Abstract
Background:
Burkitt lymphoma (BL) accounts for 90% of pediatric lymphomas in sub-Saharan Africa. Plasmodium falciparum malaria is considered an etiological factor of BL. We describe the geographic distribution of pediatric BL in Malawi and association with P. falciparum malaria prevalence rate (PfPR).
Methods:
We enrolled 220 pathologically confirmed incident pediatric BL cases (2013–2018) into an observational clinical cohort at Kamuzu Central Hospital (KCH) in Lilongwe district. KCH is the main tertiary cancer referral center serving the central and northern regions of Malawi. Using an ecological study design, we calculated district-level annual BL incidence rate using census population estimates. District-level PfPR was extracted from the National Malaria Control Program 2010 report. BL incidence and PfPR maps were constructed in QGIS. Moran’s I test was used to identify BL spatial clusters. Pearson’s correlation and multiple linear regression analyses were used to statistically examine the relationship between PfPR and BL.
Results:
BL incidence was higher in central region districts (8.2 cases per million) than northern districts (2.9 cases per million) and was elevated in lakeshore districts. Districts with elevated PfPR tended to have elevated BL incidence. A low-risk BL cluster was detected in the north. Statistically, BL incidence was positively correlated with PfPR (r = .77, p < .01). A 1% increase in PfPR predicted an increase in BL incidence of 0.2 cases per million (p = .03), when controlling for travel time from referral district hospital to KCH.
Conclusion:
Our study supports evidence for an association between P. falciparum and BL and highlights a need to improve geographic accessibility to tertiary cancer services in Malawi’s northern region.
Keywords: lymphoma Malawi, malaria, pediatric, pediatric Burkitt lymphoma, pediatric hematological malignancies, spatial analysis Burkitt lymphoma
1 │. INTRODUCTION
Burkitt lymphoma (BL), an aggressive B-cell non-Hodgkin lymphoma is the most common pediatric cancer in sub-Saharan Africa (SSA),1 accounting for up to 90% of pediatric lymphomas in the region.2 In the early 1960s, Dr. Dennis Burkitt published a map displaying the geographic distribution of documented BL cases in SSA.3,4 The map showed that BL cases originated in areas with climatic and topographic characteristics favoring endemic Plasmodium falciparum malaria transmission. This finding provided principal epidemiological evidence for the role of P. falciparum malaria, leading to holoendemic P. falciparum malaria becoming widely considered a co-factor in BL pathogenesis5–10 together with Epstein–Barr virus (EBV).
In SSA children, it is proposed that pediatric BL pathogenesis is driven by extensive expansion of a monoclonal EBV-infected B-cell population, suppression of EBV-specific T-cell immunity, reactivation and massive propagation of EBV, and activation-induced cytidine deaminase (AID)-dependent genomic translocation.5,6,9–12 This occurs more frequently in children who experience repeated P. falciparum malaria co-infection overtime before the age of 5 years, leading to a peak age incidence of BL clinical presentation at age 8 years in SSA.13
Contemporary studies in SSA have also reported increased BL risk in areas with hyperendemic P. falciparum transmission,1,2,14–16 corroborating Dr. Burkitt’s early findings. In Kenya, children in areas with chronic P. falciparum transmission were found to be 3.48–7.30 times more likely to develop BL than those living in low P. falciparum transmission areas.1,16 In Mozambique, higher rates of BL were observed in the northern region where malaria transmission is elevated.2 However, studies in Uganda and Cameroon observed increased BL risk in both high P. falciparum endemicity areas and low P. falciparum endemicity areas.14,17–20
In Malawi, a 1993 study by van den Bosch et al.21 identified time–space clusters for BL cases diagnosed between 1987 and 1989. Since then, however, there have been no contemporary studies that describe the spatial distribution of BL in Malawi and its potential association with P. falciparum malaria, despite BL accounting for half of all pediatric cancers22 and there being an estimated four million cases of P. falciparum malaria that occur annually in the country.23,24
Herein, we generate a district-level map of pathologically confirmed pediatric BL cases enrolled between 2013 and 2018 into the Kamuzu Central Hospital (KCH) Lymphoma Study, an observational clinical cohort based at the main referral center for cancer treatment in central and northern Malawi. Using an ecological study design, we assess the association between pediatric BL cases and district-level P. falciparum malaria prevalence, hypothesizing that BL risk will be elevated in districts with higher P. falciparum malaria prevalence. We also examine the potential influence of geographic accessibility from referring sites to KCH on BL incidence estimates.
2 │. METHODS
2.1 │. Geographic context
Malawi has five main climatic zones; the northern zone, lakeshore zone, central zone, Shire Valley zone, and southern zone25 (Figure S1). P. falciparum malaria intensity varies by elevation and rainfall, with the most severely affected areas located in the Shire Valley and lakeshore zones, followed by the central zone. EBV is ubiquitous.26 Administratively, Malawi is divided into three regions, the north, south, and central regions. The regions are subdivided into 28 districts (Figure 1).
FIGURE 1.
