Abstract
The nucleolus is a multifunctional nuclear body. To tease out the roles of nucleolar structure without resorting to the use of multi-action drugs, we knocked down the RNA polymerase I subunit RPA194 in HeLa cells by siRNA. Loss of RPA194 resulted in nucleolar-structural segregation and effects on both nucleolus-proximal and distal-nuclear components. The perinucleolar compartment was disrupted, centromere clustering around nucleoli was significantly reduced, and the intranuclear locations of specific genomic loci were altered. Moreover, Cajal bodies, distal from nucleoli, underwent morphological and some compositional changes. In comparison, when the preribosomal RNA-processing factor, UTP4, was knocked down, neither nucleolar segregation nor the intranuclear effects were observed, demonstrating that the changes of nucleolar proximal and distal nuclear domains in RPA194 knockdown cells unlikely arise from a cessation of ribosome synthesis, rather from the consequence of nucleolar-structure alteration. These findings point to a commutative system that links nucleolar structure to the maintenance and spatial organization of certain nuclear domains and genomic loci.
INTRODUCTION
The nucleus is highly compartmentalized, with interphase chromosomes organized into specific territories and the interchromatin space occupied by various nonmembrane enclosed organelles, such as Cajal and PML bodies, and interchromatin-granule clusters (Misteli and Spector, 1998; Misteli and Spector, 2011; Pederson, 2011b; Pederson, 2011a). The nucleolus is the most prominent nuclear body and is the site of ribosome synthesis as well as other functions that have come into view, including assembly of the signal-recognition particle, control of cell-cycle progression, macromolecular trafficking, vicinal-heterochromatin interactions and their regulation, and surveillance of genotoxic and nucleolar stress (Pederson and Tsai, 2009; Pederson, 2011a; Lin et al., 2014; Baserga and DiMario, 2018; Nemeth and Grummt, 2018; Baserga et al., 2020; Gupta and Santoro, 2020). The rDNA repeats, rDNA transcription, and other components have been found critical to the formation of nucleoli (McClintock, 1934; Hernandez-Verdun, 2011; Caudron-Herger et al., 2015; Feric et al., 2016; Riback et al., 2020; Lafontaine et al., 2021). Here we explore whether the nucleolar structure itself might have roles beyond ribosome synthesis, particularly within the nucleus. Classically, a frequently deployed tool to disrupt the nucleolus has been exposure of cells to a low concentration of actinomycin D. A pioneering study using this approach correlated a reduction of 3Huridine labeling of the nucleolus with a biochemical demonstration of impaired ribosomes synthesis (Perry, 1962), and was one of the key steps in defining the nucleolus as the site of ribosome biogenesis. It was subsequently found that exposure of mammalian cells to low concentrations of actinomycin D also leads to alterations of nucleolar morphology, in which its three ultrastructurally defined components, the fibrillar centers (FC), the dense fibrillar component (DFC) and the granular component (GC), undergo repositioning, (Clark et al., 1967). At these low concentrations, actinomycin D preferentially intercalates between adjacent GC base pairs, and also triggers alkylation of adjacent DNA (Sengupta et al., 1988; Gallego et al., 1997). Because the ribosomal RNA genes of eukaryotic cells are GC-rich (at least 65–70% in the case of most mammalian genomes), there has been a tendency to regard low actinomycin as a highly specific experimental intervention. However, there are numerous other GC-rich sites throughout mammalian genomes. Indeed, our bioinformatics survey of the human-reference genome revealed 320 sites that are 65% or more GC, including some that are 85% or more (Supplemental Table S1). Thus, it is possible that nucleolar disruption by actinomycin D and concurrently observed effects could be attributable to the drug’s action at one or more of these hundreds of genomic sites or to other, yet to be defined actions of the drug.
To circumvent issues attending the use of low actinomycin D, we disrupted the Rdna-transcription machinery by siRNA-knockdown of the RNA polymerase I large subunit RPA194 to determine whether the resulting disruption of rDNA transcription has an impact on nucleolar architecture as actinomycin D does, and whether nucleolar-structural integrity plays a role in the organization of other nuclear domains.
RESULTS
Knockdown of RPA194 phenocopies low actinomycin D treatment
We used siRNA knockdown of RPA194, the largest subunit of RNA polymerase I (Russell and Zomerdijk, 2005), to disrupt rDNA transcription. We chose to work with HeLa cells because they lack p53 in which the disruption of ribosome synthesis does not induce p53 mediated apoptosis within the time frame of the study. Seventy-two h after transfection, the level of RPA194 was reduced by more than 95% while the levels of another rDNA-transcription factor, UBF, remained unchanged (Figure 1A). Knockdown of RPA194 led to an inhibition of rDNA transcription as measured both by pulse-labeling cells with BrU and by qRT-PCR of the 5’ ETS region of the nascent pre-rRNA (Supplemental Figure S1A and B). Although pol I transcription was halted, pol II transcription in RPA194 knockdown cells was not significantly changed as evaluated by measuring and comparing the average fluorescence intensity of BrU incorporation signals after 5 min labeling in the nucleoplasm of cells with and without RPA194 knockdown (Supplemental Figure S1C). In addition, the distribution of nuclear speckles, known to be sensitive to pol II transcription inhibition, was not altered in RPA194 knockdown cells compared with α-amanitin-treated cells (Supplemental Figure S1D). To confirm ribosome-synthesis inhibition in RPA194 knockdown cells, an inducible GFP-tagged ribosomal protein RPL29 HeLa cell line was used (Wild et al., 2010). As expected, there was a resultant-reduction of ribosome synthesis (Figure 1B). In untreated and control siRNA transfected cells, GFP-RPL29 was localized in both nucleoli and the cytoplasm within 24 h of its activated expression, reflecting its assembly into new ribosomes and localization to the cytoplasm. In comparison, in RPA194 knocked-down cells, GFP-RPL29 was detected only in nucleoli and not in the cytoplasm 24 h after activation, indicating a lack of newly synthesized ribosomal particles bearing GFP tagged RPL29 entering the cytoplasm (Figure 1B). The robust expression and localization of the inducible GFP-RPL29 in the nucleoli also indicates that transcription by pol II, the activity of translational machinery, and the nucleolar translocation of newly synthesized GFP-RPL29 remained uninterrupted. In comparison, when the preribosomal RNA processing factor UTP4 (Freed et al., 2012) was knocked down, ribosome synthesis was similarly blocked (Figure 1B), as also represented by a similar lack of cytoplasmic GFP-RPL29 signal. These results demonstrate the inhibition of ribosome synthesis by knockdown of either RPA194 or UTP4.
