Abstract
Background
Excess weight gain is an important health concern among people with HIV (PWH) on antiretroviral therapy (ART). The extent to which ART contributes to body mass index (BMI) changes is incompletely understood.
Methods
We conducted a retrospective study of PWH initiating ART and demographically matched people without HIV (PWoH). Data on baseline BMI (kg/m2; categorized as underweight/normal, overweight, or obese) and ART class (integrase strand transfer inhibitor [INSTI], non-nucleoside reverse transcriptase inhibitor [NNRTI], protease inhibitor [PI]) were obtained from electronic health records. BMI was evaluated longitudinally using piecewise linear splines in mixed effects models by HIV status, baseline BMI, and ART class. Models were adjusted for sociodemographics, comorbidities, and substance use.
Results
The study included 8256 PWH and 129 966 PWoH (mean baseline age, 40.9 and 42.2 years, respectively; 88% men). In adjusted models, the average annual change in BMI in the first 2 years after ART initiation was 0.53 for PWH and 0.12 for PWoH (P < .001). BMI increases among PWH were observed for all ART classes: 0.69 for INSTIs, 0.69 for PIs, and 0.40 for NNRTIs vs 0.12 among PWoH. For PWH initiating INSTIs, BMI increases were observed regardless of baseline BMI. Overall BMI changes >2 years after ART initiation were similar by HIV status (0.02 average annual increase for PWH and PWoH).
Conclusions
PWH initiating ART gained excess weight in the first 2 years, emphasizing the importance of monitoring weight and cardiometabolic health among ART-treated PWH.
Keywords: antiretroviral therapy, body mass index, cardiovascular disease, return to health, weight
People with HIV experienced greater increases in body mass index in the first 2 years of antiretroviral therapy use when compared with people without HIV. Body mass index increases were observed across antiretroviral therapy classes. For people with HIV initiating integrase strand transfer inhibitors, increases were similar for those who started as underweight/normal weight, overweight, or obese.
Excess weight gain and obesity are increasingly recognized as major health concerns among people with HIV (PWH) on antiretroviral therapy (ART) [1, 2]. Over the past 3 decades, the incidence and prevalence of obesity among PWH have steadily increased [3, 4]. Factors contributing to these trends are incompletely understood. Recent studies have focused on the cardiometabolic adverse effects of ART [5–10], but these are difficult to disentangle from other influences, including aging-related weight gain, systemic HIV-related inflammation, and concurrent non–HIV-related factors driving the obesity epidemic in the US population [11]. While the overall prevalence of obesity among PWH remains lower than among people without HIV (PWoH), weight gain in the HIV setting confers greater risk of metabolic disease, with a disproportionate burden borne by women and minorities [1].
Metabolic complications among PWH on ART are not a new issue. Older ART regimens (ie, early nucleoside reverse transcriptase inhibitors [NRTIs] and protease inhibitors [PIs]) affected adipose distribution, increasing visceral fat in the midsection and decreasing peripheral adiposity, even in the absence of clinically apparent lipodystrophy or change in total body fat [1, 12]. Other side effects of these therapies included dysregulation of glucose and lipid metabolism, which increased risk of insulin resistance, dyslipidemia, and metabolic disease [13, 14]. With current-generation ART regimens, generalized weight gain has been associated with use of integrase strand transfer inhibitor (INSTI)-based and tenofovir alafenamide (TAF)-based regimens, which have become more broadly used as first-line ART [1, 4, 8, 15–24]. While weight gain among PWH who are underweight or normal weight at ART initiation is associated with reduced risk of mortality [25], excess weight gain and obesity are associated with increased risk of diabetes and cardiovascular disease [1, 26].
Better characterizing weight gain among PWH in the context of interrelated HIV and non-HIV factors, as well as weight gain due to aging, is important given the pathophysiologic link of obesity with serious cardiometabolic health conditions and premature death [27]. The objectives of this study were to evaluate baseline and longitudinal differences in body mass index (BMI) in PWH initiating ART and comparator PWoH receiving care in 3 US integrated healthcare systems.
METHODS
Study Design, Setting, and Population
We conducted a retrospective cohort study of adults (≥21 years old) who were members of Kaiser Permanente in Northern California (KPNC), Southern California, and Mid-Atlantic States (includes Maryland, Virginia, and Washington, DC). These integrated healthcare systems provide comprehensive medical services to a diverse patient population that is demographically representative of the general insured population in its service areas [28]. Electronic health record (EHR) databases at Kaiser Permanente capture data from healthcare visits, laboratory tests, and pharmacy fills. HIV registries include all known cases of HIV infection in the Kaiser Permanente patient population.
Study participants were drawn from an established cohort of PWH and PWoH who were frequency matched 1:10 by age (±2 years), sex, race/ethnicity, and first year of follow-up. People were eligible for inclusion if they had at least 2 BMI values (kg/m2) in the EHR between 2005 and 2016. PWH were further restricted to those who initiated an ART regimen on or after 1 January 2005 and did not previously have an HIV RNA level <200 copies/mL (which could indicate prior ART).
Patient Consent
The study was approved by the KPNC institutional review board with a waiver for informed consent. Kaiser Permanente in Southern California and Mid-Atlantic States ceded to the KPNC institutional review board.
Participant Follow-up
Study follow-up occurred from 1 January 2005 to 31 December 2016. For PWH, baseline was defined as date of first ART prescription fill. For PWoH, baseline was 1 January of the year in which they were selected as part of the frequency-matching process. Follow-up ended at health plan disenrollment, death, or last day of the study.
Measures
BMI was measured at baseline (defined as the BMI measure closest to baseline in the 4 years preceding baseline) and longitudinally (all available BMI values in the EHR during follow-up). Categories of BMI were defined as follows: underweight (<18.5), normal (18.5–24.9), overweight (25.0–29.9), and obese (≥30).
