Abstract
S-fimbriated Escherichia coli strains cause sepsis and meningitis in newborns and are known to recognize the carbohydrate sequence sialyl-(α2-3)-galactoside. We show that adhesion of cloned S-fimbriated E. coli to human epithelial cells is inhibited Fab independently by sialyloligosaccharides on secretory immunoglobulin A (s-IgA). This indicates an anti-infective function of s-IgA (Fc), particularly in early human milk.
Secretory immunoglobulin A (s-IgA) forms the predominant antibody fraction in human milk, with a mean concentration of about 2 mg/ml postpartum and characteristic phase-dependent variations during lactation (4). Its regular function is seen in the Fab-mediated neutralization of viruses, bacteria, enzymes, and toxins, thereby providing passive immunoprotection of newborns against gastrointestinal infections. Human s-IgA carries N- and O-linked oligosaccharides (total carbohydrate content, 8.7%), most (63%) of which are bound to the heavy chains (16). While the IgA1 subtype is exclusively N glycosylated, the protease-resistant IgA2 subtype is additionally characterized by five O-glycosidic chains localized in the hinge region of the molecule (2, 3, 17). A possible role of these carbohydrates in antiadhesion effects of s-IgA on human pathogens has previously been suggested and supported by experimental evidence (1, 18). In this context, mannose residues, which are a regular component of N-linked oligosaccharides on s-IgA, have been reported to be receptors for type 1 fimbriae of Escherichia coli (18). Since other types of fimbriae, equipped with S- or P-type adhesins, also bind to carbohydrate receptors, the model study by Wold et al. (18) was extended to S-fimbriated E. coli, which is of major importance in newborns as an agent of sepsis and meningitis (7). S fimbriae bind specifically to the terminal oligosaccharide sequence sialyl-(α2-3)galactoside (8, 10), which is found on many epithelial surfaces as a structural component of glycoproteins or glycolipids. Besides this, S fimbriae have been reported to bind most strongly to sialylated β-galactosides carrying N-glycolylneuraminic acid or the (α2-8)-linked dimer of N-acetylneuraminic acid, as found on gangliosides of the b series or fetal glycopeptides (5). Potential receptors for S adhesins on human s-IgA have been characterized in the complex fraction of O-glycosidically linked oligosaccharides exhibiting (α2-3)-sialylated core2 structures (11). Since sialic acid represents about 1 to 1.5% of the total dry mass of s-IgA (11, 16), its capacity to inhibit bacterial adhesion to buccal epithelial cells in vitro should have a biological significance for infant nutrition.
Inhibition assays were performed with recombinant S-fimbriated E. coli HB101(pANN801-4) and buccal epithelial cells obtained from healthy adult nonsmokers. Bacteria were prepared as described previously (12) and labelled with fluorescein isothiocyanate (Sigma, München, Germany). In brief, the cells were washed in borate buffer (20 mM)–NaCl (150 mM) (pH 9.0) and treated with fluorescein isothiocyanate (1 mg/ml) for 30 min at room temperature. After washing, the cell suspension was diluted to an A540 of 0.32, which corresponds to 2 × 108 cells/ml. Buccal epithelial cells were washed and adjusted to 105 cells/ml. Preincubation of bacteria (30 min) with various concentrations of s-IgA from human colostrum (Sigma) or α-methylmannoside (0.5 to 2.5%; Sigma) in a total volume of 100 μl was followed by addition of an equal volume of a buccal epithelial cell suspension. After shaking for 1 h at 4°C, nonadherent bacteria were separated by three successive centrifugations (100 × g, 5 min, 4°C), and the adherent cells were counted under a fluorescence microscope by inspection of 50 buccal epithelial cells (12).