FIGURE 1:

siRPA194 and siUTP4 treatment in HeLa cells significantly reduced ribosome synthesis. A) The level of RPA194 protein was significantly reduced while the UBF level was unchanged at 72 h after transfection. B) Both siRPA194 and siUTP4 treatment inhibited ribosome synthesis as assayed in an inducible GFP-RPL29 stably transfected HeLa cell line. Activation of the fusion-protein expression 48 h after transfection showed little presence of GFP-RPL29 in the cytoplasm in siRPA194 and siUTP4 treated cells compared with control cells, demonstrating lack of new ribosome-synthesis. Bars = 5 µm. A minimum of 50 cells were examined in all cases.
Electron microscopy (EM) revealed that knockdown of RPA194 resulted in a reorganization of nucleolar architecture. The nucleolus normally contains three distinct ultrastructural components: 1) FC (Figure 2A, arrows), 2) a DFC, Figure 2A, arrowheads) and 3) a GC (Figure 2A, asterisk; Raska, 2003; Pederson, 2011a). Knockdown of RPA194 induced segregation of the fibrillar components out to the periphery of the GC (Figure 2A, left panel; compare with untreated, right panel) when examined by EM. These findings are consistent with previous studies using actinomycin D treatment (Goldblatt et al., 1970) or the loss of function of Pol I transcription factor, TIF-IA (Yuan et al., 2005). The nucleolar segregation corresponds to the redistribution of the FC marker protein, UBF (Figure 2B, arrowheads) and the DFC-marker protein fibrillarin (Supplemental Figure S2) from their normal-nucleolar location into nucleolar caps (Figure 2B, arrows) at the light microscopy level. Quantitative analyses revealed that the loss of RPA194 in siRNA treated cells closely paralleled-nucleolar segregation (Figure 2C), similar to that observed in cells treated with low actinomycin D (Supplemental Figure S3; Shav-Tal et al., 2005). Nucleolar segregation was also observed after knockdown of UBF (Supplemental Figure S4). However, in contrast to the knockdown of RPA194, UTP4 knockdown, while blocking ribosome synthesis as shown (Figure 1B), did not lead to nucleolar segregation (Figure 2, A–C) even through it did increase nucleolar size and reduce nucleolar number (Freed et al., 2012). These findings demonstrate that nucleolar segregation is unlikely the result of ribosome synthesis loss, but rather is due the loss of pol I transcription, leading to the reduction of pre-rRNA which has been considered important in maintaining nucleolar structure in cells (Szaflarski et al., 2022).
FIGURE 2:
RPA194 knockdown induced nucleolar segregation. A) EM demonstrated the RPA194 knockdown (left panel) in HeLa cells changed nucleolar structure where the fibrillar component (arrows) was segregated from the GC (*). In comparison, the nucleolus in UTP4 knockdown-cells (middle panel) maintained the typical organization of the three structural components, fibrillar center (arrow), DFC (arrowhead), and GC (*) as was the case in the Control siRNA or untreated cells (right panels). B) Correspondingly, siRPA194 treated, but not siUTP4 treated or control cells, showed segregated caps of UBF as detected by immunostaining. C) Quantification of RPA194 knockdown and nucleolar segregation showed that the segregation of nucleoli occurs mostly in RPA194 knockdown HeLa cells (a minimum of 100 cells were examined in all cases; p < 0.005 between siRPA194 and all other conditions). Bars = 5 μm.
The spatial redistribution of the FC upon nucleolar segregation implies that the rDNA undergoes a similar repositioning. To determine whether this was the case, we deployed CRISPR-dCas9 mediated-labeling of rDNA clusters in control versus RPA194 knocked down cells (Ma et al. 2015, 2016). Sixteen sets of CRISPR-Cas9 gsRNA were designed to label rDNA (see Material and Methods). The specificity of the labeling was validated by simultaneous labeling with an anti-UBF antibody on mitotic Nucleolar Organizer Regions (NORs; Supplemental Figure S5) as it has been shown that rDNA-transcription factors remain bound to active NORs throughout mitosis (Roussel et al., 1996). In untreated, control siRNA or siRNA-UTP4 treated cells, the labeled rDNA was localized in the typical pattern (Figure 3A). But after RPA194 knockdown, the rDNA signals became redistributed and colocalized with the repositioned fibrillarin caps (Figure 3A and C). This was also the case with low actinomycin treatment (Figure 3B and C), consistent with findings from a recent study (Mangan and McStay, 2021). In both cases, nearly all the rDNA signal was associated with the fibrillarin protein-localized caps (Figure 3, A–C). These findings together with those in Figure 1 show that RPA194 knockdown recapitulates the classical phenomenon of nucleolar segregation observed with low-actinomycin treatment.
