Abstract
Introduction
Proton craniospinal irradiation (pCSI) is a treatment option for leptomeningeal disease (LMD), which permits whole neuroaxis treatment while minimizing toxicity. Despite this, patients inevitably experience progression. Adding systemic therapy to pCSI may improve outcomes.
Methods
In this single-institution retrospective case series, we present the feasibility of treatment with pCSI (30Gy, 10 fractions) and an immune checkpoint inhibitor (ICI) in two sequential patients with LMD from melanoma.
Results
The first patient developed LMD related to BRAF V600E-mutant melanoma after prior ICI and BRAF-targeted therapy. After pCSI with concurrent nivolumab, the addition of relatlimab, and BRAF-targeted therapy, he remained alive 7 months after LMD diagnosis despite central nervous system progression. The second patient developed LMD related to BRAF-wildtype melanoma after up-front ICI. He received pCSI with concurrent ipilimumab and nivolumab, then nivolumab maintenance. Though therapy was held for ICI hepatitis, the patient remained progression-free 5 months after LMD diagnosis.
Conclusion
Adding an ICI to pCSI is feasible for patients with LMD and demonstrates a tolerable toxicity profile. While prospective evaluation is ultimately warranted, pCSI with ICI may confer survival benefits, even after prior ICI.
Keywords: leptomeningeal disease, proton therapy, craniospinal irradiation, immune checkpoint inhibitors, immunotherapy
INTRODUCTION
Leptomeningeal disease (LMD), the spread of cancer into the pia mater and arachnoid membrane encasing the brain and spinal cord, is a devastating pattern of cancer progression.[1] The prognosis of LMD from solid tumors is poor, with a median overall survival (OS) of approximately 3 to 6 months.[1,2] Although individuals whose tumors have actionable mutations for which targeted therapies are available may be exceptions to the dismal prognosis, these patients represent a minority of cases.[3] Survival 6 months after LMD diagnosis is rare.[4]
Historically, LMD has been treated with photon-based whole-brain radiotherapy with focal spinal radiotherapy (RT) to symptomatic areas or craniospinal irradiation (CSI).[5] Photon CSI is associated with toxicities, such as weight loss, myelosuppression, and esophagitis.[6] Proton CSI (pCSI) has emerged as a novel technique to treat the entire neuroaxis while limiting normal tissue toxicities.[7] Rather than the progressive dose fall off seen with photon radiation, protons deposit most of their energy within a few millimeters of their end range, effectively eliminating exit radiation dose.[8] This allows therapeutic radiation dose delivery to the craniospinal axis, sparing the vertebral bone marrow and the anterior torso organ system and demonstrating an improved toxicity profile.[8] The efficacy of proton compared with photon RT has been evaluated across multiple clinical trials for a diverse range of pediatric and adult solid tumors.[9] Though evidence is not yet definitive and multiple studies are ongoing, available data suggest similar efficacy but decreased toxicity of proton compared with photon therapy for prostate, lung, and esophageal cancers.[9]
The safety and tolerability of pCSI for patients with LMD were established in a phase I clinical trial. 7 In this study, 24 patients with LMD were treated with pCSI. Median central nervous system (CNS) progression-free survival (PFS) was 7 months, and median OS was 8 months. The study included a heterogeneous population with 11 (46%) patients having non–small cell lung cancer (NSCLC) with actionable EGFR, ALK, or ROS1 mutations.[7] None of the patients in this trial had melanoma.
Another clinical trial compared pCSI with involved field photon radiation.[10] Forty-two patients were treated with pCSI and 21 with photon-involved field RT, defined as whole-brain radiotherapy and/or focal spinal radiotherapy. Time to CNS tumor progression, PFS, and OS were improved in the pCSI arm compared with the photon RT arm. A significant benefit in CNS PFS was observed with pCSI (median 7.5 months; 95% CI, 6.6 months to not reached) compared with photon RT (2.3 months; 95% CI, 1.2–5.8). Median OS for pCSI was 9.9 months (95% CI, 7.5–NA) compared with 6.0 months for photon RT (95% CI, 3.9–NA).[10] Six patients in this trial had melanoma, but patient-level survival data were not provided for these individuals. Nevertheless, many patients in the study experienced disease progression after treatment and succumbed to LMD. The addition of systemic therapy to pCSI may improve outcomes.
