Abstract
Contamination of feed with mycotoxins has become a severe issue worldwide. Among the most prevalent trichothecene mycotoxins, T-2 toxin is of particular importance for livestock production, including poultry posing a significant threat to animal health and productivity. This review article aims to comprehensively analyze the pathological consequences, metabolism, and toxic effects of T-2 toxin in poultry. Trichothecene mycotoxins, primarily produced by Fusarium species, are notorious for their potent toxicity. T-2 toxin exhibits a broad spectrum of negative effects on poultry species, leading to substantial economic losses as well as concerns about animal welfare and food safety in modern agriculture. T-2 toxin exposure easily results in negative pathological consequences in the gastrointestinal tract, as well as in parenchymal tissues like the liver (as the key organ for its metabolism), kidneys, or reproductive organs. In addition, it also intensely damages immune system-related tissues such as the spleen, the bursa of Fabricius, or the thymus causing immunosuppression and increasing the susceptibility of the animals to infectious diseases, as well as making immunization programs less effective. The toxin also damages cellular processes on the transcriptional and translational levels and induces apoptosis through the activation of numerous cellular signaling cascades. Furthermore, according to recent studies, besides the direct effects on the abovementioned processes, T-2 toxin induces the production of reactive molecules and free radicals resulting in oxidative distress and concomitantly occurring cellular damage. In conclusion, this review article provides a complex and detailed overview of the metabolism, pathological consequences, mechanism of action as well as the immunomodulatory and oxidative stress-related effects of T-2 toxin. Understanding these effects in poultry is crucial for developing strategies to mitigate the impact of the T-2 toxin on avian health and food safety in the future.
Key words: mycotoxin, chicken, poultry, T-2 toxin, trichothecene
INTRODUCTION
In the contemporary world, contamination of feed and food by various mold species is increasingly becoming a severe issue. As secondary metabolites, these fungi can produce chemicals that, while not essential for their survival, may profoundly impact the physiological status of the organism producing them. The presence of these molds in feed is common, but the quantity of secondary metabolites they release is not directly proportional to the level of fungal contamination. This discrepancy arises because molds spend a significant proportion of their lifecycle producing primary metabolites—less harmful molecules, necessary for their development and reproduction—under favorable living conditions (Zhao et al., 2021; Liu et al., 2022). However, during certain phases or due to sudden changes in temperature, nutrient availability, or oxygen levels, fungi begin synthesizing secondary metabolites (Edite Bezerra da Rocha et al., 2014). Such metabolites include for example, plant growth factors, chemicals combating microorganisms, specific pigments, as well as mycotoxins (Mukherjee et al., 2017; Janevska and Tudzynski, 2018; Keswani et al., 2019). The latter are toxic compounds, that can influence the metabolic and health status of animal cells, particularly protein and nucleic acid metabolism as well as other biochemical processes within cellular components. Recent research suggests that there are hundreds of poisonous compounds produced by fungi, although the number of mycotoxins relevant to animal feeding is substantially lower (Omotayo et al., 2019; Awuchi et al., 2022).
Extensive research on mycotoxin-related damage and their exact mechanism of action commenced in the 1960s. This discovery followed an unexplained and unknown ailment (turkey X disease), which led to the death of more than 100,000 turkeys in England, and was attributed to high levels of aflatoxin contamination in peanut meal in the feed (Bradburn et al., 1994). In contrast to less frequent acute mycotoxicoses, there is now a much higher incidence of low-level, longer-term mycotoxin contamination, leading to the development of chronic and less specific symptoms in animals (Bryden, 2012). Immunosuppressive effects of mycotoxins have been observed wherein even concentrations below the acute intoxication dose significantly increased the animals' susceptibility to complex multifactorial diseases (Awad et al., 2013; Pierron et al., 2016). Mold presence is often an unrecognized problem causing substantial economic damage to crops, livestock and food production globally. Recent surveys indicate that in Europe, approximately 60 to 70% of all crop samples are contaminated with mycotoxins. In some parts of Asia and America, the percentage exceeds 80% (Luo et al., 2021; Nešić et al., 2023; Sun et al., 2023). Only a fraction of this contaminated amount, that poses a severe risk, is destroyed, while the remainder is used as food or feed. The significance of mycotoxins for food hygiene and safety is underscored by their accumulation in animals, entering the food chain, and potentially causing harm to humans through the ingestion of animal-derived food (Hussain et al., 2010; Bansal et al., 2023).
Mold contamination does not invariably imply mycotoxin presence, as their production can be influenced by various external factors. These factors may include temperature, humidity, pH conditions, specific climatic elements, the fungi species infecting the feed, the timing of infection or the extent of damage to the grain. It is crucial to note that, depending on environmental factors, many fungal species may produce entirely different types of mycotoxins (Rodríguez and Núñez, 2020). Studies on mycotoxins in feed also suggest that higher levels of mycotoxin contamination can be expected under more extreme weather conditions (Van der Fels-Klerx et al., 2016). Grains most commonly contaminated with T-2 and HT-2 toxin include wheat, maize, barley, and oats. Detailed information of contaminated unprocessed grains according to recent European Food Safety Authority (EFSA) results are presented in Table 1 (European Food Safety Authority et al., 2017). The incidence of T-2 and HT-2 toxin varies greatly between depending on the geographical regions. According to recent results, prevalence of the toxin is the highest in oat samples. Examples for highly affected areas are southern Finland and northern Italy where dangerous amounts of contaminations were reported (Luo et al., 2021). T-2 and HT-2 toxins were found in more than 60% of oat samples in Finland, with average concentrations of T-2 and HT-2 as high as 60.1μg/kg and 159 μg/kg, respectively (Nathanail et al., 2015). Among the most contaminated samples, the sum of T-2 and HT-2 toxin (1,860 μg/kg) exceeded the current threshold applied by the European Commission (200 μg/kg) by 8 times (Luo et al., 2021). Similarly, durum wheat in Italy had T-2 values as high as 149 μg/kg and 486 μg/kg (Juan et al., 2016). These results emphasize the great importance of implementing comprehensive meteorological surveillance together with strict quality control measures to effectively decrease the health hazards connected to trichothecene exposure (Nada et al., 2022).
Table 1.
Summary statistics of the levels of individual T2, HT2, and sum of the T2 and HT2 in unprocessed grain samples according to EFSA results.
| Commodity | N | %LC | Concentration range (μg/kg) |
|||
|---|---|---|---|---|---|---|
| Mean | Median | P75 | P95 | |||
| T-2 toxin | ||||||
| Grains as crops, unspecified | 1 | 100 | 0.00–19.0 | 0–19.0 | 0–19.0 | – |
| Wheat grain crop | 85 | 98 | 0.32–19.8 | 0–25.0 | 0–25.0 | 0–25.0 |
| Barley grain | 63 | 98 | 0.70–80.8 | 0–100 | 0–100 | 0–100 |
| Corn grain | 17 | 76 | 8.92–20.3 | 0–6.40 | 0–24.6 | – |
| Rye grain | 17 | 100 | 0.00–91.2 | 0–100 | 0–100 | – |
| Spelt grain | 3 | 100 | 0.00–2.00 | 0–2.00 | 0–2.00 | – |
| Buckwheat grain | 4 | 100 | 0.00–87.5 | 0–100 | 0–100 | – |
| Millet grain | 2 | 100 | 0.00–75.0 | 0–75.0 | 0–100 | – |
| Oats, grain | 91 | 87 | 1.52–33.7 | 0–50.0 | 0–50.0 | 10.4–150 |
| Rice | 191 | 100 | 0.00–93.2 | 0–100 | 0–100 | 0–100 |
| HT-2 toxin | ||||||
| Grains as crops, unspecified | 1 | 100 | 0.00–47.5 | 0–47.5 | 0–47.5 | – |
| Wheat grain crop | 68 | 99.5 | 0.13–20.2 | 0–25.0 | 0–25.0 | 0–25.0 |
| Barley grain | 61 | 98 | 0.89–95.6 | 0–100 | 0–100 | 0–100 |
| Corn grain | 7 | 100 | 0.00–8.29 | 0–7.60 | 0–10.0 | – |
| Rye grain | 17 | 100 | 0.00–100 | 0–100 | 0–100 | – |
| Spelt grain | 3 | 100 | 0.00–0.00 | 0.00–0.00 | 0.00–0.00 | – |
| Buckwheat grain | 4 | 100 | 0.00–100 | 0–100 | 0–100 | – |
| Millet grain | 2 | 100 | 0.00–100 | 0–100 | 0–100 | – |
| Oats, grain | 91 | 74 | 11.1–65.4 | 0–70.0 | 2.8–100 | 90–250 |
| Rice | 191 | 100 | 0.00–100 | 0–100 | 0–100 | 0–100 |
| Sum of T-2 and HT-2 toxins | ||||||
| Grains as crops, unspecified | 1 | 100 | 0.00–19.0 | 0–19.0 | 0–19.0 | – |
| Wheat grain crop | 150 | 98 | 1.32–35.8 | 0–50.0 | 0–50.0 | 0–50.0 |
| Barley grain | 14 | 100 | 0.00–113 | 0–22.8 | 0–75.0 | – |
| Corn grain | 14 | 64 | 40.3–52.5 | 0–25.0 | 35.0–35.0 | – |
| Spelt grain | 3 | 100 | 0.00–0.00 | 0.00–0.00 | 0.00–0.00 | – |
| Oats, grain | 49 | 90 | 13.4–109 | 0–75.0 | 0–75.0 | – |
N: number of analytical results; LC: left-censored data; P75: 75th percentile; P95: 95th percentile; LB: lower bound; UB: upper bound (European Food Safety Authority et al., 2017).
There is also increasing attention recently regarding the so-called masked and hidden mycotoxins in addition to their intact form. According to the currently accepted classification, the different mycotoxin derivatives can be divided into 3 groups. These are mycotoxins in their free form, mycotoxins bound to the so-called matrix and toxins that have been chemically modified for some reason. These latter derivatives are often also highly toxic, but their importance is increased by the fact that they can be converted back to their original structure or to other molecules that are significantly more toxic than the initial one. This can happen either by detoxification processes in humans or animals or as the result of metabolism by the intestinal microbiota (Berthiller et al., 2013; Rychlik et al., 2014; Tan et al., 2022).
General Characteristics of Trichothecenes Focusing on T-2 Toxin
Field fungi of the Fusarium genus, encompassing both saprobiontic and plant pathogenic species, are widely distributed and can inflict significant economic damage on agriculture globally, particularly in temperate climatic zones (Rampersad, 2020). Trichothecenes, produced by this genus, are relatively small, amphipathic sesquiterpene molecules. These compounds can passively traverse the cell membrane, and can be absorbed through the gastrointestinal tract, skin surface, or via inhalation, from the lungs (Adhikari et al., 2017; Huang et al., 2023). Additionally, other fungal species apart from Fusarium, such as Cephalosporium, Trichotecium, Stachybotrys, Myrothecium, or Trichoderma species also produce trichothecene mycotoxins, albeit with lesser significance (Song et al., 2023). This group comprises over 200 fungal toxins. Trichothecenes can be classified into subgroups A, B, C, and D based on their structure (Liu et al., 2023a,b). They share the common 12,13-epoxy ring as a fundamental structure and contain various substitution groups depending on the side chains of the specific toxin. According to the aforementioned classification, the T-2 toxin is categorized as a non-macrocyclic type-A trichothecene (Ji et al., 2019). It is an extremely stable molecule that primarily contaminates feed during storage, exhibiting high resistance to heat exposure and UV radiation. Consequently, it is not degraded during common food and feed production processes or even after autoclaving; approximately 30 to 40 min at around 200°C are required to inactivate the toxin (Chen et al., 2019a).