Map of Malawi’s central and northern regions and districts. Map includes district names and boundaries
2.2 │. Burkitt lymphoma data
The KCH Lymphoma Study is an observational clinical cohort that enrolled pediatric patients (0–19 years) with incident pathologically confirmed lymphoproliferative disorders between 2013 and 2018 at KCH, a tertiary hospital in the Malawian capital of Lilongwe. The capital Lilongwe is situated in Lilongwe district in the central region of Malawi. KCH is one of the country’s main referral centers for cancer treatment, serving approximately 10 million people with a catchment area that includes Malawi’s northern and central regions. The KCH Lymphoma Study was approved by the University of North Carolina (UNC) Institutional Review Board and Malawi National Health Sciences Research Committee. All cases were diagnosed with real-time evaluation through weekly telepathology conferences involving pathologists in Malawi and the United States. The pathologists rendered a consensus diagnosis using local tissue biopsy and immunohistochemistry stains.27 In addition, all specimens were shipped to the UNC in the United States for diagnostic confirmation using additional immunophenotyping with a larger panel of immunohistochemistry stains if needed.27,28
Demographic information, including district of residence, was recorded for each participant at enrollment. For the analysis, patients who reported from the southern region were excluded because there is a competing tertiary facility that provides cancer treatment in Blantyre district in the southern region. It is therefore less common for patients from this region to travel to Lilongwe for treatment.
2.3 │. Malaria data
District-level P. falciparum malaria prevalence rates (PfPR) for 2010 were extracted from an epidemiological malaria profile published by the Malawi National Malaria Control Program.29 PfPR estimates from 2010 are used in order to take into account the temporal lag between recurrent EBV reactivation driven by repeated malaria co-infection over time, and BL pathogenesis.13 The National Malaria Control Program malaria profile bases PfPR estimates on quantitative cross-sectional community malaria data from published and unpublished sources. PfPR data were standardized to the classical age range of 2–10 years (PfPR2–10) and transformed into district-level estimates. The original profile fully describes the statistical approaches used to produce district-level PfPR2–10 estimates.29
2.4 │. Population data
Childhood population (0–19 years) was estimated for 2013 based on population projections from the Malawi National Statistics Office.30 The projections were calculated considering various population components, including mortality, fertility, and migration. Childhood population was estimated for 2018 based on findings from the Malawi Population and Housing Census published by the National Statistics Office.31 The population structure for 0–19-year olds was similar between study districts when broken down into age groups 0–4, 4–9, 10–14, and 15–19 years (Table S2). We calculated 2013–2018 mid-study childhood population estimates for each district. To calculate the 6-year average BL incidence rate for each study district, we used the mid-study childhood population as the denominator. Six-year average BL crude cases were used as the numerator.
2.5 │. Travel time to KCH
KCH is the only hospital that provides pediatric cancer care services for the central and northern regions of Malawi. Referral travel time from other district hospitals in the central and northern regions to KCH varies from district to district and is considerably longer for districts in the northern region than for districts in the central region. Referral travel to KCH may be delayed due to the financial burden and long travel times for BL cases, who reside in districts that are further away. If those cases die prior to reporting to KCH for pathological BL diagnosis, the BL incidence rate for these districts may be underestimated. We therefore calculated and controlled for geographic accessibility to KCH in the analysis. Access Mod 5,32 an open-source spatial modeling program, was used to estimate travel time to KCH as a proxy for geographic accessibility to KCH. The program accounts for landscape restraints, elevation, land use, travel mode, and travel speed to produce a raster map in which each grid represents the minimum travel time from that grid to a specified destination (KCH). The travel speeds used in the model were extracted from existing literature.33–35
2.6 │. Data visualization and analysis
We employed an ecological study design to assess the association between pediatric BL and P. falciparum prevalence. All maps were constructed in QGIS3.36 Class breaks for the BL incidence and PfPR2–10 maps were determined using four-tier quantiles. Quantitative data analysis was conducted in STATA version 12.0 (College Station, TX, USA).37 Non-normally distributed demographic data were described with medians and interquartile ranges (IQRs). Pearson’s test for correlation and multiple linear regression was used to statistically examine the association between PfPR2–10 and referral travel time from district hospital to KCH and BL incidence. A two-sided alpha of .05 was considered statistically significant. GeoDa,38 an open-source spatial cluster detection program, was used to conduct spatial analyses. Global Moran’s I test was used to estimate the degree of spatial autocorrelation between districts for BL incidence and P. falciparum prevalence.39 Positive autocorrelation is indicated by Moran’s I statistic between 0 and 1, and suggests clusters of similar values (low rates or high rates) for a particular variable. Moran’s I statistic close to zero indicates random or no clustering. Queen’s contiguity was selected as the specified spatial weight for the spatial analyses.40 A pseudo p-value was calculated to assess significance based on 999 conditional randomizations.41 In addition, a local univariate Moran’s I test was run to identify the specific districts that formed BL and P. falciparum malaria clusters.
3 │. RESULTS
3.1 │. Study population
From July 2013 to November 2018, a total of 237 pediatric patients with incident BL were enrolled into the KCH study cohort. We excluded 17 participants from the analytic sample based on the following criteria: the participant reported from Mozambique (n = 6), the participant reported from the southern region of Malawi (n = 2), the participant reported from Likoma Island district (this island is situated in Lake Malawi more than 69 km from the mainland) (n = 1), or the participant had no district of residence recorded (n = 8). Of the 220 participants remaining in the analytic sample, 76 (34.6%) were female and 144 (65.4%) were male. Median age at enrollment was 9 years (IQR: 6–12). Four (1.8%) participants were HIV-positive and HIV status was unknown for 16 participants (7.3%). Twenty-one (9.5%) participants reported from the northern region, of these 11 (52.4%) reported from districts along the lakeshore of Lake Malawi. One hundred ninety-nine (90.5%) participants reported from the central region, of these 28 (14%) reported from districts along the lakeshore. The overall 6-year average annual BLIR for the entire study population was 6.3 cases per million.
