Abstract
Background:
Per- and polyfluoroalkyl substances (PFAS) encompass a class of chemically and structurally diverse compounds that are extensively used in industry and detected in the environment. The US Environmental Protection Agency (US EPA) 2021 PFAS Strategic Roadmap describes national research plans to address the challenge of PFAS.
Objectives:
Systematic Evidence Map (SEM) methods were used to survey and summarize available epidemiological and mammalian bioassay evidence that could inform human health hazard identification for a set of 345 PFAS that were identified by the US EPA’s Center for Computational Toxicology and Exposure (CCTE) for in vitro toxicity and toxicokinetic assay testing and through interagency discussions on PFAS of interest. This work builds from the 2022 evidence map that collated evidence on a separate set of PFAS. Like our previous work, this SEM does not include PFAS that are the subject of ongoing or completed assessments at the US EPA.
Methods:
SEM methods were used to search, screen, and inventory mammalian bioassay and epidemiological literature from peer-reviewed and gray literature sources using manual review and machine-learning software. For each included study, study design details and health end points examined were summarized in interactive web-based literature inventories. Some included studies also underwent study evaluation and detailed extraction of health end point data. All underlying data is publicly available online as interactive visuals with downloadable metadata.
Results:
More than 13,000 studies were identified from scientific databases. Screening processes identified 121 mammalian bioassay and 111 epidemiological studies that met screening criteria. Epidemiological evidence (available for 12 PFAS) mostly assessed the reproductive, endocrine, developmental, metabolic, cardiovascular, and immune systems. Mammalian bioassay evidence (available for 30 PFAS) commonly assessed effects in the reproductive, whole-body, nervous, and hepatic systems. Overall, 41 PFAS had evidence across mammalian bioassay and epidemiology data streams (roughly 11% of searched chemicals).
Discussion:
No epidemiological and/or mammalian bioassay evidence were identified for most of the PFAS included in our search. Results from this SEM, our 2022 SEM on PFAS, and other PFAS assessment products from the US EPA are compiled into a comprehensive PFAS dashboard that provides researchers and regulators an overview of the current PFAS human health landscape including data gaps and can serve as a scoping tool to facilitate prioritization of PFAS-related research and/or risk assessment activities. https://doi.org/10.1289/EHP13423
Introduction
There is a growing need to understand the human health effects of per- and polyfluoroalkyl substances (PFAS) because human exposure to these persistent and bioaccumulative compounds is widespread.1 PFAS are a large and complex class of synthetic chemicals consisting of a fluorinated carbon chain. Although there are many definitions for PFAS,2–4 this research follows the definition used in the US EPA CompTox Chemicals Dashboard,5 which includes substances containing specific substructural elements that “are designed to be simple, reproducible and transparent, yet general enough to encompass the largest set of structures having sufficient levels of fluorination to potentially impart PFAS-type properties.”6 As of August 2022, the CompTox Chemicals Dashboard contains over 14,735 PFAS (PFAS Struct v5).7 Of the thousands of PFAS cataloged in the CompTox Chemicals Dashboard, most toxicity data exist for a relatively small number of legacy PFAS (i.e., long-chain PFAS, such as perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS), that have been phased out of commercial production in the United States).8,9 However, less is known about the toxicity of the thousands of other PFAS.
The research presented in this systematic evidence map (SEM) is one component of the US EPA’s PFAS Strategic Roadmap, a comprehensive approach to research, restrict, and remediate PFAS contamination.10,11 This SEM expands on our previous effort that collated the mammalian bioassay and epidemiological evidence base for a set of PFAS.12 In this SEM, we apply the same systematic review approaches used in our previous work to characterize toxicity data for hundreds of additional procurable, DMSO-solubilized PFAS inventoried by the US EPA’s Center for Computational Toxicology and Exposure (CCTE) in a PFAS testing library.13,14 As described in Patlewicz et al.,14 the list of PFAS chemicals (termed “PFAS 430”) formed the CCTE’s PFAS library and included considerations both for grouping as well as procurement and analytical considerations. A total of 303 PFAS from the PFAS 430 are included in this evidence map, because some of the PFAS 430 were already analyzed in the 2022 SEM.12 In addition, this SEM also characterizes the evidence base for an additional 42 PFAS (termed “additional PFAS”) that were identified as compounds of interest in interagency discussions during the period 2017–2018, resulting in a grand total of 345 PFAS included in this SEM. Because these efforts were completed during distinct temporal windows, some of the literature search results are presented separately rather than pooled (PFAS 430 and additional PFAS).
The goal of this evidence map is to use systematic review methods to catalog the available hazard information and critically evaluate studies for a large number of PFAS, but not to synthesize the data or reach hazard conclusions on the causal relationships between PFAS exposures and health effects. This evidence map’s primary objective is to assemble and summarize a previously uncharacterized literature base of health outcome data. The evidence map can highlight evidence bases with more or less evidence that could inform research efforts on topics that are not well studied. Of note, this SEM does not include PFAS that are the subject of ongoing or completed assessments at the US EPA (Table S1), specifically, perfluorobutanoic acid (PFBA),15 perfluorobutanesulfonic acid (PFBS),16 perfluorohexanoic acid (PFHxA),17 PFOA,18–20 perfluorononanoic acid (PFNA),21 perfluorodecanoic acid (PFDA),22 perfluorohexanesulfonic acid (PFHxS),23 PFOS,19,24,25 and perfluoro-2-methyl-3-oxahexanoic acid (GenX chemicals).26 Therefore, we also developed a comprehensive PFAS dashboard to display data extracted through various US EPA PFAS assessments products [including Office of Water (OW) Health Effects Support Documents, Integrated Risk Information System (IRIS) Assessments, and our PFAS SEMs]. We hope that this comprehensive PFAS dashboard provides researchers and regulators with the evidence available to inform specific analysis questions including risk assessments, PFAS categorization, and novel research to address data gaps. If resourcing allows, this dashboard might be updated as additional US EPA PFAS projects are made publicly available or as projects included within the Dashboard are revised or updated.
Methods
The Office of Research and Development (ORD) Staff Handbook for Developing IRIS Assessments (referred to as the “IRIS Handbook”)27 and a published evidence map template28,29 outlines the systematic review methods used to develop the evidence map. The methods reproduced below are taken directly from our earlier SEMs on PFAS,12,30 because this SEM is a continuation of that work. The methods below have been adjusted, where appropriate, for the specific needs of this SEM (e.g., chemical names, literature search dates, other resources consulted). As described in the introduction, this expanded PFAS includes a list of PFAS chemicals (termed “PFAS 430”)14 and included considerations both for grouping as well as procurement and analytical considerations. A total of 303 PFAS from the PFAS 430 are included in this evidence map, because some of the PFAS 430 were already analyzed in the 2022 SEM.12 In addition, this SEM also characterizes the evidence base for an additional 42 PFAS (termed “additional PFAS”) that were identified as compounds of interest in interagency discussions during the period 2017–2018, resulting in a grand total of 345 PFAS included in this SEM. Because these efforts were completed during distinct temporal windows, some of the literature search results are presented separately rather than pooled (“PFAS 430” and “additional PFAS”).
Populations, Exposures, Comparators, and Outcomes (PECO) Criteria and Supplemental Material Tagging
PECO criteria (presented in Table 1) are used to focus the scope of an evidence map or systematic review by defining the research question(s), search terms, and inclusion/exclusion criteria. In addition to PECO-relevant studies, studies that did not meet PECO criteria but contained “potentially relevant” supplemental material were tracked during the literature screening process. Supplemental material was tagged by category, as outlined in Table 2. Note that “supplemental material” does not refer to findings contained in the supplemental materials of the papers identified. Figure 1 provides a visual overview of our workflow process for evidence map development.
Table 1.
PECO criteria.
| PECO element | Description |
|---|---|
| Populations | Human: Any population and life stage (occupational or general population, including children and other potentially sensitive populations). Animal: Nonhuman mammalian animal species (whole organism) of any life stage (including preconception, in utero, lactation, peripubertal, and adult stages). |
| Exposures | Relevant forms: PFAS chemicals represented by structures and substances identified in the Excel File (Excel Table S1 and Excel Table S21). Human: Any exposure to PFAS via the oral and inhalation routes because these are the most relevant routes of human exposure and typically the most useful for developing human health toxicity values. Studies are also included if biomarkers of PFAS exposure are evaluated (e.g., measured PFAS or metabolite in tissues or bodily fluids) but the exposure route is unclear or reflects multiple routes. Other exposure routes, including dermal, and mixture-only studies (i.e., without effect estimates for individual PFAS of interest) are tracked during title and abstract screening and are tagged as “potentially relevant” supplemental material. Animal: Any exposure to PFAS via oral or inhalation routes. Studies involving exposures to mixtures are included only if a treatment group consists of exposure to a PFAS alone. Other exposure routes, including dermal or injection, and mixture-only studies are tagged as “potentially relevant” supplemental material. |
| Comparators | Human: A comparison or referent population exposed to lower levels (or no exposure/exposure below detection limits) or exposed for shorter periods of time. However, worker surveillance studies are considered to meet PECO criteria even if no referent group is presented. Case reports describing findings in 1–3 people in nonoccupational or occupational settings are tracked as “potentially relevant” supplemental material. Animal: A concurrent control group exposed to vehicle-only treatment and/or untreated control (control could be a baseline measurement). |
| Outcomes | All health outcomes (cancer and noncancer). |
Note: The definitions in the table follow standard template language that is used in systematic evidence maps developed by the US EPA27,28,32 and have only been adjusted, where appropriate, for the specific needs of this SEM. CASRN, Chemical Abstract Service registry number; DTXSID, DSSTox substance identifier; PECO, Populations Exposures Comparators Outcomes; PFAS, per- and polyfluoroalkyl substances; SEM, Systematic Evidence Map.
Full chemical lists of included Expanded PFAS chemicals, DTXSIDs, CASRNs, and synonyms are provided in the supplemental material (Excel Table S1 is the “PFAS 430” list and Excel Table S2 is the “Additional PFAS” list).
Table 2.
Major categories of “potentially relevant” supplemental material.
| Category | Description |
|---|---|
| In vitro, ex vivo, or in silico “mechanistic” studies | In vitro, ex vivo, or in silico studies reporting measurements related to a health outcome that inform the biological or chemical events associated with phenotypic effects, in both mammalian and nonmammalian model systems. |
| ADME | ADME studies are primarily controlled experiments where defined exposures usually occur by intravenous, oral, inhalation, or dermal routes, and the concentration of particles, a chemical, or its metabolites in blood or serum, other body tissues, or excreta are then measured. These data are used to estimate the amount absorbed (A), distributed to different organs (D), metabolized (M), and/or excreted/eliminated (E) through urine, breathe, feces, etc.
|
| Classical PK Model Studies, or PBPK Model studies | Classical PK or Dosimetry Model Studies: Classical PK or dosimetry modeling usually divides the body into just one or two compartments, which are not specified by physiology, where movement of a chemical into, between, and out of the compartments is quantified empirically by fitting model parameters to ADME data. PBPK or Mechanistic Dosimetry Model Studies: PBPK models represent the body as various compartments (e.g., liver, lung, slowly perfused tissue, richly perfused tissue) in order to quantify the movement of chemicals or particles into and out of the body (compartments) by defined routes of exposure, metabolism and elimination, and thereby estimate concentrations in blood or target tissues. |
| Nonmammalian model systems | Studies in nonmammalian model systems, e.g., Xenopus, fish, birds, C. elegans. |
| Transgenic mammalian model systems | Transgenic studies in mammalian model systems. |
| Non-oral or noninhalation routes of administration | Studies in which humans or animals (whole organism) were exposed via a non-oral or noninhalation route (e.g., injection, dermal exposure). |
| Exposure characteristics (no health outcome assessment) | Exposure characteristic studies which include data that are unrelated to health outcomes, but which provide information on exposure sources or measurement properties of the environmental agent (e.g., demonstrate a biomarker of exposure). |
| Mixture studies | Mixture studies that are not considered PECO-relevant because they do not contain an exposure or treatment group assessing only the chemical of interest. This category is generally used for experimental studies and generally does not apply to epidemiological studies where the exposure source may be unclear. |
| Case reports | Case reports describing health outcomes after exposure will be tracked as potentially relevant supplemental information when the number of subjects is . |
| Records with no original data | Records that do not contain original data, such as other agency assessments, informative scientific literature reviews, editorials or commentaries. |
| Conference abstracts | Records that do not contain sufficient documentation to support study evaluation and data extraction. |
| ECHA read-across | Data from ECHA on a nonrelevant chemical that makes inferences about a relevant PFAS chemical. |
| Presumed duplicate | Duplicate studies (e.g., published vs. unpublished reports) identified during data extraction and study quality evaluation. |
Note: “Potentially relevant” supplemental material are studies that do not meet the PECO criteria but may still contain information of interest that was tracked during screening. The definitions in the table follow standard template language that is used in systematic evidence maps developed by the US EPA27,28,32 and have only been adjusted, where appropriate, for the specific needs of this SEM. ADME, absorption, distribution, metabolism, and excretion; ECHA, European Chemicals Agency; PBPK, physiologically-based pharmacokinetic; PK, pharmacokinetic; SEM, Systematic Evidence Map.
Figure 1.
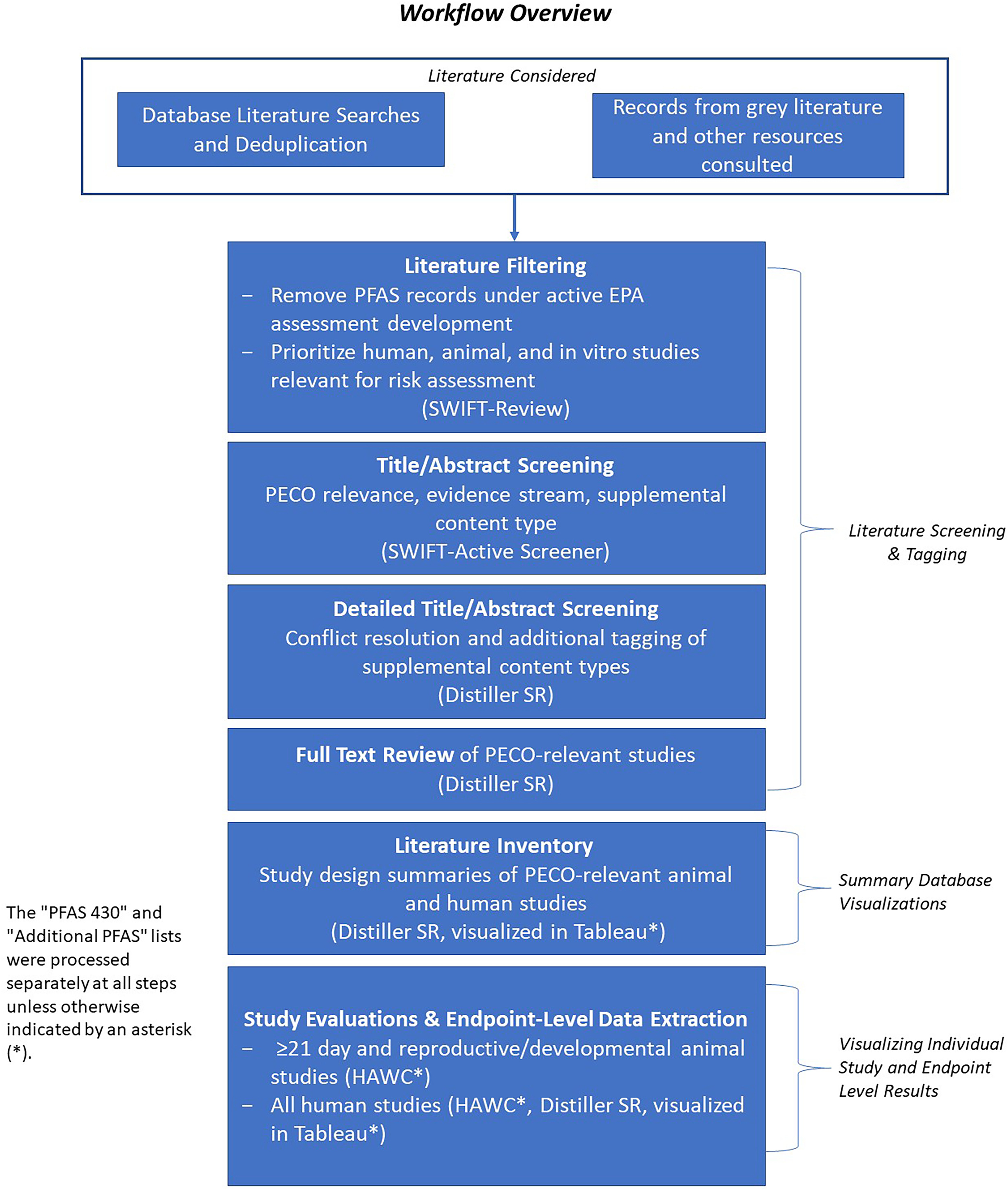
Project workflow overview. Visual flow chart of the literature identification, filtering, screening, review, inventory, and study evaluation and extraction processes that are the summary steps of evidence map development. Note: HAWC, Health Assessment Workspace Collaborative; PECO, population, exposure, comparator, outcome; SR, systematic review.
Literature Search and Screening Strategies
Database search term development.
Chemical search terms were used to search for relevant literature in the databases listed below. The detailed search strategies for each database are presented in the Supplemental Materials, including specific synonyms (Excel Tables S1 and S2) and search strings (Excel Tables S3 through S8).
PubMed [National Library of Medicine (NLM)]
Web of Science (Thomson Reuters)
ToxLine via the ToxNet (included in the 2019 search; no longer operational in the 2020, 2021, and 2022 search updates)
The literature search for the included PFAS consisted only of the chemical name, synonyms, and trade names, and no additional limits with exception of the Web of Science (WoS) search strategy were included. Due to the specifics of searching the WoS, a chemical name-based search can retrieve a very large number of off-topic references. Given the number of PFAS included in this screening effort, a more targeted WoS search strategy was used to identify the records most likely applicable to human health (see Excel Tables S3 through S8). Chemical synonyms for PFAS were identified by using synonyms in the CompTox Chemicals Dashboard31 indicated as “valid” or “good.” The preferred chemical name (as presented in the CompTox Chemicals Dashboard), Chemical Abstract Services registry number (CASRN), and synonyms were then shared with US EPA information specialists who used these inputs to develop search strategies tailored for PubMed, WoS, and ToxLine (see Excel Tables S3 through S8).
Database searches.
