Abstract
Background
Patients living in rural settings have poorer access to care and more frequent readmissions after treatment for acute coronary syndrome (ACS) than patients in urban settings. It is unclear what types of medication-related issues are encountered by this cohort and whether pharmacist-led care could resolve them.
Objectives
To describe the issues related to cardiac medications encountered by rural patients after treatment for ACS and the impact of a pharmacist-led virtual follow-up pilot program in this population.
Methods
A quality improvement initiative was developed whereby a cardiology pharmacist provided follow-up to post-ACS rural patients in Alberta, Canada, between March and May 2022. For each patient, the pharmacist identified and resolved cardiac medication–related issues through regular telephone visits over a 30-day period following hospital discharge. The primary outcome was the number of cardiac medication–related issues identified. Secondary outcomes included the types of medication-related issues identified and actions taken by the pharmacist to resolve them.
Results
During the 15-week program, 40 patients received care, and 139 virtual visits were completed. The median time spent per visit was 60 (interquartile range [IQR] 50–80) minutes. In total, 255 cardiac medication–related issues (6 per patient, IQR 3.75–8.25) were identified, of which 233 (91%) were resolved by the pharmacist. Prescription errors, adverse effects, and drug therapy optimization were the most common issues identified on days 1, 10, and 30, respectively. The pharmacist commonly undertook patient counselling (n = 126, 54%) and medication prescribing (n = 63, 27%) to address medication-related issues.
Conclusions
A substantial number of cardiac medication–related issues were identified and resolved through a pharmacist-led virtual follow-up program in rural post-ACS patients. These findings could assist in the development of future follow-up programs to improve care for this high-risk population.
Keywords: pharmacist-led care, pharmacist follow-up, rural patients, acute coronary syndrome, post-acute coronary syndrome care, virtual care
RÉSUMÉ
Contexte
L’accès des patients vivant en milieu rural aux soins est plus difficile et leur réadmission plus fréquente après un traitement pour le syndrome coronarien aigu (SCA) que les patients vivant en milieu urbain. On ne sait pas exactement quels types de problèmes liés aux médicaments rencontre cette cohorte et si les soins dispensés par les pharmaciens pourraient les résoudre.
Objectifs
Décrire les problèmes liés aux médicaments cardiaques que rencontrent les patients vivant en milieu rural après un traitement pour le SCA et les effets d’un programme pilote de suivi virtuel dirigé par un pharmacien dans cette population.
Méthodes
Une initiative d’amélioration de la qualité a été développée dans le cadre de laquelle un pharmacien en cardiologie a assuré le suivi des patients vivant en milieu rural après un SCA en Alberta, au Canada, entre mars et mai 2022. Pour chaque patient, le pharmacien a identifié et résolu les problèmes liés aux médicaments cardiaques grâce à des visites téléphoniques régulières sur une période de 30 jours après le congé de l’hôpital. Le critère de jugement principal était le nombre de problèmes identifiés liés aux médicaments cardiaques. Les critères de jugement secondaires comprenaient les types de problèmes liés aux médicaments identifiés et les mesures prises par le pharmacien pour les résoudre.
Résultats
Au cours du programme de 15 semaines, 40 patients ont reçu des soins et 139 visites virtuelles ont été réalisées. La durée médiane de chaque visite était de 60 minutes (intervalle interquartile [IQR] 50–80). Au total, 255 problèmes liés aux médicaments cardiaques (6 par patient, IQR 3,75–8,25) ont été identifiés, dont 233 (91 %) ont été résolus par le pharmacien. Les erreurs de prescription, les événements indésirables et l’optimisation du traitement médicamenteux étaient les problèmes les plus fréquents les jours 1, 10 et 30, respectivement. Le pharmacien offrait généralement du counseling aux patients (n = 126, 54 %) et prescrivait des médicaments (n = 63, 27 %) pour résoudre les problèmes liés aux médicaments.
Conclusions
Un nombre important de problèmes liés aux médicaments cardiaques ont été identifiés et résolus grâce à un programme de suivi virtuel dirigé par un pharmacien chez les patients vivant en milieu rural après un SCA. Ces résultats pourraient aider à élaborer de futurs programmes de suivi pour améliorer les soins dans cette population à haut risque.
