Abstract
Objective:
Contingency management (CM) is the gold standard treatment for stimulant use disorder but typically requires twice- to thrice-weekly in-person treatment visits to objectively verify abstinence and deliver therapeutic incentives. There has been growing interest in telehealth-based delivery of CM to support broad access to this essential intervention—a need that has been emphatically underscored by the COVID-19 pandemic. Herein, we present observations from initial efforts to develop and test a protocol for telehealth-based delivery of prize-based CM treatment incentivizing stimulant abstinence.
Method:
Four participants engaged in hybrid courses of CM, including one or more telehealth-based treatment sessions, involving self-administered oral fluid testing to confirm abstinence. Observations from initial participants informed iterative improvements to telehealth procedures, and a 12-week course of telehealth-based CM was subsequently offered to two additional participants to further evaluate preliminary feasibility and acceptability.
Results:
In most cases, participants were able to successfully join telehealth treatment sessions, self-administer oral fluid testing, and share oral fluid test results to verify stimulant abstinence. However, further improvements in telehealth-based toxicology testing may be necessary to interpret test results accurately and reliably, especially when colorimetric immunoassay results reflect substance concentrations near the cutoff for point-of-care testing devices.
Conclusions:
Preliminary findings suggest that telehealth-based CM is sufficiently feasible and acceptable to support future development, in particular through improved methods for remote interpretation and verification of test results. This is especially important in CM, wherein accurate and reliable detection of both early and sustained abstinence is crucial for appropriate delivery of therapeutic incentives.
Contingency management (CM) is an evidence-based behavioral intervention for substance use disorders, providing time-limited incentives to reinforce abstinence and treatment engagement. Although broadly effective to support behavior change, CM is a particularly crucial intervention for individuals with stimulant use disorder given limited pharmacotherapy options (Chan et al., 2018) and abundant empirical support (Benishek et al., 2014; Prendergast et al., 2006). In the United States, a nationwide implementation effort within the Veterans Health Administration serves as a model for expanding access to CM (Khazanov et al., 2022), and California has recently attained coverage through Medicaid.
Improving access to CM has become a growing priority with the increasing prevalence of stimulant use and stimulant-related overdose deaths—issues further catalyzed by circumstances presented by the COVID-19 pandemic (Ciccarone, 2021). Negative impacts of the pandemic include factors that broadly contribute to substance use such as social isolation, psychiatric symptoms, and reduced access to treatment. Concerning stimulants specifically, pandemic-related changes in drug supply chains may also contribute to co-occurring use of stimulants and opioids, as well as adulteration of stimulants with fentanyl and fentanyl analogs (Park et al., 2021), thus increasing overdose risk.
Unfortunately, as the pandemic brought a new landscape of need into focus, it also highlighted barriers to substance use treatment access. Most notably, disruptions to in-person treatment imposed by infection prevention precautions have prompted the historic ascendance of telehealth, including mental health and substance use treatment services (Cantor et al., 2022; Doraiswamy et al., 2020; Zangani et al., 2022), but some interventions pose unique challenges. Specific medications (e.g., depot formulations, opioid agonists) required ongoing in-person administration or supervised dosing throughout the pandemic (Dunlop et al., 2020; Forster et al., 2022). Likewise, requirements of some psychological interventions, including CM, have also posed unique problems for delivery via telehealth (Moring et al., 2020; Wells et al., 2020; Zastepa et al., 2020). Although telehealth can potentially expand access to CM, issues including the validity and confidentiality of remotely administered drug testing are clear concerns.