ART regimen information was ascertained through pharmacy dispensation records and categorized by ART class: integrase strand transfer inhibitor [INSTI], non-nucleoside reverse transcriptase inhibitor [NNRTI], and protease inhibitor [PI]. All regimens had an NRTI backbone (predominantly tenofovir disoproxil fumarate [TDF] during the study period).
Covariates
Data on sociodemographic and clinical factors that could be associated with weight change were obtained from the EHR. Sociodemographic covariates were age, sex, race/ethnicity (White, Black, Hispanic, Asian, other), census-based education and income (estimated with US Census residential block-level data), and insurance type (ie, commercial, Medicaid, Medicare, other/unknown). Comorbidities were cancer (any type), chronic kidney disease, cardiovascular disease (unstable angina, acute myocardial infarction, and stroke), hypertension, dyslipidemia, diabetes mellitus, and liver disease (chronic liver disease, cirrhosis, hepatitis B infection, and hepatitis C infection). Comorbidities were identified by ICD codes, CPT codes, and laboratory results based on approaches previously described [29–31] and were included in time-updated analyses (ie, incorporated new diagnoses during follow-up). Substance use factors were also time updated and included ICD-coded alcohol use disorder and other substance use disorder diagnoses as well as patient-reported smoking status.
Analyses
Baseline characteristics were compared descriptively by HIV status. Changes in BMI were evaluated longitudinally via linear mixed effects models with an autoregression covariance structure. Models were constructed to evaluate average annual BMI change (1) by HIV status; (2) by HIV status and baseline BMI category; and (3) by HIV status, baseline BMI category, and ART class at time of ART initiation. Models included piecewise linear splines with a knot at 2 years to evaluate BMI change in the first 2 years following ART initiation and >2 years after ART initiation. This approach was based on prior studies suggesting the biphasic nature of weight gain following ART initiation, with substantial weight gain occurring within the first 1 to 2 years [3, 32]. Average annual BMI change was modeled for a 12-year period in analyses by HIV status and by HIV status and baseline BMI category but only for a 7-year period in analyses by HIV status, baseline BMI category, and ART class, since INSTIs were not available until the latter part of the study period. Analyses did not account for ART class switching during follow-up. Since the proportion of study participants who were underweight at baseline was small (3% of PWH and 1% of PWoH), the “underweight” category was combined with the “normal weight” category in all analyses. Multivariable models included adjustment for baseline year (ie, 2005–2007, 2008–2009, 2010–2011, 2012–2013, 2014–2015, 2016), current age, race/ethnicity, sex, education, income, insurance type, comorbidities, substance use disorders, and smoking. To evaluate potential sex-specific differences in BMI change, subanalyses evaluated average annual BMI change by HIV status and sex. A sensitivity analysis was done excluding people who were underweight at baseline. Analyses were performed with SAS version 9.4 (SAS Institute).
RESULTS
Participant Characteristics at Baseline
The study included 8256 PWH and 129 966 demographically matched PWoH. PWH were followed for a mean ± SD 3.3 ± 2.8 years, and PWoH were followed for 3.5 ± 2.6 years. The mean baseline age was 40.9 ± 11.7 years for PWH and 42.2 ± 12.1 for PWoH (Table 1). PWH were mostly men (88%), and 33% were White, 26% Black, 26% Hispanic, and 6% Asian. When compared with PWoH, a higher proportion of PWH had cancer, chronic kidney disease, and liver disease, and a lower proportion had diabetes mellitus, dyslipidemia, and hypertension. A higher proportion of PWH than PWoH had a history of smoking, alcohol use disorder, or other substance use disorder. First ART regimens filled following HIV diagnosis included NNRTIs (48.9%), INSTIs (26.6%), and PIs (24.5%).
Table 1.
Characteristics of Study Participants at Baseline by HIV Status
| Participants, No. (%) | ||
|---|---|---|
| Characteristic | With HIV (n = 8256) | Without HIV (n = 129 966) |
| Male | 7229 (87.6) | 112 501 (86.6) |
| Age, y, mean (SD) | 40.9 (11.7) | 42.2 (12.1) |
| Race/ethnicity | ||
| White | 2725 (33.0) | 49 033 (37.7) |
| Black | 2180 (26.4) | 33 886 (26.1) |
| Hispanic | 2124 (25.7) | 31 747 (24.4) |
| Asian | 474 (5.7) | 6763 (5.2) |
| Other/unknown | 753 (9.1) | 8537 (6.6) |
| Body mass index, kg/m2 | ||
| Underweight, <18.5 | 227 (2.8) | 877 (0.7) |
| Normal, 18.5–24.9 | 3625 (43.9) | 31 161 (24.0) |
| Overweight, 25.0–29.9 | 2927 (35.5) | 49 602 (38.2) |
| Obese, ≥30.0 | 1477 (17.9) | 48 326 (37.2) |
| Neighborhood-level factors | ||
| Lower educationa | 1946 (27.4) | 28 637 (23.1) |
| Lower incomeb | 1040 (14.6) | 11 703 (9.5) |
| Insurance type | ||
| Commercial | 7609 (92.2) | 121 242 (93.3) |
| Medicare | 358 (4.3) | 5128 (4.0) |
| Medicaid | 256 (3.1) | 3134 (2.4) |
| Other/unknown | 33 (0.4) | 462 (0.4) |
| History of cancer, any type | 213 (2.6) | 1161 (0.9) |
| Cardiovascular diseasec | 46 (0.6) | 815 (0.6) |
| Hypertension | 1338 (16.2) | 26 955 (20.7) |
| Chronic kidney disease | 194 (2.4) | 2216 (1.7) |
| Diabetes mellitus | 435 (5.3) | 10 646 (8.2) |
| Dyslipidemia | 1436 (17.4) | 38 491 (29.6) |
| Liver disease | 632 (7.7) | 3854 (3.0) |
| Current or past smoking | 3893 (47.2) | 48 328 (37.2) |
| Alcohol use disorderd | 912 (11.1) | 9175 (7.1) |
| Other substance use disorderd | 1323 (16.0) | 7215 (5.6) |
| HIV risk group | … | |
| Men who have sex with men | 4285 (51.9) | |
| Heterosexual | 1528 (18.5) | |
| Injection drug use | 428 (5.2) | |
| Other/unknown | 2015 (24.4) | |
| First ART regimen | … | |
| NNRTI | 4037 (48.9) | |
| INSTI | 2199 (26.6) | |
| PI | 2020 (24.5) | |
Abbreviations: ART, antiretroviral therapy; INSTI, integrase strand transfer inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
aLower neighborhood-level education defined as ≥25% of the population in the patient's census block group having no high school diploma.