The database searches for PFAS from the PFAS 430 were conducted by a US EPA information specialist, and searches were updated in November 2019, November 2021, and December 2022. Searches for the additional PFAS of interest were conducted in November 2019, December 2021, and December 2022. All records were stored in the US EPA’s Health and Environmental Research Online (HERO) database.33,34 The HERO database35 is used to provide access to the references used in the US EPA’s scientific assessments, including this effort. After deduplication in HERO using unique identifiers (e.g., PMID, WoSID, or DOI) and citations, the references went through an additional round of deduplication using the ICF International DeDuper tool (described in detail in Supplemental Material, “DeDuper”) that uses a two-phase approach to identify duplicates by a) locating duplicates using automated logic and b) employing machine learning built from Python’s Dedupe package to predict likely duplicates, which are then verified manually.36 Following deduplication, SWIFT-Review software (version 1.061; Sciome LLC)37 was used to identify which of the unique references were most relevant for human health risk assessment. In brief, SWIFT-Review was used to filter the unique references based on the software’s preset literature search strategies (titled “evidence stream”). These evidence streams were developed by information specialists and can be used to separate the references most relevant to human health from those that are not (e.g., environmental fate studies). References are tagged to a specific evidence stream if the search terms from that evidence stream appear in the title, abstract, keyword and/or medical subject headings (MeSH) fields of that reference. For this SEM, the following SWIFT-Review evidence streams were applied: human, animal models for human health, and in vitro studies. Specific details on the evidence stream search strategies are available through Sciome’s SWIFT-Review documentation.38 Studies not retrieved using the search strategies were not considered further.
Other resources consulted.
The literature search strategies described above are intentionally broad; however, it is still possible that some studies were not captured (e.g., cases where the specific chemical is not mentioned in title, abstract, or keyword content; “gray” literature that is not indexed in the databases listed above). For the sources discussed below, if incomplete citation information was provided in a reference list or database (e.g., if reference lists searched did not include titles), no additional searching was conducted. Thus, in addition to the databases identified above, the sources below were used to identify studies that may not have been captured in the database searches. Table 3 describes the other resources consulted.
Table 3.
Summary of references identified from other sources consulted.
| Source name | Source citation | Search terms | Search date | Total number of results retrieveda | Unique records that were screened in DistillerSRb |
|---|---|---|---|---|---|
| Review of reference lists studies considered relevant to PECO based on full-text screening. | NA | NA | NA | 1,054 | 572 |
| Reference list from the Health Effects Chapter of the draft ATSDR Toxicological Profiles | ATSDR 2018, 202239,40 | NA | NA | 460 | 330 |
| US EPA CompTox (Computational Toxicology Program) Chemicals Dashboard (ToxVal) | US EPA 202131 | Provided by CCTE | Provided by CCTE | 7 | 7 |
| ECHA | ECHA 202041 | (CASRN) | 11/27/2019–12/18/2019 | 371 | 364 |
| 2019, 2020 PFAS-Tox Database | PFAS ToxDatabase 202142 | NA | NA | 1,521 | 1,122 |
| AEGL | US EPA 201843 | (CASRN) | 11/2019 | 32 | 32 |
| ECOTOX Knowledgebase | US EPA 202144 | (CASRN) | 11/2019 | 18 | 15 |
Note: AEGL, Acute Exposure Guideline Levels for Airborne Chemicals; ATSDR, Agency for Toxic Substances and Disease Registry; CCTE, Center for Computational Toxicology and Exposure; CASRN, Chemical Abstract registry number; ECHA, European Chemicals Agency; NA, not applicable; PECO, populations exposures comparators outcomes; PFAS, per- and polyfluoroalkyl substances.
“Total number of results retrieved” were the unique records identified from each of the other sources consulted as listed in the “Source Name” column.
“Unique records that were screened in DistillerSR” were the unique records identified from each of the other sources consulted as listed in the “Source Name” column after deduplication with peer-reviewed references and references identified from other sources in the “Source Name” column.
Reference list from the Health Effects Chapter of the draft Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles for one PFAS included in this expanded SEM [perfluorododecanoic acid (CASRN 307-55-1)].39,40
Reference list from the PFAS-Tox Database, a 2019 evidence map of 29 PFAS.42,45,46
Reference lists from all PECO-relevant mammalian bioassay and epidemiological studies identified in the database searches meeting PECO criteria (see Excel Table S9)
Acute Exposure Guideline Levels for Airborne Chemicals (AEGL)43
ECOTOX Knowledgebase44
- References from the US EPA CompTox Chemicals Dashboard ToxValDB (Toxicity Values Database) to identify studies or assessments that present point of departure (POD) information.31 ToxValDB collates publicly available toxicity dose–effect related summary values typically used in risk assessments. Many of the PODs presented in ToxValDB are based on gray literature studies or assessments not available in databases such as PubMed, WoS, etc. It is important to note that ToxValDB entries have not undergone quality control (QC) to ensure accuracy or completeness and may not include recent studies.
- ToxValDB include POD data collected from data sources within ACToR (Aggregated Computational Toxicology Resource) and ToxRefDB (Toxicity Reference Database) and no-observed and lowest-observed (adverse) effect level (NOEL, NOAEL, LOEL, LOAEL) data extracted from repeated dose toxicity studies submitted under Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). Also included are reference dose and concentration values (RfDs and RfCs) from the US EPA IRIS Program and dose descriptors from the US EPA Provisional Peer-Reviewed Toxicity Values (PPRTV) documents. Acute toxicity information in ToxValDB comes from a number of different sources, including Organization for Economic Cooperation and Development (OECD) eChemPortal, NLM, Hazardous Substances Data Bank (HDSB), ChemIDplus via the US EPA Toxicity Estimation Software Tool (TEST), and the EU Joint Research Centre (JRC) AcutoxBase and the EU COSMOS project and the European Chemicals Agency (ECHA) registration dossiers to identify data submitted by registrants.41
Results from Searching Other Resources: ECHA and ToxValDB
The specific methods and results for searching each source are in Carlson et al.12 Searching of these sources is summarized to include the source type or name, the search string (when applicable), the number of results present within the resource, and the URL (when available and applicable). A search of the ECHA registered substances database was conducted as described in Carlson et al.12 using the CASRN. In addition, ToxValDB data were retrieved for the PFAS chemicals from the US EPA CompTox Dashboard47 as described in Carlson et al.12 Records from these other sources were uploaded into DistillerSR (version 2.29.0; Evidence Partners Inc.) and annotated with respect to source of the record.
Screening and tagging process.
The studies identified from the evidence streams in SWIFT-Review were imported into SWIFT-Active Screener for title and abstract screening (TIAB). SWIFT-Active Screener is a web-based collaborative software application that uses active machine-learning approaches to reduce the screening effort.48 The screening process was designed to prioritize records that appeared to meet PECO criteria or included supplemental material content based on TIAB content (i.e., both types of records were screened as “include” for active-learning purposes). Studies were screened in SWIFT-Active Screener until the software indicated a likelihood of 95% that all relevant studies had been captured. This threshold is comparable to human error rates37,49,50 and is used as a metric to evaluate machine-learning performance. Any studies in “partially screened” status at the time of reaching the 95% threshold were fully screened.
Studies that met these criteria from TIAB screening were then imported into DistillerSR for more specific TIAB tagging (i.e., to separate studies meeting PECO criteria vs. supplemental content and to tag the specific category of supplemental content and, if necessary, the chemical). Supplemental content tags are described in Table 2. For studies meeting PECO criteria at the DistillerSR TIAB level, full text articles were retrieved through the US EPA HERO database. References that were not able to be retrieved within 45 d were not considered further.
Studies identified via the gray literature searches were imported directly into DistillerSR at the TIAB phase. References identified in the gray searches that had previously been screened as not relevant to PECO at either the SWIFT-Review or SWIFT-Active stage were rescreened in Distiller.
Both TIAB and full-text screening were conducted by two independent reviewers. At all levels (SWIFT-Active Screener TIAB, DistillerSR TIAB, and DistillerSR full-text review), any conflicts in screening were resolved by discussion between the two independent reviewers; a third reviewer was consulted if any conflicts remained thereafter. Conflicts between screeners in applying the supplemental tags were resolved by discussion at both the TIAB and full-text levels, erring on the side of overtagging at the TIAB level. At the TIAB level, articles without an abstract were screened based on title (title should indicate clear relevance) and number of pages (articles two pages in length or less were assumed to be records with no original data). For additional information, please see Table 2 for supplemental categorization information. All studies identified as supplemental material at the TIAB and full-text levels were tagged to their respective chemical(s) using the preferred chemical names. All studies identified as PECO relevant were tagged to the preferred chemical name after the full text screening stage. Of note, supplemental references that did not list a specific PFAS in the TIAB (i.e., included terms like “PFAS”) were tagged to “chemical not specified.” For these supplemental references, if any PFAS were specified in the abstract, references were tagged only to those chemicals, even though it was possible that additional PFAS chemicals were reported in the Full Text. All chemical tagging was reviewed by an expert in chemistry (with a Ph.D. or similar credential). Where chemical identity was unclear, the study authors were contacted to resolve the chemical species. A full report of the literature tagging is available in HAWC51 and in Excel Table S10. Note that many studies have complex designs, so a single study can be tagged and extracted to represent all health outcome data reported (for example, studies may include information on pregnant women and maternal–child data, etc.).
Complex projects summarizing large manually curated databases will inevitably contain some data entry errors, even when QC measures are taken. As errors are identified and corrected, they will be recorded in a changelog and added to the HAWC project page for this publication.51 The study counts and figures presented in this paper represent a snapshot in time of a repository that may be updated as future analyses are conducted. For the most up-to-date and accurate information, users should consult the web-based inventory and project pages (https://hawc.epa.gov/assessment/100500256/).51
Literature Inventory
Studies that met PECO criteria after full-text review were summarized using custom forms [Word Supplemental Material “Distiller literature inventory standard operating procedure (SOP) for PFAS Evidence Map (abbreviated)”] in DistillerSR. For mammalian bioassay studies, the following study summary information was captured in a literature inventory: PFAS assessed, study type [acute ( h), short term (1–30 d), subchronic (30–90 d), chronic ( d), developmental, peripubertal, multigenerational], route of exposure, species, sex, and health system(s) assessed (described in Table S2). For epidemiological studies, the following study summary information was captured in a literature inventory: PFAS assessed, sex, population, study design (Table S3), exposure measurement (e.g., blood, feces), and health system(s) assessed. Summaries were then extracted into DistillerSR by one team member, and the extracted data were quality checked by at least one other team member. The data from these summary literature inventories were exported from DistillerSR to an Excel format and were then modified and transformed using Excel’s “Get and Transform” features for import into Tableau visualization software (version 2019.4; Tableau Software LLC). These data transformations include pivoting multiple columns of data to single columns, appending data from multiple literature inventories, and merging detailed reference information and chemical ID information into the dataset.
The survey of available evidence presented in the Tableau “heatmaps” is also available for download as an Excel file.51
The literature inventory was used to prioritize mammalian bioassay studies with exposure to the PFAS 430 chemicals for nonacute durations ( h). Studies meeting these exposure timing and duration parameters were moved forward for study evaluation (described in next section). All studies, regardless of exposure duration, were considered for end points level data extraction if they had enough information to extract. Studies with limited information (e.g., only providing an median lethal dose) were not extracted. All epidemiological studies proceeded to study evaluation and data extraction.
Study Evaluation
Study evaluation was conducted for nonacute mammalian bioassay studies and all epidemiology studies to identify important deficiencies that should be considered when interpreting the study results.
Mammalian bioassay studies.
Study evaluation was conducted for nonacute mammalian bioassay studies by two reviewers using the US EPA’s version of HAWC.52 Acute studies were not prioritized for study evaluation. Reviews were made by toxicologists with multiple years of experience in developing chemical human health assessments. For each study evaluation domain, at least two reviewers reached a consensus rating of “Good,” “Adequate,” “Deficient,” “Not Reported,” or “Critically Deficient,” as defined in HAWC. Key study evaluation considerations included potential sources of bias (factors affecting the magnitude or direction of an effect in a systematic way) and insensitivity (factors limiting detection of a true effect). The evaluated domains for toxicology studies included: Reporting Quality, Allocation, Blinding, Confounding/Variable Control, Selective Reporting/Attrition, Chemical Administration & Characterization, Study Design Applicability (exposure timing, frequency, and duration), Outcome Assessment, and Results Presentation. Core and prompting questions used to guide the judgment for each domain (e.g., reporting quality, confounding, outcome assessment) are described in more detail in the IRIS Handbook,27 and the approach applied here has been used in several other published publications, including Yost, Dishaw, Radke, and Carlson et al.12,30,53–57 After a consensus rating was reached, the reviewers considered the identified strengths and limitations to reach an overall study confidence rating of “High,” “Medium,” “Low,” or “Uninformative” for each health outcome. The ratings, which reflect a consensus judgment between reviewers, are defined in the IRIS Handbook.27 The definitions below follow standard template language that is used in systematic evidence maps developed by the US EPA28,32 and have only been adjusted, where appropriate, for the specific needs of this SEM.
High: A well-conducted study with no notable deficiencies or concerns identified for the outcome(s) of interest; the potential for bias is unlikely or minimal, and the study used sensitive methodology. “High” confidence studies generally reflect judgments of “Good” across all or most evaluation domains.
Medium: A study where some deficiencies or concerns were noted for the outcome(s) of interest, but the limitations are unlikely to be of a notable degree. Generally, “medium” confidence studies will include “Adequate” or “Good” judgments across most domains, with the impact of any identified limitation not being judged as severe.
Low: A study where one or more deficiencies or concerns were noted for the outcome(s) of interest, and the potential for bias or inadequate sensitivity could have a significant impact on the study results or their interpretation. Typically, “Low” confidence studies would have a “Deficient” evaluation for one or more domains, although some “medium” confidence studies may have a “Deficient” rating in domain(s) considered to have less influence on the magnitude or direction of the results. Generally, in an assessment context (or a full systematic review), “Low” confidence results are given less weight in comparison with high or medium confidence results during evidence synthesis and integration and are generally not used as the primary sources of information for hazard identification or derivation of toxicity values unless they are the only studies available. Studies rated as “Low” confidence only because of sensitivity concerns about biases toward the null would require additional consideration during evidence synthesis.
Uninformative: A study where serious flaw(s) make the results unusable for informing hazard identification for the outcome(s) of interest. Studies judged “Critically deficient” in any evaluation domain will almost always be classified as “Uninformative” (see explanation above). Studies with multiple “Deficient” judgments across domains may also be considered “Uninformative.” As mentioned above, although outside the scope of this SEM, in an assessment or full systematic review, uninformative studies would not be considered during the synthesis and integration of evidence for hazard identification or for dose response but might be used to highlight possible research gaps. Thus, data from studies deemed “Uninformative” are not depicted in the results displays included in this SEM.
Rationales for each study evaluation classification, including a description of how domain ratings impacted the overall study confidence rating are available in Excel Table S11 and are documented and retrievable in HAWC.51
Epidemiology studies.
The same approach was used for evaluation of all epidemiology studies. These methods have been summarized from Radke.30 For epidemiological studies, the top two rating levels (“Good” or “Adequate” and “High” or “Medium” confidence) were combined for the final evaluation, so there were ultimately three rating levels instead of four. This was done because the majority of studies had been evaluated for previous systematic review or evidence mapping projects and the evaluations were compiled from multiple projects. The evaluations were updated to reflect appropriate ratings for the PFAS included in this SEM, but because evaluation decisions may have differed slightly across projects due to between-reviewer variability, project goals, and evolving evaluation criteria, the merging of the top two levels is intended to improve consistency across studies within this project. The evaluated domains for epidemiology studies were participant selection, exposure measurement, outcome ascertainment, confounding, analysis, selective reporting, and sensitivity; detailed considerations for each domain are available in the Systematic Review Protocol for PFAS IRIS Assessments.21
Data Extraction of Study Methods and Findings
A detailed data extraction was conducted for mammalian bioassays with sufficient data to extract and all epidemiological studies. The purpose of conducting data extractions was to provide users of this SEM a database of health-related PFAS data that can expedite future analyses.
Mammalian bioassay studies.
Data extraction was conducted for mammalian bioassay studies by two members of the evaluation team using the US EPA’s version of Health Assessment Workspace Collaborative (HAWC),51 a free and open source web-based software application that facilitates the management of literature assessments for environmental pollutants. Data extracted included basic study information (e.g., full citation, funding, author-reported conflicts of interest); experiment details (e.g., study type, chemical name, chemical source, and purity); animal group specifics (species, strain, sex, age at exposure and assessment, husbandry); dosing regimen; end points evaluated; and results (qualitative or quantitative) by end point. In addition, end point–level no observed (adverse) effect levels [NO(A)ELs] and lowest observed (adverse) effect levels [LO(A)ELs] were determined by the extractors based on statistical significance reported by the study authors and are noted in the HAWC data extractions. Authors were not contacted for information that was not reported in a study. Data extraction was performed by one member of the evaluation team (primary extractor) and checked by a second member for completeness and accuracy (secondary extractor). Data extraction results for nonacute studies were used to create HAWC visualizations (e.g., exposure–response arrays) by health system and effect for each of the PFAS chemicals. Although outside the scope of this SEM, once in HAWC, the extracted data can be used for evidence synthesis and benchmark dose (BMD) modeling on an end point–by–end point basis at the discretion of the user. The detailed HAWC extractions for mammalian bioassays are available for download in Excel format from HAWC51 and are presented in Excel Table S12. The data extraction output will also be available as an excel file from the CompTox Chemicals Dashboard ToxValDB database in a future release.
Subsequent to HAWC data extraction, US EPA study authors and subject matter experts (SMEs) (M.A., X.A., L.C., A.D., L.D., A.K., P.K., L.L., J.C., P.N., A.S., and M.T.) reviewed studies of durations d and studies focusing on exposure windows targeting reproduction or development to identify study- and system-level (e.g., hepatic, urinary, etc.) NO(A)ELs and LO(A)ELs (Excel Table S13). These judgements were made at the individual study level and focused on duration of d because these study designs were considered most suitable for identifying a subchronic or chronic POD. This review was conducted to ensure consistent annotation of the mammalian bioassay studies in CCTE analyses that will compare in vitro with in vivo potencies and effects. Although in most cases, determinations of NO(A)ELs and LO(A)ELs were consistent with statistically significant findings as reported by authors, judgments were based on the biological significance and evaluation in the context of other, related findings. For example, findings of noncancer hepatic adversity were evaluated considering recommendations presented in Hall et al.58 These determinations were made by SMEs, each of whom has multiple years of experience in developing chemical human health assessments. Judgments were made independently by two SMEs, with additional discussions and/or review by a third SME to resolve conflicts (if any).
Epidemiology studies.
The epidemiological studies that were determined to meet PECO criteria after full-text review underwent data extraction for all included PFAS using a structured form in DistillerSR as described in Radke et al.30 The form captured citation information, study design characteristics (population, study design, country, year of data, exposure measurement type, exposure levels, outcome measures, sample size), whether the paper presented correlations across PFAS (though the actual correlations were not extracted), covariates included in statistical modeling, and quantitative results (effect estimate, confidence interval). Data extraction was performed by a trained member of the team (primary extractor) and checked by a second member for completeness and accuracy (QC extractor). Authors were not contacted for information that was not reported in a study unless clarifications were needed for apparent typos. The data from these extractions were exported from DistillerSR to an Excel format and then transformed for import into Tableau visualization software. The data transformations included pivoting multiple columns of data to single columns and merging detailed reference information and chemical ID information into the data set. The available evidence was visualized in Tableau, and the full extraction file is available in Excel Table S14.