FIGURE 3:
rDNA cosegregated with the fibrillar-center proteins (fibrillarin or UBF) in cap-structures in segregated nucleoli. A) Eleven guide RNAs complementary to the rDNA were coexpressed with dCas9-GFP (Ma et al., 2015). The labeled rDNA redistributed with the fibrillar components to nucleolar caps in RPA194 knockdown cells (arrows) similarly to those subjected to low actinomycin D (arrows). B) rDNA and UBF localization after actinomycin treatment. C) Quantitative evaluation demonstrated the copartitioning of rDNA with the fibrillar components in the majority of cells after RPA194 knockdown or low actinomycin D treatment. Bars = 5 µm. A minimum of 100 cells were examined in all cases.
Nucleolus-proximal nuclear domains are altered by knockdown of RPA194, but not UTP4
With siRPA194 knockdown as a selective tool to disrupt nucleolar structure without the drawbacks of actinomycin, we proceeded to investigate whether nucleolar structure might play a role in global-nuclear organization. The perinucleolar compartment (PNC) is a distinct structure in many cancer-cell types, including HeLa cells, and is physically associated with the nucleolus (Norton et al., 2008; Pollock and Huang, 2009). In a previous study, knockdown of RPA194 resulted in disassembly of PNCs (Frankowski et al., 2018). To investigate whether PNC disruption is the consequence of ribosome synthesis or rather due to nucleolar-structure alteration, we repeated such experiments but including UTP4 knockdown as a control. Antibodies against PTB (the polypyrimidine tract binding protein, a fiduciary marker of the PNC) were used to immunolabel PNCs. As shown in Figure 4A and B, while the RPA194 knockdown reduced PNC prevalence, knockdown of UTP4 did not significantly impact PNC’s. Additionally, most of the remaining PNCs in RPA194 knocked-down cells underwent structural alteration, becoming crescent shaped (Figure 4A, arrows). Thus, PNC integrity is dependent on the nucleolar structure or rDNA-transcription machinery, rather than ribosome synthesis.
FIGURE 4:

PNC structure and prevalence were altered after RPA194 but not UTP4 knockdown. A) The shape of most PNCs was changed, becoming extended (arrows) in RPA194 knockdown, but not after UTP4 knockdown or in control cells. B) Quantitative analyses demonstrated a significant reduction of PNC prevalence in RPA194 knockdown cells. A minimum of 100 cells were examined in all cases. Bars = 5 µm; ****p < 0.001.
Centromeres are known to cluster around nucleoli (Padeken and Heun, 2013), with an average 60% of the centromeres associated with nucleoli in the HeLa cells we have used here (Foltz et al., 2009). Our recent studies showed that nucleoli-centromere associations change through differentiation, and that the association does not correspond to nucleolar number or size (Rodrigues et al., 2023). To evaluate whether nucleolar segregation impacts nucleolus-centromere proximity, we immunolabeled centromeres (of all chromosomes) and nucleoli with anti-CENPA and anti-UBF antibodies respectively. As shown in Figure 5A and B, upon RPA194-knockdown centromeres were less frequently located to the nucleolar periphery and were more dispersed throughout the nucleus. In contrast, UTP4 knockdown did not change nucleolus-centromere associations (Figure 5A and B). These findings indicate that the nucleolus-proximal centromere arrangement is contingent on normal nucleolar structure or pol I transcription.
FIGURE 5:

RPA194 knockdown changed nucleoli-centromere proximity A) The clustering of centromeres (green) around nucleoli (red) decreased in RPA194 knockdown cells but not in UTP4 knockdown or control cells. B) Quantification showed that the changes were statistically significant; ****p < 0.001. Bars = 5 µm. A minimum of 50 cells were examined in all cases.
Repositioning of genomic loci after RPA194 knockdown
We next asked whether there are more wide-ranging effects throughout the 3D nucleome upon RPA194 knockdown. We chose to localize gene loci that had altered-gene expression in RPA194 knocked-down cells (Figure 6). One of these, the HIST1H2BN locus, became more centrally located following RPA194 knockdown in a substantial portion of cells (Figure 6B). This repositioning was associated with an increase in the transcription of this locus, as shown by RNA-Seq (Figure 6A). Conversely, in a substantial portion of cells, the MALL locus, typically more centrally located, became repositioned more to the nuclear periphery (Figure 6C), which corresponded to its reduced expression (Figure 6A). These positional and expression changes of the two genomic loci were not observed in either UTP4 knocked down or control siRNA-treated cells, nor in untreated cells (Figure 6B and C), and thus are specifically attributable to RPA194 knockdown-coupled nucleolar segregation or pol I disruption. However, not all the loci with expression changes were altered in their spatial distribution in the nucleus. For example, two other gene loci with increased expression, SNORD46 and PAR5, did not undergo significant changes in their nuclear positioning upon RPA194 knockdown (unpublished data).
FIGURE 6:
RPA194 knockdown changed spatial distributions of selected genes. A) Changes in the RNA levels of the HIST1H2BN and MALL loci in RPA194 knockdown cells comparing to control cells. B) HIST1H2BN loci became more centrally localized after RPA194 knockdown in contrast with UTP4 knockdown or control cells (arrowheads). C) MALL loci became more peripherally localized in RPA194 knockdown cells (arrowheads). The respective histograms show the significant differences in the central versus peripheral localization of the two loci in RPA194 comparing with UTP4 knockdown, or control siRNA treated cells and untreated cells. P < 0.01. Bars = 5 µm. A minimum of 100 cells were examined in all cases.
