SYNOPSIS
Prolonged air leak or alveolar-pleural fistula is common after lung resection and can usually be managed with continued pleural drainage until resolution. Further management options include blood patch administration, chemical pleurodesis, and one-way endobronchial valve placement. Bronchopleural fistula is rare but is associated with high mortality, often due to development of concomitant empyema. Bronchopleural fistula should be confirmed with bronchoscopy, which may allow for bronchoscopic intervention; however, transthoracic stump revision or window thoracostomy may be required.
Keywords: Bronchopleural fistula, air leak, thoracic surgery, perioperative management
PROLONGED AIR LEAK
BACKGROUND
The most common postoperative complication after elective lung resection is an alveolar-pleural fistula, or air leak1. An air leak is defined as a communication between the alveoli of the pulmonary parenchyma distal to a segmental bronchus with the pleural space2,3. Prolonged air leak (PAL) is defined by the Society of Thoracic Surgeons (STS) General Thoracic Surgery Database (GTSD) as an air leak persisting longer than 5 days postoperatively. The incidence of air leak after lung resection is 25 to 50% on postoperative day 1 and up to 20% on day 24,5. Though the majority of air leaks resolve spontaneously with chest tube drainage, the incidence of PAL after lung cancer resection was 10% over the past decade within the STS GTSD,6 and 15 to 25% in other reports7,8.
PAL negatively impacts other perioperative outcomes. Patients with PAL have significantly increased length of stay, leading to increased cost. Among non-pneumonectomy lung resection patients, those with PAL, compared to those without, had a mean length of stay of 7.2 vs 4.8 days (p<0.001) and a 30% increase in the inpatient costs ($26,070 vs $19,558, p<0.001)9. Similar results were demonstrated in a cohort of video-assisted thoracoscopic surgery (VATS) lung cancer resection patients, with mean length of stay nearly twice as long compared to those without a PAL (11.7 vs 6.5 days, p<0.001)10. These results are corroborated in the National Emphysema Treatment Trial data (11.8 vs 7.6 days, p<0.001)11. Medicare patients with PAL for 7–10 days after lung resection and >10 days after lung resection had a 30% and 100%, respectively, greater inpatient hospital costs compared to those with PAL <7 days (p<0.001)12. Postoperative intensive care unit readmission rates may be higher with PAL (9 vs 5%, p=0.05)13, likely due to associated complications such as pneumonia and empyema11,14. The incidence of empyema is 10.4% with PAL>7 days, compared to 1% with air leaks ≤7 days (p=0.01)15. PAL requires prolonged chest tube drainage, which increases postoperative pain1,16, respiratory splinting leading to increased pneumonia risk11, venous thromboembolic risk due to diminished mobility16 and necessity for additional procedures such as chemical or mechanical pleurodesis17. Additionally, the PAL rate was twice as high amongst readmitted lobectomy patients compared with those who did not require readmission (21.4 vs 10.2%, p<0.001)18. PAL also is associated with increased in-hospital mortality13. Those with an air leak have a 3.4 times greater risk of death than those without (95% CI 1.9–6.2).9
PREOPERATIVE RISK FACTORS
Demographic Factors
Patients undergoing lung resection who develop PAL are often older than those who do not19, with many PAL predictive tools utilizing an age cut-off of >65 years20. Men are 11–39% more likely than women to have a PAL6,9.
Clinical Factors
Patients with a lower body mass index are at increased risk of PAL, with cut-off ≤25 kg/m2 commonly studied6,20. Emphysematous disease processes such as COPD dramatically increase the odds of PAL9. Resection through emphysematous bullous tissue can make adequate sealing of parenchymal transection lines with staplers more challenging. Decreased forced expiratory volume in one second (FEV1) is a strong independent predictor for PAL6,11,20,21. Lower diffusion capacity of the lung for carbon monoxide (DLCO) also increases the risk of PAL11,20. Several case series of patients with pulmonary disease associated with infectious agents such as tuberculosis and aspergillosis have demonstrated a high risk of PAL22–24.
INTRAOPERATIVE RISK FACTORS
Larger parenchymal resections tend to increase the risk of PAL. Fissure dissection during lobectomy and bilobectomy, particularly in the setting of an incomplete fissure, can cause parenchymal tears leading to air leaks1,25. Using a fissureless dissection technique significantly reduces the risk of PAL (OR 0.32, 95% CI 0.22–0.51)25. Additionally, longer staple lines required for lobectomies over sublobar resections increase the length over which a staple line air leak can potentially occur. As such, lobectomy has been shown to have a 1.5–2.0x increased odds of PAL compared to segmentectomy or wedge resection9,13. When comparing types of lobar resections, resection of upper lobes regardless of laterality has been shown to increase the odds of PAL13. Concordantly, review of the Cleveland Clinic experience in lobectomies found that resection of the left lower lobe was an independent predictor for protection against PAL26.
Presence of pleural adhesions, which can be highly vascular and require extensive adhesiolysis, is a substantial risk factor for PAL11. Pleural adhesions were the only independent risk factor for PAL in a recent cohort of 1,051 lung cancer resection patients (OR 2.38, 95% CI 1.43–3.95)10, and are an important intraoperative risk factor in several PAL prediction scores20,27,28.
POSTOPERATIVE RISK FACTORS
Postoperative mechanical ventilation is the only postoperative risk factor identified for development of PAL29, with up to a 19% incidence of air leak in pneumonectomy patients requiring postoperative ventilation30.
PAL PREDICTIVE SCORES
Numerous investigators have proposed scoring systems to predict the risk of PAL6,20,27,28,31–36. Brunelli et al. proposed a PAL risk score in 2004 including FEV1, pleural adhesions and upper lobe resections20. Their group revised and validated their score in 2010, based on 4 factors: age >65 years (1 point); pleural adhesions (1 point); FEV1 <80% (1.5 points); and body mass index (BMI) <25.5 kg/m2 (2 points)32 (Table 1). PAL risk increased step-wise with each class: Class A (0 points), 1.4%; Class B (1 point), 5.0%; Class C (1.5–3 points), 12.5%; Class D (>3 points), 29.0%. Lee et al. devised a PAL prediction tool based on the Canadian experience that similarly included pleural adhesions, FEV1 and DLCO27, and a more complex index of PAL model was produced by French investigators including male sex, BMI, dyspnea score, pleural adhesions, lobectomy or segmentectomy, bilobectomy, bullae resection, pulmonary volume reduction and upper lobe resection28,34. Brunelli et al. have updated their own European Society of Thoracic Surgeons risk score, finding that male gender, FEV1 <80 and BMI ≤18.5 kg/m2 better predict PAL in VATS patients33.
Table 1.
Aggregate Prolonged Air Leak (PAL) Risk Score derived by Brunelli et al.
From Brunelli A, Varela G, Refai M, et al. A scoring system to predict the risk of prolonged air leak after lobectomy. Ann Thorac Surg. 2010;90(1):206; with permission.
| Points | |
|---|---|
| Age >65 years | 1 |
| Presence of pleural adhesions | 1 |
| Forced expiratory volume in one second <80% | 1.5 |
| Body mass index <25.5 kg/m2 | 2 |
An STS GTSD study of 52,198 patients formulated a PAL Score (PALS) dichotomizing patients as either high or low risk. The score includes all variables easily determined preoperatively: BMI ≤25 kg/m2 (7 points); lobectomy or bilobectomy (6 points); FEV1 ≤70% (5 points); male sex (4 points); and right upper lobe (3 points) (Table 2). A score >17 points predicted a high PAL risk compared to ≤ 17 points as a low PAL risk (19.6 vs 9% incidence, respectively), with a sensitivity of 30%, specificity of 85%, negative predictive value of 91%, and positive predictive value of 19%.
Table 2.
Prolonged Air Leak Score (PALS) derived by Seder et al.
From Seder CW, Basu S, Ramsay T, et al. A prolonged air leak score for lung cancer resection: an analysis of the STS GTSD. Ann Thorac Surg. 2019;108(5):1480; with permission.
| Points | |
|---|---|
| Body mass index ≤25 kg/m2 | 7 |
| Lobectomy or bilobectomy | 6 |
| Forced expiratory volume in one second ≤70% | 5 |
| Male sex | 4 |
| Right upper lobe procedure | 3 |
AIR LEAK EVALUATION
An air leak is identified by observing air bubbling into the water seal chamber of the pleural drainage cannister. Such a finding warns that removal of a chest tube is likely to result in continued parenchymal air leak with subsequent pneumothorax development. Recently, digital drainage systems have been developed to better objectively evaluate air leaks37. Such drainage systems can provide real-time monitoring of continuous air flow and pleural pressure as well as accurate drainage volume measurements38. A recent Japanese study found that persistent air flow ≥20 milliters/minute at 36 hours postoperatively was highly predictive of PAL, with sensitivity and specificity of 91% and 73%, respectively and receiver operating characteristic c-statistic of 0.88 (95% CI 0.80–0.96)39. A Canadian group used modeling of digital drainage system data to accurately predict air leak recurrence after chest tube removal with sensitivity of 80% and specificity of 88%40. Other studies have found no difference in chest tube duration nor length of stay with use of digital drainage systems5. While widespread implementation of digital pleural drainage systems to improve chest tube removal decision making has been slow to gain traction, this may change in the future as health systems attempt to identify ways to reduce prolonged lengths of stay.
PRINCIPLES OF MANAGEMENT
Most uncomplicated alveolar-pleural fistulae will resolve with chest tube drainage and expectant management41. Though chest tube management strategies vary, many surgeons advocate keeping chest tubes on −20 cm of water suction until the morning of postoperative day 1, at which time tubes are transitioned to water seal4,42,43. A small air leak at this time may be best managed on water seal, but a new or enlarging pneumothorax or development of subcutaneous emphysema should prompt return to suction44. A meta-analysis of seven randomized trials found no differences in the incidence of PAL, chest tube duration or hospital stay when comparing initial postoperative chest tube management on suction versus waterseal45.