Comprehensive PFAS Dashboard
Of note, this SEM does not include some of the PFAS for which US EPA assessments are already underway or completed (e.g., PFBA, PFBS, PFHxA, PFOA, PFNA, PFHxS, PFDA, PFOS, GenX chemicals, and HQ-115).,15–18,21–24,26,59 However, for users to more easily and efficiently evaluate the evidence base across all PFAS for which the agency has conducted assessments, we created a separate, comprehensive PFAS dashboard60 to display literature inventory-level data across these multiple PFAS projects. The Comprehensive PFAS Dashboard compiles information from various publicly available human health assessment products published by the US EPA during the past several years. These projects include our evidence maps12,30 and this publication, ORD assessments on PFBA,15 PFHxA,17 PFHxS,23 PFDA,22 HQ-115,59 PFPrA,61 and PFBS,16 and Office of Water’s (OW) assessments on PFOA/PFOS18,24 and GenX chemicals.26 In addition, the comprehensive dashboard includes missed literature results for six PFAS that were inadvertently omitted from the 2020 and 2021 literature updates for our 2022 evidence map12 and to correct a small number of tagging or other errors that were identified since publication.62 These updated results can be viewed in the comprehensive dashboard and extracted data and study evaluations are available in the PFAS 150 HAWC project.63
Data extractions included in the comprehensive PFAS dashboard were conducted as part of their respective parent projects. Additional information was extracted, and data transformations were performed where necessary to harmonize the extracted data across projects for visualization purposes. As a result of each project’s unique scoping criteria and goals, there may be inconsistencies in the way some data are presented. For example, sex was not extracted for human studies in the IRIS PFAS parent projects. In addition, for the OW Assessments, all studies were tagged to the acid form of the PFAS regardless of whether the acid or related salt was studied. For the PFOA18 and PFOS24 assessments, the scope of the systematic review for these toxicity assessments was focused on health effects outcomes with the strongest weight of evidence and, therefore, may not encompass literature search results for all health effects outcomes. Parent PFAS projects are indicated in the comprehensive PFAS dashboard “data sources” tab with a parenthetical date indicating the month and year of the literature search for PFAS included in that project. Readers should refer to individual assessments to learn more about the literature search strategies or scoping criteria of the original parent project. Additional details and navigation tips are available in the “read me” tab of the interactive dashboard, along with a searchable chemical list that allows users to easily query for specific chemicals.
Results
Literature Screening Results
Please note that the study counts and figures presented in this paper represent a snapshot in time of a repository that may evolve as this project is updated or revisions are made. For the most current information, please visit the web-based inventory and project pages in HAWC and HERO.33,34 The US EPA anticipates releasing an update of the Comprehensive PFAS dashboard to include additional PFAS. However, the US EPA does not currently have plans to routinely update the SEMs moving forward. As priorities are identified and additional resources become available, the US EPA may conduct updates, most likely targeted to certain PFAS when specific assessments are undertaken, building from the existing evidence maps.
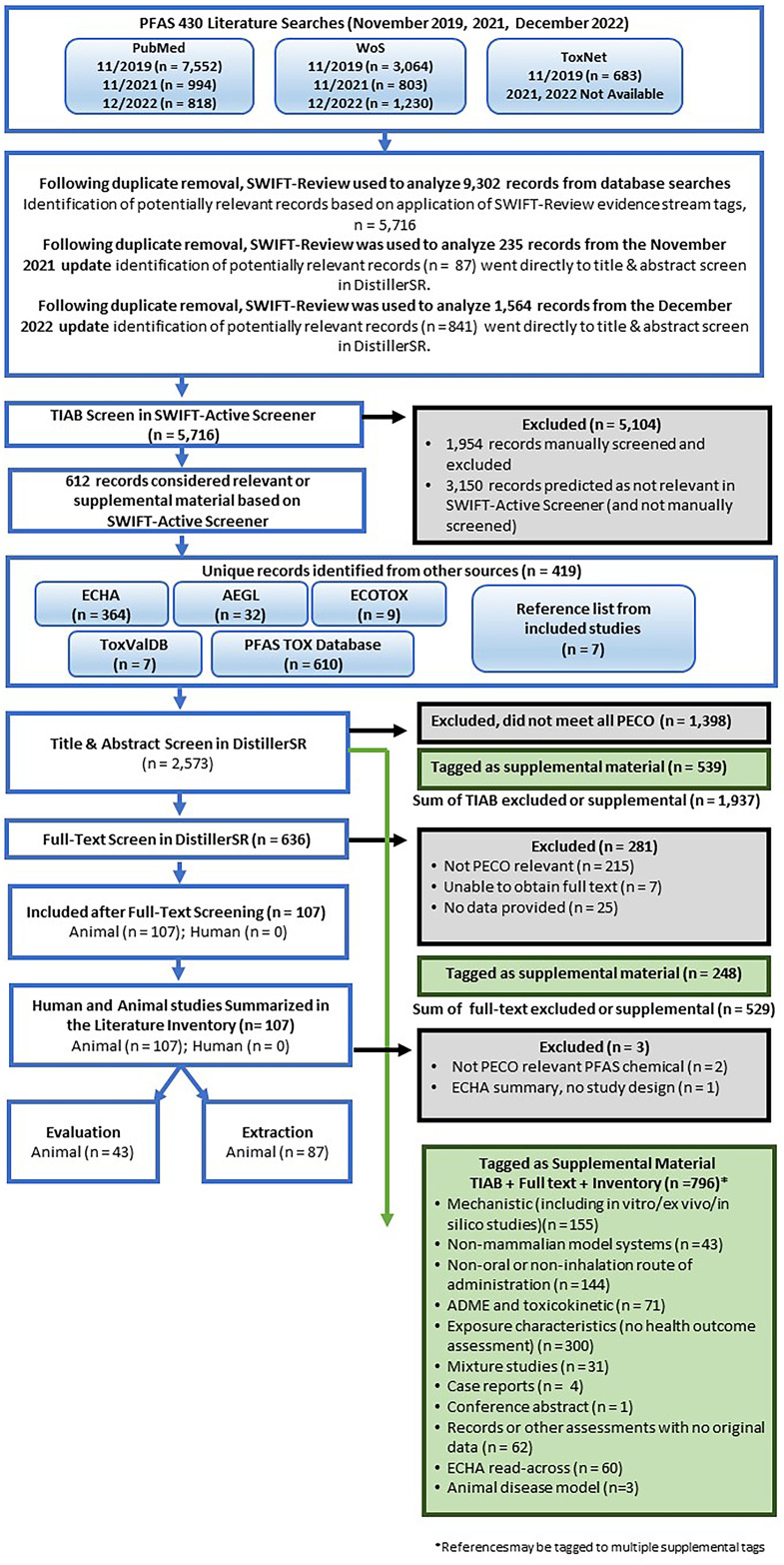
PFAS 430.
The 2019 database searches of the list of PFAS that formed the CCTE’s PFAS library yielded 9,302 records after duplicate removal (Figure 2). All references identified are available in HERO.33,34 After application of the SWIFT-Review evidence stream filters for human, animal (human health models), and in vitro evidence, the total number of studies for consideration was reduced to 5,716 references. These studies were screened in SWIFT-Active Screener using predictive relevance, resulting in 2,566 studies being manually screened to identify studies that were considered potentially PECO-relevant or supplemental (“included” for the purposes of machine learning) and records that were excluded. After manually reviewing the records, screening was stopped because SWIFT-Active Screener indicated that it was likely that 95% of the relevant studies were identified.
Figure 2.
PFAS 430 study flow diagram (3 November 2023). Literature searches and results are pooled across years. References identified from other sources joined screening at Distiller SR title-abstract review. Some references may have multiple supplemental or exclusion tags. Note: AEGL, Acute Exposure Guideline Level; ADME, absorption, distribution, metabolism, excretion; ECHA, European Chemicals Agency; ECOTOX, US EPA Ecotoxicology Knowledgebase; HAWC, Health Assessment Workspace Collaborative; PECO, populations, exposure, comparator, outcome criteria; PFAS, per- and polyfluoroalkyl substances; PFAS Tox Database, 2019 PFAS evidence map42,45,46; TIAB, title/abstract screening; ToxValDB, US EPA CompTox Chemicals Dashboard; WoS, Web of Science.
Literature search updates conducted in November 2021 and December 2022 yielded 1,799 records after duplicate removal. All references identified are available in HERO. After application of the same SWIFT-Review evidence stream filters, the total number of studies from the 2021 and 2022 searches was reduced to 928 references, those 928 studies went directly to title-abstract review in DistillerSR.
An additional 419 unique studies were identified from the gray literature and other sources searched (ECHA, AEGL, ECOTOX, ToxValDB, PFAS TOX database), including 7 studies that came from reviewing the reference lists of studies considered PECO-relevant after full-text review. These studies identified from other sources also proceeded to title and abstract screening in DistillerSR.
During title and abstract screening in DistillerSR, 636 studies were included for full-text review, 539 were tagged as supplemental material, and 1,398 were excluded as not relevant to PECO.
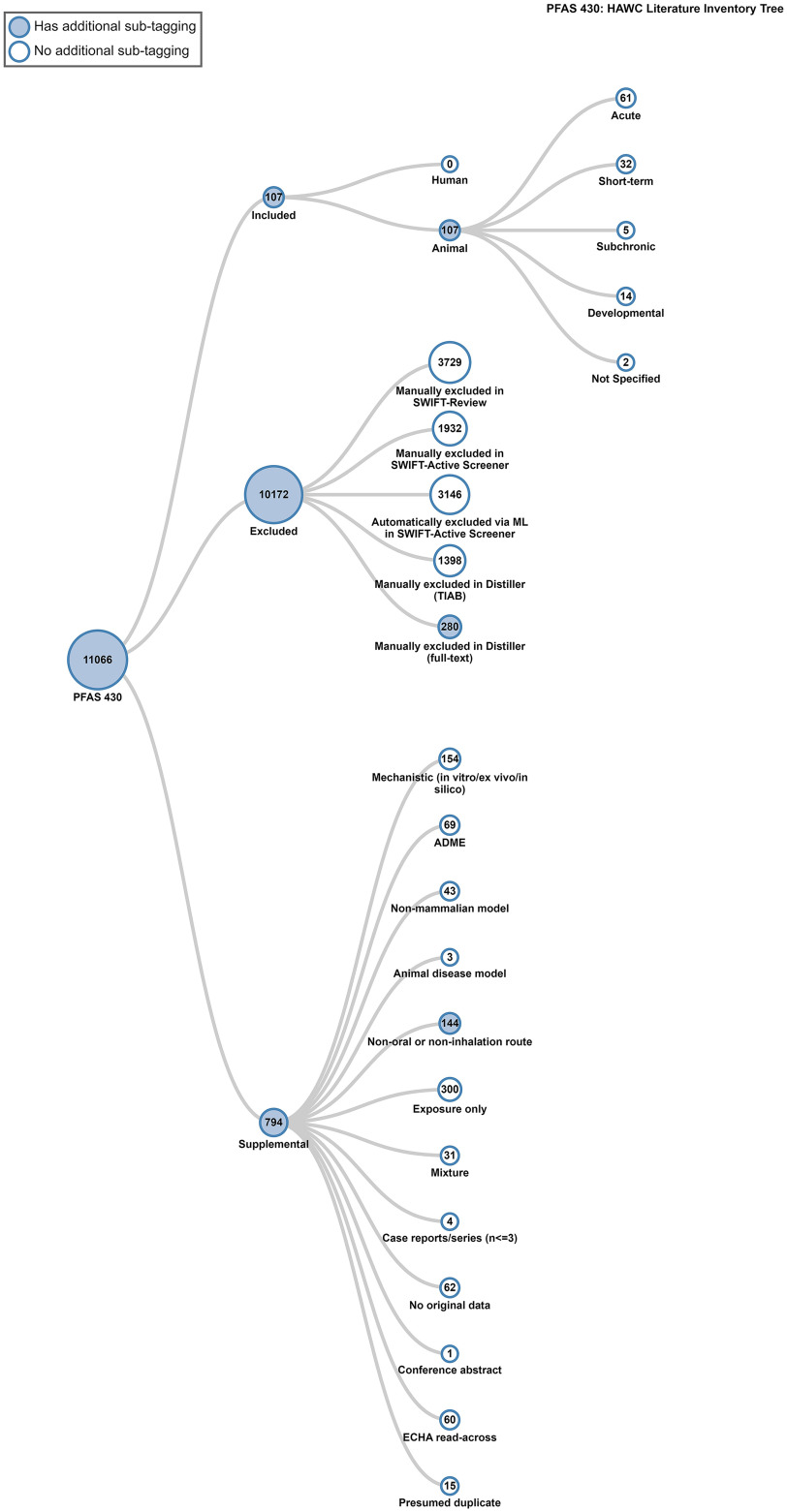
During full-text review, 107 studies were considered PECO-relevant (107 mammalian bioassay; 0 epidemiological), 281 studies were excluded, and 248 studies were tagged as supplemental material. Of the mammalian bioassay studies, 43 primarily nonacute studies were prioritized for study evaluation and 87 studies (including some acute studies) had enough information to be extracted. Literature search results pooled from the 2019–2022 searches are summarized graphically in Figure 2. Chemical-specific literature trees (Figure 3) are also available as interactive graphics in HAWC51 (filter by visualization type “literature tagtree”). References are available in the literature trees by Ctrl-clicking or Command-clicking on a node.
Figure 3.
PFAS 430 literature inventory tree. Screenshot from interactive image. This is a thumbnail image of the interactive visual (https://hawc.epa.gov/summary/visual/assessment/100500256/PFAS-430-HAWC-Literature-Inventory-Tree/) accessed 3 November 2023.51 References are available in the literature inventory tree by Ctrl-clicking or Command-clicking on a node. A full download of the literature review and study tagging can be found in Excel Table S103. Note: ADME, absorption, distribution, metabolism, and excretion studies; ECHA, European Chemicals Agency; HAWC, Health Assessment Workspace Collaborative; ML, machine learning; PFAS, per- and polyfluoroalkyl substances; TIAB, title/abstract screening.
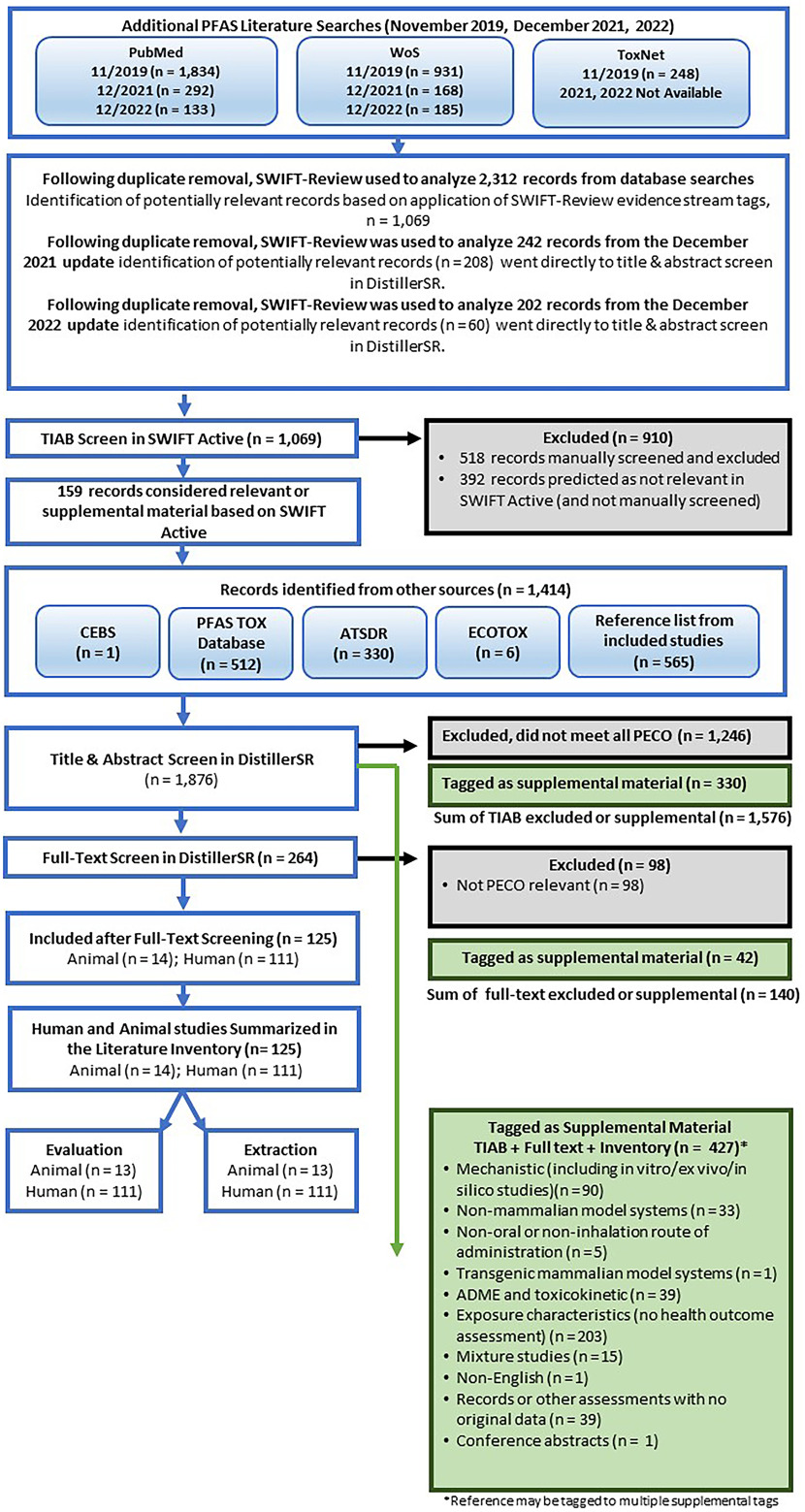
Additional PFAS.
The 2019 literature search of additional PFAS identified as compounds of interest in interagency discussions around 2017–2018 found 2,312 records from database searches after duplicate removal (Figure 4). After application of the SWIFT-Review evidence stream filters for human, animal (human health models), and in vitro evidence, the total number of studies for consideration was reduced to 1,069 references. These studies were screened in SWIFT-Active Screener, as described above, resulting in 677 studies being manually screened before reaching the 95% threshold. At this point, screening in SWIFT-Active Screener was stopped.
Figure 4.
Additional PFAS study flow diagram (3 November 2023). Literature Searches and results are pooled across years. References identified from other sources joined screening at Distiller SR title-abstract review. Some references may have multiple supplemental or exclusion tags. Note: ADME, absorption, distribution, metabolism, excretion; ATSDR, Agency for Toxic Substances and Disease Registry; CEBS, National Toxicology Program Chemical Effects in Biological Systems; ECOTOX, US EPA Ecotoxicology Knowledgebase; HAWC, Health Assessment Workspace Collaborative; PBPK, physiologically-based pharmacokinetic; PECO, populations, exposure, comparator, outcome criteria; PFAS, per- and polyfluoroalkyl substances; PFAS Tox Database, 2019 PFAS evidence map42,45,46; TIAB, title and abstract screening; WoS, Web of Science.