Immune checkpoint inhibitors (ICI) have proven efficacy in numerous systemic malignancies, including melanoma.[11] Given its prior successes, ICI monotherapy was studied for patients with LMD.[12,13] In one study, 13 patients with LMD from solid tumors were treated with pembrolizumab. Median CNS PFS was 2.9 months, and median OS was 4.9 months.[12] In a second study, 20 patients with LMD were treated with pembrolizumab monotherapy. Median CNS PFS was 2.6 months, and median OS was 3.6 months, with the study meeting its primary endpoint with a 3-month OS of 60%.[13]
The utility of combining RT with immunotherapy is promising in the field of cancer treatment.[14] While RT has been regarded as immunosuppressive and associated with lymphopenia, RT-mediated killing of neoplastic tissue can also act as a functional in situ vaccination, generating a systemic immune response.[15] This is evidenced by the abscopal effect, where nonirradiated tissue reduces in size after RT treatment of a separate lesion.[16] By treating an otherwise ICI-resistant tumor with RT, this functional vaccination may prime the immune system to a heightened response from subsequent immunotherapy.[14] This combination was efficacious in the PACIFIC phase III clinical trial of NSCLC, with a median OS of 47.5 months for patients treated with chemoradiation followed by anti–PD-L1 ICI durvalumab compared with 29.1 months for patients treated with placebo after chemoradiation.[17] The KEYNOTE-799 phase II trial for stage III unresectable NSCLC with anti–PD-1 ICI pembrolizumab showed a similar benefit.[18]
The combination of stereotactic radiosurgery (SRS) with ICIs nivolumab or ipilimumab was studied in patients with brain metastases related to melanoma.[19] The results from the combination of SRS with anti–CTLA-4 ICI ipilimumab were inconsistent, with some studies demonstrating improved intracranial PFS and OS from combination therapy while others finding no such benefit.[19] In a study of 80 consecutive patients with melanoma brain metastases, the 12-month intracranial PFS rate was 42% (95% CI, 24%–65%) for patients receiving SRS and anti–PD-1 ICI nivolumab and 17% (95% CI, 5%–31%) for those treated with SRS and ipilimumab, suggesting increased benefit from anti–PD-1 compared with anti-CTLA4–targeted ICI.[20] We hypothesized that combining pCSI with ICI may improve outcomes for patients with LMD from melanoma.
METHODS
The Mayo Clinic institutional review board approved this study and granted a waiver of consent for retrospective analysis. In this single-institution retrospective case series, we present the feasibility of treatment with pCSI and ICI in two sequential patients with LMD from melanoma. Both patients were treated at Mayo Clinic in Rochester, Minnesota, with a combination of pCSI and ICI. pCSI was administered as 30 Gy in 10 fractions using a Hitachi proton therapy system, with ICI administered at the discretion of the treating oncologists. Data were retrieved and recorded for each patient from the clinic’s medical records, including patient demographics, disease characteristics, treatment, procedures, evaluations related to LMD treatment, and response. No exclusions were made relating to patient sex, race, or age. Patient follow-up was through June 2023.