With the advent of portable pleural drainage systems, outpatient management of PAL is feasible and common, given that most will resolve with adequate visceral and parietal pleural apposition46. Thus, patients can be safely discharged with chest tube in place for outpatient leak testing and removal47,48. Such strategies may in part contribute to increasing postoperative day 1 discharges after anatomic lung resections, without increased risk of mortality or readmission49–51. In fact, 4% of STS GTSD contributing centers discharge >20% of anatomic lung resection patients on postoperative day 150. However, this must be balanced with recent data indicating a 25% readmission rate and nearly 17% incidence of empyema in patients discharged with a chest tube after pulmonary resection, with over 12% requiring decortication52.
More aggressive management strategies have been explored for PAL such as chemical pleurodesis (with tetracycline, talc, iodine or silver nitrate)17,53, blood patch administration54, and endobronchial one-way valve placement55–58, which have shown some efficacy. None of these techniques have been compared in a randomized fashion, but case series have demonstrated PAL resolution rates of >95% with chemical pleurodesis, >92% with autologous blood patches, and >93% with endobronchial valve placement17.
BRONCHOPLEURAL FISTULA
BACKGROUND
In contrast to alveolar-pleural fistulae, a bronchopleural fistula (BPF) is defined as a communication between a main stem bronchus, lobar or sublobar bronchus with the pleural space59. The incidence of BPF is ≤1% for lobectomy and sublobar resections and 4–20% after pneumonectomy60–62.
Historically, the mortality rate associated with BPF ranged from 20% to 50%60,63–67. Modern series demonstrate a mortality rate of 11% to 18% for early BPF (within 30 days of surgery)62,68,69 and 0 to 7% for late BPF (beyond 30 days of surgery)68–70. BPF mortality risk is particularly high after pneumonectomy because there is often concomitant empyema due to failure to control the bronchial stump leak resulting in pneumonia of the remaining contralateral lung. Empyema after lobectomy likely occurs as a combination of PAL, percutaneous drain as a potential infectious nidus and persistent pleural space71. Conversely, over 75% of postpneumonectomy empyemas occur in the setting of a bronchial stump BPF62,72,73.The etiology of BPF-induced empyema is from direct pleural space contamination by mucocutaneous, respiratory or digestive tract microbes. BPF-associated empyema carries a significant risk of cardiopulmonary complications, upwards of 61.5% versus 11.4% in patients without BPF (p<0.001), and a mortality risk of 30.8% versus 3.9% in patients without BPF (p<0.001)29. BPF in conjunction with postpneumonectomy empyema has repeatedly been shown to be an independent predictor of mortality67,74, especially early in the postoperative course when mortality ranges from 11.6 to 18% compared to late BPF from 0 to 7.1%68,69. More recent data from France reported early (within two weeks of surgery) BPF-associated empyema mortality rates of 19% compared to 5% when empyema occurs later (after postoperative day 14)63. Survival differences become even more pronounced over time, with 1 year survival of 80% versus 47% for late versus early postpneumonectomy empyema (p=0.01)63. As such, this complication that is primarily seen in pneumonectomy patients must be recognized and addressed early to prevent significant morbidity and mortality.
PREOPERATIVE RISK FACTORS
Demographic Factors
Similar to alveolar-pleura fistulae, advanced age increases the risk of BPF. Age cut-offs of >60 and >70 years have been shown to dramatically increase the risk of BPF development, with ORs of 1.18 (95% CI 1.12–1.62) to 2.14 (95% CI 1.14–3.93), respectively68,69. A recent French BPF prediction model found that men had a 2.63x greater odds of postpneumonectomy BPF than women (p<0.001)75.
Clinical Factors
Diabetic microangiopathy causes small vessel ischemia throughout the end organs of the body, and the bronchial stump circulation is particularly prone to poor wound healing secondary to ischemia71,76. A recent meta-analysis found that diabetic patients undergoing pulmonary resection had a pooled increased odds of BPF of 1.97 (95% CI 1.39 – 2.80) compared to non-diabetic patients77, which is corroborated in other BPF risk models68. Preoperative albumin <3.5 gram/deciliter is an independent predictor of BPF after pneumonectomy (p=0.02), suggesting that poor wound healing of the bronchial stump lead to BPF development78. Additionally, low BMI has been shown to increase BPF risk, with each additional 1 kg/m2 decrease in BMI increasing the odds of BPF by 1.7x (p<0.001)75.
Benign Lung Disease
In general, the risk of BPF after pneumonectomy is higher for benign pulmonary disease, primarily infectious, rather than for cancer resections. Most case series analyzing BPF describe patients undergoing completion pneumonectomy (during which the risk of operative complications is invariably higher), as primary pneumonectomy for benign disease is rare79–84. Analysis of the STS GTSD pneumonectomy experience demonstrates a 2.8x greater odds of major complication, including empyema and BPF, for those with benign disease versus lung cancer (95% CI 1.35–5.82)85. The French experience found that of 5,975 pneumonectomies over a decade, only 3.4% and 2.0% underwent pneumonectomy and completion pneumonectomy, respectively for benign conditions86. However, these patients had a significantly higher complication rate (53% vs 39%) and in-hospital mortality rate (22% vs 5%) compared to those undergoing pneumonectomy for malignancy (p<0.001). Certainly other factors contribute to this increased risk of BPF and mortality in pneumonectomy patients with benign pathology. In fact, 37% of the pneumonectomies for benign disease were done in a nonelective fashion (compared to only 1.6% for malignant disease), which is a known risk factor for operative complications. Additionally, pulmonary decortications and resections for infectious disease are fraught with complication risk due to dense adhesions and an infected operative field71,87. Highly vascularized adhesions can cause both significant bleeding and increase the risk of bronchial ischemia intraoperatively. Finally, the proinflammatory state of acute infections such as pneumonia have been shown to increase the risk of BPF69,88.
Neoadjuvant Therapy
For patients with malignancy, there are mixed results on the risk of BPF associated with induction chemotherapy. One purported effect is the risk of poor wound healing associated with chemotherapy80. One study from MD Anderson reported zero incidence of BPF or empyema in lobectomy and pneumonectomy patients who received neoadjuvant chemotherapy89. This was corroborated by more recent data from Pittsburgh where investigators found similar BPF and empyema rates between patients receiving neoadjuvant chemotherapy versus upfront pneumonectomy (8.8% vs 7.3%, p=0.61). Analysis by Hu et al. of 684 patients undergoing pneumonectomy found neoadjuvant therapy to be an independent predictor of BPF (HR 2.48, 95% CI 0.05–0.28)68.
To this end, a recent meta-analysis of 30 studies of 14,912 lung cancer resection patients found that neoadjuvant chemotherapy alone did not increase the risk of BPF (OR 1.86, 95% CI 0.88–3.91)90. Rather, neoadjuvant radiotherapy alone (OR 3.91, 95% CI 1.40–10.94) or as combination chemoradiotherapy (OR 2.53, 95% CI 1.35–4.74) significantly increased the risk of BPF. Similarly, neoadjuvant radiotherapy was an independent predictor of late (but not early) BPF in the Shanghai experience (OR 2.83, 95% CI 3.12 – 30.96)68. Radiotherapy induces bronchial mucosa ischemia91, but the mucosal blood flow can recover in as little as 8 to 10 days after completion of therapy92. Early radiation can cause mucosal edema and inhibit capillary angiogenesis, but late effects can cause fibrotic small vessel disease through radiation vasculopathy91. Additionally, radiation-induced mucosal ischemia may be exacerbated by the ischemia from bronchial vessel disruption associated with lymphadenectomy during lung cancer resection71.
POSTOPERATIVE RISK FACTORS
Immediate or early extubation should be the goal as prolonged positive pressure ventilation is an independent risk factor for early BPF29. The incidence of BPF can be as high as 19% in patients requiring mechanical ventilation postoperatively30,61.
DIAGNOSIS
The signs and symptoms of BPF after lung resection can be varied and nonspecific, therefore it is important to have a high index of suspicion. Signs of empyema (leukocytosis, fever, pleural fluid on imaging, and purulence fluid on thoracentesis) should raise the concern for an underlying BPF. Continued air leak is not uncommon after lung resection, but a large continuous air leak should immediately raise the suspicion for air leaking from a bronchial rather than a parenchymal source. Development of a pneumothorax after chest tube removal could represent a continued parenchymal PAL, but a large pneumothorax days or weeks after resection is highly concerning for a BPF.