3.2 │. Distribution of BL cases
District-level 6-year average annual BL incidence is presented in Table 1. In general, districts in Malawi’s central region had higher BL incidence than districts in Malawi’s northern region, with an average BL incidence rate of 8.2 cases per million for districts in the central region compared to an average BLIR of 2.9 cases per million for districts in the northern region. BL incidence was highest in Mchinji district (13.6 cases per million), followed by central region districts in the lakeshore climatic zone, Nkhotakota (10.0 cases per million) and Salima (9.8 cases per million). In the northern region, BL incidence was highest in districts in the lakeshore climatic zone, Nkhata Bay and Karonga, with 6.6 cases per million and 4.2 cases per million, respectively. Overall, northern districts Rumphi and Chitipa had the lowest BL incidence (0.0 cases per million and 1.3 cases per million, respectively).
TABLE 1.
Six-year average annual incidence rate of pediatric BL per 1,000,000 children by district in central, northern, and lakeshore climatic zones of Malawi, 2013–2018
| n (%) | BL crude cases (n = 220) | 6-year BL case averagea (2013–2018) | 6-year average annual BLIR per 1,000,000b |
|---|---|---|---|
| Northern highland districts | |||
| Mzimba | 9 (3.2) | 1.50 | 2.5 |
| Chitipa | 1 (0.5) | 0.17 | 1.3 |
| Rumphi | 0 (0.0) | 0.00 | 0.0 |
| Central districts | |||
| Dedza | 23 (10.5) | 3.83 | 8.9 |
| Dowa | 19 (8.7) | 3.17 | 7.8 |
| Kasungu | 20 (9.1) | 3.33 | 7.2 |
| Ntcheu | 9 (4.1) | 1.50 | 4.4 |
| Lilongwe | 70 (31.8) | 11.67 | 9.0 |
| Ntchisi | 3 (1.4) | 0.50 | 3.0 |
| Mchinji | 27 (12.3) | 4.50 | 13.6 |
| Lakeshore districts | |||
| Nkhata Bay (N) | 6 (2.7) | 1.00 | 6.6 |
| Karonga (N) | 5 (2.3) | 0.83 | 4.2 |
| Salima (C) | 15 (6.8) | 2.50 | 9.8 |
| Nkhotakota (C) | 13 (5.9) | 2.17 | 10.0 |
Note: BLIR 6-year average annual incidence rate of pediatric Burkitt lymphoma.
Abbreviations: BL, Burkitt lymphoma; BLIR, Burkitt lymphoma incidence rate; C, central; N, north.
Total number of crude cases divided by 6.
Six-year BL case average divided by mid-study average population × 1,000,000.
3.3 │. Spatial clustering of BL and malaria
The global Moran’s I test detected moderate levels of spatial autocorrelation for BL incidence, indicating that BL incidence was more spatially clustered than would be expected if the distribution were random (Moran’s I = .387, p < .01). A moderate level of spatial autocorrelation was also detected for malaria prevalence (Moran’s I = .403, p < .01). The local Moran’s I detected a significant (p < .05) low-value BL cluster in the northern-most region of Malawi comprising Chitipa, Karonga, and Rumphi districts (Figure 2C). A coinciding low-value malaria cluster was also identified (Figure 2D). In the central region, Lilongwe was detected as a high-value BL cluster (p < .01) and high-value malaria cluster (p < .10), indicating that Lilongwe has a high BL incidence rate and malaria prevalence, and is situated among districts with high BL incidence and malaria prevalence. Ntchisi was detected as a low-value outlier (p < .10) for BL, indicating that it has a relatively low BL incidence, although surrounded by districts with relatively high BL incidence. For malaria however, Ntchisi was detected as a high-value cluster (p < .05).