CM typically requires in-person treatment sessions to (a) objectively verify abstinence (e.g., via urine toxicology testing) and (b) deliver therapeutic incentives. To address these considerations, recommendations for virtual CM during the pandemic suggested reinforcing treatment attendance rather than abstinence and delivering incentives via prepaid debit cards (Zastepa et al., 2020). Technologies to support remote verification of abstinence also have the promise to expand options for virtual CM. Although previous work in this domain has primarily targeted alcohol abstinence and smoking cessation (Coughlin et al., 2023; Dallery et al., 2023; Kurti et al., 2016; McPherson et al., 2018), technology-enhanced virtual CM appears to be quite effective and has recently been extended to target illicit drug use in alcohol and opioid use disorders (DeFulio et al., 2021; Hammond et al., 2021). For example, the DynamiCare Health smartphone app (DynamiCare Health, Boston, MA) was recently used to provide incentives for alcohol and drug abstinence verified via digitally submitted breath alcohol analysis and oral fluid test results, with the latter represented by videos of self-administered testing, reviewed and interpreted by DynamiCare staff (Hammond et al., 2021). However, no previous work has used oral fluid testing to deliver CM targeting stimulant abstinence through live telehealth interactions.
Here, we aim to develop and test a protocol for virtual prize-based contingency management (PBCM) targeting stimulant abstinence using accessible, existing technologies. We specifically considered the feasibility of patient-self-administered “point-of-care” oral fluid testing devices (OFT-Ds) to verify stimulant abstinence in treatment sessions delivered via clinical video teleconferencing. This case series highlights successes and challenges while specifically addressing (a) logistical and technical considerations and (b) acceptability of telehealth-based CM, including example contexts in which this option may be preferred.
Method
Participants and setting
Individuals referred for CM targeting stimulant abstinence at Veterans Affairs (VA) Pittsburgh Healthcare System (VAPHS) were informed of the project upon disclosing barriers to in-person attendance. All participants were male Veterans of the United States Army with cocaine use disorder (Table 1). Each participant provided written informed consent to participate in the institutional review board–approved protocol.
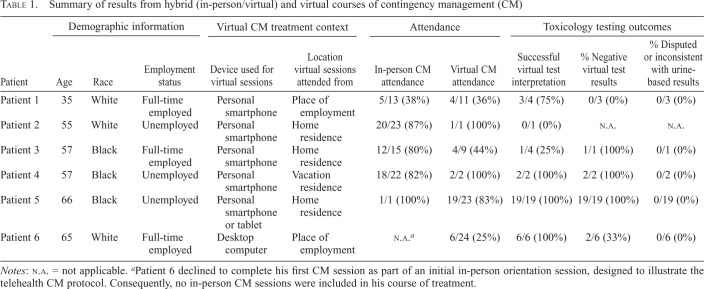
Table 1.
Summary of results from hybrid (in-person/virtual) and virtual courses of contingency management (CM)
| Patient | Demographic information | Virtual CM treatment context | Attendance | Toxicology testing outcomes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | Race | Employment status | Device used for virtual sessions | Location virtual sessions attended from | In-person CM attendance | Virtual CM attendance | Successful virtual test interpretation | % Negative virtual test results | % Disputed or inconsistent with urine-based results | |
| Patient 1 | 35 | White | Full-time employed | Personal smartphone | Place of employment | 5/13 (38%) | 4/11 (36%) | 3/4 (75%) | 0/3 (0%) | 0/3 (0%) |
| Patient 2 | 55 | White | Unemployed | Personal smartphone | Home residence | 20/23 (87%) | 1/1 (100%) | 0/1 (0%) | N.A. | N.A. |
| Patient 3 | 57 | Black | Full-time employed | Personal smartphone | Home residence | 12/15 (80%) | 4/9 (44%) | 1/4 (25%) | 1/1 (100%) | 0/1 (0%) |
| Patient 4 | 57 | Black | Unemployed | Personal smartphone | Vacation residence | 18/22 (82%) | 2/2 (100%) | 2/2 (100%) | 2/2 (100%) | 0/2 (0%) |
| Patient 5 | 66 | Black | Unemployed | Personal smartphone or tablet | Home residence | 1/1 (100%) | 19/23 (83%) | 19/19 (100%) | 19/19 (100%) | 0/19 (0%) |
| Patient 6 | 65 | White | Full-time employed | Desktop computer | Place of employment | N.A. a | 6/24 (25%) | 6/6 (100%) | 2/6 (33%) | 0/6 (0%) |
Notes: N.A. = not applicable.
Patient 6 declined to complete his first CM session as part of an initial in-person orientation session, designed to illustrate the telehealth CM protocol. Consequently, no in-person CM sessions were included in his course of treatment.