Nucleolar organization influences Cajal body structures
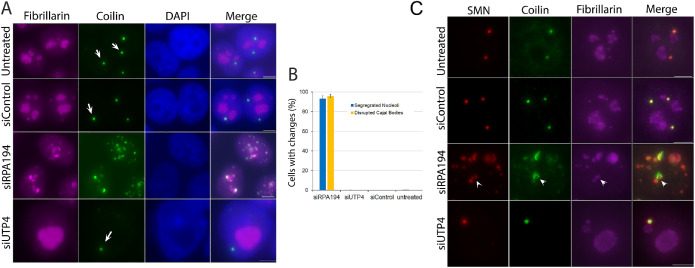
Given these results, we wondered whether normal-nucleolar organization might play a role not only in the intranuclear-spatial location of centromeres and certain genomic loci, but perhaps also in the maintenance of other nuclear bodies, even ones lying further away We thus examined Cajal bodies (Verheggen et al., 2002; Gall, 2003; Nizami et al., 2010; Wang et al., 2016). We found that knockdown of RPA194, but not of UTP4, led to Cajal body disruption as defined by the localization of p80 coilin, a Cajal body marker, with colabeling of fibrillarin or UBF (Figure 7A; and Supplemental Figure S6). The images of RPA194 knocked-down cells reveal different stages of nucleolar-structure disruption, possibly reflecting differences in the levels of transfected siRNAs. Indeed, there appeared to be a degree of correlation between the alteration of nucleoli and Cajal bodies. For example, the lower-left cell in Figure 7A had slight changes in nucleolar structure with a very minor alteration of Cajal bodies, which became associated with the altering nucleoli. In comparison, the top-right cells went through full-nucleolar segregation and significant Cajal body disruptions. The nucleolar segregation and disruptions of Cajal bodies were closely coupled as quantified in Figure 7B. This same effect on Cajal bodies was observed with knockdown of UBF (Supplemental Figure S7). These changes have been shown in previous studies using low actinomycin D treatment (Shav-Tal et al., 2005). The dispersion of Cajal bodies was also observed when examined with another marker protein SMN (Figure 7C). In addition to the bodies’ structural changes, the Cajal body proteins SMN and coilin no longer perfectly colocalized as shown in Figure 7C. These findings demonstrate that the nucleolus, in its normal operation, not only impacts 3D genomic organization, but also Cajal bodies usually situated distant from it. However, the histone-locus body (Nizami et al., 2010), as monitored by immunolabeling of FLASH, a protein involved in histone-locus body function (Bongiorno-Borbone et al., 2010), was not affected (Supplemental Figure S8) in RPA194 knockdown cells, demonstrating that not all nuclear bodies are governed by the nucleolus.
FIGURE 7:
RPA194 knockdown but not UTP4 knockdown induced disruption of Cajal bodies. A) After RPA194 knockdown, Cajal bodies became irregularly shaped and some juxtaposed segregated nucleoli as detected using anticoilin antibodies. Cajal bodies displayed their typical round appearance in UTP4 knockdown cells, or control siRNA treated cells and untreated cells. B) Quantitative evaluation showing the close-correlation between nucleolar segregation and disruption of Cajal bodies. A minimum of 100 cells were examined in all cases; (p < 0.0001 between RPA194 knockdown and all others). C) Cajal body components, SMN and coilin, no longer showed perfect colocalization after RPA194 knockdown (Merge; arrowhead). Bars = 5 µm
DISCUSSION
To circumvent the lack of specificity of low actinomycin D treatment or other drugs as tools to investigate the possible roles of nucleolar structure in global-nuclear organization, we deployed siRNA knockdown of the RNA polymerase I large subunit, RPA194, and of a pre-rRNA processing factor, UTP4, both leading to ribosome-synthesis inhibition. However, of these two experimental interventions, only RPA194 knockdown induced nucleolar segregation, indicating that ribosome synthesis itself is not likely to be responsible for maintenance of the classical tripartite-nucleolar ultrastructure. This gave us an opportunity to specifically address the impact of nucleolar structure per se on other nuclear components. We found that nucleolar segregation leads to changes throughout the 3D nucleome, in which nucleolus-proximal and distal components change their structural and spatial organization. Centromere clusters reorganize significantly, certain genomic loci change their spatial distribution from nucleolus-proximal to central nuclear locations or from mostly nucleus-central locations to the nuclear periphery. In addition, nucleolar segregation disrupts Cajal bodies, but not histone locus bodies.
The formation of the nucleolus is a highly dynamic and regulated process. Nucleoli disassemble as cells enter mitosis and reassemble as cells exit mitosis. NORs and rDNA transcription as well as other components have classically been shown to play key roles in nucleolar assembly (McClintock, 1934; Hernandez-Verdun, 2011). Over the past decade, there have been further studies regarding the formation of the nucleolus. Ideas that nucleoli form through liquid–liquid phase separation brings a new perspective of nucleolar-structural organization (Berry et al., 2015; Feric et al., 2016; Riback et al., 2020). However, how nucleoli interact and coordinate with their most proximal-nuclear components (e.g., heterochromatin) and other more distal-nuclear sites under various physiological and pathological conditions remains underexplored. In the present investigation we asked whether nucleolar structure is important in maintaining other structural and functional components in the nucleus. A previous study demonstrated that low actinomycin D and genotoxic drugs inhibit pol I transcription and induce nucleolar segregation, accompanied by a nucleolar cap accumulation of certain nucleoplamsic proteins (Shav-Tal et al., 2005). However, it was not clear whether the reported effect was due to the changes of nucleolar structure, to the multifunctionality of the drugs or to the ribosome-synthesis inhibition. Here we used siRNA knockdown of RPA194 in p53-null HeLa cells which allowed us to see effects without apoptosis being triggered. We found that the segregation of nucleoli induced by RPA194 knockdown is associated with reduced centromere association with nucleoli, relocations of certain genomic loci as well as disruption of nucleolar distal Cajal bodies. However, these changes did not occur when UTP4 was knocked down. These findings demonstrate that the global nuclear effects we have observed upon RPA194 knockdown are not due to a general inhibition of ribosome synthesis, but rather to the segregation of the nucleolar structure itself.