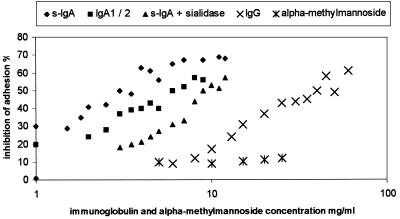
Quantitative analysis in the above-described assay system revealed that s-IgA from human colostrum was able to reduce bacterial adhesion in a dose-dependent manner (Fig. 1). Fifty percent inhibition was achieved with 3 mg/ml. At higher concentrations, a plateau was reached in the range of 70% inhibition. Since α-methylmannoside had no measurable effect on the adhesion of S-fimbriated E. coli, a contribution of mannose residues in N-linked glycans of s-IgA can be excluded (Fig. 1).
FIG. 1.
Dose-dependent inhibition of bacterial binding to buccal epithelial cells. Percent inhibition of bacterial adhesion (mean values) is plotted against the concentration of immunoglobulin inhibitors and α-methylmannoside on a logarithmic scale. The 50% inhibition value of each inhibitor is the mean of three independent measurements: s-IgA, 3 ± 0.5 mg/ml; IgA1 or IgA2, 6 ± 0.5 mg/ml; sialidase-treated s-IgA, 9 ± 1 mg/ml; IgG, 40 ± 5 mg/ml.
The subclasses and subtypes of immunoglobulins exhibit various contents of sialic acid. While the values measured for s-IgA reach 1.5 g/100 g (11, 16), plasmatic IgA1 (1.38 g/100 g) and IgA2 (1.27 g/100 g) (9) or IgG (0.23 g/100 g) (15) is characterized by similar or lower sialic acid content. To assess, whether the different amounts of protein-bound sialic acid affect the capacity of different immunoglobulin fractions to inhibit bacterial adhesion, the assays described above were repeated in the presence of IgA1 or IgA2 (Dunn-Biodesign, Asbach, Germany) and s-IgA or IgG (Sigma). As expected, the measured 50% inhibitory doses of the immunoglobulin fractions varied with their sialic acid contents: s-IgA, 3 mg/ml; IgA1 and IgA2, 6 mg/ml; IgG, 40 mg/ml (Fig. 1).
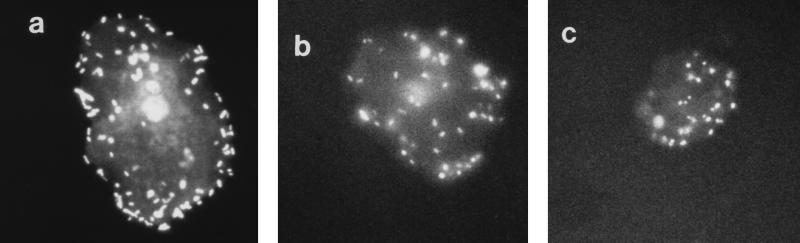
To prove the assumption that protein-bound sialic acid is responsible for the inhibitory effect of s-IgA on bacterial adhesion, the acidic sugar was cleaved by enzymatic hydrolysis with Vibrio cholerae sialidase (immobilized on beaded agarose [Sigma]) prior to the inhibition assay (37°C, 18 h). This treatment resulted in a significant decrease in inhibitory capacity, since 9 mg/ml was necessary to reduce bacterial adhesion to 50% (Fig. 1). The inhibitory effects of various concentrations of s-IgA on the binding of S-fimbriated bacteria to buccal epithelial cells are also documented in Fig. 2, showing the reduction of fluorescent particles by 0, 50, and 70% in the presence of increasing inhibitor concentrations. Even at the highest inhibitor concentration, the cells did not exhibit microscopically detectable morphological changes (Fig. 2).
FIG. 2.
Binding of S-fimbriated E. coli to human buccal epithelial cells in the presence of s-IgA. The cells were incubated with fluorescent bacteria (1,000:1) in the presence of s-IgA at 0 (a), 3 (b), or 8 (c) mg/ml and, after separation of the unbound bacteria by centrifugation, inspected microscopically at a magnification of ×400.
