Abstract
The results of earlier investigations that tested whether CD8+ T cells are required in the defense against Leishmania major have been inconsistent. We used CD8-deficient mice to directly address this issue. After primary infection with L. major, CD8-deficient mice controlled the infection for over 1 year and mounted strong T helper 1 cell responses. Thus, CD8+ T cells are not required for the long-term control of a primary infection with L. major.
T-cell immunity mediated by both CD4+ T cells and CD8+ T cells plays a crucial role in the host defense against many intracellular microbes. The immune response to the intracellular protozoan Leishmania major has been extensively studied in the mouse model of cutaneous leishmaniasis. Ample evidence has demonstrated the central role of CD4+ helper T (Th) cells in the control of this infection, including the findings that Th1 cells have been associated with cure and Th2 cells have been associated with progressive disease (18).
The role of CD8+ cells in cutaneous leishmaniasis is less well defined. Although CD8+ cells appear to be important for resistance to a secondary challenge with L. major (5, 15–17), their role in primary infection is not clear. By using antibody-mediated cell depletion (19), thymectomy plus cell depletion (8), or in vitro assays (4, 9), a number of studies have suggested a contribution of CD8+ cells to the control of primary leishmaniasis.
In contrast, experiments with β2-microglobulin-deficient (β2-m−/−) mice demonstrated the capacity of these mice to mount strong Th1 cell responses and to heal their primary infections with L. major (21). Because these mice carry no CD8+ T cells, such data suggested no functional requirement for these cells in the control of an L. major infection. However, β2-m−/− mice lack not only CD8+ T cells (24) but also a small population of CD4+ T cells expressing the NK1.1+ marker (NKT cells) (1, 2). NKT cells represent a unique lineage of cells capable of producing large amounts of interleukin 4 (IL-4) within hours after activation in vivo and are candidates for directing the immune response towards the Th2 cell type (22, 23). Consequently, the lack of these cells in β2-m−/− mice may influence the course of cutaneous leishmaniasis. Therefore, the interpretation of the importance of CD8+ cells for the course of L. major infection may be limited by the types of experiments done with these mice. To circumvent this difficulty, we used CD8-deficient mice (6), which are not deficient in NKT cells, to further analyze the role of CD8+ cells in the immunity to a primary infection with L. major.
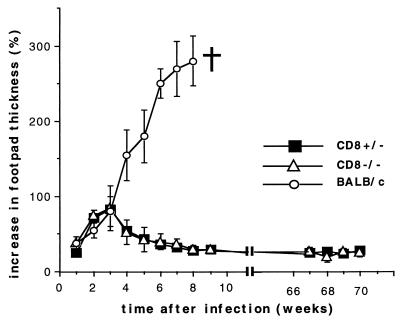
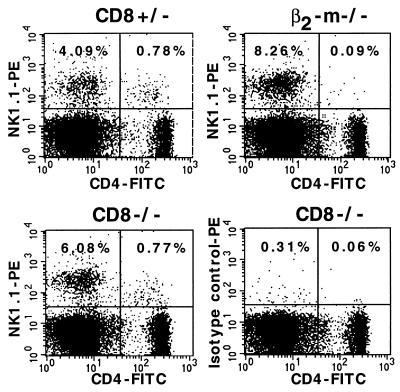
Mice which had a C57BL/6 genetic background (seventh backcross) and which were either heterozygous (CD8+/−) or homozygous (CD8−/−) for the disrupted CD8α gene were infected subcutaneously in the right hind footpad with 2 × 107 stationary-phase promastigotes of the L. major strain MHOM/IL/81/FEBNI (13) in 50 μl of buffer. The course of the infection was monitored by weekly measurements of the thicknesses of the infected and the contralateral uninfected footpads for 70 weeks. CD8+/− and CD8−/− mice controlled the long-term infection with no discernible difference (Fig. 1). A similar course of disease occurred in wild-type C57BL/6 mice with intact CD8α loci (data not shown). After the 70-week test period, the animals were killed. A fluorescence-activated cell sorter analysis of lymph node (LN) and spleen cells confirmed that CD8+ T cells were absent in CD8−/− mice and were present at normal levels in CD8+/− mice (data not shown). In contrast, the levels of NKT cells were comparable in CD8−/− and CD8+/− mice, while β2-m−/− mice contained no NKT cells, as expected (Fig. 2).
FIG. 1.
Time-dependent lesion development in CD8−/− and CD8+/− mice after infection with L. major. Mice were infected in the right hind footpad with L. major promastigotes. At the indicated time points, the thicknesses of the infected and the contralateral uninfected footpads were measured with a vernier caliper, and the increases in footpad thicknesses were calculated as described previously (13). The bars denote the standard deviations. Results shown are representative of two experiments involving four CD8−/− mice, three CD8+/− mice, and four susceptible control BALB/c mice.