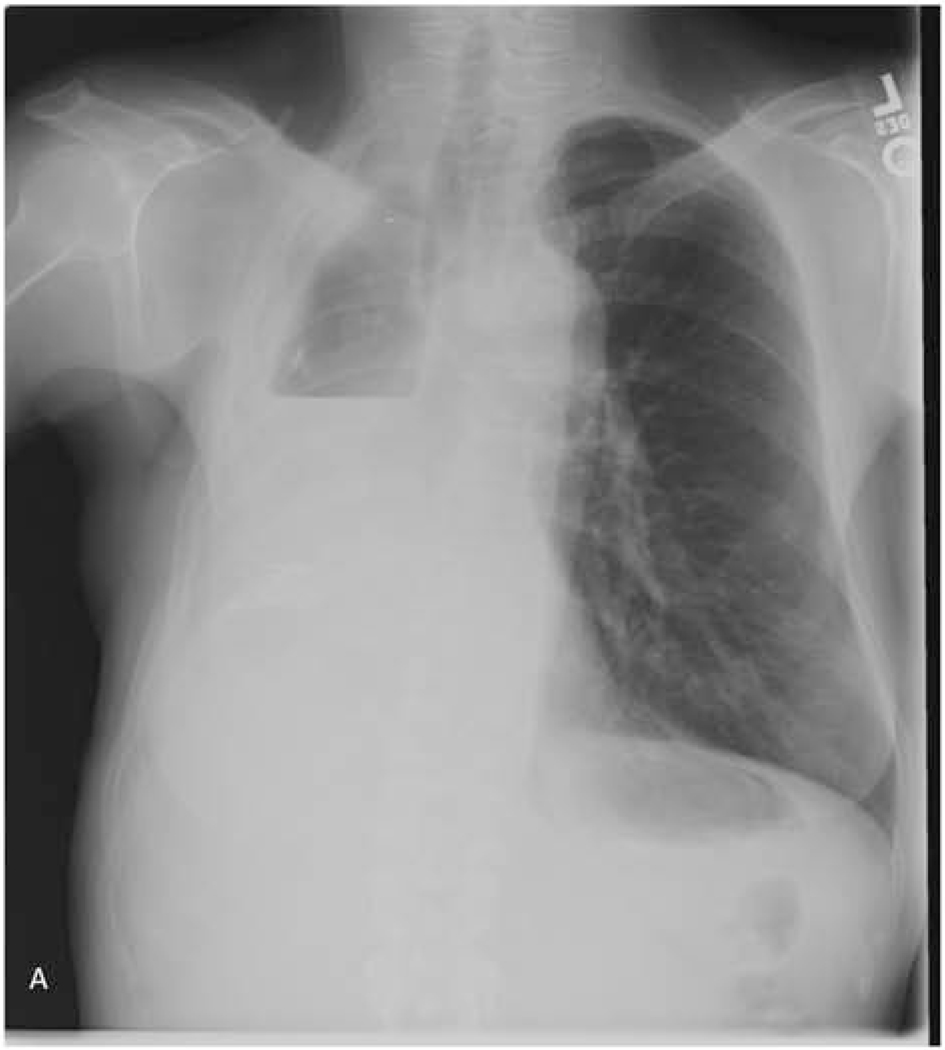
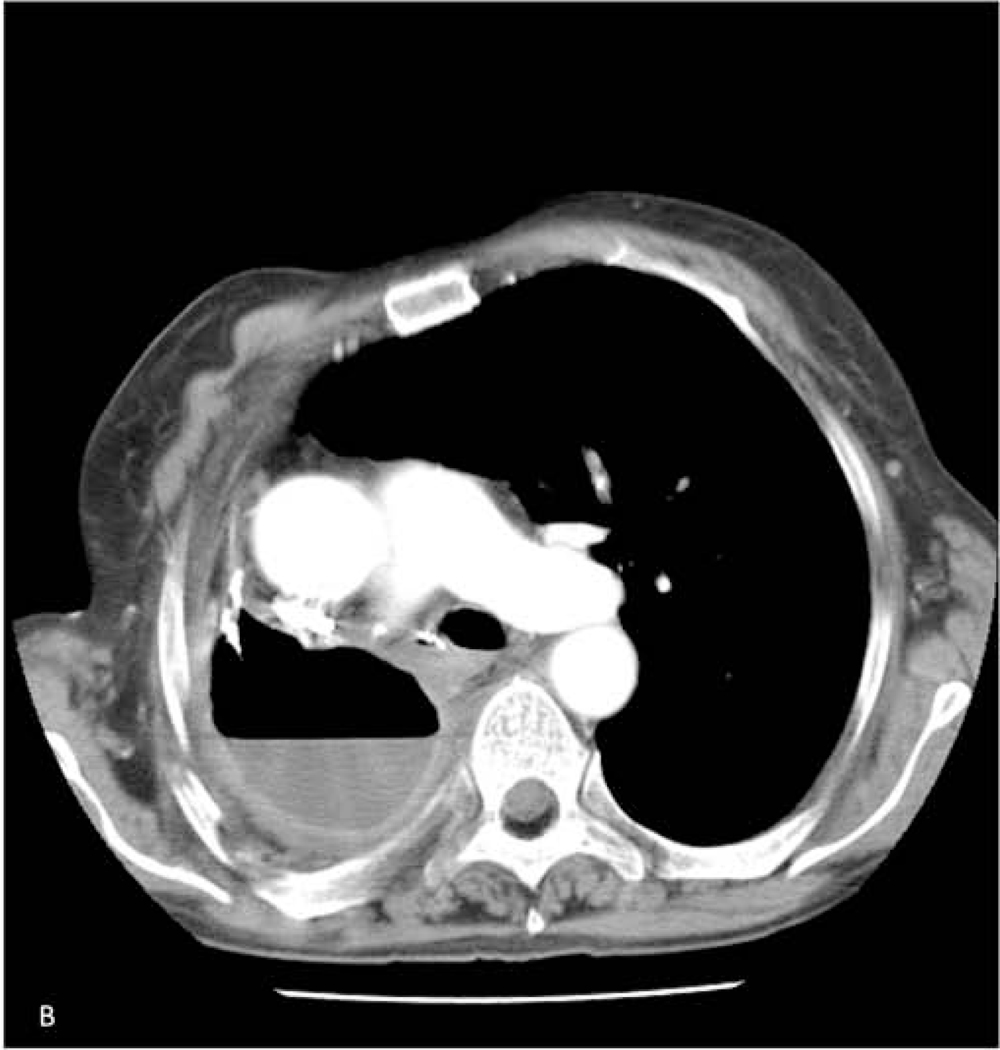
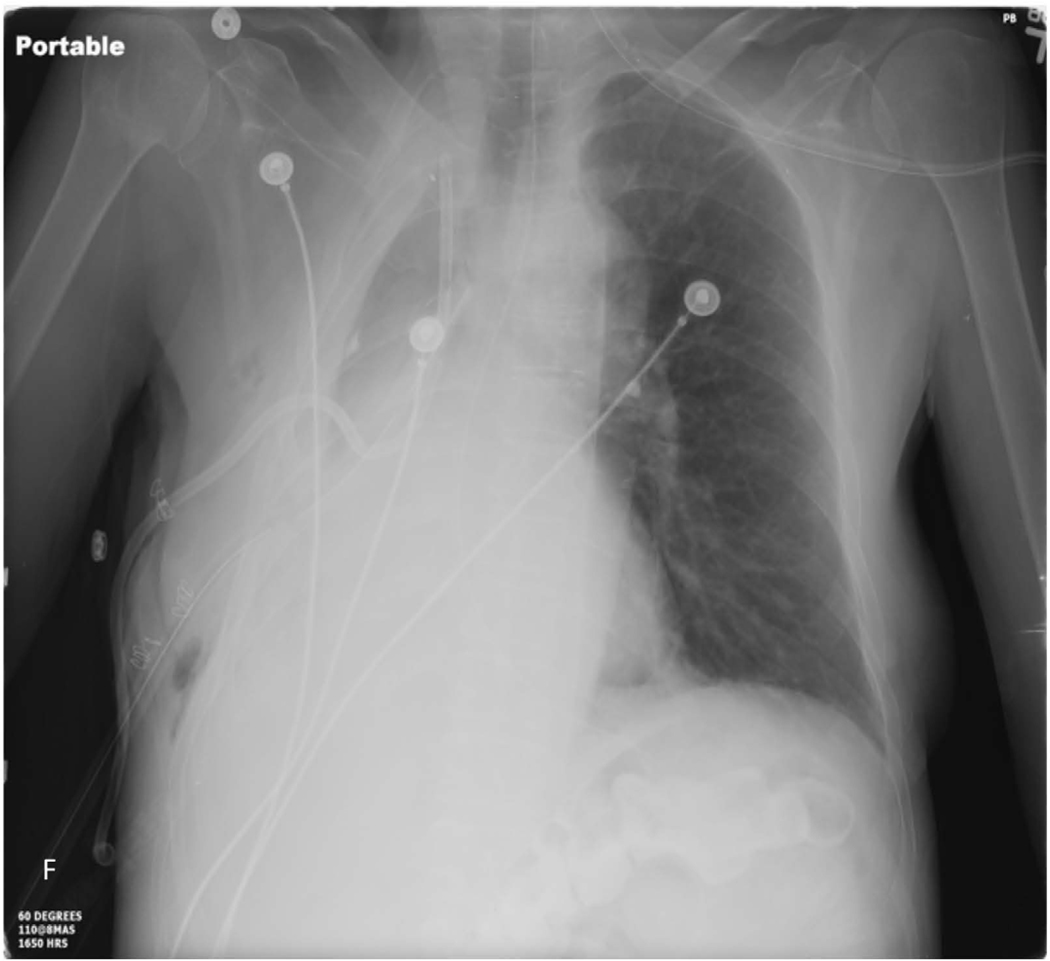
The classic radiographic sign of postpneumonectomy BPF is a decreasing air-fluid level over time (≥2 cm), indicating displacement of the postoperative pleural fluid (Figure 1). During this time the patient will often have of a persistent and worsening cough, and is at risk of developing pneumonia in the contralateral lung71. All patients suspected of having a BPF should be evaluated with a chest computed tomography scan and flexible bronchoscopy. Saline can be instilled during bronchoscopy to look for bubbling at the staple line. If radiographic and bronchoscopic findings are still equivocal, transthoracic exploration and submersion of the stump under saline for a bubble test under positive pressure ventilation can make the definitive diagnosis.
