Abstract
Background:
Standard ECG criteria for left ventricular (LV) hypertrophy rely on QRS amplitudes. However, in the setting of left bundle branch block (LBBB), ECG correlates of LV hypertrophy are not well established. We sought to evaluate quantitative ECG predictors of LV hypertrophy in the presence of LBBB.
Methods:
We included adult patients with typical LBBB having ECG and transthoracic echocardiogram performed within 3 months of each other in 2010–2020. Orthogonal X, Y, Z leads were reconstructed from digital 12-lead ECGs using Kors’s matrix. In addition to QRS duration, we evaluated QRS amplitudes and voltage-time-integrals (VTIs) from all 12 leads, X, Y, Z leads and 3D (root-mean-squared) ECG. We used age, sex and BSA-adjusted linear regressions to predict echocardiographic LV calculations (mass, end-diastolic and end-systolic volumes, ejection fraction) from ECG, and separately generated ROC curves for predicting echocardiographic abnormalities.
Results:
We included 413 patients (53% women, age 73 ± 12 years). All 4 echocardiographic LV calculations were most strongly correlated with QRS duration (all p < 0.00001). In women, QRS duration ≥ 150 ms had sensitivity/specificity 56.3%/64.4% for increased LV mass and 62.7%/67.8% for increased LV end-diastolic volume. In men, QRS duration ≥ 160 ms had a sensitivity/specificity 63.1%/72.1% for increased LV mass and 58.3%/74.5% for increased LV end-diastolic volume. QRS duration was best able to discriminate eccentric hypertrophy (area under ROC curve 0.701) and increased LV end-diastolic volume (0.681).
Conclusions:
In patients with LBBB, QRS duration (≥ 150 in women and ≥ 160 in men) is a superior predictor of LV remodeling esp. eccentric hypertrophy and dilation.
Keywords: ECG, Left ventricular dilation, Left ventricular hypertrophy, Left ventricular remodeling, Left bundle branch block, QRS duration, QRS amplitude, QRS area, QRS voltage-time-integral
Introduction
Scientific and clinical background:
Left ventricular hypertrophy (LVH) is the increase in the mass of the left ventricle (LV) and is usually detected with echocardiography. LVH can be concentric (increase in LV wall thickness) or eccentric (dilation of the LV chamber) and usually occurs respectively in response to a chronic increase in pressure or volume load within LV [1]. LVH has consistently been shown to be an independent predictor of worse prognosis and early identification is an important first step in mitigating adverse cardiovascular outcomes [2]. Electrocardiography (ECG) is a simple, non-invasive and widely available modality to screen for cardiac abnormalities [3]. A multitude of reasonably specific ECG diagnostic criteria for LVH in setting of narrow QRS complex have been developed, such as Sokolow and Lyon, point score of Romhilt and Estes, Cornell criteria, and Peguero and Lo Presti, although they have limited sensitivity [4–7]. As LVH results in an overall increase in the QRS voltage, these criteria rely on different combinations of R and S amplitudes in the standard limb and/or precordial ECG leads [8]. However, none of these criteria are valid in the presence of left bundle branch block (LBBB), though prior publications have assessed the utility of these criteria in presence of LBBB [9,10]. The sequential anterior-to-posterior depolarization of the LV due to LBBB results in additive effects on the QRS voltage independent of LV hypertrophy, and we do not have established voltage-based criteria to detect LV hypertrophy in the setting of LBBB [9,11,12].
Published ECG criteria relying on QRS amplitudes for LVH in the setting of LBBB were obtained from small patient sample sets and have yielded limited sensitivity and specificity. Klein et al. developed the criteria S amplitude in lead V2 + R amplitude in lead V6 > 4.5 mV [13]. Kafka et al. published a complex sequential 4 component criteria (i) R amplitude in lead aVL ≥11 mV, (ii) QRS axis ≤ −40°, (iii) S amplitude in lead V1 + R amplitude in V5 or V6 ≥ 40 mV, (iv) and S in lead V2 ≥ 30 mV and in V3 ≥ 25 mV [14]. Subsequently, Haskell et al. determined that voltages were not sensitive or specific for LVH diagnosis in setting of LBBB [15]. Instead, they concluded that QRS duration was the best correlate of LVH, 160 ± 12 ms with LVH and 148 ± 11 ms without LVH. When Fragola et al. tried to validate different criteria for LVH in 100 patients with LBBB, they concluded that all aforementioned criteria were unreliable in predicting LVH [12].
QRS 3D-voltage-time-integral (VTIQRS-3D) is a novel summary metric obtained by the integrating, over the duration of the QRS, the instantaneous absolute 3D ECG voltage (root-mean-square of orthogonal X, Y, Z coordinate instantaneous voltages) [16–20]. 3D QRS area, an approximation of VTIQRS-3D, has been proposed as a marker for patient selection and evaluating response to resynchronization therapy [21–24]. VTI of the QRS can be obtained for any standard ECG lead, the reconstructed orthogonal X, Y and Z axes leads or the absolute 3D ECG.