Mots-clés: soins dispensés par un pharmacien, suivi par un pharmacien, patients vivant en milieu rural, syndrome coronarien aigu, soins post-syndrome coronarien aigu, soins virtuels
INTRODUCTION
The first 30 days after an acute coronary syndrome (ACS) is a vulnerable period with the highest risk for recurrent cardiovascular events relative to any other period following an ACS.1,2 Previous Canadian research has shown that patients living in rural locations have higher rates of repeat ACS than patients residing in urban centres.3 This disparity may be the result of reduced access to care and cardiovascular follow-up in rural locations.4,5 Delayed outpatient follow-up after ACS has also been shown to result in lower prescription fill rates, which have been associated with adverse events, medication errors, and additional use of the health care system.6–9
As medication experts, pharmacists play a unique role in mitigating barriers to medication-taking, resolving therapy-related issues, and managing drug regimens. Numerous pharmacist-led programs in cardiology have demonstrated improved prescription fill rates and better management of risk factors and cardiovascular conditions.10–13 Furthermore, although access to care in rural locations remains a limiting factor, virtual care programs have been shown to provide an efficient, sustainable, and cost-saving alternative to in-person cardiac programs.14,15 Several meta-analyses of virtual post-ACS programs in urban locations have demonstrated improvements in lifestyle modification and risk factor management.16–18 Studies assessing virtual programs in rural settings are limited, but improved risk factor management and resource utilization have been demonstrated in the setting of virtual cardiac rehabilitation.19
Although pharmacist-led programs and virtual care have each shown clinical benefit in the management of cardiovascular conditions, a combination of these approaches has not, to our knowledge, been assessed for rural post-ACS patients in Canada. Furthermore, the types of medication-related issues encountered in this patient population and the pharmacist actions required to resolve them have not been explicitly explored. Identifying these factors is necessary to develop an effective post-ACS follow-up program, which could ultimately help in closing the care gap for rural patients.
The PLURAL-ACS (Pharmacist-Led Follow-Up Program for Rural Acute Coronary Syndrome Patients) pilot program was a 30-day pharmacist-led virtual follow-up pilot program for rural post-ACS patients in Alberta. The program objectives were to identify and resolve any cardiac medication–related issues encountered by post-ACS patients in an outpatient setting for 30 days after hospital discharge.
METHODS
Program Design and Setting
The PLURAL-ACS pilot program was a program evaluation/quality improvement initiative undertaken at the Mazankowski Alberta Heart Institute, a tertiary cardiac care institution in Edmonton, Alberta. Given the nature of the study, ethics approval was not required. Discharged patients entered the program between March 9 and May 25, 2022, and were followed virtually for 30 days each as outpatients.
Program Participants
Patients 18 years of age or older from the Central and Northern Alberta zones (Alberta Health Services) who were admitted to the Mazankowski Alberta Heart Institute with ACS (i.e., ST-elevation myocardial infarction or non-ST-elevation acute coronary syndrome) were eligible for the pilot program. Patients were excluded if they did not have telephone access, did not speak English, had a diagnosis of dementia or significant cognitive dysfunction following anoxic brain injury during the index event, had been discharged to a rehabilitation or long-term care facility, had undergone coronary artery bypass surgery for the index event, or had experienced myocardial infarction with non-obstructive coronary artery disease. All patients received medication review and teaching by the inpatient pharmacist before discharge, as part of the unit protocol.
Program Service
The program pharmacist (H.E.B.) was an advanced year-2 cardiology pharmacy resident with prescribing privileges who had prior clinical experience as a staff pharmacist in inpatient cardiology. Eligible patients were identified before discharge by the program pharmacist and members of the inpatient cardiology care teams. The program pharmacist introduced the program to the patients in person, and gave each patient a handout detailing the program.
All patients who agreed to participate received 3 scheduled telephone visits with the program pharmacist, as follows: at 24 hours (1 day), 10 days, and 30 days after discharge. These time points were chosen to encompass the different types of issues that patients might encounter over the first 30 days after discharge. Specifically, the early intervention (day 1) was intended to ensure that the patient had timely access to prescribed medications, given that provincial data for Alberta have indicated delays in prescription fill times for rural ACS patients following hospital discharge (Shlakhter O. AMI/STEMI/NSTEMI and ED visit rates. Alberta Health Services Cardiovascular Health and Stroke Strategic Clinical Network; 2021. Unpublished report). Similarly, the 30-day time point was selected because the same report indicated an increased rate of 30-day readmission for ACS among these rural patients.
The pharmacist’s telephone visits were structured and standardized. If the pharmacist failed to reach the patient for a scheduled visit, 2 additional contact attempts were made, after which the pharmacist left a telephone voice message. The program pharmacist also provided patients with the number for a direct phone line that was monitored during weekdays (0800–1600) and scheduled issue-focused follow-up telephone calls, in addition to the scheduled protocol phone calls, as required. For each patient, all available data were included in the analysis, but patients could drop out of the pilot project at any time; as such, data may be incomplete for later time points.
