Abstract
OBJECTIVE:
To examine obstetric physicians’ beliefs about using professional or regulatory guidelines, opioid risk-screening tools, and preferences for recommending nonanalgesic therapies for postpartum pain management.
METHODS:
A qualitative study design was used to conduct semi-structured interviews with obstetric and maternal–fetal medicine physicians (N=38) from two large academic health care institutions in central Pennsylvania. An interview guide was used to direct the discussion about each physicians’ beliefs in response to questions about pain management after childbirth.
RESULTS:
Three trends in the data emerged from physicians’ responses: 1) 71% of physicians relied on their clinical insight rather than professional or regulatory guidelines to inform decisions about pain management after childbirth; 2) although many reported that a standard opioid patient screening tool would be useful to inform clinical decisions about pain management, nearly all (92%) physician respondents reported not currently using one; and 3) 63% thought that nonpharmacologic pain management therapies should be used whenever possible to manage pain after childbirth. Key physician barriers (eg, lack time and evidence, being unaware of how to implement) and patient barriers (eg, take away from other responsibilities, no time or patience) to implementation were also identified.
CONCLUSION:
These findings suggest that obstetric physicians’ individual beliefs and clinical insight play a key role in pain management decisions for women after childbirth. Practical and scalable strategies are needed to: 1) encourage obstetric physicians to use professional or regulatory guidelines and standard opioid risk-screening tools to inform clinical decisions about pain management after childbirth, and 2) educate physicians and patients about nonopioid and nonpharmacologic pain management options to reduce exposure to prescription opioids after childbirth.
Cesarean birth is the most common major surgery in the United States.1,2 As part of postoperative care, women are commonly given opioids in greater quantities than needed to adequately manage postpartum pain.3–7 For example, Osmundson and colleagues7 found that in a cohort of 246 patients who underwent cesarean birth, 75% had unused opioids resulting in an excess of more than 2,500 unused 5 mg oxycodone tablets. There also is evidence to suggest that 5,800 opioid-naïve new mothers per year in the United States develop persistent opioid use after cesarean (4,200)3,8 and vaginal (1,600) births.4 Opioid exposure after birth potentially increases the risk for not only long-term chronic opioid and other substance use, but also postpartum depression and difficulty bonding with the newborn.9,10 Despite this growing body of evidence, limited research has been conducted to understand why rates of opioid prescriptions are so high after childbirth and what strategies may effectively reduce prescription opioid use in this setting.
One explanation for the high rates of filled opioid prescriptions may be the unintended consequence of The Joint Commission’s recognizing pain as the fifth vital sign and recommending that physicians consider treating pain with opioids.11 Another explanation may be individual differences in physicians’ beliefs about patient expectations12 and implementation of recommendations. For example, the American College of Obstetricians and Gynecologists (ACOG) released guidelines10 in 2018 for postpartum pain management that mirrored the use of the longstanding stepwise analgesic ladder introduced by the World Health Organization for treatment of cancer pain.13 Step 1 recommends nonopioid analgesics (eg, acetaminophen or nonsteroidal antiinflammatory drugs); step 2 incorporates milder options (eg, codeine, hydrocodone, oxycodone, tramadol); and step 3 adds stronger options (eg, fentanyl). In Pennsylvania, state guidelines14 released in 2018 suggest that opioids should be prescribed only when “deemed necessary” (Box 1). To date, however, little to no research has specifically examined obstetric physicians’ use of these recommendations in practice. Given this gap in the literature, the purpose of this study was to assess obstetric physicians’ beliefs about the use of professional and regulatory guidelines and risk-screening tools to guide clinical decision making for prescribing opioids after delivery, and nonanalgesic therapies for postpartum pain management. Understanding physicians’ preferences will help to inform the design of an effective intervention to reduce opioid exposure after childbirth.
Box 1. 2018 Commonwealth of Pennsylvania Guidelines for Childbirth and Postpartum Pain Management.
All pregnant and postpartum women are recommended to receive a brief screening for substance use disorder
NSAIDs or acetaminophen are recommended as the first line agent for moderate pain
-
Nonpharmacologic therapies (eg, heat, relaxation, light physical activity) for mild pain
NSAIDs, nonsteroidal antiinflammatory drugs.
METHODS
A qualitative study design was used to conduct semi-structured interviews with obstetric and maternal–fetal medicine physicians recruited from two large academic health care systems in central Pennsylvania. Interviews were conducted from May 2019 to March 2020. The study was approved by the Pennsylvania State University Institutional Review Board (CATS #00009937). Participants were attending and resident physicians who were identified by study team liaisons, recruited by email and verbal contact, and screened for eligibility. Physicians were eligible to participate in this study if they were employed by one of two health care systems in central Pennsylvania, currently treating patients after delivery, and able to prescribe opioids. Exclusion criteria were failure to meet inclusion criteria. If eligible and interested, participants were scheduled for an in-person (onsite at health care facility) or phone call interview that was expected to take 30 minutes.