FIG. 2.
Staining of NKT cells in the spleens of CD8−/−, CD8+/−, and β2-m−/− mice. Spleen cells were stained with fluorescein isothiocyanate (FITC)-labeled anti-CD4 and phycoerythrin (PE)-labeled anti-NK1.1 or isotype control phycoerythrin-labeled mouse immunoglobulin G2a antibodies (all from Pharmingen). NKT cells are represented in the upper right quadrant. The numbers represent the percentages relative to the total splenocyte population.
For direct quantification of the parasite burden in CD8+/− and CD8−/− mice, cultures were set up from the spleens and lesion-draining popliteal LNs 70 weeks after L. major infection. Tissues were homogenized, and serial dilutions (1:3) of single-cell suspensions obtained from individual mice were pipetted into 96-well flat-bottomed culture plates (24 wells per dilution), in medium generated according to a previously published formula (12). After 10 days, the growth of parasites was determined microscopically. In accordance with Poisson statistics (11), the cell dilution at which 37% of the wells were negative for parasite growth was taken to represent the original plating of one Leishmania organism. The number of parasites per LN or spleen was calculated by multiplying the frequency of parasites by the number of cells in each organ. The results are presented in Table 1. Consistent with their healed local lesions, CD8−/− mice carried small numbers of parasites within their LNs and spleens. These numbers were comparable to those obtained by measuring parasites carried in the respective organs of CD8+/− mice. Thus, CD8 deficiency had no effect on the clinical course of the infection with L. major and on the parasite burden in C57BL/6 mice.
TABLE 1.
Parasite burden in organs of CD8+/− and CD8−/− mice 70 weeks after infection with L. major
| Organ | CD8 genotype | No. of Leishmania parasites/organa |
|---|---|---|
| LN | +/− | 4.63 ± 0.95 |
| −/− | 3.75 ± 0.65 | |
| Spleen | +/− | <2 |
| −/− | <2 |
Parasite numbers in the popliteal LNs draining the infected footpads or in the spleens of CD8+/− and CD8−/− mice were determined 70 weeks after L. major infection by limiting dilution assays. Results are given as the logs of parasites (means ± standard deviations for three to four mice per group) in total LNs and spleens of infected animals. The absolute cell numbers of LNs and spleens were comparable in CD8+/− and CD8−/− mice. The data are representative of two separate experiments.
Previous studies have shown a correlation between the production of Th1 cytokines by T lymphocytes and the control of L. major infection. At the same time, the appearance of Th2 cytokines correlated with progressive disease (18). To assess the Th cell subset differentiation during an L. major infection in CD8-deficient mice, spleen and lesion-draining popliteal LN cells of CD8+/− and CD8−/− mice were analyzed 70 weeks after infection for cytokine production. Triplicate cultures of single-cell suspensions (2 × 105 cells/well) were incubated in 96-well plates together with uninfected, irradiated (20 Gy) syngeneic spleen cells (3 × 105/well) and with or without L. major antigens (freeze-thawed lysates of 3 × 105 promastigotes/well). After 48 h, culture supernatants were harvested and tested for IL-4 and gamma interferon (IFN-γ) by enzyme-linked immunosorbent assay with commercial antibody pairs (Pharmingen, San Diego, Calif.). The measured values were standardized with recombinant IL-4 and IFN-γ. The results (Table 2) show that cells from CD8−/− mice as well as from CD8+/− mice produced the typical low-IL-4 and high-IFN-γ profile characteristic of healer mice. The levels of IL-4 and IFN-γ were comparable in both CD8−/− and CD8+/− mice. These data demonstrate a strong Th1 cell response against L. major and the long-term maintenance of this response in CD8-deficient mice.
TABLE 2.
Cytokine production by total LN and spleen cells of CD8+/− and CD8−/− mice 70 weeks after infection with L. majora
| Type of cell | Genotype | Stimulus | Amt of IL-4 in culture supernatant (ng/ml) | Amt of IFN-γ in culture supernatant (ng/ml) |
|---|---|---|---|---|
| LN | CD8+/− | None | <0.05 | <0.05 |
| LmAg | <0.05 | 13 ± 7.3 | ||
| CD8−/− | None | <0.05 | <0.05 | |
| LmAg | <0.05 | 27 ± 18 | ||
| Spleen | CD8+/− | None | <0.05 | <0.05 |
| LmAg | 0.35 ± 0.06 | 21 ± 6.5 | ||
| CD8−/− | None | <0.05 | <0.05 | |
| LmAg | 0.56 ± 0.09 | 15 ± 2 |
Amounts of IFN-γ and IL-4 secreted in response to L. major antigens (LmAg) (freeze-thawed lysates of 3 × 105 promastigotes/well) in the presence of irradiated (20 Gy) syngeneic spleen cells (3 × 105/well) by total popliteal LN and spleen cells (2 × 105 cells/well) of CD8+/− and CD8−/− mice were determined 70 weeks after infection with L. major. Results are representative of two separate experiments and give the means of three to four individually tested mice per group ± standard deviations.