Metabolism of T-2 Toxin in Poultry
The precise mechanism of the toxin's degradation in the body is an area of intensive research. Following absorption, liver serves as the primary organ for toxin metabolism as trichothecenes are usually metabolized by the hepatic microsomal xenobiotic transforming enzymes (Bócsai et al., 2016). It is also important to mention, that in different animal species sometimes different enzymes might play a central role in the detoxification process. Consequently, there are also distinct avian-specific characteristics compared to mammals (Wu et al., 2020; Li et al., 2022). As the result of the complex degradation process, a total of 19 different metabolites were detected in the feces and bile of chickens. Moreover, approximately 80% of orally administered T-2 toxin is metabolized and eliminated in the excreta within 48 h (Li et al., 2022). Half-life of the toxin in blood plasma is generally short, primarily influenced by the mode of application, the ingested amount, and species-specific characteristics. In contrast to other mycotoxins such as aflatoxin, the ingested toxin does not accumulate significantly in the tissues (Kuca et al., 2008; Li et al., 2011).
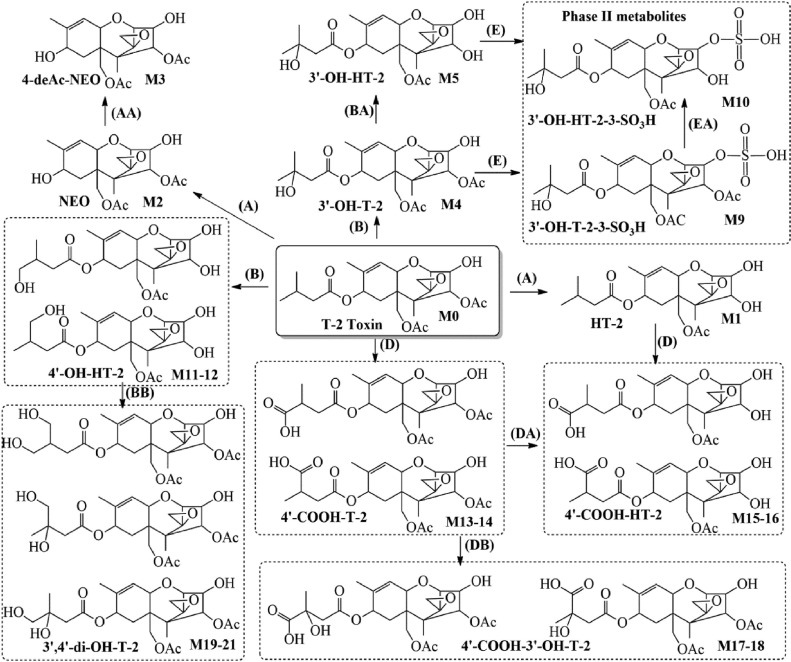
In the complex degradation process, HT-2 toxin and thereafter neosolaniol (NEO), T-2 triol and tetraol, as well as a number of further intermediates can be synthesized from T-2 toxin (Yang et al., 2017a). Major metabolic pathways of T-2 toxin involve hydrolysis, hydroxylation, and sulfate conjugation. De-epoxidation also stands as a crucial step in the detoxification (Yang et al., 2013). Major degradation pathways and hypothesized metabolism of T-2 toxin in chickens are presented in Figure 1 (Yang et al., 2017a).
Figure 1.
Degradation pathway and metabolism of T-2 toxin in chickens according to Yang et al. (2017). Major chemical reactions: hydrolysis (A); hydroxylation (B); carboxylation (D); and sulfation (E) (Yang et al., 2017a).
There is considerable variability in the toxicity among different metabolites. HT-2 toxin, the primary metabolite of T-2 toxin, is considered highly toxic, while others are mainly less harmful molecules (Meneely et al., 2023). T-2 toxin is rapidly metabolized into HT-2 toxin, indicating that HT-2 toxin contributes at least partially to its toxic effects (Edwards et al., 2009). They are commonly found together in contaminated grains (Yang et al., 2017b).
Research to date has shown that besides carboxylesterase 1 (Ces1), members of the cytochrome P450 (CYP) enzyme family such as CYP1A4, CYP1A5, CYP2H1, CYP2C18, and CYP3A37 enzymes, play a pivotal role in the degradation process in chickens (Osselaere et al., 2013; Shang et al., 2013; Yuan et al., 2013; Lin et al., 2015; Dai et al., 2020). Additionally, the xenobiotic-transforming capacity of the liver cells can be altered by the toxin. This alteration may occur due to the inhibition of the microsomal monooxygenase enzyme system, including cytochrome P450 (CYP) enzymes, affecting physiological drug metabolism and causing changes in the withdrawal period of animal products following medication (Osselaere et al., 2013). It was hypothesized, that activation of farnesoid X receptor (FXR) also promotes T-2 toxin xenobiotic metabolism by increasing CYP3A37 expression. Therefore, its induction plays a cytoprotective role in liver injury induced by T-2 toxin through hydroxylation (Dai et al., 2020). Furthermore, the action of inducible CYPs may lead to protective or even toxic effects via the metabolism of their substrate or the production of reactive oxygen species (ROS), highlighting their complex role in the exertion of toxic effects related to xenobiotics (Nebert and Dalton, 2006). According to previous results, T-2 toxin also affects the expression and the activity of the aforementioned CYP enzymes by either upregulating or downregulating them in chicken hepatocytes. Expression was found to be mediated by aryl hydrocarbon receptors (AhR) in case of several CYP enzymes (Wang et al., 2011; Shang et al., 2013; Liu et al., 2019). AhR expression and its translocation into the nucleus is induced following T-2 toxin exposure which thereafter is attached to the proximal xenobiotic-responsive element (XRE) in the 5′-flanking region of CYP1A5. Since chicken CYP1A5 can be considered as the ortholog of human CYP1A2, effect of T-2 was found to be similar to other, already known inductor molecules like β-naphthoflavone (BNF), 3-methylcholanthrene (3MC) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). The mechanism affects the basal expression of CYP1A5 as well as leads to its induced transcriptional activation and results in the catalysis of T-2 toxin degradation into 3′-OH-T-2, which is an even more toxic metabolite (Liu et al., 2019). For this reason, activation of CYP1A5 is considered to increase the cytotoxic effects of T-2 toxin correlating with consequently reduced cell viability, increased DNA damage and oxidative stress, ultimately leading to apoptosis (Liu et al., 2019). There are also some species-specific characteristics regarding detoxification mechanisms comparing chicken with other poultry species; however, this field and the relationship with T-2 toxin metabolism is yet poorly understood (Grizzle et al., 2005).
General Pathologic Findings in Poultry Caused by T-2 Toxin
The most common symptoms of T-2 toxin consumption in poultry include reduced feed intake and growth rate, altered immune function, neurological and reproductive disorders, depigmentation of the skin of the feet, deterioration of feather quality and lesions on the mucous membranes of the digestive track (Sokolovic et al., 2008).
According to currently available results, the median lethal dose 50 (LD50) for broiler chickens was described at 4.97 mg per kilogram of body weight (mg/[kg·bw]), while for laying hens, the LD50 was determined to be 6.27 mg/(kg·bw) (Liu et al., 2019; Gu et al., 2023). In bobwhite quails, on the other hand, LD50 was registered remarkably higher, as 14.7 mg/kg bw (Grizzle et al., 2004). The degree of damage caused by T-2 toxin depends on various parameters, such as the mode and frequency of intake, duration of exposure, dose of the toxin, the age, sex and health status of the animal, as well as the presence of other mycotoxins. The latter is particularly important because co-existing mycotoxins may have additive or even synergistic effects on the organism (Palumbo et al., 2020; Kulcsár et al., 2023). Similar synergism has been found in instances where aflatoxin, ochratoxin-A and cyclopiazonic acid co-occurred together with T-2 toxin in chickens (Huff et al., 1988; Pál et al., 2009; Manafi et al., 2012; Stefanović et al., 2023). In 2011, a comprehensive meta-analysis of over 100 previous publications described several sometimes contradictory results, including cases of antagonistic effects between different mycotoxins (Grenier and Oswald, 2011).
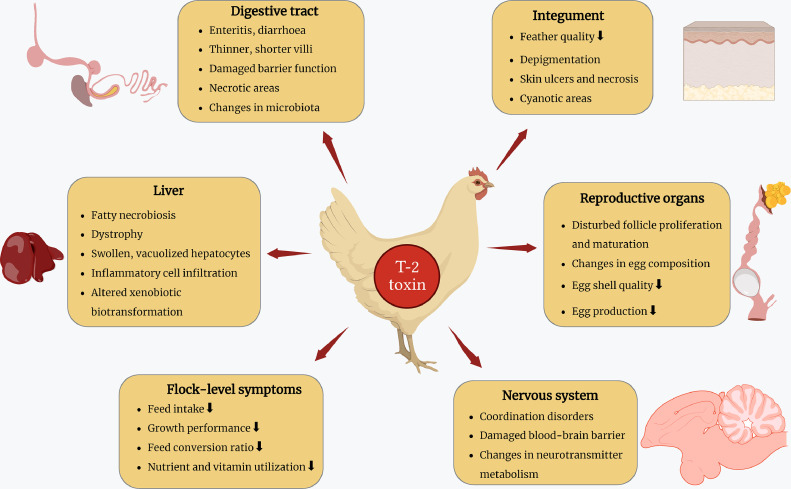
Previous research and observations indicate that the toxin significantly affects other fungal species, plants, insects and all domestic animal species including avians (Haque et al., 2020; Berenbaum et al., 2021). The symptoms of T-2 toxicosis closely resemble to those seen in mammals, differing mainly in frequency and severity. In poultry, initial signs of toxicosis may manifest as a decline in body weight gain, weight loss, skin and feather abnormalities, diarrhea, and coordination problems (Yang et al., 2017a; Salah et al., 2021). Figure 2 presents a detailed summary of major pathological findings in poultry.
Figure 2.
Detailed summary of the major pathological findings in poultry resulting from exposure to T-2 toxin. The figure was fully prepared by the authors.
Necrotizing dermatitis and other localized external lesions, such as depigmentation of the legs or skin cyanosis have been observed in birds, albeit less frequently and at significantly higher toxin doses compared to mammals (Sokolovic et al., 2008). Animals may also develop feathering disorders leading to a shaggy appearance. The lesions are primarily observed on the wings (Raju and Devegowda, 2000; Gu et al., 2023). Increased susceptibility to the dermatotoxic effects of T-2 toxin has also been observed in Muscovy ducks (Rafai et al., 2000).
The toxin was found to induce musculoskeletal abnormalities in poultry. Additionally, in White Roman geese, both the incidence and severity score of angel wing syndrome were reported to increase due to the effect of T-2 toxin-containing diet (Lin et al., 2018). On the other hand, no significant histological findings were observed in the heart muscle (Grizzle et al., 2004).