The 3 program-specified follow-up telephone visits focused on the identification and timely resolution of any barriers to medication-taking (e.g., drug unavailability in pharmacy) and cardiac medication–related issues (e.g., adverse effects related to a medication). The need to optimize drug therapy for medical issues outside management of the patient’s index ACS event was considered to be a medication-related issue; however, such issues were not addressed during program follow-up, unless the situation was urgent and action was deemed medically necessary.
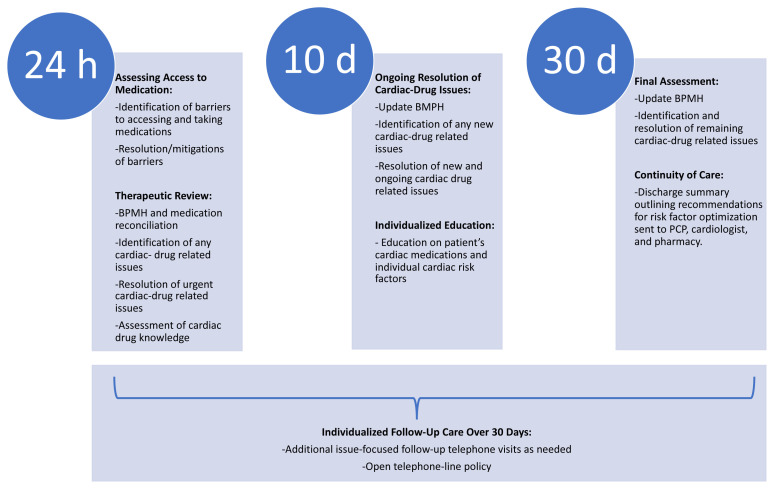
Figure 1 outlines the services provided by the program pharmacist during each visit. The first visit involved a comprehensive review of the patient’s discharge medications, as well as assessment of their medication-taking behaviours and baseline medication knowledge. Barriers to accessing cardiac medications were addressed promptly, within 24 hours after discharge, and any cardiac medication–related issues were identified and resolved at each visit. To further improve their medication-taking behaviour, patients also received individualized education on cardiac medications and their own particular cardiac risk factors, according to the initial assessment of need. At the end of the 30-day follow-up, the program pharmacist provided a written discharge summary to the patient’s primary care provider (PCP), cardiologist, and community pharmacist; this summary outlined the patient’s cardiac-specific issues, details of care and interventions provided during the program, and any unresolved cardiac medication–related issues that required further follow-up for optimization of therapy. Full details of the clinical services, patient assessment, and discharge summary templates are provided in Supplement 1, parts 1, 2, and 3, respectively (available from https://www.cjhp-online.ca/index.php/cjhp/article/view/3472).
FIGURE 1.
Overview of types of care provided by the program pharmacist at various time points in the PLURAL-ACS pilot program. BPMH = best possible medication history, PCP = primary care provider.
Outcomes
The primary outcome was the total number of cardiac medication–related issues identified by the program pharmacist during each patient’s 30-day follow-up. Cardiac medication–related issues were defined as any medication-related issue (e.g., drug interactions, adverse effects, medication non-adherence) that pertained to patients’ post-ACS medications, as listed in Supplement 1, part 4. For any scheduled visit that a patient did not attend, cardiac medication–related issues were not identified.
The secondary outcomes were the total number of cardiac medication–related issues that were resolved by the end of the program, the types of cardiac medication–related issues identified, the actions taken by the pharmacist to resolve each issue, and the types of unresolved cardiac medication–related issues remaining at the end of the pilot period. If appropriate, resolution of previously identified cardiac medication–related issues was determined at the next scheduled visit or the issues were marked as unresolved if resolution was indeterminate, according to chart review and patient interview at the end of the program. The time spent, per visit and by type of activity, was also recorded.
All outcomes were documented by the program pharmacist after each telephone visit, including after the 30-day visit assessment, using standardized clinical documentation. The types of cardiac medication–related issues and pharmacist actions were documented according to a prespecified categorization adapted from previous studies.20,21
Cardiac medication–related issues were divided into 3 categories, each comprising various subcategories (further definitions are provided in Supplement 1, part 5):
General cardiac medication issues: adverse effects, patients’ medication concerns, contraindicated therapy, drug or food interactions, requirement for assistance with medication adherence, requirement for therapy optimization, and requirement for follow-up on previously ordered blood work.
Patient-level medication issues: non-intentional medication non-adherence, intentional medication non-adherence, continuation of discontinued preadmission medication, discharge medication not obtained from pharmacy, and medication discontinued by patient.
System-level medication issues: insufficient prescription duration, non-indicated therapy, omission of needed medication from discharge prescription, insufficient supply of pass-medications (supply of new medications provided to patient at discharge to ensure continuity of therapy until the patient is able to fill prescription in community pharmacy), drug cost creating a barrier to medication-taking, conflicting information between discharge documents, unavailability of medication at pharmacy, and failure to reconcile home medications.