RESULTS
Patient 1
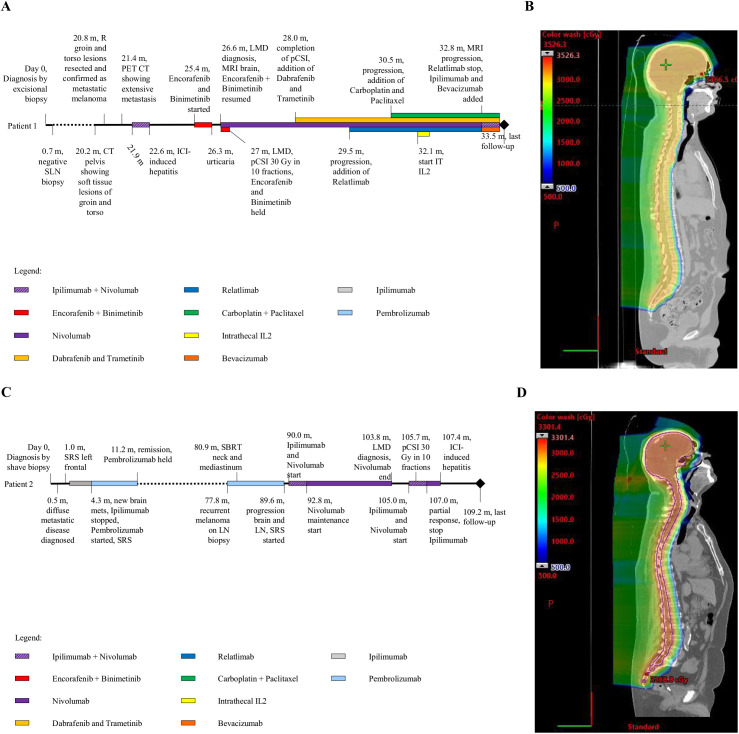
A male patient in his 30s was diagnosed with BRAF V600E-mutant melanoma, initially presenting as a right, lower back skin lesion (Fig. 1A). Excisional biopsy confirmed the diagnosis with negative sentinel lymph node biopsies. The patient received no adjuvant therapy and was followed clinically. Twenty months after the initial melanoma diagnosis, he was seen for right middle back and right groin soft tissue lesions. The lesions were resected, and the pathology was consistent with metastatic melanoma. Fludeoxyglucose F18 (FDG) positron-emission tomography (PET) revealed hypermetabolic lesions involving the liver, right groin, and left posterior chest wall. The patient was started on ipilimumab (anti–CTLA4 ICI) and nivolumab (anti–PD1 ICI). After two treatment cycles, the patient had stable disease but developed immune-related hepatitis refractory to prednisone, requiring the addition of mycophenolate and discontinuation of ICI. Three months later, he was started on encorafenib and binimetinib, which was stopped after 1 month due to urticaria.
Figure 1.
Timeline of patient disease course and proton craniospinal irradiation treatment plans. (A) Patient 1 timeline of disease course. (B) Patient 1, sagittal view, proton craniospinal irradiation plan. Prescribed 30 Gy in 10 fractions. (C) Patient 2 timeline of disease course. (D) Patient 2, sagittal view, proton craniospinal irradiation plan. Prescribed 30 Gy in 10 fractions.
SLN: sentinel lymph node; R: right; CT: computerized tomography; PET: positron-emission tomography; ICI: immune checkpoint inhibitor; Enc: encorafenib; Bin: binimetinib; LMD: leptomeningeal disease; MRI: magnetic resonance imaging; pCSI: proton craniospinal irradiation; Gy: Gray; PC: paclitaxel and carboplatin; IT: intrathecal; IL2: interleukin-2; SRS: stereotactic radiosurgery; Ipi: ipilimumab; pembro: pembrolizumab; LN: lymph node; SBRT: stereotactic body radiotherapy; nivo: nivolumab.
At 27 months after diagnosis, FDG PET demonstrated a systemic response to BRAF-targeted therapy. However, the patient presented with nausea and exertional headache. A brain MRI revealed diffuse intracranial LMD, and cerebrospinal fluid cytology was positive for malignant cells. He was started on nivolumab and briefly resumed on encorafenib and binimetinib, the latter of which was then held for pCSI (30 Gy in 10 fractions, Fig. 1B). The patient tolerated pCSI well with no toxicities other than Common Terminology Criteria for Adverse Events grade 1 fatigue dysgeusia.[21] After completing pCSI, the patient was started on dabrafenib and trametinib. Anti–LAG-3 ICI relatlimab was later added. Three months after the completion of pCSI, diffuse leptomeningeal CNS radiographic progression was noted. Carboplatin and paclitaxel were started. The patient also underwent Ommaya catheter placement and received intrathecal interleukin-2 (IL2). One month after intrathecal IL2 therapy initiation, an MRI of the brain demonstrated further LMD progression. IL2 and relatlimab were stopped, and bevacizumab and ipilimumab were added. The patient remained alive 7 months after LMD diagnosis and continued combination systemic therapy.