In the digestive tract, even small doses of the toxin, can irritate and damage the mucosa, leading to reduced absorption of nutrients and decreased feed efficiency. The toxin exposure causes necrotic areas not only around the beak, oral cavity, and pharyngeal mucosa but also in the gizzard or other regions of the gastrointestinal tract, along with potential effects on the liver (Chi and Mirocha, 1978; Kubena et al., 1995; Richard, 2007). Alterations in the histology of the gastrointestinal tract are frequently observed, including shorter and thinner villi, reduced migration rate and damaged barrier function (Sklan et al., 2003). In the glandular stomach of chicks, T-2 induces mucosal edema, increases inflammatory cell counts together with epithelial desquamation and necrosis (Luo et al., 2019). Toxin contamination of the feed has significant negative impact on the utilization of vitamins, such as vitamin E in broiler chickens (Weber et al., 2007). Due to enterohepatic circulation, increased damage to the intestinal tract and liver is often observed (Yuan et al., 2013). In mild cases of exposure, mucosal irritation occurs, while in more severe cases, acute enteritis with watery diarrhea may develop (Wei et al., 2019). Changes in the microbiota were also reported following T-2 exposure. The toxin significantly decreased Firmicutes, and increased Bacteroidetes and Proteobacteria abundance on the phylum level. On genus level, there were increased abundances of Brachybacterium, Blautia, Dorea, Ruminococcus, Bradyrhizobium, and Sphingomonas following treatment, which indicating an impairment of intestinal health (Liu et al., 2023b).
Under in vivo conditions, the main target of the toxin is the liver, where a significant proportion of protein synthesis occurs. Exposure may result in fatty necrobiosis, hepatic dystrophy and other regressive disorders (Krishnamoorthy et al., 2007). Regarding histologic findings, hepatocytes exhibit swelling and tend to show balloon-like deformation. In most cases, the cytoplasm is vacuolated, and the nucleus appears to be located either in the center of the vacuole or squeezed on the side of the cell. Furthermore, exposure to the T-2 toxin in chickens and bobwhite quails resulted in stenosis of hepatic sinuses, deposits of red blood cells, focal inflammatory cell infiltration and intense interlobular bile duct epithelial cell proliferation (Grizzle et al., 2004; Yin et al., 2020a). It is also presumed that the toxin affects wound healing, angiogenesis and blood vessel remodeling, contributing to its widespread negative effects in poultry (Luo et al., 2019).
Furthermore, reduced egg production, changes in egg weight, shell structure and pigmentation can also be results of T-2 toxin uptake (Dazuk et al., 2020). Other signs include alterations in egg composition such as lower vitamin, protein and mineral content, reduced hatchability, slower follicle maturation, follicle proliferation and rupture and consequent peritonitis (Ványi et al., 1994; Yang et al., 2020; Dazuk et al., 2020). Interestingly, no egg production and quality-related negative effects were observed in bobwhite quails following prepuberal exposure. Conversely, the investigated parameters were significantly affected if the toxin was in presence already in the egg yolk (Grizzle et al., 2005).
In some cases, T-2 toxin may also have neurotoxic effects, which may occur partly due to the damage of the blood-brain barrier and to changes in serotonin levels in the central nervous system (Wang et al., 1998; Wan et al., 2016; Salah et al., 2021). It has also been described that dopamine and norepinephrine concentrations in the brain can be modified by the effects of the toxin (Smith, 1992). Clinical changes in neurotransmitter levels, such as food refusal, unsteady movements, and incoordination typically manifest at the herd level (Zhang et al., 2020).
Regarding general hematologic parameters, the toxin induces anemia as indicated by significant decrease in total erythrocyte counts, hemoglobin levels, and cell volume values in chickens. Additionally, exposed birds often exhibit leucopenia, lymphocytopenia, heterophilia, and thrombocytopenia (Yohannes et al., 2013). Hypoproteinemia along with hypoalbuminemia and hyperglobulinemia were also observed concurrently. Moreover, the toxin increased alkaline phosphatase (ALP) enzyme activity (Yohannes et al., 2013). Observations revealed that serum uric acid, cholesterol and hemoglobin concentrations as well as aspartate transaminase (AST) and alanine transaminase (ALT) activities and heterophil/lymphocyte ratio values were significantly higher in T-2 treated chickens compared to those of the control groups (Singh et al., 2020; Jiang et al., 2023).
Highly significant differences were also observed in total protein, albumin, globulin, cholesterol and glucose concentrations along with AST, ALT, ALP and gamma glutamyl transferase (GGT) activities in Japanese quails (Madheswaran et al., 2004). On the contrary, T-2 toxin treatment only resulted in alterations regarding ALP activity in White Roman geese and did not cause any detrimental metabolic disturbances in broiler ducks even following 7 wk of treatment (Kutasi et al., 2012). In ducklings, T-2 toxin induced hypocalcemia and hypomagnesemia, and decreased activities of creatine phosphokinase (CPK) and ALP, while in correlation with the aforementioned findings in chickens, it increased blood urea nitrogen concentration and AST activity (Tso et al., 2021).
In addition, a metabolomic study revealed changes in several plasma, liver, kidney and spleen metabolites after intravenous T-2 toxin injection. These changes are involved in energy, amino acid, and nucleotide metabolism as well as oxidative stress. Notably, tryptophan levels were increased during this experiment, potentially explaining the neurotoxic effect of the toxin (Wan et al., 2016).
Immunomodulatory Effects
Once trichothecenes penetrate the intestinal epithelium, their subsequent target is the immune system (Maresca, 2013). These agents can cause damage to various organs by upregulating pro-inflammatory factors and triggering immunotoxicity (Minervini et al., 2005). T-2 toxin has been found to exert an immunosuppressive effect on the animal organism in several cases. However, some studies have reported an apparently opposite immunostimulatory effect (Pestka et al., 2004). The observed differences depend on the route of administration, the quantity of toxin and the duration of exposure. According to certain studies, immunosuppression occurs in almost all cases when the organism is exposed to higher levels of T-2 toxin. Degeneration, atrophy of bone marrow, lymph nodes, as well as lymphocyte depletion and hemorrhages in the spleen were also observed in connection with the immunomodulatory effects in chickens (Kamalavenkatesh et al., 2005; Venkatesh et al., 2005; Xue et al., 2010; Yohannes et al., 2012). Increased numbers of necrotic cells and spleen peripheral lymphocytes may also indicate the extensive necrosis of B and T cells, which can affect the spleen's ability to function properly as a key organ in the immune response of the body (Chen et al., 2019a). Similarly, marked lymphocyte necrosis as well as depletion throughout the thymus, bursa of Fabricius, spleen, and gut-associated lymphoid tissue (GALT) were observed in bobwhite quails due to exposure to 50% lethal dose (LD50) of T-2 toxin. On the other hand no negative histological finding was observed in bone marrow samples (Grizzle et al., 2004).
T-2 toxin in combination with ochratoxin-A (OTA) decreased the weight of the spleen, thymus and bursa of Fabricius (Wang et al., 2009). In the same study, flow cytometry results showed that this toxin combination treatment significantly decreased CD4+ cell count along with CD4+/CD3+ and CD4+/CD8+ ratios, while increased CD8+/CD3+ ratio (Wang et al., 2009). Similar observations were described following T-2 toxin treatment alone and also in combination with cyclopiazonic acid, where both CD4+ and CD8+ lymphocyte counts were decreased in the thymus and spleen of chickens (Kamalavenkatesh et al., 2005; Wang et al., 2009; Chen et al., 2019a). This finding has been further supported by a recent study where T-2 toxin induced the decrement in CD4+ and CD8+ lymphocyte counts, although it did not cause any consequences regarding these parameters applied in smaller concentrations (Chen et al., 2019b). CD4+ and CD8+ T cells are key components of the acquired immune system and are of pivotal importance for successful vaccination. In general, CD4+ T lymphocytes correspond to the helper T cell population, while CD8+ T lymphocytes are antigen-specific cytotoxic T lymphocytes responsible for the recognition and elimination of cells infected by intracellular pathogens. Under physiological circumstances, the ratio of CD4+/ CD8+ T cells is about 2:1 (Muller et al., 2015). Deviations from this ratio can significantly reduce immune function and the normal immune response (Obar and Lefrançois, 2010; McBride and Striker, 2017; Sekelova et al., 2017; Lee et al., 2018). Increased jejunal levels of CD3+ and Goblet cells were also described along with alterations in circulating macrophage and suppressor macrophage, B-lymphocyte and T-lymphocyte numbers. Virgin cytotoxic T-lymphocyte and terminally activated cytotoxic T-lymphocyte counts have been also increased simultaneously (Koppenol et al., 2019). In broiler ducks, T-2 toxin depressed the blastogenic response of lymphocytes by nonspecific mitogens (Kutasi et al., 2012). There are contradictory results available about the effects of T-2 on the vaccination of poultry. According to some results, T-2 toxin lowered antibody titers post-vaccination against Newcastle disease (ND) and infectious bursal disease (IBD) (Girish and Devegowda, 2006; Xue et al., 2010), while on the contrary, another study showed that antibody formation against ND was not suppressed by the short-term sublethal toxin load (Weber et al., 2008).
T-2 toxin affected the defensive function of chicken HD-11 macrophages against Aspergillus fumigatus infection in vitro. The toxin impaired antifungal activity against conidial infection and promoted a pro-inflammatory response in infected macrophages, which might compensate for the caused macrophage-related functional damage (Li et al., 2013). In response to A. fumigatus infection, the expression of pro-inflammatory cytokines, including chemokine (C-X-C motif) ligand 1 and 2 (CXCLi1, CXCLi2; respectively), interleukin (IL)-1β, IL-6, IL-12β and IL-18 and chemokine ligand 2 (CCLi2) was upregulated and transforming growth factor (TGF)-β4, IL-2 and interferon-γ (IFN-γ) was downregulated in T-2 toxin-exposed HD-11 macrophages compared with the non-T-2 toxin-exposed macrophages (Li et al., 2013). Accordingly, experimental inoculation of chickens with A. fumigatus in vivo revealed that exposure to the toxin significantly exacerbated aspergillosis in chickens exposed to dietary T-2 toxin (Li et al., 2015). Regarding IL-6 and IL-8 concentrations, similar findings were described using chicken in vitro hepatocyte and hepatocyte—non-parenchymal cell (mostly macrophage Kupffer cells) models (Mackei et al., 2020).
A major cause of the harmful effects on immune cells and the reduction of CD4+/CD8+ T cell ratio is supposed to be the increased oxidative stress induced by the toxin (Chen et al., 2019b). Furthermore, type A trichothecenes, such as T-2 toxin, were found to be even more genotoxic to chicken spleen leukocytes than the type B trichothecene deoxynivalenol (DON) (Frankic et al., 2006). As a consequence, susceptibility to pathogens such as infectious bronchitis virus (IBV) or Salmonella Typhimurium is increased, resulting in further damage, possibly death, and significant production losses (Kubena et al., 2001; Yohannes et al., 2012, 2013). It is also highly relevant that the presence of T-2 toxin may also affect the efficacy of vaccination. In such cases, reduced antibody titers may be observed in diseases of high economic importance such as avian influenza or Marek's disease (Kamalavenkatesh et al., 2005; Kufuor-Mensah et al., 2015). Other studies also suggest that exposure to sublethal doses of T-2 toxin may influence the development of turkey herpesvirus viremia and Marek's disease-related lesions and mortality, but only in unvaccinated chickens (Kufuor-Mensah et al., 2015).
In contrast to the results of high concentration T-2 toxin exposure, other researchers have reported that prolonged effects of lower toxin concentrations may have an immunostimulatory effect. In this case, serum immunoglobulin (Ig)A and IgE concentrations increase significantly, which may be explained by a transient activation of gene sequences involved in the inflammatory response and in the function of certain immune processes (Pestka et al., 2004). It is important to note; however, that most of the research on this topic has been carried out so far mainly in laboratory experimental animals, so that the precise mechanisms by which T-2 toxin exerts its immunomodulatory effects in poultry, either by impairing or by promoting the mechanisms of action, are not completely understood.