Statistical Analysis
Given the descriptive nature of this analysis, as well as the small sample size and non-normal distribution of certain results, the outcomes are expressed as medians with interquartile ranges (IQRs) or percentages.
RESULTS
Program Participants
A total of 40 patients entered the PLURAL-ACS pilot program. Two of these patients did not complete the full 30-day program. One patient had a non–cardiac-related readmission and died several days after completing the day 1 telephone visit; the second patient could not be reached for the 30-day visit.
The baseline characteristics of all participants are presented in Table 1. The median age was 65.5 years, and 83% of the participants were male. Almost all participants (95%) had a history of dyslipidemia, 65% had a history of hypertension, and 48% were current cigarette smokers. More than half (65%) of the participants were admitted with ST-elevation myocardial infarction, and 95% received percutaneous coronary intervention. All of the program participants were initiated on new cardiac medications during their index hospitalization (median of 5 new cardiac medications per participant, IQR 4–5). For all of the participants, an outpatient cardiology follow-up appointment was planned for 4–8 weeks after discharge. Although 88% (n = 35) of the participants had an established PCP before starting the program, 98% (n = 39) had received post-discharge follow-up with a PCP by the end of the program.
TABLE 1.
Baseline Characteristics of Patients in the PLURAL-ACS Pilot Program
| Variable | No. (%) of Patientsa (n = 40) |
|---|---|
| Age, years (median and IQR) | 65.5 (59.0–74.3) |
|
| |
| Sex, male | 33 (83) |
|
| |
| Area of residence | |
| Central Zone | 21 (53) |
| North Zone | 19 (48) |
|
| |
| Cardiovascular risk factors | |
| Dyslipidemia | 38 (95) |
| Hypertension | 26 (65) |
| Cigarette smoker | 19 (48) |
| Type 2 diabetes mellitus | 14 (35) |
| Prior coronary artery disease | 9 (23) |
| Positive family history | 9 (23) |
|
| |
| Admission diagnosis | |
| STEMI | 26 (65) |
| NSTEACS | 14 (35) |
|
| |
| Managementb | |
| PCI | 38 (95) |
| Outpatient staged PCI | 4 (10) |
|
| |
| LVEF after acute coronary syndrome | |
| 40%–50% | 13 (33) |
| < 40% | 9 (23) |
|
| |
| Discharge factors | |
| No. of new CV medications (median and IQR) | 5 (4–5) |
| Length of stay, days (median and IQR) | 3.5 (4–5) |
|
| |
| Drug insurance | |
| Government | 23 (58) |
| Private | 12 (30) |
| None | 5 (13) |
|
| |
| Follow-up | |
| Established PCP before pilot program | 35 (88) |
| Assigned cardiologist at discharge | 40 (100) |
CV = cardiovascular, IQR = interquartile range, LVEF = left ventricular ejection fraction, NSTEACS = non-ST elevation acute coronary syndrome, PCI = percutaneous coronary intervention, PCP = primary care provider, STEMI = ST-elevation myocardial infarction.
Except where indicated otherwise.
Some patients received both types of management.
Telephone Visits
Over the course of the 15-week program, a total of 139 telephone visits were completed: 117 standard (scheduled) visits, 12 participant-initiated visits, and 10 pharmacist-initiated visits. Overall, the median time spent per visit was 60 (IQR 50–80) minutes, which included the completion of any required chart reviews, patient care notes, and correspondence with other health care professionals. More specifically, the median time spent per visit was 80 (IQR 70–95) minutes, 60 (IQR 45–60) minutes, and 60 (IQR 50–80) minutes for the day 1, day 10, and day 30 visits, respectively. The activities requiring the most time were patient counselling and education (median 30 [IQR 25–30] minutes), patient assessments (median 20 [IQR 20–30] minutes), and completing discharge summaries (median 20 [IQR 15–30] minutes).
Primary Outcome
The pharmacist identified a total of 255 cardiac medication–related issues during the pilot program. Over the entire 30-day follow-up period, the median number of cardiac medication–related issues identified per participant was 6 (IQR 3.75–8.25). More specifically, the median numbers of cardiac medication–related issues identified per participant were 3.5 (IQR 2–5) at the day 1 visit, 1 (IQR 1–2) at the day 10 visit, and 0.5 (IQR 0–1.25) at the day 30 visit.