Semi-structured interviews were conducted by a physician and medical student trained in qualitative research methods. All participants were consented and given a copy of the interview questions before the interview. The consent form indicated that no individual would be identified in the interview responses, participants were given the option to refrain from responding to a question if they did not want to answer it, and nonidentifying results from the interviews would be published. An interview guide was used to elicit the physicians’ thoughts and beliefs in response to six questions about clinical recommendations for providing pain management prescriptions after delivery, familiarity with current opioid prescribing guidelines for the Commonwealth of Pennsylvania (Box 1),14 available opioid-screening tools, and nonpharmacologic pain management options. Following the established qualitative interview guidelines of Adams,15 participants were encouraged to discuss their responses to each question as openly as possible, and the moderator prompted the participant with clarifying questions as needed based on their responses. This process resulted in the moderator asking a subgroup of physicians clarifying questions about motives, beliefs, and barriers to using nonpharmacologic therapies.
All interviews were recorded with a digital voice recorder. MP3 recordings were sent by secure file transfer to secure data storage ([REDCap] Research Electronic Data Capture)16 and transcribed verbatim by trained research staff using standard procedures described in our prior studies.17–19 Transcribed interviews were coded using NVivo20 software and final coded data sets were stored in REDCap16 and SPSS.21 Inductive thematic analysis was used to allow themes to emerge naturally from the data.22 Interviews were coded separately by two independent trained coders in the following steps drawn from phases proposed by Vaismoradi and colleagues22: 1) transcribed interviews were read thoroughly to obtain a sense for interview content; 2) sentences or text blocks with information relevant to the themes were highlighted; 3) highlighted sentences and text blocks were condensed and labeled with a code; 4) codes (subthemes) were clustered to create categories (themes) to present a condensed but broad description, and coding between independent coders was merged once discrepancies were resolved and there was 100% agreement; and 5) preliminary tables with themes and subthemes were constructed and reviewed by the independent coders and the first author during frequent discussions until all themes were finalized. Representative quotations for each theme were selected and reviewed by two trained coders following the recommendations of Lingard.23 Quotes were deemed authentic and selected if they were illustrative of the point, reasonably succinct, and representative of the patterns in the data. Final themes were presented as a total sample because responses of the attending and resident physicians were similar.
RESULTS
One hundred ten physicians were contacted, of whom 60 responded. From this group, 18 declined participation (eg, lack of time, no longer interested) and 42 agreed to participate in the interview (70% response rate). Forty-one interviews were conducted face-to-face, and one interview was completed by phone. Four of the interviews (10%) were excluded owing to poor recording quality, resulting in a final sample of 38 physicians. Twenty-two interviews were conducted at one institution and 16 at the second institution. Interviews took an average of 15 minutes per participant (range 7–39 minutes). Participants were attending physicians (76%) and residents (24%) who practiced medicine for an average of 11.8 years, with 11.3 years of experience in obstetrics and gynecology, and had been in their current position for 5.9 years (Table 1). The mean age was 41.7 years, and 63% of participants were female. All participants (N=38) completed the six interview questions (Table 2), and 68% (26/38) provided responses to the three supplemental questions about nonpharmacologic therapies (Table 3).
Table 1.
Participant Demographics (N = 38 Obstetric Physicians)
| Descriptive | Mean±SD | Range | n (%) |
|---|---|---|---|
|
| |||
| Sex | |||
| Female | — | — | 24 (63) |
| Male | — | — | 14 (37) |
| Current position | |||
| Attending | — | — | 29 (76) |
| Resident | — | — | 9 (24) |
| Age | 41.7±13.2 | 28–70 | — |
| Years in current position | 5.9±7.2 | 12–26 | — |
| Attending | 5.9±7.1 | 0.13–26 | 29 (76) |
| Resident | 3±0.7 | 2–4 | 9 (24) |
| Years practicing medicine | 11.8±12.0 | 0.5–41 | — |
| Attending | 14.1±13.0 | 0.5–41 | 29 (76) |
| Resident | 3.1±0.8 | 2–4 | 9 (24) |
| Years practicing obstetrics and gynecology | 11.3±11.5 | 0.5–40 | — |
| Attending | 13.7±12.3 | 0.5–40 | 29 (76) |
| Resident | 3±0.7 | 2–4 | 9 (24) |
Table 2.
