Abstract

This study aims to investigate the impact of semaglutide on the expression of liver cancer proteins in obese mice induced by a high-fat diet. Sixteen obese mice were randomly divided into two groups: the high-fat diet group and the semaglutide group, each consisting of eight mice. Additionally, eight normal male mice were included as the control group. Serum samples were collected, and a differential expression analysis of total proteins in adipose tissue was performed using quantitative tandem mass spectrometry (TMT) in combination with liquid chromatography–tandem mass spectrometry (LC-MS/MS). Significant differential proteins were identified and subjected to a bioinformatics analysis. The findings revealed that these differential proteins, namely, integrin αV (ITGAV), laminin γ1 (LAMC1), fatty acid-binding protein 5 (FABP5), and lipoprotein lipase (LPL), regulate the occurrence and development of liver cancer by participating in the extracellular matrix (ECM) signaling pathway and the peroxisome proliferator-activated receptor (PPAR) signaling pathway. Notably, semaglutide can decelerate the progression of liver cancer by inducing the expression of ITGAV, LAMC1, FABP5, and LPL in the adipose tissue of obese mice.
Keywords: LIHC, obesity, cancer, semaglutide, adipose tissue
1. Introduction
The incidence of obesity and obesity-related cancers has been increasing globally.1 Epidemiological studies have shown that obesity is associated with an increased risk of 13 types of cancer, including endometrial cancer, esophageal cancer, kidney cancer, pancreatic cancer, liver cancer, cardiac cancer, meningioma, multiple myeloma, colorectal cancer, postmenopausal breast cancer, ovarian cancer, gallbladder cancer, and thyroid cancer.2
The World Health Organization (WHO) and various cancer research organizations have identified obesity as a leading modifiable risk factor for cancer. One of the mechanisms by which obesity contributes to liver cancer is through the accumulation of fat in the liver, a condition known as nonalcoholic fatty liver disease (NAFLD).3 NAFLD is strongly associated with obesity and metabolic syndrome, and it can progress to more severe liver conditions such as nonalcoholic steatohepatitis (NASH) and, eventually, liver cancer.4
Liver hepatocellular carcinoma (LIHC) is indeed the most common type of liver cancer, accounting for more than 90% of all liver cancer cases.5 Several risk factors contribute to the development of LIHC, including hepatitis B virus (HBV), hepatitis C virus (HCV) infections, alcoholic liver disease, aflatoxin, type 2 diabetes, obesity, and previous liver cirrhosis. In certain regions, such as China, chronic HBV infection and aflatoxin exposure are the primary determinants of liver cancer occurrence and development.6 The example of lactate production in mice fed a high-fat diet is relevant to the discussion of liver cancer development. It suggests that a high-fat diet can lead to metabolic changes in the liver, including increased lactate production. Therefore, more and more data support that liver fat increases the risk of liver cancer.7
NAFLD appears to be most closely associated with obesity, insulin resistance (including diabetes), and other features of metabolic syndrome, such as elevated triglycerides and low high-density lipoprotein cholesterol levels.8 ITGAV, which interacts with the extracellular matrix (ECM), is expressed in the liver and plays an important role in regulating smooth muscle cell proliferation, migration, and endothelial cell function.9 Lamc1 is an important component of the ECM and is involved in cellular processes, such as tumor cell proliferation and metabolism. FABP5 is a specific subtype of FABPs that enhances the transcriptional activity of the nuclear receptor peroxisome proliferator-activated receptor β/δ. Metabolic abnormalities of FABP5 can lead to hepatic steatosis, fatty liver disease, liver fibrosis, and, ultimately, LIHC.10 Increased uptake of lipoproteins mediated by LPL can lead to alterations in lipid metabolism in LIHC cells.11
Glucagon-like peptide-1 (GLP-1) is an incretin hormone that offers a promising therapeutic approach for individuals with type 2 diabetes and obesity, providing improved glycemic control, weight management, and potential cardiovascular benefits.12 While semaglutide has shown promising results in improving liver transaminases and reducing metabolic dysfunction associated with nonalcoholic fatty liver disease, its specific impact on liver cancer development in this context is currently unknown. The hepatic metabolomic profile was determined by liquid chromatography/mass spectrometry (LC-MS) method to explore the regulatory targets and underlying mechanisms of semaglutide on liver cancer.
2. Materials and Methods
2.1. Ethical Statement
This study followed the guidelines outlined in the NIH Guide for the Care and Use of Laboratory Animals and other relevant guidelines. Additionally, it received approval from the Animal Ethics Committee of Hebei General Hospital, with approval No. 202173.
2.2. Animal Model
In this study, a total of 24 6-week-old male C57BL/6 mice were obtained from Hebei Shengwu Technology Co., Ltd. The company holds a license (license No. SYXK) (June 2015-0004) for the sale and distribution of laboratory animals. The mice were then housed in the Experimental Animal Center of Hebei Provincial People’s Hospital, where they were kept under standard laboratory conditions. These conditions included a temperature of 22 ± 2 °C, humidity of 55 ± 10%, a 12 h light-dark cycle, and free access to water. These standardized conditions ensure that the mice were provided with a suitable environment for the duration of the study. Following a 1-week acclimation period, the mice were randomly assigned to one of three groups: the normal chow diet group (NCD), the high-fat diet group (HFD), and the high-fat diet plus semaglutide group (Sema group). It is important to note that all experimental procedures were approved by the Animal Ethics Committee of Hebei General Hospital and conducted in accordance with the “Regulations for the Administration of Experimental Animals in Hebei Province.” In this study, the NCD group was provided with a normal diet (D1035, Beijing Huafukang Biotechnology Co., LTD, China) consisting of 4% fat, 20% protein, 20% carbohydrates, and a total energy content of 34.8 kcal/100 g. The HFD group, on the other hand, was fed a high-fat diet (H10060, Beijing Huafukang Biotechnology Co., LTD, China) containing 60% fat, 20% protein, 20% carbohydrates, and a total energy content of 524 kcal/100 g. The Sema group followed a similar high-fat diet for 12 weeks but also received daily subcutaneous injections of semaglutide at a dose of 30 nmol/kg/day (Novo Nordisk, Bagsvaerd, Denmark). The highest dose of semaglutide was selected on the basis of previously published studies in mice.13
After 12 weeks of treatment, glucose tolerance tests and metabolic measurements were conducted. The mice were fasted for 12 h prior to euthanasia. At the end of the experiment, the mice were intraperitoneally injected with 1% pentobarbital sodium (60 mg/kg) for anesthesia. Blood samples were collected from the retro-orbital sinus and placed in sterile tubes containing 1 mm ethylenediaminetetraacetic acid (EDTA). Euthanasia was then performed on the mice. Brown adipose tissue (iBAT) and epididymal white adipose tissue (eWAT) were collected from the interscapular region, weighed, and either stained with hematoxylin and eosin or frozen in liquid nitrogen and stored at −80 °C for further analysis.