Our observations demonstrate a connection between nucleolar structure and a subset of various intranuclear components or genomic loci. The changes in the PNCs or of the nucleolus-to-centromere association, we observed are not difficult to envision as arising from changes in the nucleolar surface. But the more distant intranuclear effects we have found require other explanations. There is extensive evidence for shuttling of proteins between the nucleolus and the nucleoplasm (Phair and Misteli, 2000; Chen and Huang, 2001) and it is possible that this is part of a regulatory network important to nuclear homeostasis, one that is perturbed by an alteration of nucleolar structure. Further work will be needed to test this and other possibilities for the control factors that underly the nuclear homeostasis network our present findings have revealed.
MATERIALS AND METHODS
Cell culture
HeLa cells were maintained in DMEM (ThermoFisher Scientific) with 10% FBS (GEMINI Bio Products) and 100 units/ml each of penicillin and streptomycin (ThermoFisher Scientific). RPL29-GFP cells were cultured in DMEM with 0.3 mg/ml hygromycin and 0.5 µg/ml puromycin. Cells were plated 24 h before treatment with siRNA.
RNA interference
The siRNA targeting human RPA194 was obtained from Invitrogen (catalogue#: 10620318) as was a scrambled-sequence negative control siRNA (catalogue#: 12935-300). The siRNA targeting human UTP4 was obtained from GE Dharmacon (catalogue#: M-015011-01-0005). siRNAs’ were transfected into cells with Lipofectamine RNAiMAX Transfection Reagent (ThermoFisher Scientific; catalogue#: 13778) according to the manufacturer’s instructions. The reported experiments were performed 72 h posttransfection.
Immunofluorescence
Cells were fixed in 4% paraformaldehyde for 15 min and washed in phosphate-buffered saline (PBS) three times of 10-min each before permeabilized by 0.2% Triton-X 100/PBS for 10 min and additional PBS wash three times. Primary antibodies were then incubated for 1 h at room temperature. They are from the indicated sources and used at the stated dilutions: RPA194, Santa Cruz Biotechnology, catalogue#sc-48385, 1:100; PTB (S. Huang laboratory), 1:300; UBF, Santa Cruz Biotechnology, catalogue#: sc-13125, 1:50; fibrillarin, Sigma, catalogue#: ANA-N, 1:10; CENPA (Thermo Fisher #MA1-20832, 1:100; coilin, Santa Cruz Biotechnology, catalogue#: sc-32860, 1:300; Flash histone locus protein (Joseph Gall, Carnegie Institution for Science), 1:500; SMN, Santa Cruz Biotechnology, catalogue# sc-15320, 1:300, SC-35 1:200 (David Spector, Cold Spring Harbor Laboratory). After incubation with primary antibodies, the fluorescein-conjugated (Jackson ImmunoResearch) and Alexa Fluor-labeled (ThermoFisher Scientific ) secondary antibodies were incubated with cells at a dilution of 1:200 for 1 h at room temperature. Cell bearing coverslips were then mounted with Vector mounting media. Slides were visualized on a Nikon Eclipse Ti-E inverted fluorescence microscope using NIS-Elements AR 4.2 software (Nikon).
BrU labeling
The BrU labeling protocol was as described previously (Wang et al., 2008). Untreated or siRNA-transfected cells were labeled with five-bromouridine (BrU) at 0.2 mM for 5 min. The incorporation was measured by staining with an anti-BrdU antibody that also recognizes BrU (Sigma, catalogue# B8434) at a 1:50 dilution, followed by an Alexa Fluor-conjugated second antibody (Thermo Fisher). Images were obtained with a Nikon Eclipse Ti-E inverted fluorescence microscope using NIS-Elements AR 3.2 software (Nikon).
Quantification of BrU nucleoplasmic labeling
To directly assess the effect of RPA194 knockdown on nucleoplasmic BrU labeling as a reflection of pol II and pol III transcription, we took advantage of the fact that not all the cells on a given coverslip undergo knockdown of RPA194 after exposure to the siRNA. Thus, we interrogated single coverslips exposed to siRNA for RPA194, measuring and comparing the nucleoplasmic BrU labeling in cells that did or did not display knockdown. The RPA194 knockdown cells were defined as those which lost most of the RPA194 labeling coupled with nucleolar segregation. Cells without RPA194 were defined as those with normal RPA194 labeling signal and normal nucleolar labeling patterns. The comparisons were made among cells from the same clover glass so as to avoid labeling heterogeneity among different coverslips.
qPCR
Real time PCR was performed with ABI PRISM 7900HT instrument and Power SYBR Green PCR Master Mix (Life technologies catalogue# 4367659) in the Northwestern University Core facility. The gene-specific primers used for qPCR were: 5′ETS (5′ETS-F: cctccagtggttgtcgactt 5′ETS-R: gaacgacacaccaccgttc), GAPDH (Integrated DNA Technologies Ready-Made Primers). The qPCR was run on the QuantStudio 7 Flex Real-Time PCR system at the NUseq Core facility at Northwestern University.