Obstetric Physicians’ Interview Questions, Themes, and Responses
| Theme | Response*† |
|---|---|
|
| |
| Question 1: What clinical recommendations do you use to guide your decision process for writing a prescription? | |
| No guidelines, relied on clinical insight | 27 (71) |
| Medical training experience | 12 (32) |
| Evidence from literature | 6 (16) |
| Patient history or check prescription drug monitoring program | 5 (13) |
| No guidelines | 3 (8) |
| Urine drug screen | 1 (3) |
| Used guidelines | 10 (26) |
| ACOG | 8 (21) |
| CDC | 2 (5) |
| Did not answer appropriately | 1 (3) |
| Question 2: How familiar are you with the obstetrics and gynecology opioid prescribing guidelines recently developed by the Commonwealth of Pennsylvania? | |
| Not familiar | 33 (87) |
| Familiar | 5 (13) |
| Question 3: These guidelines recommend use of the safest drug available (eg, acetaminophen) for most mild to moderate pain, and suggest that opioids can be used for 3–5 days after cesarean birth or severe perineal trauma. What are your thoughts about these recommendations? | |
| In line with current practice | 34 (89) |
| Recommendations are strict | 4 (11) |
| Question 4: What patient screening tools do you administer to assess a woman’s readiness to receive an opioid prescription after delivery? | |
| No standardized tool | 35 (92) |
| No routine use of screening tool | 13 (34) |
| No screening tool but provide clinical insight | 13 (34) |
| No screening tool but ask patient or review history | 5 (13) |
| Pennsylvania Prescription Drug Monitoring Program | 3 (8) |
| Did not answer question appropriately | 1 (3) |
| Question 5: What screening tools do you administer to examine for substance use disorder? | |
| No standardized tool | 35 (92) |
| No screening tool but talk with patient or review patient history | 24 (63) |
| No routine use of a screening tool | 11 (29) |
| Screen for other drugs | 5 (13) |
| NIDA Drug Use Screening Tool: Quick Screen | 1 (3) |
| Pennsylvania Prescription Drug Monitoring Program | 1 (3) |
| 5Ps (Parents, Peers, Partner, Past, Present) | 1 (3) |
| Question 6: The Commonwealth of Pennsylvania guidelines also suggest nonpharmacologic therapies should be used whenever possible to manage pain during pregnancy. What are your thoughts/beliefs on using these therapies after delivery? | |
| Like to use | 24 (63) |
| Use on a case-by-case basis | 9 (24) |
| Not sure if therapies work | 2 (5) |
| Not familiar with these guidelines | 1 (3) |
| Did not answer question appropriately | 2 (5) |
ACOG, American College of Obstetricians and Gynecologists;CDC, Centers for Disease Control and Prevention.
Data are n (%).
Participants may have provided responses to more than one theme so some frequency totals may exceed the number of physicians that were interviewed.
Thirty-eight physicians completed questions 1–6.
Table 3.
Obstetric Physicians’ Motives, Beliefs, and Barriers to Recommending Nonpharmacologic Therapies for Pain Management After Childbirth
| Theme | Response*† |
|---|---|
|
| |
| Question 7: How motivated are physicians to recommend these nonpharmacologic therapies? | |
| Open-minded | 6 (23) |
| Would consider using if there is evidence | 5 (19) |
| If patient wants | 1 (4) |
| Depends on culture and beliefs of physicians | 1 (4) |
| Not open or motivated (imagery and mindfulness would not be useful) | 3 (12) |
| Question 8: What do you think about recommending low levels of physical activity in the days after birth for managing pain? | |
| Recommend | 18 (69) |
| Good idea | 17 (65) |
| Depends on pain level | 1 (4) |
| Would not recommend | 5 (19) |
| Did not answer the question appropriately | 3 (12) |
| Question 9: Are there barriers to recommending nonpharmacologic strategies? | |
| Barriers for physicians | 25 (96) |
| Lack of time | 9 (35) |
| Lack of evidence | 7 (27) |
| Unaware of existing evidence | 5 (19) |
| Unaware of how to implement | 2 (8) |
| Unwillingness to change | 2 (8) |
| Lack of knowledge | 2 (8) |
| Need more staff to recommend | 1 (4) |
| Barriers for patients | 7 (28) |
| May take away from responsibilities (eg, childcare) | 3 (12) |
| Lack of patient openness | 2 (8) |
| New mothers have no time or patience to use | 1 (4) |
| Might be too much information for patient | 1 (4) |
Data are n (%).
Participants may have provided responses to more than one theme so some frequency totals may exceed the number of physicians that were interviewed.
Twenty-six physicians offered additional feedback for questions 7–9.
When asked about the use of recommendations to guide decisions for prescribing pain medications after delivery, two primary themes emerged from the responses: 1) “none, rely on clinical insight” (71%) and 2) “used guidelines” (26%). There were seven subthemes for the primary theme of relying on clinical insight, with 32% of physicians indicating that they depended on their medical training and experiences followed by evidence from the literature (16%), patient history or checking the Pennsylvania Prescription Drug Monitoring Program24 (13%), no guidelines (8%), and urine drug screens (3%). One physician responded, “We typically don’t [use guidelines], it’s more of a clinical judgment.” Only 21% and 5% of physicians reported using the ACOG10 and Centers for Disease Control and Prevention (CDC) guidelines,25 respectively. One physician stated, “My default is to go by the current CDC guidelines as far as prescribing, so trying to stay under the 90 morphine equivalence per day of dosing to try to do the shortest course as possible.” When asked about familiarity with the opioid prescribing guidelines for the Commonwealth of Pennsylvania,14 two themes emerged: 1) “not familiar” (87%) and 2) “familiar” (13%). One physician who was not familiar responded, “Unfamiliar. I didn’t know it existed.” Another who responded as familiar stated, “I’ve read it, but I don’t have it memorized.”