bLower neighborhood-level income defined as census block group having an annual average income <$35 000 (https://www.payingforseniorcare.com/federal-poverty-level).
cCardiovascular disease included unstable angina, acute myocardial infarction, and stroke.
dAlcohol and other substance use disorders were identified according to ICD-9 and ICD-10 diagnoses in the electronic health record.
BMI at Baseline and Changes Over Time by HIV Status
At baseline, mean BMI was lower for PWH (26.2 ± 5.3) than for PWoH (29.2 ± 6.2, P < .001). Among PWH, 35.5% were overweight and 17.9% were obese at baseline. In comparison, 38.2% of PWoH were overweight and 37.2% were obese at baseline.
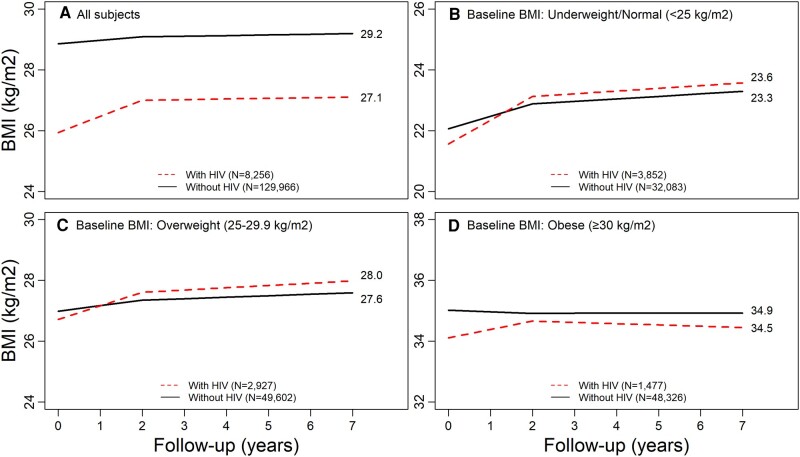
In adjusted models, the average annual change in BMI in the first 2 years after ART initiation was 0.53 (95% CI, .47–.59) per year for PWH as compared with 0.12 (95% CI, .10–.13) per year for PWoH (P < .001; Figure 1A, Appendix Table 1). In the period >2 years after ART initiation, BMI change was similar by HIV status (in PWH, 0.02 [95% CI, −.02 to .07]; in PWoH, 0.02 [95% CI, .01–.04]). At the end of follow-up, the average BMI was 27.1 for PWH and 29.2 for PWoH. Excluding underweight people had minimal impact on results (data not shown).
Figure 1.
Adjusted changes in BMI by HIV status and baseline BMI. Adjusted for age, sex, race/ethnicity, calendar period, neighborhood-level education, neighborhood-level income, insurance type, history of cancer (any type), cardiovascular disease, hypertension, chronic kidney disease, diabetes mellitus, dyslipidemia, liver disease, smoking, alcohol use disorders, and other substance use disorders. A, all participants; B, underweight/normal; C, overweight; D, obese. BMI, body mass index.
In adjusted models stratified by sex, the average annual change in BMI in the first 2 years after ART initiation was 0.53 (95% CI, .56–.59) per year for male PWH as compared with 0.13 (95% CI, .11–.14) per year for male PWoH (P < .001); it was 0.67 (95% CI, .48–.86) per year for female PWH vs 0.16 (95% CI, .11–.21) per year for female PWoH (P < .001). In the period >2 years after ART initiation, the average annual change in BMI was 0.02 (95% CI, −.02 to .07) per year for male PWH and 0.03 (95% CI, .01–.04) per year for male PWoH (P = .33); it was 0.04 (95% CI, −.10 to .18) per year for female PWH as compared with 0.04 (95% CI, .004–.08) per year for female PWoH (P = .54).
BMI Changes Over Time by HIV Status and Baseline BMI
In the first 2 years after ART initiation, PWH in all baseline BMI categories had greater adjusted average annual increases in BMI than PWoH (Figure 1B–D, Appendix Table 1). Among people who were underweight or had normal weight at baseline, average annual increases in BMI in the first 2 years were 0.78 (95% CI, .73–.83) per year for PWH as opposed to 0.41 (95% CI, .39–.43) per year for PWoH, after adjustment for the same covariates as in prior models (P < .001). Among people who were overweight at baseline, BMI increased on average 0.44 (95% CI, .38–.50) per year in the first 2 years for PWH as compared with 0.18 (95% CI, .16–.20) per year for PWoH (P < .001). Among people who were obese at baseline, BMI increased 0.28 (95% CI, .11–.44) per year in the first 2 years for PWH but decreased slightly among PWoH (−0.05 [95% CI, −.08 to −.02]; P = .01). In the period >2 years after ART initiation, BMI change was similar by HIV status in all BMI categories.