Literature search updates conducted in December 2021 and December 2022 yielded 444 records in HERO after duplicate removal. After application of the same SWIFT-Review evidence stream filters the total number of studies from the 2021 and 2022 searches was reduced to 268 references; these 268 studies went directly to title-abstract review in DistillerSR. A total of 1,069 studies from the 2019 search were identified for screening in SWIFT-Active Screener.
An additional 1,414 unique studies were identified from the gray literature sources searched, including 565 that came from reviewing the reference lists of studies considered PECO-relevant after full-text review. During TIAB screening in DistillerSR, 264 were included for full-text review, 330 were tagged as supplemental material, and 1,246 were excluded as not relevant to PECO.
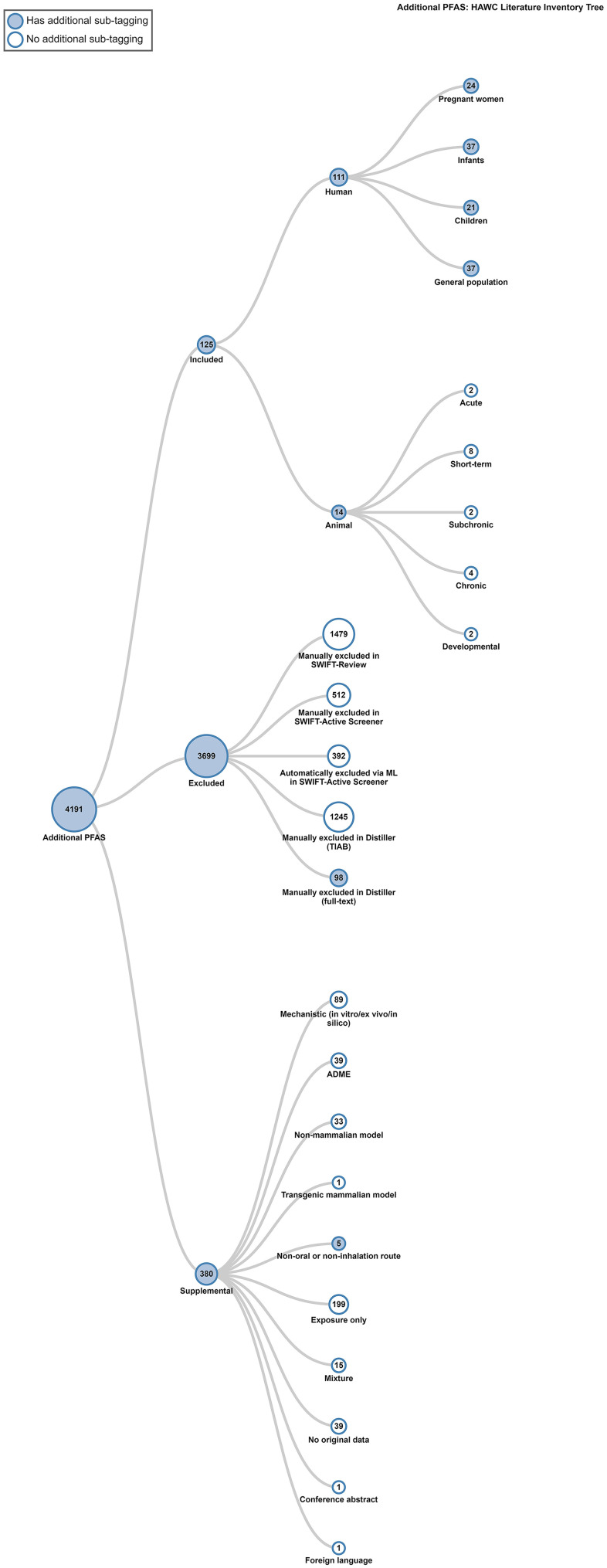
During full text review, 125 studies were considered PECO-relevant (14 mammalian bioassay; 111 epidemiological), 98 studies were excluded, and 42 studies were tagged as supplemental material. Of the 14 mammalian bioassay studies, 13 were evaluated and had enough information to be extracted. Literature search results are summarized graphically in Figure 4. Chemical-specific literature trees (Figure 5) are also available as interactive graphics in HAWC51 (filter by visualization type “literature tagtree”). References are available in the literature trees by Ctrl-clicking or Command-clicking on a node.
Figure 5.
Additional PFAS literature inventory tree. This is a thumbnail image of the interactive visual (https://hawc.epa.gov/summary/visual/assessment/100500256/PFAS-XAgency-HAWC-Literature-Inventory-Tree/) accessed 3 November 2023.64 References are available in the literature inventory tree by Ctrl-clicking or Command-clicking on a node. A full download of the literature review and study tagging can be found in Excel Table S10. Note: ADME, absorption, distribution, metabolism, and excretion studies; HAWC, Health Assessment Workspace Collaborative; ML, machine learning; PFAS, per- and polyfluoroalkyl substances; TIAB, title/abstract screening.
Details of Identified Mammalian Bioassay and Epidemiological Studies
In this review, a total of 232 mammalian bioassay and epidemiological studies on PFAS exposure were mapped to 15 health effect systems. There were 121 mammalian bioassay studies evaluating end points in a variety of species for different durations and life stages and 111 epidemiological studies conducted using different populations and study designs. Although mammalian bioassay and epidemiological data are available for some chemicals, other chemicals and end points of interest have not been extensively studied. More detailed analyses of mammalian bioassay and epidemiological studies are described below.
Mammalian bioassay studies.
Literature inventory.
There were 121 mammalian bioassay studies that met PECO criteria. A survey of the animal model systems, study designs, and health effects is provided in Figure 6. Further details on specific studies, chemicals, and routes of exposure are available in an interactive graphic in HAWC.65 Table 4 also provides a high-level summary of which PFAS (pooled across both the “PFAS 430” and “additional PFAS” lists) had at least one mammalian bioassay study identified during literature inventory.
Figure 6.
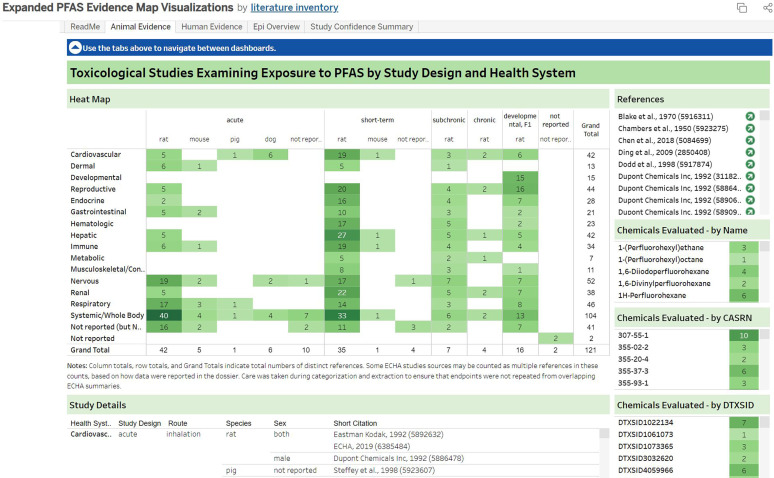
Survey of mammalian bioassay studies that met PECO criteria by study design, species, and health systems. This is a thumbnail image of the interactive visual (https://hawc.epa.gov/summary/visual/assessment/100500256/PFAS-430-XAgency-Evidence-Map-Dashboard/) accessed on 3 November 2023 that is filterable by health system, study design, PFAS name, CASRN, and DTXSID.65 The numbers in the heat map inset indicate the distinct number of studies that investigated a health system within a particular study design. If a study evaluated multiple health outcomes or presented several experiments, it is shown here multiple times, though totals reflect distinct numbers of studies. The study design panel includes information on animal model, exposure duration, route of administration, and dose level(s) tested. CASRN, Chemical Abstracts Service registry number; DTXSID, DSSTox substance identifier; PECO, population, exposure, comparator, outcome; PFAS, per- and polyfluoroalkyl substances.
Table 4.
PFAS chemicals identified in this systematic evidence map that had at least one mammalian bioassay or epidemiological study summarized in the literature inventory.
| Chemical name | CASRN | Animal evidence | Human evidence |
|---|---|---|---|
| (E)-Perfluoro(4-methyl-2-pentene) | 3709-71-5 | 8 | — |
| 1-(Perfluorohexyl)ethane | 80793-17-5 | 3 | — |
| 1-(Perfluorohexyl)octane | 133331-77-8 | 1 | — |
| 1,6-Diiodoperfluorohexane | 375-80-4 | 4 | — |
| 1,6-Divinylperfluorohexane | 1800-91-5 | 2 | — |
| 11-chloroeicoafluoro-3-oxaundecane-1-sulfonate | 763051-92-9 | — | 8 |
| 11-H-Perfluoroundecanoic acid | 1765-48-6 | 1 | — |
| 1H,1H,5H-Perfluoropentyl methacrylate | 355-93-1 | 3 | — |
| 1H-Perfluorohexane | 355-37-3 | 6 | — |
| 2-(N-Ethyl-perfluorooctanesulfonamido)acetate | 909405-49-8 | — | 15 |
| 2-(N-Ethylperfluorooctanesulfonamido)acetic acid | 2991-50-6 | — | 7 |
| 2-(N-Methylperfluorooctanesulfonamido)acetate | 909405-48-7 | — | 21 |
| 2-(N-Methylperfluorooctanesulfonamido)acetic acid | 2355-31-9 | — | 11 |
| 2-(Perfluorohexyl)ethanethiol | 34451-26-8 | 2 | — |
| 2,2,2-Trifluoroethyl triflate | 6226-25-1 | 3 | — |
| 2,3-Dichlorooctafluorobutane | 355-20-4 | 2 | — |
| 2:1 Fluorotelomer alcohol | 422-05-9 | 6 | — |
| 2H,3H-Decafluoropentane | 138495-42-8 | 16 | — |
| 2H-Perfluoro-2-propanol | 920-66-1 | 7 | — |
| 2-Methoxy-2H-perfluoropropane | 13171-18-1 | 3 | — |
| 2-Perfluorohexyl ethanoic acid | 53826-12-3 | 1 | — |
| 2-Vinyl(1-bromoperfluoroethane) | 18599-22-9 | 2 | — |
| 3-(Perfluoroethyl)propanol | 148043-73-6 | 2 | — |
| 3-Ethoxyperfluoro(2-methylhexane) | 297730-93-9 | 6 | — |
| 4,8-Dioxa-3H-perfluorononanoic acid | 919005-14-4 | — | 1 |
| 6:2 Fluorotelomer phosphate diester | 57677-95-9 | — | 3 |
| 8:2 Fluorotelomer phosphate diester | 678-41-1 | — | 1 |
| Perfluoro(2-((6-chlorohexyl)oxy)ethanesulfonic acid) | 756426-58-1 | — | 11 |
| Ammonium 4,8-dioxa-3H-perfluorononanoate | 958445-44-8 | 1 | — |
| Dichloromethyl((perfluorohexyl)ethyl)silane | 73609-36-6 | 1 | — |
| Heptafluoro-2-iodopropane | 677-69-0 | 1 | — |
| Heptafluoropropyl iodide | 754-34-7 | 1 | — |
| Hexaflumuron | 86479-06-3 | 2 | — |
| Perfluoro-1,4-diiodobutane | 375-50-8 | 7 | — |
| Perfluoro-2-methyl-3-pentanone | 756-13-8 | 15 | — |
| Perfluorodecanesulfonate | 126105-34-8 | — | 7 |
| Perfluorodecanesulfonic acid | 335-77-3 | — | 2 |
| Perfluorododecanoic acid | 307-55-1 | 10 | 83 |
| Perfluoromethylcyclohexane | 355-02-2 | 3 | — |
| Trifluoroacetate | 14477-72-6 | 2 | — |
| Trimethoxy((perfluorohexyl)ethyl)silane | 85857-16-5 | 1 | — |
Note: An interactive visual summary of the information extracted in the literature inventory can be found in Tableau66 and the ORD Expanded SEM HAWC project.52 —, no studies summarized in the literature inventory; CASRN, Chemical Abstracts Service registry number; HAWC, Health Assessment Workspace Collaborative; ORD, US EPA Office of Research and Development; PFAS, per- and polyfluoroalkyl substances; SEM, Systematic Evidence Map.
These studies evaluated exposure to 30 unique PFAS administered orally (via gavage, diet, or water) or through inhalation (list of chemicals provided in Table 4). Of these, 2H,3H-Decafluoropentane ( studies), perfluoro-2-methyl-3-pentanone ( studies), perfluorododecanoic acid ( studies), and (E)-Perfluoro(4-methyl-2-pentene) ( studies) were the most frequently studied compounds. Most studies were conducted in rats and mice, but data were also available for rabbits, pigs, and dogs.
Study evaluation.
Figure 7 summarizes the study evaluations of the mammalian bioassay studies. Specific rationales for each domain, as well as the overall confidence ratings are available in an interactive graphic in HAWC.51 The final study evaluation report from HAWC is available in Excel Table S12, and additional notes and multiple reviewer ratings can be found directly in HAWC. Similar to our findings in our previous effort involving PFAS,12 the majority of the mammalian bioassay studies identified were from ECHA summaries (37/56). Most of these ECHA summaries (28/37) had “Uninformative” or “Low” confidence ratings for specific end points or overall due to a lack of reporting on methods and results. The use of ECHA summaries in an assessment context is limited because of a lack of transparency arising from limited study details and no access to the primary study. However, ECHA summaries may be useful on a case-by-case basis in assessments and can be used to inform read-across approaches or for use in screening-level analyses. The use of ECHA summaries in an assessment should depend on the decision context and the level of uncertainty in the results. As our evidence maps have demonstrated, there is little toxicity data on many PFAS, and ECHA summaries can help further our understanding of this complex class of chemicals.
Figure 7.
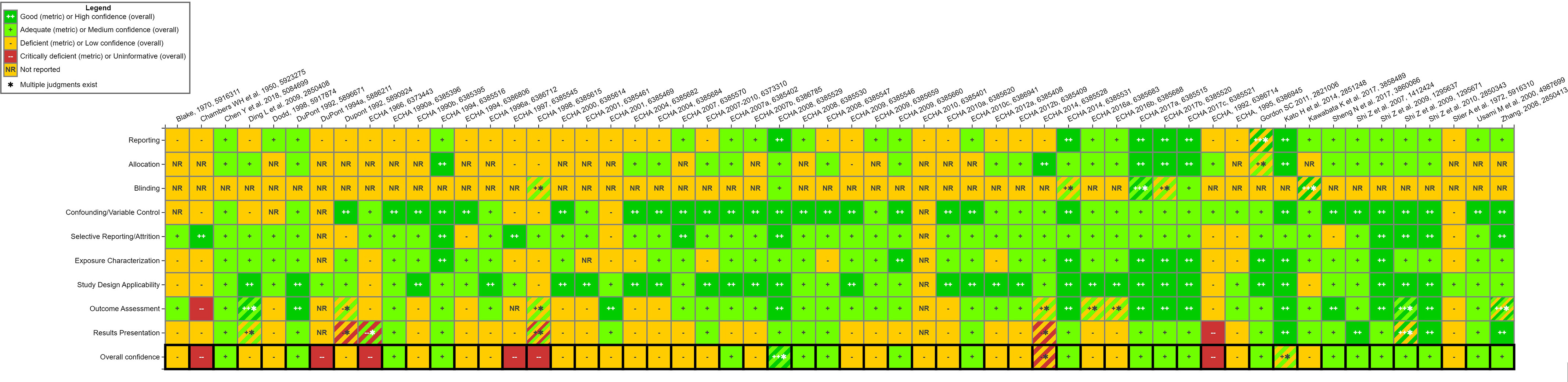
Study evaluation for all mammalian bioassay studies. This is a thumbnail image of the interactive visual (https://hawc.epa.gov/summary/visual/assessment/100500256/Animal-SQE-Heatmap/) accessed on 3 November 2023.66 the study evaluation approach follows standard methods that are used in systematic evidence maps developed by the US EPA27,28,32 and have only been adjusted, where appropriate, for the specific needs of this SEM. A full download of detailed evaluation summaries is available through HAWC, or in Excel Table S11. Note: ECHA, European Chemicals Agency; HAWC, Health Assessment Workspace Collaborative; SEM, Systematic Evidence Map.
Study evaluations for non-ECHA studies are displayed in Figure 8. Of these studies, a little over 50% (11/19) were “medium” confidence overall or for specific end points. These studies were considered well conducted with “Good” or “Adequate” ratings for most of the study evaluation domains. Of note, none of the non-ECHA studies were “High” confidence due to a lack of reporting of study details or deficiencies in the study design or data analysis. For example, while Kato et al.68 had “Good” ratings across most domains, significant mortality in high-dose females (11/12 animals) impacted interpretation of study results in this group. Therefore, the overall study confidence was “Medium” confidence rather than “High.”
Figure 8.
