well as mosaicism.
-
6.
Clustered regularly interspaced short palindromic repeat (CRISPR)-Cas9 gene editing: The development of CRISPR-Cas9 gene-editing technology has led to new possibilities for correcting genetic abnormalities in embryos. This technology can potentially be used in conjunction with PGT to correct genetic mutations before implantation.
-
7.
Non-invasive prenatal testing: Non-invasive prenatal testing (NIPT) is a type of genetic testing that can be performed on the mother's blood during pregnancy to detect chromosomal abnormalities in the fetus. This technology has become more widely available and could potentially be used in conjunction with PGT to provide additional information regarding the health of developing embryos.
-
8.
Artificial intelligence: The use of artificial intelligence (AI) in PGT is an emerging area of research that may lead to more accurate and efficient testing. AI algorithms can potentially be used to analyze large amounts of genomic data and identify patterns that are difficult for humans to detect.
-
9.
Mitochondrial DNA analysis: Mitochondrial DNA (mtDNA) analysis is an emerging area of research in PGT that can provide additional information about the health of developing embryos. Mutations in mtDNA have been associated with a variety of genetic diseases, including hereditary cancers.
-
10.
Improved bioinformatics tools: The development of improved bioinformatics tools has made it easier to analyze large amounts of genomic data, leading to more accurate and efficient PGT testing. This includes the use of cloud-based computing platforms that can handle large datasets and provide real-time data analysis.
-
11.
Gene expression analysis: Gene expression analysis is an emerging area of research in PGT that could provide additional information about the health of developing embryos. By analyzing the levels of gene expression in individual cells, it may be possible to identify genetic abnormalities that are not detectable by traditional PGT methods.
Challenges and limitations of preimplantation genetic testing in hereditary cancer cases
The first challenge observed in the use of PGT for hereditary cancer cases was the late onset of these genetic conditions, where the disease phenotype does not appear until puberty or later.6 The other challenge was examining the embryos via PGT for pathological conditions that are characterized by low penetrances, such as certain hereditary cancers, in which the probability of occurrence ranged from approximately 30–80%. Although these diseases may be controlled early through periodic examinations or treated, a child or adult who carries the pathogenic variants responsible for these diseases may never show symptoms.6
In some cases, PGT may not be an option because it does not have the ability to accurately diagnose the case clinically in terms of the timing, severity, and number of tumors or the potential for further cancers. Moreover, although individuals born through IVF will not be at risk of specific cancer that is tested by PGT, they still have the opportunity to suffer from another type of cancer because nearly one in three of the world's population are at risk of developing some type of cancer over their lifetime.58 One of the pitfalls of PGT-M is the unknown long-term effect of biopsy on embryos, although this might be solved by non-invasive assessments using approaches other than embryo biopsy, including slight aspiration of the blastocoel fluid (BF) or analysis of spent culture media.56
Although our knowledge and understanding of genetics have increased greatly, the question remains about how to determine whether the danger of a pathological variant to the health of a child or adult is imminent or significant.59
In cases where the pathogenic variants have not been identified or haplotype studies have not provided specific information on a pathogenic variant in families, PGT may be excluded as a reproductivity option. Although a pathogenic variant may have been identified in a germline, such as in RB1, the gene for the pathogenic variant may not be identifiable in a single cell in such families.7
Moreover, a limited number of medical centers offer this test in some countries because of the technical challenges of working in the PGT field. Therefore, couples who are more likely to pass the mutated gene to their offspring may have limited opportunities to benefit from this technology.7,60
The decision on whether to continue performing PGT examinations leads to certain psychological dilemmas; this represents one of the hidden challenges in PGT work. Such decisions often lead to great psychological pressure and emotional upheaval. The decision to proceed with the examination has positive and negative effects on the involved individuals, including the couple seeking a child and their family, as well as society.61
Ethical and legal boundaries
These techniques also present certain legal and ethical challenges.59 Although the European Society for Human Reproduction and Embryology (ESHRE) and HFEA have accepted the use of PGT for adult-onset and multifactorial diseases, such as cancer, this approval remains controversial because these diseases are not evident in the early stages and may never appear at all.10,62
In many countries, the use of PGT is restricted by law, although the degree of restriction varies. In addition, clear guidelines or mechanisms regarding the limits of permitted and prohibited work by this technology are lacking. Moreover, the use of PGT for special indications has sparked many additional concerns.5,63
Currently, PGT is performed on a large scale in specialized centers worldwide. These above ethical issues occur more frequently as the number of applications increases.64 In certain countries that do not have governmental laws that limit its use, PGT has become a routine procedure. Recent scientific reviews have shown that countries differ in the way that they deal with this technology. For example, explicit and clear legislation has been enacted in Spain, France, the UK, and the Netherlands, laws governing the process are lacking in the US and Belgium, and PGT is limited by strict laws in Sweden, Italy, Germany, and Austria.17,19,63
Local and national laws allow PGT to be applied for diseases in which there is no effective treatment available; however, for some diseases, PGT procedures are permitted within a limited framework and usually have more restrictions. For example, in Belgium, the law necessitates agencies implementing PGT diagnostics to determine the motives of couples who intend to perform a PGT test for HLA before giving approval.5
Therefore, one of the persistent problems with PGT is the type of disease that should be diagnosed, and considerable disagreement has been observed regarding this point. In the UK, all PGT must be approved by the HFEA. When PGT was initially approved for BC, the HFEA prepared a consultation document and offered the first license to perform PGT for BC to the University College London Centre (UCL) in 2007.3
The decision of the ESHRE Preimplantation Genetic Diagnosis (PGD) Consortium to begin collecting data on the use of PGT to determine the sex of a fetus was met with controversy, some important figures in the PGT field felt that these data should not be disclosed. This debate will continue, especially after performing comprehensive genome analyses, such as WGS techniques to determine the entire sequence of the human genome or SNP arrays to diagnose many disorders from a single cell.3
Ethical issues have been raised in certain scenarios, such as a couple who requests IVF because of infertility but then one member has a late-onset disorder or cancer predisposition syndrome. In cases where all embryos are affected, the couple may decide to transfer the affected embryo because healthy embryos are not available. This choice is due to the fact that the need for a child outweighs the need to have children free of that disorder.3
Examples of ethically controversial applications of PGT cases include gender selection for social reasons, the so-called “intermediate” cases of PGT diagnosis, especially the PGT/HLA classification, late-onset disorders and low-penetrance pathogenic variants, and non-medical purposes. As mentioned earlier, the use of PGT for HBOC is more controversial for Huntington's disease because penetration is uncertain and medical interventions may prevent them, such as performing preventive surgery, and effectively reducing morbidity and mortality rates in carriers of this disease.65,66
Moreover, the process of determining cancer predisposition genes involves assessing the risk and not providing an absolute finding of the occurrence of disease during the life of the examined embryo. More importantly, the option of using PGT to have a child with special characteristics such as hair and eye color, intelligence, and athletic ability is very limited because of the need for a genetic basis for these traits and the possibility of being traced genetically. In addition, these traits should be present at a high frequency in the parents and sufficient to make the selection of the appropriate embryo possible. Moreover, some traits such as intelligence, are affected by the environment and are complex traits that depend on multiple genes.67
Nevertheless, decisions based on incomplete conclusions should be avoided.68 Although these pathogenic variants are not fully penetrating, they still present a high risk for carriers, such as 80% in the case of BC and 50% in the case of OC. A question regarding the penetrance of cancer: why does the penetrance of the variant have to be complete to give permission for PGT? Therefore, the evaluation criteria may be unstable.3 For example, in the case of X-linked disorders, 50% of male fetuses are healthy; thus, these fetuses are not chosen. Second, ethically relevant questions remain regarding the effectiveness of the available preventive and/or curative measures and the burdens they involve.69 Reports have indicated that the effectiveness of medical surveillance is currently far from ideal. Although the effectiveness of preventive bilateral mastectomy appears to be high, longer follow-up studies with a greater number of BC carriers are necessary to determine the preventive value of this procedure.70 Moreover, preventive surgeries for mastectomy have side effects on the quality of life for women.71