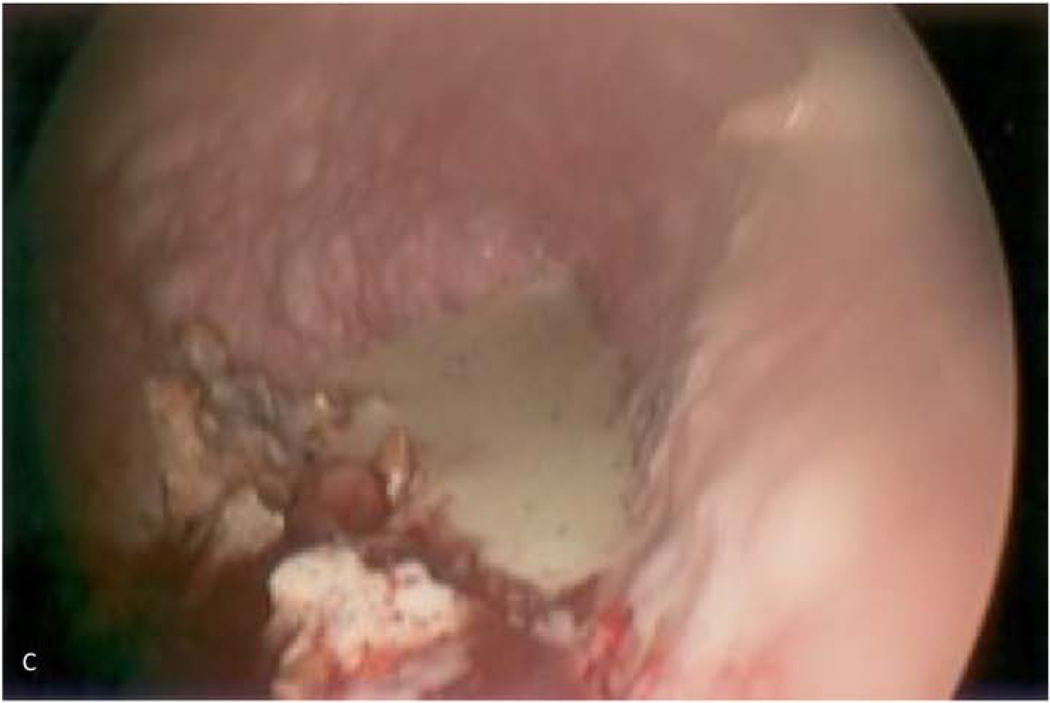
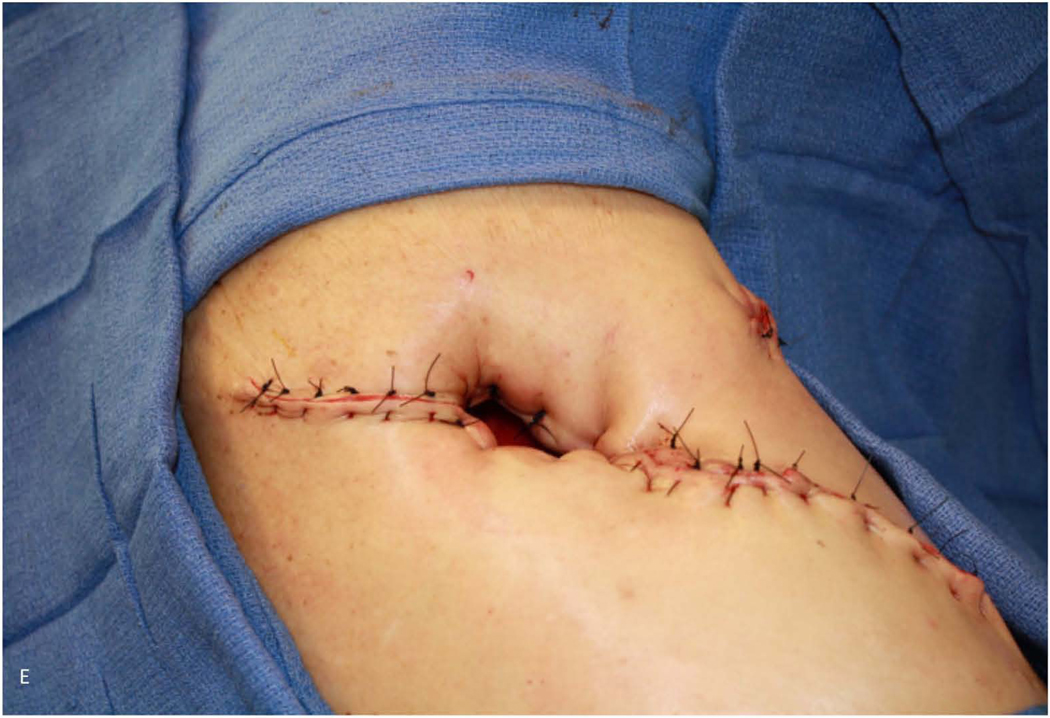
Figure 1.
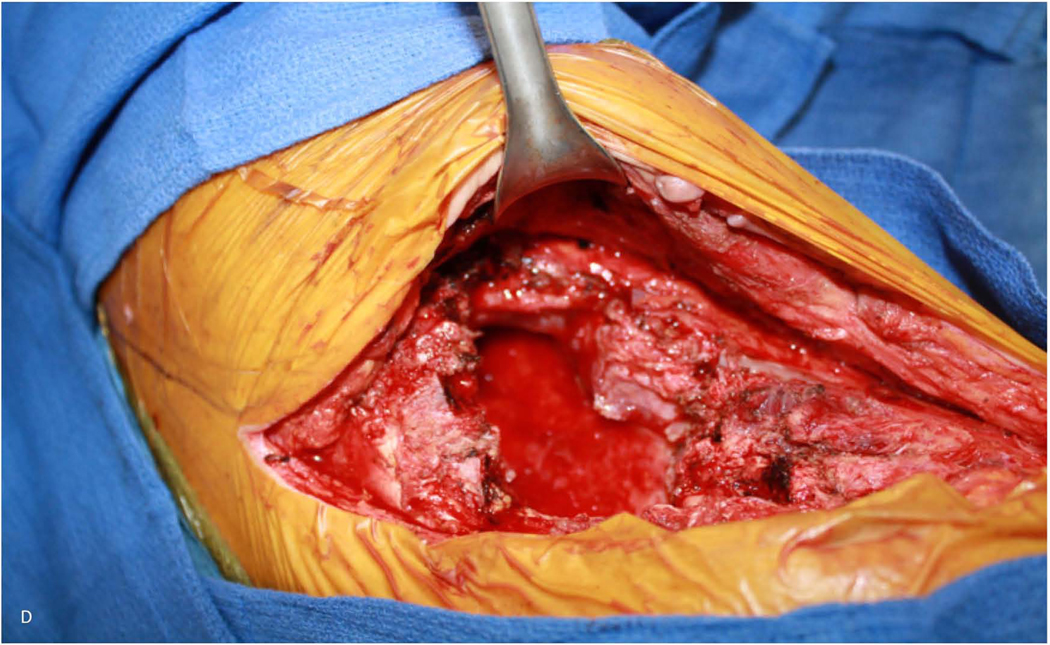
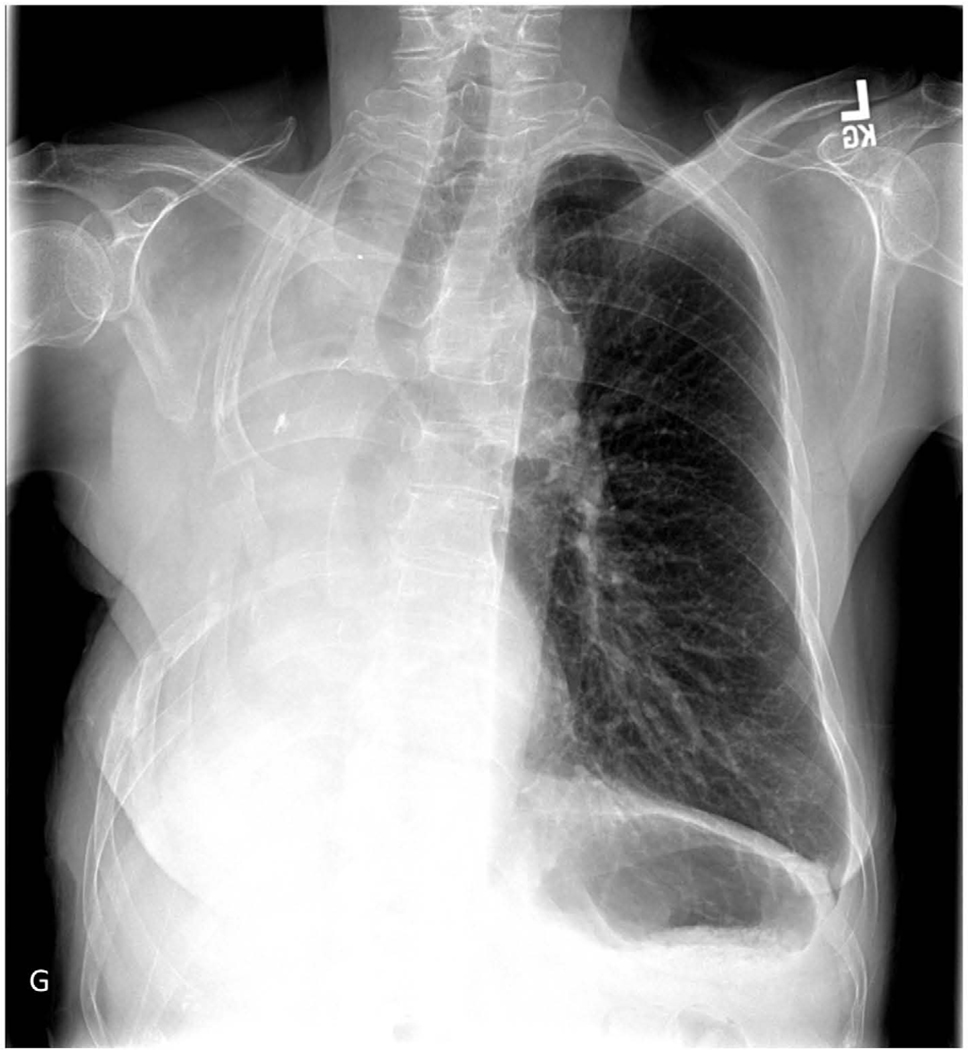
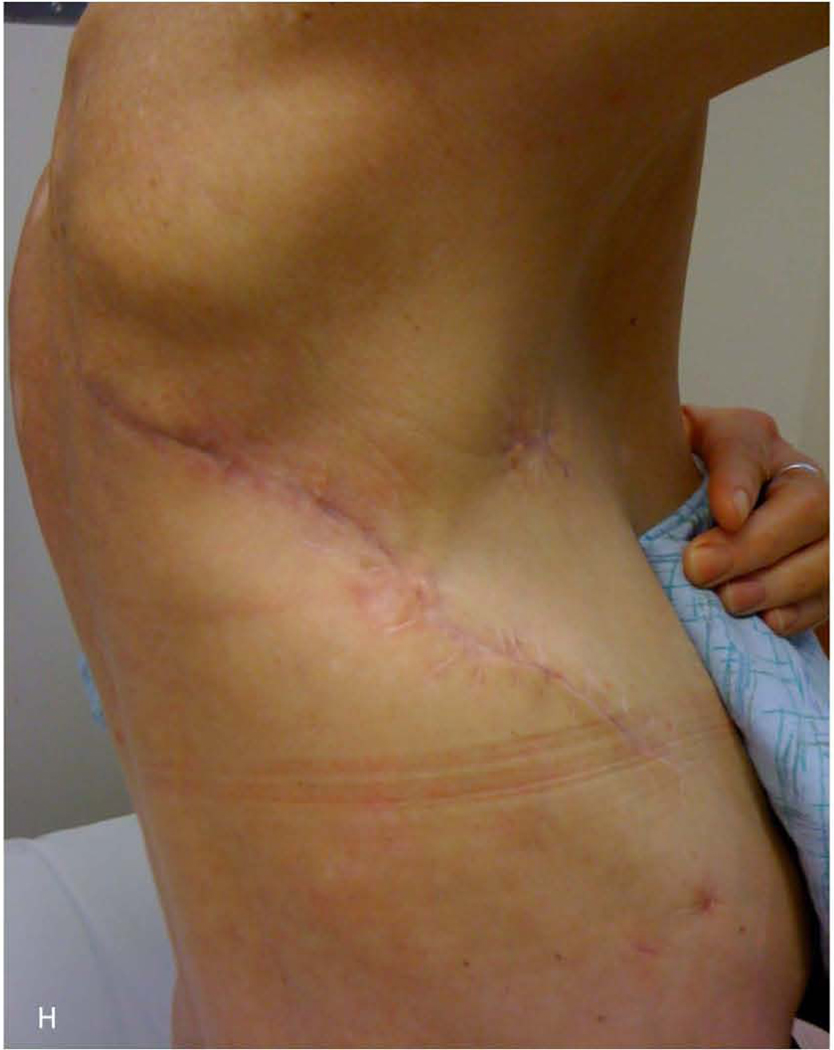
59-year-old woman who underwent right pneumonectomy for adenocarcinoma and 16 years later developed failure to thrive secondary to chronic post-pneumonectomy empyema. Chest radiograph at presentation with air-fluid level (A). Computed tomography imaging showing BPF and empyema (B). Empyema intraoperatively (C) during bronchial stump closure with Eloesser thoracostomy window intraoperative dissection (D) and creation (E). She then underwent omental flap and partial chest wall closure with a pleural drainage system 8 weeks after Eloesser thoracostomy window, as seen on chest radiograph (F). She eventually had the drainage system removed with resolution of the BPF and empyema on chest radiograph (G), and chest wall wound closure with latissimus dorsi flap coverage 18 weeks after initial Eloesser thoracostomy window creation (H).