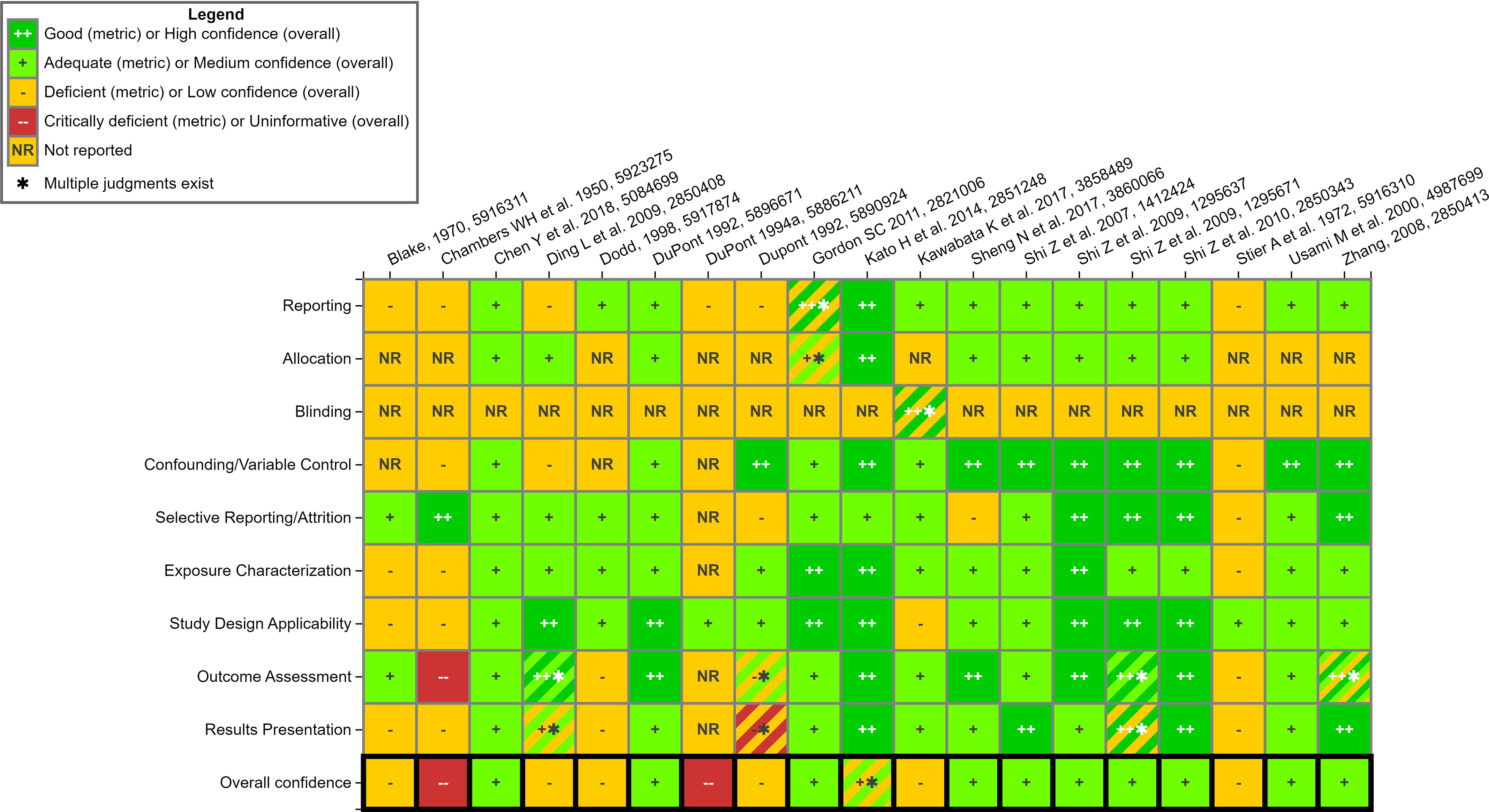
Study evaluation for mammalian bioassay studies- non-ECHA reports. This is a thumbnail image of the interactive visual (https://hawc.epa.gov/summary/visual/assessment/100500256/Animal-SQE-Heatmap_nonECHA/) accessed on 3 November 2023.67 The study evaluation approach follows standard methods that are used in systematic evidence maps developed by the US EPA27,28,32 and have only been adjusted, where appropriate, for the specific needs of this SEM. A full download of detailed evaluation summaries is available through HAWC, or in Excel Table S11. Note: ECHA, European Chemicals Agency; HAWC, Health Assessment Workspace Collaborative; SEM, Systematic Evidence Map.
Just as was noted in our previous evidence map, information on blinding was rarely reported and is reflected by low scores across this domain for all the mammalian bioassay studies. Outcome-specific judgments sometimes varied within a study for a particular domain, usually due to presentation of results, and this variation is indicated by hashing in the visualizations. Specific details regarding the outcome-specific judgments can be viewed by clicking on individual cells in the interactive HAWC visualization.51
Summary of extracted data.
Of the 121 PECO-relevant studies, a subset of 100 studies had enough information to extract end points–level data. The majority of these studies had exposure durations d. Only 36 studies for 15 PFAS focused on 21 d or longer exposure durations (or were reproductive or developmental studies). These study designs were considered most suitable for identifying a subchronic or chronic POD. Overall, the most commonly assessed health systems included the whole body (e.g., body weight); reproductive (e.g., male and female reproductive organ weights, fertility); developmental (e.g., pup weight and viability, lactation index, skull malformation); renal (e.g., kidney weight, organ function measurements such as blood urea nitrogen); immune (e.g., spleen and/or thymus weight; blood components such as monocytes, eosinophils, and lymphocytes); and hepatic (e.g., liver weight, enzyme activity, cholesterol, and lipid metabolism, total bilirubin, nonneoplastic lesions such as hepatocellular hypertrophy) systems.
The US EPA’s final drinking water health advisories for hexafluoropropylene oxide (HFPO) dimer acid and its ammonium salt (referred to as “GenX chemicals”)69 and potassium perfluorobutane sulfonate (PFBS)70 are based on final toxicity assessments.16,26 The toxicity assessment for GenX chemicals reported health effects on hepatic, renal, immune, and developmental systems, as well as cancer. The most sensitive health effect was an adverse liver effect (constellation of liver lesions) that was used for derivation of toxicity values.26 For PFBS, animal studies reported health effects on endocrine, reproductive, developmental, and renal systems, and the final toxicity assessment used decreased levels of serum total thyroxine as the critical adverse effect following a gestational exposure in newborn mice.16 For the IRIS assessment of PFBA and related salts, health effects were reported for endocrine, hepatic, and developmental systems.15 The critical end points used to derive a lifetime toxicity value were hepatic effects (increased hepatocellular hypertrophy) and endocrine effects (decreased total thyroxine).15 For context, in this SEM, there are endocrine studies (on 13 PFAS), and hepatic studies (on 18 PFAS), 38 renal studies (on 15 PFAS), and 15 developmental studies (on 9 PFAS). Although there are some studies that assessed end points identified as sensitive targets for other completed health assessments of PFAS (PFBA, GenX, PFBS), for a subset of PFAS examined there were no data available for the vast majority of PFAS included in this SEM. Furthermore, many of the mammalian toxicology studies available were gray literature (e.g., ECHA reports), which often have limited methods and results reporting and can limit their use in assessment development.
Figure 9, Figure 10, Figure 11, and Figure 12 are example visualizations of the data available for some of the PFAS mammalian bioassay studies. More than 45 exposure–response arrays (data pivot visualizations) on 21 PFAS across 16 health effect systems are available in HAWC, which are not fully discussed here because of space limitations. Instead, we have presented a representative subset of visuals for exploring the different data available. Users can view visualizations by chemical name or by health effect system in HAWC. Table S4 also describes the inventory of HAWC data pivot visuals available by PFAS chemical and health system. Perfluorododecanoic acid’s oral toxicity data are shown in Figure 9, presenting an example of a comparatively data-rich PFAS chemical. Figure 10 and Figure 11 present the inhalation and oral data, respectively, for 2:1 fluorotelomer alcohol, which include data reported from both peer-reviewed studies and ECHA summaries. Figure 12 displays the developmental toxicity end points for Ammonium 4,8-dioxa-3H-perfluorononanoate (ADONA), a shorter chain replacement PFAS. All data extraction and visualizations (more than 75 unique figures) are available in HAWC by selecting visualization type “Data pivot (animal bioassay).”51
Figure 9.
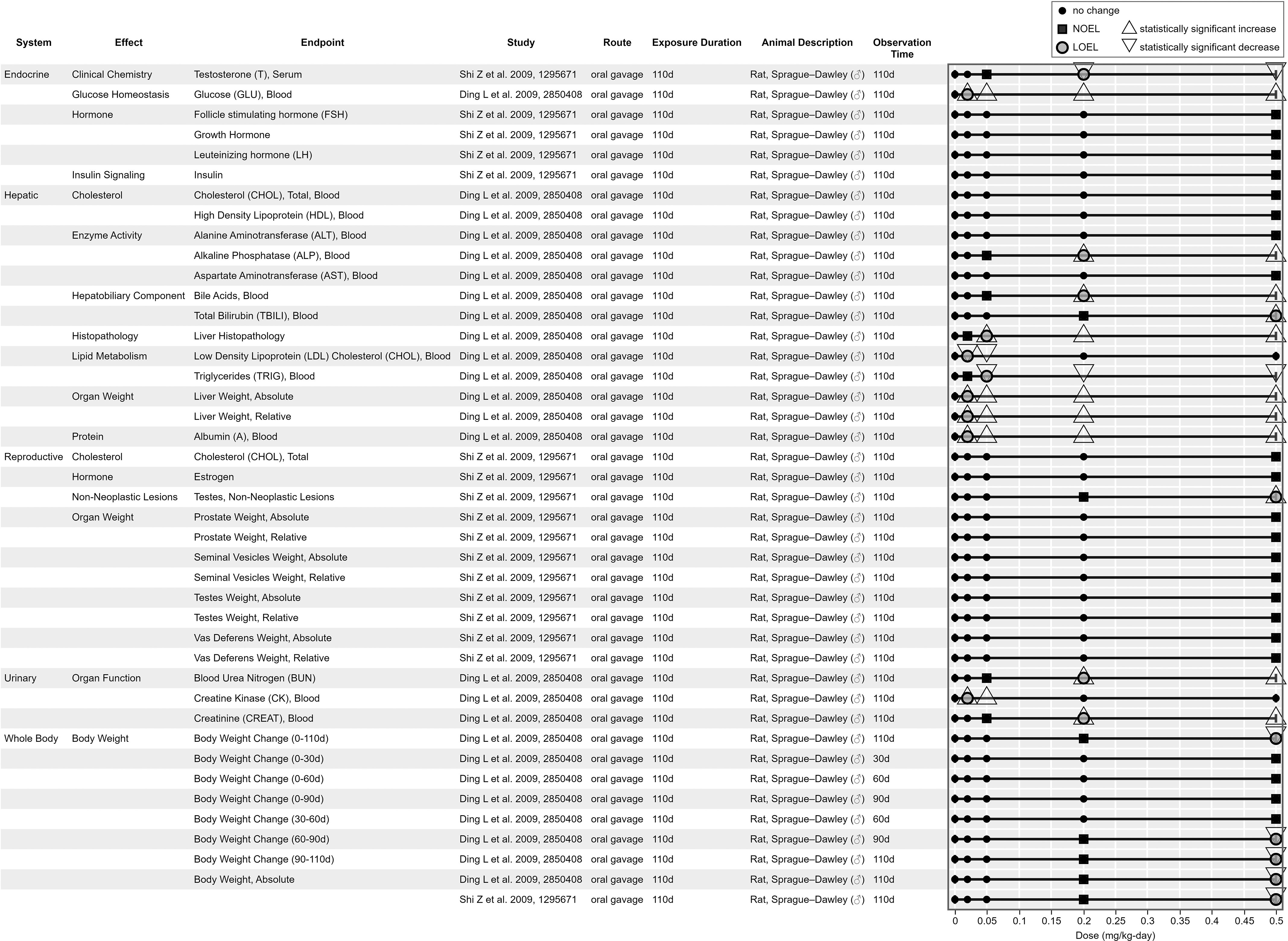
Survey of oral toxicology findings for perfluorododecanoic acid. This is a thumbnail image of the interactive visual (https://hawc.epa.gov/summary/data-pivot/assessment/100500256/Figure-21-Perfluorododecanoic-acid_OralChronic/) accessed on 3 November 2023.71 Data are also available in Excel Table S12 (filter by chemical name). Six-digit number in “study” column is Health and Environmental Research Online identification. Note: d, days; LOEL, lowest observed effect level; NOEL, no observed effect level.
Figure 10.
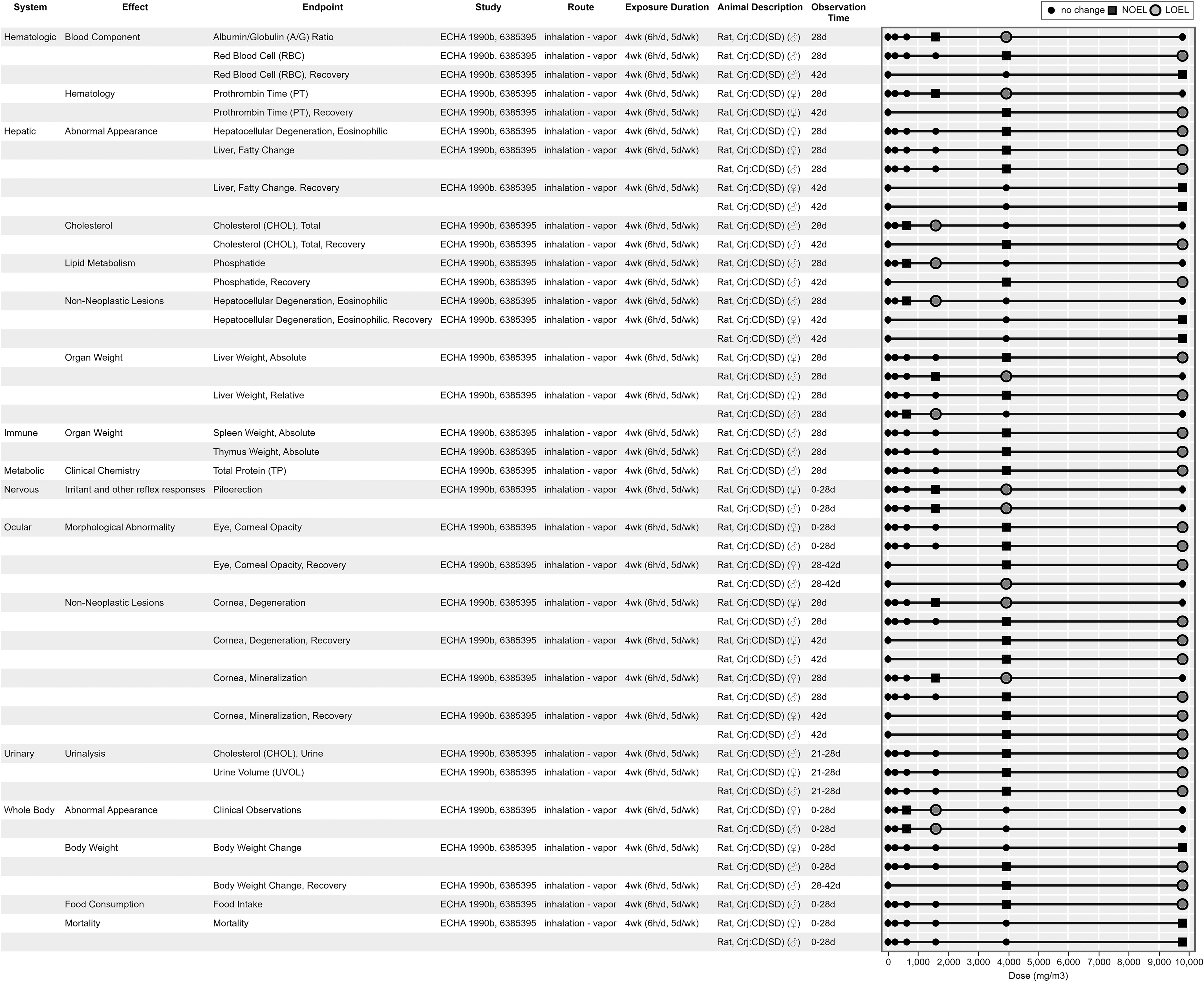
Survey of inhalation toxicology data for 2:1 fluorotelomer alcohol. This is a thumbnail image of the interactive visual (https://hawc.epa.gov/summary/data-pivot/assessment/100500256/Figure-24-21-Fluorotelomer-alcohol_Inhalation_All/) accessed on 3 November 2023.72 Data are also available in Excel Table S12 (filter by chemical name). Six-digit number in study column, Health and Environmental Research Online identification. Note: d, days; h, hours; LOEL, lowest observed effect level; NOEL, no observed effect level; wk, weeks.
Figure 11.
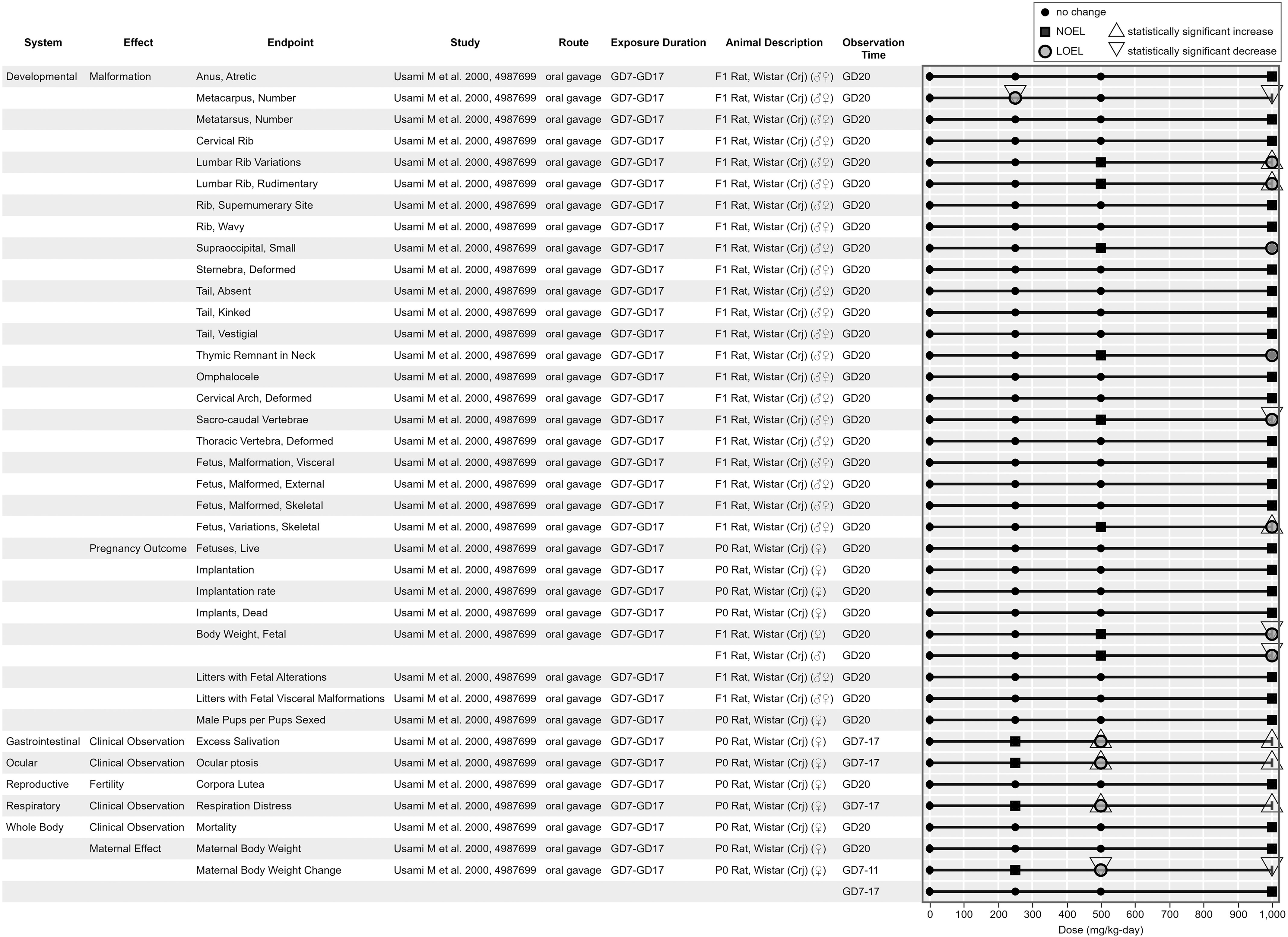
Survey of oral toxicology data for 2:1 fluorotelomer alcohol. This is a thumbnail image of the interactive visual (https://hawc.epa.gov/summary/data-pivot/assessment/100500256/Figure-6-21-Fluorotelomer-alcohol_Oral-All/) accessed on 3 November 2023.73 Data are also available in Excel Table S12 (filter by chemical name). Six-digit number in study column, Health and Environmental Research Online identification. Note: d, days; GD, gestation day; LOEL, lowest observed effect level; NOEL, no observed effect level.