Western blots
These were performed according to manufacturers’ protocols (Millipore). The primary antibodies and dilutions used were RPA194 (see above, 1:500); UBF (see above, 1:500); and β-actin (Sigma, catalogue# A5060), 1:3000, as loading controls. LI-COR IRDye secondary antibodies (1:10,000) were used for visualization. Protein bands were detected using LI-COR Odyssey Image Studio (LI-COR Biosciences).
Electron microscopy (EM)
Cells were fixed in 2% paraformaldehyde 2.5% glutaraldehyde in 0.1M cacodylate buffer, pH 7.3, processed and examined at the Center for Advanced Microscopy & Nikon Imaging Center in the Northwestern University Feinberg School of Medicine.
Inducible expression of RPL29-GFP HeLa cell line
The tetracycline-inducible RPL29-GFP cell line was maintained in hygromycin and puromycin. Before each experiment the cells were transferred to medium without the antibiotics and transfected with the desired siRNA or left untreated. After 48 h, tetracycline was added at 125 ng/ml to induce RPL29-GFP expression followed by examination 20 h later.
In situ hybridization
HeLa cells were seeded to six well-plates 1 d before transfection of siRNAs. Three days posttransfection, the genomic loci HIST1H2BN and MALL were detected using BACs obtained from bac.chori.org (catalogue#’s RP-11-958P15 and RP11-349F11, respectively, and labeled by nick translation Abbott Laboratories (catalogue# 07J00-001). Standard DNA in situ hybridization protocol was followed (Frey and Matera, 1995). The hybridization signals were measured using the Z-series photos taken on a Nikon Eclipse Ti-E inverted fluorescence microscope. The gene-loci spatial positions were analyzed in their relation to the nuclear envelope and nucleoli, and the space between.
CRISPR dCas9 labeling of rDNA
The CRISPR Cas9 rDNA labeling constructs were “Nm dCas9 3XGFP” as detailed in (Ma et al., 2015). The guide RNAs were U6 promoter plasmids containing a pool of the following16 sequences complementary to rDNA, as follows:
rDNA-F1: ACCGCGCGTGTTCTCATCTAGAAG;
rDNA-R1: AAACCTTCTAGATGAGAACACGCG;
rDNA-F2: ACCGTGGGCAGAACGAGGGGGACC;
rDNA-R2: AAACGGTCCCCCTCGTTCTGCCCA;
rDNA-F3: ACCGCGGACACAGCACTGACTACC;
rDNA-R3: AAACGGTAGTCAGTGCTGTGTCCG;
rDNA-F4: ACCGCCACCTCCTTGACCTGAGTC;
rDNA-R4: AAACGACTCAGGTCAAGGAGGTGG;
rDNA-F5: ACCGTCGCTCTGTCACCCAGGCTG;
rDNA-R5: AAACCAGCCTGGGTGACAGAGCGA;
rDNA-F6: ACCGTCTTCGGTAGCTGGGATTAC;
rDNA-R6: AAACGTAATCCCAGCTACCGAAGA;
rDNA-F7: ACCGTCCTGGGCCTCCCAAAGTGC;
rDNA-R7: AAACGCACTTTGGGAGGCCCAGGA;
rDNA-F8: ACCGTAGGCCATTGCACTGTAGCC;
rDNA-R8: AAACGGCTACAGTGCAATGGCCTA;
rDNA-F9: ACCGACGTCTGTCATCCCGAGGTC;
rDNA-R9: AAACGACCTCGGGATGACAGACGT;
rDNA-F10: ACCGCGAAATGGAGTCAGGCGCCG;
rDNA-R10: AAACCGGCGCCTGACTCCATTTCG;
rDNA-F11: ACCGTGTAACCCCAGCTACTCGGG;
rDNA-R11: AAACCCCGAGTAGCTGGGGTTACA;
rDNA-F12: ACCGAATTGCTTGAACCTGGCAGG;
rDNA-R12: AAACCCTGCCAGGTTCAAGCAATT;
rDNA-F13: ACCGCTGGGCGACAGAGTGAGACC;
rDNA-R13: AAACGGTCTCACTCTGTCGCCCAG;
rDNA-F14: ACCGACTGTATTGCTACTGGGCTA;
rDNA-R14: AAACTAGCCCAGTAGCAATACAGT;
rDNA-F15: ACCGCCGAGAGGAATCTAGACAGG;
rDNA-R15: AAACCCTGTCTAGATTCCTCTCGG;
rDNA-F16: ACCGGGTCCCCGCTTGGATGCGAG;
rDNA-R16: AAACCTCGCATCCAAGCGGGGACC.
HeLa cells were seeded onto six well-plates followed by siRNA transfection 24 h later. After another 24 h, the cells were cotransfected with the dCas9 and guide RNA plasmids using Lipofectamine 2000 according to the manufacturer’s instructions and at a concentration of 200 ng of dCas9 plasmid and 750 ng of the guide RNA plasmids pool. Signals were evaluated 42 h posttransfection using Nickon fluorescence microscope.
Centromere-nucleolar association measurement
Immunolabeled cells were imaged using a Nikon Eclipse Ti fluorescence microscope with 60× objective 1.4NA and four channels of fluorescence. Cells were imaged through Z-stack with 500-nm intervals. The top and bottom limits were set to include the entire nucleus. The Z-stack of images were maximally projected, and centromeres physically associated with nucleoli (immunostained with UBF, or fibrillarin, or NPM1, combined with phase contrast images) in those projected images were hand-scored and counted using Element imaging tools.