BMI Changes Over Time by HIV Status, Baseline BMI, and First ART Class
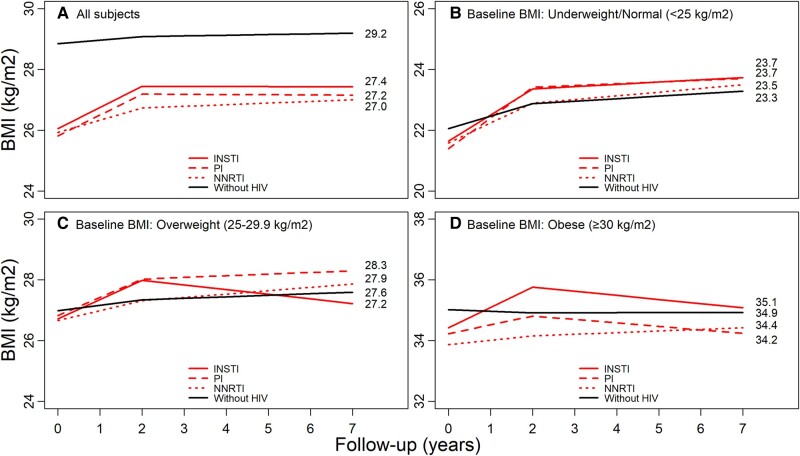
PWH on each ART regimen had greater average annual increases in BMI during the first 2 years of follow-up when compared with PWoH. PWoH (reference) had an average annual change in BMI of 0.12. In the first 2 years after ART initiation, average annual increases in BMI among PWH were 0.69 (95% CI, .56–.83) for PWH initiating INSTIs, 0.40 (95% CI, .32–.48) for PWH initiating NNRTIs, and 0.69 (95% CI, .57–.81) for PWH initiating PIs, after adjustment for covariates (Figure 2A, Appendix Table 1). In the period >2 years after ART initiation, BMI was stable among PWH and increased slightly among PWoH.
Figure 2.
Adjusted changes in BMI by HIV status, baseline BMI, and first ART class. Adjusted for age, sex, race/ethnicity, calendar period, neighborhood-level education, neighborhood-level income, insurance type, history of cancer (any type), cardiovascular disease, hypertension, chronic kidney disease, diabetes mellitus, dyslipidemia, liver disease, smoking, alcohol use disorders, and other substance use disorders. A, all participants; B, underweight/normal; C, overweight; D, obese. ART, antiretroviral therapy; BMI, body mass index; INSTI, integrase strand transfer inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
In analyses stratified by baseline BMI and ART class, PWH who were underweight/normal weight or overweight at ART initiation had significant average annual increases in BMI in the first 2 years after ART initiation (Figure 2B and 2C, Appendix Table 1; P < .001 for all ART classes). During this time frame, obese PWH initiating INSTIs had significant BMI increases (0.67; 95% CI, .30–1.04), but PWH initiating NNRTIs or PIs had nonsignificant BMI increases (0.14 [95% CI, −.09 to .37] and 0.29 [95% CI, −.04 to .62], respectively; Figure 2D).
In the period >2 years after ART initiation, PWH who were overweight at INSTI initiation experienced significant decreases in BMI (−0.15; 95% CI, −.29 to −.02). In contrast, overweight PWH initiating NNRTIs had increasing BMI (0.11; 95% CI, .07–.15); PWH initiating PIs had stable BMI (0.06; 95% CI, −.0003 to .11); and PWoH had slightly increasing BMI (0.05; 95% CI, .04–.06) during this same period (Figure 2C and 2D). All unadjusted results are in Appendix Table 2. All adjusted results are in Appendix Table 1.
DISCUSSION
In this study of weight gain among PWH and demographically matched PWoH receiving health care in the same setting, we report several key findings. First, PWH experienced greater increases in BMI during the first 2 years after ART initiation than PWoH, consistent with hypotheses that HIV-specific factors such as ART use may contribute to initial weight gain. Second, PWH initiating ART gained weight in the first 2 years regardless of baseline BMI, emphasizing the importance of monitoring relative weight change, not just development of obesity. Last, early increases in BMI among PWH were consistently observed among PWH initiating INSTIs, which highlights the importance of monitoring weight change following ART initiation, especially since INSTIs are now the primary first-line ART regimen. Importantly, even though PWH initiating ART had lower BMIs than PWoH, they ended up with BMIs similar to those of PWoH, which could represent healthy weight gain for some individuals but development of obesity for others.
In the first 2 years after ART initiation, BMI increased on average >4 times as fast for PWH (0.53 per year) vs PWoH (0.12 per year). This finding of weight gain following ART initiation is consistent with results of many prior studies [16–18, 20], though not all [2, 3, 33]. Differences in weight gain detected among PWH across cohorts may be due to differences in demographics, access to care, and/or background prevalence of obesity in the general population, which could obscure characterization of obesity among PWH. Indeed, there are wide geographic differences in risk of obesity given disparities in healthcare resources, healthy food environments, and other non–HIV-related factors. By comparing BMI change against matched PWoH with access to care in the same setting and adjusting analytically for confounding factors for weight change, our study extends findings in prior studies conducted among PWH only [2, 3, 16–18] and strengthens the hypothesis that ART use may contribute to weight gain.
In the period >2 years after ART initiation, BMI stabilized for most PWH and PWoH. However, PWH who were overweight at INSTI initiation experienced significant BMI decreases during this period, which could reflect discontinuation of INSTI, improved weight management, or a combination of these factors. Investigating the extent to which ART regimen changes contributed to BMI changes over time was beyond the scope of this study but would be important to investigate in future studies.
Even though PWH started off at a lower average BMI than PWoH, by 12 years of follow-up, average BMI was in the overweight category for PWH (27.1) and PWoH (29.2). While more rapid weight gain among PWH accounts for much of this trend, changes in HIV diagnosis and management may contribute in part. In recent years, HIV testing has been expanded, and rapid ART initiation has become standard practice. As a result, a greater proportion of PWH are being diagnosed earlier and initiating ART at higher CD4 counts and BMI. Among PWH in our cohort, 35.5% were overweight and 17.9% were obese at baseline, aligning more closely with the prevalence of high BMI in the general population than HIV-associated weight loss and wasting. This is consistent with other studies showing an increasing likelihood of PWH being overweight or obese at HIV diagnosis and ART initiation [2, 4]. As ART use becomes more widespread and PWH remain on lifelong treatment starting from younger ages and higher baseline BMI, better understanding the weight-related consequences of long-term ART and distinguishing healthy from unhealthy weight gain will be important.