PRINCIPLES OF MANAGEMENT
If empyema is suspected or confirmed, antibiotics are necessary. The majority of BPF associated empyema are monomicrobial, with the most common pathogens being staphylococcus and streptococcus species63. Next, adequate drainage should be established by placement of a thoracostomy tube and instillation of fibrinolytics if the empyema is loculated78.
In postpneumonectomy BPF, care should be made to avoid spillage of any empyema into the contralateral lung by keeping the patient upright at least at 45 degrees and decubitus on the operative side down if able71. After drainage of the pleural space, bronchoscopy should be used to identify the BPF and to assess the viability and length of the bronchial stump. As above, thoracoscopy can be paired with bronchoscopy to identify occult BPFs with a saline leak test under positive pressure.
In appropriately selected patients, BPF can be treated via endobronchial therapy, avoiding a major reoperation. Small defects (<5 mm) in patients without sepsis can often be managed with endoscopic fibrin glue93,94or silver nitrate64,95. Fibrin glue can have high rates of success, up to 100% after 2–3 applications94. Silver nitrate has been shown to have success rates ranging from 80 to 100%64,95. A Japanese case series of 7 patients showed 100% success with bronchoscopic instillation of a polyglycolic acid mesh with fibrin glue over the fistula area96. Most recently, airway stenting has shown considerable success, with 97% first attempt and 100% second attempt success rates in a series of 148 patients from China97.
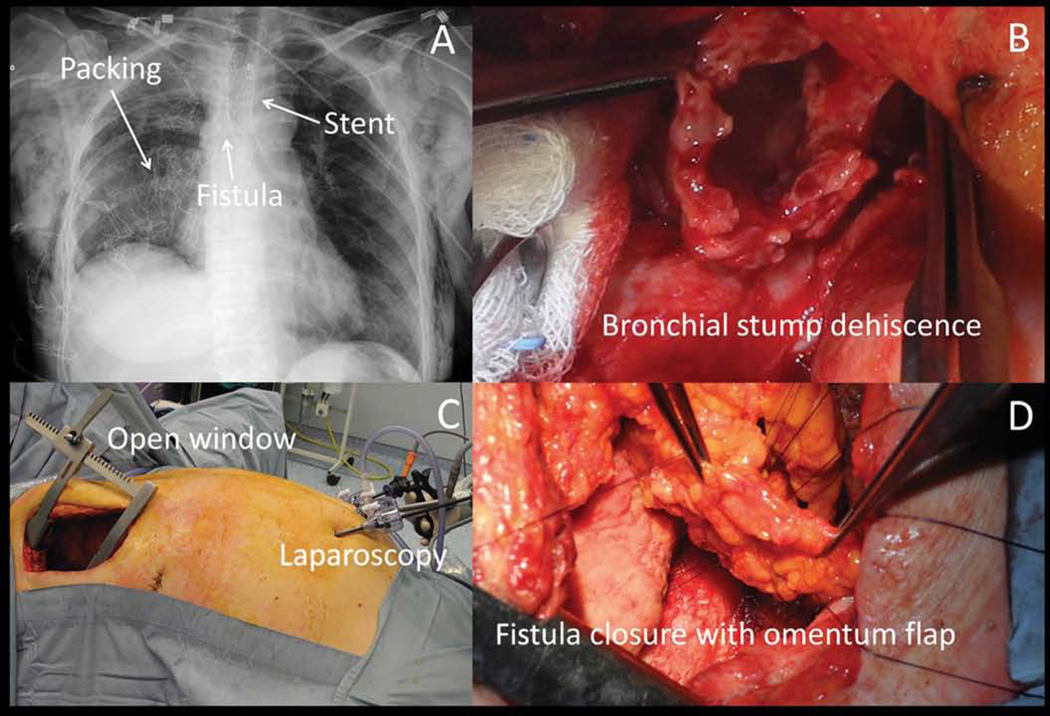
Nevertheless, the need for operative re-exploration is common, with historical case series indicating rates of >90% and reclosure of the bronchial stump in nearly half of those cases61. The need for and success of reoperative interventions is dependent upon many factors including early versus late presentation, dehiscence size, length of the bronchial stump, quality of the remnant stump tissue, presence of remnant malignancy at the stump site, and extent of contamination of the ipsilateral pleural cavity or infectious involvement of the contralateral lung30. Generally, early dehiscence tends to be more amenable to immediate repair or stump revision, while late dehiscence can be more technically challenging to repair due to diminished tissue quality, development of a matured fistula tract and significant pleural contamination and scarring71. Longer stumps should be trimmed back to healthier tissue leaving a minimum of 3 mm of remnant bronchial stump length98, and if there is insufficient length for a new staple line, then direct suture repair should be performed with absorbable, monofilament, pledgeted sutures with vascularized tissue buttressing71. Dehiscence of greater than 50% of the bronchial stump is associated with dramatically delayed time to successful stump closure99. Indeed, a recent South Korean case suggests empiric musculocutaneous flap coverage of any BPF with dehiscence of 1 cm or greater100. Repair of a late dehiscence is often impractical as extensive dissection of the stump can be risky. Tissue transfers into the pleural cavity (muscle or omental flaps) can cover the bronchial stump and eliminate the persistent pleural space (Figure 2).
Figure 2.
Repair with of a bronchopleural fistula in a staged fashion starting with placement of a conical stent to exclude the fistula from airflow (A), open thoracotomy pleural washout and packing with antibiotic soaked gauzes (B), followed by laparoscopic omental harvesting (C) and bronchopleural fistula primary closure buttressed with an omental flap (D). (From Andreetti C, Menna C, D’Andrilli A, et al. Multimodal treatment for post-pneumonectomy bronchopleural fistula associated with empyema. Ann Thorac Surg. 2018;106(6):e338; with permission.)
In patients unable to tolerate bronchial stump revision or if early repair fails, open window thoracostomy should be considered as adequate drainage may allow most fistulae to close over time. An open window thoracostomy (OWT) such as the Eloesser flap is created by removal of a portion of two or three ribs at the most dependent portion of the empyema cavity with marsupialization of the subcutaneous tissues/skin flaps to the pleura, with success rates as high as 60 to 90%22,101,102 (Figure 1). The original Eloesser flap was expanded to a two-stage Clagett procedure wherein after adequate drainage, serial operative debridements and local wound care, intrapleural antibiotic solution is instilled with definitive chest wall closure103. Obliteration of the pleural cavity can also be aided with muscular or omental flap transposition. Almost any nearby vascularized tissue pedicle can be used for buttressing, but common options include: muscle flaps (latissimus dorsi, serratus anterior, intercostal),104 parietal pleura,105 pericardium,106 pericardial fat,107 pericardiophrenic graft,107 azygos vein on the right side,105 rectus abdominus myocutaneous flaps,108 and omentum109,110, which can be harvested from a thoracotomy through a transdiaphragmatic approach111.
The duration of OWT is patient dependent and depends on response to antibiotic therapy, obliteration of the empyema cavity, nutrition status, and strict adherence to tobacco cessation efforts98. A recent Italian case series had a median duration of OWT of 5 months (range 3–9) and found that early OWT creation increased the success of BPF healing101. Despite these encouraging results, other contemporary case series report dismal long term survival after OWT of only 8% at 4 years78, and ongoing packing of an open thoracic wound is often poorly tolerated by patients112.
Recently, open window thoracostomy has been paired with vacuum-assisted closure devices to improve patient tolerance of wound care and healing time113. Additionally, the recent Swiss experience has shown success in reducing mean time to OWT closure to 8 days using a modified Clagett process with povidone-iodine-soaked sponge packing changed in the operating room every 48 hours to allow for serial debridements, leading to a 100% OWT closure success rate with 0% 3-month mortality66. For BPF after partial lung resection, a last resort can be completion lobectomy or bilobectomy to ensure bronchial stump closure at a level of the bronchial tree with healthy tissue. Main bronchial stump revision sometimes is best accomplished through a transsternal transpericardial approach to the carina, especially for left sided and long stump BPFs. This approach provides an uninfected and noninflamed operative field114. More recent case series have shown considerable success in managing BPF with thoracoscopic debridement and stump revision, obviating the need for open window thoracostomy in many patients70,115,116.