2.3. Assessment of Body Weight and Food Intake
Throughout the entire experiment, the body weight and food consumption of the mice were measured on a weekly basis. These measurements can provide valuable data to evaluate the effects of the drug intervention on the mice’s physiology and metabolism.
2.4. Assessment of Plasma Levels
The plasma insulin levels of the mice were measured using the Elabscience Mouse Insulin ELISA Kit, which is manufactured by Wuhan Elarite Biotech Co., Ltd. This kit is specifically designed to measure insulin levels in mouse plasma samples. The levels of serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), total cholesterol (TC), triglycerides (TG), and inflammatory cytokine (IL-6) and superoxide dismutase (SOD) were measured using COD-PAP (colorimetric method with 4-aminoantipyrine), glycerol-3-phosphate oxidase-peroxidase (GPO-PAP), and commercially available kits from Nanjing Jiancheng. All measurements were detected using a fully automated ELISA reader called VERSAmax, which is manufactured in the USA. The data obtained from the ELISA reader was analyzed using SOFTmax PRO 4.3LS software.
2.5. Protein Extraction and Digestion
Nine epididymal white adipose tissue samples (3 tissues per group) were ground into cell powder using liquid nitrogen, lysed, and extracted using SDT buffer [4% SDS, 100 mM Tris-HCl, 1 mM dithiothreitol (DTT), pH 7.6]. The protein concentration was determined using the bicinchoninic acid (BCA) protein assay kit (Bio-Rad). Protein digestion was performed using trypsin according to the filter-aided sample preparation (FASP) protocol described by Mann (18). The resulting digested peptides from each sample were desalted on a C18 Empore SPE cartridge (C18 standard density, Sigma), concentrated by vacuum centrifugation, and reconstituted in 40 μL of 0.1% (v/v) formic acid. The purity of the proteins was assessed using a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) system, followed by labeling of each sample’s 100 μg peptide mixture using iTRAQ reagent (Applied Biosystems) or tandem mass spectrometry (TMT) reagent (Thermo Scientific) according to the manufacturer’s instructions. Please translate, proofread, and check for accuracy.
2.6. Mass Spectrometry Analysis
The dissolved protein samples were separated by using a C18 reverse-phase analytical column. This column is specifically designed for separating peptides based on their hydrophobicity using a reverse-phase chromatography technique. The column used in this experiment was the Thermo Scientific Easy column, which has a length of 10 cm, an inner diameter of 75 mm, and contains a 3 mm resin. To facilitate the separation, the samples were dissolved in buffer A, which contains 0.1% formic acid, and buffer B, which consists of 84% acetonitrile and 0.1% formic acid. Buffer A helps to maintain the pH of the solution, while buffer B provides the necessary organic solvent for the chromatographic separation. The separated peptides were then analyzed using a Q Exactive mass spectrometer, which is a high-resolution mass spectrometer manufactured by Thermo Scientific. The mass spectrometer was coupled to Easy nLC, which is a liquid chromatography system also manufactured by Thermo Scientific. The mass spectrometer was operated for either 60 or 90 min, depending on the specific experimental setup. During the analysis, MS data was obtained using a top 10 data-dependent higher-energy collisional dissociation (HCD) method. This method involves selecting the top 10 most intense precursor ions for fragmentation using higher-energy collisions, allowing for the generation of fragment ions for identification and quantification. The study utilized TMT combined with LC-MS/MS to analyze the alterations in protein expression in adipose tissue of mice that were fed a high-fat diet and subsequently treated with semaglutide. This approach allowed researchers to observe the effects of semaglutide intervention on adipose tissue at a molecular level. By investigating the functional and mechanistic effects of semaglutide on adipose tissue in obese individuals, this study provides valuable insights into the potential prevention and treatment of obesity and its associated complications. These findings contribute to our understanding of the therapeutic potential of semaglutide in managing obesity and related health issues.
2.7. Enrichment Analysis
Gene ontology (GO) aims to establish a standardized language vocabulary applicable to various species, defining and describing the functions of genes and proteins, which can be updated as research progresses. GO is divided into three main categories or ontologies: molecular function (MF), biological process (BP), and cellular component (CC). The Kyoto Encyclopedia of Genes and Genomes (KEGG) is a comprehensive database that provides information on the systematic analysis of metabolic pathways and functions of gene products in cells.14 The Entrez database is a bioinformatics database maintained by the National Center for Biotechnology Information (NCBI). It integrates a wide range of biological data, including DNA and protein sequences, gene expression data, and literature citations. Entrez provides comprehensive biological functional annotation information for large-scale genes or proteins, allowing researchers to access and analyze various types of biological data in a unified platform.