Secondary Outcomes
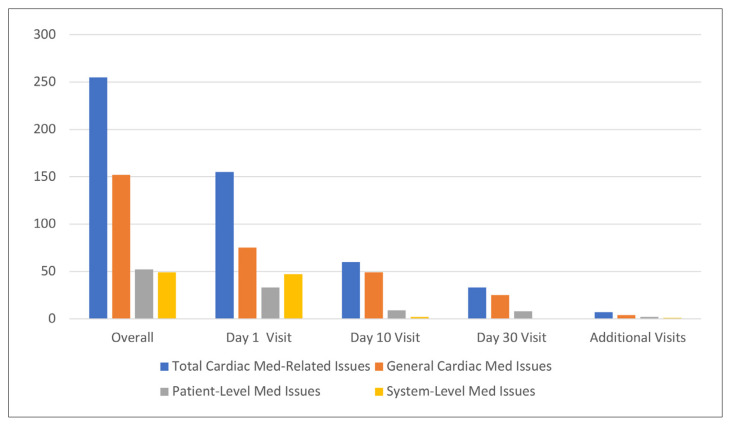
Of the 255 cardiac medication–related issues identified during the program, 233 (91%) were resolved by the program pharmacist. Overall, general cardiac medication–related issues were the most common, followed by patient-level and system-level medication issues (Figure 2). The absolute number and percentage of each cardiac medication–related issue can be found in Supplement 1, part 6. Errors in the discharge prescription were most commonly identified on day 1, adverse effects related to cardiac medications were most common on day 10, and requirement for therapy optimization was most common on day 30.
FIGURE 2.
Numbers of issues related to cardiac medications, categorized by type of issue (general, patient-level, system-level), overall and at 1, 10, and 30 days. Med = medication.
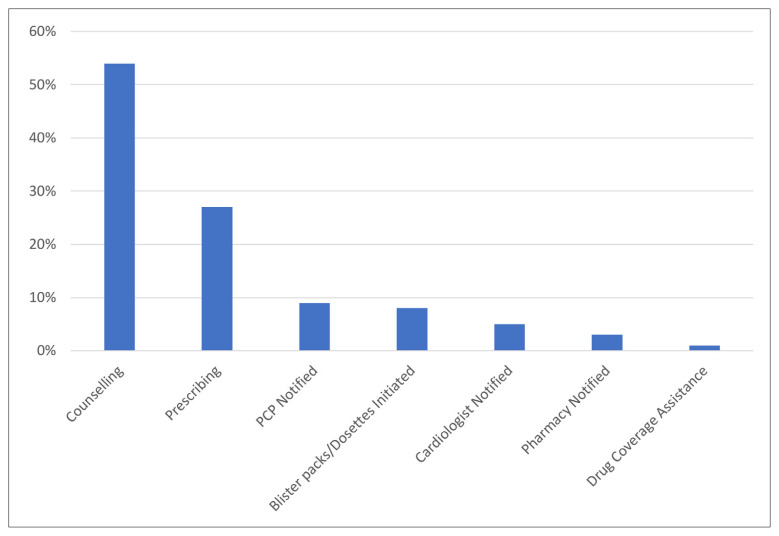
The most common pharmacist actions during the program were patient counselling about a particular cardiac medication–related issue (54%), direct prescribing of cardiac medication (27%), and notifying the PCP of a cardiac medication–related issue that required in-person assessment (9%) (Figure 3).
FIGURE 3.
Types of actions taken by the program pharmacist to resolve issues related to cardiac medications during the PLURAL-ACS pilot program (n = 233). PCP = primary care provider.
Drug therapy optimization was the issue that most commonly remained unresolved by the end of the program, with 20% (n = 8), 13% (n = 5), and 8% (n = 3) of the 40 participants requiring optimization for smoking cessation, hypertension, and diabetes management, respectively. However, given the short duration of this pilot study, active optimization of therapy was not a focus of the intervention. In addition, 8% (n = 3) of the participants were found to have medication non-adherence at the 30-day visit (reported by the patient during interview with the pharmacist).
DISCUSSION
Here, we have reported the number and types of cardiac medication–related problems encountered by rural ACS patients in the 30 days after discharge and the pharmacist actions required to resolve them, as determined during a pharmacist-led virtual follow-up pilot program. A substantial number of cardiac medication–related issues were identified by the program pharmacist, and 91% of them were resolved, even though most study participants were also seen by their PCPs within the 30-day follow-up period. This finding highlights the potential benefit of a transitional pharmacist-led follow-up program for a rural ACS cohort, especially in the short term, when these patients are most vulnerable and at higher risk of adverse outcomes.