Study objectives and hypothesis:
The presence of LBBB signifies conduction system disease. This can occur in association with structural heart disease, heart failure or cardiac ischemia, but can also occur independently related to age-related conduction system degeneration. The identification of LBBB may warrant evaluation for structural heart disease with cardiac imaging e.g. echocardiogram or magnetic resonance imaging (MRI). Regardless, there remains is a large subset of patients with LBBB who are asymptomatic and have no identifiable structural cardiac abnormality on imaging [25]. Such patients, however, remain at risk of developing eccentric hypertrophy and systolic cardiomyopathy. Therefore, it would be useful to have ECG criteria to identify which patients with LBBB have LVH.
The increased myocardial mass in LVH would generate increased voltage, and it would take longer to activate the enlarged LV. We therefore hypothesized that presence of LV adverse remodeling with hypertrophy and dilation in presence of LBBB would increase the QRS duration, amplitude and the VTI. We sought to evaluate such quantitative ECG variables as predictors of structural LV abnormalities on echocardiography in a larger dataset of patients with LBBB, and compare to previously published criteria [26].
Methods
Study data and patient selection
All adult patients evaluated between January 1, 2010 to December 31, 2020 at The University of Kansas Medical Center with a diagnosis code for left bundle branch block who had a 12-lead ECG and comprehensive transthoracic echocardiogram within 3 months of each other were potential subjects. We utilized HERON (Healthcare Enterprise Repository for Ontological Narration), a search discovery tool that facilitates searches on various hospital electronic data sources, to query and retrospectively identify eligible subjects [27,28]. The study was conducted under an approval from the Institutional Review Board.
ECG criteria for LBBB:
On manual review of the ECGs, only patients with typical LBBB per ACC/AHA criteria were included and those with atypical LBBB or non-specific intraventricular conduction delay were excluded. ACC/AHA criteria for left bundle branch block consist of QRS duration ≥120 ms, broad notched or slurred R waves in left-sided leads, small r waves in right-sided leads, R peak time > 60 ms in V5 and V6, and ST and T waves in opposite direction to the QRS [29]. Of the remainder we further excluded patients who had a QRS duration less than the sex-specific 10th percentile (130 ms for women and 136 ms for men) as these may be incomplete LBBB.
Data extraction
ECG:
The 12-lead ECGs were downloaded and were manually reviewed together by the AD, SR and AN to ensure they meet ACC/AHA criteria for LBBB and all disagreements resolved by mutual consensus. Subsequently, digital ECGs were processed in Python and converted to vectorcardiograms utilizing the Kors’s regression matrix [30]. The root-mean-square of the instantaneous voltages from the orthogonal leads (X, Y and Z) was integrated over the QRS duration to obtain the VTIQRS-3D (see Fig. 1). The QRS amplitude was obtained from the root-mean-square (3D) ECG (amplitudeQRS-3D). Similarly, VTIs and peak-to-peak amplitudes were obtained for all 12 standard ECG leads and the reconstructed X, Y and Z leads.
Fig. 1.
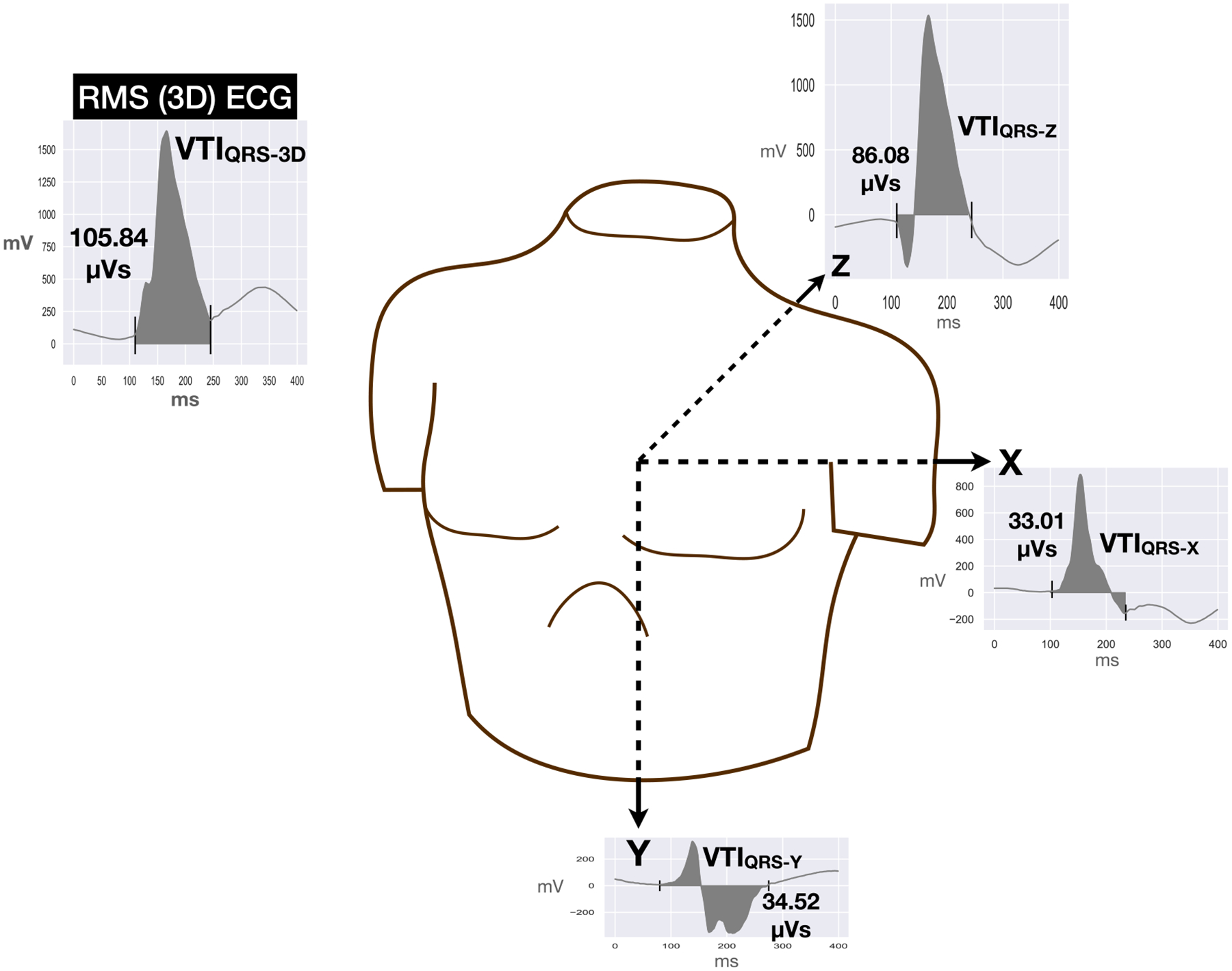
Example of vectorcardiographic reconstruction from 12-lead ECG with X, Y and Z lead ECGs and root-mean-squared (3D) ECG. Voltage-time-integrals (VTIs) for the QRS in X, Y and Z leads and VTIQRS-3D are shown.