2.8. Expression Levels of Several Proteins in Liver Cancer
In our study, we utilized two databases, GEPIA (gene expression profiling interactive analysis) and the Xiangtan database, to compare the expression levels of several protein mRNAs in liver cancer tissue. To further investigate the protein expression patterns, we collected immunohistochemical (IHC) staining images of liver cancer (LIHC) and normal tissues from the Human Protein Atlas (HPA) database. HPA is a comprehensive resource that combines transcriptomics and proteomics data to provide protein profiles of different tissues and cell types. It includes the tissue atlas, cell atlas, and pathology atlas, which offer valuable insights into protein expression patterns in various contexts.
2.9. Survival Prognosis Analysis of DEGs
In our study, we utilized the Kaplan–Meier Plotter, a web-based tool available at (http://kmplot.com/analysis/), to evaluate the impact of gene expression on the survival rates of various types of cancer. The database sources used in the Kaplan–Meier plotter include the Gene Expression Omnibus (GEO), the European Genome-phenome Archive (EGA), and The Cancer Genome Atlas (TCGA). By utilizing this tool, we specifically focused on the “Liver Hepatocellular Carcinoma” module to analyze the correlation between the expression levels of several proteins and the survival rates of patients with liver cancer.
2.10. Statistical Analysis
The experimental data obtained in this study were subjected to statistical analysis using GraphPad Prism 8.0 software. The results were presented as mean ± standard deviation (SD), which provides information about the central tendency and variability of the data. Statistical significance was set at a threshold of P < 0.05, denoted by (*), indicating that the observed differences between groups were unlikely to occur by chance alone. Additionally, a more stringent significance level of P < 0.01, denoted by (**), was used to indicate highly significant differences.
3. Results
3.1. Changes in Mouse Body Weight, Blood Glucose, Insulin, and Inflammatory Markers in Three Groups of Mice
In summary, the study initially observed no significant difference in body weight among the three groups of mice. However, after 12 weeks of high-fat feeding, the mice in the HFD group showed a significant increase in body weight compared to the NCD group (Figure 1A). Following intervention with semaglutide, there was a significant decrease in body weight, indicating the potential effectiveness of semaglutide in reducing weight gain. This decrease in body weight was statistically significant when compared to the HFD group (P < 0.05) (Figure 1A,B). Furthermore, the study found that mice in the HFD group exhibited significantly increased glucose tolerance (OGTT) compared to the NCD group. However, after intervention with semaglutide, the OGTT of the mice significantly decreased, suggesting a positive effect of semaglutide on glucose regulation (P < 0.05) (Figure 1C). Regarding fasting insulin (INS) levels, mice in the HFD group showed a significant increase compared to the NCD group. After intervention with semaglutide, there was a decrease in fasting insulin levels, although this decrease was not statistically significant (Figure 1D).
Figure 1.
Effects of semaglutide treatment on the metabolic parameters in HFD-induced obese mice. (A) Changes of body weight of mice in the NCD group, the HFD group, and the Sema group; (B) high-fat diet increased the body weight of mice in the HFD group, while the application of semaglutide intervention significantly decreased the body weight of mice in the Sema group (A: 6 weeks, B: 12 weeks, C: 24 weeks); (C) comparison of the OGTT (oral glucose tolerance test) of mice in different groups; (D) comparison of the INS (insulin) of mice in different groups; (E) comparison of the IL-6 of mice in different groups; (F) comparison of the SOD of mice in different groups (*p < 0.05, **p < 0.01, ***p < 0.001).
In terms of inflammatory factors, mice in the HFD group had significantly higher levels of IL-6 compared to the NCD group. However, after intervention with semaglutide, the levels of IL-6 significantly decreased, indicating a potential anti-inflammatory effect of semaglutide. Additionally, the study found that mice in the HFD group had significantly higher levels of superoxide dismutase (SOD) compared to the NCD group. After intervention with semaglutide, the levels of SOD significantly decreased, suggesting a potential improvement in oxidative stress with semaglutide treatment (P < 0.05) (Figure 1E,F).
3.2. Changes in Blood Lipids in Three Groups of Mice
We observed changes in AST, ALT, TC, and TG levels in the three groups of mice. Compared with the NCD group, the AST level in the HFD group increased significantly, but without statistical significance. After intervention with semaglutide, the AST level significantly decreased compared to the HFD group, indicating a potential improvement in liver function. This decrease was statistically significant (P < 0.001), suggesting a significant effect of semaglutide on reducing AST levels (Figure 2A). Compared to the NCD group, the HFD group showed a significant increase in ALT levels, indicating potential liver damage due to high-fat feeding. However, after intervention with semaglutide, the ALT level significantly decreased, suggesting a potential improvement in liver health. This decrease was statistically significant (P = 0.001) (Figure 2B). Similarly, the TC and TG levels were significantly increased in the HFD group compared to the NCD group, indicating dyslipidemia associated with high-fat feeding. However, after intervention with semaglutide, both the TC and TG levels significantly decreased, suggesting an improvement in lipid profiles. The decrease in both TC and TG levels was statistically significant (P < 0.001 for TC and P < 0.05 for TG) (Figure 2C,D).
Figure 2.
Comparison of AST (A), ALT (B), TC (C), and TG (D) in mice of groups NCD, HFD, and Sema. (*p < 0.05, **p < 0.01, ***p < 0.001).
3.3. Qualitative and Quantitative Analysis of Adipose Tissue Identification Proteins
The mass spectrometry analysis yielded a total of 1,038,748 secondary spectra. By retrieving theoretical protein data, we determined that 130,933 of these secondary spectra were effective for further analysis. Through spectral analysis, we were able to identify a total of 53,911 peptide segments. Among these, 48,914 were specific peptide segments, meaning they were unique to a particular protein. Additionally, we identified a total of 7590 proteins, out of which 7553 were quantifiable, meaning their abundance or concentration could be measured.