Regarding physicians’ thoughts about the recommendation in the guidelines to use the safest drug available (eg, acetaminophen) for mild-moderate pain and opioids only as needed (eg, 3–5 days after cesarean birth or sever perinatal trauma), the most frequent response given by 89% of the sample was that this guideline was “In line with current practice”, followed by “Recommendations are strict” (11%). One physician said, “I feel like I practice that.” Another stated, “I think it’s a little harsh to say that three to five days after a C-section for the narcotic cut off. I think certain patients have higher pain requirements. I think it’s a little strict, honestly.”
For the question about screening tools to assess a woman’s readiness to receive an opioid prescription after delivery, two primary themes emerged: 1) “no standardized tool” and 2) “Pennsylvania Prescription Drug Monitoring Program.”24 The majority (92%) of physicians reported that they did not use a standardized tool. There were three subthemes under this theme: 34% of the participants indicated no routine use of a screening tool, 34% indicated no use of a screening tool but provided clinical insight, and 13% indicated no screening tool but asked the patient about their history or reviewed their chart. One physician responded, “I don’t think I use any formal screening tools. We review their history and if they have a history of substance abuse or history of opioid addiction, we would ask them if they have a plan for what they are going to do with this prescription…If someone says they don’t want them I don’t give them, to them.” The remaining 8% of physicians responded that they used the Pennsylvania Prescription Drug Monitoring Program24 software as a screening tool.
When asked about screening tools for substance use disorder, five primary themes emerged: 1) “no standardized tool” (92%), 2) “urine screen for other drugs” (13%), 3) “NIDA Drug Use Screening Tool: Quick Screen”26 (3%), 4) “Pennsylvania Prescription Drug Monitoring Program”24 (3%), and 5) “5Ps (Parents, Peers, Partner, Past, Present)”27,28 (3%). The primary theme of no standardized tool had two subthemes: 1) no screening tool but talk with the patient about history of drug use or review patient history (63%), and 2) no routine use of a screening tool (29%). One physician said, “We don’t have anything that we do routinely here in the office. It’s normally obtained from the history…any current drug use, any history of drug use, any history of abuse during our regular questions that we admit patients to the hospital with.”
For the question about nonpharmacologic therapies to manage pain after delivery, the majority of physicians (63%) responded that they “Liked to use” or “Use on a case-by-case basis” (24%). One physician said, “I like local heat, massage, actually sometimes even alternative methods, even chiropractic therapy, things like that.” However, 5% reported they were not sure the therapies would work, and 3% reported they were unfamiliar with the guidelines about these methods.
When further prompted about nonpharmacologic therapies after delivery, physicians said they were “open-minded” to recommending these therapies (23%), “would consider using if there is evidence” (19%), would recommend “if patient wants” (4%), and “depends on culture and beliefs of physicians” (4%). One physician said, “I think it’s a great idea because there is no harm and if patients are interested, it would be wonderful to give them information about those things.” However, 12% of the physicians said they were not open or motivated to recommend nonpharmacologic strategies (eg, imagery and mindfulness would not be useful). With respect to recommending physical activity in the days after birth for managing pain, the majority of physicians (69%) said they would recommend (eg, good idea, depends on pain level), and 19% said they would not recommend. When asked about potential barriers to recommending nonpharmacologic strategies, most of the physicians (96%) said there were barriers for physicians, and some (28%) noted there were barriers for patients. Specifically, lack of time (35%), lack of evidence (27%), and unawareness of existing evidence (19%) were the most frequently cited barriers for physicians, followed by being unaware of how to implement (8%), unwillingness to change (8%), lack of knowledge (8%), and need more staff to recommend (4%). One physician said, “If it’s been your practice routinely to not use those things, it’s going to be harder to implement them.” Barriers identified by the physicians for patients included the ideas that nonpharmacologic therapies may take away from responsibilities (eg, childcare; 12%), lack of patient openness (8%), new mothers have no time or patience to use (4%), and might be too much information for patient (4%). One physician said, “They sound great but immediate postpartum, new moms don’t really have the time or patience to take things like that, so I do not believe that they may be as helpful with a screaming baby next to you, having pain from a C-section.”