Contingency management treatment program
PBCM is offered through VAPHS as part of a national implementation effort, in accordance with the protocol described by DePhilippis et al. (2018). The program typically involves twice-weekly, in-person sessions over 12 weeks, offering an escalating schedule of probabilistic rewards, determined by drawing from a fishbowl. Prize draws are awarded for objectively verified abstinence from targeted substances, typically confirmed through urine-based testing. Prizes are Veterans Canteen Service (VCS) vouchers.
Development and evaluation of telehealth-based contingency management
Four Veterans participated in a hybrid course of CM, including one or more telehealth sessions. Initial efforts focused on identifying and troubleshooting barriers to delivery of CM via telehealth and exploring contexts in which telehealth CM was a desirable treatment option. Observations from initial participants subsequently informed development of a fully remote telehealth-based CM protocol, which was offered to two Veterans who were unable to attend in-person appointments. Evidence of feasibility was provided by successful execution of telehealth treatment sessions, and patient willingness to participate in telehealth sessions was considered to demonstrate acceptability.
Telehealth sessions were conducted via the HIPAA-compliant VA Video Connect (VVC) platform, using standard procedures to ensure safety, privacy, and information security. Telehealth-based toxicology testing was conducted using Oratect 6-Panel OFT-Ds (Branan Medical Corporation, Irvine, CA), remotely self-administered by the patient with guidance from the telehealth provider. Each OFT-D provided colorimetric results for targeted stimulants (cocaine, amphetamine, methamphetamine) and three nontargeted substances (tetrahydrocannabinol, benzodiazepines, opiates). Although oral fluids generally support a shorter detection time than urine, the advertised detection window for OFT targeting stimulants was comparable (0–72 hours) to that for point-of-care urine testing (2–72 hours).
For hybrid courses, OFT-Ds were provided at the time of the preceding in-person session. For fully remote courses of treatment, OFT-Ds for all sessions were provided on initiation of treatment. In each case, one to three surplus OFT-Ds were provided to account for potential device failures. Veterans independently managed testing supplies and were engaged in a discussion of potential privacy and confidentiality concerns regarding storage, use, and disposal during the informed consent process.
Participants shared results by positioning the OFT-D in front of the camera used for synchronous video during the session or transmitting a digital image via the VVC platform. When applicable, prize draws were conducted by (a) the telehealth provider drawing from a fishbowl or (b) the participant choosing numbers corresponding to entries in a spreadsheet that were randomly reshuffled to reveal prize outcomes (i.e., the “electronic fishbowl”; DePhilippis & Motoyama, 2020).
Results
Indications for telehealth contingency management
Patients 1 and 2 were scheduled for one or more weekly in-person clinic visits for medication management targeting opioid use disorder and expressed interest in attending weekly telehealth CM sessions in combination with in-person sessions (coinciding with medication-related clinic visits) to limit work absences (Patient 1) and transportation burden (Patient 2). Patient 3 initiated a course of in-person CM but elected to switch to twice-weekly telehealth-based sessions for Weeks 8–12 after new employment precluded further in-person attendance. Patient 4 similarly initiated a course of in-person CM but sought to participate in CM Sessions 21 and 22 (of 24) via telehealth because of out-of-town travel. Participants engaged in fully remote courses of treatment (Patients 5 and 6) reported roundtrip travel time exceeding 1.5 hours, lacked a vehicle for transportation, and did not have viable public transportation options. Patient 6 also reported that his work schedule precluded twice-weekly in-person attendance.