Related to this concept is our observation that PWH gained more weight than matched PWoH across all categories of baseline BMI, although the type of ART initiated played an important role. To some extent, normal aging contributes to weight gain [34]: increases in BMI were observed among PWH and PWoH over time, even after adjustment for risk factors for weight gain. However, weight gain was slowest among PWoH who were obese, possibly due to heightened medical attention and prioritization of weight loss in this group [35, 36]. Our findings highlight the importance of tracking weight change among PWH initiating ART, even among those at a healthy weight to start, particularly since some other studies suggest that obesity-related health risks may occur at a lower BMI among PWH [11].
We found that PWH initiating INSTIs had >5-fold greater annual weight gain in the first 2 years after ART initiation than PWoH. These findings are similar to INSTI-related weight gain reported in clinical trials of INSTIs [5], observational cohorts of treatment-naive PWH initiating INSTIs [16, 17, 22], and studies of PWH switching from other regimens to INSTIs [9, 10, 23, 24, 37]. More recent studies report heterogeneity in presence and magnitude of weight gain across population subgroups [38, 39], including one study that found no significant weight gain among people aged >65 years who initiated INSTIs [38–41]. Now that INSTI-based regimens are recommended first-line treatment for most PWH [42] and oftentimes prescribed with TAF, which is also associated with excess weight gain [23, 24, 43], it is especially critical to monitor for clinically relevant weight change among PWH undergoing ART [9, 15]. Our study was conducted with data from 2005 to 2016 and primarily included regimens with TDF and not TAF (which was not approved in the United States until November 2015) [1].
The clinical implications of excess weight gain among PWH are multiple. In the context of ART use in an aging HIV population, obesity could lead to significant cardiometabolic health complications and related health challenges [14]. Of particular concern is that the metabolic effects of excess weight and obesity appear to be compounded in the HIV setting [14]. This may be due to the contributing effects of HIV-related chronic inflammation and unique pathophysiologic processes and pathways underlying the development of metabolic syndromes in PWH [14]. As compared with PWoH, weight gain, increased central adiposity, and other metabolic risk factors among PWH confer a heightened risk for health issues such as neurocognitive impairment [44], diabetes [7, 45], cardiovascular disease [46], and nonalcoholic fatty liver disease [1, 47]. Obesity is also associated with reduced quality of life, excess medical care expenditures, discrimination, and social stigmatization [48].
This study had some limitations. First, analyses were based on information collected during routine health care encounters. We were unable to account for some lifestyle and behavioral factors, such as caloric intake and level of physical activity, that were not routinely recorded in the EHR but could have affected BMI. Second, we recognize that BMI is an imprecise measure of body composition [49–51]. The use of BMI as a proxy anthropometric measure for overall body composition allowed us to conduct longitudinal analyses on a large sample of PWH and PWoH. Future studies of cardiometabolic risk may consider use of central adiposity or waist circumference measures. Third, we did not evaluate concomitant usage of medications that could have contributed to weight changes, but models were adjusted for common comorbidities and would likely have accounted for some of these confounding effects. Future studies evaluating the metabolic effects of individual drugs would be important since polypharmacy is common among PWH [13]. Fourth, we could not fully account for instances where participants may have switched ART regimens or become less adherent to ART following noticeable weight gain. However, we note that switching to abacavir, an NRTI that has not been associated with weight gain, would have been unlikely since clinicians at KPNC were concerned about its cardiovascular effects [52]. Future studies that capture ART switching would be important to address full ART exposure history and its effects on BMI. Also, while analyses were stratified by ART class, we were unable to substratify by individual ART type or evaluate the weight-related side effects of regimen components. Last, our study period did not include bictegravir, the most prescribed INSTI used in practice today, since it was not approved by the US Food and Drug Administration until 2018 and the study period ended in 2016.
This study builds on prior work in several key ways:
Use of HIV registries that contain comprehensive data on ART prescription fills for all PWH in 3 large health systems
Comparison with PWoH in the same setting to better understand weight gain independent of aging and other structural non–HIV-related risk factors that could affect BMI
Use of EHR data to account for multiple individual-level cardiometabolic risk factors that were insufficiently captured in some prior studies
Longitudinal follow-up in a contemporary ART-treated cohort of PWH, which enabled evaluation of weight change over time by ART class.
In conclusion, PWH initiating ART gained more weight over time when compared with PWoH receiving care in the same setting and after adjustment for confounding sociodemographic and clinical risk factors. Weight gain during ART could offset some of the benefits of ART, such as virologic suppression and reduced inflammation. INSTIs are well tolerated and offer convenient dosing, but the weight gain associated with these agents could have important implications for cardiometabolic health. The findings from this study highlight the need to investigate remaining uncertainties regarding ideal target weight among ART-treated PWH, approaches for weight management, and the extent that individual ART classes contribute to long-term metabolic changes and cardiometabolic health.
Appendix
Table 1.