DISCUSSION
In this study, three trends in the data emerged from the participants. First, although some (26%) physicians used regulatory or professional guidelines, the majority (71%) of physicians reported that they relied on their clinical knowledge and prior experiences to inform decisions about pain management after childbirth. Second, although physicians reported that a standard opioid patient screening tool would be useful to inform clinical decisions about pain management, nearly all participants (92%) said that they did not currently use a formal tool to assess a woman’s readiness to receive an opioid prescription after delivery or to screen for substance use disorder. Third, although most (63%) physicians liked to recommend the use of nonanalgesic pain management therapies after childbirth, they perceived important clinical or physician barriers (eg, lack of time, lack of evidence, unaware of existing evidence or how to implement) and patient barriers (eg, compete with childcare responsibilities, lack of patient willingness, no time or patience to use). These findings show that individual clinical insight, knowledge, and experience of the physicians interviewed in this study played a key role in pain management decisions for women after childbirth. This study also uncovered important areas of opportunity for future interventions and potential health care system refinements to reduce opioid exposure and misuse after childbirth. Professional or regulatory guidelines for prescribing opioids aim to improve patient outcomes by reducing the risk of exposure and misuse of opioids. Our findings suggest that few (26%) participants used professional or regulatory guidelines to make decisions about pain management after childbirth. Awareness may be a barrier to using guidelines, as our findings also showed that only 13% of the participants were familiar with the Commonwealth of Pennsylvania14 guidelines for childbirth and postpartum pain management, and none of the physicians specifically noted these guidelines when making pain management decisions for their patients. There is an important need to disseminate existing opioid-related guidelines (eg, ACOG, CDC, and Commonwealth of Pennsylvania)10,14,25 to all health care professionals (eg, nurses, physicians) and trainees (eg, nursing and medical students) at the academic institutions included in this study. Educational materials can be disseminated through multiple avenues, such as continuing medical education, participation in grand rounds presentations, and mandatory training required by the health care institutions. Moreover, future studies can use these study findings to develop systems to assess implementation of guidelines (eg, Commonwealth of Pennsylvania)14 across health care centers. Research is also needed to examine how obstetric physicians across the United States make decisions about pain management after delivery to determine whether our study findings are unique or representative of potential national trends. Our findings indicate that pain management decisions after delivery are driven by individual physicians’ clinical insight and beliefs rather than by application of specific guidelines. If these findings are replicated on a national level, these results would support implementation of standardized training by national organizations (eg, ACOG)10 to close this gap in knowledge and practice.
There are also several available screening tools to assess a patient’s readiness to receive an opioid prescription and to screen for substance use disorder. Most (92%) of the participants did not use a routine screening tool, but instead relied on clinical insight, patient history, and discussions with the patient about potential risks. One explanation for these findings may be the lack of system-level functions that streamline use of these tools.29 For example, screening tools can be built into the electronic medical record as patient-reported data. Given that few physicians reported using the most common tools such as the NIDA Quick Screen26 or 5Ps,27,28 it may also be useful to educate all postpartum care professionals about these specific tools and others that may be available. Importantly, workflows for collecting these data must be optimized, and physicians need to know where to access the data in electronic medical records, perhaps as a mandatory step before a prescription can be written for a postpartum patient. The academic institutions involved in this study are currently exploring systems to optimize the implementation of universal screening for obstetric patients. Moreover, identifying the extent to which opioid-screening tools are used nationally to inform postpartum pain management is an important area for further research.
Most of the physicians in this study also were open to prescribing nonanalgesic forms of pain management such as mindfulness training, cold and hot compresses, and light physical activity either generally or on a case-by-case basis to manage postpartum pain. However, it is important to acknowledge the physicians perceived several key physician barriers (ie, lack of time, lack of evidence, unaware of how to implement therapies) and patient barriers (eg, may take away from responsibilities such as childcare, lack of patient openness, new mothers have no time or patience to use therapies) that stand in the way of routine implementation in practice. Given these barriers, it may be useful to educate maternity care professionals about the growing body of evidence30–35 to support the use of nonpharmacologic pain management therapies and practical training on how to incorporate these approaches into a pain management treatment plan.
There were several strengths of this study, including the novelty and public health importance of the topic, valuable insight from obstetric physicians about pain management after childbirth, and implications of these study findings for patient care and quality care improvements that can be implemented. The study findings highlight an important gap between the availability and implementation of standard guidelines, use of screening tools, and recommending nonpharmacologic therapies for pain management after childbirth. For example, based on our study findings, we have developed a physician toolbox (Fig. 1) with key information from the trends in the data. We are currently exploring strategies for disseminating the toolbox at the academic institutions. Despite these strengths, there are some limitations. Participants in this study were limited to physicians practicing obstetrics in two academic health care organizations in central Pennsylvania. There is a need to replicate these study findings with physicians from varied organizations (eg, community and academic health care organizations) and locations around the country to improve generalizability. Also, the sample included both attending and resident physicians. Although we did not observe any obvious differential themes in their responses, some variation is still possible given that education and supervision of care could vary from attending and resident physicians. Our study findings suggest there is a need to conduct a system-level evaluation at these institutions to address concerns and promote training and education on guidelines and screening tools during initial medical training and through continuing education at these institutions. In addition, despite the fact that we implemented several procedures to ensure rigor (eg, moderators were trained in qualitative methods, consent form clearly indicated a participant could decline to answer any question, ensuring confidentiality of responses), there is, nevertheless, the chance of social desirability response bias because the questions asked about prescribing opioid pain medications and the moderators were associated with the academic institutions.