FIGURE 2.
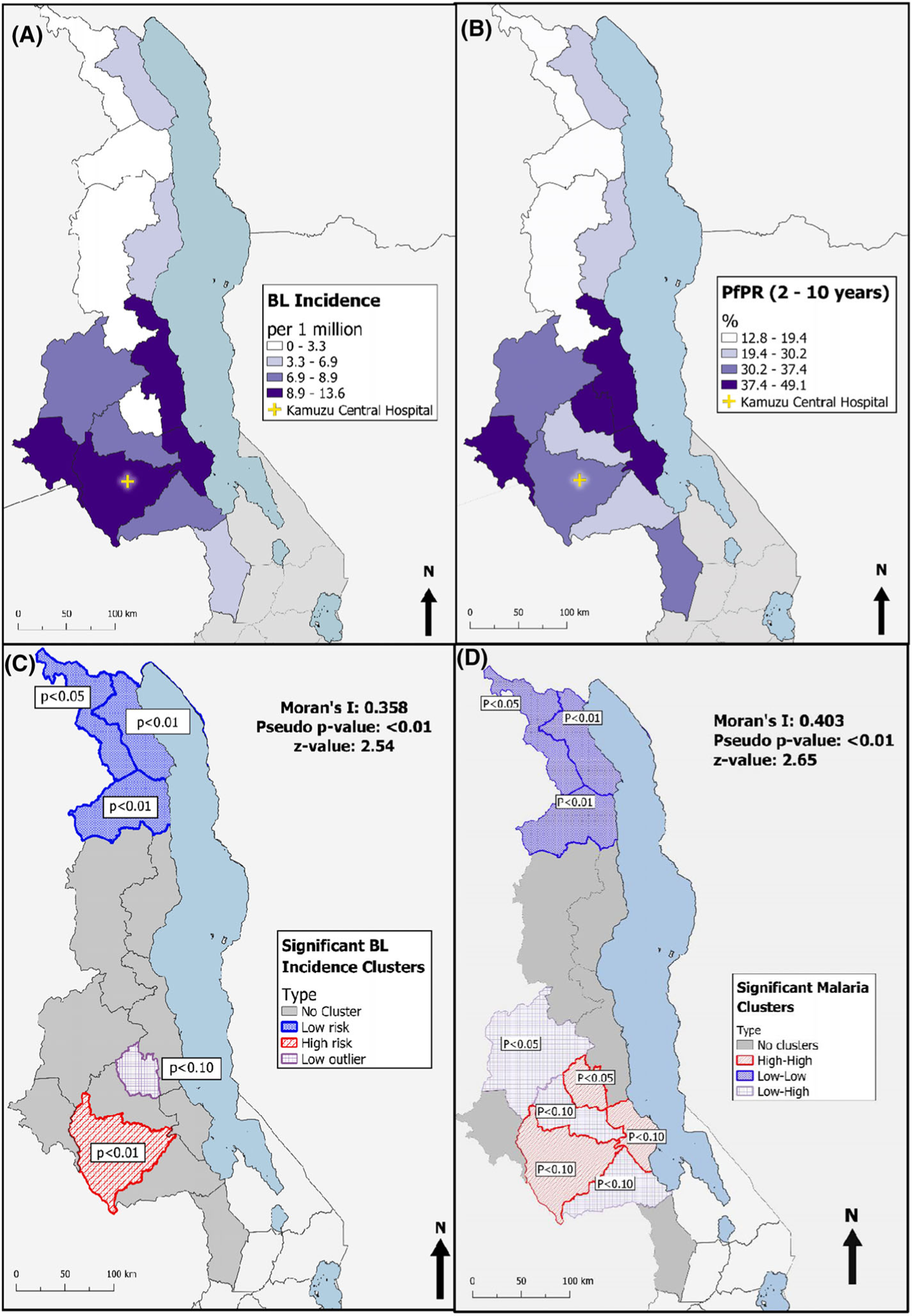
(A) Map of 6-year pediatric Burkitt lymphoma (BL) incidence rate per 1,000,000 by district for 2013–2018. (B) Map of Plasmodium falciparum malaria prevalence by district for year 2010. (C) Map of significant (p < .10) clusters of BL identified by Anselin’s Local Moran test for spatial autocorrelation in GeoDa. (D) Map of significant (p < .10) clusters of Plasmodium falciparum malaria prevalence rate (PfPR) identified by Anselin’s Local Moran test for spatial autocorrelation in GeoDa
3.4 │. Association of BL incidence with malaria and travel time to KCH
To examine the association between BL incidence and PfPR2–10, we visually compared a map displaying district-level 6-year annual BL incidence (Figure 2A) with a corresponding map showing district-level PfPR2–10 (Figure 2B). A similar pattern is observed between the two maps; districts with elevated PfPR2–10 also tend to have elevated BL incidence. Mchinji, Nkhotakota, Salima, and Ntchisi districts are in the top quartile for PfPR2–10, indicated by the darkest fill shade in Figure 2B. Similarly, these districts are in the top quartile for BL incidence (indicated by the same fill shade in Figure 2A), with the exception of Ntchisi, which is in the lowest quartile for BL incidence (indicated by the faintest fill shade). The remaining districts in the central region fall within the second and third quartiles for both PfPR2–10 and BL incidence, although the degree of correspondence between specific quartile level varies by district. In the northern region, districts in the lakeshore climatic zone (Karonga and Nkhata Bay) are in the second lowest quartile for both PfPR2–10 and BL incidence. The three remaining northern districts Chitipa, Rhumpi, and Mzimba are in the lowest quartile for both PfPR2–10 and BL incidence.
To examine the influence of geographic accessibility to KCH on BL incidence estimates, we visually compared a map displaying travel time to KCH (Figure 3) with the BL incidence map (Figure 2A). A general pattern can be observed between the maps. In central region districts (Lilongwe, Mchinji, Dowa, Dedza, Salima, and Ntchisi), where it takes between 0 and 4 hours to travel to KCH (depending on the starting point), BL incidence falls within the top two quartiles with the exception of Ntchisi. In central region districts that have areas where travel times to KCH exceed 4 hours, such as in the outskirts of Kasungu district, the southernmost part of Ntcheu district, and most of Nkhotakota district, BL incidence falls within the second lowest quartile, with the exception of Nkhotakota. In the northern region where travel time to KCH exceeds 4 hours in most areas, BL incidence falls within the two lowest quartiles.
FIGURE 3.