Adjusteda Annual BMI Change and 95% Confidence Intervals, by HIV Status, Baseline BMI, and ART Class
| First 2 years After ART Initiation | >2 years After ART Initiation | |||
|---|---|---|---|---|
| With HIV | Without HIV | With HIV | Without HIV | |
| N = 8256 | n = 129 966 | N = 8256 | n = 129 966 | |
| Overall (Figure 1, panel a) | 0.53 (0.47, 0.59) | 0.12 (0.10, 0.13) | 0.02 (−0.02, 0.07) | 0.02 (0.01, 0.04) |
| By baseline BMI (Figure 1, panels b to d) | ||||
| Underweight/normal | 0.78 (0.73, 0.83) | 0.41 (0.39, 0.43) | 0.09 (0.06, 0.12) | 0.08 (0.07, 0.10) |
| Overweight | 0.44 (0.38, 0.50) | 0.18 (0.16, 0.20) | 0.07 (0.04, 0.11) | 0.05 (0.04, 0.06) |
| Obese | 0.28 (0.11, 0.44) | −0.05 (−0.08, −0.02) | −0.04 (−0.16, 0.07) | 0.002 (−0.02, 0.02) |
| By ART class (Figure 2, panel a) | 0.12 (0.10, 0.13) | 0.02 (0.01, 0.04) | ||
| INSTI | 0.69 (0.56, 0.83) | −0.003 (−0.18, 0.17) | ||
| NNRTI | 0.40 (0.32, 0.48) | 0.05 (−0.006, 0.11) | ||
| PI | 0.69 (0.57, 0.81) | −0.007 (−0.09, 0.08) | ||
| By ART class, among underweight/normal (Figure 2, panel b) | 0.41 (0.39, 0.43) | 0.08 (0.07, 0.10) | ||
| INSTI | 0.86 (0.74, 0.98) | 0.07 (−0.05, 0.20) | ||
| NNRTI | 0.65 (0.58, 0.73) | 0.12 (0.08, 0.16) | ||
| PI | 1.01 (0.90, 1.11) | 0.06 (0.004, 0.11) | ||
| By ART class, among overweight (Figure 2, panel c) | 0.18 (0.16, 0.20) | 0.05 (0.04, 0.06) | ||
| INSTI | 0.64 (0.50, 0.77) | −0.15 (−0.29, −0.02) | ||
| NNRTI | 0.32 (0.24, 0.40) | 0.11 (0.07, 0.15) | ||
| PI | 0.60 (0.49, 0.72) | 0.06 (−0.0003, 0.11) | ||
| By ART class, among obese (Figure 2, panel d) | −0.05 (−0.08, −0.02) | 0.002 (−0.02, 0.02) | ||
| INSTI | 0.67 (0.30, 1.04) | −0.14 (−0.58, 0.31) | ||
| NNRTI | 0.14 (−0.09, 0.37) | 0.05 (−0.09, 0.20) | ||
| PI | 0.29 (−0.04, 0.62) | −0.11 (−0.31, 0.09) | ||
aAdjusted for age, sex, race/ethnicity, calendar period, neighborhood-level education, neighborhood-level income, insurance type, history of cancer (any type), cardiovascular disease, hypertension, chronic kidney disease, diabetes mellitus, dyslipidemia, liver disease, smoking, alcohol use disorder, and other substance use disorders.
Table 2.
Unadjusted Annual BMI Change and 95% Confidence Intervals, by HIV Status, Baseline BMI, and ART Class
| First 2 Years After ART Initiation | >2 Years After ART Initiation | |||
|---|---|---|---|---|
| With HIV | Without HIV | With HIV | Without HIV | |
| N = 8256 | n = 129 966 | N = 8256 | n = 129 966 | |
| Overall | 0.61 (0.55, 0.67) | 0.19 (0.17, 0.20) | 0.08 (0.04, 0.13) | 0.09 (0.07, 0.10) |
| By baseline BMI | ||||
| Underweight/normal | 0.81 (0.76, 0.86) | 0.43 (0.41, 0.45) | 0.10 (0.07, 0.14) | 0.10 (0.08, 0.11) |
| Overweight | 0.46 (0.40, 0.52) | 0.19 (0.17, 0.20) | 0.08 (0.05, 0.11) | 0.05 (0.04, 0.06) |
| Obese | 0.29 (0.12, 0.46) | −0.04 (−0.07, −0.01) | −0.04 (−0.15, 0.08) | 0.02 (−0.004, 0.04) |
| By ART class | 0.19 (0.17, 0.20) | 0.09 (0.07, 0.10) | ||
| INSTI | 0.78 (0.65, 0.92) | 0.06 (−0.11, 0.24) | ||
| NNRTI | 0.47 (0.39, 0.56) | 0.11 (0.05, 0.17) | ||
| PI | 0.77 (0.65, 0.89) | 0.06 (−0.02, 0.15) | ||
| By ART class, among underweight/normal | 0.43 (0.41, 0.45) | 0.10 (0.08, 0.11) | ||
| INSTI | 0.89 (0.77, 1.01) | 0.09 (−0.04, 0.21) | ||
| NNRTI | 0.68 (0.61, 0.76) | 0.13 (0.10, 0.17) | ||
| PI | 1.03 (0.92, 1.14) | 0.07 (0.01, 0.12) | ||
| By ART class, among overweight | 0.19 (0.17, 0.20) | 0.05 (0.05, 0.06) | ||
| INSTI | 0.65 (0.52, 0.79) | −0.16 (−0.29, −0.02) | ||
| NNRTI | 0.34 (0.26, 0.42) | 0.11 (0.07, 0.16) | ||
| PI | 0.62 (0.50, 0.73) | 0.06 (0.01, 0.12) | ||
| By ART class, among obese | −0.04 (−0.07, −0.01) | 0.02 (−0.004, 0.04) | ||
| INSTI | 0.70 (0.33, 1.07) | −0.14 (−0.60, 0.31) | ||
| NNRTI | 0.15 (−0.08, 0.38) | 0.05 (−0.10, 0.20) | ||
| PI | 0.31 (−0.02, 0.64) | −0.09 (−0.29, 0.12) | ||
Contributor Information
Jennifer O Lam, Division of Research, Kaiser Permanente Northern California, Oakland, California, USA.
Wendy A Leyden, Division of Research, Kaiser Permanente Northern California, Oakland, California, USA.
Stacey Alexeeff, Division of Research, Kaiser Permanente Northern California, Oakland, California, USA.
Alexandra N Lea, Division of Research, Kaiser Permanente Northern California, Oakland, California, USA.
Rulin C Hechter, Department of Research and Evaluation, Kaiser Permanente Southern California, Pasadena, California, USA; Department of Health Systems Science, Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, California, USA.
Haihong Hu, Mid-Atlantic Permanente Research Institute, Kaiser Permanente Mid-Atlantic States, Rockville, Maryland, USA.