Telehealth treatment context, attendance, and toxicology testing outcomes
A case-by-case summary of hybrid (Patients 1–4) and fully remote (Patients 5 and 6) courses of treatment is provided in Table 1. Two participants elected to attend telehealth CM sessions from their place of employment, three attended from home, and one attended from a vacation residence. Participants attending from work connected to sessions from a private office or vehicle to ensure privacy and confidentiality. Although attendance was variable, participants in hybrid courses generally exhibited similar attendance rates for in-person and telehealth CM sessions. Patient 3 exhibited a decline in attendance after transitioning from in-person (80% attended) to telehealth sessions (44% attended), but this also corresponded with resumption of cocaine use in the context of new employment. Patient 5 attended all but four telehealth sessions in his fully remote course of treatment. One session was an excused absence, and Sessions 22–24 were declined because the patient lost access to a private, internet-enabled space to attend from at the transitional housing facility where he was residing. Patient 6 attended six telehealth sessions over the first month of treatment but was subsequently lost to follow-up. Rates of successful OFT-D interpretation and negative results were also variable, ranging from 0% to 100%. Importantly, 100% successful OFT-D interpretation was achieved for patients in fully remote courses of treatment, after integrating improvements from earlier hybrid courses. There were no instances of disputed OFT-D results or inconsistencies between OFT-D results and urine-based testing. For hybrid courses, urine-based testing was conducted at the time of in-person sessions (Patients 1–4), as well as through a transitional housing program in the case of Patient 3. Participants in fully remote courses of CM were also subject to drug–alcohol urinalysis through their housing (Patient 5) or local VA community-based outpatient clinic (Patient 6), thus providing a secondary means of objectively verifying abstinence during treatment.
Prize determination and delivery
Patients 3–6 achieved negative OFT-D results during telehealth sessions for which prize draws were awarded and Patients 4–6 won prize vouchers. For Patient 4 (hybrid course), winnings were distributed at the next in-person treatment session. To accommodate prompt transmission of prizes to Patient 5 (fully remote course), prize vouchers were transferred to the participant's VA-affiliated housing facility and released upon each win. Efforts were also made to arrange for release of prize vouchers to Patient 6 via his local VA community-based outpatient clinic but were unsuccessful. Consequently, vouchers were mailed following each win.
Problems encountered and modifications introduced
During initial hybrid courses, OFT-D results could not be obtained and/or interpreted in 5/11 (45%) of cases. In three such instances, the participant was unable to connect via VVC and unwilling to reschedule. In a fourth instance, a participant declined to self-administer OFT because of recent cocaine use and an expected presumptive positive result. In a final instance, Patient 2 was unable to clearly display his OFT-D via synchronous video using his front-facing smartphone camera and was unable to switch to his rear-facing camera to capture a higher resolution image (reporting that damage to his smartphone touchscreen prevented necessary interface interactivity). An initial in-person orientation session was subsequently developed to proactively identify and troubleshoot technical issues. During this session, participants were asked to bring devices they planned to use for telehealth treatment visits (if possible) and complete all necessary activities (e.g., connecting via VVC, self-administering OFT, displaying OFT results) while the telehealth provider was available to offer onsite technical support and guidance. Tips for successful visualization of OFT results (e.g., use of rear-versus front-facing camera for improved image resolution, displaying the OFT on a white background to improve contrast) were also shared during this initial session and reviewed, as needed, during subsequent telehealth sessions. Of note, Patient 6 declined to take part in Session 1 at the time of his in-person orientation visit and repeatedly experienced significant difficulty presenting OFT-D results for interpretation, which appeared to reduce his satisfaction and confidence in objective testing methods. These difficulties likely reflected (a) presentation via webcam synchronous video and (b) less robust colorimetric findings because of recent cocaine use.
Discussion
This case series supports the feasibility and acceptability of telehealth-based CM using OFT-Ds for objective verification of stimulant abstinence with some caveats. All patients offered telehealth-based CM were agreeable to this option as a solution to self-identified barriers to in-person attendance, including transportation issues and employment-related scheduling restrictions. Patients were able to self-administer OFT-Ds with guidance from the provider, and no privacy or confidentiality concerns were voiced related to the storage, use, or disposal of OFT supplies. Occasional problems connecting via the VVC platform could generally be resolved but occasionally required session cancellation and/or rescheduling. Technical problems and limitations related to review of OFT-D results were also noted but rarely prevented session delivery. These preliminary findings serve to encourage future research supporting development of telehealth-based CM.