Detailed Mechanism of Action
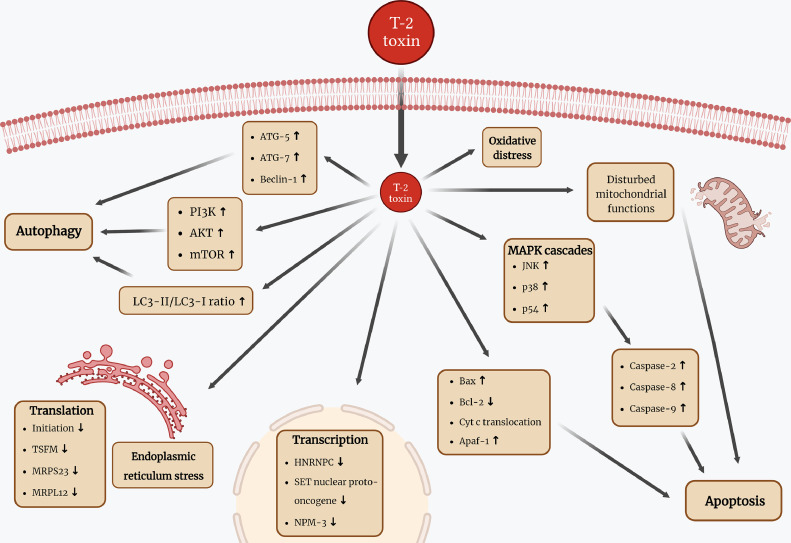
In both poultry and mammalian species, wide variety of detrimental effects were described connected to T-2 toxin exposure. The genotoxic effects were described also in broiler chicken red blood cells and leukocytes (Rezar et al., 2007; Szabó et al., 2019), while general cytotoxicity was detected in rat and human, as well as in several farm animal species such as rabbit, chicken and pig (Wu et al., 2014). Furthermore, teratogenic effects and developmental toxicity were detected in studies using zebrafish embryos, but according to our knowledge, no further study is available in poultry (Yuan et al., 2014). The toxin disrupts DNA, RNA and protein synthesis, acting mainly at the ribosomal level. Trichothecenes inhibit protein synthesis by binding to the peptidyl transferase enzyme. In contrast to DON and trichodermin—2 other trichothecene mycotoxins that has an effect on the termination and chain formation of the protein synthesis—T-2 toxin inhibits the initiation step (Shifrin and Anderson, 1999; Li and Pestka, 2008). Proteomic analysis also revealed that other translational proteins are also affected by the toxin, such as the Ts translation elongation factor, mitochondrial (TSFM), the mitochondrial ribosomal protein S23 (MRPS23) and the mitochondrial ribosomal protein L12 (MRPL12). Furthermore, transcription proteins, including SET nuclear proto-oncogene, heterogeneous nuclear ribonucleoprotein C (HNRNPC) and nucleoplasmin-3 (NPM3) are also affected, resulting in the inhibition of RNA synthesis (Mu et al., 2013). It also impairs mitochondrial electron transport and affects the cell cycle, inducing apoptosis both in vivo and in vitro (Figure 3) (Fang et al., 2012; Liu et al., 2017; Dai et al., 2019).
Figure 3.
Schematic summary of T-2 toxin exposure-related cellular effects in poultry focusing on apoptosis, autophagy, as well as translation and transcription. Abbreviations used in the figure are all explained and can be found in the main text. The figure was fully prepared by the authors.
Actively dividing cells such as those found in the digestive tract, spleen, liver, bone marrow or bursa of Fabricius are more sensitive to the presence of T-2 toxin (Konjević et al., 2004; Krishnamoorthy et al., 2007; Xue et al., 2010). Some mitogen-activated protein kinase (MAPK) cascades, such as the c-Jun N-terminal kinase (JNK), p38 and p54 protein kinase pathways, participate in the induction of cell death in different species. However, the exact background of this process remains incompletely understood, especially in case of poultry species (Kong et al., 2021; Liu et al., 2023b; Lee and Park, 2023). It is also noteworthy that the expression levels of certain genes are significantly affected by the toxin through these MAPK and JNK signaling pathways (El Golli et al., 2006; Wu et al., 2011; Fang et al., 2012). Further results in U937 cells of human origin revealed, that the caspase-2 pathway plays a key role in T-2 toxin-induced apoptosis, and that the process is not solely regulated by the mitochondrial pathway but also by the caspase-8 and caspase-3 mediated cascades (Huang et al., 2007). Recent studies in poultry emphasize the caspase-related apoptotic effects of T-2 toxin, demonstrating that the exposure leads to decreased concentrations of pro-caspase-3 and pro-caspase-9, coupled with increased levels of caspase-3 and caspase-9 in a dose-dependent manner (Figure 3) (Yin et al., 2020b). The proteomic analysis mentioned previously also found T-2 toxin interference with several proteins involved in the cellular redox homeostasis, regulation of transcription and translation, lipid and carbohydrate metabolism, transport processes and protein degradation in chickens (Mu et al., 2013). Recent findings indicate that the toxin disrupts physiological nucleotide, phospholipid and energy metabolism in chicken intestinal and hepatic samples (Mackei et al., 2020; Liu et al., 2023b).
OXIDATIVE STRESS
The oxidative stress-inducing effects of T-2 toxin have also been described in a number of tissues and cell types. It is often hypothesized to be the major underlying cause of T-2 toxin induced cytotoxicity and apoptosis (Yang et al., 2016). T-2 toxin can increase the formation of ROS causing oxidative stress leading to DNA damage, increased lipid peroxidation and subsequently compromising membrane integrity (Sokolovic et al., 2007; Awuchi et al., 2022; Liu et al., 2023b). In chickens, akin to mammalian species, T-2 exposure significantly increases malondialdehyde (MDA) concentrations, a specific marker indicating the terminal phase of lipid peroxidation. It also elevates glutathione peroxidase (GPx), superoxide dismutase (SOD) and catalase (CAT) activities, 3 enzymes crucial for maintaining the oxidative homeostasis. Concurrently, T-2 toxin decreased the levels of reduced glutathione (GSH), α-tocopherol, carotenoids, and ascorbic acid. These findings were predominantly described in hepatocytes in both in vivo and in vitro studies (Leal et al., 1998; Dvorska et al., 2007; Mu et al., 2013; Balogh et al., 2015; Yang et al., 2016, 2019; Nakade et al., 2018; Yin et al., 2020b; Liu et al., 2023b; Jiang et al., 2023). Similar results were found in the intestines of chicks, where besides the abovementioned parameters, T-2 toxin also reduced antioxidant capacity and increased protein carbonyl content (Liu et al., 2023b). Other research groups also pointed out an age-dependent manner regarding the effects of the toxin on glutathione system (Pelyhe et al., 2018). These detrimental effects on the redox homeostasis were also described in quails, resulting in lower levels in antioxidants such as tocopherols, ascorbic acid, retinol and retinyl esters (Dvorska and Surai, 2001). On the other hand, further studies found that acute exposure did not result in elevated ROS production, though, metabolic activity was intensely decreased and interleukin production such as IL-6 and IL-8 were increased in hepatic cell culture models of chicken origin (Mackei et al., 2020).
In chicken splenocytes, the elevation of ROS, SOD, CAT and GPx along with a decrease in MDA concentration was observed similarly to the liver (Chen et al., 2019a). Studies involving chicken leukocytes described that T-2 toxin causes DNA fragmentation together with lipid peroxidation (Frankic et al., 2006; Rezar et al., 2007). It was also shown that in chicken chondrocytes, T-2 toxin increased ROS and MDA levels in a dose-dependent manner (He et al., 2012). In addition, T-2 toxin induced the formation of heterophil extracellular traps (HETs) composed of DNA, elastase and citrullinated H3 (citH3). The same experiment also demonstrated a potential link between their formation and toxin-stimulated ROS production (Liu et al., 2021).
Regarding species-specific characteristics, it is also important to highlight, that according to some results, the most sensitive species to oxidative stress were geese, followed by ducks and chickens (Mezes et al., 1999). Pheasants, compared to other poultry species appeared to exhibit higher tolerance to T-2 toxin, possibly correlated with the slightly different function of the glutathione redox system against oxidative damage (Fernye et al., 2018).
ROS accumulation can also lead to an increased mitochondrial mass indicating potential oxidative stress (Chen et al., 2019a; Yin et al., 2020b). The mitochondrial apoptotic pathway plays a pivotal role in the initiation of apoptosis due to toxic effects. Its activation requires the translocation of cytochrome c (cyt c) into the cytoplasm via the Bcl-2 associated X protein (Bax)/B-cell lymphoma 2 (Bcl-2) pathway (Figure 3). The relative levels of the pro-apoptotic Bax and anti-apoptotic Bcl-2 proteins determine the activation of the caspase system, as they form heterodimers influencing the cell-death-inducing effects of Bax (Yang et al., 2017b; Yin et al., 2020b). In vivo observations indicated that T-2 toxin downregulated Bcl-2 while upregulated Bax levels, consequently increasing the Bax/Bcl-2 ratio. In addition, cyt c was also translocated into the cytoplasm, resulting in an apoptosome with the apoptotic protease activating factor 1 (Apaf-1) and the caspase-9 apoptotic proteases (Yin et al., 2020b). Similar results were observed in chicken splenocytes, where the concentration of the cytosolic cyt c increased, while the mitochondrial cyt c level decreased (Chen et al., 2019a).
Furthermore, it has also been described in chickens in vivo, that T-2 toxin can induce autophagy via the phosphoinositide 3-kinase (PI3K)/ Protein kinase B (AKT)/ Mechanistic target of rapamycin (mTOR) pathway (Figure 3). Autophagy plays a crucial role in maintaining the cell homeostasis and is associated with apoptosis by inducing caspase-dependent cell death and enhancing cell survival, thus providing protection against the adverse effects of the toxin. In correlation with this hypothesis, T-2 toxin was found to induce the expression levels of Autophagy related 5 and 7 (ATG5, ATG7 respectively) and Beclin-1 genes. Further, ratio of LC3-II/LC3-I increased depending on the T-2 toxin dosage, while the concentration of p62 protein decreased significantly (Yin et al., 2020b). Endoplasmic reticulum (ER) stress is also frequently associated with oxidative stress (Yao et al., 2015; Burban et al., 2018). Newly synthesized proteins undergo folding and modification in the ER facilitated by various chaperones and folding enzymes, including members of the heat shock protein 70 (HSP70) family as well as further heat shock proteins. Correspondingly, levels of HSP70 were also found to increase upon exposure to T-2 toxin in chicken hepatic cell culture models (Mackei et al., 2020).
ACKNOWLEDGMENTS
The work was supported by the Hungarian National Research, Development and Innovation Office (grant number OTKA FK 134940). Project no. RRF-2.3.1-21-2022-00001 has been implemented with the support provided by the Recovery and Resilience Facility (RRF), financed under the National Recovery Fund budget estimate, RRF-2.3.1–21 funding scheme.
DISCLOSURES
The authors of the following manuscript declare no conflict of interest.