Evidence on the benefit of endobronchial valve (EBV) placement in aiding BPF closure is emerging though the data are limited to small case series. These one-way valves limit airflow into the pleural space while allowing backflow of mucus and air117. EBV placement is most commonly used for persistent pneumothorax secondary to PAL as opposed to BPF with concomitant empyema, however use in BPF is gaining traction118. One series of 3 critically ill mechanically ventilated patients with BPF found immediate air leak resolution after EBV placement followed by BPF resolution and extubation within 5 to 13 days and good long term survival119. Other case reports have demonstrated recovery from BPF in patients on extracorporeal membrane oxygenation120 as well as in severe cystic fibrosis as a bridge to lung transplantation121.
CONCLUSION:
PAL is common after lung resection but is usually managed with continued pleural drainage until resolution. Additional management options include blood patch administration, chemical pleurodesis, and one-way endobronchial valve placement. BPF is rarer but significant because it is associated with a high mortality rate due to development of concomitant empyema. BPF should be confirmed with bronchoscopy which may allow for bronchoscopic intervention. However early operative intervention, especially when diagnosed early, with transthoracic stump revision or open window thoracostomy may ultimately expediate BPF closure and improve survival.
KEY POINTS.
Prolonged air leak or bronchoalveolar fistula is common and can usually be managed with continued pleural drainage until resolution
Bronchopleural fistula is rare but is associated with high mortality, often due to development of concomitant empyema
Bronchopleural fistula should be confirmed with bronchoscopy and often can be treated endoscopically, but may require operative stump revision or window thoracostomy
Footnotes
DISCLOSURE STATEMENT
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mueller MR, Marzluf BA. The anticipation and management of air leaks and residual spaces post lung resection. J Thorac Dis. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kozower BD. 42 - Complications of Thoracic Surgical Procedures. In: LoCicero J, Feins RH, Colson YL, Rocco G, ed. Shields’ General Thoracic Surgery. 8th Editio. Philadelphia: Lippincott Williams & Wilkins; 2019:573–585. [Google Scholar]
- 3.Cerfolio RJ. Advances in thoracostomy tube management. Surg Clin North Am. 2002. [DOI] [PubMed] [Google Scholar]
- 4.Cerfolio RJ, Bass C, Katholi CR. Prospective randomized trial compares suction versus water seal for air leaks. Ann Thorac Surg. 2001;71(5):1613–1617. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert S, McGuire AL, Maghera S, et al. Randomized trial of digital versus analog pleural drainage in patients with or without a pulmonary air leak after lung resection. J Thorac Cardiovasc Surg. 2015;150(5):1243–1251. [DOI] [PubMed] [Google Scholar]
- 6.Seder CW, Basu S, Ramsay T, et al. A Prolonged Air Leak Score for Lung Cancer Resection: An Analysis of the STS GTSD. Ann Thorac Surg. July 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cerfolio RJ, Pickens A, Bass C, et al. Fast-tracking pulmonary resections. J Thorac Cardiovasc Surg. 2001. [DOI] [PubMed] [Google Scholar]
- 8.Abolhoda A, Liu D, Brooks A, Burt M. Prolonged air leak following radical upper lobectomy: An analysis of incidence and possible risk factors. Chest. 1998. [DOI] [PubMed] [Google Scholar]
- 9.Yoo A, Ghosh SK, Danker W, Kassis E, Kalsekar I. Burden of air leak complications in thoracic surgery estimated using a national hospital billing database. Clin Outcomes Res. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao K, Mei J, Xia C, et al. Prolonged air leak after video-assisted thoracic surgery lung cancer resection: Risk factors and its effect on postoperative clinical recovery. J Thorac Dis. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeCamp MM, Blackstone EH, Naunheim KS, et al. Patient and Surgical Factors Influencing Air Leak After Lung Volume Reduction Surgery: Lessons Learned From the National Emphysema Treatment Trial. Ann Thorac Surg. 2006. [DOI] [PubMed] [Google Scholar]
- 12.Wood D, Lauer L, Layton A, Tong K. Prolonged length of stay associated with air leak following pulmonary resection has a negative impact on hospital margin. Clin Outcomes Res. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elsayed H, McShane J, Shackcloth M. Air leaks following pulmonary resection for lung cancer: Is it a patient or surgeon related problem? Ann R Coll Surg Engl. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varela G, Jiménez MF, Novoa N, Aranda JL. Estimating hospital costs attributable to prolonged air leak in pulmonary lobectomy. In: European Journal of Cardio-Thoracic Surgery. ; 2005. [DOI] [PubMed] [Google Scholar]
- 15.Brunelli A, Xiume F, Al Refai M, Salati M, Marasco R, Sabbatini A. Air leaks after lobectomy increase the risk of empyema but not of cardiopulmonary complications: A case-matched analysis. Chest. 2006. [DOI] [PubMed] [Google Scholar]
- 16.Sánchez PG, Vendrame GS, Madke GR, et al. Lobectomy for treating bronchial carcinoma: Analysis of comorbidities and their impact on postoperative morbidity and mortality. J Bras Pneumol. 2006. [DOI] [PubMed] [Google Scholar]
- 17.Dugan KC, Laxmanan B, Murgu S, Hogarth DK. Management of Persistent Air Leaks. Chest. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown LM, Thibault DP, Kosinski AS, et al. Readmission after Lobectomy for Lung Cancer. Ann Surg. August 2019:1. http://insights.ovid.com/crossref?an=00000658-90000000094927. Accessed November 16, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang L, Jiang G, Zhu Y, Hao W, Zhang L. Risk factors predisposing to prolonged air leak after video-assisted thoracoscopic surgery for spontaneous pneumothorax. Ann Thorac Surg. 2014. [DOI] [PubMed] [Google Scholar]
- 20.Brunelli A, Monteverde M, Borri A, Salati M, Marasco RD, Fianchini A. Predictors of prolonged air leak after pulmonary lobectomy. Ann Thorac Surg. 2004. [DOI] [PubMed] [Google Scholar]
- 21.Ciccone AM, Meyers BF, Guthrie TJ, et al. Long-term outcome of bilateral lung volume reduction in 250 consecutive patients with emphysema. J Thorac Cardiovasc Surg. 2003. [DOI] [PubMed] [Google Scholar]
- 22.Zanotti G, Mitchell JD. Bronchopleural Fistula and Empyema After Anatomic Lung Resection. Thorac Surg Clin. 2015;25(4):421–427. [DOI] [PubMed] [Google Scholar]
- 23.Olcmen A, Gunluoglu MZ, Demir A, Akin H, Kara HV., Dincer SI. Role and outcome of surgery for pulmonary tuberculosis. Asian Cardiovasc Thorac Ann. 2006. [DOI] [PubMed] [Google Scholar]
- 24.Lang-Lazdunski L, Offredo C, Le Pimpec-Barthes F, Danel C, Dujon A, Riquet M. Pulmonary resection for Mycobacterium xenopi pulmonary infection. Ann Thorac Surg. 2001. [DOI] [PubMed] [Google Scholar]
- 25.Gómez-Caro A, Calvo MJR, Lanzas JT, Chau R, Cascales P, Parrilla P. The approach of fused fissures with fissureless technique decreases the incidence of persistent air leak after lobectomy. Eur J Cardio-thoracic Surg. 2007. [DOI] [PubMed] [Google Scholar]
- 26.Okereke I, Murthy SC, Alster JM, Blackstone EH, Rice TW. Characterization and importance of air leak after lobectomy. Ann Thorac Surg. 2005. [DOI] [PubMed] [Google Scholar]
- 27.Lee L, Hanley SC, Robineau C, Sirois C, Mulder DS, Ferri LE. Estimating the risk of prolonged air leak after pulmonary resection using a simple scoring system. J Am Coll Surg. 2011. [DOI] [PubMed] [Google Scholar]
- 28.Rivera C, Bernard A, Falcoz PE, et al. Characterization and prediction of prolonged air leak after pulmonary resection: A nationwide study setting up the index of prolonged air leak. Ann Thorac Surg. 2011. [DOI] [PubMed] [Google Scholar]
- 29.Algar FJ, Alvarez A, Aranda JL, Salvatierra A, Baamonde C, López-Pujol FJ. Prediction of early bronchopleural fistula after pneumonectomy: A multivariate analysis. Ann Thorac Surg. 2001. [DOI] [PubMed] [Google Scholar]
- 30.Wright CD, Wain JC, Mathisen DJ, Grillo HC, Miller JI, Putnam J. Postpneumonectomy bronchopleural fistula after sutured bronchial closure: Incidence, risk factors, and management. J Thorac Cardiovasc Surg. 1996. [DOI] [PubMed] [Google Scholar]
- 31.Viti A, Socci L, Congregado M, et al. The everlasting issue of prolonged air leaks after lobectomy for non-small cell lung cancer: A data-driven prevention planning model in the era of minimally invasive approaches. J Surg Oncol. 2018. [DOI] [PubMed] [Google Scholar]
- 32.Brunelli A, Varela G, Refai M, et al. A Scoring System to Predict the Risk of Prolonged Air Leak After Lobectomy. Ann Thorac Surg. 2010. [DOI] [PubMed] [Google Scholar]
- 33.Pompili C, Falcoz PE, Salati M, Szanto Z, Brunelli A. A risk score to predict the incidence of prolonged air leak after video-assisted thoracoscopic lobectomy: An analysis from the European Society of Thoracic Surgeons database. In: Journal of Thoracic and Cardiovascular Surgery. ; 2017. [DOI] [PubMed] [Google Scholar]