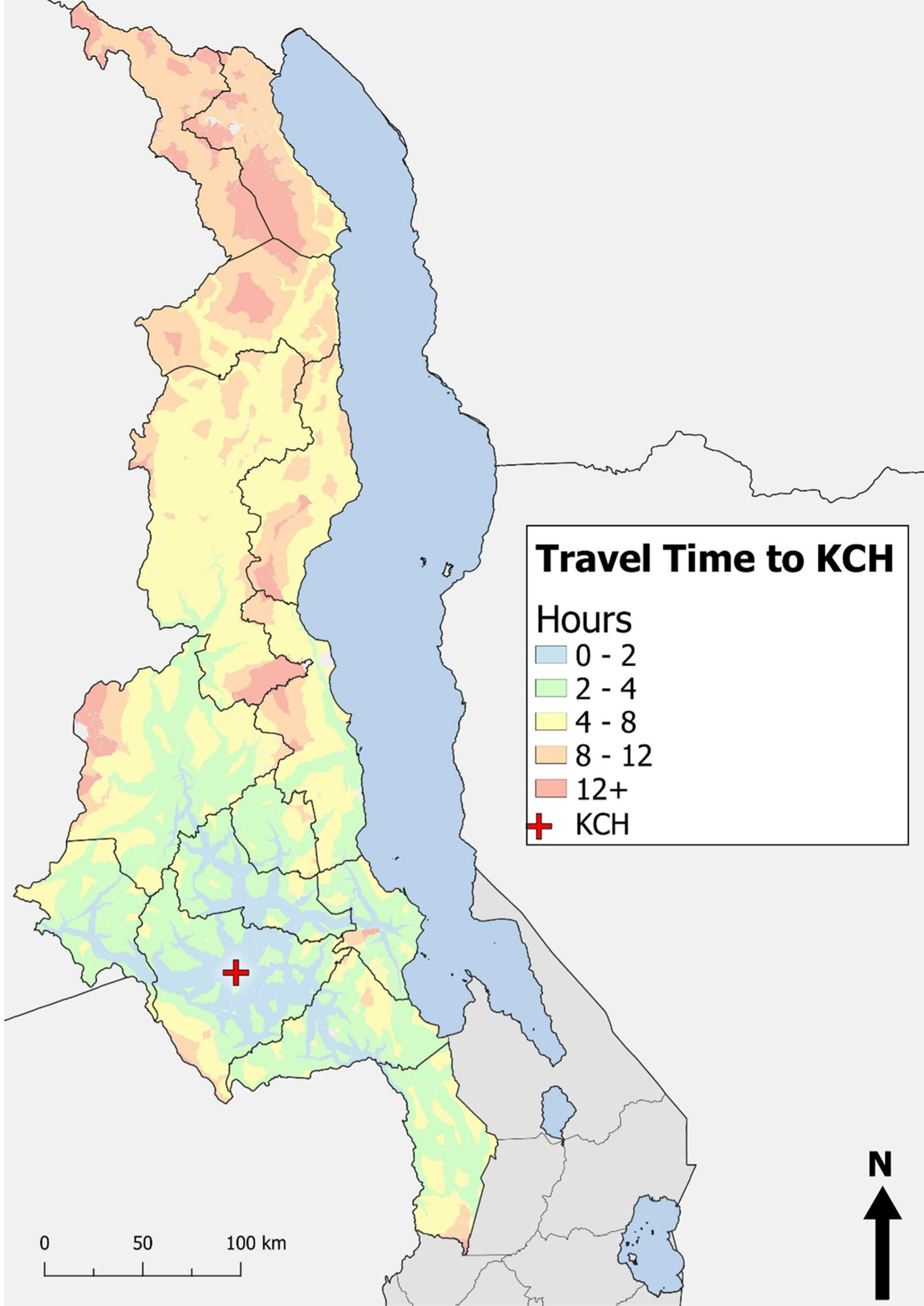
Raster map of travel time from referring sites to Kamuzu Central Hospital
Pearson’s correlation test was conducted to statistically examine the visual patterns we observed between BL incidence and PfPR2–10 (Table 2) and between BL incidence and travel time to KCH (Table 2). The results indicate a strong positive correlation between BL incidence and PfPR2–10 (r = .77, p < .01), and a strong negative correlation between BL incidence and referral travel time to KCH from district hospital (r =−.67, p < .01). When excluding outlier Ntchisi, the positive correlation between BL incidence and PfPR2–10 increases (r = .87, p < .01). A strong negative correlation is also present between PfPR2–10 and referral travel time to KCH from district hospital (r =−.65, p = .01).
TABLE 2.
Correlations between BL incidence rate per million, PfPR2–10, and referral travel time to KCH from district hospital
| Variables | Correlation coefficient | p-Value |
|---|---|---|
| BL incidence | ||
| PfPR2–10 | 0.77 | <.01 |
| Travel time to KCH from district hospital | −0.67 | <.01 |
| PfPR2–10 | ||
| Travel time to KCH from district hospital | −0.65 | .01 |
Abbreviations: BL, Burkitt lymphoma; KCH, Kamuzu Central Hospital; PfPR2–10, Plasmodium falciparum malaria prevalence rate standardized to the classical age range of 2–10 years.
A multiple linear regression model was constructed to assess the independent role of district-level PfPR2–10 on BL incidence while controlling for referral travel time from district hospital to KCH (Table 3). A 1% increase in PfPR2–10 predicted a rise in BL incidence of 0.2 cases per million (standard error [SE] = 0.078, p = .03) when controlling for referral travel time to KCH from district hospital. A 1-hour increase in travel time to KCH from district hospital predicts a decrease in BL incidence of 0.37 cases per million when controlling for PfPR2–10 (SE = 0.32, p = .26). The overall model was statistically significant (R2 = .646, p =< .01).
TABLE 3.
Association between 6-year average pediatric BLIR per million and independent PfPR2–10 and referral travel time to KCH from district hospital
| Variables | Coefficient (SE)a | p-Value |
|---|---|---|
| PfPR2–10 | .20 (.078) | .03 |
| Referral time to KCH from district hospital | −.37 (.32) | .26 |
Abbreviations: BLPR, Burkitt lymphoma incidence rate; KCH, Kamuzu Central Hospital; PfPR2–10, Plasmodium falciparum malaria prevalence rate standardized to the classical age range of 2–10 years; SE, standard error.
Linear regression model: Burkitt lymphoma incidence as dependent variable, PfPR2–10 and referral time to KCH from district hospital as independent variables. R2 = .646, p =< .01, F(2, 11) = 10.0.
4 │. DISCUSSION
This is the first study since 1993 to map the distribution of pediatric BL cases and evaluate the association between BL and P. falciparum malaria in Malawi. We analyzed incident pediatric BL cases diagnosed from 2013 to 2018 from Malawi’s northern and central regions. We observed a nonrandom distribution of BL cases, identifying one low-risk cluster in the northern region and one high-risk cluster and low-risk outlier in the central region. Supporting our hypothesis, we observed a small but positive association between BL incidence and PfPR2–10, finding that districts with a higher PfPR2–10 were likely to have a higher BL incidence when controlling for referral travel time from district hospital to KCH.