Julia L Marcus, Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, Massachusetts, USA.
Lakecia Pitts, Division of Research, Kaiser Permanente Northern California, Oakland, California, USA.
Qing Yuan, Department of Research and Evaluation, Kaiser Permanente Southern California, Pasadena, California, USA.
William J Towner, Department of Research and Evaluation, Kaiser Permanente Southern California, Pasadena, California, USA; Department of Clinical Science, Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, California, USA.
Michael A Horberg, Department of Health Systems Science, Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, California, USA; Mid-Atlantic Permanente Research Institute, Kaiser Permanente Mid-Atlantic States, Rockville, Maryland, USA.
Michael J Silverberg, Division of Research, Kaiser Permanente Northern California, Oakland, California, USA; Department of Health Systems Science, Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, California, USA; Department of Epidemiology and Biostatistics, University of California, San Francisco, California, USA.
Notes
Financial support. This work was supported by grants from Gilead, Inc (to W. J. T., M. A. H., and M. J. S.); and the National Institute of Allergy and Infectious Diseases (grantK01 AI157849 to J. O. L.).
References
- 1. Bailin SS, Gabriel CL, Wanjalla CN, Koethe JR. Obesity and weight gain in persons with HIV. Curr HIV/AIDS Rep 2020; 17:138–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Crum-Cianflone N, Roediger MP, Eberly L, et al. Increasing rates of obesity among HIV-infected persons during the HIV epidemic. PLoS One 2010; 5:e10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hasse B, Iff M, Ledergerber B, et al. Obesity trends and body mass index changes after starting antiretroviral treatment: the Swiss HIV Cohort Study. Open Forum Infect Dis 2014; 1:ofu040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koethe JR, Jenkins CA, Lau B, et al. Rising obesity prevalence and weight gain among adults starting antiretroviral therapy in the United States and Canada. AIDS Res Hum Retroviruses 2016; 32:50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sax PE, Erlandson KM, Lake JE, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis 2020; 71:1379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koethe JR, Jenkins CA, Lau B, et al. Body mass index and early CD4 T-cell recovery among adults initiating antiretroviral therapy in North America, 1998–2010. HIV Med 2015; 16:572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rebeiro PF, Jenkins CA, Bian A, et al. Risk of incident diabetes mellitus, weight gain, and their relationships with integrase inhibitor-based initial antiretroviral therapy among persons with human immunodeficiency virus in the United States and Canada. Clin Infect Dis 2021; 73:e2234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Norwood J, Turner M, Bofill C, et al. Brief report: weight gain in persons with HIV switched from efavirenz-based to integrase strand transfer inhibitor-based regimens. J Acquir Immune Defic Syndr 2017; 76:527–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Erlandson KM, Carter CC, Melbourne K, et al. Weight change following antiretroviral therapy switch in people with viral suppression: pooled data from randomized clinical trials. Clin Infect Dis 2021; 73:1440–51. [DOI] [PubMed] [Google Scholar]
- 10. Lake JE, Wu K, Bares SH, et al. Risk factors for weight gain following switch to integrase inhibitor-based antiretroviral therapy. Clin Infect Dis 2020; 71:e471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lake JE. The fat of the matter: obesity and visceral adiposity in treated HIV infection. Curr HIV/AIDS Rep 2017; 14:211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koethe JR, Lagathu C, Lake JE, et al. HIV and antiretroviral therapy–related fat alterations. Nat Rev Dis Primers 2020; 6:48. [DOI] [PubMed] [Google Scholar]
- 13. Srinivasa S, Grinspoon SK. Metabolic and body composition effects of newer antiretrovirals in HIV-infected patients. Eur J Endocrinol 2014; 170:R185–202. [DOI] [PubMed] [Google Scholar]
- 14. Nix LM, Tien PC. Metabolic syndrome, diabetes, and cardiovascular risk in HIV. Curr HIV/AIDS Rep 2014; 11:271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lake JE, Trevillyan J. Impact of integrase inhibitors and tenofovir alafenamide on weight gain in people with HIV. Curr Opin HIV AIDS 2021; 16:148–51. [DOI] [PubMed] [Google Scholar]
- 16. Bourgi K, Jenkins CA, Rebeiro PF, et al. Weight gain among treatment-naïve persons with HIV starting integrase inhibitors compared to non-nucleoside reverse transcriptase inhibitors or protease inhibitors in a large observational cohort in the United States and Canada. J Int AIDS Soc 2020; 23:e25484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bakal DR, Coelho LE, Luz PM, et al. Obesity following ART initiation is common and influenced by both traditional and HIV-/ART-specific risk factors. J Antimicrob Chemother 2018; 73:2177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bourgi K, Rebeiro PF, Turner M, et al. Greater weight gain in treatment-naive persons starting dolutegravir-based antiretroviral therapy. Clin Infect Dis 2020; 70:1267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen YW, Hardy H, Pericone CD, Chow W. Real-world assessment of weight change in people with HIV-1 after initiating integrase strand transfer inhibitors or protease inhibitors. J Health Econ Outcomes Res 2020; 7:102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guehi C, Badjé A, Gabillard D, et al. High prevalence of being overweight and obese HIV-infected persons, before and after 24 months on early ART in the ANRS 12136 Temprano Trial. AIDS Res Ther 2016; 13:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Han WM, Law MG, Choi JY, et al. Weight changes, metabolic syndrome and all-cause mortality among Asian adults living with HIV. HIV Med 2022; 23:274–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu KS, Anderson C, Little SJ. Integrase strand transfer inhibitors play the main role in greater weight gain among men with acute and early HIV infection. Open Forum Infect Dis 2021; 8:ofaa619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sjaarda A, Bernstein A, Sparks A, Saber S, Siegel M. Comparison of weight gain after antiretroviral switch to integrase strand transfer inhibitor or tenofovir alafenamide-based therapy. Infection 2022; 50:407–12. [DOI] [PubMed] [Google Scholar]
- 24. Palella FJ, Hou Q, Li J, et al. Weight gain and metabolic effects in persons with HIV who switch to ART regimens containing integrase inhibitors or tenofovir alafenamide. J Acquir Immune Defic Syndr 2023; 92:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yuh B, Tate J, Butt AA, et al. Weight change after antiretroviral therapy and mortality. Clin Infect Dis 2015; 60:1852–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lake JE, Stanley TL, Apovian CM, et al. Practical review of recognition and management of obesity and lipohypertrophy in human immunodeficiency virus infection. Clin Infect Dis 2017; 64:1422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA 2013; 309:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gordon NP. Similarity of adult Kaiser Permanente members to the adult population in Kaiser Permanente's Northern California service area: Comparisons based on the 2017/2018 cycle of the California Health Interview Survey. Report prepared for the Kaiser Permanente Division of Research, Oakland, CA, November 8, 2020. Available at: https://divisionofresearch.kaiserpermanente.org/projects/memberhealthsurvey/SiteCollectionDocuments/compare_kp_ncal_chis2017-18.pdf. Accessed 24 December 2020.