In cases where surgical removal is not successful or appropriate, such as for cancer-prone organs, e.g., testicular cancer, the patient may also suffer from a high-penetrance pathogenic variant. Such families likely will not accept the birth of a child if it means that the child will undergo sterilizing surgery to avoid possible cancer. Therefore, parents' concerns about the lack of a suitable alternative treatment strategy are valid.72
An important point regarding this approach is that the surgical intervention does not protect against other diseases related to this pathogenic variant. For example, in medullary thyroid cancer (MTC), which is caused by pathogenic variants in the MEN2A gene, the surgical removal of the cancer tissue does not remove the risk that the disease will recur and complications may still occur after the operation.11
PGT-M is a powerful tool in reproductive genetics. Although the complete penetrance criterion is restrictive for allowing IVF and PGT, it is crucial to determine the minimum degree of penetrance for each disease. Testing for diseases with complete penetrance and a minimum degree of penetrance is important because it helps to identify whether an individual requires PGT or ART assistance. In addition, the use of PGT is desirable and permissible for individuals who require ART regardless of whether they are at risk of developing cancer. For individuals carrying genes that put them at risk of developing cancer and who need to preserve their sperm or oocytes, PGT is a promising option. As a result, careful evaluations of patients and their families along with multidisciplinary counseling and support are essential for determining the best course of action for using PGT for monogenic disorders.66,73
The most common potential ethical scenario that may affect the use of PGT in cancer diseases in which the penetration potential is high is that the parents are unable to care for the child because of illness or death from the tested disease. Such scenarios apply to life-threatening diseases, such as human immunodeficiency virus (HIV), or embryo or gamete cryopreservation before cancer treatment. However, the potential loss of parents or caretakers for the expected child does not obviate the need for PGT.6 In such cases, although the child may lose a parent earlier than expected and thus will suffer grief and loss, the other parent or a relative or caregiver may be able to support the child. Additionally, this risk is unavoidable. Psychological trauma resulting from the death or illness of a parent is not a sufficient reason to prevent the birth of a child. Moreover, the child may be a source of happiness for parents suffering from the disease. Thus, parents with the risk of cancer and doctors who help them have a healthy child cannot be judged on the pretext that they may cause unnecessary suffering to their children.74
Lastly, the use of PGT for non-medical issues has raised many ethical and social questions, including whether parents' reproductive concerns give them the authority to create and destroy embryos and whether these tests may cause harm to the children who are conceived, denigrate the workers, or even cause social harm.67 Despite the legal and ethical controversy surrounding such cases, a large number of patients consider PGT a preferred option and sometimes the only option to achieve a pregnancy with a healthy child.17
Ethical comments and speculation about current and expanded uses of preimplantation genetic testing
Advances in array technology and NGS will help reduce the effort and introduce automation in the PGT process; however, they will also create challenges based on the generation of a huge amount of genetic information, which will lead to ethical challenges for genetic counseling. Technological development generates an enormous amount of information reflected in the increased possibility of controlling the targeting of traits in the field of PGT. This must be paralleled by changes in ethical thinking and requires in-depth and comprehensive discussions associated with these new capabilities, which will extend from diagnosing monogenic diseases to predicting the risk of polygenic disorders, such as hereditary cancer.42
Therefore, ethical comments and speculation about future increases in genetically based selection and genetic manipulation through the exploitation of PGT represent legitimate points of discussion. This process must be organized so that it does not deviate from the acceptable ethical framework for such practices because work environments around the world differ in how they adhere to ethical standards.
In general, ethical objections to the application of PGT and its controversial extension proposals can be categorized into two groups: the first group objects to the principle of generating embryos, selecting them on chromosomal or genetic grounds, and then disposing of unselected embryos; and the second group criticizes the selection process itself, regardless of the reason. The background of these objections is that some people consider the fetus to be an individual and therefore object to the principle of creation and destruction; therefore, they are opposed to most of the uses of PGT. Other people consider the preimplantation embryos as primitive in their stage of development; thus, they believe the embryos have no interests or rights. However, they believe that they deserve respect as the first stage of the formation of a new human being.74
There is another category of people who object to the selection itself and the dangers of expanding the selection of embryos in the future. Their view stems from a religious belief surrounding the nature of human reproduction: they believe it is wrong to select offspring traits regardless of whether the intentions are noble. This group considers any form of selection as turning the child into a “manufactured product” and thus impairs human individuality established by diversity and random selection of traits.75 Some religions, such as Christianity and Catholicism, firmly oppose the manipulation of embryos and the use of PGT for genetic selection. This has led to debates and conflicts regarding the ethical implications of PGT, particularly in countries where religion plays a significant role in public policy and healthcare decisions.
The second source of concern arises from the extension of genetic testing to non-medical purposes, which will inevitably invite comparisons to eugenics and children “designers” who are more concerned with estimating the genotype of children than their inherent characteristics, which would lead to genetic engineering of humans as a routine practice. These concerns present scientists with the challenge of defining the line between what is ethical, legitimate, and consistent with the applicable regulatory guidelines in the field of PGT. Considering the aforementioned diversity of adherence to these ethical standards across the world, PGT may result in a breach of undesirable consequences.74
Termination of pregnancy is considered a very stressful and difficult choice for couples and causes practical, psychological, and ethical issues; therefore, PGT for embryos represents an alternative to the prenatal examination of embryos and subsequent termination. Moreover, preimplantation embryos are less controversial than embryos in the mother's womb because they are less developed, regardless of their developmental stages. Therefore, the process that occurs during PGT from creating and destroying embryos to producing a healthy child is not considered an arrogant or frivolous way of dealing with embryos, and it respects embryos at this stage of development. Accordingly, every case that requires an abortion, such as for diseases that may affect the fetus, is governed by laws that permit abortion. In such cases, it is a fortiori that the embryo is selected using the PGT technique.
However, diseases whose symptoms begin late in life should not be judged differently from diseases that arise from Mendelian disorders and whose symptoms begin in the early stages of life. Having a child with genetic susceptibility to cancer constitutes a major source of suffering for the parents and child and may negatively affect the couple's decision to have children. Husbands' fear that their children will suffer from genetic diseases may lead them not to have children to spare their children from suffering or the need for special care that constitutes a great financial and moral burden.76
An effective ethics committee is necessary for any laboratory or medical center that provides PGT services.74 Whenever a new technology becomes available, it must be intensively evaluated from all ethical perspectives. Moreover, clear and realistic ethical and laboratory guidelines that control these applications are required to ensure the rights of embryos, ensure that professional ethics are not violated, and ensure that the methods are acceptable to those concerned with producing healthy children.
Legal restrictions according to the country
With regard to the PGT legal restrictions in these countries, wide variations are observed in the policies and regulations in different countries. Some countries have clear and specific laws that specify what is permissible and what is forbidden in terms of PGT cases, whereas other countries leave the matter open to jurisprudence and do not prove specific regulatory mechanisms for eligible cases.63 However, this can lead to ambiguity and uncertainty for clinicians, patients, and researchers in the field of PGT. An example of a country with a flexible approach to PGT is Denmark, where the decision to provide PGT services is based on the severity of the disease and performed by clinical geneticists on a case-by-case basis. This is in contrast to some other countries that limit the use of PGT to specific diseases that have been approved for testing.11,77 The American Thyroid Association (ATA) released a review of MTC guidelines and recommended that physicians inform patients with MEN2A about the possibility of undergoing PGT-M testing and refer them to a genetic specialist clinic; accordingly, patients appeared to show interest in using PGT technology to have healthy babies.11,78,79
In addition, the use of PGT for hereditary cancerous variations is still impacted by legal and moral reservations. Legal restrictions represent an obstacle to the spread and expansion of this technology because practitioners may not want to risk breaking the law.5,19 Overall, the legal landscape surrounding PGT is complex and constantly evolving. As new technologies and techniques emerge, it is important for researchers and clinicians to remain up to date on the latest legal and regulatory developments to ensure that their work is conducted in an ethical and responsible manner.