BL incidence was generally higher in districts with elevated P. falciparum malaria risk, particularly in the low-lying central region of Malawi, and in lakeshore districts, which tend to have hotter, wetter, and more humid climates. In the highland areas of the northern region where elevation is higher and temperatures are colder, BL risk was significantly lower. Although BL incidence was more elevated in northern lakeshore districts than in northern highland districts, it remained low compared to central lakeshore districts. The differences in BL incidence between lakeshore districts in the northern region and lakeshore districts in the central region may be attributed to the high level of ecological and topographical diversity present in northern lakeshore districts. Within the northern lakeshore districts, as proximity to the lakeshore increases, conditions become increasingly favorable for P. falciparum malaria transmission. In central lakeshore districts, conditions for P. falciparum malaria transmission are consistent and favorable throughout the district.
Varying referral travel times to KCH may also have contributed to the differences in BL incidence between the northern and central lakeshore districts. For central region lakeshore districts, travel time to KCH falls primarily between 2 and 4 hours, with travel from some areas exceeding 4 hours but rarely exceeding 8 hours. For northern lakeshore districts, travel time to KCH is considerably longer, falling primarily between 4 and 8 hours for Nkhata Bay and a minimum of 8 hours from almost every starting point in Karonga. Long travel times may prevent some BL cases from traveling to KCH to seek diagnostic and treatment services. Thus, the number of incident pediatric BL cases in northern lakeshore districts may be underestimated.
In our analysis, when controlling for PfPR2–10, travel time from district hospital to KCH was negatively associated with BL incidence, but this relationship was not statistically significant. Nonetheless, our findings highlight a need for tertiary cancer treatment services in the northern region of Malawi, as a considerable number of pediatric BL cases originate from the northern lakeshore regions. Improving geographic accessibility to cancer services could potentially lead to earlier presentation and reduce the likelihood of underdiagnosed incident BL cases.
We encountered one outlier in our analysis, the central region district of Ntchisi. Ntchisi district is in the top quartile for PfPR2–10 and travel time from the district to KCH is primarily less than 4 hours, such that we hypothesized the district would have a relatively high BL incidence. Contrary to our hypothesis, Ntchisi district was in the lowest quartile for BL incidence, surrounded by two lakeshore districts in the highest quartile and three inland districts in the second and third quartile for BLIR. It is possible that Nchitsi’s BL incidence is underestimated, or that Ntchitsi has an underlying cofounding factor that may account for the low BLIR observed, warranting further investigation in the future.
Study strengths include high-quality pathologically confirmed diagnoses and recruitment of participants from one of the main cancer treatment facilities in Malawi, which serves approximately 60% of the country’s population. Our study also has some limitations; first, our study was based at a single center at a national teaching hospital, it is not reflective of a population-based cancer registry. However, KCH is the only hospital that provides pediatric cancer care services in Malawi’s central and northern regions. There are no private hospitals in Malawi that offer pediatric cancer care and very few Malawians can afford to travel abroad for cancer treatment. Therefore, it is reasonably expected that we captured most cases that seek treatment from the central and northern regions, except those who die at home prior to referral and those that did not agree to participate in the clinical cohort.
We do not have BL case data from the tertiary cancer treatment facility in the southern region of Malawi where the majority of pediatric BL cases who reside in the south report for diagnosis and treatment. Therefore, we can only provide a partial examination of Malawi’s geographic trends in pediatric BL.
Geographic information for our BL cohort was incomplete beyond the district level, resulting in a sample size of only 14. This small sample size may affect the margin of error of our statistical analyses, specifically the spatial cluster analysis and regression model. Thus, the results of the cluster analysis and regression analysis should be interpreted with caution. That being said, malaria control programs in Malawi are often delivered at the district level, and national malaria statistics are presented at the district level. Thus, conducting our analyses at the district administrative level is appropriate for policy-related applications of our findings.
Because we used an ecological design for this analysis, all comparisons are at the district level, and we cannot make assumptions about the association between P. falciparum and BL incidence for individual participants. Finally, the model that we used to estimate travel time to KCH for each district does not take into account cost of travel or availability and regularity of public transport.
5 │. CONCLUSION
Our study supports continued evidence for the relationship between P. falciparum malaria transmission and BL development, despite significant malaria control efforts in recent years, highlighting the value of better collaboration between malaria and BL programs. Perhaps even more important, our study highlights the need to improve geographic accessibility to cancer services in the northern region of Malawi, as it currently experiences very long travel times to cancer services despite having two lakeshore districts with elevated pediatric BL risk. Finally, further elucidation of etiologic factors related to BL occurrence is important, for example, as illustrated by the unexpectedly low BL incidence despite high malaria prevalence in Ntchisi.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (K01TW011191, K01TW009488, R21CA180815, U54CA190152, P01CA019014), grants from the Lineberger Comprehensive Cancer Center (P30CA016086), and grants from the Fogarty Global Health Fellows Program (R25TW009340). This work was also partly supported by the Fogarty International Center of the National Institutes of Health (D43TW010060). We are exceedingly grateful to the study participants and their families, and for the leadership from Kamuzu Central Hospital, the Malawi Ministry of Health (Lilongwe, Malawi), and the University of North Carolina Project Malawi (Lilongwe, Malawi) for their support in conducting this study.