The antiadhesion effect of s-IgA on S-fimbriated E. coli could be mediated partially by specific binding of the Fab fragments to the sugar. To exclude a contribution of adaptive immunity to the observed inhibition of bacterial adhesion, IgA was cleaved into Fab and Fc fragments and the cleavage products were tested separately for their antiadhesion effects. Plasmatic IgA1 was cleaved within the hinge region by using the proline-specific protease from Neisseria gonorrhoeae (Boehringer, Mannheim, Germany) acting on the sequence Ser-Thr-Pro-Pro-Thr (6). Since IgA2 lacks this motif, it was omitted from the experiment. Human plasmatic IgA1 (2 mg) in 50 mM Tris-HCl (pH 7.7) containing 1 mM Na2-EDTA and 50-μg/ml gentamicin was treated with the protease (50 μg/ml) for 20 h at 37°C. The formation of Fab and Fc fragments from IgA1 was verified by sodium dodecyl sulfate–17% polyacrylamide gel electrophoresis as described by Laemmli, and the binding of isolated S fimbriae to the separated proteins was tested after their transfer to polyvinylidene difluoride membranes (14). Two major bands were visible after staining of the proteins: a 62-kDa Fc fragment and a 48-kDa Fab fragment (Fig. 3, lane a). In overlay assays of the blotted proteins, it was demonstrated that both fragments were able to bind isolated S fimbriae (Fig. 1, lane b). This finding supports the assumption that at least part of the observed inhibitory effect of s-IgA should be mediated by the supposed mechanism.
FIG. 3.
Electrophoretic separation of Fab and Fc fragments derived from human IgA combined with Western blot overlay analysis with isolated S fimbriae. Lane a, sodium dodecyl sulfate-polyacrylamide gel electrophoresis of IgA1 protease-digested and Coomassie brilliant blue-stained Fab and Fc fragments. The IgA-specific protease formed a band at 120 kDa. MW, molecular mass. Lane b, Western blot of IgA1 protease digestion after overlay with isolated S fimbriae, immunodetection with an anti-S fimbrial monoclonal antibody and a phosphatase-conjugated secondary antibody, and development with nitroblue tetrazolium. The bands in the higher-molecular-mass range correspond to undigested (dimeric and monomeric) IgA or to partially digested IgA. Due to the small amounts, their presence is apparent only on the Western blot.
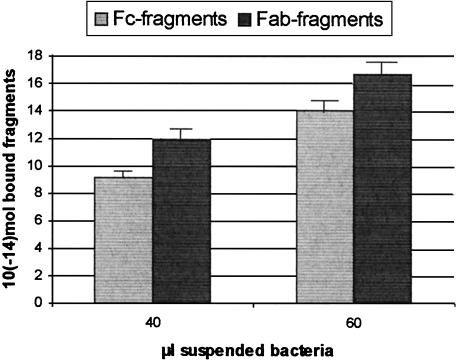
To assess the relative affinities of Fab and Fc fragment binding to the bacterial surface, a semiquantitative enzyme immunoassay was established. Bacteria (2 × 108 cells/ml) were mixed in phosphate-buffered saline with Fab and Fc fragments (final concentration of digested IgA1, 0.5 mg/ml), and after incubation for 1 h at 0°C, the cells were separated from unbound fragments by centrifugation. The sediment was resuspended in carbonate buffer (0.5 M), pH 9.4, and used to coat microtitration plates dried at 37°C. After blocking of the active surface with 1% bovine serum albumin–0.1% Tween 20 (40 mM Tris-HCl [pH 7.4], 150 mM NaCl) for 1 h at 37°C, the primary antibodies (anti-Fab and anti-Fc, 2 mg/ml) were incubated at a dilution of 1:250 for 2 h. A secondary antibody, a goat anti-mouse immunoglobulin-phosphatase conjugate (diluted 1:250), was added, the mixture was incubated for 1 h, and the plate was developed with p-nitrophenylphosphate in 10% diethanolamine buffer, pH 9.3. The molar ratio of bound Fab to Fc fragments was found to be 1.2 (mean value of triplicate assays after subtraction of the buffer control value) (Fig. 4). Taking into account that the molar concentration of Fab fragments was twice as high as that of Fc fragments, the latter should exhibit significantly higher affinity for the bacterial surface.