Non-disclosure of the couples’ status
In families with a history of disease, such as cancers that appear late in life, carriers of the pathogenic variant who want to have healthy children or children free of the pathogenic variant by applying PGT technology may not know that they are carriers of the variant.80 The use of PGT technology for such cases created problems during genetic counseling because individuals who learn that they have these variants may experience negative psychological and social effects.62 The process of discovering that one partner has the variant and may develop the disease leads to questions as to who will provide care for the child in the future in this situation, they may need to discuss the care that the affected partner needs.3
Therefore, in some hereditary cancer diseases caused by an autosomal dominant pathogenic variant, some parents ask for non-disclosure testing or do not have the test, often when the probability of developing the disease is 50% because one of the parents had the disease. One of the most famous diseases to which this example applies is Huntington's disease, also known as late-onset disease.81,82
This situation could also occur for hereditary cancer diseases; therefore, there are two options available in such cases. The first option is to directly examine the pathogenic variant in the fetus without knowing the status of the parents in terms of carrying the disease or being at risk of exposure to the disease, or to indirectly examine the pathogenic variant in the grandparents.17 Such indirect testing involves the transfer of embryos carrying the haplotype of their unaffected grandparents and the exclusion of embryos carrying the haplotype of grandparents because they have a 50% chance of being affected. The second option is exclusion screening, which includes excluding all embryos with a 50% risk probability based on linked markers. This process aims to exclude the transmission of the pathological variant from the affected grandparents to the offspring.5
The main disadvantage of such an option is that it requires the parents to go through IVF, which includes some complex and somewhat dangerous procedures. However, parents may not need to go through IVF if their father or mother is not a carrier of the pathogenic variant.82 Therefore, direct examination of these parents may prevent complicated and expensive procedures.
Here, several ethical and behavioral questions arise. For example, although the disease status and exposure risk of the parents may be known by the institution that performed the PGT examination, the institution may be committed to not disclosing these results and completing the rest of the IVF and PGT procedures.81,83 Non-disclosure of the PGT is applied because it may be the only alternative for couples. Moreover, the ESHRE has recommended that the non-disclosure test should not be preferred over the PGD exclusion test because the exclusion method is considered morally more acceptable.62,84
Challenges to preimplantation genetic testing counseling in cancer predisposition cases
One of the most urgent tasks for those providing genetic counseling and oncology services is to inform patients that they are at high risk of developing a hereditary cancer disease but have the ability to avoid having children with a genetic predisposition to that particular cancer. Likewise, clinics should highlight the options available for couples who may have chosen to remain without children earlier because of their concern about exposure to a PNT and the possibility of termination of pregnancy because of the possibility of having embryos with these diseases.17
Therefore, similar to other genetic testing applications, intensive counseling and education must be provided to concerned couples regarding the benefits and limitations of this test, which necessitates obtaining informed consent before they start the process of benefiting from ARTs, such as IVF and PGT.85 This information includes the costs, the possibility of a multiple pregnancy, and the possibility of obtaining affected embryos that are not suitable for uterine implantation. Moreover, controversy remains because of the increased possibility of developing childhood RB, childhood cancers, birth defects, and developmental disorders in children conceived by IVF technology.86,87 These are some topics that need to be discussed with the medical team when a couple applies for this option to have children to ensure that they making an informed choice. Therefore, potential candidates for this procedure must consider the risks and benefits of using this technique.7
A question regarding the role of PGT healthcare professionals in patient decision-making has been posed. Many personal, geographic, and social factors influence the decision to accept PGT. These factors include medical history, income, qualification level of health care providers, religious beliefs, gender, and age; considering the religious belief factor, it undoubtedly influences the decision related to PGT acceptance.20,78,88,89 Some clerics have suggested that this procedure is similar to abortion, although it is acceptable when the other option is to terminate the pregnancy or donate gametes, which are not morally or religiously acceptable.90
Regarding the economic factor, PGT is an expensive method, which influences the decision to apply the method.88 However, a comparison of the economic costs of PGT testing with the treatment and traditional diagnosis of genetic diseases reveals that this test saves more money in the long term.90 It is worth noting that in some countries, the costs of these examinations are covered by official governmental health care authorities or health insurance, while in others, they are not covered by any of these bodies.11
Population awareness about preimplantation genetic testing applications in hereditary cancer diseases
A review of previous studies showed that awareness of PGT is low among patients who carry genes that may predispose them or their offspring to cancer at an old age.6
Although the utilization of PGT is a practical and important option in the reproductive process for couples who are at risk of having children inherit a pathogenic variant of hereditary cancer, awareness and knowledge of this technology is lacking, even in highly developed countries such as the US, where PGT therapy has been offered for a long time.19,34,91,92
More knowledge about PGT must be available to the general public at risk of developing hereditary cancers. They must be provided with sufficient information about this technology and the available capabilities so that people who need this technology will have the ability to use this option to receive appropriate counseling.22 This requires that health practitioners (medical doctors, gynecologists, and genetic counselors) be made should be aware of the required information to provide more details to patients when requesting medical or genetic counseling.
In the study by Hanson et al., the couple received genetic counseling by a clinical geneticist and was given information about options for childbearing, including PGT-M, spontaneous pregnancy with prenatal screening, obtaining a sperm donation, or adoption.11
A session with a clinical geneticist is necessary to present all the options available for conceiving a healthy child predisposed to diseases that appear in adulthood, and it is an important part of the clinical management for patients with diseases that predispose them to hereditary cancer syndrome.11
Population and practitioner acceptance of preimplantation genetic testing applied for hereditary cancer
Although many people support the application of PGT for serious cases of genetic diseases that show symptoms at the beginning of life, it is more difficult to support the application of PGT to select an embryo and destroy another because of the possibility of a disease affecting the child in the fifth decade of life, such as hereditary cancer diseases or other conditions, e.g., Alzheimer's and Huntington's disease.93
A study in the Netherlands showed that PGT is an acceptable option for couples undergoing HBOC.23 The study by Menon et al. reported that the majority of BRCA gene pathogenic variant carriers endorse the decision to use PGT for this variant, even if they did not choose PGT for themselves.94 Franklin and Roberts reported that couples felt as if they should do anything to meet their desire for a healthy child.95 These answers apply to all other hereditary cancers.36,96
Practitioners in the field were surveyed in the UK and indicated that the use of PGT application to treat low-penetration genes should be up to the parents themselves. They acknowledge that having a child prone to hereditary cancer that may lead to death at an early age is a source of suffering for both parents and the child.6
Franklin and Roberts also mentioned that respondents who were concerned about using PGT to obtain embryos free from a genetic disease wanted to eliminate a genetic disease but did not want ‘designed’ children. They also discussed the image that applying PGT is a slippery slope and was developed before society could control its use. This argument disregards the long history of critical evaluation and controversy that revolves around PGT in society and suggests that PGT workers mainly help to form restrictions on this technology, at least within the UK. As per this viewpoint, a serious and concerted endeavor has been initiated to make the process as accountable as possible to public scrutiny.95
The HFEA committee conducted a survey on the opinions of the public and professionals in this field before authorizing such measures.97 Through this survey, it was concluded that it is appropriate for PGT to be performed for serious diseases, which are characterized by low penetrance and appearance at old age, such as breast, colon, and ovary cancer. Initially, these requests had to be evaluated independently, although this procedure or requirement was ended in 2010 when approval was given to apply PGT for low penetrance cases.6