3.4. Identification of Differentially Expressed Proteins
The full protein quantification experiment using mass spectrometry was repeated three times to ensure the reliability of the results. The significance threshold for differential protein expression was set at P < 0.05. For upregulated proteins, the fold change had to be greater than 1.2, while for downregulated proteins, the fold change had to be less than 1.2. Based on these criteria, we compared the protein expression levels among the three treatment groups and obtained quantitative information on differentially expressed proteins. In the Sema/NCD group, a total of 772 differentially expressed proteins were identified, with 446 proteins being upregulated and 326 proteins being downregulated (Figure 3A). In the Sema/HFD group, there were 640 differentially expressed proteins, with 292 proteins being upregulated and 348 proteins being downregulated (Figure 3B). In the NCD/HFD group, a total of 683 differentially expressed proteins were found, with 342 proteins being upregulated and 341 proteins being downregulated (Figure 3C).
Figure 3.
Quantitative information on differential protein identification from XIANTAO database. (A–C) Number distribution of differentially expressed proteins in NCD/HFD, HFD/Sema, and Sema/NCD, three different comparison groups; (D–F) volcano plot of differentially expressed proteins. The horizontal axis is the relative quantitative value of protein after Log2 logarithm conversion, and the vertical axis is the p-value of difference significance test after Log10 logarithm conversion. The red dots indicate the significantly different expressions of upregulated proteins, while the blue dots indicate the significantly different expressions of downregulated proteins. (*p < 0.05, **p < 0.01, ***p < 0.001).
To visually represent the differentially expressed proteins, volcano plots were generated. Volcano plots are commonly used to display the fold change (x-axis) and statistical significance (y-axis) of differentially expressed genes or proteins between two samples. In these volcano plots, red dots represent proteins with significantly upregulated expression differences, while blue dots represent proteins with significantly downregulated expression differences (Figure 3D–F).
3.5. GO Analysis of Differential Proteins
We conducted an analysis to determine the biological processes (BP), molecular functions (MF), and cellular components (CC) associated with the differentially expressed proteins (DEPs) that we screened. In terms of biological processes (BP), the DEPs in the HFD/NCD group were found to be primarily involved in ribonucleotide metabolism, organic compound oxidation–reduction reactions, cell respiration, and electron transport chain. Regarding cellular components (CC), the DEPs were mainly distributed in oxidoreductase complexes, mitochondrial protein complexes, mitochondrial inner membranes, and organelle membranes. In terms of molecular functions (MF), the DEPs were primarily associated with coenzyme binding, NADH dehydrogenase activity, and oxidoreductase activity (Figure 4A). In the Sema/HFD group, the DEPs were found to be primarily associated with several BP. These processes include oxidative stress response, alcohol metabolism, extracellular protein localization, and protein membrane localization. In terms of CC, the DEPs were mainly related to cell–extracellular matrix adhesion, cell front, enrichment in collagen-containing extracellular matrix, and extracellular matrix. Regarding MF, the DEPs were primarily associated with lipid binding, enzyme activator activity, purine nucleotide metabolism, and phosphatidylinositol binding (Figure 4B).
Figure 4.
Gene Ontology functional analysis of differentially expressed proteins from the XIANTAO database. (A) Differential protein domain in NCD/HFD group; (A1) domain of upregulated protein in NCD/HFD group; (A2) domain of downregulated protein in NCD/HFD group; (B) differential protein domain in HFD/Sema group; (B1) domain of upregulated protein in HFD/Sema group; (B2) domain of downregulated protein in HFD/Sema group. NCD normal diet; HFD, high-fat diet; Sema, high-fat diet + semaglutide.
3.6. KEGG Pathway Analysis of Differential Proteins
According to the KEGG pathway analysis, the differentially expressed proteins in the NCD/HFD group were found to be involved in several pathways. These pathways include rubella, nonalcoholic fatty liver disease, oxidative phosphorylation, degradation of valine, leucine, and isoleucine, and propanoate metabolism (Figure 5A). Among these pathways, the upregulated proteins were primarily associated with EB virus infection, phagosome, lysosome, B cell receptor signaling pathway, and FCY-mediated phagocytosis (Figure 5A1). On the other hand, the downregulated proteins were mainly involved in rubella, Parkinson’s disease, fever, nonalcoholic fatty liver disease, and oxidative phosphorylation (Figure 5A2). In the Sema/HFD group, the differentially expressed proteins were predominantly enriched in focal adhesion, fluid shear stress and atherosclerosis, ECM signaling pathway, FCY-mediated phagocytosis, and bacterial invasion of epithelial cells (Figure 5B). The upregulated proteins in this group were mainly concentrated in virus infection, human cytomegalovirus infection, fluid shear stress and atherosclerosis, natural-killer-cell-mediated cytotoxicity, and antigen processing and presentation (Figure 5B1). Conversely, the downregulated proteins were primarily associated with focal adhesion, ECM signaling pathway, PPAR signaling pathway, small cell lung cancer, and bacterial invasion of epithelial cells (Figure 5B2). Based on these results, we selected several differentially expressed proteins that are mainly concentrated in the extracellular matrix (ECM) and peroxisome proliferator-activated receptor (PPAR) signaling pathways (Figures 6 and 7).
Figure 5.
KEGG pathway enrichment of differentially expressed proteins from the XIANTAO database. (A) In the NCD/HFD group, differentially expressed proteins were involved in Prion disease, nonalcoholic fatty liver disease, oxidative phosphorylation, degradation of valine, leucine, and isoleucine, and propanoate metabolism; (A1) pathway of upregulated proteins; and (A2) pathway of downregulated proteins. (B) In the HFD/Sema group, the differential proteins were mainly concentrated in focal adhesion, fluid shear stress and atherosclerosis, ECM signaling pathway, FCY-mediated phagocytosis, and bacterial invasion of epithelial cells; (B1) pathway of upregulated proteins; (B2) pathway of downregulated proteins; NCD, normal diet; HFD, high-fat diet; Sema, high-fat diet + semaglutide.