REFERENCES
- Adhikari M., Negi B., Kaushik N., Adhikari A., Al-Khedhairy A.A., Kaushik N.K., Choi E.H. T-2 mycotoxin: toxicological effects and decontamination strategies. Oncotarget. 2017;8:33933. doi: 10.18632/oncotarget.15422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad W., Ghareeb K., Böhm J., Zentek J. The toxicological impacts of the fusarium mycotoxin, deoxynivalenol, in poultry flocks with special reference to immunotoxicity. Toxins. 2013;5:912–925. doi: 10.3390/toxins5050912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awuchi C.G., Ondari E.N., Nwozo S., Odongo G.A., Eseoghene I.J., Twinomuhwezi H., Ogbonna C.U., Upadhyay A.K., Adeleye A.O., Okpala C.O.R. Mycotoxins’ toxicological mechanisms involving humans, livestock and their associated health concerns: a review. Toxins. 2022;14:167. doi: 10.3390/toxins14030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balogh K., Bócsai A., Pelyhe C., Zándoki E., Erdélyi M., Szabó-Fodor J., Mézes M. Effects of long-term feeding of graded levels of T-2 toxin-contaminated diets on performance, some lipid peroxide and glutathione redox status parameters of broiler chickens. J. Poult. Sci. 2015;52:176–182. [Google Scholar]
- Bansal A., Sharma M., Pandey A., Shankar J. In: Pages 565–594 in Fungal Resources for Sustainable Economy: Current Status and Future Perspectives. Singh I., Rajpal V.R., Navi S.S., editors. Springer Nature; Singapore: 2023. Aflatoxins: occurrence, biosynthesis pathway, management, and impact on health. [Google Scholar]
- Berenbaum M.R., Bush D.S., Liao L.-H. Cytochrome P450-mediated mycotoxin metabolism by plant-feeding insects. Curr. Opin. Insect Sci. 2021;43:85–91. doi: 10.1016/j.cois.2020.11.007. [DOI] [PubMed] [Google Scholar]
- Berthiller F., Crews C., Dall'Asta C., Saeger S.D., Haesaert G., Karlovsky P., Oswald I.P., Seefelder W., Speijers G., Stroka J. Masked mycotoxins: a review. Mol. Nutr. Food Res. 2013;57:165–186. doi: 10.1002/mnfr.201100764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bócsai A., Pelyhe C.s., Zándoki E., Ancsin Z.s., Szabó-Fodor J., Erdélyi M., Mézes M., Balogh K. Short-term effects of T-2 toxin exposure on some lipid peroxide and glutathione redox parameters of broiler chickens. J. Anim. Physiol. Anim. Nutr. 2016;100:520–525. doi: 10.1111/jpn.12399. [DOI] [PubMed] [Google Scholar]
- Bradburn N., Coker R.D., Blunden G. The aetiology of turkey ‘x’ disease. Phytochemistry. 1994;35:817. [Google Scholar]
- Bryden W.L. Mycotoxin contamination of the feed supply chain: Implications for animal productivity and feed security. Anim. Feed Sci. Technol. 2012;173:134–158. [Google Scholar]
- Burban A., Sharanek A., Guguen-Guillouzo C., Guillouzo A. Endoplasmic reticulum stress precedes oxidative stress in antibiotic-induced cholestasis and cytotoxicity in human hepatocytes. Free Radic. Biol. Med. 2018;115:166–178. doi: 10.1016/j.freeradbiomed.2017.11.017. [DOI] [PubMed] [Google Scholar]
- Chen Y., Han S., Wang Y., Li D., Zhao X., Zhu Q., Yin H. Oxidative stress and apoptotic changes in broiler chicken splenocytes exposed to T-2 toxin. Biomed. Res. Int. 2019;2019 doi: 10.1155/2019/5493870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Han S., Wang Y., Li D., Zhao X., Zhu Q., Yin H. Oxidative stress and apoptotic changes in broiler chicken splenocytes exposed to T-2 toxin. Biomed. Res. Int. 2019;2019:1–9. doi: 10.1155/2019/5493870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi M.S., Mirocha C.J. Necrotic oral lesions in chickens fed diacetoxyscirpenol, T-2 toxin, and crotocin. Poult. Sci. 1978;57:807–808. doi: 10.3382/ps.0570807. [DOI] [PubMed] [Google Scholar]
- Dai C., Xiao X., Sun F., Zhang Y., Hoyer D., Shen J., Tang S., Velkov T. T-2 toxin neurotoxicity: role of oxidative stress and mitochondrial dysfunction. Arch. Toxicol. 2019;93:3041–3056. doi: 10.1007/s00204-019-02577-5. [DOI] [PubMed] [Google Scholar]
- Dai D., Pan Y., Zeng C., Liu S., Yan Y., Wu X., Xu Z., Zhang L. Activated FXR promotes xenobiotic metabolism of T-2 toxin and attenuates oxidative stress in broiler chicken liver. Chem. Biol. Interact. 2020;316 doi: 10.1016/j.cbi.2019.108912. [DOI] [PubMed] [Google Scholar]
- Dazuk V., Boiago M.M., Rolim G., Paravisi A., Copetti P.M., Bissacotti B.F., Morsch V.M., Vedovatto M., Gazoni F.L., Matte F., Gloria E.M., Da Silva A.S. Laying hens fed mycotoxin-contaminated feed produced by Fusarium fungi (T-2 toxin and fumonisin B1) and Saccharomyces cerevisiae lysate: impacts on poultry health, productive efficiency, and egg quality. Microbial Pathogen. 2020;149 doi: 10.1016/j.micpath.2020.104517. [DOI] [PubMed] [Google Scholar]
- Dvorska J.E., Pappas A.C., Karadas F., Speake B.K., Surai P.F. Protective effect of modified glucomannans and organic selenium against antioxidant depletion in the chicken liver due to T-2 toxin-contaminated feed consumption. Compar. Biochem. Physiol. C Toxicol. Pharmacol. 2007;145:582–587. doi: 10.1016/j.cbpc.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Dvorska J.E., Surai P.F. Effects of T-2 toxin, zeolite and mycosorb on antioxidant systems of growing quail. Asian-Australas. J. Anim. Sci. 2001;14:1752–1757. [Google Scholar]
- Edite Bezerra da Rocha M., Freire F.d.C.O., Erlan Feitosa Maia F., Izabel Florindo Guedes M., Rondina D. Mycotoxins and their effects on human and animal health. Food Control. 2014;36:159–165. [Google Scholar]
- Edwards S., Barrier-Guillot B., Clasen P.-E., Hietaniemi V., Pettersson H. Emerging issues of HT-2 and T-2 toxins in European cereal production. World Mycotoxin J. 2009;2:173–179. [Google Scholar]
- El Golli E., Hassen W., Bouslimi A., Bouaziz C., Ladjimi M.M., Bacha H. Induction of Hsp 70 in Vero cells in response to mycotoxins: cytoprotection by sub-lethal heat shock and by vitamin E. Toxicol. Lett. 2006;166:122–130. doi: 10.1016/j.toxlet.2006.06.004. [DOI] [PubMed] [Google Scholar]
- European Food Safety Authority. Arcella D., Gergelova P., Innocenti M.L., Steinkellner H. Human and animal dietary exposure to T-2 and HT-2 toxin. EFSA J. 2017;15:e04972. doi: 10.2903/j.efsa.2017.4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H., Wu Y., Guo J., Rong J., Ma L., Zhao Z., Zuo D., Peng S. T-2 toxin induces apoptosis in differentiated murine embryonic stem cells through reactive oxygen species-mediated mitochondrial pathway. Apoptosis. 2012;17:895–907. doi: 10.1007/s10495-012-0724-3. [DOI] [PubMed] [Google Scholar]
- Fernye C., Ancsin Z., Bócsai A., Balogh K., Mézes M., Erdélyi M. Role of glutathione redox system on the T-2 toxin tolerance of pheasant (Phasianus colchicus) Toxicol. Res. 2018;34:249–257. doi: 10.5487/TR.2018.34.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankic T., Pajk T., Rezar V., Levart A., Salobir J. The role of dietary nucleotides in reduction of DNA damage induced by T-2 toxin and deoxynivalenol in chicken leukocytes. Food Chem. Toxicol. 2006;44:1838–1844. doi: 10.1016/j.fct.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Girish C.K., Devegowda G. Efficacy of glucomannan-containing yeast product (Mycosorb®) and hydrated sodium calcium aluminosilicate in preventing the individual and combined toxicity of aflatoxin and T-2 toxin in commercial broilers. Asian-Australas. J. Anim. Sci. 2006;19:877–883. [Google Scholar]
- Grenier B., Oswald I. Mycotoxin co-contamination of food and feed: meta-analysis of publications describing toxicological interactions. World Mycotoxin J. 2011;4(3):285–313. [Google Scholar]
- Grizzle J.M., Kersten D.B., Houston A.E., Saxton A.M. Effect of chronic vs. intermittent exposure to T-2 toxin on reproductive performance in bobwhite quail. Int. J. Poult. Sci. 2005;4:71–75. [Google Scholar]
- Grizzle J.M., Kersten D.B., McCracken M.D., Houston A.E., Saxton A.M. Determination of the acute 50% lethal dose T-2 toxin in adult bobwhite quail: additional studies on the effect of T-2 mycotoxin on blood chemistry and the morphology of internal organs. Avian Dis. 2004;48:392–399. doi: 10.1637/7100. [DOI] [PubMed] [Google Scholar]
- Gu W., Bao Q., Weng K., Liu J., Luo S., Chen J., Li Z., Cao Z., Zhang Y., Zhang Y., Chen G., Xu Q. Effects of T-2 toxin on growth performance, feather quality, tibia development and blood parameters in Yangzhou goslings. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2022.102382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque M.A., Wang Y., Shen Z., Li X., Saleemi M.K., He C. Mycotoxin contamination and control strategy in human, domestic animal and poultrya A review. Microbial Pathogen. 2020;142 doi: 10.1016/j.micpath.2020.104095. [DOI] [PubMed] [Google Scholar]
- He S., Hou J., Dai Y., Zhou Z., Deng Y. N-acetyl-cysteine protects chicken growth plate chondrocytes from T-2 toxin-induced oxidative stress. J. Appl. Toxicol. 2012;32:980–985. doi: 10.1002/jat.1697. [DOI] [PubMed] [Google Scholar]
- Huang P., Akagawa K., Yokoyama Y., Nohara K., Kano K., Morimoto K. T-2 toxin initially activates caspase-2 and induces apoptosis in U937 cells. Toxicol. Lett. 2007;170:1–10. doi: 10.1016/j.toxlet.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Huang T.-Y., Song W.-X., Wang Y.-S., Liu Y., Chen F.-J., Chen Y.-H., Jiang Y.-B., Zhang C., Yang X. A review of anorexia induced by T-2 toxin. Food Chem. Toxicol. 2023;179 doi: 10.1016/j.fct.2023.113982. [DOI] [PubMed] [Google Scholar]
- Huff W.E., Harvey R.B., Kubena L.F., Rottinghaus G.E. Toxic synergism between aflatoxin and T-2 toxin in broiler chickens. Poult. Sci. 1988;67:1418–1423. doi: 10.3382/ps.0671418. [DOI] [PubMed] [Google Scholar]
- Hussain Z., Khan M.Z., Khan A., Javed I., Saleemi M.K., Mahmood S., Asi M.R. Residues of aflatoxin B1 in broiler meat: effect of age and dietary aflatoxin B1 levels. Food Chem. Toxicol. 2010;48:3304–3307. doi: 10.1016/j.fct.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Janevska S., Tudzynski B. Secondary metabolism in Fusarium fujikuroi: strategies to unravel the function of biosynthetic pathways. Appl. Microbiol. Biotechnol. 2018;102:615–630. doi: 10.1007/s00253-017-8679-5. [DOI] [PubMed] [Google Scholar]