In a study conducted at the University of Texas, a questionnaire was distributed to people with inherited MEN2 cancer syndrome who visited a genetic counselor at that institution, and they were asked about their awareness and acceptance of using PGT technology.78 Twenty-four percent of the respondents showed that they were aware and knowledgeable about PGT, whereas people with low socioeconomic status showed a lack of knowledge about this technology. These results indicate that awareness and knowledge are lacking among people who may benefit from this technology. The study also showed that 72% of the respondents felt that PGT should be used and implemented and 43% looked to benefit from the technology themselves. This study had limitations in terms of the weak response rate to the questionnaire, with only 38% of the respondents providing their opinions. It is worth noting that an extensive and structured scientific review with a meta-analysis showed the same results as the University of Texas survey.78,89
The variability in cancer syndromes treated by PGT reflects the level of acceptance of PGT among those at risk. This acceptability corresponded to parents’ awareness of the severity of cancer.6 In addition, this acceptance was affected by the cost of the test, technical doubts, and long-term procedures.85
Practical challenges facing preimplantation genetic testing in hereditary cancer cases
Practical issues associated with using PGT technique for hereditary cancer cases have been studied by many researchers. Direct and indirect methods are two widely used approaches for analyzing one-gene disorders. The direct method, which is the preferred method, involves performing fragment analysis for small deletions, additions, or duplications, whereas mini-sequencing is used for point mutations. The indirect method, however, uses a set of short tandem repeat (STR) markers linked to the gene to be tested, but it requires two affected individuals within the family to determine the haplotype associated with the disease. Moreover, the applicability of these methods varies according to the specific mutation.49,98,99
For example, duplication or large deletion mutations cannot be identified by conventional PCR and require other techniques, such as multiplex ligation-dependent probe amplification (MLPA) or Southern blot analysis. Nonetheless, these two procedures have limitations, such as requiring a large amount of DNA and being unsuitable for analyzing single-cell samples. When the pathogenic variants cannot be detected by fragment analysis, the haplotype must be identified by performing an analysis of polar body I versus polar body II or by an evaluation of spermatozoa.8 However, the standard indirect protocol using multiplex PCR to analyze STR markers requires at least two informative STRs on both sides of the gene to accurately detect chromosome recombination events, which can make the protocol more complicated and time-consuming. Therefore, a special protocol is often used for each pair depending on the nature of the existing mutation.50,100,101
Recent guidelines, such as ESHRE's 2020 Good Practice Recommendations for PGT-M, provide a comprehensive overview of best practices for PGT-M. These guidelines emphasize the importance of analyzing both sides of the flanking region of the gene to detect recombination and minimize the risk of misdiagnosis because relying solely on one side can result in undetected recombination events. Additionally, guidelines recommend using several PCR replicates to identify any ADO or amplification efficacy issues because both can vary depending on the cell being analyzed.102 For instance, in the D11S1298 marker was ADO was 20% in the pre-PGT study but 0% in the PGT cycle, whereas, in the D11S1313 genotype, the amplification efficacy was approximately 70% in the pre-PGT study but 90% in the PGT cycle.103
The second method is the indirect method, which occurs when the affected family members are not available and the haplotype must be identified by performing an analysis of polar body I versus polar body II or from a spermatozoon.104,105 In these cases, the precise risk associated with the identified haplotype may not be known because the specific variant cannot be tested. However, it is important to note that even in cases where the risk cannot be precisely determined, the use of indirect PGT can still provide valuable information that can help guide clinical decision-making. For example, in cases in which a high-risk haplotype is identified, this information can be used to inform patients about the potential risks and benefits of pursuing pregnancy. In some cases, patients may choose to proceed with pregnancy despite the known risks, while in other cases, they may choose to explore alternative options, such as donor eggs or adoption.
In cases where a low-risk haplotype is identified, this information can provide reassurance to patients and may help alleviate concerns regarding the potential for passing on a genetic condition to their offspring.
Nevertheless, in some cases, such as the one mentioned by Bautista-Llacer et al., the indirect method is not applicable because the pathogenic variant cannot be detected by PCR.103 The indirect method is the standard PGT method for performing this technique, since the same multiplex PCR comprises the same microsatellite markers that can be applied to other parents or families.106
However, it is very difficult to create multiplex PCR when at least two informative STRs are required in all cases. In addition, the STRs need to be in flanking regions on both sides of the gene to detect recombination events in the chromosomes.107
Thus, the standard indirect protocol is an ad hoc protocol. A special protocol has been developed for each pair, and it depends on the nature of the existing pathogenic variant.103 Overall, while the use of indirect PGT methods may not provide a complete picture of the potential risks associated with a particular genetic variant, it can still provide valuable information that can help guide decision-making and inform patients about their options for family planning. It is important for clinicians to carefully consider the specific circumstances of each case and use appropriate testing and analysis methods to ensure that the information provided is accurate and reliable.
Bautista-Llacer et al. performed fluorescent multiplex heminested PCR to identify the haplotype that carried the pathogenic variant, and they also indicated that the use of multiplex PCR is a reliable method for detecting contamination in samples, the occurrence of allelic dropout, or the recombination of chromosomes.103
Diagnosis should not be based solely on STR markers at one side of the flanking region of the gene because recombinations may not be detected and misdiagnoses may occur.100,108
The recombination between one STR marker and the gene to be investigated was further studied and described by Kakourou et al.109
PGT-M is available for monogenic disorders for which disease-causing variant(s) have been unequivocally identified. These loci are nuclear (X-linked, autosomal dominant, or recessively inherited) or mitochondrial (maternally inherited) and involve pathogenic germline genetic variant(s).110
Azim et al. indicated that ovarian stimulation performed during IVF to obtain more oocytes may increase the concentration of estradiol, which may theoretically increase the risk of recurrence of BC. Therefore, letrozole has been used to inhibit estradiol during IVF and has proven to be effective. However, it is worth noting that a number of case reports demonstrated that the rate of BC recurrence is similar between patients who underwent IVF treatment and those who did not.111,112 Moreover, a comparative study conducted on patients and healthy controls showed that without the use of letrozole, IVF treatment did not increase the recurrence rate of BC originating from a pathogenic variant in the BRCA gene.113 ESHRE guidelines also recommend testing embryos for cancer predisposition genes in certain situations, including when one or both partners are carriers of a cancer predisposition gene or have a family history of cancer.84
Overall, incorporating the ESHRE guidelines into PGT-M practice could help address many of the practical issues faced in the application of the technique to hereditary cancer cases. These guidelines recommend best practices that can lead to accurate diagnoses, reduced risk of misdiagnosis, and improved efficacy of the technique.
Conclusion
This review aimed to shed light on the potential benefits and challenges of PGT for individuals predisposed to hereditary cancer. In addition, the modern technologies used in this field will improve the ability to accurately diagnose these diseases, identify the location of variants, and consequently choose embryos that are free of hereditary cancerous disease.
Despite the challenges and controversies surrounding PGT, its potential to help couples conceive healthy children cannot be ignored. As technology continues to develop and become more advanced, it is important for scientific and medical communities to consider the ethical and social implications of its use. Stakeholders must engage in open and transparent discussions, understand the concerns and perspectives of all parties involved, and develop guidelines and policies to balance the benefits and risks of PGT. In the future, the range of PGT applications will likely expand to include the detection of more genetic abnormalities and diseases. As such, it is important for scientific and medical communities to continue to work together to ensure that PGT is used in a responsible and ethical manner to benefit individuals and society as a whole.
PGT may be the preferred solution for cases in which the hereditary cancer disease is not serious enough to warrant PNT or terminating a pregnancy faces ethical, psychological, or social obstacles; however, these conditions require careful consideration on a case-by-case basis. Therefore, proper selection in every case and multidisciplinary support for patients and their families would make this examination an acceptable option. Such consideration would also ensure that all legal and ethical concerns are met.
Advances in PGT used for hereditary cancer are likely to continue to improve the accuracy and efficiency of testing and provide more information about the health of the developing embryo. As new technologies and research emerge, PGT will likely become an increasingly important tool in the prevention and treatment of hereditary cancers. These potential advances could lead to significant improvements in the prevention and treatment of genetic diseases. As the field continues to evolve and further research is performed, new technologies and approaches are likely to emerge, which will lead to more accurate and efficient PGT testing. These advancements will lead to significant improvements in the safety and efficacy of PGT for hereditary cancers and the continued growth in this field.