Figure 6.
Network of ECM pathways from the KEGG database.
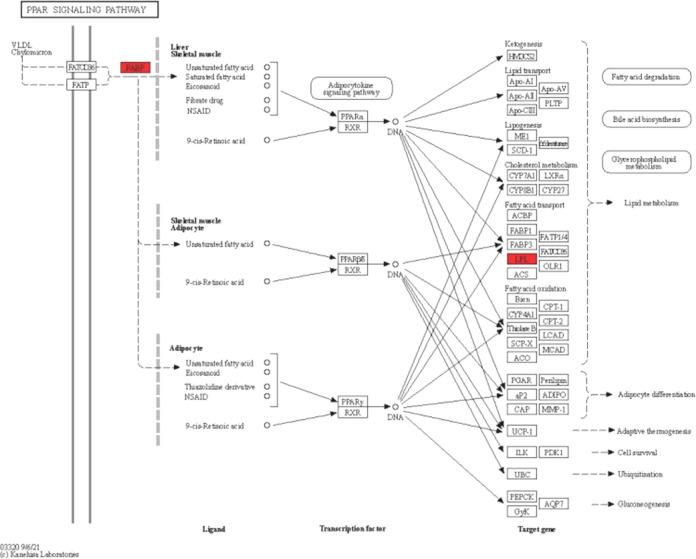
Figure 7.
Network of PPAR pathways from the KEGG database.
3.7. Expression Levels of Several mRNAs in Liver Cancer
We identified four proteins, including ITGAV, FABP5, LPL, and LAMC1 in, in mouse adipose tissue. This finding suggests that ITGAV, FABP5, LPL, and LAMC1 may be overexpressed in LIHC tissues compared to normal tissues (Figure 8A). We also performed immunohistochemical (IHC) analysis to detect the expression of these proteins in LIHC tissues and their corresponding normal tissues. We found that the expression levels of ITGAV, FABP5, and LAMC1 proteins were higher in LIHC tissues than in normal tissues (Figure 8B).
Figure 8.
mRNA expression profile of ITGAV, LAMC1, FABP5, and LPL (A) ITGAV, LAMC1, FABP5, and LPL expression level in different tumors from XIANTAO database. (*p < 0.05, **p < 0.01, ***p < 0.001). (B) Immunohistochemistry images of ITGAV, LAMC1, FABP5, and LPL expression in normal (left) and tumor (right) tissues analyzed by the Human Protein Atlas.
3.8. Prognostic Analysis of the Expression of Several Proteins
The analysis of the GEPIA database revealed that the mRNA levels of ITGAV, FABP5, LPL, and LAMC1 were significantly correlated with the overall survival (OS) of LIHC patients. The Kaplan–Meier curves demonstrated that an increase in the levels of these proteins was associated with a poorer overall survival (OS). The log-rank test analysis further confirmed the statistical significance of these correlations (p < 0.05) (Figure 9).
Figure 9.
Correlation between the expression of ITGAV FABP5 LPL and LAMC1 and overall survival (OS) in liver cancer from the XIANTAO database. (A) ITGAV, (B) FABP5, (C) LPL, (D) LAMC1.
3.9. Semaglutide Downregulates the Levels of ITGAV, FABP5, LPL, and LAMC1
We observed a significant increase in the mRNA expression levels of ITGAV, FABP5, LPL, and LAMC1 proteins in the adipose tissue of the HFD group compared to the NCD group, with statistical significance (P < 0.05) (Figure 10A). Interestingly, after intervention with semaglutide, the expression levels of ITGAV, FABP5, LPL, and LAMC1 in the serum were significantly decreased compared to the HFD group (P ≤ 0.05) (Figure 10B).
Figure 10.
Semaglutide downregulated the mRNA expression of ITGAV, FABP5, LPL, and LAMC1. (A) mRNA expression of ITGAV. (B) mRNA expression of FABP5. (C) mRNA expression of LPL. (D) mRNA expression of LAMC1.
4. Discussion
Semaglutide is a glucagon-like peptide-1 (GLP-1) analogue that is not only used to treat type 2 diabetes but also reduces the incidence of cardiovascular events and has been approved for obesity treatment.14 In this study, the researchers used TMT combined with LC-MS/MS to investigate the effects of semaglutide on protein changes in the adipose tissue of obese mice induced by a high-fat diet. The aim was to explore the potential mechanisms of semaglutide in relation to LIHC. This approach can provide valuable insights into the molecular pathways and proteins involved in the beneficial effects of semaglutide in obesity and LIHC.15
The rise in obesity rates has led to an alarming increase in obesity-related diseases, including type 2 diabetes and cancer, posing significant health risks to individuals.8 In a study conducted by P.H. et al., it was observed that treatment with semaglutide resulted in a substantial average weight reduction of 14.9% in mice. This reduction was significantly higher compared to the baseline weight reduction of 4.0–10.9% observed in the group treated with approved antiobesity drugs. It is worth noting that a high-fat diet can contribute to obesity, metabolic disorders, hypertension, and associated vascular and renal damage, leading to morphological changes in various organs.18 These findings highlight the potential of semaglutide as a promising therapeutic option for addressing obesity-related complications and improving overall health outcomes.
In this study, an obese mouse model was established by feeding NCD group mice with a high-fat diet for 12 weeks. The administration of semaglutide significantly reduced the weight of the mice, indicating its weight-reducing effect. Additionally, it was observed that a high-fat diet increased blood glucose levels in mice, but after semaglutide intervention, these levels were significantly reduced. Furthermore, insulin levels, as well as serum levels of AST, ALT, TC, TG, IL-6, and SOD, were found to increase after a high-fat diet but significantly decreased after semaglutide intervention. These findings provide a comprehensive conclusion, suggesting the potential treatment of obese patients and highlighting obesity as an important factor in the development of LIHC. Moreover, the study suggests that treatment with semaglutide may reduce the incidence of LIHC.