- Ji F., He D., Olaniran A.O., Mokoena M.P., Xu J., Shi J. Occurrence, toxicity, production and detection of Fusarium mycotoxin: a review. Food Prod. Process Nutr. 2019;1:6. [Google Scholar]
- Jiang Y., Qian Y., Hong H., Gao X., Liu W., Jin Q., Chen M., Jin Z., Liu Q., Wei Z. Morin protects chicks with T-2 toxin poisoning by decreasing heterophil extracellular traps, oxidative stress and inflammatory response. Br. Poult. Sci. 2023;0:1–11. doi: 10.1080/00071668.2023.2226083. [DOI] [PubMed] [Google Scholar]
- Juan C., Covarelli L., Beccari G., Colasante V., Mañes J. Simultaneous analysis of twenty-six mycotoxins in durum wheat grain from Italy. Food Control. 2016;62:322–329. [Google Scholar]
- Kamalavenkatesh P., Vairamuthu S., Balachandran C., Manohar B.M., raj G.D. Immunopathological effect of the mycotoxins cyclopiazonic acid and T-2 toxin on broiler chicken. Mycopathologia. 2005;159:273–279. doi: 10.1007/s11046-004-7321-0. [DOI] [PubMed] [Google Scholar]
- Keswani C., Singh H.B., Hermosa R., García-Estrada C., Caradus J., He Y.-W., Mezaache-Aichour S., Glare T.R., Borriss R., Vinale F., Sansinenea E. Antimicrobial secondary metabolites from agriculturally important fungi as next biocontrol agents. Appl. Microbiol. Biotechnol. 2019;103:9287–9303. doi: 10.1007/s00253-019-10209-2. [DOI] [PubMed] [Google Scholar]
- Kong L., Zhu L., Yi X., Huang Y., Zhao H., Chen Y., Yuan Z., Wen L., Wu J., Yi J. Betulinic acid alleviates spleen oxidative damage induced by acute intraperitoneal exposure to T-2 toxin by activating Nrf2 and inhibiting MAPK signaling pathways. Antioxidants. 2021;10:158. doi: 10.3390/antiox10020158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konjević D., Srebočan E., Gudan A., Lojkić I., Severin K., Sokolović M. A pathological condition possibly caused by spontaneous trichotecene poisoning in Brahma poultry: first report. Avian Pathol. 2004;33:377–380. doi: 10.1080/0307945042000220714. [DOI] [PubMed] [Google Scholar]
- Koppenol A., Branco Beirão B.C., Ingberman M., Caron L.F. Measuring peripheral and some mucosal immune cells to better understand immunomodulation by T-2 toxin in broilers. J. Appl. Poult. Res. 2019;28:837–845. [Google Scholar]
- Krishnamoorthy P., Vairamuthu S., Muralimanohar B. Pathology of chlorpyriphos and T-2 toxin on broiler chicken. Vet. Arhiv. 2007;77:47–57. [Google Scholar]
- Kubena L.F., Bailey R.H., Byrd J.A., Young C.R., Corrier D.E., Stanker L.H., Rottinghaust G.E. Cecal volatile fatty acids and broiler chick susceptibility to Salmonella Typhimurium colonization as affected by aflatoxins and T-2 toxin. Poult. Sci. 2001;80:411–417. doi: 10.1093/ps/80.4.411. [DOI] [PubMed] [Google Scholar]
- Kubena L.F., Edrington T.S., Kamps-holtzapple C., Harvey R.B., Elissalde M.H., Rottinghaus G.E. Influence of Fumonisin B1, present in Fusarium moniliforme culture material, and T-2 toxin on turkey poults. Poult. Sci. 1995;74:306–313. doi: 10.3382/ps.0740306. [DOI] [PubMed] [Google Scholar]
- Kuca K., Dohnal V., Jezkova A., Jun D. Metabolic pathways of T-2 toxin. Curr. Drug Metab. 2008;9:77–82. doi: 10.2174/138920008783331176. [DOI] [PubMed] [Google Scholar]
- Kufuor-Mensah E., Reed W.M., Sleight S., Pestka J., Fadly A.M., Dunn J.R. Effects of T-2 toxin on turkey herpesvirus–induced vaccinal immunity against Marek's disease. Avian Dis. 2015;60:56–62. doi: 10.1637/11245-072815-Reg.1. [DOI] [PubMed] [Google Scholar]
- Kulcsár S., Kövesi B., Balogh K., Zándoki E., Ancsin Z., Erdélyi M., Mézes M. The co-occurrence of T-2 toxin, deoxynivalenol, and fumonisin B1 activated the glutathione redox system in the EU-limiting doses in laying hens. Toxins. 2023;15:305. doi: 10.3390/toxins15050305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutasi J., Papp Z., Jakab L., Brydl E., Rafai P. Deactivation of T-2 toxin in broiler ducks by biotransformation. J. Appl. Poult. Res. 2012;21:13–20. [Google Scholar]
- Leal M., De Mejía E.G., Ruíz F., Shimada A. Effect of carotenoids on cytotoxicity of T-2 toxin on chicken hepatocytes in vitro. Toxicol. In Vitro. 1998;12:133–139. doi: 10.1016/s0887-2333(97)00110-0. [DOI] [PubMed] [Google Scholar]
- Lee I.K., Gu M.J., Ko K.H., Bae S., Kim G., Jin G.-D., Kim E.B., Kong Y.-Y., Park T.S., Park B.-C., Jung H.J., Han S.H., Yun C.-H. Regulation of CD4+CD8−CD25+ and CD4+CD8+CD25+ T cells by gut microbiota in chicken. Sci. Rep. 2018;8:8627. doi: 10.1038/s41598-018-26763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W.-Y., Park H.-J. T-2 mycotoxin Induces male germ cell apoptosis by ROS-mediated JNK/p38 MAPK pathway. Ecotoxicol. Environ. Saf. 2023;262 doi: 10.1016/j.ecoenv.2023.115323. [DOI] [PubMed] [Google Scholar]
- Li M., Pestka J.J. Comparative induction of 28S ribosomal RNA cleavage by ricin and the trichothecenes deoxynivalenol and T-2 toxin in the macrophage. Toxicol. Sci. 2008;105:67–78. doi: 10.1093/toxsci/kfn111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.-J., Dhaenens M., Garmyn A., Verbrugghe E., Van Rooij P., De Saeger S., Eeckhout M., Ducatelle R., Croubels S., Haesebrouck F., Deforce D., Pasmans F., Martel A. Exposure of Aspergillus fumigatus to T-2 toxin results in a stress response associated with exacerbation of aspergillosis in poultry. World Mycotoxin J. 2015;8:323–333. [Google Scholar]
- Li S.-J., Pasmans F., Croubels S., Verbrugghe E., Van Waeyenberghe L., Yang Z., Haesebrouck F., Martel A. T-2 toxin impairs antifungal activities of chicken macrophages against Aspergillus fumigatus conidia but promotes the pro-inflammatory responses. Avian Pathol. 2013;42:457–463. doi: 10.1080/03079457.2013.822958. [DOI] [PubMed] [Google Scholar]
- Li S.-J., Zhang G., Xue B., Ding Q., Han L., Huang J., Wu F., Li C., Yang C. Toxicity and detoxification of T-2 toxin in poultry. Food Chem. Toxicol. 2022;169 doi: 10.1016/j.fct.2022.113392. [DOI] [PubMed] [Google Scholar]
- Li Y., Wang Z., Beier R.C., Shen J., De Smet D., De Saeger S., Zhang S. T-2 toxin, a trichothecene mycotoxin: review of toxicity, metabolism, and analytical methods. J. Agric. Food Chem. 2011;59:3441–3453. doi: 10.1021/jf200767q. [DOI] [PubMed] [Google Scholar]
- Liu M., Zhang L., Mo Y., Li J., Yang J., Wang J., Karrow N.A., Wu H., Sun L. Ferroptosis is involved in deoxynivalenol-induced intestinal damage in pigs. J. Anim. Sci. Biotechnol. 2023;14:29. doi: 10.1186/s40104-023-00841-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Zhao L., Gong G., Zhang L., Shi L., Dai J., Han Y., Wu Y., Khalil M.M., Sun L. Invited review: remediation strategies for mycotoxin control in feed. J. Anim. Sci. Biotechnol. 2022;13:19. doi: 10.1186/s40104-021-00661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Zhao L., Wei J.-T., Huang Y.-X., Khalil M.M., Wu W.-D., Kuča K., Sun L.-H. T-2 toxin-induced intestinal damage with dysregulation of metabolism, redox homeostasis, inflammation, and apoptosis in chicks. Arch. Toxicol. 2023;97:805–817. doi: 10.1007/s00204-023-03445-z. [DOI] [PubMed] [Google Scholar]
- Lin M.-J., Chang S.-C., Tso K.-H., Lin W.-C., Chang C.-L., Lee T.-T. Effect of T-2 toxin and antioxidants on angel wing incidence and severity in white roman geese. J. Appl. Anim. Res. 2018;46:340–348. [Google Scholar]
- Lin N.-N., Chen J., Xu B., Wei X., Guo L., Xie J.-W. The roles of carboxylesterase and CYP isozymes on the in vitro metabolism of T-2 toxin. Mil. Med. Res. 2015;2:1–7. doi: 10.1186/s40779-015-0041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Wen J., Zhu J., Zhang T., Deng Y., Jiang J. Aromatic hydrocarbon receptor regulates chicken cytochrome P450 1A5 transcription: a novel insight into T-2 toxin-induced gene expression and cytotoxicity in LMH cells. Biochem. Pharmacol. 2019;168:319–329. doi: 10.1016/j.bcp.2019.07.023. [DOI] [PubMed] [Google Scholar]
- Liu W., Wu D., Li S., Xu J., Li P., Jiang A., Zhang Y., Liu Z., Jiang L., Gao X., Yang Z., Wei Z. Glycolysis and reactive oxygen species production participate in T-2 toxin-stimulated chicken heterophil extracellular traps. J. Agric. Food Chem. 2021;69:12862–12869. doi: 10.1021/acs.jafc.1c05371. [DOI] [PubMed] [Google Scholar]
- Liu X., Guo P., Liu A., Wu Q., Xue X., Dai M., Hao H., Qu W., Xie S., Wang X., Yuan Z. Nitric oxide (NO)-mediated mitochondrial damage plays a critical role in T-2 toxin-induced apoptosis and growth hormone deficiency in rat anterior pituitary GH3 cells. Food Chem. Toxicol. 2017;102:11–23. doi: 10.1016/j.fct.2017.01.017. [DOI] [PubMed] [Google Scholar]
- Luo J.-J., Zhang Y., Sun H., Wei J.-T., Khalil M.M., Wang Y.-W., Dai J.-F., Zhang N.-Y., Qi D.-S., Sun L.-H. The response of glandular gastric transcriptome to T-2 toxin in chicks. Food Chem. Toxicol. 2019;132 doi: 10.1016/j.fct.2019.110658. [DOI] [PubMed] [Google Scholar]
- Luo S., Du H., Kebede H., Liu Y., Xing F. Contamination status of major mycotoxins in agricultural product and food stuff in Europe. Food Control. 2021;127 [Google Scholar]
- Mackei M., Orbán K., Molnár A., Pál L., Dublecz K., Husvéth F., Neogrády Z., Mátis G. Cellular effects of T-2 toxin on primary hepatic cell culture models of chickens. Toxins. 2020;12:46. doi: 10.3390/toxins12010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madheswaran R., Balachandran C., Murali Manohar B. Influence of dietary culture material containing aflatoxin and T2 toxin on certain serum biochemical constituents in Japanese quail. Mycopathologia. 2004;158:337–341. doi: 10.1007/s11046-005-8399-8. [DOI] [PubMed] [Google Scholar]
- Manafi M., Umakantha B., Mohan K., Swamy H.D.N. Synergistic effects of two commonly contaminating mycotoxins (aflatoxin and T-2 toxin) on biochemical parameters and immune status of broiler chickens. World Appl. Sci. J. 2012;17:364–367. [Google Scholar]