Funding information
National Institutes of Health, Grant/Award Numbers: K01TW011191, K01TW009488, R21CA180815, U54CA190152, P01CA019014; Lineberger Comprehensive Cancer Center, Grant/Award Number: P30CA016086; Fogarty Global Health Fellows Program, Grant/Award Number: R25TW009340; Fogarty International Center of the National Institutes of Health, Grant/Award Number: D43TW010060
Abbreviations:
- BL
Burkitt lymphoma
- EBV
Epstein–Barr virus
- IQR
interquartile range
- KCH
Kamuzu Central Hospital
- PfPR
Plasmodium falciparum malaria prevalence rate
- PfPR2–10
Plasmodium falciparum malaria prevalence rate standardized to the classical age range of 2–10 years
- SSA
sub-Saharan Africa
- UNC
University of North Carolina
Footnotes
CONFLICT OF INTEREST
No commercial support was provided for this study. This work was completed while Dr. Satish Gopal was employed at the University of North Carolina at Chapel Hill. The opinions expressed in this article are the authors’ own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States Government.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Rainey JJ, Omenah D, Sumba PO, Moormann AM, Rochford R, Wilson ML. Spatial clustering of endemic Burkitt’s lymphoma in high-risk regions of Kenya. Int J Cancer. 2007;120(1):121–127. 10.1002/ijc.22179 [DOI] [PubMed] [Google Scholar]
- 2.O’Callaghan-Gordo C, Casabonne D, Carrilho C, et al. Incidence of endemic burkitt lymphoma in three regions of Mozambique. Am J Trop Med Hyg. 2016;95(6):1459–1462. 10.4269/ajtmh.16-0424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugden B. Epstein–Barr virus: the path from association to causality for a ubiquitous human pathogen. PLoS Biol. 2014;12(9):e1001939. 10.1371/journal.pbio.1001939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magrath I. Denis Burkitt and the African lymphoma. Ecancermedicalscience. 2009;3:159. 10.3332/ecancer.2009.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rochford R, Moormann A. Current topics in microbiology and immunology Barr virus. Burkitt’s Lymphoma; 1:267–280. 10.1007/978-3-319-22822-8_11 [DOI] [PubMed] [Google Scholar]
- 6.Moormann AM, Snider C, Chelimo K. The company malaria keeps: how co-infection with Epstein–Barr virus leads to endemic Burkitt lymphoma. Curr Opin Infect Dis. 2012;24(5):435–441. 10.1097/QCO.0b013e328349ac4f.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peprah S, Ogwang MD, Kerchan P, et al. Risk factors for Burkitt lymphoma in East African children and minors: a case-control study in malaria-endemic regions in Uganda, Tanzania, and Kenya. Int J Cancer. 2020;146(4):953–969. 10.1002/ijc.32390.Risk [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moormann AM, Bailey JA. Malaria — how this parasitic infection aids and abets EBV-associated Burkitt lymphomagenesis. Curr Opin Virol. 2016;20:78–84. 10.1016/j.coviro.2016.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molyneux EM, Rochford R, Griffi B, et al. Burkitt’s lymphoma. Lancet. 2012;379(9822):1234–1244. 10.1016/S0140-6736(11)61177-X [DOI] [PubMed] [Google Scholar]
- 10.del Quintana MP, Smith-Togobo C, Moormann A, Hviid L. Endemic Burkitt lymphoma – an aggressive childhood cancer linked to Plasmodium falciparum exposure, but not to exposure to other malaria parasites. APMIS. 2020;128(2):129–135. 10.1111/apm.13018 [DOI] [PubMed] [Google Scholar]
- 11.Thorley-Lawson D, Deitsch KW, Duca KA, Torgbor C. The link between Plasmodium falciparum malaria and endemic Burkitt’s lymphoma—new insight into a 50-year-old enigma. PLoS Pathog. 2016;12(1):12–16. 10.1371/journal.ppat.1005331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Tong H, Brindley PJ, Meyer CG, Velavan TP. Parasite infection, carcinogenesis and human malignancy. EBioMedicine. 2017;15:12–23. 10.1016/j.ebiom.2016.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rochford R, Moormann AM. Burkitt’s lymphoma. Curr Top Microbiol Immunol. 2015;390:267–285. 10.1007/978-3-319-22822-8_11 [DOI] [PubMed] [Google Scholar]
- 14.Ogwang MD, Bhatia K, Biggar RJ, Mbulaiteye SM. Incidence and geographic distribution of endemic Burkitt lymphoma in northern Uganda revisited. Int J Cancer. 2008;123(11):2658–2663. 10.1002/ijc.23800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kreis C, Doessegger E, Lupatsch JE, Spycher BD. Space–time clustering of childhood cancers: a systematic review and pooled analysis. Eur J Epidemiol. 2019;34(1):9–21. 10.1007/s10654-018-0456-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rainey JJ, Mwanda WO, Wairiumu P, Moormann AM, Wilson ML, Rochford R. Spatial distribution of Burkitt’s lymphoma in Kenya and association with malaria risk. 2007;12(8):936–943. 10.1111/j.1365-3156.2007.01875.x [DOI] [PubMed] [Google Scholar]
- 17.Pike MC, Smith PG, Ziegler JL, Kisuule A. Burkitt’s lymphoma: a time–space cluster of cases in Bwamba county of Uganda. Br Med J. 1971;2(5760):491. 10.1136/bmj.2.5760.491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams EH, Smith PG, Day NE, Geser A, Ellice J, Tukei P. Space–time clustering of Burkitt’s lymphoma in the West Nile district of Uganda: 1961–1975. Br J Cancer. 1978;37(1):109–122. 10.1038/bjc.1978.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams EH, Spit P, Pike MC. Further evidence of space-time clustering of Burkitt’s lymphoma patients in the West Nile district of Uganda. Br J Cancer. 1969;23(2):235–246. 10.1038/bjc.1969.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright NJ, Hesseling PB, Mccormick P, Tchintseme F. The incidence, clustering and characteristics of Burkitt lymphoma in the Northwest province of Cameroon. Trop Doct. 2009;39(4):228–230. 10.1258/td.2009.080373 [DOI] [PubMed] [Google Scholar]