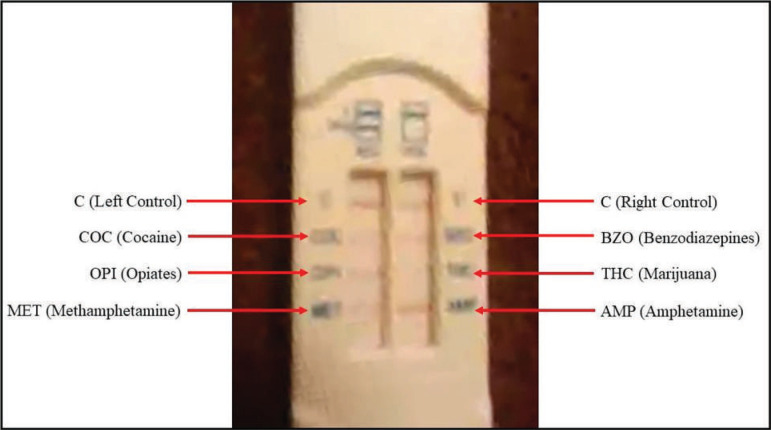
Initial test cases informed the design of an in-person orientation with the patient's preferred telehealth device(s) to (a) proactively identify and troubleshoot device-specific issues and (b) practice steps for connecting to sessions and sharing OFT-D results. However, successful interpretation of colorimetric results via telehealth (see Figure 1 for example) may still prove challenging when environmental (e.g., lighting), technical (e.g., camera resolution), and patient-related (e.g., recency of use) conditions are less favorable. Under such conditions, visualization and interpretation of OFT-Ds often require multiple attempts, which may cause frustration and erode confidence in testing. Despite these challenges, 100% of OFT results were consistent with patient self-report and urine-based testing results. External devices that standardize visualization conditions with respect to lighting, resolution, and image stability, as well as computer vision algorithms for colorimetric result classification (Kim et al., 2017), may further improve the accuracy, reliability, and convenience of toxicology testing via telehealth in the future.
Figure 1.
Screen capture of an oral fluid test result transmitted by Patient 5 via synchronous video. Despite somewhat poor resolution, it was possible to interpret this image as depicting a visible test line for all targeted stimulants, indicating a presumptive negative result for cocaine, methamphetamine, and amphetamine. Patient 5 was also subject to weekly drug–alcohol urinalysis and consistently tested negative for all substances, including tetrahydrocannabinol (THC), throughout the course of treatment. Based on both patient self-report and secondary objective testing, it is noted that a test line for THC should be visible (indicating a presumptive negative result) but is difficult to detect in this image.
Optimizing OFT-Ds and virtual testing protocols for CM also requires consideration of test performance characteristics. Although shorter detection intervals afforded by OFT versus urine testing provide opportunities to reliably detect (and reward) early evidence of abstinence, individual excretion patterns for stimulants may vary considerably by amount and frequency of use (Jufer et al., 2006). Additional empirical research is needed to determine ideal test characteristics and testing schedules (e.g., frequency, predictability) to reliably and accurately detect evidence of substance use and abstinence via OFT and remote visualization methods. Future research is also needed to examine potential benefits of hybrid and fully remote CM protocols with respect to treatment attendance and abstinence outcomes. Although it is possible that improved attendance will be achieved by integrating telehealth-based treatment options in accordance with patient preference (or other factors), such effects require investigation in a large, representative sample. Telehealth-based treatment options also have potential to benefit outcomes by supporting continuity of care and consistent delivery of CM at an evidence-based “dose” and should be examined in future pragmatic trials.
Timely delivery of CM rewards is also important to treatment effectiveness (Lussier et al., 2006). Although we considered several mechanisms for delivering rewards from telehealth-based treatment sessions (e.g., remote release by local VA affiliate, mail-based delivery), options were limited by the available reward modality (i.e., VCS vouchers). As described elsewhere (Khazanov et al., 2022; Zastepa et al., 2020), remotely allocating funds to debit or gift cards would further minimize delays in reward transmission while also expanding options for reward redemption. Preferences for reward delivery were not specifically assessed herein and would ideally be considered in relation to possible treatment modalities including in-person, hybrid, and fully remote courses of treatment.