A number of reports have suggested a role for CD8+ cells in mediating successful immunity against a primary infection with L. major in mice. For example, it has been shown that antibody-mediated depletion of CD8+ cells resulted in increased footpad swelling and parasite burden in genetically resistant and susceptible mice (19). Further, there have been several in vitro analyses of the capacity of CD8+ cells isolated from mice after primary infection to be activated by or to lyse Leishmania-infected macrophages. The conclusions of these in vitro studies were inconsistent. Some reports showed that CD8+ cells derived from mice with resolved primary L. major infections efficiently lysed sensitized syngeneic macrophages (4, 9). In contrast, other reports failed to demonstrate lytic activity of CD8+ cells against macrophages infected with Leishmania (3, 7, 14). In accordance with these negative results, the experiments with β2-m−/− mice suggested no functional requirement for CD8+ cells in the control of an L. major infection (21). Our results show that L. major-infected CD8-deficient mice developed a typical healing Th1 cell response with small, transient footpad lesions: even after long-term infection, small numbers of parasites were detectable in the LNs and spleens of infected animals, while LN cells and spleen cells produced large amounts of IFN-γ after antigen-specific stimulation in vitro. Therefore, CD8+ T cells are not required for the long-term maintenance of a Th1-cell-mediated immunologic control of an infection with L. major. These findings extend the results reported for L. major-infected β2-m−/− mice by demonstrating that even in the presence of NKT cells, which could provide IL-4 for Th2 cell differentiation, CD8+ cells are not decisive in the maintenance and control of Th1 cell immunity to L. major in C57BL/6 mice. Our results are also consistent with those of von der Weid et al. (20), who found that, in BALB/c mice, the presence or lack of NKT cells does not affect the generation of Th2 cell responses against L. major. Accordingly, it has been shown by Launois et al. (10) that NK1.1− CD4+ T cells rather than NKT cells are responsible for the early burst of L. major-induced IL-4 mRNA expression in BALB/c mice.
Acknowledgments
We acknowledge the skillful assistance of Susanne Bischof and of Claudia Gießler at our institute.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (SFB 263) and by the Johannes und Frieda Marohn Stiftung, Erlangen, Germany.
REFERENCES
- 1.Bendelac A, Killeen N, Littman D L, Schwartz R H. A subset of CD4+ thymocytes selected by MHC class I molecules. Science. 1994;263:1774–1778. doi: 10.1126/science.7907820. [DOI] [PubMed] [Google Scholar]
- 2.Coles M C, Raulet D H. Class I dependence of development of CD4+ CD8− NK1.1+ thymocytes. J Exp Med. 1994;180:395–399. doi: 10.1084/jem.180.1.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coutinho S G, Louis J A, Mauel J, Engers H D. Induction by specific T lymphocytes of intracellular destruction of Leishmania major in infected murine macrophages. Parasite Immunol. 1984;6:157–170. doi: 10.1111/j.1365-3024.1984.tb00789.x. [DOI] [PubMed] [Google Scholar]
- 4.da Conceicao-Silva F, Perlaza B L, Louis J A, Romero P. Leishmania major infection in mice primes for specific major histocompatibility complex class I-restricted CD8+ cytotoxic T cell responses. Eur J Immunol. 1994;24:2813–2817. doi: 10.1002/eji.1830241135. [DOI] [PubMed] [Google Scholar]
- 5.Farell J P, Müller I, Louis J A. A role for Lyt-2+ T cells in resistance to cutaneous leishmaniasis in immunized mice. J Immunol. 1989;142:2052–2056. [PubMed] [Google Scholar]
- 6.Fung-Leung W-P, Schilham M W, Rahemtulla A, Kündig T M, Vollenweider M, Potter J, van Ewijk W, Mak T W. CD8 is needed for development of cytotoxic T cells but not helper T cells. Cell. 1991;65:443–449. doi: 10.1016/0092-8674(91)90462-8. [DOI] [PubMed] [Google Scholar]
- 7.Garcia M R, Graham S, Harris R A, Beverley S M, Kaye P M. Epitope cleavage by Leishmania endopeptidase(s) limits the efficiency of the exogenous pathway of major histocompatibility complex class I-associated antigen presentation. Eur J Immunol. 1997;27:1005–1013. doi: 10.1002/eji.1830270430. [DOI] [PubMed] [Google Scholar]