Supplementary Material
Acknowledgments
This study was funded by National Institutes of Health grants 2 R01 GM078555-09 and U01 CA260699 to S.H., 1 R35 GM131687 to S.J.B and U01 DA040588 to T.P. and Paul Kaufman. We thank Lihua Julie Zhu and Paul Kaufman at UMass Chan Medical School for their expertise on the bioinformatics search of GC-rich loci in the human genome. We also thank Dr. Samuel Sondalle in the laboratory of S.J.B. for performing some of the protein-expression analyses. In addition, we thank Ulrike Kutay (Institute of Biochemistry, ETH Zurich, Zurich, Switzerland) for the inducible GFP-RPL29 HeLa cell line, Zehra Nizami and Joseph Gall (Department of Embryology, Carnegie Institution for Science) for the anti-FLASH antibody, and David Spector (Cold Spring Harbor Laboratory) for SC-35 antibody. We would like to thank Tom Volpe, Northwestern University (Evanston) for critical reading of the manuscript. We would also like to thank the Center for Advanced Microscopy & Nikon Imaging Center at Northwestern Univerisity for EM imaging assistance.
Abbreviations used:
- BrU
bromouridine
- CRISPR
clustered regularly interspaced short palindromic repeats
- DFC
dense fibrillar component
- ETS
external transcribed spacer
- FC
fibrillar center
- GC
granular component
- NOR
nucleolar organizer region
- PNC
perinucleolar compartment.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E23-02-0062) on August 23, 2023.
REFERENCES
- Baserga SJ, Dimario PJ (2018). Emerging roles for the nucleolus 2017. Mol Biol Cell 29, 773–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baserga SJ, Dimario PJ, Duncan FE (2020). Emerging roles for the nucleolus 2019. J Biol Chem 295, 5535–5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry J, Weber SC, Vaidya N, Haataja M, Brangwynne CP (2015). RNA transcription modulates phase transition-driven nuclear body assembly. Proc Natl Acad Sci USA 112, E5237–5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongiorno-Borbone L, De Cola A, Barcaroli D, Knight RA, Di Ilio C, Melino G, De Laurenzi V (2010). FLASH degradation in response to UV-C results in histone locus bodies disruption and cell-cycle arrest. Oncogene 29, 802–810. [DOI] [PubMed] [Google Scholar]
- Caudron-Herger M, Pankert T, Seiler J, Németh A, Voit R, Grummt I, Rippe K (2015). Alu element-containing RNAs maintain nucleolar structure and function. EMBO J 34, 2758–2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Huang S (2001). Nucleolar components involved in ribosome biogenesis cycle between the nucleolus and nucleoplasm in interphase cells. J Cell Biol 153, 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AM, Love R, Studzinski GP, Ellem KA (1967). A correlated morphological and functional study of the effects of actinomycin D on HeLa cells. I. Effects on the nucleolar and cytoplasmic ribonucleoproteins. Exp Cell Res 45, 106–119. [DOI] [PubMed] [Google Scholar]
- Feric M, Vaidya N, Harmon TS, Mitrea DM, Zhu L, Richardson TM, Kriwacki RW, Pappu RV, Brangwynne CP (2016). Coexisting liquid phases underlie nucleolar subcompartments. Cell 165, 1686–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltz DR, Jansen LE, Bailey AO, Yates JR, 3rd, Bassett EA, Wood S, Black BE, Cleveland DW (2009). Centromere-specific assembly of CENP-a nucleosomes is mediated by HJURP. Cell 137, 472–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankowski KJ, Wang C, Patnaik S, Schoenen FJ, Southall N, Li D, Teper Y, Sun W, Kandela I, Hu D, et al. (2018). Metarrestin, a perinucleolar compartment inhibitor, effectively suppresses metastasis. Sci Transl Med 10, eaap8307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed EF, Prieto JL, Mccann KL, Mcstay B, Baserga SJ (2012). NOL11, implicated in the pathogenesis of North American Indian childhood cirrhosis, is required for pre-rRNA transcription and processing. PLoS Genet 8, e1002892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey MR, Matera AG (1995). Coiled bodies contain U7 small nuclear RNA and associate with specific DNA sequences in interphase human cells. Proc Natl Acad Sci USA 92, 5915–5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall JG (2003). The centennial of the Cajal body. Nat Rev Mol Cell Biol 4, 975–980. [DOI] [PubMed] [Google Scholar]
- Gallego J, Ortiz AR, De Pascual-Teresa B, Gago F (1997). Structure-affinity relationships for the binding of actinomycin D to DNA. J Comput Aided Mol Des 11, 114–128. [DOI] [PubMed] [Google Scholar]
- Goldblatt PJ, Verbin RS, Sullivan RJ (1970). Induction of nucleolar segregation by actinomycin D following inhibition of protein synthesis with cycloheximide. Exp Cell Res 63, 117–123. [DOI] [PubMed] [Google Scholar]
- Gupta S, Santoro R (2020). Regulation and roles of the nucleolus in embryonic stem cells: from ribosome biogenesis to genome organization. Stem Cell Reports 15, 1206–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Verdun D (2011). Assembly and disassembly of the nucleolus during the cell cycle. Nucleus 2, 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafontaine DLJ, Riback JA, Bascetin R, Brangwynne CP (2021). The nucleolus as a multiphase liquid condensate. Nat Rev Mol Cell Biol 22, 165–182. [DOI] [PubMed] [Google Scholar]