- 29. Go AS, Hsu CY, Yang J, et al. Acute kidney injury and risk of heart failure and atherosclerotic events. Clin J Am Soc Nephrol 2018; 13:833–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lam JO, Lee C, Gilsanz P, et al. Comparison of dementia incidence and prevalence between individuals with and without HIV infection in primary care from 2000 to 2016. AIDS 2022; 36:437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Go AS, Reynolds K, Avula HR, et al. Human immunodeficiency virus infection and variation in heart failure risk by age, sex, and ethnicity: the HIV HEART Study. Mayo Clin Proc 2022; 97:465–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tate T, Willig AL, Willig JH, et al. HIV infection and obesity: where did all the wasting go? Antivir Ther 2012; 17:1281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Esser S. No difference in changes of the body mass index categories over 5 years in people living with HIV and the general population from the German Ruhr area. Presented at: European AIDS Conference 2021; London, UK; 27–30 October 2021. [Google Scholar]
- 34. Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012; 307:491–7. [DOI] [PubMed] [Google Scholar]
- 35. QuickStats: percentage of adults with a visit to a health professional in the past 12 months who received dietary advice, by obesity status and age group—National Health Interview Survey, United States, 2014. MMWR Morb Mortal Wkly Rep 2016; 65:405. [Google Scholar]
- 36. QuickStats: percentage of adults aged ≥20 years who reported being told by a doctor or health professional to increase their physical activity, by age group and obesity status—National Health and Nutrition Examination Survey, United States, 2011–2014. MMWR Morb Mortal Wkly Rep 2017; 66:1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Debroy P, Feng H, Miao H, et al. Changes in central adipose tissue after switching to integrase inhibitors. HIV Res Clin Pract 2020; 21:168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Verboeket SO, Boyd A, Wit FW, et al. Generally rare but occasionally severe weight gain after switching to an integrase inhibitor in virally suppressed AGEhIV cohort participants. PLoS One 2021; 16:e0251205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Verburgh ML, Wit FWNM, Boyd A, Verboeket SO, Reiss P, van der Valk M. One in 10 virally suppressed persons with HIV in the Netherlands experiences ≥10% weight gain after switching to tenofovir alafenamide and/or integrase strand transfer inhibitor. Open Forum Infect Dis 2022; 9:ofac291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guaraldi G, Calza S, Milic J, et al. Dolutegravir is not associated with weight gain in antiretroviral therapy experienced geriatric patients living with HIV. AIDS 2021; 35:939–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Taramasso L, Stapleton JT, Siedner MJ. Weight gain and aging in people with HIV. AIDS 2021; 35:987–9. [DOI] [PubMed] [Google Scholar]
- 42. US Department of Health and Human Services . Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. 2022. https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/whats-new-guidelines. Accessed 29 June 2022.
- 43. Mallon PWG, Brunet L, Hsu RK, et al. Weight gain before and after switch from TDF to TAF in a US cohort study. J Int AIDS Soc 2021; 24:e25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pasipanodya EC, Montoya JL, Campbell LM, et al. Metabolic risk factors as differential predictors of profiles of neurocognitive impairment among older HIV+ and HIV– adults: an observational study. Archiv Clin Neuropsychol 2021; 36:151–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Herrin M, Tate JP, Akgün KM, et al. Weight gain and incident diabetes among HIV-infected veterans initiating antiretroviral therapy compared with uninfected individuals. J Acquir Immune Defic Syndr 2016; 73:228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013; 173:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mohammed SS, Aghdassi E, Salit IE, et al. HIV-positive patients with nonalcoholic fatty liver disease have a lower body mass index and are more physically active than HIV-negative patients. J Acquir Immune Defic Syndr 2007; 45:432–8. [DOI] [PubMed] [Google Scholar]
- 48. Massie DC, Amaro A, Kaplan M. Patient well-being and the clinical and economic burdens associated with obesity in the United States. Am J Manag Care 2022; 28(15):S279–87. [DOI] [PubMed] [Google Scholar]
- 49. Camhi SM, Bray GA, Bouchard C, et al. The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: sex and race differences. Obesity (Silver Spring) 2011; 19:402–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Camhi SM, Must A, Gona PN, et al. Duration and stability of metabolically healthy obesity over 30 years. Int J Obes (Lond) 2019; 43:1803–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dimala CA, Ngu RC, Kadia BM, Tianyi FL, Choukem SP. Markers of adiposity in HIV/AIDS patients: agreement between waist circumference, waist-to-hip ratio, waist-to-height ratio and body mass index. PLoS One 2018; 13:e0194653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Marcus JL, Neugebauer RS, Leyden WA, et al. Use of Abacavir and risk of cardiovascular disease among HIV-infected individuals. J Acquir Immune Defic Syndr 2016; 71:413–9. [DOI] [PubMed] [Google Scholar]