Echocardiogram:
The echocardiographic calculations were obtained from the HERON search. These include LV mass, LV end diastolic volume (LVEDV), LV end systolic volume (LVESV) and LV ejection fraction (LVEF). American Society of Echocardiography definitions were used for all echocardiographic calculations including LV mass and concentric/eccentric hypertrophy based on relative wall thickness (RWT) [31]. We used the American Society of Echocardiography recommended formula for LV mass calculation from 2D echocardiographic measurements in parasternal long axis view i.e. 0.8 × 1.04[(LVIDd + IVSd + PWd)3 − LVIDd3] + 0.6 grams [32]. When LV mass is elevated, relative wall thickness is defined 2 × PWd/LVIDd. RWT > 0.42 defines concentric hypertrophy and RWT ≤ 0.42 classifies eccentric hypertrophy.
Patient demographics
Patient demographics were also obtained from HERON.
Statistical analysis
Baseline demographics and echocardiographic continuous variables among men and women subjects are reported as medians with interquartile ranges. Unadjusted correlation coefficients and multivariate linear regressions adjusted for age, sex, and body surface area (BSA) were used to assess the associations of ECG QRS predictors with echocardiographic calculations (LV mass, LVEDV, LVESV and LVEF). In addition to the overall QRS duration, peak-to-peak QRS amplitudes and voltage-time-integrals (VTIs) from all 12 standard ECG leads, X, Y, Z vectorcardiographic leads and 3D ECG were individually evaluated as predictors for each of the 4 echocardiographic calculations. Two-tailed p-values <0.05 were considered statistically significant. For each of the ECG predictors, we separately obtained receiver operator characteristic (ROC) curves to predict each of the sex-specific BSA-indexed echocardiographic calculation being increased out of normal range (decreased for LVEF). ROC curves were generated for the entire study population and separately for men and women. The statistical analyses were performed in JMP Pro 16.0.0 (SAS Institute, Cary, NC).
Results
We included 413 patients in our study, with a median age of 73 years with 53% women. The baseline characteristics are shown in Table 1. The absolute median difference between dates of ECG and echocardiogram was 3 (interquartile range 1–19) days. All 4 echocardiographic calculations were correlated with each other (Supplementary Table 1).
Table 1.
Study population characteristics.
| Variable | Women (N = 219) | Men (N = 194) |
|---|---|---|
| Median (IQR) | Median (IQR) | |
| Age, years | 74 (65–83) | 72.5 (66–80) |
| BSA, m2 | 1.9 (1.7–2.1) | 2.1 (2.0–2.3) |
| LV Mass, g | 164 (133–197) | 227 (187–275) |
| LVEDV, ml | 101 (76–138) | 147 (113–199) |
| LVESV, ml | 45 (31–71) | 68 (48–105) |
| LVEF, % | 54 (45–63) | 53 (38–63) |
| QRS duration, ms | 149 (140–158) | 157 (150–168) |
| AmplitudeQRS-3D, mV | 1.4 (1.2–1.7) | 1.7 (1.3–1.9) |
| VTIQRS-3D, μVs | 102 (85–123) | 124 (99–150) |
After adjusting for age, sex, and BSA, among all ECG variables that were assessed, QRS duration was found to be the strongest predictor of all 4 echocardiographic calculations i.e., LV mass, LVEDV, LVESV and LVEF (p < 0.00001 for all, Table 2). Associations with LV calculations were weaker with QRS VTIs and weakest with QRS amplitudes. VTIQRS-3D and amplitudeQRS-3D respectively were generally the strongest VTI and amplitude predictors of LV calculations among the 16 ECG leads evaluated (12 standard, X, Y, Z and 3D ECG) and are also shown along with QRS duration in Table 2. VTIQRS-3D was statistically associated with LV mass and volumes, but not LVEF. AmplitudeQRS-3D was only associated with LV mass.