Patient 2
A male patient in his 70s was diagnosed with BRAF-wildtype melanoma after a biopsy of a nodular lesion involving their right thigh (Fig. 1C). The initial PET demonstrated multiple hypermetabolic lesions involving right upper extremity, thoracic, and bilateral lower extremity musculature, as well as multiple cervical, thoracic, and retroperitoneal lymph nodes. A brain MRI identified a left frontal lobe lesion, which was treated with stereotactic radiosurgery (SRS). He started systemic therapy with ipilimumab. A surveillance MRI of the brain identified additional metastases, treated with SRS. Five months after the initial diagnosis, a PET scan demonstrated systemic progression with an FDG-avid lymph node biopsy confirming metastatic melanoma. The patient was treated with pembrolizumab for 6 months with a radiographic response on PET imaging. Ninety months after the initial diagnosis, a brain MRI identified two new left frontal metastases, again treated with SRS. After this, the patient was started on ipilimumab and nivolumab.
Almost 9 years after the initial diagnosis, a brain MRI demonstrated enhancement involving bilateral internal auditory canals consistent with LMD, which was asymptomatic. An MRI 1 month later demonstrated increasing findings of LMD. Owing to rapid radiographic progression and high clinical suspicion for LMD, cerebrospinal fluid analysis was deferred, and the patient was urgently treated with pCSI (30 Gy in 10 fractions, Fig. 1D) with concurrent nivolumab and ipilimumab. The patient had a Common Terminology Criteria for Adverse Events grade 1 headache and fatigue during pCSI.[21] Approximately 2 months later, the clinical course was complicated by the development of ICI hepatitis. The patient improved after discontinuing ICI and administering corticosteroids and mycophenolate mofetil. He remained progression free and asymptomatic from LMD 5 months after diagnosis, with a resolution of leptomeningeal enhancement on the most recent imaging.
DISCUSSION
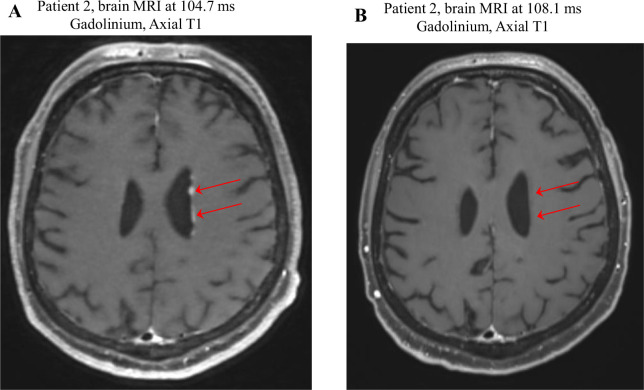
We report two consecutive patients with LMD related to melanoma treated with pCSI and ICI, showing that this approach is feasible in clinical practice with a signal of possible improved survival. One of our patients had a radiographic response (Fig. 2), further supporting the effectiveness of therapy.
Figure 2.
MRI shows Patient 2’s response to proton craniospinal irradiation and immune checkpoint inhibitor therapy. (A) 3- to 4-mm low-density subependymal nodules (arrows) were observed along the body of the left lateral ventricle and anterior horn of the left lateral ventricle, representing metastatic disease. (B) Resolution of low-density subependymal nodules (arrows) after treatment with ipilimumab and nivolumab, proton craniospinal irradiation, and nivolumab maintenance therapy.
Both patients received ICI before diagnosis of LMD and later received additional ICI in combination with pCSI for LMD. While patient 1 experienced CNS radiographic progression despite the combination of pCSI, ICI, and BRAF-targeted therapy, he remained alive 7 months after LMD diagnosis and has tolerated multiple ICIs targeting PD1, CTLA4, and LAG-3. Patient 2 stabilized after pCSI and ICI treatment with evidence of radiographic response and remained progression free and asymptomatic from LMD 5 months after diagnosis (Fig. 2).