Fig. 1.
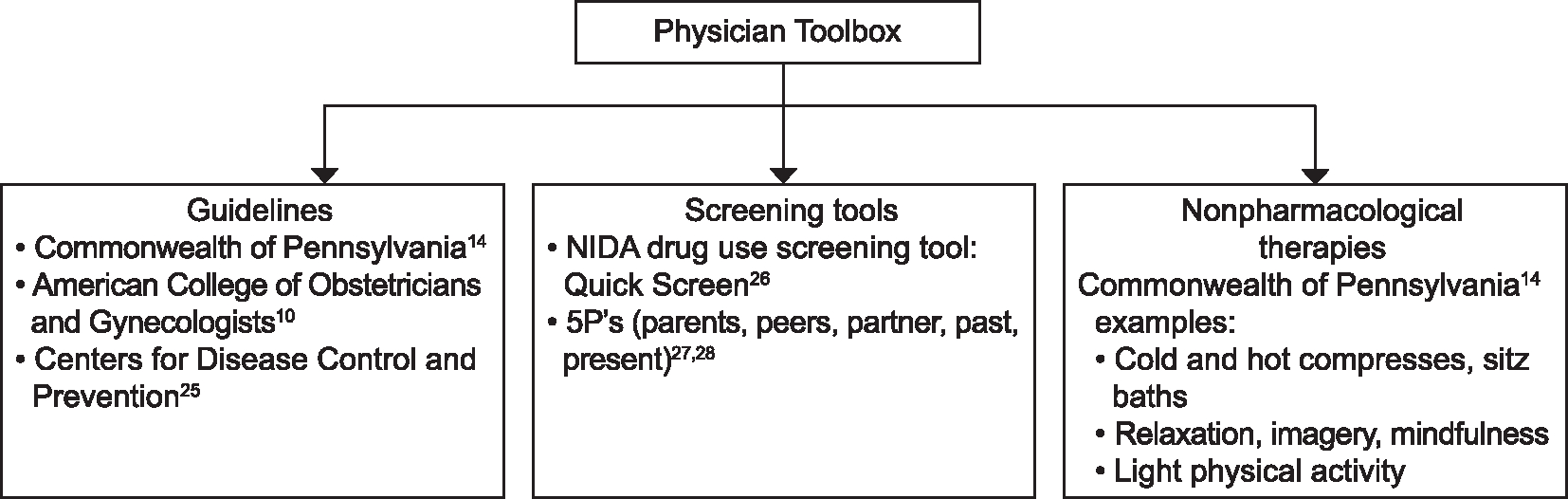
Physician toolbox for postpartum pain management.
Downs. Beliefs on Opioid Screening Tools and Guidelines. Obstet Gynecol 2021.
In summary, our study findings suggest that obstetric physicians’ individual beliefs and clinical knowledge and insight play a key role in pain management decisions for women after childbirth, and they provide an important baseline from which to design future interventions. For example, the physicians in this study can benefit from education on current opioid prescribing guidelines, use of risk-screening tools, and nonopioid pain management strategies for the postpartum period. Such an intervention is one strategy that can be easily scalable and widely disseminated through national organizations to address similar deficiencies at other institutions. Educating women about their pain management options after childbirth may also be a useful strategy to reduce the risk of unnecessary or prolonged exposure to opioid medications. Given the barriers noted by the physicians, it may be more challenging to develop interventions to promote nonpharmacologic pain management strategies (eg, mindfulness, imagery, physical activity). However, the noted benefits of these treatments for managing other patient pain conditions (eg, cancer, chronic back pain, joint replacement),36–39 suggest there is, nevertheless, a need to explore feasibility and user acceptability of these strategies after childbirth. Lastly, the ever-changing landscape of technology advancements provides the opportunity to explore the utility of innovative care delivery strategies (eg, virtual counseling, telehealth programs) to deliver nonpharmacologic therapies to manage pain in postpartum and reduce exposure to prescription opioids after childbirth.
Supplementary Material
Acknowledgments
The project described was supported by The Pennsylvania State University Social Science Research Institute Consortium to Combat Substance Abuse and the Clinical and Translational Science Institute, National Center for Advancing Translational Sciences, and National Institutes of Health, through Grant UL1 TR002014. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Financial Disclosure
Mohamed Satti disclosed that Geisinger received subcontracts for projects related to this work, both from The Pennsylvania State University grants noted above in the acknowledgements. Nicole Cumbo disclosed receiving a Russell C. and Ann E. Seward Work Study Award in Women’s Health. Richard S. Legro’s disclosures are available as Appendix 1, online at http://links.lww.com/AOG/C168. The other authors did not report any potential conflicts of interest.