Studies assessing in-person and virtual post-ACS programs have not routinely included a pharmacist to provide patient care.22,23 As medication experts, pharmacists not only play an important role in assessing pharmacotherapy and providing direct therapy interventions, but also facilitate medication adherence and education.12,13,24 Our program entailed 3 phone visits per patient (lasting about 60 minutes each), over a 1-month period, during which a clinical pharmacist identified and resolved medication-related issues, which highlights the efficiency and potential feasibility of a short-term post-discharge follow-up program. Lack of feasibility and sustainability have been limitations observed in other rural health follow-up programs, primarily due to the costs of staffing and provision of in-person assessments.25
The types of cardiac medication–related issues identified in this program also highlight the need for a multifaceted follow-up approach. Similar to previous transition-of-care studies,20,26 seamless care issues were commonly identified within 24 hours after discharge in our pilot program, despite the fact that each of the study participants had been discharged from a tertiary cardiac care centre that was supported by a large care team, including an inpatient pharmacist and transition-of-care nurse. Therapy management issues, such as medication adverse effects, were most common on day 10, whereas therapy optimization was most commonly required on day 30. The finding on day 10 might be explained by newly prescribed medications having reached steady state and patients having had more time to recognize adverse effects while settling back into their usual home routines. Conversely, by the 30-day (1-month) mark, patients might have started to relapse in terms of lifestyle modifications and therapy adherence, potentially leading to requirements for therapy optimization. In addition, every patient in the program was started on new cardiac medications during their index hospitalization, and these new medications often required optimization during the outpatient follow-up period.
To our knowledge, no other pharmacist-led follow-up program involving rural ACS patients has categorized the types of cardiac medication–related issues encountered over the follow-up period. The findings reported here could help delineate the types of services and the time required for specific post-discharge services to ensure successful program implementation and patient care optimization. Given the variety of issues identified, a multifaceted approach to providing optimal cardiac drug therapy would be required for a comprehensive outpatient follow-up program.
Our program’s setting and target population were novel. The majority of previous pharmacist-led post-ACS follow-up programs have included in-person care in urban centres, which would limit access for rural patients. Our program took a rather general approach by addressing various cardiac medication–related issues over 30 days, whereas most other pharmacist-led follow-up programs for ACS patients have focused on managing seamless care issues (e.g., medication reconciliation) or performing medication counselling.12,25 These studies mainly found benefit in terms of medication adherence and medication beliefs at follow-up assessment.12,26,27 Referrals to other health care professionals after hospital discharge have led to delayed or failed therapy implementation; therefore, clinically effective programs have often involved trained cardiology pharmacists with prescribing authority or ability to implement timely recommendations.28,29 In our program, the second most common pharmacist action was direct prescribing of cardiac therapy, which allowed for timely and efficient resolution of cardiac medication–related issues and appropriate follow-up on new therapy regimens.
A major strength of this study was the collection and categorization of the various pharmacist services required to resolve the medication-related issues encountered over each participant’s 30-day follow-up period. These outcomes have often been insufficiently collected in similar studies, yet they are critical for understanding a program’s feasibility and reproducibility. Another strength of our study was its external validity, given that the study population was not restricted to high-risk or referral-only ACS patients, which has been the case for many previous pharmacist-led programs.20,26 The inclusion of all consecutively discharged patients who met the eligibility criteria also limited potential referral bias. Finally, the study had almost complete follow-up, with data being incomplete for only 2 participants, one who failed to attend the third follow-up visit and another who passed away from non–cardiac-related issues after the first follow-up visit.
Our study had several limitations that warrant discussion. Participants might not have been forthcoming or accurate when the program pharmacist asked about certain medication-related issues (e.g., adherence), which could have introduced bias. Restricting the study to English-speaking patients limited the population that could be assessed. Data collection was completed by the program pharmacist who implemented the service, which could have led to assessment bias. Furthermore, although the categorization of cardiac medication–related issues was adapted from prior studies, the categorization scheme was not validated.20,21 Therefore, it is difficult to determine the clinical significance of each cardiac medication–related issue encountered. The study lacked a control group, which would be required to determine an association between the program service and outcomes. However, a follow-up study is planned, the PLURAL-ACS Outcomes study, in which this group of participants will be compared with a matched control group. This study will assess clinically relevant end points, including time to filling of discharge prescriptions and the occurrence of cardiac emergency department visits and cardiac rehospitalizations, to shed further light on the potential benefits of this program.
Although therapy optimization was not the focus of this short-term intervention and despite PCP follow-up, the pilot study clearly identified a gap in this aspect of post-ACS care. Future studies are needed to examine whether therapy optimization is a role that pharmacists can play in this high-risk population.
CONCLUSION
In the high-risk rural post-ACS population participating in this study, cardiac medication–related issues were common, occurring early in the post-discharge period and continuing to at least 30 days. Most of these issues were promptly assessed and addressed by a cardiac pharmacist using a structured intervention and supported by pharmacist prescribing. Future studies are required to determine the impact of this type of service on patient outcomes.
Supplementary Information
Footnotes
Competing interests: None declared.
Funding: None received.