The development of guidelines will help stakeholders and practitioners determine the optimal utilization of PGT for a given condition or predisposition. Although almost all genetic applications have experienced certain objections; such concerns have been endured and overcome.
Funding
None.
Authors contribution
Mohammed Albujja: Conception and design of the study, data collection, drafting of the article, and final approval of the version to be published; Maher Al Ghedan: Drafting of the article, critical revision of the article, and final approval of the version to be published; Lakshmidev Dakshnamoorthy: Drafting the article and critical revision of the article; and Josep Pla Victori: Critical revision of the article and final approval of the version to be published.
Ethics statement
None.
Data availability statement
The datasets used in the current study are available from the corresponding author on reasonable request.
Conflict of interest
None.
Acknowledgments
None.
Managing Editor: Peng Lyu
References
- 1.Handyside A.H., Kontogianni E.H., Hardy K., Winston R.M. Pregnancies from biopsied human preimplantation embryos sexed by Y-specific DNA amplification. Nature. 1990;344:768–770. doi: 10.1038/344768a0. [DOI] [PubMed] [Google Scholar]
- 2.Konstantopoulou I., Pertesi M., Fostira F., Grivas A., Yannoukakos D. Hereditary cancer predisposition syndromes and preimplantation genetic diagnosis: where are we now? J BUON. 2009;14(Suppl 1):S187–S192. [PubMed] [Google Scholar]
- 3.Harper J. Cambridge University Press; Cambridge: 2009. Preimplantation genetic diagnosis. [Google Scholar]
- 4.Hardy T. The role of prenatal diagnosis following preimplantation genetic testing for single-gene conditions: a historical overview of evolving technologies and clinical practice. Prenat Diagn. 2020;40:647–651. doi: 10.1002/pd.5662. [DOI] [PubMed] [Google Scholar]
- 5.Griffin D.K., Harton G. CRC Press; Boca Raton: 2020. Preimplantation genetic testing recent advances in reproductive medicine. [Google Scholar]
- 6.El-toukhy T., Braude P. Springer-Verlag; London: 2014. Preimplantation genetic diagnosis in clinical practice. [Google Scholar]
- 7.Xu K., Rosenwaks Z., Beaverson K., Cholst I., Veeck L., Abramson D.H. Preimplantation genetic diagnosis for retinoblastoma: the first reported liveborn. Am J Ophthalmol. 2004;137:18–23. doi: 10.1016/s0002-9394(03)00872-9. [DOI] [PubMed] [Google Scholar]
- 8.Kokkali G., Coticchio G., Bronet F., et al. Consortium and SIG-Embryology Biopsy Working Group. ESHRE PGT Consortium and SIG Embryology good practice recommendations for polar body and embryo biopsy for PGT. Hum Reprod Open. 2020;2020:hoaa020. doi: 10.1093/hropen/hoaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernández R.M., Peciña A., Lozano-Arana M.D., et al. Experience of preimplantation genetic diagnosis with HLA matching at the University Hospital Virgen del Rocío in Spain: technical and clinical overview. BioMed Res Int. 2014;2014 doi: 10.1155/2014/560160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Human Fertilisation and Embryology Authority PGD conditions. https://www.hfea.gov.uk/treatments/embryo-testing-and-treatments-for-disease/approved-pgt-m-and-ptt-conditions/ Published 2006. Available from:
- 11.Würgler Hansen A., Sønderberg Roos L.K., Løssl K., Godballe C., Mathiesen J.S. Preimplantation genetic testing of multiple endocrine neoplasia type 2A. Front Endocrinol. 2020;11 doi: 10.3389/fendo.2020.572151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yandell D.W., Campbell T.A., Dayton S.H., et al. Oncogenic point mutation in the human retinoblastoma gene: their application to genetic counseling. N Engl J Med. 1989;321:1689–1695. doi: 10.1056/NEJM198912213212501. [DOI] [PubMed] [Google Scholar]
- 13.Lima A.D., Alves V.R., Rocha A.R., et al. Preimplantation genetic diagnosis for a patient with multiple endocrine neoplasia type 1: case report. JBRA Assist Reprod. 2018;22:67–70. doi: 10.5935/1518-0557.20180010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thakker R.V. Molecular and cellular endocrinology multiple endocrine neoplasia type 1 (MEN1) and type 4 (MEN4) Mol Cell Endocrinol. 2014;386:2–15. doi: 10.1016/j.mce.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor M.D., Gokgoz N., Andrulis I.L., Mainprize T.G., Drake J.M., Rutka J.T. Familial posterior fossa brain tumors of infancy secondary to germline mutation of the hSNF5 gene. Am J Hum Genet. 2000;66:1403–1406. doi: 10.1086/302833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Rycke M., Staessen C. In: Hoboken: Elsevier Ltd. Molecular diagnostics. Patrinos G.P., editor. 2017. Preimplantation genetic diagnosis; pp. 407–421. [Google Scholar]
- 17.Kuliev A., Rechitsky S., Simpson J.L. Springer Nature Switzerland AG; Basel: 2020. Practical preimplantation genetic testing. [Google Scholar]
- 18.Cram D. Preimplantation genetic diagnosis for familial cancer. Reprod Biomed Online. 2001;3:3–4. doi: 10.1016/s1472-6483(10)61954-2. [DOI] [PubMed] [Google Scholar]
- 19.Bayefsky M.J. Comparative preimplantation genetic diagnosis policy in Europe and the USA and its implications for reproductive tourism. Reprod Biomed Soc Online. 2016;3:41–47. doi: 10.1016/j.rbms.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lammens C., Bleiker E., Aaronson N., et al. Attitude towards pre-implantation genetic diagnosis for hereditary cancer. Fam Cancer. 2009;8:457–464. doi: 10.1007/s10689-009-9265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rechitsky S., Kuliev A. Preimplantation genetic testing (PGT) for non-traditional indication. Reprod Biomed Online. 2019;38:e2–e3. doi: 10.1016/j.rbmo.2019.03.006. [DOI] [Google Scholar]
- 22.Lee V.C., Chow J.F., Lau E.Y., et al. Preimplantation genetic diagnosis for hereditary cancer syndrome: local experience. Hong Kong Med J. 2016;22:289–291. doi: 10.12809/hkmj144499. [DOI] [PubMed] [Google Scholar]
- 23.Derks-Smeets I.A., de Die-Smulders C.E., Mackens S., et al. Hereditary breast and ovarian cancer and reproduction: an observational study on the suitability of preimplantation genetic diagnosis for both asymptomatic carriers and breast cancer survivors. Breast Cancer Res Treat. 2014;145:673–681. doi: 10.1007/s10549-014-2951-5. [DOI] [PubMed] [Google Scholar]
- 24.Ao A., Wells D., Handyside A.H., Winston R.M., Delhanty J.D. Preimplantation genetic diagnosis of inherited cancer: familial adenomatous polyposis coli. J Assist Reprod Genet. 1998;15:140–144. doi: 10.1023/a:1023008921386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verlinsky Y., Rechitsky S., Verlinsky O., et al. Preimplantation diagnosis for p53 tumour suppressor gene mutations. Reprod Biomed Online. 2001;2:102–105. doi: 10.1016/s1472-6483(10)62233-x. [DOI] [PubMed] [Google Scholar]
- 26.Harper J.C., de Die-Smulders C., Goossens V., et al. ESHRE PGD consortium data collection VII: cycles from January to December 2004 with pregnancy follow-up to October 2005. Humanit Rep. 2008;23:741–755. doi: 10.1093/humrep/dem354. [DOI] [PubMed] [Google Scholar]