FIG. 4.
Quantitative studies of IgA fragment (Fab and Fc) binding to S-fimbriated E. coli. Binding of Fab or Fc fragments to suspended bacteria was measured after incubation in the cold, centrifugation, and immobilization of E. coli in the plastic wells of an enzyme-linked immunosorbent assay plate. The bound Fab and Fc fragments were quantified by detection with specific anti-Fc or anti-Fab antibody, followed by an alkaline phosphatase-conjugated secondary antibody. The molar amounts shown were derived from a standard curve measured with plate-immobilized immunoglobulin fragments. Statistical evaluation of the triplicate assays revealed standard deviations between ±0.5 × 10−14 and ±1.5 × 10−14 mol.
To summarize the above-reported findings, it can be stated that s-IgA in human milk is a potent inhibitor of adhesion by S-fimbriated E. coli to human buccal epithelial cells. The mechanism of inhibition was shown to involve specific interactions of sialyloligosaccharides on s-IgA with the bacterial adhesins. Alternative mechanisms mediated by mannose-specific type 1 adhesins could be excluded, and Fab-mediated effects resulting from maternal immunization were demonstrated to have a lesser role in the observed inhibition. Together with our previous findings that mucin-bound sialyloligosaccharides of human milk fat globule membranes are potent inhibitors of adhesion of S-fimbriated E. coli (12), these results can be presumed to have potential significance for pediatric research in the context of infant nutrition. As milk fat globule membrane mucins and s-IgA are relatively resistant to digestion, their protective effects may be important for the entire intestinal system. Secretory and membrane-bound IgA on milk fat globules (13) are presumed to act together as an anti-infective agent by two independent mechanisms: Fab-mediated and glycosylation-mediated inhibition of bacterial adhesion. Further studies will have to elucidate whether the proposed alternative mechanism of bacterial neutralization by s-IgA has any relevance in vivo.
Acknowledgments
We acknowledge K. Jann for providing monoclonal antibody A21356 against S fimbriae.
REFERENCES
- 1.Anthony B F, Concepion N F, Puentes S M, Payne N R. Nonimmune binding of human immunoglobulin A to type II group B streptococci. Infect Immun. 1990;58:1789–1795. doi: 10.1128/iai.58.6.1789-1795.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baenziger J, Kornfeld S. Structure of the carbohydrate units of IgA1 immunoglobulin. I. Composition, glycopeptide isolation, and structure of the asparagine-linked oligosaccharide units. J Biol Chem. 1974;249:7260–7269. [PubMed] [Google Scholar]
- 3.Despont J P J, Abel C. Glycopeptides of heavy chains from human IgA myeloma proteins. J Immunol. 1974;112:1623–1627. [PubMed] [Google Scholar]
- 4.Goldman A S, Goldblum R M. Immunologic system in human milk. In: Lebenthal E, editor. Textbook of gastroenterology and nutrition in infancy. 2nd ed. New York, N.Y: Raven Press; 1989. pp. 135–142. [Google Scholar]
- 5.Hanisch F-G, Hacker J, Schroten H. Specificity of S fimbriae on recombinant Escherichia coli: preferential binding to gangliosides expressing NeuGcα(2-3)Gal and NeuAcα(2-8)NeuAc. Infect Immun. 1993;61:2108–2115. doi: 10.1128/iai.61.5.2108-2115.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kilian M, Thomsen B, Petersen H, Bleeg H S. Occurrence and nature of IgA proteases. Ann N Y Acad Sci. 1983;83:612–624. doi: 10.1111/j.1749-6632.1983.tb26903.x. [DOI] [PubMed] [Google Scholar]