Footnotes
Each author has confirmed compliance with the journal’s requirements for authorship.
REFERENCES
- 1.Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Matthews TJ. Births: final data for 2012. Natl Vital Stat Rep 2013;62:1–68. [PubMed] [Google Scholar]
- 2.Peahl AF, Smith R, Johnson T, Morgan D, Pearlman M. Better late than never: why obstetricians must implement enhanced recovery after cesarean. Am J Obstet Gynecol2019;221:117. e1–7. doi: 10.1016/j.ajog.2019.04.030 [DOI] [PubMed] [Google Scholar]
- 3.Bateman BT, Franklin JM, Bykov K, Avorn J, Shrank WH, Brennan TA, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid naïve women. Am J Obstet Gynecol 2016;215:353.e1–18. doi: 10.1016/j.ajog.2016.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jarlenski M, Bodnar LM, Kim JY, Donohue J, Krans EE, Bogen DL. Filled prescriptions for opioids after vaginal delivery. Obstet Gynecol 2017;129:431–7. doi: 10.1097/AOG.0000000000001868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavoie A, Toledo P. Multimodal postcesarean delivery analgesia. Clin Perinatol 2013;40:443–55. doi: 10.1016/j.bpa.2017.01.003 [DOI] [PubMed] [Google Scholar]
- 6.Bateman BT, Cole NM, Maeda A, Burns SM, Houle TT, Huybrechts KF, et al. Patterns of opioid prescription use after cesarean delivery. Obstet Gynecol 2017;130:29–35. doi: 10.1097/AOG.0000000000002093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osmundson SS, Schornack LA, Grash JL, Zuckerwise LC, Young JL, Richardson MG. Postdischarge opioid use after cesarean delivery. Obstet Gynecol 2017;130:36–41. doi: 10.1097/AOG0000000000002095 [DOI] [PubMed] [Google Scholar]
- 8.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK. Births: final data from 2018. Natl Vital Stat Rep 2019;68:1–47. [PubMed] [Google Scholar]
- 9.Chapman SLC, Wu L. Postpartum substance use and depressive symptoms: a review. Women Health 2013;53:479–503. doi: 10.1080/03630242.2013.804025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Postpartum pain management. ACOG Committee Opinion No. 742. American College of Obstetricians and Gynecologists. Obstet Gynecol; 2018;132:e35–43. doi: 10.1097/AOG.0000000000002683 [DOI] [PubMed] [Google Scholar]
- 11.Baker DW. History of the joint commission’s pain standards: lessons for today’s prescription opioid epidemic. JAMA 2017; 317:1117–8. doi: 10.1001/jama.2017.0935 [DOI] [PubMed] [Google Scholar]
- 12.Onishi E, Kobayashi T, Dexter E, Marino M, Maeno T, Deyo R. Comparison of opioid prescribing patterns in the United States and Japan: primary care physicians’ attitudes and perceptions. J Am Board Fam Med 2017;30:248–54. doi: 10.3122/jabfm.2017.02.160299 [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Cancer pain relief. Accessed August 7, 2020. https://apps.who.int/iris/bitstream/handle/10665/43944/9241561009_eng.pdf [Google Scholar]
- 14.Ashburn M, Boateng S, Booker S, Cleaver M, Flicker AB, Julius RJ, et al. Obstetrics and gynecology opioid prescribing guidelines. Accessed July 8, 2020. http://www.overdosefreepa.pitt.edu/wp-content/uploads/2015/12/OB-GYN-FINAL-12-14-15.pdf [Google Scholar]
- 15.Adams WC. Conducting semi-structured interviews. In: Newcomer KE, Hatry HP, Wholey JS, editors. Handbook of Practical Program Evaluation, Fourth ed. Accessed October 22, 2020. 10.1002/9781119171386.ch19 [DOI] [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Symons Downs D, Smyth JM, Heron KE, Feinberg ME, Hillemeier M, Materia FT. Beliefs about using smartphones for health behavior change: an elicitation study with overweight and obese rural women. J Technol Behav Sci 2019;4;33–41. [PMC free article] [PubMed] [Google Scholar]
- 18.Rauff EL, Symons Downs D. Mobile health technology in prenatal care: understanding OBGYN providers’ beliefs about using technology to manage gestational weight gain. J Technol Behav Sci 2019;4:17–24. doi: 10.1007/s41347-018-0068-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis LB, Kling SMR, Cochran WJ, Hassink S, Hess L, Hosterman JF, et al. Integrating and coordinating care between the Women, Infants, and Children Program and pediatricians to improve patient-centered preventive care for health growth. Transl Behav Med 2018;8:944–52. doi: 10.1093/tbm/ibx046 [DOI] [PubMed] [Google Scholar]
- 20.QSR International. NVivo qualitative data analysis software. Accessed August 16, 2020. https://www.qsrinternational.com/nvivo-qualitative-data-analysis-software/home