Conclusion
Telehealth-based CM has excellent potential to expand access to this important treatment option. Observations from this case series suggest that telehealth-based CM targeting stimulant abstinence is feasible and acceptable. However, future research is needed to optimize methods for virtual interpretation of patient-self-administered OFT-Ds and clarify opportunities to improve treatment access and outcomes by integrating telehealth options.
Conflict-of-Interest Statement
The authors have no conflicts of interest to disclose.
Acknowledgments
The authors thank the VA Pittsburgh Healthcare System Behavioral Health Service Line for supporting the larger research effort of which the current work is a part.
Footnotes
This work was supported by funding from IK2 CX001807/CX/CSRD VA (principal investigator: Sarah E. Forster). The contents of this article do not represent the views of the Department of Veterans Affairs, Department of Defense, or the U.S. Government.
References
- Benishek L. A., Dugosh K. L., Kirby K. C., Matejkowski J., Clements N. T., Seymour B. L., Festinger D. S. Prize-based contingency management for the treatment of substance abusers: A meta-analysis. Addiction. 2014;109(9):1426–1436. doi: 10.1111/add.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor J., McBain R. K., Kofner A., Hanson R., Stein B. D., Yu H. Telehealth adoption by mental health and substance use disorder treatment facilities in the COVID-19 pandemic. Psychiatric Services. 2022;73(4):411–417. doi: 10.1176/appi.ps.202100191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan B., Kondo K., Ayers C., Freeman M., Montgomery J., Paynter R., Kansagara D. VA evidence-based synthesis program reports. Washington, DC: Department of Veterans Affairs; 2018. Pharmacotherapy for stimulant use disorders: A systematic review. [PubMed] [Google Scholar]
- Ciccarone D. The rise of illicit fentanyls, stimulants and the fourth wave of the opioid overdose crisis. Current Opinion in Psychiatry. 2021;34(4):344–350. doi: 10.1097/YCO.0000000000000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin L. N., Salino S., Jennings C., Lacek M., Townsend W., Koffarnus M. N., Bonar E. E. A systematic review of remotely delivered contingency management treatment for substance use. Journal of Substance Use and Addiction Treatment. 2023;147:208977. doi: 10.1016/j.josat.2023.208977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallery J., Ives L., Knerr A. Toward an era of impact of digital contingency management in the treatment of substance use disorders. Policy Insights from the Behavioral and Brain Sciences. 2023;10(1):107518. doi: 10.1016/j.ypmed.2023.107518. [DOI] [PubMed] [Google Scholar]
- DeFulio A., Rzeszutek M. J., Furgeson J., Ryan S., Rezania S. A smartphone-smartcard platform for contingency management in an inner-city substance use disorder outpatient program. Journal of Substance Abuse Treatment. 2021;120:108188. doi: 10.1016/j.jsat.2020.108188. [DOI] [PubMed] [Google Scholar]
- DePhilippis D., Petry N. M., Bonn-Miller M. O., Rosenbach S. B., McKay J. R. The national implementation of contingency management (CM) in the Department of Veterans Affairs: Attendance at CM sessions and substance use outcomes. Drug and Alcohol Dependence. 2018;185:367–373. doi: 10.1016/j.drugalcdep.2017.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doraiswamy S., Abraham A., Mamtani R., Cheema S. Use of telehealth during the COVID-19 pandemic: Scoping review. Journal of Medical Internet Research. 2020;22(12):e24087. doi: 10.2196/24087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop A., Lokuge B., Masters D., Sequeira M., Saul P., Dunlop G., Ryan J., Hall M., Ezard N., Habor P., Lintzeris N., Maher L. Challenges in maintaining treatment services for people who use drugs during the COVID-19 pandemic. Harm Reduction Journal. 2020;17(1) doi: 10.1186/s12954-020-00370-7. Article no. 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster S. E., Gancz N. N., Gaither M. L., Haas G. L., Starver K. D., Steinhauer S. R. Barriers to long-acting injectable antipsychotic adherence during the COVID-19 pandemic: Observations from one site. Journal of Psychiatric Practice. 