Recent evidence has shed light on various molecular pathways that link obesity to the development of LIHC. These pathways include insulin resistance, which leads to elevated levels of insulin and insulin-like growth factors, chronic inflammation, remodeling of adipose tissue, secretion of pro-inflammatory cytokines and adipokines, and alterations in gut microbiota.19,20 These mechanisms align with the inflammatory and metabolic processes observed in nonalcoholic fatty liver disease, which can contribute to the initiation and progression of cancer. Studies conducted by Aleksandrova et al. have demonstrated that overweight individuals have an 18% increased risk of LIHC, while obese individuals have an 83% higher risk compared to those with normal weight. Moreover, emerging evidence suggests that abdominal obesity, rather than the general obesity indicator BMI, is more strongly associated with the risk of primary liver cancer. This association has been observed in men, individuals with underlying liver disease, and white populations.16 Furthermore, the accumulation of metabolic factors related to visceral fat is also recognized as a significant risk factor for LIHC during long-term weight gain.17 The extracellular matrix (ECM) plays a crucial role in maintaining the tissue structure and function. Changes in the composition and structure of the ECM can have significant effects on vascular compliance, vascular stiffness, and cellular processes such as migration and proliferation.18 Integrin α V (ITGAV) is a cell surface receptor that interacts with ECM proteins and initiates signaling pathways involved in various cellular functions. It has been implicated in regulating processes such as proliferation, survival, invasion, and metastasis. ITGAV serves as a critical adhesion receptor that physically connects the cell cytoskeleton to ECM proteins like vitronectin, fibronectin, fibrinogen, collagen, osteopontin, and platelet response protein.19 Given its role in mediating cell-ECM interactions and its involvement in various cellular processes, ITGAV has emerged as a potential target for cancer treatment. Targeting ITGAV could potentially disrupt the signaling pathways that promote cancer cell proliferation, invasion, and metastasis. Therefore, understanding the role of ITGAV in the context of cancer and its interaction with ECM is important for developing novel therapeutic strategies.
Obesity has been shown to have significant effects on the structure of the ECM and cell signaling in blood vessels.20 One consequence of obesity is the alteration of ECM composition, leading to increased deposition and cross-linking of vascular collagen. This can contribute to vascular stiffness and impaired vascular function. The ECM also plays a crucial role in regulating smooth muscle cell proliferation, migration, and endothelial cell function through interactions with integrins. Integrin α V (ITGAV) is one such integrin that interacts with ECM proteins and activates signaling pathways that impact various cellular functions, including adhesion, migration, invasion, metastasis, proliferation, angiogenesis, and apoptosis.25 In the context of LIHC, the expression of ITGAV and ECM proteins in the liver has been found to be closely associated with hepatitis B virus (HBV) infection and the development of LIHC. This suggests that the interaction between ITGAV and ECM proteins may play a role in the pathogenesis of LIHC, potentially influencing tumor progression and metastasis.
Indeed, ITGAV has been implicated in promoting metastasis by facilitating the adhesion of tumor cells to endothelial cells during their passage through the bloodstream.21 It has been shown to contribute to the formation of actin stress fibers, which are important for the migration and invasion of LIHC cells. Obesity, on the other hand, can have profound effects on liver pathology and metabolism. It promotes inflammation and can lead to the development of nonalcoholic fatty liver disease (NAFLD). NAFLD is characterized by the accumulation of fat in the liver and can progress to nonalcoholic steatohepatitis (NASH), which is characterized by significant steatosis (fat accumulation) and inflammation. NASH can further progress to liver cirrhosis, a condition characterized by the replacement of healthy liver tissue with scar tissue. Ultimately, liver cirrhosis increases the risk of LIHC development. The interplay between obesity, NAFLD, NASH, and LIHC highlights the complex relationship between metabolic disorders, inflammation, and liver cancer.
Laminin γ1 (Lamc1) is a gene that encodes for laminin subunit γ 1, an important component of the ECM.22 Laminin is involved in various cellular processes, including tumor cell proliferation and metabolism. Studies have shown that Lamc1 is highly expressed in tumor tissues of LIHC patients. In vitro studies have demonstrated that downregulation of Lamc1 can inhibit LIHC cell proliferation by promoting cell death and reducing glucose consumption and lactate production. Furthermore, semaglutide, a medication used for the treatment of obesity and diabetes, has been shown to reduce the expression of ITGAV and LAMC1. By reducing the levels of these ECM proteins, semaglutide may inhibit ECM proliferation and potentially delay the progression of LIHC. These findings highlight the potential therapeutic implications of targeting ECM proteins such as Lamc1 and ITGAV in the treatment of LIHC.9
The peroxisome proliferator-activated receptor (PPAR) is a key regulator of lipid metabolism in the liver, playing a crucial role in maintaining overall nutrition and energy homeostasis in the body.23 Abnormalities in liver lipid metabolism can lead to hepatic steatosis, fatty liver disease, fibrosis, and, ultimately, LIHC. Fatty acid-binding proteins (FABPs) are a group of small intracellular lipid-binding proteins that can bind to various types of lipids, including vitamin A and long-chain fatty acids. Fatty acid-binding protein 5 (FABP5) is a specific subtype of FABPs that enhances the transcriptional activity of the nuclear receptor peroxisome proliferator-activated receptor β/δ. This activation promotes cell migration, proliferation, and survival and has been associated with pro-cancer activity. Studies have shown that FABP5 is overexpressed in various cancers and is correlated with the malignant potential of tumor growth and metastasis.24 In the case of LIHC, patients with FABP5 overexpression have been found to have a worse prognosis, higher recurrence rates, and a positive correlation with distant metastasis, tumor size, and vascular invasion.25 These findings suggest that FABP5 plays a significant role in the LIHC progression and metastasis. Understanding the mechanisms underlying FABP5′s involvement in LIHC can potentially lead to the development of targeted therapies aimed at inhibiting its activity and improving patient outcomes.