Figure 12.
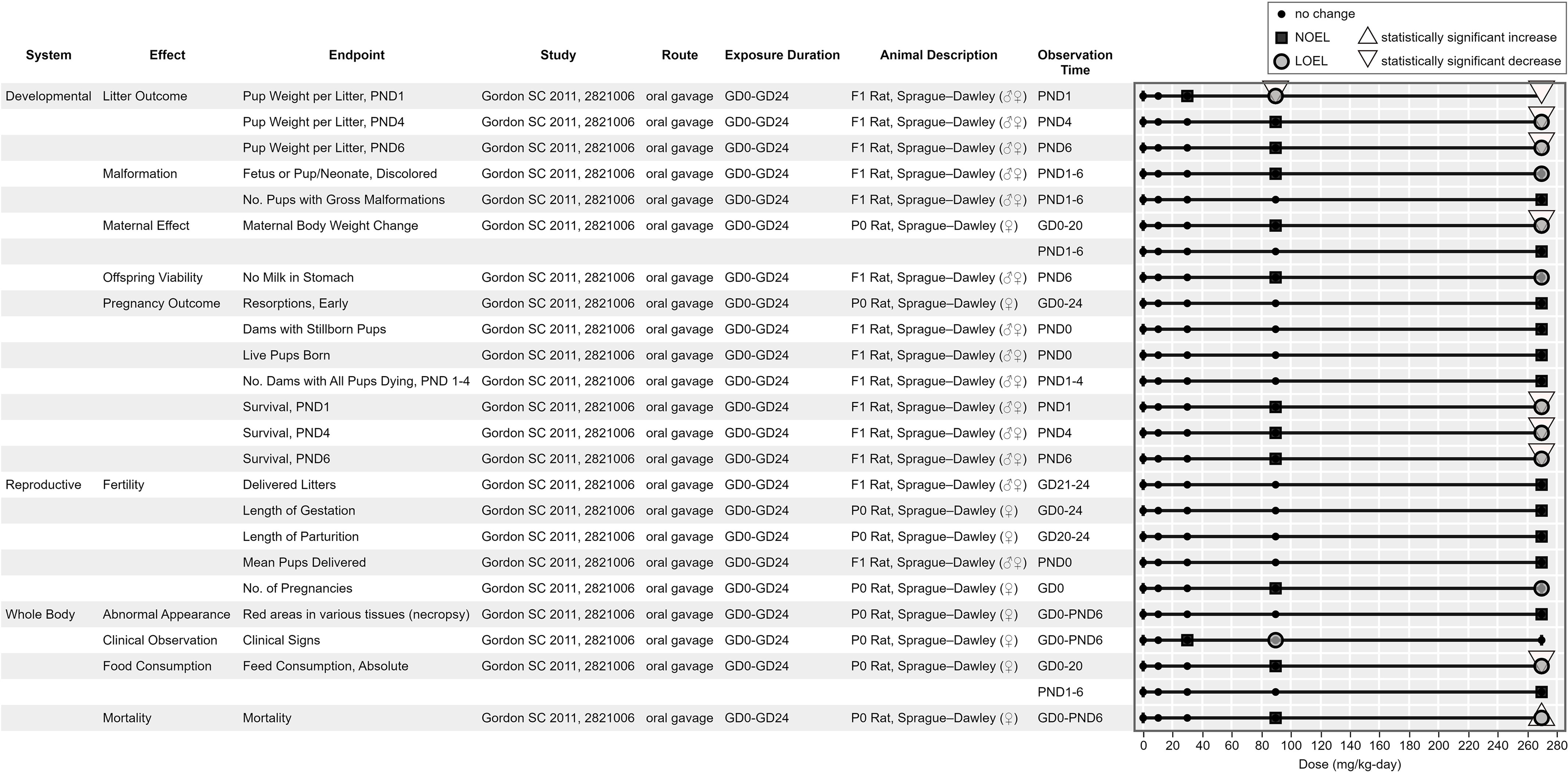
Ammonium 4,8-dioxa-3H-perfluorononanoate (ADONA) oral developmental toxicity screening end points. This is a thumbnail image of the interactive visual (https://hawc.epa.gov/summary/data-pivot/assessment/100500256/Figure-11-ADONA_Oral-devtoxscreen/) accessed on November 3, 2023.74 Data are also available in Excel Table S12 (filter by chemical name). Six-digit number in study column, Health and Environmental Research Online identification. Note: d, days; GD, gestation day; LOEL, lowest observed effect level; NOEL, no observed effect level; PND, postnatal day.
Epidemiology studies.
Literature inventory.
The epidemiological studies that met PECO criteria are summarized by study design, population, and health systems in Figure 13. Further details on the specific studies, exposure measurements, and chemicals evaluated are available in an interactive graphic.51 Table 4 also provides a high-level summary of which PFAS (pooled across both the “PFAS 430” and “additional PFAS” lists) had at least one epidemiological study identified during literature inventory.
Figure 13.
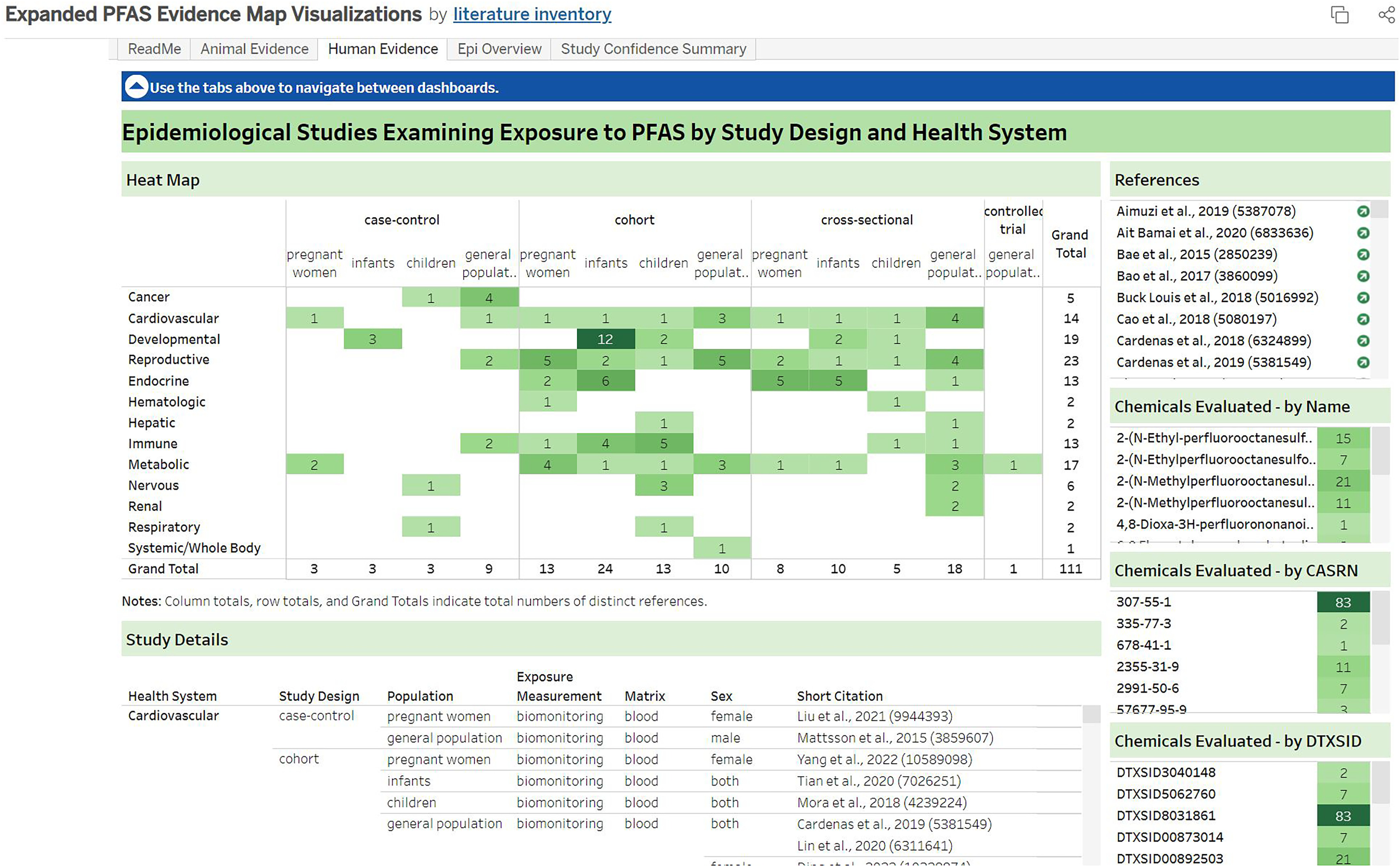
Survey of human studies that met PECO criteria summarized by study design, population, and health systems assessed. This is a thumbnail image of the interactive visual (https://hawc.epa.gov/summary/visual/assessment/100500256/PFAS-430-XAgency-Evidence-Map-Dashboard/) accessed on 3 November 2023.65 The numbers indicate the distinct number of studies that investigated a health system within a particular study design and population, not the number of studies that observed an association with PFAS exposure. If a study evaluated multiple health outcomes or populations, it is shown here multiple times, though totals reflect distinct numbers of studies. Interactive sorting allows searching by name, Chemical Abstracts Service registry number, and DSSTox substance identifier, exposure measurement information, and sex. Note: PECO, population, exposure, comparator, outcome; PFAS, per- and polyfluoroalkyl substances.
A total of 111 epidemiological studies were identified that included information on 12 different PFAS. The most frequently studied chemicals, with more than 10 studies available, were perfluorododecanoic acid ( studies), 2-(N-Methylperfluorooctanesulfonamido)acetate (21), 2-(N-Ethyl-perfluorooctanesulfonamido)acetate (15), 2-(N-Methylperfluorooctanesulfonamido)acetic acid (11), and perfluoro(2-((6-chlorohexyl)oxy)ethanesulfonic acid) (11). The vast majority of the searched PFAS had zero epidemiological studies available.
Study evaluation.
Each study was rated for the outcomes assessed, sometimes resulting in more than one rating because of methods used for different outcomes. A “High”/“Medium” confidence rating for at least one outcome was given for 83 (75%) studies. The remaining 28 studies were “Low” confidence or “Uninformative” for all outcomes. All study evaluation ratings/rationales are available in an interactive visual (Human Study Confidence Summary tab), which is filterable by health system, outcome, rating, and chemical identifier. In addition, an overview of overall study confidence by health systems is provided in Figure 14 and in Excel Table S11.
Figure 14.
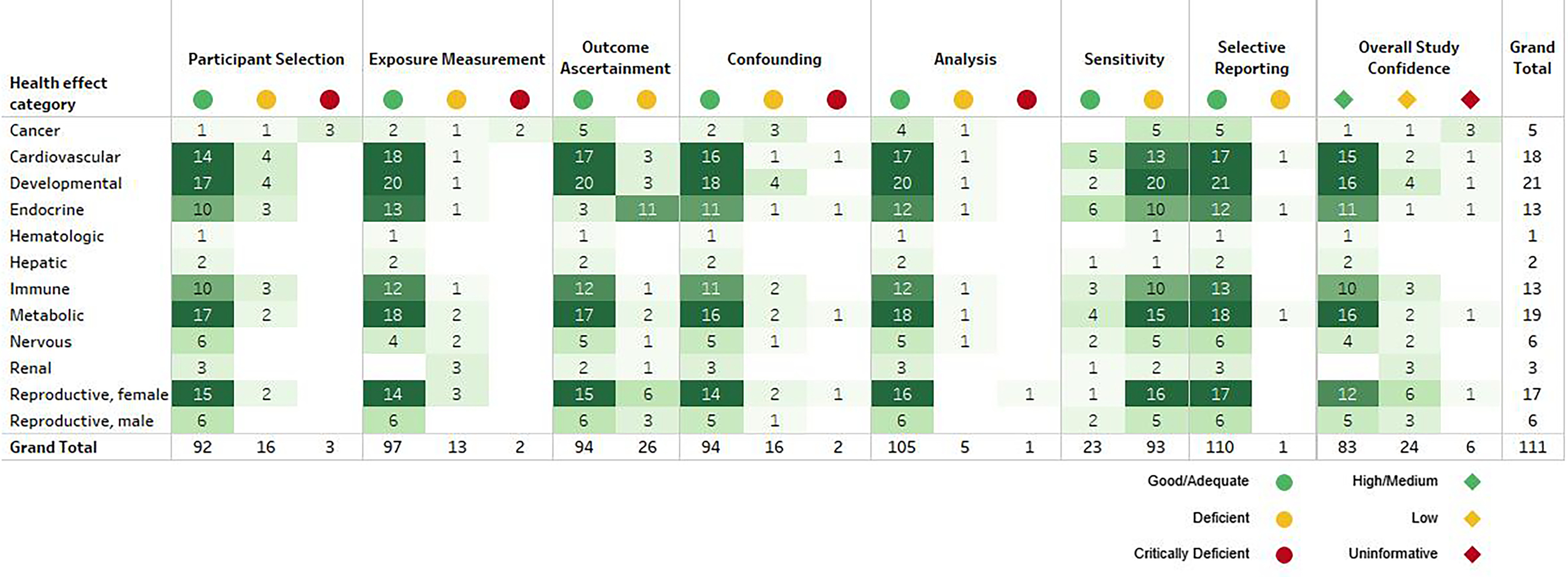
Summary of study evaluations for epidemiological studies by health effect category. This is a thumbnail image of the interactive visual accessed on 3 November 2023. In the interactive dashboard (https://hawc.epa.gov/summary/visual/assessment/100500256/PFAS-430-XAgency-Evidence-Map-Dashboard/), “Study Confidence Summary” tab, each domain/overall confidence rating and rationale is available, filterable by PFAS, health effect category, outcome, and rating.65 The study evaluation approach follows standard methods that are used in systematic evidence maps developed by the US EPA18,28 and have only been adjusted, where appropriate, for the specific needs of this SEM.27,32 A full download of detailed evaluation summaries is available in Excel Table S14. Note: PFAS, per- and polyfluoroalkyl substances; SEM, Systematic Evidence Map.
Most studies were well conducted with “Good”/“Adequate” ratings in the majority of domains. However, 93 studies (84%) were rated as “Deficient” for study sensitivity for at least one PFAS; 88 (79%) were “Deficient” for all the PFAS considered in this effort. Just as was noted in our previous evidence map,30 these ratings were primarily due to concerns of limited exposure contrast. However, the study sensitivity domain goes beyond study design and conduct to identify factors that may reduce a study’s capacity to detect an association. This is an important point for the interpretation of the results, especially with null results and understanding across study heterogeneity. The included PFAS had limited exposure contrast in most cases, which reduces the ability of the studies to detect a true effect. The specific exposure levels are available in the data extracted into the interactive visual (Figure 13). It is important to note that there was no evidence that the lower exposure levels observed in these studies (one or more orders of magnitude lower than most epidemiology studies of PFOA and PFOS) were not toxicologically relevant, but null findings should not be interpreted as indication of a lack of adverse health effect (i.e., null findings may be explained by limited sensitivity).
Summary of extracted data.
Across PFAS in this SEM, the most studied health systems, with a wide range of specific health outcomes considered, were developmental ( studies), cardiovascular (18), female reproductive (17), metabolic (19), endocrine (13), and immune (13) effects. The other health effect categories had fewer than 10 studies each (nervous system, cancer, hepatic, hematologic, renal, male reproductive). The most frequent study designs were cohort ( studies) and cross-sectional (), followed by case–control studies (). One study was a controlled crossover trial of vitamin C treatment as a modifier of the association between PFAS exposure and insulin resistance. All the available studies measured PFAS exposure using biomarkers, most commonly in serum (43 studies), plasma (18), maternal blood (34), or cord blood (20). The studies were all in the general population, sometimes including children ( y of age); [ studies (38 included both adults and children)] and pregnant women ().
The full quantitative results for each included study are available in the interactive visual (Epi Overview tab) and in Excel Table S14. This is intended to allow readers to filter the results to explore any specific questions of interest and make their own interpretations of the data, because drawing conclusions is outside the scope of this effort. Still, when examining the health systems that have been considered for dose response for other PFAS with completed health assessments, there are a few key points to make from these data. Immune effects have been observed for multiple PFAS and immune suppression measured by reduced vaccine response was selected as the critical effect in the EPA Interim Drinking Water Health Advisories for PFOA and PFOS.75,76 In this SEM, studies of immune effects are primarily available only for perfluorododecanoic acid ( of 13 studies), and only 5 examine immune suppression [four examining infectious diseases (inconsistent findings) and 1 examining antibody levels following vaccination in adults (statistically significant association)]. It would thus be difficult to draw conclusions about immune effects for these PFAS. In contrast, for reduced birth weight, which has also been commonly reported with PFAS exposure, 16 studies were available for seven PFAS in this SEM, with mostly consistent results indicating an association, so some PFAS may have adequate data to support conclusions. For hepatic effects, only two studies were available for these PFAS, with only one study each for four different PFAS; for serum lipids, eight studies were available. For cancer, studies were primarily limited to breast cancer, with no studies available for kidney and testicular cancer, which had the strongest evidence for PFOA.76
Comprehensive PFAS Dashboard
At the time of publication of this manuscript, the comprehensive PFAS dashboard consists of 370 mammalian bioassay studies for 87 PFAS and 631 epidemiological studies for 35 PFAS across multiple projects. As noted in the “data sources” tab of the Comprehensive dashboard, each project was initiated on different schedules and has unique goals, and therefore the literature search dates for each base project vary (2021–2023). Figure 15 is a static image of the comprehensive PFAS dashboard. Further details on specific studies, chemicals, and routes of exposure are available in an interactive graphic in HAWC.60 Mammalian bioassay studies and human studies are presented in separate tabs. In addition to presenting the chemicals for which relevant data were found, the “read me” tab includes a searchable chemical list and data sources across the included parent PFAS projects. By compiling all of these extractions into one dashboard, we hope to facilitate efforts to evaluate the potential human health effects of PFAS across the class.
Figure 15.