- 27.Ramón Y., Cajal T., Polo A., et al. Preimplantation genetic diagnosis for inherited breast cancer: first clinical application and live birth in Spain. Fam Cancer. 2012;11:175–179. doi: 10.1007/s10689-011-9497-z. [DOI] [PubMed] [Google Scholar]
- 28.Davis T., Song B., Cram D.S. Preimplantation genetic diagnosis of familial adenomatous polyposis. Reprod Biomed Online. 2006;13:707–711. doi: 10.1016/s1472-6483(10)60662-1. [DOI] [PubMed] [Google Scholar]
- 29.Marra G., Boland C.R. Hereditary nonpolyposis colorectal cancer: the syndrome, the genes, and historical perspectives. J Natl Cancer Inst. 1995;87:1114–1125. doi: 10.1093/jnci/87.15.1114. [DOI] [PubMed] [Google Scholar]
- 30.Dewanwala A., Chittenden A., Rosenblatt M., et al. Attitudes toward childbearing and prenatal testing in individuals undergoing genetic testing for Lynch Syndrome. Fam Cancer. 2011;10:549–556. doi: 10.1007/s10689-011-9448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ilic M.P., Kiralj A., Markov B., Mijatov I., Mijatov S., Vučković N. Li-Fraumeni syndrome: a case report. Vojnosanit Pregl. 2014;71:1159–1162. doi: 10.2298/vsp1412159i. [DOI] [PubMed] [Google Scholar]
- 32.Abou-Sleiman P.M., Apessos A., Harper J.C., Serhal P., Winston R.M., Delhanty J.D. First application of preimplantation genetic diagnosis to neurofibromatosis type 2 (NF2) Prenat Diagn. 2002;22:519–524. doi: 10.1002/pd.393. [DOI] [PubMed] [Google Scholar]
- 33.Huq A., Kentwell M., Tirimacco A., Rossini J., Rawlings L., Winship I. Vestibular schwannoma in a patient with neurofibromatosis type 1: clinical report and literature review. Fam Cancer. 2014;14:157–160. doi: 10.1007/s10689-014-9763-y. [DOI] [PubMed] [Google Scholar]
- 34.Quinn G., Vadaparampi lS., King L., Miree C., Friedman S. Conflict between values and technology: perceptions of preimplantation genetic diagnosis among women at increased risk for hereditary breast and ovarian cancer. Fam Cancer. 2009;8:441–449. doi: 10.1007/s10689-009-9263-7. [DOI] [PubMed] [Google Scholar]
- 35.Sagi M., Weinberg N., Eilat A., et al. Preimplantation genetic diagnosis for BRCA1/2—a novel clinical experience. Prenat Diagn. 2009;29:508–513. doi: 10.1002/pd.2232. [DOI] [PubMed] [Google Scholar]
- 36.Spits C., De Rycke M., Van Ranst N., et al. Preimplantation genetic diagnosis for cancer predisposition syndromes. Prenat Diagn. 2007;27:447–456. doi: 10.1002/pd.1708. [DOI] [PubMed] [Google Scholar]
- 37.Vadaparampil S.T., Quinn G.P., Knapp C., Malo T.L., Friedman S. Factors associated with preimplantation genetic diagnosis acceptance among women concerned about. Genet Med. 2009;11:757–765. doi: 10.1097/GIM.0b013e3181b3f451. [DOI] [PubMed] [Google Scholar]
- 38.Sinilnikova O.M., Mazoyer S., Bonnardel C., Lynch H.T., Narod S.A., Lenoir G.M. BRCA1 and BRCA2 mutations in breast and ovarian cancer syndrome: reflection on the Creighton University historical series of high risk families. Fam Cancer. 2006;5:15–20. doi: 10.1007/s10689-005-2571-7. [DOI] [PubMed] [Google Scholar]
- 39.Kallioniemi A., Kallioniemi O.P., Sudar D., et al. Comparative genomic hybridization for molecular cytogenetic analysis of solid tumors. Science. 1992;258:818–821. doi: 10.1126/science.1359641. [DOI] [PubMed] [Google Scholar]
- 40.Stern H.J. Preimplantation genetic diagnosis: prenatal testing for embryos finally achieving its potential. J Clin Med. 2014;3:280–309. doi: 10.3390/jcm3010280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Che H., Villela D., Dimitriadou E., et al. Noninvasive prenatal diagnosis by genome-wide haplotyping of cell-free plasma DNA. Genet Med. 2020;22:962–973. doi: 10.1038/s41436-019-0748-y. [DOI] [PubMed] [Google Scholar]
- 42.De Rycke M., Berckmoes V., De Vos A., et al. Preimplantation genetic testing: clinical experience of preimplantation genetic testing. Reproduction. 2020;160:A45–A58. doi: 10.1530/REP-20-0082. [DOI] [PubMed] [Google Scholar]
- 43.Rycke M., De Goossens V., Kokkali G., Coonen E., Moutou C. ESHRE PGD consortium data collection XIV-XV: cycles from january 2011 to december 2012 with pregnancy follow-up to october 2013. Humanit Rep. 2017;32:1974–1994. doi: 10.1093/humrep/dex265. [DOI] [PubMed] [Google Scholar]
- 44.Treff N.R., Marin D., Lello L., Hsu S., Tellier L.C.A.M. Preimplantation genetic testing for polygenic disease risk. Reproduction. 2020;160:A13–A17. doi: 10.1530/REP-20-0071. [DOI] [PubMed] [Google Scholar]
- 45.Zimmerman R.S., Eccles J., Jalas C., Treff N.R., Scott R.T., Jr. Molecular testing for preimplantation genetic diagnosis of single gene disorders. Methods Mol Biol. 2018;1885:61–71. doi: 10.1007/978-1-4939-8889-1_4. [DOI] [PubMed] [Google Scholar]
- 46.Yanfei Y.C., Yu Q., Ma M., et al. Variant haplophasing by long-read sequencing: a new approach to preimplantation genetic testing workups. Fertil Steril. 2021;116:774–783. doi: 10.1016/j.fertnstert.2021.04.012. [DOI] [PubMed] [Google Scholar]
- 47.Parikh F.R., Athalye A.S., Kulkarni D.K., et al. Evolution and utility of preimplantation genetic testing for monogenic disorders in assisted reproduction - a narrative review. J Hum Reprod Sci. 2021;14:329–339. doi: 10.4103/jhrs.jhrs_148_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verlinsky Y., Rechitsky S., Verlinsky O., et al. Preimplantation diagnosis for neurofibromatosis. Reprod Biomed Online. 2002;4:218–222. doi: 10.1016/s1472-6483(10)61809-3. [DOI] [PubMed] [Google Scholar]
- 49.Fiorentino F., Magli M.C., Podini D., et al. The minisequencing method: an alternative strategy for preimplantation genetic diagnosis of single gene disorders. Mol Hum Reprod. 2003;9:399–410. doi: 10.1093/molehr/gag046. [DOI] [PubMed] [Google Scholar]
- 50.Girardet A., Hamamah S., Anahory T., et al. First preimplantation genetic diagnosis of hereditary retinoblastoma using informative microsatellite markers. Mol Hum Reprod. 2003;9:111–116. doi: 10.1093/molehr/gag014. [DOI] [PubMed] [Google Scholar]
- 51.Rechitsky S., Verlinsky O., Chistokhina A., et al. Preimplantation genetic diagnosis for cancer predisposition. Reprod Biomed Online. 2002;5:148–155. doi: 10.1016/s1472-6483(10)61617-3. [DOI] [PubMed] [Google Scholar]
- 52.Sutterlin M., Sleiman P.A., Onadim Z., Delhanty J. Single cell detection of inherited retinoblastoma predisposition. Prenat Diagn. 1999;19:1231–1236. doi: 10.1002/(SICI)1097-0223(199912)19:13<1231::AID-PD725>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 53.Xu K., Shi Z., Yuan H., Cholst I., Rosenwaks Z. Diagnosis of retinoblastoma mutation by both gel electrophoresis and fluorescent fragment analysis. Fertil Steril. 2001;763:S237–S238. doi: 10.1016/S0015-0282(01)02705-4. [DOI] [Google Scholar]