- Maresca M. From the gut to the brain: journey and pathophysiological effects of the food-associated trichothecene mycotoxin deoxynivalenol. Toxins. 2013;5:784–820. doi: 10.3390/toxins5040784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride J.A., Striker R. Imbalance in the game of T cells: what can the CD4/CD8 T-cell ratio tell us about HIV and health? PLOS Pathogens. 2017;13 doi: 10.1371/journal.ppat.1006624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneely J., Greer B., Kolawole O., Elliott C. T-2 and HT-2 toxins: toxicity, occurrence and analysis: a review. Toxins. 2023;15:481. doi: 10.3390/toxins15080481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezes M., Barta M., Nagy G. Comparative investigation on the effect of T-2 mycotoxin on lipid peroxidation and antioxidant status in different poultry species. Res. Vet. Sci. 1999;66:19–23. doi: 10.1053/rvsc.1998.0233. [DOI] [PubMed] [Google Scholar]
- Minervini F., Fornelli F., Lucivero G., Romano C., Visconti A. T-2 toxin immunotoxicity on human B and T lymphoid cell lines. Toxicology. 2005;210:81–91. doi: 10.1016/j.tox.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Mu P., Xu M., Zhang L., Wu K., Wu J., Jiang J., Chen Q., Wang L., Tang X., Deng Y. Proteomic changes in chicken primary hepatocytes exposed to T-2 toxin are associated with oxidative stress and mitochondrial enhancement. Proteomics. 2013;13:3175–3188. doi: 10.1002/pmic.201300015. [DOI] [PubMed] [Google Scholar]
- Mukherjee G., Mishra T., Deshmukh S.K. Pages 525–541 in Developments in Fungal Biology and Applied Mycology. Springer; Singapore: 2017. Fungal pigments: an overview. [Google Scholar]
- Muller G.C., Gottlieb M.G.V., Correa B.L., Filho I.G., Moresco R.N., Bauer M.E. The inverted CD4:CD8 ratio is associated with gender-related changes in oxidative stress during aging. Cell. Immunol. 2015;296:149–154. doi: 10.1016/j.cellimm.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Nada S., Nikola T., Bozidar U., Ilija D., Andreja R. Prevention and practical strategies to control mycotoxins in the wheat and maize chain. Food Control. 2022;136 [Google Scholar]
- Nakade M., Pelyhe C., Kövesi B., Balogh K., Kovács B., Szabó-Fodor J., Zándoki E., Mézes M., Erdélyi M. Short-term effects of T-2 toxin or deoxynivalenol on glutathione status and expression of its regulatory genes in chicken. Acta Vet. Hung. 2018;66:28–39. doi: 10.1556/004.2018.004. [DOI] [PubMed] [Google Scholar]
- Nathanail A.V., Syvähuoko J., Malachová A., Jestoi M., Varga E., Michlmayr H., Adam G., Sieviläinen E., Berthiller F., Peltonen K. Simultaneous determination of major type A and B trichothecenes, zearalenone and certain modified metabolites in Finnish cereal grains with a novel liquid chromatography-tandem mass spectrometric method. Anal. Bioanal. Chem. 2015;407:4745–4755. doi: 10.1007/s00216-015-8676-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert D.W., Dalton T.P. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat. Rev. Cancer. 2006;6:947–960. doi: 10.1038/nrc2015. [DOI] [PubMed] [Google Scholar]
- Nešić K., Habschied K., Mastanjević K. Modified mycotoxins and multitoxin contamination of food and feed as major analytical challenges. Toxins. 2023;15:511. doi: 10.3390/toxins15080511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obar J.J., Lefrançois L. Memory CD8+ T cell differentiation. Ann. N. Y. Acad. Sci. 2010;1183:251–266. doi: 10.1111/j.1749-6632.2009.05126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omotayo O.P., Omotayo A.O., Mwanza M., Babalola O.O. Prevalence of mycotoxins and their consequences on human health. Toxicol. Res. 2019;35:1–7. doi: 10.5487/TR.2019.35.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osselaere A., Li S.J., De Bock L., Devreese M., Goossens J., Vandenbroucke V., Van Bocxlaer J., Boussery K., Pasmans F., Martel A., De Backer P., Croubels S. Toxic effects of dietary exposure to T-2 toxin on intestinal and hepatic biotransformation enzymes and drug transporter systems in broiler chickens. Food Chem. Toxicol. 2013;55:150–155. doi: 10.1016/j.fct.2012.12.055. [DOI] [PubMed] [Google Scholar]
- Pál L., Dublecz K., Weber M., Balogh K., Erdélyi M., Szigeti G., Mézes M. Effect of combined treatment with aflatoxin B 1 and T-2 toxin and metabolites on some production traits and lipid peroxide status parameters of broiler chickens. Acta Vet. Hung. 2009;57:75–84. doi: 10.1556/AVet.57.2009.1.8. [DOI] [PubMed] [Google Scholar]
- Palumbo R., Crisci A., Venâncio A., Cortiñas Abrahantes J., Dorne J.-L., Battilani P., Toscano P. Occurrence and co-occurrence of mycotoxins in cereal-based feed and food. Microorganisms. 2020;8:74. doi: 10.3390/microorganisms8010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelyhe C.S., Kövesi B., Szabó-Fodor J., Zándoki E., Erdélyi M., Kovács B., Mézes M., Balogh K. Age-dependent effects of short-term exposure of T-2 toxin or deoxynivalenol on lipid peroxidation and glutathione redox system in broiler chickens. World Mycotoxin J. 2018;11:611–624. [Google Scholar]
- Pestka J.J., Zhou H.-R., Moon Y., Chung Y.J. Cellular and molecular mechanisms for immune modulation by deoxynivalenol and other trichothecenes: unraveling a paradox. Toxicol. Lett. 2004;153:61–73. doi: 10.1016/j.toxlet.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Pierron A., Alassane-Kpembi I., Oswald I.P. Impact of mycotoxin on immune response and consequences for pig health. Anim. Nutr. 2016;2:63–68. doi: 10.1016/j.aninu.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafai P., Pettersson H., Bata A., Papp Z., Glávits R., Tuboly S., Ványi A., Soós P. Effect of dietary T-2 fusariotoxin concentrations on the health and production of white Pekin duck broilers. Poult. Sci. 2000;79:1548–1556. doi: 10.1093/ps/79.11.1548. [DOI] [PubMed] [Google Scholar]
- Raju M.V.L.N., Devegowda G. Influence of esterified-glucomannan on performance and organ morphology, serum biochemistry and haematology in broilers exposed to individual and combined mycotoxicosis (aflatoxin, ochratoxin and T-2 toxin) Br. Poult. Sci. 2000;41:640–650. doi: 10.1080/713654986. [DOI] [PubMed] [Google Scholar]
- Rampersad S.N. Pathogenomics and management of fusarium diseases in plants. Pathogens. 2020;9:340. doi: 10.3390/pathogens9050340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezar V., Frankič T., Narat M., Levart A., Salobir J. Dose-dependent effects of T-2 toxin on performance, lipid peroxidation, and genotoxicity in broiler chickens. Poult. Sci. 2007;86:1155–1160. doi: 10.1093/ps/86.6.1155. [DOI] [PubMed] [Google Scholar]
- Richard J.L. Some major mycotoxins and their mycotoxicoses—an overview. Int. J. Food Microbiol. 2007;119:3–10. doi: 10.1016/j.ijfoodmicro.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Rodríguez M., Núñez F. Novel approaches to minimizing mycotoxin contamination. Toxins. 2020;12:216. doi: 10.3390/toxins12040216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rychlik M., Humpf H.-U., Marko D., Dänicke S., Mally A., Berthiller F., Klaffke H., Lorenz N. Proposal of a comprehensive definition of modified and other forms of mycotoxins including “masked” mycotoxins. Mycotoxin Res. 2014;30:197–205. doi: 10.1007/s12550-014-0203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salah A.S., Ahmed-Farid O.A., Nassan M.A., El-Tarabany M.S. Dietary curcumin improves energy metabolism, brain monoamines, carcass traits, muscle oxidative stability and fatty acid profile in heat-stressed broiler chickens. Antioxidants. 2021;10:1265. doi: 10.3390/antiox10081265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekelova Z., Polansky O., Stepanova H., Fedr R., Faldynova M., Rychlik I., Vlasatikova L. Different roles of CD4, CD8 and γδ T-lymphocytes in naive and vaccinated chickens during Salmonella Enteritidis infection. Proteomics. 2017;17 doi: 10.1002/pmic.201700073. 13–14:1700073. [DOI] [PubMed] [Google Scholar]
- Shang S., Jiang J., Deng Y. Chicken cytochrome P450 1A5 is the key enzyme for metabolizing T-2 toxin to 3’OH-T-2. Int. J. Mol. Sci. 2013;14:10809–10818. doi: 10.3390/ijms140610809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifrin V.I., Anderson P. Trichothecene mycotoxins trigger a ribotoxic stress response that activates c-Jun N-terminal kinase and p38 mitogen-activated protein kinase and induces apoptosis. J. Biol. Chem. 1999;274:13985–13992. doi: 10.1074/jbc.274.20.13985. [DOI] [PubMed] [Google Scholar]
- Singh R., Park S., Koo J.S., Kim I.H., Balasubramanian B. Significance of varying concentrations of T-2 toxin on growth performance, serum biochemical and hematological parameters in broiler chickens. J. Anim. Sci. Technol. 2020;62:468–474. doi: 10.5187/jast.2020.62.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklan D., Shelly M., Makovsky B., Geyra A., Klipper E., Friedman A. The effect of chronic feeding of diacetoxyscirpenol and T-2 toxin on performance, health, small intestinal physiology and antibody production in turkey poults. Br. Poult. Sci. 2003;44:46–52. doi: 10.1080/0007166031000085373. [DOI] [PubMed] [Google Scholar]
- Smith T.K. Recent advances in the understanding of Fusarium trichothecene mycotoxicoses. J. Anim. Sci. 1992;70:3989–3993. doi: 10.2527/1992.70123989x. [DOI] [PubMed] [Google Scholar]
- Sokolovic M., Garaj-Vrhovac V., Ramic S., Simpraga B. Chicken nucleated blood cells as a cellular model for genotoxicity testing using the comet assay. Food Chem. Toxicol. 2007;45:2165–2170. doi: 10.1016/j.fct.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Sokolovic M., Garaj-Vrhovac V., Simpraga B. T-2 toxin: incidence and toxicity in poultry. Arhiv za higijenu rada i toksikologiju. 2008;59:43–52. doi: 10.2478/10004-1254-59-2008-1843. [DOI] [PubMed] [Google Scholar]