Table 2.
Linear prediction of LV echocardiographic parameters by ECG (N = 413).
| QRS Duration | VTIQRS-3D | AmplitudeQRS-3D | |||||||
|---|---|---|---|---|---|---|---|---|---|
| r * | t-score † | p-value † | r * | t-score † | p-value † | r * | t-score † | p-value † | |
| LV Mass | 0.49 | 8.19 | <0.00001 | 0.27 | 5.78 | <0.00001 | 0.13 | 3.64 | 0.0003 |
| LVEDV | 0.48 | 8.52 | <0.00001 | 0.22 | 4.12 | 0.00005 | 0.09 | 1.84 | 0.07 |
| LVESV | 0.42 | 8.04 | <0.00001 | 0.22 | 3.51 | 0.0005 | 0.11 | 1.38 | 0.2 |
| LVEF | −0.20 | −4.48 | <0.00001 | −0.15 | −1.84 | 0.07 | −0.12 | −0.88 | 0.4 |
VTI, voltage-time-integral; LV, left ventricular; EDV, end-diastolic volume; ESV, end-systolic volume; EF, ejection fraction.
Unadjusted correlation coefficients.
From linear regression models adjusted for age, sex, BSA.
Among its associations with the echocardiographic LV calculations, QRS duration was most strongly correlated with LV mass and LVEDV (Table 2). Fig. 2 shows residual plots for QRS duration, VTIQRS-3D and amplitudeQRS-3D for predicting LV mass and LVEDV.
Fig. 2.
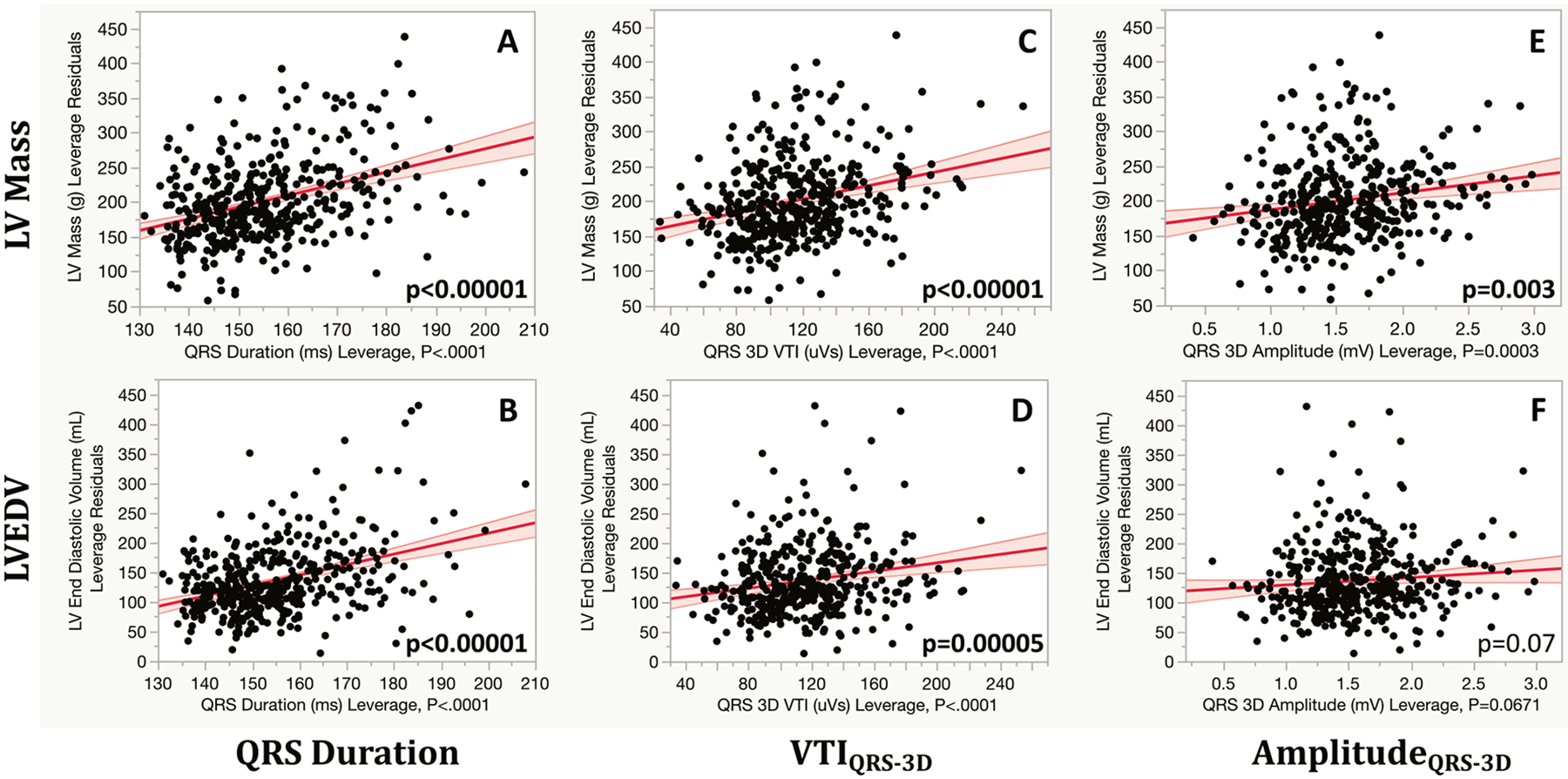
Leverage plots of QRS duration (Panels A and B), QRS 3D-voltage-time-integral (Panels C and D), and QRS 3D voltage (Panels E and F) with LV mass (top panels) and LVEDV (bottom panels) plotting residuals from multivariate linear regressions adjusting for age, sex and BSA.