- Lin T, Meng L, Lin TC, Wu LJ, Pederson T, Tsai RY (2014). Nucleostemin and GNL3L exercise distinct functions in genome protection and ribosome synthesis, respectively. J Cell Sci 127, 2302–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Naseri A, Reyes-Gutierrez P, Wolfe SA, Zhang S, Pederson T (2015). Multicolor CRISPR labeling of chromosomal loci in human cells. Proc Natl Acad Sci USA 112, 3002–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Tu L, Naseri A, Huisman M, Zhang S, Grunwald D, Pederson T (2016). Multiplexed labeling of genomic loci with dCas9 and engineered sgRNAs using CRISPRainbow. Nat Biotechnol 34, 528–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan H, Mcstay B (2021). Human nucleoli comprise multiple constrained territories, tethered to individual chromosomes. Genes Dev 35, 483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcclintock B (1934). The relation of a particular chromosomal element to the development of the nucleoi in Zea mays. Zellforsch Mikrosk. Anat. 21, 294–328. [Google Scholar]
- Misteli T, Spector DL (1998). The cellular organization of gene expression. Curr Opin Cell Biol 10, 323–331. [DOI] [PubMed] [Google Scholar]
- Misteli T, Spector DL (2011). The Nucleus. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Nemeth A, Grummt I (2018). Dynamic regulation of nucleolar architecture. Curr Opin Cell Biol 52, 105–111. [DOI] [PubMed] [Google Scholar]
- Nizami Z, Deryusheva S, Gall JG (2010). The Cajal body and histone locus body. Cold Spring Harb Perspect Biol 2, a000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton JT, Pollock CB, Wang C, Schink JC, Kim JJ, Huang S (2008). Perinucleolar compartment prevalence is a phenotypic pancancer marker of malignancy. Cancer 113, 861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padeken J, Heun P (2013). Centromeres in nuclear architecture. Cell Cycle 12, 3455–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson T (2011a). The nucleolus. Cold Spring Harb Perspect Biol 3, 209–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson T (2011b). The nucleus introduced. Cold Spring Harb Perspect Biol 3, 209–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson T, Tsai RY (2009). In search of nonribosomal nucleolar protein function and regulation. J Cell Biol 184, 771–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RP (1962). The cellular sites of synthesis of ribosomal and 4s RNA. Proc Natl Acad Sci USA 48, 2179–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phair RD, Misteli T (2000). High mobility of proteins in the mammalian cell nucleus. Nature 404, 604–609. [DOI] [PubMed] [Google Scholar]
- Pollock C, Huang S (2009). The perinucleolar compartment. J Cell Biochem 107, 189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raska I (2003). Oldies but goldies: searching for Christmas trees within the nucleolar architecture. Trends Cell Biol 13, 517–525. [DOI] [PubMed] [Google Scholar]
- Riback JA, Zhu L, Ferrolino MC, Tolbert M, Mitrea DM, Sanders DW, Wei M-T, Kriwacki RW, Brangwynne CP (2020). Composition-dependent thermodynamics of intracellular phase separation. Nature 581, 209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues A, Macquarrie KL, Freeman E, Lin A, Willis AB, Xu Z, Alvarez AA, Ma Y, White BEP, Foltz DR, et al. (2023). Nucleoli and the nucleoli-centromere association are dynamic during normal development and in cancer. Mol Biol Cell 34, br5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel P, Andre C, Comai L, Hernandez-Verdun D (1996). The rDNA transcription machinery is assembled during mitosis in active NORs. J Cell Biol. 133, 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J, Zomerdijk JC (2005). RNA-polymerase-I-directed rDNA transcription, life and works. Trends Biochem Sci 30, 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta SK, Rosenbaum DP, Sehgal RK, Almassian B, Blondin J (1988). Enantiomers of 7-(2,3-epoxypropoxy)actinomycin D as dual-action DNA-acting antitumor agents. J Med Chem 31, 1540–1547. [DOI] [PubMed] [Google Scholar]
- Shav-Tal Y, Blechman J, Darzacq X, Montagna C, Dye BT, Patton JG, Singer RH, Zipori D (2005). Dynamic sorting of nuclear components into distinct nucleolar caps during transcriptional inhibition. Mol Biol Cell 16, 2395–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski W, Lesniczak-Staszak M, Sowinski M, Ojha S, Aulas A, Dave D, Malla S, Anderson P, Ivanov P, Lyons SM (2022). Early rRNA processing is a stress-dependent regulatory event whose inhibition maintains nucleolar integrity. Nucleic Acids Res 50, 1033–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheggen C, Lafontaine DL, Samarsky D, Mouaikel J, Blanchard JM, Bordonné R, Bertrand E (2002). Mammalian and yeast U3 snoRNPs are matured in specific and related nuclear compartments. EMBO J 21, 2736–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Norton JT, Ghosh S, Kim J, Fushimi K, Wu JY, Stack MS, Huang S (2008). Polypyrimidine tract-binding protein (PTB) differentially affects malignancy in a cell line-dependent manner. J Biol Chem 283, 20277–20287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Sawyer IA, Sung MH, Sturgill D, Shevtsov SP, Pegoraro G, Hakim O, Baek S, Hager GL, Dundr M (2016). Cajal bodies are linked to genome conformation. Nat Commun 7, 10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild T, Horvath P, Wyler E, Widmann B, Badertscher L, Zemp I, Kozak K, Csucs G, Lund E, Kutay U (2010). A protein inventory of human ribosome biogenesis reveals an essential function of exportin 5 in 60S subunit export. PLoS Biol 8, e1000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Zhou Y, Casanova E, Chai M, Kiss E, Gröne H-J, Schütz G, Grummt I (2005). Genetic inactivation of the transcription factor TIF-IA leads to nucleolar disruption, cell cycle arrest, and p53-mediated apoptosis. Molecular cell 19, 77–87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.