References
- 1. Hess CN, Clare RM, Neely ML, Tricoci P, Mahaffey KW, James SK, et al. Differential occurrence, profile, and impact of first recurrent cardiovascular events after an acute coronary syndrome. Am Heart J. 2017;187:194–203. doi: 10.1016/j.ahj.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 2. Fitchett DH, Theroux P, Brophy JM, Cantor WJ, Cox JL, Gupta M, et al. Assessment and management of acute coronary syndromes (ACS): a Canadian perspective on current guideline-recommended treatment – Part 2: ST-segment elevation myocardial infarction. Can J Cardiol. 2011;27(Suppl A):S402–S412. doi: 10.1016/j.cjca.2011.08.107. [DOI] [PubMed] [Google Scholar]
- 3. Donio PJ, Freitas C, Austin PC, Ross HJ, Abdel-Qadir HM, Wijeysundera HC, et al. Comparison of readmission and death among patients with cardiac disease in Northern vs Southern Ontario. Can J Cardiol. 2019;35(3):341–51. doi: 10.1016/j.cjca.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 4. Gamble JM, Eurich DT, Ezekowitz JA, Kaul P, Quan H, McAlister FA. Patterns of care and outcomes differ for urban versus rural patients with newly diagnosed heart failure, even in a universal healthcare system. Circ Heart Fail. 2011;4(3):317–23. doi: 10.1161/CIRCHEARTFAILURE.110.959262. [DOI] [PubMed] [Google Scholar]
- 5. Tran C, Wijeysundera HC, Qui F, Tu JV, Bhatia RS. Comparing the ambulatory care and outcomes for rural and urban patients with chronic ischemic heart disease: a population-based cohort study. Circ Cardiovasc Qual Outcomes. 2014;7(6):835–43. doi: 10.1161/CIRCOUTCOMES.114.001076. [DOI] [PubMed] [Google Scholar]
- 6. Faridi KF, Peterson ED, McCoy LA, Thomas L, Enriquez J, Wang TY. Timing of first postdischarge follow-up and medication adherence after acute myocardial infarction. JAMA Cardiol. 2016;1(2):147–55. doi: 10.1001/jamacardio.2016.0001. [DOI] [PubMed] [Google Scholar]
- 7. Daugherty SL, Ho PM, Spertus JA, Jones PG, Bach RG, Krumholz HM, et al. Association of early follow-up after acute myocardial infarction with higher rates of medication use. Arch Intern Med. 2008;168(5):485–91. doi: 10.1001/archinte.168.5.485. [DOI] [PubMed] [Google Scholar]
- 8. Weir DL, Motulsky A, Abrahamowicz M, Lee TC, Morgan S, Buckeridge DL, et al. Failure to follow medication changes made at hospital discharge is associated with adverse events in 30 days. Health Serv Res. 2020;55(4):512–23. doi: 10.1111/1475-6773.13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sheehy O, LeLorier J, Rinfret S. Restrictive access to clopidogrel and mortality following coronary stent implantation. CMAJ. 2008;178(4):413–20. doi: 10.1503/cmaj.070586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kang JE, Han NY, Oh JM, Jin HK, Kim HA, Son IJ, et al. Pharmacist-involved care for patients with heart failure and acute coronary syndrome: a systematic review with qualitative and quantitative meta-analysis. J Clin Pharm Ther. 2016;41(2):145–57. doi: 10.1111/jcpt.12367. [DOI] [PubMed] [Google Scholar]
- 11. Koshman SL, Charrois TL, Simpson SH, McAlister FA, Tsuyuki RT. Pharmacist care of patients with heart failure: a systematic review of randomized trials. Arch Intern Med. 2008;168(7):687–94. doi: 10.1001/archinte.168.7.687. [DOI] [PubMed] [Google Scholar]
- 12. Cai H, Dai H, Hu Y, Yan X, Xu H. Pharmacist care and the management of coronary heart disease: a systematic review of randomized controlled trials. BMC Health Serv Res. 2013;13 doi: 10.1186/1472-6963-13-461. Article 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Santschi V, Chiolero A, Burnand B, Colosimo AL, Paradis G. Impact of pharmacist care in the management of cardiovascular disease risk factors: a systematic review and meta-analysis of randomized trials. Arch Intern Med. 2011;171(16):1441–53. doi: 10.1001/archinternmed.2011.399. [DOI] [PubMed] [Google Scholar]