Similarly, unadjusted correlations with all 4 echocardiographic LV calculations were strongest for QRS duration (correlation coefficient with LV mass 0.49, LVEDV 0.48, LVESV 0.42 and LVEF −0.20, see Table 2).
Out of all ECG predictors, QRS duration had the highest area under the ROC curve (AUC) to detect echocardiographic LV abnormalities, i.e. increased sex-specific BSA-indexed mass and volumes, and reduced LVEF (Table 3). For QRS duration, the highest AUCs were for detecting eccentric hypertrophy (0.701) and increased sex-specific LVEDVi (0.681). On the other hand, QRS duration was worse than VTIQRS-3D and amplitudeQRS-3D in predicting concentric hypertrophy (AUCs 0.567, 0.631 and 0.621 respectively), although this finding was inconsistent between men and women (Table 4A, 4B). In men, AUCs of QRS duration were reliably higher than AUCs of VTIQRS-3D and amplitudeQRS-3D for all echocardiographic LV abnormalities. However, in women, AUC of QRS duration was lower than AUCs of VTIQRS-3D and amplitudeQRS-3D for concentric hypertrophy (0.585, 0.687 and 0.686 respectively).
Table 3.
Area under curve (AUC) from ROC curves for prediction of LV echocardiographic abnormalities by ECG in the overall population.
| Echocardiographic Abnormality | AUC for Overall Population (N = 413) | ||
|---|---|---|---|
| QRS Duration | VTIQRS-3D | AmplitudeQRS-3D | |
| Increased LVMi | 0.646 | 0.636 | 0.603 |
| (F > 95, M > 115 g/m2) | |||
| Concentric Hypertrophy | 0.567 | 0.631 | 0.621 |
| (LVH & RWT > 0.42) | |||
| Eccentric Hypertrophy | 0.701 | 0.569 | 0.514 |
| (LVH & RWT ≤ 0.42) | |||
| Increased LVEDVi | 0.681 | 0.592 | 0.542 |
| (F > 61, M > 74 mL/m2) | |||
| Increased LVESVi | 0.640 | 0.591 | 0.561 |
| (F > 24, M > 31 mL/m2) | |||
| Decreased LVEF | 0.659 | 0.558 | 0.525 |
| (≤ 40%) | |||
Table 4A.
Area under curve (AUC) from ROC curves for prediction of LV echocardiographic abnormalities by ECG for women.
| Echocardiographic Abnormality | AUC for Women (n = 219) | ||
|---|---|---|---|
| QRS Duration | VTIQRS-3D | AmplitudeQRS-3D | |
| Increased LVMi | 0.657 | 0.676 | 0.640 |
| (> 95 g/m2) | |||
| Concentric Hypertrophy | 0.585 | 0.687 | 0.686 |
| (LVH & RWT > 0.42) | |||
| Eccentric Hypertrophy | 0.714 | 0.535 | 0.554 |
| (LVH & RWT ≤ 0.42) | |||
| Increased LVEDVi | 0.668 | 0.556 | 0.501 |
| (> 61 mL/m2) | |||
| Increased LVESVi | 0.652 | 0.562 | 0.526 |
| (> 24 mL/m2) | |||
| Decreased LVEF | 0.683 | 0.508 | 0.562 |
| (≤ 40%) | |||
Table 4B.
Area under curve (AUC) from ROC curves for prediction of LV echocardiographic abnormalities by ECG for men.
| Echocardiographic Abnormality | AUC for Men (n = 194) | ||
|---|---|---|---|
| QRS Duration | VTIQRS-3D | AmplitudeQRS-3D | |
| Increased LVMi | 0.703 | 0.616 | 0.567 |
| (> 115 g/m2) | |||
| Concentric Hypertrophy | 0.651 | 0.603 | 0.548 |
| (LVH & RWT > 0.42) | |||
| Eccentric Hypertrophy | 0.680 | 0.581 | 0.562 |
| (LVH & RWT ≤ 0.42) | |||
| Increased LVEDVi | 0.699 | 0.622 | 0.569 |
| (> 74 mL/m2) | |||
| Increased LVESVi | 0.634 | 0.615 | 0.587 |
| (> 31 mL/m2) | |||
| Decreased LVEF | 0.607 | 0.573 | 0.563 |
| (≤ 40%) | |||
Among women, QRS duration ≥150 ms had a sensitivity 56.3% with specificity 64.4% for identifying increased LV mass index (>95 g/m2), and a sensitivity 62.7% with specificity 67.8% for increased LVEDVi (>61 mL/m2). In men, QRS duration ≥160 ms had a sensitivity 63.1% with specificity 72.1% for increased LV mass index (>115 g/m2) and sensitivity 58.3% with specificity 74.5% for increased LVEDVi (>74 mL/m2).