- Song W., Wang Y., Huang T., Liu Y., Chen F., Chen Y., Jiang Y., Zhang C., Yang X. T-2 toxin metabolism and its hepatotoxicity: new insights on the molecular mechanism and detoxification. Environ. Pollut. 2023;330 doi: 10.1016/j.envpol.2023.121784. [DOI] [PubMed] [Google Scholar]
- Stefanović D., Marinković D., Trailović S., Vasiljević M., Farkaš H., Raj J., Tolimir N., Radulović S., Nešić V., Trailović J.N., Petrujkić B. Evaluation of effectiveness of a novel multicomponent mycotoxins detoxification agent in the presence of AFB1 and T-2 toxin on broiler chicks. Microorganisms. 2023;11:574. doi: 10.3390/microorganisms11030574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Li R., Tai B., Hussain S., Wang G., Liu X., Xing F. Current status of major mycotoxins contamination in food and feed in Asia—a review. ACS Food Sci. Technol. 2023;3:231–244. [Google Scholar]
- Szabó R.T., Kovács-Weber M., Erdélyi M., Balogh K., Fazekas N., Horváth Á., Mézes M., Kovács B. Comet assay study of the genotoxic effect of T-2 and HT-2 toxins in chicken hepatocytes. Biol. Futura. 2019;70:330–335. doi: 10.1556/019.70.2019.37. [DOI] [PubMed] [Google Scholar]
- Tan H., Zhou H., Guo T., Zhou Y., Wang S., Liu X., Zhang Y., Ma L. Matrix-associated mycotoxins in foods, cereals and feedstuffs: a review on occurrence, detection, transformation and future challenges. Crit. Rev. Food Sci. Nutr. 2022:1–14. doi: 10.1080/10408398.2022.2131724. [DOI] [PubMed] [Google Scholar]
- Tso K.-H., Lumsangkul C., Cheng M.-C., Ju J.-C., Fan Y.-K., Chiang H.-I. Differential effects of green tea powders on the protection of brown tsaiya and kaiya ducklings against trichothecene T-2 toxin toxicity. Animals. 2021;11:2541. doi: 10.3390/ani11092541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Fels-Klerx H.j., Liu C., Battilani P. Modelling climate change impacts on mycotoxin contamination. World Mycotoxin J. 2016;9:717–726. [Google Scholar]
- Ványi A., Glávits R., Bata A., Kovács F. Pathomorphological changes caused by T-2 trichothecene fusariotoxin in geese. Acta Vet. Hung. 1994;42:447–457. [PubMed] [Google Scholar]
- Venkatesh P.K., Vairamuthu S., Balachandran C., Manohar B.M., Raj G.D. Induction of apoptosis by fungal culture materials containing cyclopiazonic acid and T-2 toxin in primary lymphoid organs of broiler chickens. Mycopathologia. 2005;159:393–400. doi: 10.1007/s11046-004-6271-x. [DOI] [PubMed] [Google Scholar]
- Wan Q., He Q., Deng X., Hao F., Tang H., Wang Y. Systemic metabolic responses of broiler chickens and piglets to acute T-2 toxin intravenous exposure. J. Agric. Food Chem. 2016;64:714–723. doi: 10.1021/acs.jafc.5b05076. [DOI] [PubMed] [Google Scholar]
- Wang G.H., Xue C.Y., Chen F., Ma Y.L., Zhang X.B., Bi Y.Z., Cao Y.C. Effects of combinations of ochratoxin A and T-2 toxin on immune function of yellow-feathered broiler chickens. Poult. Sci. 2009;88:504–510. doi: 10.3382/ps.2008-00329. [DOI] [PubMed] [Google Scholar]
- Wang J., Fitzpatrick D.W., Wilson J.R. Effect of T-2 toxin on blood-brain barrier permeability monoamine oxidase activity and protein synthesis in rats. Food Chem. Toxicol. 1998;36:955–961. doi: 10.1016/s0278-6915(98)00079-9. [DOI] [PubMed] [Google Scholar]
- Wang J., Jiang J., Zhang H., Wang J., Cai H., Li C., Li K., Liu J., Guo X., Zou G., Wang D., Deng Y., Dai J. Integrated transcriptional and proteomic analysis with in vitro biochemical assay reveal the important role of CYP3A46 in T-2 toxin hydroxylation in porcine primary hepatocytes. Mol. Cell. Proteom. 2011;10 doi: 10.1074/mcp.M111.008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M., Fodor J., Balogh K., Wágner I., Erdélyi M., Mézes M. Effect of vitamin E supplementation on the haemagglutination inhibitor titre vaccinated against Newcastle disease in T-2 toxin challenged chickens. Acta Vet. Brno. 2008;77:45–49. [Google Scholar]
- Weber M., Stiller S., Balogh K., Wágner L., Erdélyi M., Mézes M. Effect of feeding T-2 toxin contaminated feed on the utilisation of vitamin E in chickens. Acta Vet. Hung. 2007;55:21–27. doi: 10.1556/AVet.55.2007.1.3. [DOI] [PubMed] [Google Scholar]
- Wei J.-T., Wu K.-T., Sun H., Khalil M.M., Dai J.-F., Liu Y., Liu Q., Zhang N.-Y., Qi D.-S., Sun L.-H. A novel modified hydrated sodium calcium aluminosilicate (HSCAS) adsorbent can effectively reduce T-2 toxin-induced toxicity in growth performance, nutrient digestibility, serum biochemistry, and small intestinal morphology in chicks. Toxins. 2019;11:199. doi: 10.3390/toxins11040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Jing L., Yuan H., Peng S. T-2 toxin induces apoptosis in ovarian granulosa cells of rats through reactive oxygen species-mediated mitochondrial pathway. Toxicol. Lett. 2011;202:168–177. doi: 10.1016/j.toxlet.2011.01.029. [DOI] [PubMed] [Google Scholar]
- Wu Q., Qin Z., Kuca K., You L., Zhao Y., Liu A., Musilek K., Chrienova Z., Nepovimova E., Oleksak P., Wu W., Wang X. An update on T-2 toxin and its modified forms: metabolism, immunotoxicity mechanism, and human exposure assessment. Arch. Toxicol. 2020;94:3645–3669. doi: 10.1007/s00204-020-02899-9. [DOI] [PubMed] [Google Scholar]
- Wu Q.-H., Wang X., Yang W., Nüssler A.K., Xiong L.-Y., Kuča K., Dohnal V., Zhang X.-J., Yuan Z.-H. Oxidative stress-mediated cytotoxicity and metabolism of T-2 toxin and deoxynivalenol in animals and humans: an update. Arch. Toxicol. 2014;88:1309–1326. doi: 10.1007/s00204-014-1280-0. [DOI] [PubMed] [Google Scholar]
- Xue C.Y., Wang G.H., Chen F., Zhang X.B., Bi Y.Z., Cao Y.C. Immunopathological effects of ochratoxin A and T-2 toxin combination on broilers. Poult. Sci. 2010;89:1162–1166. doi: 10.3382/ps.2009-00609. [DOI] [PubMed] [Google Scholar]
- Yang L., Tu D., Wang N., Deng Z., Zhan Y., Liu W., Hu Y., Liu T., Tan L., Li Y., Guo S., Wang A. The protective effects of DL-Selenomethionine against T-2/HT-2 toxins-induced cytotoxicity and oxidative stress in broiler hepatocytes. Toxicol. In Vitro. 2019;54:137–146. doi: 10.1016/j.tiv.2018.09.016. [DOI] [PubMed] [Google Scholar]
- Yang L., Tu D., Zhao Z., Cui J. Cytotoxicity and apoptosis induced by mixed mycotoxins (T-2 and HT-2 toxin) on primary hepatocytes of broilers in vitro. Toxicon. 2017;129:1–10. doi: 10.1016/j.toxicon.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Yang L., Yu Z., Hou J., Deng Y., Zhou Z., Zhao Z., Cui J. Toxicity and oxidative stress induced by T-2 toxin and HT-2 toxin in broilers and broiler hepatocytes. Food Chem. Toxicol. 2016;87:128–137. doi: 10.1016/j.fct.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Yang S., De Boevre M., Zhang H., De Ruyck K., Sun F., Zhang J., Jin Y., Li Y., Wang Z., Zhang S., Zhou J., Li Y., De Saeger S. Metabolism of T-2 toxin in farm animals and human in vitro and in chickens in vivo using ultra high-performance liquid chromatography- quadrupole/time-of-flight hybrid mass spectrometry along with online hydrogen/deuterium exchange technique. J. Agric. Food Chem. 2017;65:7217–7227. doi: 10.1021/acs.jafc.7b02575. [DOI] [PubMed] [Google Scholar]
- Yang S., Li Y., Cao X., Hu D., Wang Z., Wang Y., Shen J., Zhang S. Metabolic pathways of T-2 toxin in in vivo and in vitro systems of wistar rats. J. Agric. Food Chem. 2013;61:9734–9743. doi: 10.1021/jf4012054. [DOI] [PubMed] [Google Scholar]
- Yang X., Liu P., Cui Y., Xiao B., Liu M., Song M., Huang W., Li Y. Review of the reproductive toxicity of T-2 toxin. J. Agric. Food Chem. 2020;68:727–734. doi: 10.1021/acs.jafc.9b07880. [DOI] [PubMed] [Google Scholar]
- Yao L., Du Q., Yao H., Chen X., Zhang Z., Xu S. Roles of oxidative stress and endoplasmic reticulum stress in selenium deficiency-induced apoptosis in chicken liver. Biometals. 2015;28:255–265. doi: 10.1007/s10534-014-9819-3. [DOI] [PubMed] [Google Scholar]
- Yin H., Han S., Chen Y., Wang Y., Li D., Zhu Q. T-2 toxin induces oxidative stress, apoptosis and cytoprotective autophagy in chicken hepatocytes. Toxins. 2020;12:90. doi: 10.3390/toxins12020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H., Han S., Chen Y., Wang Y., Li D., Zhu Q. T-2 toxin induces oxidative stress, apoptosis and cytoprotective autophagy in chicken hepatocytes. Toxins. 2020;12:90. doi: 10.3390/toxins12020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohannes T., Sharma A.K., Singh S.D., Goswami T.K. Immunopathological effects of experimental T-2 mycotoxocosis in broiler chicken co-infected with infectious bronchitis virus (IBV) Vet. Immunol. Immunopathol. 2012;146:245–253. doi: 10.1016/j.vetimm.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Yohannes T., Sharma A.K., Singh S.D., Sumi V. Experimental haematobiochemical alterations in broiler chickens fed with T-2 toxin and co-infected with IBV. Open J. Vet. Med. 2013;3:252–258. [Google Scholar]
- Yuan G., Wang Y., Yuan X., Zhang T., Zhao J., Huang L., Peng S. T-2 toxin induces developmental toxicity and apoptosis in zebrafish embryos. J. Environ. Sci. 2014;26:917–925. doi: 10.1016/S1001-0742(13)60510-0. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Zhou X., Yang J., Li M., Qiu X. T-2 toxin is hydroxylated by chicken CYP3A37. Food Chem. Toxicol. 2013;62:622–627. doi: 10.1016/j.fct.2013.09.031. [DOI] [PubMed] [Google Scholar]
- Zhang J., You L., Wu W., Wang X., Chrienova Z., Nepovimova E., Wu Q., Kuca K. The neurotoxicity of trichothecenes T-2 toxin and deoxynivalenol (DON): current status and future perspectives. Food Chem. Toxicol. 2020;145 doi: 10.1016/j.fct.2020.111676. [DOI] [PubMed] [Google Scholar]
- Zhao L., Zhang L., Xu Z., Liu X., Chen L., Dai J., Karrow N.A., Sun L. Occurrence of Aflatoxin B1, deoxynivalenol and zearalenone in feeds in China during 2018–2020. J. Anim. Sci. Biotechnol. 2021;12:74. doi: 10.1186/s40104-021-00603-0. [DOI] [PMC free article] [PubMed] [Google Scholar]