Although our patient cohort is small and requires prospective evaluation, combination treatment with pCSI and ICI has a tolerable toxicity profile. Our first patient did experience ICI-induced hepatitis before a diagnosis of LMD and pCSI. Despite this, he tolerated pCSI concurrently with nivolumab without toxicities requiring ICI therapy interruption or discontinuation. He later tolerated the addition of relatlimab, again without severe toxicity, requiring ICI discontinuation. Our second patient developed hepatitis related to ipilimumab and nivolumab, but this was 2 months after completion of pCSI and the initiation of dual ICI therapy. While it is conceivable that preceding pCSI potentiated this toxicity, hepatitis is a known adverse event associated with ICI therapy. For example, combined nivolumab and ipilimumab therapy was associated with grade 3 or greater alanine transaminase elevations in 16% of participants in a prior metastatic melanoma clinical trial.[22]
CONCLUSION
LMD is characterized by high-symptom burdens, a dismal prognosis, and inevitable and relentless disease progression. Tolerance and preliminary efficacy of pCSI have been demonstrated in two previously reported clinical trials.[4,7,10] However, disease progression still occurs after pCSI, with patients only rarely demonstrating an OS longer than 6 months. There is a great need for novel therapeutics to alleviate symptom burden and prolong survival. Our experience with these patients demonstrates that the addition of systemic therapy to pCSI is both clinically feasible and has promising initial responses.
Patients with radiation-resistant tumors may benefit from the addition of ICI. Additionally, patients previously treated with ICI may still derive benefit from combination therapy, as evidenced by the two cases reported here. Adding targeted therapy to pCSI and ICI may be feasible in select cases where such options are available. While broad conclusions regarding the safety and tolerability of this approach cannot be drawn based on this limited experience, a prior meta-analysis does support the safety of combining RT with ICI for melanoma CNS metastases.[23] This may be applicable to LMD related to melanoma or, more broadly, to other solid tumors with progressive LMD, as we have also seen benefits in a patient with lung cancer.[24] Factors such as performance status at the time of LMD diagnosis, corticosteroid use, prior systemic therapies, and prior neuroaxis RT may influence which patients derive the most benefit from pCSI ICI combination therapy and must be elucidated with further study. Prospective evaluation of this approach, including consideration of optimal radiation and ICI dosing based on underlying tumor type, is ultimately warranted given the critical need for novel therapies for LMD, the longer-than-expected survival in our patients, and the radiographic response in one of our patients (which rarely occurs in LMD). A trial evaluating the combination of pCSI and ICI is currently under development at our institution.
Footnotes
Sources of Support: This work was supported in part by funds from the National Center for Advancing Translational Sciences (grant no. UL1 TR002377) and by the United States Food and Drug Administration (grant no. R01 FD-R-07288).
Conflicts of Interest: None.
References
- 1. Sener U,, Kumthekar P,, Boire A. Advances in the diagnosis, evaluation, and management of leptomeningeal disease Neurooncol Adv 2021. 3 (Suppl 5): v86–v95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oechsle K,, Lange-Brock V,, Kruell A,, et al. Prognostic factors and treatment options in patients with leptomeningeal metastases of different primary tumors: a retrospective analysis J Cancer Res Clin Oncol 2010. 136 1729–1735 [DOI] [PubMed] [Google Scholar]