In LIHC cell lines, the upregulation of FABP5 has been shown to promote in vitro cell proliferation, invasion, and migration. Conversely, the downregulation of FABP5 can reduce these cellular processes. This suggests that FABP5 plays a significant role in promoting the aggressive behavior of LIHC cells; FABP5 has also been implicated in inducing epithelial-mesenchymal transition (EMT), a process that is associated with the progression and metastasis of LIHC. EMT is characterized by the loss of epithelial characteristics and the acquisition of mesenchymal properties, leading to increased cell motility and invasiveness extracellular lipoprotein lipase (LPL), which is typically expressed in adipocytes and muscle cells, playing a crucial role in lipid metabolism. LPL catalyzes the hydrolysis of triglycerides into free fatty acids (FFAs) and facilitates the cellular uptake of lipoproteins in a noncatalytic manner. The increased cellular uptake of lipoproteins mediated by LPL can contribute to altered lipid metabolism in LIHC cells. The dysregulation of FABP5 and the involvement of LPL in LIHC highlight the intricate relationship between lipid metabolism and the progression of this liver cancer.26 Lipoprotein lipase (LPL) is a secreted enzyme that adheres to the luminal surface of capillary endothelial cells. It can be provided by tumor cells themselves or nonmalignant cells within the tumor microenvironment. High levels of LPL have been associated with invasive tumor phenotypes and shorter patient survival, as reported by Gao et al.11 Based on our experiments, it appears that giving semaglutide, a medication used for obesity and diabetes treatment, may have potential benefits in reducing the incidence of LIHC. This effect may be achieved through the downregulation of the PPAR pathway, as well as the downregulation of FABP5 and LPL.11
This study provides a comprehensive analysis of the expression and prognostic value of ITGAV, FABP5, LPL, and LAMC1 in LIHC, shedding light on the heterogeneity and complexity of LIHC at the molecular level. The high expression of these proteins in LIHC tissues suggests their potential involvement in LIHC development and identifies them as potential therapeutic targets. Furthermore, we observed that several protein mRNAs were significantly upregulated in tumor tissues compared to normal tissues in adipose tissue. After treatment with semaglutide, these protein mRNAs were significantly downregulated, and in high-fat-fed mice, AST, ALT, TC, TG, inflammatory factor (IL-6), superoxide dismutase (SOD), and body weight were significantly increased. After treatment with semaglutide, these indicators were significantly reduced. Therefore, we predict that the application of semaglutide can improve obesity and liver function and reduce the risk of LIHC occurrence.
The lack of animal experiments to validate the functional implications of gene overexpression is indeed a limitation. Animal models can provide valuable insights into the in vivo effects of gene expression changes and help establish a more comprehensive understanding of the mechanisms involved. In future research, conducting animal experiments would be beneficial to further validate the findings and explore the functional consequences of gene overexpression. Animal models can provide a more realistic representation of the complex interactions within the tumor microenvironment and allow for the assessment of therapeutic interventions in a more physiological context. By incorporating animal experiments, researchers can gain a deeper understanding of the molecular mechanisms underlying LIHC development and progression as well as evaluate the efficacy and safety of potential therapeutic interventions. This would contribute to a more comprehensive and robust understanding of LIHC biology and aid in the development of novel treatment strategies.
Acknowledgments
The author thanks Shuchun Chen from Hebei General Hospital (approval No. 202173) for editing the manuscript.
Data Availability Statement
The mass spectrometry proteomics data have been deposited to the Proteome X change Consortium (http://proteomecentral.proteomexchange.org) via the iProX partner repository27,28 with the dataset identifier PXD047492.
Author Contributions
Conception and design: Y.L. Administrative support: S.C. Provision of study materials or patients: R.Z. Data analysis and interpretation: Y.L. and S.C. Manuscript writing: all authors. Final approval of manuscript: all authors.
The authors declare no competing financial interest.