- 8.Hill J O, Awwad M, North R J. Elimination of CD4+ suppressor T cells from susceptible BALB/c mice releases CD8+ T lymphocytes to mediate protective immunity against Leishmania. J Exp Med. 1989;169:1819–1827. doi: 10.1084/jem.169.5.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kima P E, Ruddle N H, McMahon-Pratt D. Presentation via the class I pathway by Leishmania amazonensis-infected macrophages of an endogenous leishmanial antigen to CD8+ T cells. J Immunol. 1997;159:1828–1834. [PubMed] [Google Scholar]
- 10.Launois P, Ohteki T, Swihart K, MacDonald H R, Louis J A. In susceptible mice, Leishmania major induce very rapid interleukin-4 production by CD4+ cells which are NK1.1−. Eur J Immunol. 1995;25:3298–3307. doi: 10.1002/eji.1830251215. [DOI] [PubMed] [Google Scholar]
- 11.Lefkovits I, Waldmann H. Limiting dilution analysis of the cells of immune system I. The clonal basis of the immune response. Immunol Today. 1984;5:265–268. doi: 10.1016/0167-5699(84)90137-3. [DOI] [PubMed] [Google Scholar]
- 12.Lima H C, Bleyenberg J A, Titus R G. A simple method for quantifying Leishmania in tissues of infected animals. Parasitol Today. 1997;13:80–82. doi: 10.1016/s0169-4758(96)40010-2. [DOI] [PubMed] [Google Scholar]
- 13.Lohoff M, Ferrick D, Mittrücker H-W, Duncan G S, Bischof S, Röllinghoff M, Mak T W. Interferon regulatory factor-1 is required for a T helper 1 immune response in vivo. Immunity. 1997;6:681–689. doi: 10.1016/s1074-7613(00)80444-6. [DOI] [PubMed] [Google Scholar]
- 14.Lopez J A, LeBowitz J H, Beverley S M, Rammensee H-G, Overath P. Leishmania mexicana promastigotes induce cytotoxic T lymphocytes in vivo that do not recognize infected macrophages. Eur J Immunol. 1993;23:217–223. doi: 10.1002/eji.1830230134. [DOI] [PubMed] [Google Scholar]
- 15.Müller I. Role of T cell subsets during the recall of immunologic memory to Leishmania major. Eur J Immunol. 1992;22:3063–3069. doi: 10.1002/eji.1830221206. [DOI] [PubMed] [Google Scholar]
- 16.Müller I, Kropf P, Etges R J, Louis J A. Gamma interferon response in secondary Leishmania major infection: role of CD8+ T cells. Infect Immun. 1993;61:3730–3738. doi: 10.1128/iai.61.9.3730-3738.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müller I, Kropf P, Louis J A, Milon G. Expansion of gamma interferon-producing CD8+ T cells following secondary infection of mice immune to Leishmania major. Infect Immun. 1994;62:2575–2581. doi: 10.1128/iai.62.6.2575-2581.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reiner S L, Locksley R M. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 19.Titus R G, Milon G, Marchal G, Vassalli P, Cerottini J-C, Louis J A. Involvement of specific Lyt-2+ T cells in the immunological control of experimentally induced murine cutaneous leishmaniasis. Eur J Immunol. 1987;17:1429–1433. doi: 10.1002/eji.1830171007. [DOI] [PubMed] [Google Scholar]
- 20.von der Weid T, Beebe A M, Roopenian D C, Coffman R L. Early production of IL-4 and induction of Th2 responses in the lymph node originate from an MHC class I-independent CD4+ NK1.1− T cell population. J Immunol. 1996;157:4421–4427. [PubMed] [Google Scholar]
- 21.Wang Z-E, Reiner S L, Hatam F, Heinzel F P, Bouvier J, Turck C W, Locksley R M. Targeted activation of CD8 cells and infection of β2-microglobulin-deficient mice fail to confirm a primary protective role for CD8 cells in experimental leishmaniasis. J Immunol. 1993;151:2077–2086. [PubMed] [Google Scholar]
- 22.Yoshimoto T, Bendelac A, Watson C, Hu-Li J, Paul W E. Role of NK1.1+ T cells in a Th2 response and in immunoglobulin E production. Science. 1995;270:1845–1847. doi: 10.1126/science.270.5243.1845. [DOI] [PubMed] [Google Scholar]
- 23.Yoshimoto T, Paul W E. CD4pos., NK1.1pos. T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J Exp Med. 1994;179:1285–1295. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zijlstra M, Bix M, Simister N E, Loring J M, Raulet D H, Jaenisch R. β2-microglobulin deficient mice lack CD4−CD8+ cytolytic T cells. Nature. 1990;344:742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]