- 3. Yang JCH,, Kim SW,, Kim DW,, et al. Osimertinib in patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer and leptomeningeal metastases: the BLOOM study J Clin Oncol 2020. 38 538–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lamba N,, Wen PY,, Aizer AA. Epidemiology of brain metastases and leptomeningeal disease Neuro Oncol 2021. 23 1447–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buszek SM,, Chung C. Radiotherapy in leptomeningeal disease: a systematic review of randomized and non-randomized trials. Front Oncol. 2019;9:1224. doi: 10.3389/fonc.2019.01224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barney CL,, Brown AP,, Grosshans DR,, et al. Technique, outcomes, and acute toxicities in adults treated with proton beam craniospinal irradiation Neuro Oncol 2014. 16 303–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang TJ,, Wijetunga NA,, Yamada J,, et al. Clinical trial of proton craniospinal irradiation for leptomeningeal metastases Neuro Oncol 2021. 23 134–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Song S,, Park HJ,, Yoon JH,, et al. Proton beam therapy reduces the incidence of acute haematological and gastrointestinal toxicities associated with craniospinal irradiation in pediatric brain tumors Acta Oncol 2014. 53 1158–1164 [DOI] [PubMed] [Google Scholar]
- 9. Chen Z,, Dominello MM,, Joiner MC,, Burmeister JW. Proton versus photon radiation therapy: a clinical review. Front Oncol. 2023;13:1133909. doi: 10.3389/fonc.2023.1133909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang JT,, Wijetunga NA,, Pentsova E,, et al. Randomized phase II trial of proton craniospinal irradiation versus photon involved-field radiotherapy for patients with solid tumor leptomeningeal metastasis J Clin Oncol 2022. 40 3858–3867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun. 2020;11:3801. doi: 10.1038/s41467-020-17670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Naidoo J,, Schreck KC,, Fu W,, et al. Pembrolizumab for patients with leptomeningeal metastasis from solid tumors: efficacy, safety, and cerebrospinal fluid biomarkers. J Immunother Cancer. 2021;9:e002473. doi: 10.1136/jitc-2021-002473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brastianos PK,, Lee EQ,, Cohen JV,, et al. Publisher correction: single-arm, open-label phase 2 trial of pembrolizumab in patients with leptomeningeal carcinomatosis. Nat Med. 2020;26:1309. doi: 10.1038/s41591-020-0978-1. [DOI] [PubMed] [Google Scholar]
- 14. Ko EC,, Formenti SC. Radiotherapy and checkpoint inhibitors: a winning new combination? Ther Adv Med Oncol 2018. 10 1758835918768240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pham TN,, Coupey J,, Candeias SM,, et al. Beyond lymphopenia, unraveling radiation-induced leucocyte subpopulation kinetics and mechanisms through modeling approaches. J Exp Clin Cancer Res. 2023;42:50. doi: 10.1186/s13046-023-02621-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ngwa W,, Irabor OC,, Schoenfeld JD,, et al. Using immunotherapy to boost the abscopal effect Nat Rev Cancer 2018. 18 313–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Antonia SJ,, Villegas A,, Daniel D,, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer N Engl J Med 2017. 377 1919–1929 [DOI] [PubMed] [Google Scholar]
- 18. Jabbour SK,, Lee KH,, Frost N,, et al. Pembrolizumab plus concurrent chemoradiation therapy in patients with unresectable, locally advanced, stage III non-small cell lung cancer: the Phase 2 KEYNOTE-799 Nonrandomized Trial JAMA Oncol 2021. 7 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tagliaferri L,, Lancellotta V,, Fionda B,, et al. Immunotherapy and radiotherapy in melanoma: a multidisciplinary comprehensive review Hum Vaccin Immunother 2022. 18 (3): 1903827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Minniti G,, Anzellini D,, Reverberi C,, et al. Stereotactic radiosurgery combined with nivolumab or Ipilimumab for patients with melanoma brain metastases: evaluation of brain control and toxicity. J Immunother Cancer. 2019;7:102. doi: 10.1186/s40425-019-0588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Services USDoHaH. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Accessed Aug 24, 2023. ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf.
- 22. Tawbi HA,, Forsyth PA,, Algazi A,, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain N Engl J Med 2018. 379 (8): 722–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sha CM,, Lehrer EJ,, Hwang C,, et al. Toxicity in combination immune checkpoint inhibitor and radiation therapy: a systematic review and meta-analysis Radiother Oncol 2020. 151 141–148 [DOI] [PubMed] [Google Scholar]
- 24. Webb MJ,, Breen WG,, Laack NN,, et al. Proton craniospinal irradiation with bevacizumab and pembrolizumab for leptomeningeal disease: a case report. CNS Oncol. 2023;12:CNS101. doi: 10.2217/cns-2023-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]