2022;28(6):497–504. doi: 10.1097/PRA.0000000000000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond A. S., Sweeney M. M., Chikosi T. U., Stitzer M. L. Digital delivery of a contingency management intervention for substance use disorder: A feasibility study with DynamiCare Health. Journal of Substance Abuse Treatment. 2021;126:108425. doi: 10.1016/j.jsat.2021.108425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jufer R., Walsh S. L., Cone E. J., Sampson-Cone A. Effect of repeated cocaine administration on detection times in oral fluid and urine. Journal of Analytical Toxicology. 2006;30(7):458–462. doi: 10.1093/jat/30.7.458. [DOI] [PubMed] [Google Scholar]
- Khazanov G. K., Forster S. E., DePhilippis D., McKay J. R. Increasing the impact of interventions incentivizing psychiatric treatment engagement: Challenges and opportunities. Psychiatric Services. 2022;73(5):580–583. doi: 10.1176/appi.ps.202100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Awofeso O., Choi S., Jung Y., Bae E. Colorimetric analysis of saliva–alcohol test strips by smartphone-based instruments using machine-learning algorithms. Applied Optics. 2017;56(1):84–92. doi: 10.1364/AO.56.000084. [DOI] [Google Scholar]
- Kurti A. N., Davis D., Redner R., Jarvis B., Zvorsky I., Keith D. R., Bolivar H., White T. J., Rippberger P., Markeish C., Atwood G., Higgins S. T. A review of the literature on remote monitoring technology in incentive-based interventions for health-related behavior change. Translational Issues in Psychological Science. 2016;2(2):128–152. doi: 10.1037/tps0000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier J. P., Heil S. H., Mongeon J. A., Badger G. J., Higgins S. T. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101(2):192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- McPherson S. M., Burduli E., Smith C. L., Herron J., Oluwoye O., Hirchak K., Orr M. E., McDonell M. G., Roll J. M. A review of contingency management for the treatment of substance-use disorders: Adaptation for underserved populations, use of experimental technologies, and personalized optimization strategies. Substance Abuse and Rehabilitation. 2018;9:43–57. doi: 10.2147/SAR.S138439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moring J. C., Dondanville K. A., Fina B. A., Hassija C., Chard K., Monson C., LoSavio S., Wells S. Y., Morland L. A., Kaysen D., Galovski T. E., Resick P. A. Cognitive processing therapy for post-traumatic stress disorder via telehealth: Practical considerations during the COVID-19 pandemic. Journal of Traumatic Stress. 2020;33(4):371–379. doi: 10.1002/jts.22544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. N., Rashidi E., Foti K., Zoorob M., Sherman S., Alexander G. C. Fentanyl and fentanyl analogs in the illicit stimulant supply: Results from U.S. drug seizure data, 2011–2016. Drug and Alcohol Dependence. 2021;218:108416. doi: 10.1016/j.drugalcdep.2020.108416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast M., Podus D., Finney J., Greenwell L., Roll J. Contingency management for treatment of substance use disorders: A meta-analysis. Addiction. 2006;101(11):1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Wells S. Y., Morland L. A., Wilhite E. R., Grubbs K. M., Rauch S. A. M., Acierno R., McLean C. P. Delivering prolonged exposure therapy via videoconferencing during the COVID-19 pandemic: An overview of the research and special considerations for providers. Journal of Traumatic Stress. 2020;33(4):380–390. doi: 10.1002/jts.22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangani C., Ostinelli E. G., Smith K. A., Hong J. S. W., Macdonald O., Reen G., Reid K., Vincent C., Sheriff R. S., Harrison P. J., Hawton K., Pitman A., Bale R., Fazel S., Geddes J. R., Cipriani A. Impact of the COVID-19 pandemic on the global delivery of mental health services and telemental health: Systematic review. JMIR Mental Health. 2022;9(8):e38600. doi: 10.2196/38600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zastepa E., Sun J. C., Clune J., Mathew N. Adaptation of contingency management for stimulant use disorder during the COVID-19 pandemic. Journal of Substance Abuse Treatment. 2020;118:108102. doi: 10.1016/j.jsat.2020.108102. [DOI] [PMC free article] [PubMed] [Google Scholar]