- 7.Korhonen T K, Valtonen M V, Parkkinen J, Väisänen-Rhen V, Finne J, Orksov I, Svenson S B, Mäkelä P H. Serotypes, hemolysin production, and receptor recognition of Escherichia coli strains associated with neonatal sepsis and meningitis. Infect Immun. 1985;48:486–491. doi: 10.1128/iai.48.2.486-491.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korhonen T K, Väisänen-Rhen V, Rhen M, Pere A, Parkkinen J, Finne J. Escherichia coli fimbriae recognizing sialyl galactosides. J Bacteriol. 1984;159:762–766. doi: 10.1128/jb.159.2.762-766.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mestecky J, Kilian M. Immunoglobulin A. Methods Enzymol. 1985;116:37–75. doi: 10.1016/s0076-6879(85)16005-2. [DOI] [PubMed] [Google Scholar]
- 10.Parkkinen J, Finne J, Achtman M, Väisänen V, Korhonen T K. Escherichia coli strains binding neuramyl α2-3 galactosides. Biochem Biophys Res Commun. 1983;111:456–461. doi: 10.1016/0006-291x(83)90328-5. [DOI] [PubMed] [Google Scholar]
- 11.Pierce-Crétel A, Pamblanco M, Strecker G, Monteuil J, Spik G. Primary structure of twenty-three neutral and monosialylated oligosaccharides O-glycosidically linked to the human secretory immunoglobulin A hinge region, determined by a combination of permethylation analysis and 400 MHz H-NMR spectroscopy. Eur J Biochem. 1981;182:457–476. doi: 10.1111/j.1432-1033.1989.tb14853.x. [DOI] [PubMed] [Google Scholar]
- 12.Schroten H, Hanisch F G, Plogmann R, Hacker J, Uhlenbruck G, Nobis-Bosch R, Wahn V. Inhibition of adhesion of S-fimbriated Escherichia coli to buccal epithelial cells by human milk fat globule membrane components: a novel aspect of the protective function of mucins in the nonimmunoglobulin fraction. Infect Immun. 1992;60:2893–2899. doi: 10.1128/iai.60.7.2893-2899.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schroten, H., M. Bosch, R. Nobis-Bosch, and R. Plogmann. Secretory immunoglobulin A is a part of human milk fat globule membranes, abstr. PR 512, p. 382. In Abstracts of the 16th International Congress of Nutrition. IUNS, Montreal, Quebec, Canada.
- 14.Schroten H, Plogmann R, Hanisch F G, Hacker J, Nobis-Bosch R, Wahn V. Inhibition of adhesion of S-fimbriated E. coli to buccal epithelial cells by human skim milk is predominantly mediated by mucins and depends on the period of lactation. Acta Paediatr. 1993;82:1993. doi: 10.1111/j.1651-2227.1993.tb12505.x. [DOI] [PubMed] [Google Scholar]
- 15.Sydow O. Sialic acid content in serum IgG from patients with myotonic dystrophy compared with healthy controls. Acta Neurol Scand. 1989;80:476–478. doi: 10.1111/j.1600-0404.1989.tb03913.x. [DOI] [PubMed] [Google Scholar]
- 16.Tomana M, Mestecky J, Niedermeier W. Studies on human secretory immunoglobulin A. IV. Carbohydrate composition. J Immunol. 1972;108:1631–1636. [PubMed] [Google Scholar]
- 17.Torano A, Tsuzukida Y, Liu Y S, Putman W. Location and structural significance of the oligosaccharides in human IgA1 and IgA2 immunoglobulins. Proc Natl Acad Sci USA. 1977;74:2301–2305. doi: 10.1073/pnas.74.6.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wold A, Mestecky J, Tomana M, Kobata A, Ohbayashi H, Endo T, Svanborg Edén C. Secretory immunoglobulin A carries oligosaccharide receptors for Escherichia coli type 1 fimbrial lectin. Infect Immun. 1990;58:3073–3077. doi: 10.1128/iai.58.9.3073-3077.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]