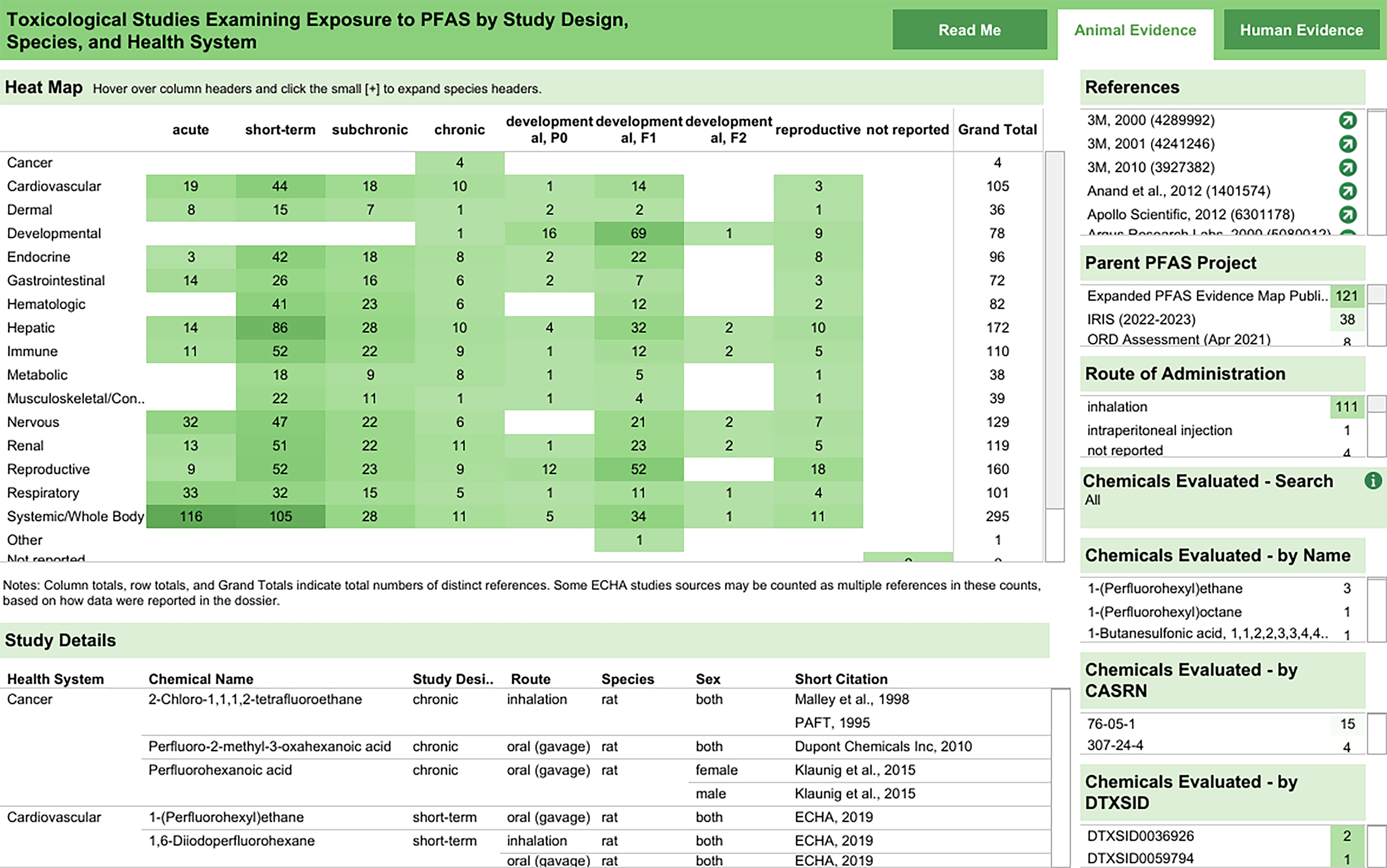
Survey of included mammalian bioassay studies included in the comprehensive PFAS dashboard. This is a thumbnail image of the interactive visual (https://hawc.epa.gov/summary/visual/assessment/100500256/Comprehensive-PFAS-Dashboard/) accessed on 3 November 2023 that is filterable by parent project, health system, study design, PFAS name, CASRN, and DTXSID.60 The numbers in the heat map inset indicate the distinct number of studies that investigated a health system within a particular study design. If a study evaluated multiple health outcomes or presented several experiments, it is shown here multiple times, though totals reflect distinct numbers of studies. The study design panel includes information on animal model, exposure duration, route of administration, and dose level(s) tested. Note: CASRN, Chemical Abstracts Service registry number; DTXSID, DSSTox substance identifier; ECHA, European Chemicals Agency; IRIS, Integrated Risk Information System; ORD, US EPA Office of Research and Development; PAFT, Program for Alternative Fluorocarbon Toxicity Testing; PFAS, per- and polyfluoroalkyl substances.
Most mammalian bioassay studies were of acute () or short-term () duration or exposed animals via oral administration (). Of the mammalian bioassay studies, 123 were of subchronic, chronic, or reproductive/developmental study designs and are considered most suitable for derivation of a subchronic or chronic POD. The most frequently studied health effects were systemic/whole body (), hepatic (), reproductive (), nervous (), and immune (). The most frequently studied PFAS were PFOA () and PFOS ().
Most epidemiological studies used cohort () or cross-sectional () study designs. The most studied health effects were endocrine (), reproductive (), cardiovascular (), developmental (), and metabolic (). The vast majority of epidemiological data were available for long-chain PFAS that are currently the subject of ongoing or completed US EPA assessments [PFOA (),18 PFOS (),24 PFHxS (),59 and PFDA ()].22
Discussion
PFAS are an important public health and environmental issue impacting many communities across the United States. An important step in understanding potential human health risks from PFAS exposure is to identify and catalog the available health effect data that currently exist from human and animal studies. The US EPA has released its PFAS Strategic Roadmap,10,11 which outlines the need to “evaluate a large number of PFAS for potential human health and ecological effects.” The evidence maps are one way in which the Agency is meeting this goal. By doing a systematic search for toxicity data and collating the data in user friendly, publicly available, web-based repositories, the evidence maps are a resource that can support the development of future toxicity assessments, research prioritization, and decision making both at the US EPA and other scientific organizations.
The goal in building this evidence map was to expand on our earlier SEM and apply systematic review methods to characterize the evidence base for an additional 345 PFAS. We conducted these evidence maps to collate currently available evidence pertinent to human health hazard identification, perform study evaluation, extract available data, and display these data in interactive visualizations. Furthermore, we conducted study evaluations and more detailed data extractions for all nonacute studies. These data are summarized in 47 visualizations (data pivots and study evaluation heatmaps) across 30 PFAS for animal evidence and across 12 PFAS for human evidence.51 A full inventory listing of the available visualizations by PFAS is available in Table S4. All data extracted can be downloaded in HAWC and are also available in the supplemental excel files. These data can serve as a resource to inform future research efforts, facilitate new systematic reviews, and be used in future analyses to compare trends in health effects across PFAS groupings or categories.
Evidence maps do not synthesize data but nonetheless are useful tools for scoping and problem formulation. For example, in response to a US EPA need, ORD used the Carlson et al.12 SEM to identify references that could support the derivation of a human health toxicity value for perfluoropropanoic acid.61 ORD relied on the systematic literature screening, study evaluations and data extractions conducted in Carlson et al.12 to support the assessment.
Furthermore, evidence maps highlight key data gaps and research needs. Our evidence maps highlighted the lack of inhalation data for PFAS and contributed to an intended research project under the Health and Environmental Risk Assessment (HERA) research program within the ORD that hopes to develop a PBPK model for route-to-route extrapolation to understand and estimate potential inhalation effects of PFAS from oral exposure data.77
Because only 41 PFAS of the 345 ( of considered PFAS) searched had any mammalian bioassay or epidemiological evidence, more studies are needed to better understand the potential toxicity of the range of chemistries and structures represented in the PFAS family. Specifically, 2H,3H-Decafluoropentane, perfluoro-2-methyl-3-pentanone, perfluorododecanoic acid, and (4-methyl-2-pentene) were the most studied PFAS chemicals in mammalian toxicology studies. Only 15 PFAS had mammalian toxicology studies that employed a 21 d or longer exposure duration (or were of a reproductive/developmental design) that would be most suitable for identifying subchronic or chronic points of departure for toxicity assessment development.
For epidemiological studies, the most frequently studied PFAS were perfluorododecanoic acid, 2-(N-Methylperfluorooctanesulfonamido)acetate, 2-(N-Ethyl-perfluorooctanesulfonamido)acetate, 2-(N-Methylperfluorooctanesulfonamido)acetic acid, and perfluoro(2-((6-chlorohexyl)oxy)ethanesulfonic acid. As more of the high-throughput toxicology (HTT) testing data being developed by the CCTE becomes available, we hope that the combination of available mammalian bioassay data and HTT data can be used to make prioritization decisions and to inform research.
There are several considerations that are key for interpreting this evidence map. Although study evaluations were completed for some PECO-relevant studies, in many cases unsatisfactory reporting quality of methodological details (for example, failure to report allocation or blinding methods) or results made it challenging to fully interpret the quality of the data. In the future, author outreach could be used for recent peer-reviewed studies to identify any missing or not-reported data that might improve study quality ratings. Gray literature studies often also had limited reporting quality, which impacted study ratings. In addition, there are ongoing efforts in the environmental health community to improve the quality of systematic reviews, both at the individual study level (making researchers more aware of key methods/experimental details to be included in publications) and at the journal level (ensuring publications meet reporting quality guidelines of a given journal).78
A similar effort to systematically characterize the evidence base for a select group of 29 PFAS was conducted by Pelch et al.46 (PFAS-Tox Database); however our evidence maps differ in some key aspects. For example, our evidence maps catalog available data for larger groups of PFAS (more than 500 PFAS across two evidence maps) and identified information from not only peer-reviewed resources, but gray literature as well. Furthermore, our evidence maps also conducted study evaluation, which facilitates use of these maps in future risk assessment and research analyses. The PFAS-Tox Database also included search terms for associated salts in the literature search strategy,46 whereas our work searched associated salts as separate chemicals. Of note, the PFAS-Tox Database includes nonmammalian animal species, dermal and injection routes of exposure, and in vitro studies as PECO-relevant, whereas our evidence maps tagged these types of studies as supplemental material. Although not directly related to our PECO, these supplemental studies may provide information to support hazard identification for PFAS. For future analyses, we have included a full study list of injection and dermal exposure studies in the supplement to this paper (Excel Table S15). These differences in our methodology make the research efforts distinct, and therefore study counts are not directly comparable across our evidence maps and the PFAS-Tox Database.
Based on a search of publicly available information through December 2023, we were unable to identify any existing or in-progress assessment work by the US EPA and other US federal agencies for the PFAS included in this SEM, except for one PFAS that was reviewed by the ATSDR [perfluorododecanoic acid (CASRN 307-55-1)].40 As noted in the “Methods” section, this SEM did not include PFAS for which relevant studies were identified as part of their respective US EPA assessments. However, we created the comprehensive PFAS dashboard that combines health-related PFAS data from across multiple US EPA projects.60 The comprehensive PFAS dashboard allows for a more holistic overview of the PFAS data landscape. As additional US EPA assessments on PFAS are finalized, we hope to update this comprehensive PFAS dashboard periodically to include data from additional projects. Our team has learned some additional lessons on how to best manage updates to a large repository, which remain a challenge for complex systematic review projects. Expansion, curation, and verification of the evidence map content is an ongoing process. Using a web-based inventory and project page allows us to update the datasets as new research is included or as corrections/changes are needed. Thus, the most current information is available on the web-based HAWC project webpage51 and the HERO project page.35 As noted above, the US EPA plans to incorporate additional PFAS in a future update of the Comprehensive PFAS dashboard. However, the US EPA does not currently have additional plans to routinely update the SEMs due to higher priorities on other projects and projected resource constraints. The US EPA expects to conduct targeted updates for certain PFAS when specific assessments are undertaken, building from the existing evidence maps.
This evidence map and the comprehensive PFAS dashboard provide a foundation for future systematic reviews and hazard assessments of PFAS and for strategic planning of research to inform areas of greater uncertainty. We hope that disseminating this research will inform strategic and targeted testing to help fill data gaps across the large and diverse PFAS chemical space. In addition, it is a potentially valuable resource for future researchers who wish to conduct additional analyses using a systematically curated database as a starting point.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Alexis Agbai, Tim DeCoff, and Lauren Johnson for support creating tables. The authors would also like to acknowledge Vicki Soto and Dahnish Shams for document production support and Brittany Jacobs, Jennifer Olker, Amanda Persad, and Samantha Jones for internal review. The authors also acknowledge Brittany Jacobs for support incorporating Office of Water assessment data into the comprehensive PFAS human health dashboard. The authors would like to acknowledge the following ICF staff for assistance in screening studies and creating Tableau visualizations and HAWC trees: Barrett Allen, Lauren Browning, Meredith Clemons, Grace Cooney, Katherine Duke, Sorina Eftim, Hannah Eglinton, Whitney Fies, Ryan Gan, Rebecca Gray, Joanna Greig, Anthony Hannani, Katie Hickok, Anna Kolanowski, Ellen Lee, Madison Lee, Alexander Lindahl, Nathan Lothrop, Denyse Marquez Sanchez, Jaycee Mayer, Kristen McKinley, Michelle Mendez, Melissa Miller, Alicia Murphy, Ashley Peppriell, Lisa Prince, Johanna Rochester, Courtney Rosenthal, Amanda Ross, Andrea Santa Rios, Alessandria Schumacher, Jared Wang, Connie Xiong, and Maricruz Zarco.
L.C. and A.S. were lead authors in developing the research project and drafting and revising the manuscript. K.A.T. played a lead role in initiating and developing the parent research project for the current effort. A.J.W., G.P., and R.S.J. identified PFAS and relevant synonyms for inclusion and participated in CCTE research efforts for grouping PFAS. R.J. conducted the literature searches. HEEAD and CPAD staff were involved with developing the PECO and previous methods, conducting tertiary reviews of epidemiology study evaluations, and revising the manuscript. EPA subject matter experts (M.A., X.A., J.C., L.C., A.D., L.D., A.K., P.K., L.L., P.N., A.S., M.T.) identified system and study level NOAEL/LOAELs. ICF authors were involved in conducting the systematic review (screening studies, data extraction, study evaluation, creating visualizations) and preparing the SEM. The ICF support was managed and coordinated by C.L.
This material has been funded in part by the US EPA under contract 68HERC19D0003 to ICF International. The views expressed are those of the authors and do not necessarily represent the views or policies of the US EPA. Any mention of trade names, products, or services does not imply an endorsement by the US Government or the US EPA. The US EPA does not endorse any commercial products, services, or enterprises.
Conclusions and opinions are those of the individual authors and do not necessarily reflect the policies or views of EHP Publishing or the National Institute of Environmental Health Sciences.
References
- 1.Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. 2019. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol 29(2):131–147, PMID: , 10.1038/s41370-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, et al. 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag 7(4):513–541, PMID: , 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Z, Buser AM, Cousins IT, Demattio S, Drost W, Johansson O, et al. 2021. A new OECD definition for per- and polyfluoroalkyl substances. Environ Sci Technol 55(23):15575–15578, PMID: , 10.1021/acs.est.1c06896. [DOI] [PubMed] [Google Scholar]
- 4.Interstate Technology and Regulatory Council. 2020. PFAS Technical and Regulatory Guidance Document and Fact Sheets. https://pfas-1.itrcweb.org/ [accessed 7 December 2021].
- 5.Williams AJ, Gaines LG, Grulke C, Lowe CN, Sinclair G, Samano V, et al. 2022. Assembly and curation of lists of per- and polyfluoroalkyl substances (PFAS) to support environmental science research. Front Environ Sci 10:1–13, PMID: , 10.3389/fenvs.2022.850019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US EPA (US Environmental Protection Agency). 2021. PFAS structures in DSSTox (update August 2021). https://comptox.epa.gov/dashboard/chemical-lists/PFASSTRUCTV4 [accessed 15 December 2021].
- 7.US EPA. 2022. PFAS|EPA: PFAS Structures in DSSTox (update August 2022). https://comptox.epa.gov/dashboard/chemical-lists/PFASSTRUCTV5 [accessed 15 December 2021].
- 8.Brase RA, Mullin EJ, Spink DC. 2021. Legacy and emerging per- and polyfluoroalkyl substances: analytical techniques, environmental fate, and health effects. Int J Mol Sci 22(3):995, PMID: , 10.3390/ijms22030995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US EPA. 2006. 2010/2015 PFOA Stewardship Program. https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/fact-sheet-20102015-pfoa-stewardship-program [accessed 15 December 2021].
- 10.US EPA. 2021. PFAS Strategic Roadmap: EPA Commitments to Action 2021–2024. EPA-100-K-21-002. https://www.epa.gov/system/files/documents/2021-10/pfas-roadmap_final-508.pdf [accessed 15 December 2021].
- 11.US EPA. 2022. EPA’s PFAS Strategic Roadmap: A Year of Progress. https://www.epa.gov/system/files/documents/2022-11/PFAS%20Roadmap%20Progress%20Report_final_Nov%2017.pdf [accessed 15 December 2022].