Discussion
Summary of our findings:
We found in our study that, among patients with LBBB, QRS duration ≥150 ms in women and ≥ 160 ms in men is a superior predictor of LV eccentric hypertrophy and dilation. QRS 3D-voltage-time-integral (VTIQRS-3D) is a better predictor of LVH than QRS amplitudes; however, it is inferior to QRS duration. QRS duration was statistically significant at predicting all 4 echocardiographic calculations i.e. LV mass, LVEDV, LVESV and LVEF; the correlation with LV mass and LVEDV being the strongest. QRS duration had a greater AUC for discriminating eccentric hypertrophy than concentric hypertrophy as opposed to VTIQRS-3D or amplitudeQRS-3D that had a higher AUC for concentric than eccentric hypertrophy, especially in women.
Mechanistic insights:
It remains unclear if the weak correlation of LVH with QRS voltages in setting of LBBB is due to the dominant effect of electrical dyssynchrony rather than hypertrophy on voltage. In addition, the heterogeneous effects of myocardial fibrosis, infiltrative cardiomyopathy, epicardial adipose tissue, pericardial effusion, body habitus and LV dilation could also impact QRS voltages [16,33]. Further analyses are required to unravel the individual contributions of such variables on the QRS voltage. VTIQRS remains a potential diagnostic and prognostic marker for LV structural abnormalities, but corrections for anthopo-metric, cardiac and extracardiac factors that impact the QRS voltage will have to be incorporated.
It intuitively makes sense that in presence of LBBB, QRS duration correlates with LV dilatation and eccentric hypertrophy. With LBBB, LV excitation is dependent on conduction within the working myocardium. Even assuming fixed intramyocardial conduction velocity, the larger the size of the LV, the longer it would take to activate it. Additionally, adverse remodeling results in impaired intraventricular conduction withing the working myocardium prolonging QRS duration, a mechanism applicable on top of presence of LBBB but also seen as non-specific intraventricular conduction delay when left bundle branch conduction is intact [34,35]. On the other hand, LV dilation may not have a consistent effect on QRS voltage. In the unipolar precordial leads, the voltage may be increased due to the increase in the activated myocardial mass, however, the voltage in the bipolar limb leads may be paradoxically reduced [33].
Conversely, it is logical that concentric hypertrophy without LV dilatation may not result in an overall increase in the time for the activation wavefront originating in the right bundle branch to travel across the LV, and thereby have smaller impact on the QRS duration. Concentric hypertrophy is more likely to be reflected as an increase in the individual ECG lead QRS amplitude as the corresponding thicker LV wall gets activated.
Increase in QRS duration has previously been reported to identify LVH in smaller studies in patients with LBBB, though sex-specific cutoffs had not been established [15]. Additionally, in LBBB, prolonged QRS duration when coexistent with left axis deviation has been correlated with systolic cardiomyopathy [26]. The correlation of QRS duration with LVEF are weaker compared to the correlation with LV mass and volumes in our analyses and may be explained on account of high degree of collinearity between LVEF and LV mass/volumes (see Supplementary Table 1).
Sex differences:
Our data is novel that we have a majority representation of women. Beyond different sex-specific cutoffs for QRS duration identifying LVH, we also found interesting modification of ECG-LVH correlates based on sex in our data. Among women, QRS duration had better discrimination of eccentric hypertrophy and QRS VTI/amplitude better discriminated concentric hypertrophy. However, among men, QRS duration always trumped QRS VTI/amplitude for all echocardiographic LV calculations. QRS duration was consistently the best discriminator of echocardiographic LV dilation but performed better in men compared to women.
Implications for practice:
This study incorporating >400 subjects with a majority women provides the largest LBBB patient population in comparison to previous studies, which ranged from 30 to 100 patients. As discussed in the introduction section, previous studies examining ECG criteria for diagnosis of LVH in the presence of LBBB have yielded conflicting results [9,10,12–15]. In our study we found sensitivities and specificities based on QRS duration cutoffs in patients with LBBB to be comparable to published ECG voltage criteria for LVH in patients with normal QRS duration [4–7]. QRS duration offers a quantitative, easy to use predictor for LV dilation (eccentric hypertrophy) with fair sensitivity and specificity. Current guidelines for screening for LVH in the presence of LBBB should be expanded to place a greater emphasis on QRS duration cutoffs. If a LBBB patient meets the QRS duration cutoff of ≥ 150 ms for women or ≥ 160 ms for men, then a follow up echocardiogram should be considered to rule out LVH and LV dilation. QRS duration dominantly trumps QRS VTI and voltage and there is no role for using measures like VTIQRS-3D or amplitudeQRS-3D for detecting adverse LV remodeling.
Limitations:
This study has many limitations. We do not have data on clinical outcomes. The LV mass formula is inherently susceptible to imprecise calculation. This involves assumption of the 3-dimensional shape and structure of the LV and relies on linear measurements made on a single parasternal long axis 2D image with slight measurement errors being compounded on account of taking cubes of the measurements. We therefore also validated the findings on LV volumes which are more precise and reproducible. We have no means to test biophysical causes for our findings and mechanistic insights remain speculative. The ECG and echocardiograms were not mandatorily obtained on the same date and there remains a possibility of temporal changes occurring between obtaining the 2 studies. Though the median absolute difference in acquiring these was 3 days, the separation was ≥19 days for a quarter of our study population. Even though we found statistically significant correlation between increased QRS duration and adverse LV remodeling, the AUC for ROC curves and sensitivity/specificity to detect echocardiographic LV abnormalities are modest, thereby limiting clinical utility.