- 21.IBM Corp. IBM SPSS statistics for windows, version 24.0. Accessed July 20, 2020. https://us.sagepub.com/en-us/nam/sage-ibm®-spss®-statistics-v240-student-version/book261635 [Google Scholar]
- 22.Vaismoradi M, Jones J, Turunen H, Snelgrove S. Theme development in qualitative content analysis and thematic analysis. J Nurs Educ Pract 2016;6:100–10. doi: 10.5430/jnep.v6n5p100 [DOI] [Google Scholar]
- 23.Lingard L Beyond the default colon: effective use of quotes in qualitative research. Perspect Med Educ 2019;8:360–4. 10.1007/s40037-019-00550-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pennsylvania Department of Health. Prescription drug monitoring program (PDMP). Accessed August 24, 2020. https://www.health.pa.gov/topics/programs/PDMP/Pages/PDMP.aspx
- 25.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain – United States, 2016. JAMA 2016;315:1624–45. doi: 10.1001/jama.2016.1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Institute on Drug Abuse. Resource guide: screening for drug use in general medical settings. Accessed August 2, 2020. https://www.drugabuse.gov/publications/resource-guide-screening-drug-use-in-general-medical-settings/nida-quick-screen
- 27.Watson E, Barnes H, Brown E, Kennedy C, Finkelstein N. Alcohol screening assessment in pregnancy: the ASAP curriculum. Institute for Health and Recovery; 2003 [Google Scholar]
- 28.Kennedy C, Finkelstein N, Hutchins E, Mahoney J. Improving screening for alcohol use during pregnancy: the Massachusetts ASAP program. Matern Child Health J 2004;8:137–47. doi: 10.1023/b:maci.0000037647.78420.e3 [DOI] [PubMed] [Google Scholar]
- 29.Porucznik CA, Johnson EM, Rolfs RT, Sauer BC. Opioid prescribing knowledge and practices: provider survey following promulgation of guidelines-Utah, 2011. J Opioid Manag 2013;9:217–24. doi: 10.5055/jom.2013.0162 [DOI] [PubMed] [Google Scholar]
- 30.Beddoe AE, Yang CP, Kennedy HP, Weiss SJ, Lee KA. The effects of mindfulness-based yoga during pregnancy on maternal psychological and physical distress. J Obstet Gynecol Neonatal Nurs 2009;38:310–9. doi: 10.1111/j.1552-6909.2009.01023.x [DOI] [PubMed] [Google Scholar]
- 31.Duncan LG, Cohn MA, Chao MT, Cook JG, Riccobono J, Bardacke N. Benefits of preparing for childbirth with mindfulness training: a randomized controlled trial with active comparison. BMC Pregnancy Childbirth 2017;17:140. doi: 10.1186/s12884-017-1319-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kantrowitz-Gordon I, Abbot S, Hoehn R. Experiences of postpartum women after mindfulness childbirth classes: a qualitative study. J Midwifery Womens Health 2018;63:462–9. doi: 10.1111/jmwh.12734 [DOI] [PubMed] [Google Scholar]
- 33.Vieten C, Astin J. Effects of a mindfulness-based intervention during pregnancy on prenatal stress and mood: results of a pilot study. Arch Womens Ment Health 2008;11:67–74. doi: 10.1007/s00737-008-0214-3 [DOI] [PubMed] [Google Scholar]
- 34.Oktaviani I Pilates workouts can reduce pain in pregnant women. Complement Ther Clin Pract 2017;31:349–51. doi: 10.1016/j.ctcp.2017.11.007 [DOI] [PubMed] [Google Scholar]
- 35.Shiri R, Coggon D, Falah-Hassani K. Exercise for the prevention of low back and pelvic girdle pain in pregnancy: a meta-analysis of randomized controlled trials. Eur J Pain 2018;22:19–27. doi: 10.1002/ejp.1096 [DOI] [PubMed] [Google Scholar]
- 36.Mehta R, Sharma K, Potters L, Wernicke AG, Parashar B. Evidence for the role of mindfulness in cancer: benefits and techniques. Cureus 2019;11:e4629. doi: 10.7759/cureus.4629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ngamkham S, Holden JE, Smith EL. A systematic review: mindfulness intervention for cancer-related pain. Asia Pac J Oncol Nurs 2019;6:161–9. doi: 10.4103/apjon.apjon_67_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeidan F, Vago DR. Mindfulness meditation-based pain relief: a mechanistic account. Ann N Y Acad Sci 2016;1373:114–27. doi: 10.1111/nyas.13153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forward JB, Greuter NE, Crisall SJ, Lester HF. Effect of structured touch and guided imagery for pain and anxiety in elective joint replacement patients: a randomized controlled trial: M-TIJRP. Perm J 2015;19:18–28. doi: 10.7812/TPP/14-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