- 54.De Rycke M., Berckmoes V. Preimplantation genetic testing for monogenic disorders. Genes. 2020;11:871. doi: 10.3390/genes11080871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Braude P. The emperor still looks naked. Reprod Biomed Online. 2018;37:133–135. doi: 10.1016/j.rbmo.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 56.Poli M., Girardi L., Fabiani M., et al. Past, present, and future strategies for enhanced assessment of embryo's genome and reproductive competence in women of advanced reproductive age. Front Endocrinol. 2019;10:1–12. doi: 10.3389/fendo.2019.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Treff N.R., Marin D., Lello L., Hsu S., Tellier L.C.A.M. Preimplantation genetic testing: preimplantation genetic testing for polygenic disease risk. Reproduction. 2020;160:A13–A17. doi: 10.1530/REP-20-0071. [DOI] [PubMed] [Google Scholar]
- 58.Sasieni P.D., Shelton J., Ormiston-Smith N., Thomson C.S., Silcocks P.B. What is the lifetime risk of developing cancer: the effect of adjusting for multiple primaries. Br J Cancer. 2011;105:460–465. doi: 10.1038/bjc.2011.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Botkin J.R., Belmont J.W., Berg J.S., et al. Points to consider: ethical, legal, and psychosocial implications of genetic testing in children and adolescents. Am J Hum Genet. 2015;97:6–21. doi: 10.1016/j.ajhg.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kanavakis E., Traeger-Synodinos J. Preimplantation genetic diagnosis in clinical practice. J Med Genet. 2002;39:6–11. doi: 10.1136/jmg.39.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pastore L.M., Mitchell C.N., Rubin L.R., Nicoloro-SantaBarbara J., Garzon M.C.G., Lobel M. Patients' preimplantation genetic testing decision-making experience: an opinion on related psychological frameworks. Hum Reprod Open. 2019;4:1–8. doi: 10.1093/hropen/hoz019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shenfield F., Pennings G., Devroey P., et al. Taskforce 5: preimplantation genetic diagnosis. Hum Reprod. 2003;18:649–651. doi: 10.1093/humrep/deg110. [DOI] [PubMed] [Google Scholar]
- 63.Council of Europe . Council of Europe; Strasbourg: 2015. Background document on preimplantation and prenatal genetic testing - clinical Situation.https://www.coe.int/t/dg3/healthbioethic/Activities/07_Human_genetics_en/(2015) Available from: [Google Scholar]
- 64.Daley G., Lovell-Badge R., Steffann J. After the storm - a responsible path for genome editing. N Engl J Med. 2019;380:897–899. doi: 10.1056/NEJMp1900504. [DOI] [PubMed] [Google Scholar]
- 65.Editorial Ethics of preimplantation genetic diagnosis for cancer. Lancet Oncol. 2006;7:611. doi: 10.1016/S1470-2045(06)70768-9. [DOI] [PubMed] [Google Scholar]
- 66.Ethics Committee of the American Society for Reproductive Medicine Use of preimplantation genetic diagnosis for serious adult onset conditions: a committee opinion. Fertil Steril. 2013;100:54–57. doi: 10.1016/j.fertnstert.2013.02.043. [DOI] [PubMed] [Google Scholar]
- 67.Handyside A.H. ‘Designer babies’ almost thirty years on. Reproduction. 2018;156:F75–F79. doi: 10.1530/REP-18-0157. [DOI] [PubMed] [Google Scholar]
- 68.Delhanty J. What are the issues surrounding preimplantation genetic diagnosis for late-onset disorders? Expert Rev Mol Diagn. 2006;6:5–6. doi: 10.1586/14737159.6.1.5. [DOI] [PubMed] [Google Scholar]
- 69.De Wert G. Ethics of predictive DNA-testing for hereditary breast and ovarian cancer. Patient Educ Counsel. 1998;35:43–52. doi: 10.1016/s0738-3991(98)00082-2. [DOI] [PubMed] [Google Scholar]
- 70.Meijers-Heijboer H., van Geel B., van Putten W.L., et al. Breast cancer after prophylactic bilateral mastectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2001;345:159–164. doi: 10.1056/NEJM200107193450301. [DOI] [PubMed] [Google Scholar]
- 71.Türk K., Yılmaz M. The effect on quality of life and body image of mastectomy among breast cancer survivors. Eur J Breast Health. 2018;14:205–210. doi: 10.5152/ejbh.2018.3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baird D.C., Meyers G.J., Hu J.S. Testicular cancer: diagnosis and treatment. Am Fam Physician. 2018;97:261–268. [PubMed] [Google Scholar]
- 73.Donnez J., Martinez-Madrid B., Jadoul P., Van Langendonckt A., Demylle D., Dolmans M.M. Ovarian tissue cryopreservation and transplantation: a review. Hum Reprod Update. 2006;12:519–535. doi: 10.1093/humupd/dml032. [DOI] [PubMed] [Google Scholar]
- 74.Robertson J.A. Extending preimplantation genetic diagnosis: the ethical debate. Ethical issues in new uses of preimplantation genetic diagnosis. Humanit Rep. 2003;18:465–471. doi: 10.1093/humrep/deg100. [DOI] [PubMed] [Google Scholar]
- 75.President’s Bioethics Commission . The President’s Bioethics Commission; Washington, D.C.: 2002. Human cloning and human dignity: an ethical inquiry.https://bioethicsarchive.georgetown.edu/pcbe/reports/cloningreport/fullreport.html Available from: [Google Scholar]
- 76.Simpson J.L. Changing indications for preimplantation genetic diagnosis (PGD) Mol Cell Endocrinol. 2001;183:S69–S75. doi: 10.1016/s0303-7207(01)00573-1. [DOI] [PubMed] [Google Scholar]
- 77.Bayefsky M. Who should regulate prdeimplantation genetic diagnosis in the United States? AMA J Ethics. 2018;20:1160–1167. doi: 10.1001/amajethics.2018.1160. [DOI] [PubMed] [Google Scholar]
- 78.Ric T.A., Liu M., Etzel C.J., et al. Comparison of attitudes regarding preimplantation genetic diagnosis among patients with hereditary cancer syndromes. Fam Cancer. 2014;13:291–299. doi: 10.1007/s10689-013-9685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wells S.A., Jr., Asa S.L., Dralle H., et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25:567–610. doi: 10.1089/thy.2014.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Van Rij M.C., De Rademaeker M., Moutou C., et al. Preimplantation genetic diagnosis (PGD) for Huntington's disease: the experience of three European centres. EJHG (Eur J Hum Genet) 2012;20:368–375. doi: 10.1038/ejhg.2011.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Braude P., De Wert G., Evers-Kiebooms G., Pettigrew R., Geraedts J. Non-disclosure preimplantation genetic diagnosis for Huntington's disease: practical and ethical dilemmas. Prenat Diagn. 1998;18:1422–1426. doi: 10.1002/(sici)1097-0223(199812)18:13<1422::aid-pd499>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 82.Sermon K., De Rijcke M., Lissens W., et al. Preimplantation genetic diagnosis for Huntington's disease with exclusion testing. Eur J Hum Genet. 2002;10:591–598. doi: 10.1038/sj.ejhg.5200865. [DOI] [PubMed] [Google Scholar]
- 83.De Die-Smulders C.E., de Wert G.M., Liebaers I., Tibben A., Evers-Kiebooms G. Reproductive options for prospective parents in families with Huntington's disease: clinical, psychological and ethical reflections. Hum Reprod Update. 2013;19:304–315. doi: 10.1093/humupd/dms058. [DOI] [PubMed] [Google Scholar]
- 84.Carvalho F., Coonen E., Goossens V., et al. ESHRE PGT Consortium Steering Committee ESHRE PGT Consortium good practice recommendations for the organisation of PGT. Hum Reprod Open. 2020;3:1–12. doi: 10.1093/hropen/hoaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lamb B., Johnson E., Francis L., et al. Pre-implantation genetic testing: decisional factors to accept or decline among in vitro fertilization patients. J Assist Reprod Genet. 2018;35:1605–1612. doi: 10.1007/s10815-018-1278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moll A.C., Imhof S.M., Cruysberg J.R., et al. Incidence of retinoblastoma in children born after in-vitro fertilisation. Lancet. 2003;361:309–310. doi: 10.1016/S0140-6736(03)12332-X. [DOI] [PubMed] [Google Scholar]