- 14. Huber D, Henriksson R, Jakobsson S, Mooe T. Nurse-led telephone-based follow-up of secondary prevention after acute coronary syndrome: one-year results from the randomized controlled NAILED-ACS trial. PLoS One. 2017;12(9):e0183963. doi: 10.1371/journal.pone.0183963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Southard BH, Southard DR, Nuckolls J. Clinical trial of an Internet-based case management system for secondary prevention of heart disease. J Cardiopulm Rehabil. 2003;23(5):341–8. doi: 10.1097/00008483-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 16. Gandhi S, Chen S, Hong L, Sun K, Gong E, Li C, et al. Effect of mobile health interventions on the secondary prevention of cardiovascular disease: systematic review and meta-analysis. Can J Cardiol. 2017;33(2):219–31. doi: 10.1016/j.cjca.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 17. Jin K, Khonsari S, Gallagher R, Gallagher P, Clark AM, Freedman B, et al. Telehealth interventions for the secondary prevention of coronary heart disease: a systematic review and meta-analysis. Eur J Cardiovasc Nurs. 2019;18(4):260–71. doi: 10.1177/1474515119826510. [DOI] [PubMed] [Google Scholar]
- 18. Turan Kavradim S, Özer Z, Boz İ. Effectiveness of telehealth interventions as a part of secondary prevention in coronary artery disease: a systematic review and meta-analysis. Scand J Caring Sci. 2020;34(3):585–603. doi: 10.1111/scs.12785. [DOI] [PubMed] [Google Scholar]
- 19. Lear SA, Singer J, Banner-Lukaris D, Horvat D, Park JE, Bates J, et al. Randomized trial of a virtual cardiac rehabilitation program delivered at a distance via the Internet. Circ Cardiovasc Qual Outcomes. 2014;7(6):952–9. doi: 10.1161/CIRCOUTCOMES.114.001230. [DOI] [PubMed] [Google Scholar]
- 20. Dempsey J, Gillis C, Sibicky S, Matta L, MacRae C, Kirshenbaum J, et al. Evaluation of a transitional care pharmacist intervention in a high-risk cardiovascular patient population. Am J Health Syst Pharm. 2018;75(17 Suppl 3):S63–S71. doi: 10.2146/ajhp170099. [DOI] [PubMed] [Google Scholar]
- 21. Pippins JR, Gandhi TK, Hamann C, Ndumele CD, Labonville SA, Diedrichsen EK, et al. Classifying and predicting errors of inpatient medication reconciliation. J Gen Intern Med. 2008;23(9):1414–36. doi: 10.1007/s11606-008-0687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clark AM, Hartling L, Vandermeer B, McAlister FA. Meta-analysis: secondary prevention programs for patients with coronary artery disease. Ann Intern Med. 2005;143(9):659–72. doi: 10.7326/0003-4819-143-9-200511010-00010. [DOI] [PubMed] [Google Scholar]
- 23. Murphy E, Vellinga A, Byrne M, Cupples ME, Murphy AW, Buckley B, et al. Primary care organisational interventions for secondary prevention of ischaemic heart disease: a systematic review and meta-analysis. Br J Gen Pract. 2015;65(636):e460–e468. doi: 10.3399/bjgp15X685681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weeda E, Gilbert RE, Kolo SJ, Haney JS, Hazard LT, Taber DJ, et al. Impact of pharmacist-driven transitions of care interventions on post-hospital outcomes among patients with coronary artery disease: a systematic review. J Pharm Pract. 2023;36(3):668–78. doi: 10.1177/08971900211064155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kakekagumick KE, Hayward MN, Harris SB, Saksvig B, Gittelsohn J, Manokeesic G, et al. Sandy Lake Health and Diabetes Project: a community-based intervention targeting type 2 diabetes and its risk factors in a First Nations community. Front Endocrinol (Lausanne) 2013;4:170. doi: 10.3389/fendo.2013.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. el Hajj MS, Jaam MJ, Awaisu A. Effect of pharmacist care on medication adherence and cardiovascular outcomes among patients post-acute coronary syndrome: a systematic review. Res Social Adm Pharm. 2018;14(6):507–20. doi: 10.1016/j.sapharm.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 27. Budiman T, Snodgrass K, Komatsu Chang A. Evaluation of pharmacist medication education and post-discharge follow-up in reducing readmissions in patients with ST-segment elevation myocardial infarction (STEMI) Ann Pharmacother. 2016;50(2):118–24. doi: 10.1177/1060028015620425. [DOI] [PubMed] [Google Scholar]
- 28. Israel EN, Farley TM, Farris KB, Carter BL. Underutilization of cardiovascular medications: effect of a continuity-of-care program. Am J Health Syst Pharm. 2013;70(18):1592–600. doi: 10.2146/ajhp120786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carter BL, Levy B, Gryzlak B, Xu Y, Chrischilles E, Dawson J, et al. Cluster-randomized trial to evaluate a centralized clinical pharmacy service in private family medicine offices. Circ Cardiovasc Qual Outcomes. 2018;11(6):e004188. doi: 10.1161/CIRCOUTCOMES.117.004188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.