- 12.Carlson LA, Angrish M, Shirke AV, Radke EG, Schulz B, Kraft A, et al. 2022. Systematic evidence map for over one hundred and fifty per- and polyfluoroalkyl substances (PFAS). Environ Health Perspect 130(5):56001, PMID: , 10.1289/EHP10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patlewicz G, Richard AM, Williams AJ, Grulke CM, Sams R, Lambert J, et al. 2019. A chemical category-based prioritization approach for selecting 75 per- and polyfluoroalkyl substances (PFAS) for tiered toxicity and toxicokinetic testing. Environ Health Perspect 127(1):14501, PMID: , 10.1289/EHP4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patlewicz G, Richard A, Williams A, Judson R, Thomas R. 2022. Towards reproducible structure-based chemical categories for PFAS to inform and evaluate toxicity and toxicokinetic testing. Comput Toxicol 24:100250, 10.1016/j.comtox.2022.100250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US EPA. 2022. IRIS Toxicological Review of Perfluorobutanoic Acid (PFBA, CASRN 375-22-4) and Related Salts. EPA/635/R-22/277F. https://cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/0701tr.pdf [accessed 3 November 2023]. [PubMed]
- 16.US EPA. 2021. Human Health Toxicity Values for Perfluorobutane Sulfonic Acid (CASRN 375-73-5) and Related Compound Potassium Perfluorobutane Sulfonate (CASRN 29420-49-3). EPA/600/R-20/345F. Washington, DC: US Environmental Protection Agency, Office of Research and Development. https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=350888 [accessed 3 November 2023]. [Google Scholar]
- 17.US EPA. 2023. Toxicological Review of Perfluorohexanoic Acid (PFHxA) and Related Salts (Final Report, 2023). EPA/635/R-23/027F. Washington, DC: Integrated Risk Information System, Center for Public Health and Environmental Assessment, Office of Research and Development, US Environmental Protection Agency. https://cfpub.epa.gov/ncea/iris_drafts/recordisplay.cfm?deid=357314 [accessed 3 November 2023]. [Google Scholar]
- 18.US EPA. 2023. Public Comment Draft: Toxicity Assessment and Proposed Maximum Contaminant Level Goal for Perfluorooctanoic Acid (PFOA) in Drinking Water. EPA/822/P-23/005. Washington, DC: US Environmental Protection Agency, Office of Water, Health and Ecological Criteria Division. https://www.epa.gov/system/files/documents/2023-03/MAIN_Proposed%20MCLG%20for%20PFOA%20in%20Drinking%20Water_3.9.23_For%20Proposal.pdf [accessed 3 November 2023]. [Google Scholar]
- 19.US EPA. 2020. Ground Water and Drinking Water: Drinking Water Health Advisories for PFOA and PFOS. https://www.epa.gov/ground-water-and-drinking-water/drinking-water-health-advisories-pfoa-and-pfos [accessed 3 November 2023]. [DOI] [PubMed]
- 20.US EPA. 2016. Drinking Water Health Advisory for Perfluorooctanoic Acid (PFOA). EPA 822-R-16-005. Washington, DC: US Environmental Protection Agency, Office of Water. https://www.epa.gov/sites/production/files/2016-05/documents/pfoa_health_advisory_final_508.pdf [accessed 15 July 2021]. [Google Scholar]
- 21.US EPA. 2021. Systematic Review Protocol for the PFAS IRIS Assessments. EPA/635/R-19/050. Washington, DC: US Environmental Protection Agency. https://cfpub.epa.gov/ncea/iris_drafts/recordisplay.cfm?deid=345065 [accessed 15 July 2021]. [Google Scholar]
- 22.US EPA. 2023. IRIS Toxicological Review of Perfluorodecanoic Acid (PFDA) and Related Salts. EPA/635/R-23/056a. Washington, DC: Integrated Risk Information System, Center for Public Health and Environmental Assessment, Office of Research and Development, US Environmental Protection Agency. https://ordspub.epa.gov/ords/eims/eimscomm.getfile?p_download_id=546623 [accessed 3 November 2023]. [Google Scholar]
- 23.US EPA. 2023. IRIS Toxicological Review of Perfluorohexanesulfonic Acid (PFHxS) and Related Salts (Public Comment and External Review Draft). EPA/635/R-23/148a. Washington, DC: Integrated Risk Information System, Center for Public Health and Environmental Assessment, Office of Research and Development, US Environmental Protection Agency. https://cfpub.epa.gov/ncea/iris_drafts/recordisplay.cfm?deid=355410 [accessed 3 November 2023]. [Google Scholar]
- 24.US EPA. 2023. Public Comment Draft: Toxicity Assessment and Proposed Maximum Contaminant Level Goal for Perfluorooctane Sulfonic Acid (PFOS) in Drinking Water. EPA/822/P-23/007. Washington, DC: US Environmental Protection Agency, Office of Water, Health and Ecological Criteria Division. https://www.epa.gov/system/files/documents/2023-03/MAIN_Proposed%20MCLG%20for%20PFOS%20in%20Drinking%20Water_3.9.23_For%20Proposal_0.pdf [accessed 3 November 2023]. [Google Scholar]
- 25.US EPA. 2016. Drinking Water Health Advisory for Perfluorooctane Sulfonate (PFOS). EPA 822-R-16-004. Washington, DC: US Environmental Protection Agency, Office of Water. https://www.epa.gov/sites/production/files/2016-05/documents/pfos_health_advisory_final_508.pdf [accessed 3 November 2023]. [Google Scholar]
- 26.US EPA. 2021. Human Health Toxicity Values for Hexafluoropropylene Oxide (HFPO) Dimer Acid and Its Ammonium Salt (CASRN 13252-13-6 and CASRN 62037-80-3). Also Known as “GenX Chemicals.” Final Report. EPA-822R-21-010. Washington, DC: US Environmental Protection Agency, Office of Water. [Google Scholar]
- 27.US EPA. 2022. ORD Staff Handbook for Developing IRIS Assessments. EPA 600/R-22/268. Washington, DC: US Environmental Protection Agency, Office of Research and Development, Center for Public Health and Environmental Assessment. https://cfpub.epa.gov/ncea/iris_drafts/recordisplay.cfm?deid=356370 [accessed 3 November 2023]. [Google Scholar]
- 28.Thayer KA, Angrish M, Arzuaga X, Carlson LM, Davis A, Dishaw L, et al. 2022. Systematic evidence map (SEM) template: report format and methods used for the US EPA Integrated Risk Information System (IRIS) program, Provisional Peer Reviewed Toxicity Value (PPRTV) (PPRTV) program, and other “fit for purpose” literature-based human health analyses. Environ Int 169:107468, PMID: , 10.1016/j.envint.2022.107468. [DOI] [PubMed] [Google Scholar]
- 29.Thayer KA, Shaffer RM, Angrish M, Arzuaga X, Carlson LM, Davis A, et al. 2022. Use of systematic evidence maps within the US Environmental Protection Agency (EPA) Integrated Risk Information System (IRIS) program: advancements to date and looking ahead. Environ Int 169:107363, PMID: , 10.1016/j.envint.2022.107363. [DOI] [PubMed] [Google Scholar]
- 30.Radke EG, Wright JM, Christensen K, Lin CJ, Goldstone AE, Lemeris C, et al. 2022. Epidemiology evidence for health effects of 150 per- and polyfluoroalkyl substances: a systematic evidence map. Environ Health Perspect 130(9):96003, PMID: , 10.1289/EHP11185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.US EPA. 2021. CompTox Chemicals Dashboard. Washington, DC. https://comptox.epa.gov/dashboard [accessed 15 July 2022]. [Google Scholar]
- 32.Keshava C, Davis JA, Stanek J, Thayer KA, Galizia A, Keshava N, et al. 2020. Application of systematic evidence mapping to assess the impact of new research when updating health reference values: a case example using acrolein. Environ Int 143:105956, PMID: , 10.1016/j.envint.2020.105956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.US EPA. 2019. Expanded PFAS SEM (formerly PFAS 430). Office of Research and Development, Center for Public Health and Environmental Assessment, Health and Environmental Research Online (HERO). https://heronet.epa.gov/heronet/index.cfm/project/page/project_id/2875 [accessed 15 July 2023].
- 34.US EPA. 2019. Additional PFAS (formerly XAgency). Office of Research and Development, Center for Public Health and Environmental Assessment, Health and Environmental Research Online (HERO). https://heronet.epa.gov/heronet/index.cfm/project/page/project_id/2877 [accessed 15 July 2023].
- 35.US EPA. 2024. Office of Research and Development, Center for Public Health and Environmental Assessment, Health & Environmental Research Online (HERO). https://heronet.epa.gov/heronet/index.cfm/content/home [accessed 15 July 2023].
- 36.Magnuson K, Cawley M, Reilly D, Varghese A. 2018. Improving Efficiency of Systematic Reviews through Machine Learning for Automated Record Deduplication and Text Analytics for Iterative Keyword Streamlining. Society of Toxicology 57th Annual Meeting and ToxExpo. 11–15 March 2018. San Antonio, Texas. [Google Scholar]
- 37.Howard BE, Phillips J, Miller K, Tandon A, Mav D, Shah MR, et al. 2016. SWIFT-Review: a text-mining workbench for systematic review. Syst Rev 5(1):87, PMID: , 10.1186/s13643-016-0263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sciome. 2019. SWIFT Review: Evidence Stream Search Strategies. https://www.sciome.com/wp-content/uploads/2019/08/SWIFT-Review-Search-Strategies-Evidence-Stream.docx [accessed 15 December 2022].
- 39.ATSDR (Agency for Toxic Substances and Disease Registry). 2018. Chapter 2: Health Effects. In: Toxicological Profile for Perfluoroalkyls. Draft for Public Comment. Atlanta, GA: US Department of Health and Human Services, Public Health Service. https://www.atsdr.cdc.gov/toxprofiles/tp200.pdf [accessed 3 November 2023]. [Google Scholar]
- 40.ATSDR. 2021. Toxicological Profile for Perfluoroalkyls. Atlanta, GA: US Department of Health and Human Services, Public Health Service. 10.15620/cdc:59198. [DOI] [Google Scholar]
- 41.ECHA (European Chemical Agency). 2020. Information on Chemicals. Helsinki, Finland: European Chemicals Agency, European Union. https://echa.europa.eu/information-on-chemicals [accessed 15 December 2020]. [Google Scholar]
- 42.Pelch KE, Reade A, Kwiatkowski CF, Merced-Nieves FM, Cavalier H, Schultz K.et al. 2021. PFAS -Tox Database. https://pfastoxdatabase.org/ [accessed 15 July 2022]. [DOI] [PubMed]
- 43.US EPA. 2018. Access Acute Exposure Guideline Levels (AEGLs) Values Database. Washington, DC: National Research Council, National Academy of Sciences. https://www.epa.gov/aegl/access-acute-exposure-guideline-levels-aegls-values#chemicals [accessed 15 December 2019]. [Google Scholar]
- 44.US EPA. 2021. ECOTOX Knowledgebase. Center for Computational Toxicology and Exposure, Great Lakes Toxicology Ecology Division. https://cfpub.epa.gov/ecotox/ [accessed 15 December 2021].
- 45.Pelch KE, Reade A, Wolffe TAM, Kwiatkowski CF. 2019. PFAS health effects database: Protocol for a systematic evidence map. Environ Int 130:104851, PMID: , 10.1016/j.envint.2019.05.045. [DOI] [PubMed] [Google Scholar]
- 46.Pelch KE, Reade A, Kwiatkowski CF, Merced-Nieves FM, Cavalier H, Schultz K, et al. 2022. The PFAS-Tox database: a systematic evidence map of health studies on 29 per- and polyfluoroalkyl substances. Environ Int 167:107408, PMID: , 10.1016/j.envint.2022.107408. [DOI] [PubMed] [Google Scholar]
- 47.Williams AJ, Grulke CM, Edwards J, Mceachran AD, Mansouri K, Baker NC, et al. 2017. The CompTox chemistry dashboard: a community data resource for environmental chemistry. J Cheminform 9(1):61, 10.1186/s13321-017-0247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Howard BE, Phillips J, Tandon A, Maharana A, Elmore R, Mav D, et al. 2020. SWIFT-active screener: accelerated document screening through active learning and integrated recall estimation. Environ Int 138:105623, PMID: , 10.1016/j.envint.2020.105623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bannach-Brown A, Przybyła P, Thomas J, Rice ASC, Ananiadou S, Liao J, et al. 2019. Machine learning algorithms for systematic review: reducing workload in a preclinical review of animal studies and reducing human screening error. Syst Rev 8(1):23, PMID: , 10.1186/s13643-019-0942-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen AM, Hersh WR, Peterson K, Yen PY. 2006. Reducing workload in systematic review preparation using automated citation classification. J Am Med Inform Assoc 13(2):206–219, PMID: , 10.1197/jamia.M1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.US EPA. 2023. Health Assessment Workspace Collaborative (HAWC) Public Assessments: ORD SEM PFAS Expanded. https://hawc.epa.gov/assessment/100500256/ [accessed 3 November 2023].
- 52.US EPA. 2021. Health Assessment Workspace Collaborative (HAWC). https://hawc.epa.gov/portal/ [accessed 15 July 2023].
- 53.Yost EE, Galizia A, Kapraun DF, Persad AS, Vulimiri SV, Angrish M, et al. 2021. Health effects of naphthalene exposure: a systematic evidence map and analysis of potential considerations for dose-response evaluation. Environ Health Perspect 129(7):76002, PMID: , 10.1289/EHP7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dishaw L, Yost E, Arzuaga X, Luke A, Kraft A, Walker T, et al. 2020. A novel study evaluation strategy in the systematic review of animal toxicology studies for human health assessments of environmental chemicals. Environ Int 141:105736, PMID: , 10.1016/j.envint.2020.105736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Radke EG, Glenn B, Galizia A, Persad A, Nachman R, Bateson T, et al. 2019. Development of outcome-specific criteria for study evaluation in systematic reviews of epidemiology studies. Environ Int 130:104884, PMID: , 10.1016/j.envint.2019.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yost EE, Euling SY, Weaver JA, Beverly BEJ, Keshava N, Mudipalli A, et al. 2019. Hazards of diisobutyl phthalate (DIBP) exposure: a systematic review of animal toxicology studies. Environ Int 125:579–594, PMID: , 10.1016/j.envint.2018.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Radke EG, Braun JM, Meeker JD, Cooper GS. 2018. Phthalate exposure and male reproductive outcomes: a systematic review of the human epidemiological evidence. Environ Int 121(Pt. 1):764–793, PMID: , 10.1016/j.envint.2018.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hall AP, Elcombe CR, Foster JR, Harada T, Kaufmann W, Knippel A, et al. 2012. Liver hypertrophy: a review of adaptive (adverse and non-adverse) changes—conclusions from the 3rd International ESTP Expert Workshop. Toxicol Pathol 40(7):971–994, PMID: , 10.1177/0192623312448935. [DOI] [PubMed] [Google Scholar]
- 59.US EPA. 2023. ORD Human Health Toxicity Value for Lithium Bis [(trifluoromethyl)sulfonyl]azanide (HQ-115) (CASRN 90076-65-6|DTXSID8044468). EPA/600/R-22/195F. Washington, DC: US Environmental Protection Agency. https://cfpub.epa.gov/si/si_public_file_download.cfm?p_download_id=547684&Lab=CPHEA [accessed 3 November 2023]. [PubMed] [Google Scholar]
- 60.US EPA. 2023. Health Assessment Workspace Collaborative (HAWC) Public Assessments: Comprehensive PFAS Dashboard. https://hawc.epa.gov/summary/visual/assessment/100500256/Comprehensive-PFAS-Dashboard/ [accessed 15 July 2023].
- 61.US EPA. 2023. ORD Human Health Toxicity Value for Perfluoropropanoic Acid (CASRN 422-64-0|DTXSID8059970). EPA/600/R-22-042F. Washington, DC: US Environmental Protection Agency. https://cfpub.epa.gov/si/si_public_file_download.cfm?p_download_id=547699&Lab=CPHEA [accessed 3 November 2023]. [PubMed] [Google Scholar]
- 62.Carlson LA, Angrish M, Shirke AV, Radke EG, Schulz B, Kraft A, et al. 2024. Erratum: systematic evidence map for over one hundred and fifty per- and polyfluoroalkyl substances (PFAS). Environ Health Perspect 132(1):019001, 10.1289/EHP14191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.US EPA. 2020. PFAS 150 (2020). https://hawc.epa.gov/assessment/100500085/ [accessed 3 November 2023].
- 64.US EPA. 2022. Health Assessment Workspace Collaborative (HAWC) Public Assessments: Additional PFAS: HAWC Literature Inventory Tree. https://hawc.epa.gov/summary/visual/assessment/100500256/PFAS-XAgency-HAWC-Literature-Inventory-Tree/ [accessed 3 November 2023].
- 65.US EPA. 2022. Health Assessment Workspace Collaborative (HAWC) Public Assessments: Expanded PFAS Evidence Map Visualizations. https://hawc.epa.gov/summary/visual/assessment/100500256/PFAS-430-XAgency-Evidence-Map-Dashboard/ [accessed 3 November 2023].
- 66.US EPA. 2022. Health Assessment Workspace Collaborative (HAWC) Public Assessments: animal SQE Heatmap. https://hawc.epa.gov/summary/visual/assessment/100500256/Animal-SQE-Heatmap/ [accessed 3 November 2023].
- 67.US EPA. 2022. Health Assessment Workspace Collaborative (HAWC) Public Assessments: Animal SQE Heatmap_nonECHA. https://hawc.epa.gov/summary/visual/assessment/100500256/Animal-SQE-Heatmap_nonECHA/ [accessed 3 November 2023].
- 68.Kato H, Fujii S, Takahashi M, Matsumoto M, Hirata-Koizumi M, Ono A, et al. 2015. Repeated dose and reproductive/developmental toxicity of perfluorododecanoic acid in rats. Environ Toxicol 30(11):1244–1263, PMID: , 10.1002/tox.21996. [DOI] [PubMed] [Google Scholar]
- 69.US EPA. 2022. Drinking Water Health Advisory: Hexafluoropropylene Oxide (HFPO) Dimer Acid (CASRN 13252-13-6) and HFPO Dimer Acid Ammonium Salt (CASRN 62037-80-3), Also Known as GenX Chemicals. EPA/822/R-22/005. https://www.epa.gov/system/files/documents/2022-06/drinking-water-genx-2022.pdf [accessed 3 November 2023].
- 70.US EPA. 2022. Drinking Water Health Advisory: Perfluorobutane Sulfonic Acid (CASRN 375-73-5) and Related Compound Potassium Perfluorobutane Sulfonate (CASRN 29420-49-3). EPA/822/R-22/006. https://www.epa.gov/system/files/documents/2022-06/drinking-water-pfbs-2022.pdf [accessed 3 November 2023].
- 71.US EPA. 2022. Health Assessment Workspace Collaborative (HAWC) Public Assessments: ORD SEM Expanded PFAS (2023): Visualizations: Figure 21. Perfluorododecanoic acid_Oral (Chronic, All). https://hawc.epa.gov/summary/data-pivot/assessment/100500256/Figure-21-Perfluorododecanoic-acid_OralChronic/ [accessed 3 November 2023].
- 72.US EPA. 2022. Health Assessment Workspace Collaborative (HAWC) Public Assessments: ORD SEM Expanded PFAS (2023): Visualizations: Figure 24. 2:1 Fluorotelomer alcohol_Inhalation_All. https://hawc.epa.gov/summary/data-pivot/assessment/100500256/Figure-24-21-Fluorotelomer-alcohol_Inhalation_All/ [accessed 3 November 2023].
- 73.US EPA. 2022. Health Assessment Workspace Collaborative (HAWC) Public Assessments: ORD SEM Expanded PFAS (2023): Visualizations: Figure 06. 2:1 Fluorotelomer alcohol_Oral All. https://hawc.epa.gov/summary/data-pivot/assessment/100500256/Figure-6-21-Fluorotelomer-alcohol_Oral-All/ [accessed 3 November 2023].
- 74.US EPA. 2022. Health Assessment Workspace Collaborative (HAWC) Public Assessments: ORD SEM Expanded PFAS (2023): Visualizations: Figure 11. ADONA_Oral (Developmental Toxicity Screening, all endpoints). https://hawc.epa.gov/summary/data-pivot/assessment/100500256/Figure-11-ADONA_Oral-devtoxscreen/ [accessed 3 November 2023].
- 75.US EPA. 2022. INTERIM Drinking Water Health Advisory: Perfluorooctane Sulfonic Acid (PFOS) CASRN 1763-23-1. EPA/822/R-22/004. Washington, DC: US Environmental Protection Agency, Office of Water, Office of Science and Technology. https://www.epa.gov/sdwa/drinking-water-health-advisories-pfoa-and-pfos [accessed 3 November 2023]. [Google Scholar]
- 76.US EPA. 2022. INTERIM Drinking Water Health Advisory: Perfluorooctanoic Acid (PFOA) CASRN 335-67-1. EPA/822/R-22/003. Washington, DC: US Environmental Protection Agency, Office of Water, Office of Science and Technology. https://www.epa.gov/sdwa/drinking-water-health-advisories-pfoa-and-pfos [accessed 3 November 2023]. [Google Scholar]
- 77.US EPA. 2022. Health and Environmental Risk Assessment (HERA) Strategic Research Action Plan. Fiscal Years 2023-2026. EPA/600/R-22/237. Washington, DC: US Environmental Protection Agency, Office of Research and Development. https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P1015SWX.txt [accessed 3 November 2023]. [Google Scholar]
- 78.Whaley P, Blaauboer BJ, Brozek J, Cohen Hubal EA, Hair K, Kacew S, et al. 2021. Improving the quality of toxicology and environmental health systematic reviews: what journal editors can do. ALTEX 38(3):513–522, PMID: , 10.14573/altex.2106111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.