Supplementary Material
Acknowledgements
Research reported in this publication was supported by the KUMC Research Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the KUMC Research Institute.
This work was supported by a CTSA grant from NCATS awarded to the Frontiers Clinical and Translational Science Institute at the University of Kansas (# UL1TR002366). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NCATS.
Abbreviations:
- AUC
Area under the curve
- ECG
Electrocardiogram
- LBBB
Left bundle branch block
- LV
Left ventricle
- LVH
Left ventricular hypertrophy
- LVEF
Left ventricular ejection fraction
- LVESV
Left ventricular end-systolic volume
- LVEDV
Left ventricular end-diastolic volume
- ROC
Receiver operator characteristic
Footnotes
Disclosures
None.
CRediT authorship contribution statement
Ashley DeBauge: Conceptualization, Methodology, Writing – original draft. Tyan Fairbank: Conceptualization, Methodology. Christopher J. Harvey: Methodology, Software. Sagar Ranka: Writing – original draft. Sania Jiwani: Writing – original draft. Seth H. Sheldon: Writing – review & editing. Madhu Reddy: Writing – review & editing. Timothy A. Beaver: Writing – review & editing. Amit Noheria: Conceptualization, Methodology, Software, Formal analysis, Writing – original draft, Supervision.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jelectrocard.2023.03.004.
References
- [1].Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16(3):233–71. [DOI] [PubMed] [Google Scholar]
- [2].Vakili BA, Okin PM, Devereux RB. Prognostic implications of left ventricular hypertrophy. Am Heart J 2001;141(3):334–41. [DOI] [PubMed] [Google Scholar]
- [3].Devereux RB. Is the electrocardiogram still useful for detection of left ventricular hypertrophy? Circulation 1990;81(3):1144–6. [DOI] [PubMed] [Google Scholar]
- [4].Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J 1949;37(2):161–86. [DOI] [PubMed] [Google Scholar]
- [5].Romhilt DW, Estes EH Jr. A point-score system for the ECG diagnosis of left ventricular hypertrophy. Am Heart J 1968;75(6):752–8. [DOI] [PubMed] [Google Scholar]
- [6].Casale PN, Devereux RB, Alonso DR, Campo E, Kligfield P. Improved sex-specific criteria of left ventricular hypertrophy for clinical and computer interpretation of electrocardiograms: validation with autopsy findings. Circulation 1987;75(3): 565–72. [DOI] [PubMed] [Google Scholar]
- [7].Peguero JG, Lo Presti S, Perez J, Issa O, Brenes JC, Tolentino A. Electrocardiographic criteria for the diagnosis of left ventricular hypertrophy. J Am Coll Cardiol 2017;69(13):1694–703. [DOI] [PubMed] [Google Scholar]
- [8].Bacharova L, Schocken D, Estes E, Strauss D. The role of ECG in the diagnosis of left ventricular hypertrophy. Curr Cardiol Rev 2014;10(3):257–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tavares CAM, Samesima N, Lazar Neto F, Hajjar LA, Godoy LC, Padrao EMH, et al. Usefulness of ECG criteria to rule out left ventricular hypertrophy in older individuals with true left bundle branch block: an observational study. BMC Cardiovasc Disord 2021;21(1):547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rodriguez-Padial L, Rodriguez-Picon B, Jerez-Valero M, Casares-Medrano J, Akerstrom FO, Calderon A, et al. Diagnostic accuracy of computer-assisted electrocardiography in the diagnosis of left ventricular hypertrophy in left bundle branch block. Rev Esp Cardiol (Engl Ed) 2012;65(1):38–46. [DOI] [PubMed] [Google Scholar]
- [11].Mehta A, Jain AC, Mehta MC, Billie M. Usefulness of left atrial abnormality for predicting left ventricular hypertrophy in the presence of left bundle branch block. Am J Cardiol 2000;85(3):354–9. [DOI] [PubMed] [Google Scholar]
- [12].Fragola PV, Autore C, Ruscitti G, Picelli A, Cannata D. Electrocardiographic diagnosis of left ventricular hypertrophy in the presence of left bundle branch block: a wasted effort. Int J Cardiol 1990;28(2):215–21. [DOI] [PubMed] [Google Scholar]
- [13].Klein RC, Vera Z, DeMaria AN, Mason DT. Electrocardiographic diagnosis of left ventricular hypertrophy in the presence of left bundle branch block. Am Heart J 1984;108(3 Pt 1):502–6. [DOI] [PubMed] [Google Scholar]
- [14].Kafka H, Burggraf GW, Milliken JA. Electrocardiographic diagnosis of left-ventricular hypertrophy in the presence of left-bundle branch-block - an echocardiographic study. Am J Cardiol 1985;55(1):103–6. [DOI] [PubMed] [Google Scholar]
- [15].Haskell RJ, Ginzton LE, Laks MM. Electrocardiographic diagnosis of left-ventricular hypertrophy in the presence of left-bundle branch-block. J Electrocardiol 1987;20(3):227–32. [DOI] [PubMed] [Google Scholar]
- [16].Noheria A, Sodhi S, Orme GJ. The evolving role of electrocardiography in cardiac resynchronization therapy. Curr Treat Options Cardiovasc Med 2019;21(12). [DOI] [PubMed] [Google Scholar]