- 87.Bradbury B.D., Jick H. In vitro fertilization and childhood retinoblastoma. Br J Clin Pharmacol. 2004;58:209–211. doi: 10.1111/j.1365-2125.2004.02109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Genoff Garzon M., Rubin L., Lobel M., Stelling J., Pastore L. Review of patient decision-making factors and attitudes regarding preimplantation genetic diagnosis. Clin Genet. 2018;94:22–42. doi: 10.1111/cge.13174. [DOI] [PubMed] [Google Scholar]
- 89.Quinn G.P., Pal T., Murphy D., Vadaparampil S.T., Kumar A. High-risk consumers' perceptions of preimplantation genetic diagnosis for hereditary cancers: a systematic review and meta-analysis. Genet Med. 2012;14:191–200. doi: 10.1038/gim.0b013e31822ddc7e. [DOI] [PubMed] [Google Scholar]
- 90.Burton A. Controversy surrounds the selection of embryos to avoid cancer. Lancet Oncol. 2009;10:545. doi: 10.1016/s1470-2045(09)70150-0. [DOI] [PubMed] [Google Scholar]
- 91.Quinn G.P., Vadaparampil S.T., Miree C.A., et al. High risk men's perceptions of pre-implantation genetic diagnosis for hereditary breast and ovarian cancer. Hum Reprod. 2010;25:2543–2550. doi: 10.1093/humrep/deq207. [DOI] [PubMed] [Google Scholar]
- 92.Verlinsky Y., Pergament E., Strom C. The preimplantation genetic diagnosis of genetic diseases. J In Vitro Fert Embryo Transf. 1990;7:1–5. doi: 10.1007/BF01133875. [DOI] [PubMed] [Google Scholar]
- 93.Klipstein S. Preimplantation genetic diagnosis: technological promise and ethical perils. Fertil Steril. 2005;83:1347–1353. doi: 10.1016/j.fertnstert.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 94.Menon U., Harper J., Sharma A., et al. Views of BRCA gene mutation carriers on preimplantation genetic diagnosis as a reproductive option for hereditary breast and ovarian cancer. Humanit Rep. 2007;22:1573–1577. doi: 10.1093/humrep/dem055. [DOI] [PubMed] [Google Scholar]
- 95.Franklin S., Roberts C. Princeton University Press; Princeton: 2006. Born and Made: an ethnography of preimplantation genetic diagnosis. [Google Scholar]
- 96.Offit K., Sagi M., Hurley K. Preimplantation genetic diagnosis for cancer syndromes: a new challenge for preventive medicine. J Am Med Assoc. 2006;296:2727–2730. doi: 10.1001/jama.296.22.2727. [DOI] [PubMed] [Google Scholar]
- 97.Krahn T. Where are we going with preimplantation genetic diagnosis? CMAJ. 2007;176:1445–1446. doi: 10.1503/cmaj.061690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Alberola T.M., Bautista-llácer R., Fernández E., Vendrell X., Pérez-Alonso M. Preimplantation genetic diagnosis of P450 oxidoreductase deficiency and Huntington disease using three different molecular approaches simultaneously. J Assist Reprod Genet. 2009;26:263–271. doi: 10.1007/s10815-009-9327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fiorentino F., Biricik A., Nuccitelli A., et al. Strategies and clinical outcome of 250 cycles of preimplantation genetic diagnosis for single gene disorders. Hum Reprod. 2006;21:670–684. doi: 10.1093/humrep/dei382. [DOI] [PubMed] [Google Scholar]
- 100.Altarescu G., Eldar Geva T., Brooks B., Margalioth E., Levy-Lahad E., Renbaum P. PGD on a recombinant allele: crossover between the gene and ‘linked’ markers impairs accurate diagnosis. Prenat Diagn. 2008;28:929–933. doi: 10.1002/pd.2070. [DOI] [PubMed] [Google Scholar]
- 101.Renwick P. Proof of principle and first cases using preimplantation genetic haplotyping – a paradigm shift for embryo diagnosis. Reprod Biomed Online. 2006;13:110–119. doi: 10.1016/s1472-6483(10)62024-x. [DOI] [PubMed] [Google Scholar]
- 102.Thornhill A.R., deDie-Smulders C.E., Geraedts J.P., et al. ESHRE PGD Consortium ‘Best practice guidelines for clinical preimplantation genetic diagnosis (PGD) and preimplantation genetic screening (PGS)’. Hum Reprod. 2005;20:35–48. doi: 10.1093/humrep/deh579. [DOI] [PubMed] [Google Scholar]
- 103.Bautista-Llacer R., Alberola T.M., Vendrell X., Fernández E., Pérez-Alonso M. Case report: first successful application of preimplantation genetic diagnosis for hereditary angiooedema. Reprod Biomed Online. 2010;21:658–662. doi: 10.1016/j.rbmo.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 104.Altarescu G., Eldar-Geva T., Varshower I., et al. Real-time reverse linkage using polar body analysis for preimplantation genetic diagnosis in female carriers of de novo mutations. Hum Reprod. 2009;24:3225–3229. doi: 10.1093/humrep/dep293. [DOI] [PubMed] [Google Scholar]
- 105.Burlet P., Gigarel N., Magen M., et al. Single-sperm analysis for recurrence risk assessment of spinal muscular atrophy. Eur J Hum Genet. 2010;18:505–508. doi: 10.1038/ejhg.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gigarel N., Frydman N., Burlet P., et al. Preimplantation genetic diagnosis for autosomal recessive polycystic kidney disease. Reprod Biomed Online. 2008;16:152–158. doi: 10.1016/s1472-6483(10)60569-x. [DOI] [PubMed] [Google Scholar]
- 107.Spits C., Sermon K. PGD for monogenic disorders: aspects of molecular biology. Prenat Diagn. 2009;29:50–56. doi: 10.1002/pd.2161. [DOI] [PubMed] [Google Scholar]
- 108.Wilton L., Thornhill A., Traeger-Synodinos J., Sermon K.D., Harper J.C. The causes of misdiagnosis and adverse outcomes in PGD. Hum Reprod. 2009;24:1221–1228. doi: 10.1093/humrep/den488. [DOI] [PubMed] [Google Scholar]
- 109.Kakourou G., Dhanjal S., Daphnis D., et al. Preimplantation genetic diagnosis for myotonic dystrophy type 1: detection of crossover between the gene and the linked marker APOC2. Prenat Diagn. 2007;27:111–116. doi: 10.1002/pd.1611. [DOI] [PubMed] [Google Scholar]
- 110.Richards S., Aziz N., Bale S., Bick D., Das S. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of medical genetics and genomics and the association for molecular pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Azim A.A., Costantini-Ferrando M., Oktay K. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: a prospective controlled study. J Clin Oncol. 2012;26:2630–2635. doi: 10.1200/JCO.2007.14.8700. [DOI] [PubMed] [Google Scholar]
- 112.Reddy J., Oktay K. Ovarian stimulation and fertility preservation with the use of aromatase inhibitors in women with breast cancer. Fertil Steril. 2012;98:1363–1369. doi: 10.1016/j.fertnstert.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 113.Kotsopoulos J., Librach C.L., Lubinski J., et al. Infertility, treatment of infertility, and the risk of breast cancer among women with BRCA1 and BRCA2 mutations: a case-control study. Cancer Causes Control. 2008;19:1111–1119. doi: 10.1007/s10552-008-9175-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used in the current study are available from the corresponding author on reasonable request.