- 34.Orsini B, Baste JM, Gossot D, et al. Index of prolonged air leak score validation in case of video-assisted thoracoscopic surgery anatomical lung resection: Results of a nationwide study based on the French national thoracic database, EPITHOR. Eur J Cardio-thoracic Surg. 2015. [DOI] [PubMed] [Google Scholar]
- 35.Attaar A, Winger DG, Luketich JD, et al. A clinical prediction model for prolonged air leak after pulmonary resection. In: Journal of Thoracic and Cardiovascular Surgery. ; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oh SG, Jung Y, Jheon S, et al. Postoperative air leak grading is useful to predict prolonged air leak after pulmonary lobectomy. J Cardiothorac Surg. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takamochi K, Imashimizu K, Fukui M, et al. Utility of Objective Chest Tube Management After Pulmonary Resection Using a Digital Drainage System. Ann Thorac Surg. 2017. [DOI] [PubMed] [Google Scholar]
- 38.Brunelli A, Cassivi SD, Salati M, et al. Digital measurements of air leak flow and intrapleural pressures in the immediate postoperative period predict risk of prolonged air leak after pulmonary lobectomy. Eur J Cardio-thoracic Surg. 2011. [DOI] [PubMed] [Google Scholar]
- 39.Goto M, Aokage K, Sekihara K, et al. Prediction of prolonged air leak after lung resection using continuous log data of flow by digital drainage system. Gen Thorac Cardiovasc Surg. 2019. [DOI] [PubMed] [Google Scholar]
- 40.Yeung C, Ghazel M, French D, et al. Forecasting pulmonary air leak duration following lung surgery using transpleural airflow data from a digital pleural drainage device. J Thorac Dis. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rocco G, Brunelli A, Rocco R. Suction or Nonsuction: How to Manage a Chest Tube After Pulmonary Resection. Thorac Surg Clin. 2017. [DOI] [PubMed] [Google Scholar]
- 42.Cerfolio RJ, Bryant AS. The Management of Chest Tubes After Pulmonary Resection. Thorac Surg Clin. 2010. [DOI] [PubMed] [Google Scholar]
- 43.Marshall MB, Deeb ME, Bleier JIS, et al. Suction vs water seal after pulmonary resection: A randomized prospective study. Chest. 2002. [DOI] [PubMed] [Google Scholar]
- 44.Cerfolio RJ, Bryant AS, Singh S, Bass CS, Bartolucci AA. The management of chest tubes in patients with a pneumothorax and an air leak after pulmonary resection. Chest. 2005. [DOI] [PubMed] [Google Scholar]
- 45.Coughlin SM, Emmerton-Coughlin HMA, Malthaner R. Management of chest tubes after pulmonary resection: A systematic review and meta-analysis. Can J Surg. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toloza EM, Harpole DH. Intraoperative techniques to prevent air leaks. Chest Surg Clin N Am. 2002. [DOI] [PubMed] [Google Scholar]
- 47.Varela G, Jiménez MF, Novoa N. Portable Chest Drainage Systems and Outpatient Chest Tube Management. Thorac Surg Clin. 2010. [DOI] [PubMed] [Google Scholar]
- 48.Brims FJH, Maskell NA. Ambulatory treatment in the management of pneumothorax: A systematic review of the literature. Thorax. 2013. [DOI] [PubMed] [Google Scholar]
- 49.Towe CW, Khil A, Ho VP, et al. Early discharge after lung resection is safe: 10-year experience. J Thorac Dis. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Linden PA, Perry Y, Worrell S, et al. Postoperative day 1 discharge after anatomic lung resection: A Society of Thoracic Surgeons database analysis. J Thorac Cardiovasc Surg. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosen JE, Salazar MC, Dharmarajan K, Kim AW, Detterbeck FC, Boffa DJ. Length of Stay From the Hospital Perspective: Practice of Early Discharge Is Not Associated With Increased Readmission Risk After Lung Cancer Surgery. Ann Surg. 2017. [DOI] [PubMed] [Google Scholar]
- 52.Reinersman JM, Allen MS, Blackmon SH, et al. Analysis of Patients Discharged From the Hospital With a Chest Tube in Place. Ann Thorac Surg. 2018. [DOI] [PubMed] [Google Scholar]
- 53.Jabłoński S, Kordiak J, Wcisło S, et al. Outcome of pleurodesis using different agents in management prolonged air leakage following lung resection. Clin Respir J. 2018. [DOI] [PubMed] [Google Scholar]
- 54.Özpolat B. Autologous blood patch pleurodesis in the management of prolonged air leak. Thorac Cardiovasc Surg. 2010. [DOI] [PubMed] [Google Scholar]
- 55.Travaline JM, McKenna RJ, De Giacomo T, et al. Treatment of persistent pulmonary air leaks using endobronchial valves. Chest. 2009. [DOI] [PubMed] [Google Scholar]
- 56.Gillespie CT, Sterman DH, Cerfolio RJ, et al. Endobronchial valve treatment for prolonged air leaks of the lung: A case series. Ann Thorac Surg. 2011. [DOI] [PubMed] [Google Scholar]
- 57.Reed MF, Gilbert CR, Taylor MD, Toth JW. Endobronchial valves for challenging air leaks. In: Annals of Thoracic Surgery. ; 2015. [DOI] [PubMed] [Google Scholar]
- 58.Firlinger I, Stubenberger E, Müller MR, Burghuber OC, Valipour A. Endoscopic one-way valve implantation in patients with prolonged air leak and the use of digital air leak monitoring. Ann Thorac Surg. 2013. [DOI] [PubMed] [Google Scholar]
- 59.Cerfolio RJ. The incidence, etiology, and prevention of postresectional bronchopleural fistula. Semin Thorac Cardiovasc Surg. 2001;13(1):3–7. [DOI] [PubMed] [Google Scholar]
- 60.Nagahiro I, Aoe M, Sano Y, Date H, Andou A, Shimizu N. Bronchopleural fistula after lobectomy for lung cancer. Asian Cardiovasc Thorac Ann. 2007. [DOI] [PubMed] [Google Scholar]
- 61.Sirbu H, Busch T, Aleksic I, Schreiner W, Oster O, Dalichau H. Bronchopleural fistula in the surgery of non-small cell lung cancer: incidence, risk factors, and management. Ann Thorac Cardiovasc Surg. 2001;7(6):330–336. [PubMed] [Google Scholar]
- 62.Fuso L, Varone F, Nachira D, et al. Incidence and Management of Post-Lobectomy and Pneumonectomy Bronchopleural Fistula. Lung. 2016. [DOI] [PubMed] [Google Scholar]
- 63.Stern JB, Fournel L, Wyplosz B, et al. Early and delayed post-pneumonectomy empyemas: Microbiology, management and prognosis. Clin Respir J. 2018. [DOI] [PubMed] [Google Scholar]
- 64.Boudaya MS, Smadhi H, Zribi H, et al. Conservative management of postoperative bronchopleural fistulas. J Thorac Cardiovasc Surg. 2013. [DOI] [PubMed] [Google Scholar]
- 65.Zaheer S, Allen MS, Cassivi SD, et al. Postpneumonectomy Empyema: Results After the Clagett Procedure. Ann Thorac Surg. 2006. [DOI] [PubMed] [Google Scholar]
- 66.Schneiter D, Grodzki T, Lardinois D, et al. Accelerated treatment of postpneumonectomy empyema: A binational long-term study. J Thorac Cardiovasc Surg. 2008. [DOI] [PubMed] [Google Scholar]
- 67.Alexiou C, Beggs D, Rogers ML, Beggs L, Asopa S, Salama FD. Pneumonectomy for nonsmall cell lung cancer: Predictors of operative mortality and survival. Eur J Cardio-thoracic Surg. 2001. [DOI] [PubMed] [Google Scholar]
- 68.Hu XF, Duan L, Jiang GN, Wang H, Liu HC, Chen C. A clinical risk model for the evaluation of bronchopleural fistula in non-small cell lung cancer after pneumonectomy. Ann Thorac Surg. 2013. [DOI] [PubMed] [Google Scholar]
- 69.Jichen Q V, Chen G, Jiang G, Ding J, Gao W, Chen C. Risk Factor Comparison and Clinical Analysis of Early and Late Bronchopleural Fistula After Non-Small Cell Lung Cancer Surgery. Ann Thorac Surg. 2009. [DOI] [PubMed] [Google Scholar]
- 70.Bribriesco A, Patterson GA. Management of Postpneumonectomy Bronchopleural Fistula: From Thoracoplasty to Transsternal Closure. Thorac Surg Clin. 2018. [DOI] [PubMed] [Google Scholar]
- 71.Brown Lisa M. Vallieres E. 57 - Postsurgical Empyema. In: LoCicero J, Feins RH, Colson YL, Rocco, ed. Shields’ General Thoracic Surgery. 8th Editio. Philadelphia: Lippincott Williams & Wilkins; 2019:746–754. [Google Scholar]
- 72.Vallières E. Management of empyema after lung resections (pneumonectomy/lobectomy). Chest Surg Clin N Am. 2002. [DOI] [PubMed] [Google Scholar]
- 73.. Wain JC Management of late postpneumonectomy empyema and bronchopleural fistula. Chest Surg Clin N Am. 1996;6(3):529–541. https://www.ncbi.nlm.nih.gov/pubmed/8818420. Accessed November 17, 2019. [PubMed] [Google Scholar]
- 74.Di Maio M, Perrone F, Deschamps C, Rocco G. A meta-analysis of the impact of bronchial stump coverage on the risk of bronchopleural fistula after pneumonectomy. Eur J Cardiothoracic Surg. 2015;48(2):196–200. [DOI] [PubMed] [Google Scholar]
- 75.Pforr A, Pagès PB, Baste JM, et al. A predictive score for bronchopleural fistula established using the French database epithor. Ann Thorac Surg. 2016. [DOI] [PubMed] [Google Scholar]
- 76.Duque JL, Ramos G, Castrodeza J, et al. Early complications in surgical treatment of lung cancer: A prospective, multicenter study. Ann Thorac Surg. 1997. [DOI] [PubMed] [Google Scholar]
- 77.Li SJ, Fan J, Zhou J, Ren YT, Shen C, Che GW. Diabetes Mellitus and Risk of Bronchopleural Fistula after Pulmonary Resections: A Meta-Analysis. Ann Thorac Surg. 2016;102(1):328–339. [DOI] [PubMed] [Google Scholar]