- [17].Parimi NK, Mahmood U, Abudan A, Sami F, Lacy S, Harvey CJ, et al. Change in QRS 3D voltage time integral (3D QRS area) Predicts left ventricular Reverse remodeling in Sacubitril-valsartan treated patients with left bundle branch block. Circulation 2021;144(Suppl_1):A13007. [Google Scholar]
- [18].Morey T, Harvey CJ, Mahmood U, Parimi N, Lacy S, DeBauge A, et al. Change in QRS 3D voltage time integral (3D QRS area) with cardiac resynchronization therapy predicts subsequent left ventricular reverse remodeling and heart failure hospitalizations. J Am Coll Cardiol 2021;77(18_Supplement_1):359. [Google Scholar]
- [19].Lacy S, Parimi N, Morey T, Mahmood U, Harvey C, Sheldon S, et al. QRS 3D voltage time integral (3D QRS area) is a predictor for pacing induced cardiomyopathy. Circulation 2021;144(Suppl_1). A13592–A13592. [Google Scholar]
- [20].Mahmood U, Parimi N, Harvey CJ, Sheldon S, Reddy M, Noheria A. QRS 3D voltage time integral (3D QRS area) in cardiomyopathy and narrow QRS complex. J Am Coll Cardiol 2021;77(18_Supplement_1). 348–348. [Google Scholar]
- [21].van Stipdonk AMW, Ter Horst I, Kloosterman M, Engels EB, Rienstra M, Crijns H, et al. QRS area is a strong determinant of outcome in cardiac resynchronization therapy. Circ Arrhythm Electrophysiol 2018;11(12):e006497. [DOI] [PubMed] [Google Scholar]
- [22].Emerek K, Friedman DJ, Sorensen PL, Hansen SM, Larsen JM, Risum N, et al. Vectorcardiographic QRS area is associated with long-term outcome after cardiac resynchronization therapy. Heart Rhythm 2019;16(2):213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Okafor O, Zegard A, van Dam P, Stegemann B, Qiu T, Marshall H, et al. Changes in QRS area and QRS duration after cardiac resynchronization therapy predict cardiac mortality, heart failure hospitalizations, and ventricular arrhythmias. J Am Heart Assoc 2019;8(21):e013539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ghossein MA, van Stipdonk AMW, Plesinger F, Kloosterman M, Wouters PC, Salden OAE, et al. Reduction in the QRS area after cardiac resynchronization therapy is associated with survival and echocardiographic response. J Cardiovasc Electrophysiol 2021;32(3):813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kusumoto FM, Schoenfeld MH, Barrett C, Edgerton JR, Ellenbogen KA, Gold MR, et al. ACC/AHA/HRS guideline on the evaluation and Management of Patients with Bradycardia and Cardiac Conduction Delay: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the heart Rhyth. Circulation 2018;2019. 140(8). [DOI] [PubMed] [Google Scholar]
- [26].Das MK, Cheriparambil K, Bedi A, Kassotis J, D ES, Reddy CVR, et al. Prolonged QRS duration (QRS ≥170 ms) and left axis deviation in the presence of left bundle branch block: a marker of poor left ventricular systolic function? Am Heart J 2001; 142(5):756–9. [DOI] [PubMed] [Google Scholar]
- [27].Murphy SN, Weber G, Mendis M, Gainer V, Chueh HC, Churchill S, et al. Serving the enterprise and beyond with informatics for integrating biology and the bedside (i2b2). J Am Med Inform Assoc 2010;17(2):124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Waitman LR, Warren JJ, Manos EL, Connolly DW. Expressing observations from electronic medical record flowsheets in an i2b2 based clinical data repository to support research and quality improvement. AMIA Annu Symp Proc 2011;2011: 1454–63. [PMC free article] [PubMed] [Google Scholar]
- [29].Hancock EW, Deal BJ, Mirvis DM, Okin P, Kligfield P, Gettes LS. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram. Circulation 2009;119(10):e251–61. [DOI] [PubMed] [Google Scholar]
- [30].Kors JA, van Herpen G, Sittig AC, van Bemmel JH. Reconstruction of the frank vectorcardiogram from standard electrocardiographic leads: diagnostic comparison of different methods. Eur Heart J 1990;11:1083–92. [DOI] [PubMed] [Google Scholar]
- [31].Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28 (1):1–39.e14. [DOI] [PubMed] [Google Scholar]
- [32].Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986;57(6):450–8. [DOI] [PubMed] [Google Scholar]
- [33].Chinitz JS, Cooper JM, Verdino RJ. Electrocardiogram voltage discordance: interpretation of low QRS voltage only in the limb leads. J Electrocardiol 2008;41 (4):281–6. [DOI] [PubMed] [Google Scholar]
- [34].Bacharova L, Szathmary V, Mateasik A. Electrocardiographic patterns of left bundle-branch block caused by intraventricular conduction impairment in working myocardium: a model study. J Electrocardiol 2011;44(6):768–78. [DOI] [PubMed] [Google Scholar]
- [35].Bacharova L, de Luna B. The primary alteration of ventricular myocardium conduction: the significant determinant of left bundle branch block pattern. Cardiol Res Pract 2022;2022:3438603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