- 21.van den Bosch C, Hills M, Kazembe P, Dziweni CKL. Time-space case clusters of Burkitt’s lymphoma in Malawi. Leukemia. 1993;7(11):1875–1878. [PubMed] [Google Scholar]
- 22.Westmoreland K, Montgomery N, Stanley C, et al. Plasma Epstein–Barr virus DNA for pediatric Burkitt lymphoma diagnosis, prognosis, and response assessment in Malawi. Int J Cancer. 2017;140(11):25092516. 10.1002/ijc.30682.Plasma [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chilanga E, Collin-Vézina D, MacIntosh H, Mitchell C, Cherney K. Prevalence and determinants of malaria infection among children of local farmers in Central Malawi. Malar J. 2020;19(1):308. 10.1186/s12936-020-03382-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaston RT, Ramroop S. Prevalence of and factors associated with malaria in children under five years of age in Malawi, using malaria indicator survey data. Heliyon. 2020;6(5):e03946. 10.1016/j.heliyon.2020.e03946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chirombo J, Ceccato P, Lowe R, et al. Childhood malaria case incidence in Malawi between 2004 and 2017: spatio-temporal modelling of climate and non-climate factors. Malar J. 2020;19(1):5. 10.1186/s12936-019-3097-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piriou E, Asito AS, Sumba PO, et al. Early age at time of primary epstein-barr virus infection results in poorly controlled viral infection in infants from western Kenya: clues to the etiology of endemic Burkitt lymphoma. J Infect Dis. 2012;205(6):906–913. 10.1093/infdis/jir872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montgomery ND, Liomba NG, Kampani C, et al. Accurate real-time diagnosis of lymphoproliferative disorders in Malawi through clinicopathologic teleconferences: a model for pathology services in sub-Saharan Africa. Am J Clin Pathol. 2016;146(4):423–430. 10.1093/ajcp/aqw118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gopal S, Krysiak R, Liomba NG, et al. Early experience after developing a pathology laboratory in Malawi, with emphasis on cancer diagnoses. PLoS One. 2013;8(8):6–13. 10.1371/journal.pone.0070361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Malaria Control Programme, KEMRI-Wellcome Trust Research Programme and London School of Hygiene & Tropical Medicine. Malawi: a Profile of Malaria Control and Epidemiology. National Malaria Control Programme; 2018. [Google Scholar]
- 30.National Statistical Office. Malawi Population Projections 2008– 2030. National Statistical Office; 2008. Accessed July 14, 2022. http://www.nsomalawi.mw/images/stories/data_on_line/demography/census_2008/MainReport/ThematicReports/PopulationProjectionsMalawi.pdf [Google Scholar]
- 31.National Statistical Office. 2018 Malawi Population & Housing Census Main Report. National Statistical Office; 2018. Accessed July 14, 2022. http://www.nsomalawi.mw/images/stories/data_on_line/demography/census_2018/2018%20Malawi%20Population%20and%20Housing%20Census%20Main%20Report.pdf [Google Scholar]
- 32.Ray N, Santiago H, Colombo R, Ebener S. User Manual AccessMod 4.0: Physical Accessibility to Health Care and Population Coverage Modeling. ArcGIS Online; 2013. Accessed July 14, 2022. http://www.arcgis.com/home/item.html?id=f64ccd70c3e045eb8ba6811033c9def6 [Google Scholar]
- 33.Palk L, Okano JT, Dullie L, Blower S. Travel time to health-care facilities, mode of transportation, and HIV eliminationation in Malawi: a geospatial modelling analysis. Lancet Glob Health. 2020;8:e1555–64. 10.1016/S2214-109X(20)30351-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Felix O, George A, Fredrick O, Erick O. The cost-implications of reaching universal coverage of maternity health services in Siaya county, Western Kenya. Public Health Res. 2020;10(1):1–11. 10.5923/j.phr.20201001.01 [DOI] [Google Scholar]
- 35.Chen YN, Schmitz MM, Serbanescu F, Dynes MM, Maro G, Kramer MR. Geographic access modeling of emergency obstetric and neonatal care in Kigoma region, Tanzania: transportation schemes and programmatic implications. Glob Health Sci Pract. 2017;5(3):430–445. 10.9745/GHSP-D-17-00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.QGIS Geographic Information System. QGIS Association; 2021. http://www.qgis.org. Accessed July 14, 2022. [Google Scholar]
- 37.Stata Statistical Software: Release 12. StataCorp; Published online 2011. [Google Scholar]
- 38.Anselin L, Syabri I, Kho Y. GeoDa: an introduction to spatial data analysis. Geogr Anal. 2006;38(1):5–22. 10.1111/j.0016-7363.2005.00671.x [DOI] [Google Scholar]
- 39.Moran PAP. Notes on continuous stochastic phenomena. Biometrika. 1950;37(1–2):17–23. [PubMed] [Google Scholar]
- 40.Anselin L. Contiguity-Based Spatial Weights. GeoDa: An Introduction to Spatial Data Science. GeoDa. Accessed December 15, 2021. https://geodacenter.github.io/workbook/4a_contig_weights/lab4a.html#fn1 [Google Scholar]
- 41.Anselin L. Global Spatial Autocorrelation (1). GeoDa: An Introduction to Spatial Data Science. GeoDa. Accessed December 15, 2021. https://geodacenter.github.io/workbook/5a_global_auto/lab5a.html#permutation-inference [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