- 78.Mazzella A, Pardolesi A, Maisonneuve P, et al. Bronchopleural Fistula After Pneumonectomy: Risk Factors and Management, Focusing on Open-Window Thoracostomy. Semin Thorac Cardiovasc Surg. 2018. [DOI] [PubMed] [Google Scholar]
- 79.Puri V, Tran A, Bell JM, et al. Completion pneumonectomy: Outcomes for benign and malignant indications. Ann Thorac Surg. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Okuda M, Go T, Yokomise H. Risk factor of bronchopleural fistula after general thoracic surgery: review article. Gen Thorac Cardiovasc Surg. 2017. [DOI] [PubMed] [Google Scholar]
- 81.Fujimoto T, Zaboura G, Fechner S, et al. Completion pneumonectomy: Current indications, complications, and results. J Thorac Cardiovasc Surg. 2001. [DOI] [PubMed] [Google Scholar]
- 82.Al-Kattan K, Goldstraw P. Completion pneumonectomy: Indications and outcome. J Thorac Cardiovasc Surg. 1995. [DOI] [PubMed] [Google Scholar]
- 83.Miller DL, Deschamps C, Jenkins GD, Bernard A, Allen MS, Pairolero PC. Completion pneumonectomy: Factors affecting operative mortality and cardiopulmonary morbidity. Ann Thorac Surg. 2002. [DOI] [PubMed] [Google Scholar]
- 84.Hamaji M, Chen-Yoshikawa TF, Date H. Completion pneumonectomy and autotransplantation for bronchopleural fistula. J Thorac Cardiovasc Surg. September 2019. [DOI] [PubMed] [Google Scholar]
- 85.Shapiro M, Swanson SJ, Wright CD, et al. Predictors of major morbidity and mortality after pneumonectomy utilizing the society for thoracic surgeons general thoracic surgery database. Ann Thorac Surg. 2010. [DOI] [PubMed] [Google Scholar]
- 86.Rivera C, Arame A, Pricopi C, et al. Pneumonectomy for benign disease: Indications and postoperative outcomes, a nationwide study. Eur J Cardio-thoracic Surg. 2015. [DOI] [PubMed] [Google Scholar]
- 87.Reed CE. Pneumonectomy for chronic infection: Fraught with danger? Ann Thorac Surg. 1995. [DOI] [PubMed] [Google Scholar]
- 88.Kobayashi S, Karube Y, Nishihira M, et al. Postoperative pyothorax a risk factor for acute exacerbation of idiopathic interstitial pneumonia following lung cancer resection. Gen Thorac Cardiovasc Surg. 2016. [DOI] [PubMed] [Google Scholar]
- 89.Siegenthaler MP, Pisters KM, Merriman KW, et al. Preoperative chemotherapy for lung cancer does not increase surgical morbidity. Ann Thorac Surg. 2001. [DOI] [PubMed] [Google Scholar]
- 90.Li S, Fan J, Liu J, et al. Neoadjuvant therapy and risk of bronchopleural fistula after lung cancer surgery: A systematic meta-analysis of 14 912 patients. Jpn J Clin Oncol. 2016. [DOI] [PubMed] [Google Scholar]
- 91.Yamamoto R, Tada H, Kishi A, Tojo T. Effects of preoperative chemotherapy and radiation therapy on human bronchial blood flow. J Thorac Cardiovasc Surg. 2000. [DOI] [PubMed] [Google Scholar]
- 92.Inui K, Takahashi Y, Hasegawa S, et al. Effect of preoperative irradiation on wound healing after bronchial anastomosis in mongrel dogs. J Thorac Cardiovasc Surg. 1993. [PubMed] [Google Scholar]
- 93.Hollaus PH, Lax F, Janakiev D, et al. Endoscopic treatment of postoperative bronchopleural fistula: Experience with 45 cases. Ann Thorac Surg. 1998. [DOI] [PubMed] [Google Scholar]
- 94.Tsunezuka Y, Sato H, Tsukioka T, Hiranuma C. A new instrument for endoscopic gluing for bronchopleural fistulae. In: Annals of Thoracic Surgery. ; 1999. [DOI] [PubMed] [Google Scholar]
- 95.Stratakos G, Zuccatosta L, Porfyridis I, et al. Silver nitrate through flexible bronchoscope in the treatment of bronchopleural fistulae. J Thorac Cardiovasc Surg. 2009. [DOI] [PubMed] [Google Scholar]
- 96.Yamamoto S, Endo S, Minegishi K, Shibano T, Nakano T, Tetsuka K. Polyglycolic acid mesh occlusion for postoperative bronchopleural fistula. Asian Cardiovasc Thorac Ann. 2015. [DOI] [PubMed] [Google Scholar]
- 97.Han X, Yin M, Li L, et al. Customized airway stenting for bronchopleural fistula after pulmonary resection by interventional technique: single-center study of 148 consecutive patients. Surg Endosc. 2018. [DOI] [PubMed] [Google Scholar]
- 98.Litle VR. Management of Post-pneumonectomy Bronchopleural Fistula: A Roadmap for Rescue. Semin Thorac Cardiovasc Surg. 2018. [DOI] [PubMed] [Google Scholar]
- 99.Gómez JMN, Carbajo MC, Concha DV, De La Cruz JLCC. Conservative treatment of postlobectomy bronchopleural fistula. Interact Cardiovasc Thorac Surg. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Park JS, Eom JS, Choi SH, Kim YH, Kim EK. Use of a serratus anterior musculocutaneous flap for surgical obliteration of a bronchopleural fistula. Interact Cardiovasc Thorac Surg. 2015. [DOI] [PubMed] [Google Scholar]
- 101.Massera F, Robustellini M, Della Pona C, Rossi G, Rizzi A, Rocco G. Open window thoracostomy for pleural empyema complicating partial lung resection. Ann Thorac Surg. 2009;87. 10.1016/j.athoracsur.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 102.Regnard JF, Alifano M, Puyo P, Fares E, Magdeleinat P, Levasseur P. Open window thoracostomy followed by intrathoracic flap transposition in the treatment of empyema complicating pulmonary resection. J Thorac Cardiovasc Surg. 2000. [DOI] [PubMed] [Google Scholar]
- 103.CLAGETT OT, GERACI JE. A procedure for the management of postpneumonectomy empyema. J Thorac Cardiovasc Surg. 1963. [PubMed] [Google Scholar]
- 104.Babu AN, Mitchell JD. Technique of Muscle Flap Harvest for Intrathoracic Use. Oper Tech Thorac Cardiovasc Surg. 2010. [Google Scholar]
- 105.Anderson TM, Miller JI. Use of pleura, azygos vein, pericardium, and muscle flaps in tracheobronchial surgery. Ann Thorac Surg. 1995. [DOI] [PubMed] [Google Scholar]
- 106.Taghavi S, Marta GM, Lang G, et al. Bronchial stump coverage with a pedicled pericardial flap: An effective method for prevention of postpneumonectomy bronchopleural fistula. Ann Thorac Surg. 2005. [DOI] [PubMed] [Google Scholar]
- 107.Anderson TM, Miller JI. Surgical technique and application of pericardial fat pad and pericardiophrenic grafts. Ann Thorac Surg. 1995. [DOI] [PubMed] [Google Scholar]
- 108.Fricke A, Bannasch H, Klein HF, et al. Pedicled and free flaps for intrathoracic fistula management. Eur J Cardiothorac Surg. 2017. [DOI] [PubMed] [Google Scholar]
- 109.Shrager JB, Wain JC, Wright CD, et al. Omentum is highly effective in the management of complex cardiothoracic surgical problems. J Thorac Cardiovasc Surg. 2003. [DOI] [PubMed] [Google Scholar]
- 110.Boulton BJ, Force S. The technique of omentum harvest for intrathoracic use. Oper Tech Thorac Cardiovasc Surg. 2010. [Google Scholar]
- 111.D’Andrilli A, Ibrahim M, Andreetti C, Ciccone AM, Venuta F, Rendina EA. Transdiaphragmatic Harvesting of the Omentum Through Thoracotomy for Bronchial Stump Reinforcement. Ann Thorac Surg. 2009. [DOI] [PubMed] [Google Scholar]
- 112.Begum SSS, Papagiannopoulos K. The use of vacuum-assisted wound closure therapy in thoracic operations. Ann Thorac Surg. 2012. [DOI] [PubMed] [Google Scholar]
- 113.Karapinar K, Saydam Ö, Metin M, et al. Experience with Vacuum-Assisted Closure in the Management of Postpneumonectomy Empyema: An Analysis of Eight Cases. Thorac Cardiovasc Surg. 2016. [DOI] [PubMed] [Google Scholar]
- 114.Ginsberg RJ, Saborio DV. Management of the recalcitrant postpneumonectomy bronchopleural fistula: The transsternal transpericardial approach. Semin Thorac Cardiovasc Surg. 2001. [DOI] [PubMed] [Google Scholar]
- 115.Galetta D, Spaggiari L. Video-Thoracoscopic Management of Postpneumonectomy Empyema. Thorac Cardiovasc Surg. 2018. [DOI] [PubMed] [Google Scholar]
- 116.Scarci M, Abah U, Solli P, et al. EACTS expert consensus statement for surgical management of pleural empyema. Eur J Cardio-thoracic Surg. 2015. [DOI] [PubMed] [Google Scholar]
- 117.Leiter N, Pickering EM, Sangwan YS, Burrows WM, Friedberg JS, Sachdeva A. Intrapleural Therapy for Empyema in the Setting of a Bronchopleural Fistula: A Novel Use of an Intrabronchial Valve. Ann Thorac Surg. 2018. [DOI] [PubMed] [Google Scholar]
- 118.Ding M, Gao Y dong, Zeng XT, Guo Y, Yang J. Endobronchial one-way valves for treatment of persistent air leaks: A systematic review. Respir Res. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kalatoudis H, Nikhil M, Zeid F, Shweihat Y. Bronchopleural Fistula Resolution with Endobronchial Valve Placement and Liberation from Mechanical Ventilation in Acute Respiratory Distress Syndrome: A Case Series. Case Reports Crit Care. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brichon PY, Poquet C, Arvieux C, Pison C. Successful treatment of a life-threatening air leakage, complicating severe abdominal sepsis, with a one-way endobronchial valve. In: Interactive Cardiovascular and Thoracic Surgery. ; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fischer W, Feller-Kopman D, Shah A, Orens J, Illei P, Yarmus L. Endobronchial valve therapy for pneumothorax as a bridge to lung transplantation. J Hear Lung Transplant. 2012. [DOI] [PubMed] [Google Scholar]