References
- Aleksandrova K.; Stelmach-Mardas M.; Schlesinger S. Obesity and Liver Cancer. Recent Results Cancer Res. 2016, 208, 177–198. 10.1007/978-3-319-42542-9_10. [DOI] [PubMed] [Google Scholar]
- Kulik L.; El-Serag H. B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156 (2), 477–491.e1. 10.1053/j.gastro.2018.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänzelmann S.; Castelo R.; Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinf. 2013, 14, 7. 10.1186/1471-2105-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. L.; Taaffe D. R.; Newton R. U.; Hart N. H.; Lyons-Wall P.; Galvão D. A. Obesity and prostate cancer: A narrative review. Crit Rev. Oncol. Hematol. 2022, 169, 103543 10.1016/j.critrevonc.2021.103543. [DOI] [PubMed] [Google Scholar]
- Kang C. L.; Qi B.; Cai Q. Q.; et al. LncRNA AY promotes hepatocellular carcinoma metastasis by stimulating ITGAV transcription. Theranostics 2019, 9 (15), 4421–4436. 10.7150/thno.32854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. G.; Zhang S. W. Liver cancer epidemic in China: past, present and future. Semin. Cancer Biol. 2011, 21 (1), 59–69. 10.1016/j.semcancer.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Broadfield L. A.; Duarte J.; Schmieder R.; et al. Fat Induces Glucose Metabolism in Nontransformed Liver Cells and Promotes Liver Tumorigenesis. Cancer Res. 2021, 81 (8), 1988–2001. 10.1158/0008-5472.CAN-20-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marengo A.; Rosso C.; Bugianesi E. Liver Cancer: Connections with Obesity, Fatty Liver, and Cirrhosis. Annu. Rev. Med. 2016, 67, 103–117. 10.1146/annurev-med-090514-013832. [DOI] [PubMed] [Google Scholar]
- Lee S. K.; Kim M. H.; Cheong J. Y.; Cho S. W.; Yang S. J.; Kwack K. Integrin alpha V polymorphisms and haplotypes in a Korean population are associated with susceptibility to chronic hepatitis and hepatocellular carcinoma. Liver Int. 2009, 29 (2), 187–195. 10.1111/j.1478-3231.2008.01843.x. [DOI] [PubMed] [Google Scholar]
- Liu F.; Liu W.; Zhou S.; et al. Identification of FABP5 as an immunometabolic marker in human hepatocellular carcinoma. J. Immunother. Cancer 2020, 8 (2), e000501 10.1136/jitc-2019-000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H.; Deng Q.; Ni T.; et al. Targeted Inhibition of LPL/FABP4/CPT1 fatty acid metabolic axis can effectively prevent the progression of nonalcoholic steatohepatitis to liver cancer. Int. J. Biol. Sci. 2021, 17 (15), 4207–4222. 10.7150/ijbs.64714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwabo Kamdje A. H.; Seke Etet P. F.; Kipanyula M. J.; et al. Insulin-like growth factor-1 signaling in the tumor microenvironment: Carcinogenesis, cancer drug resistance, and therapeutic potential. Front. Endocrinol. 2022, 13, 927390 10.3389/fendo.2022.927390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalbøge L. S.; Christensen M.; Madsen M. R.; et al. Nephroprotective Effects of Semaglutide as Mono- and Combination Treatment with Lisinopril in a Mouse Model of Hypertension-Accelerated Diabetic Kidney Disease. Biomedicines 2022, 10 (7), 1661. 10.3390/biomedicines10071661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheang J. Y.; Moyle P. M. Glucagon-Like Peptide-1 (GLP-1)-Based Therapeutics: Current Status and Future Opportunities beyond Type 2 Diabetes. ChemMedChem 2018, 13 (7), 662–671. 10.1002/cmdc.201700781. [DOI] [PubMed] [Google Scholar]
- Mahapatra M. K.; Karuppasamy M.; Sahoo B. M. Therapeutic Potential of Semaglutide, a Newer GLP-1 Receptor Agonist, in Abating Obesity, Non-Alcoholic Steatohepatitis and Neurodegenerative diseases: A Narrative Review. Pharm. Res. 2022, 39 (6), 1233–1248. 10.1007/s11095-022-03302-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojtahedi Z.; Farjadian S. Awareness of Obesity-Related Cancers: A Complex Issue. Int. J. Environ. Res. Public Health 2022, 19 (11), 6617 10.3390/ijerph19116617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlynn K. A.; Petrick J. L.; El-Serag H. B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (1), 4–13. 10.1002/hep.31288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi-Sunyer F. X. The obesity epidemic: pathophysiology and consequences of obesity. Obes. Res. 2002, 10 (2), 97S–104S. 10.1038/oby.2002.202. [DOI] [PubMed] [Google Scholar]
- Seo J.; Jeong D. W.; Park J. W.; Lee K. W.; Fukuda J.; Chun Y. S. Fatty-acid-induced FABP5/HIF-1 reprograms lipid metabolism and enhances the proliferation of liver cancer cells. Commun. Biol. 2020, 3 (1), 638. 10.1038/s42003-020-01367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X.; Kong M.; Thurmond D. C. Editorial: Connecting the Dots Between Obesity, Diabetes and Cancer. Front. Endocrinol. 2020, 11, 583456 10.3389/fendo.2020.583456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu T.; Budzinska M. A.; Maczurek A. E.; et al. Novel aspects of the liver microenvironment in hepatocellular carcinoma pathogenesis and development. Int. J. Mol. Sci. 2014, 15 (6), 9422–9458. 10.3390/ijms15069422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Y.; Keogh A.; Waldt A.; et al. Single-cell and bulk transcriptomics of the liver reveals potential targets of NASH with fibrosis. Sci. Rep. 2021, 11 (1), 19396 10.1038/s41598-021-98806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Suárez A. Burden of cancer attributable to obesity, type 2 diabetes and associated risk factors. Metabolism 2019, 92, 136–146. 10.1016/j.metabol.2018.10.013. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Nakajima T.; Gonzalez F. J.; Tanaka N. PPARs as Metabolic Regulators in the Liver: Lessons from Liver-Specific PPAR-Null Mice. Int. J. Mol. Sci. 2020, 21 (6), 2061 10.3390/ijms21062061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding J. Managing patients with type 2 diabetes and obesity. Practitioner 2015, 259 (1778), 25–28. [PubMed] [Google Scholar]
- Wilding J. P.; Batterham R. L.; Calanna S.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384 (11), 989–1002. 10.1056/NEJMoa2032183. [DOI] [PubMed] [Google Scholar]
- Ma J.; Chen T.; Wu S.; Yang C.; Bai M.; Shu K.; Li K.; Zhang G.; Jin Z.; He F.; Hermjakob H.; Zhu Y. iProX: an integrated proteome resource. Nucleic Acids Res. 2019, 47, D1211–D1217. 10.1093/nar/gky869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T.; Ma J.; Liu Y.; Chen Z.; Xiao N.; Lu Y.; Fu Y.; Yang C.; Li M.; Wu S.; Wang X.; Li D.; He F.; Hermjakob H.; Zhu Y. iProX in 2021: connecting proteomics data sharing with big data. Nucleic Acids Res. 2022, 50, D1522–D1527. 10.1093/nar/gkab1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The mass spectrometry proteomics data have been deposited to the Proteome X change Consortium (http://proteomecentral.proteomexchange.org) via the iProX partner repository27,28 with the dataset identifier PXD047492.