Abstract
BrkA confers resistance to killing by complement in Bordetella pertussis. Complement resistance in Bordetella bronchiseptica was examined. Four B. bronchiseptica strains possessed the brkA gene; however, only three expressed the protein. Only the strain lacking BrkA was susceptible to complement. Introduction of the B. pertussis brkA gene restored BrkA expression to this strain but did not confer resistance. brkA was mutated in the strains that naturally expressed BrkA, and loss of BrkA did not confer sensitivity to complement. As a species, B. bronchiseptica is more resistant to complement than B. pertussis, and BrkA does not mediate resistance.
Previously, we described BrkA, a virulence factor for Bordetella pertussis (8, 18). BrkA is synthesized as a 103-kDa precursor that is proteolytically processed to a 73-kDa N-terminal domain and a 30-kDa C-terminal domain which remains in the outer membrane (8). It is homologous to two other B. pertussis proteins, pertactin (12) and tracheal colonization factor (10). These proteins mediate attachment to cells (8, 10, 12), but only BrkA confers resistance to killing by the antibody-dependent pathway of complement (8). Complement is a defense associated with serum and blood-borne pathogens; however, it is also extruded to mucosal surfaces. Normal mucosa has approximately 10% as much complement as serum, and this amount increases during infection (16). A mechanism of complement resistance may be essential for every mucosal pathogen.
Bordetella species are mucosal pathogens. B. pertussis causes human whooping cough, B. parapertussis causes less-severe human disease and more-serious infections in sheep, and B. bronchiseptica causes kennel cough in dogs and atrophic rhinitis in pigs (17). Members of this genus are closely related (72 to 94% homologous by DNA hybridization analysis) and share several toxins and adhesins (2, 17). However, some genes are differentially expressed. Pertussis toxin, which causes many of the symptoms unique to whooping cough, is expressed only by B. pertussis. B. bronchiseptica and B. parapertussis strains possess defective copies of the pertussis toxin genes or lack them entirely (4). Only B. bronchiseptica cells express flagella and are motile (2, 7, 17). They also differ in the structures of their lipopolysaccharides (LPS).
B. bronchiseptica and B. parapertussis possess brkA (8). Since genes may be present but not expressed, we examined four isolates of B. bronchiseptica for expression of BrkA and its role in complement resistance. 110H was from a dog (14), RB50 was from a rabbit (7), and P-4609 was from a pig (1). The source of strain 213 is not known (14), but it was chosen because it is unusual in that it lacks the genes for pertussis toxin (data not shown). All four strains, like those described in our previous report (8), possessed a single chromosomal band that hybridized with the brkA gene of B. pertussis by Southern blot analysis (data not shown), indicating that all four strains possessed the brkA gene.
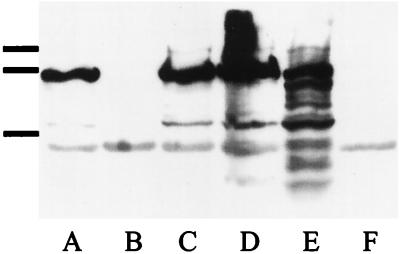
Western analysis was used to detect expression of BrkA. Bacterial cells were harvested from Bordet-Gengou agar (BGA), and proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membranes as described elsewhere (5), and probed with a polyclonal antibody from a rat immunized with a purified histidine-tagged fusion protein containing amino acids 289 to 595 of BrkA expressed in pRSETC. The blot was developed by chemiluminescence (Renaissance kit; NEN Research Products, Boston, Mass.). BrkA was detected in the B. pertussis wild-type strain BP338 (Fig. 1, lane E), as evidenced by a major band corresponding to the 73-kDa processed form and larger and smaller bands corresponding to unprocessed (103-kDa form) and degraded forms of BrkA not present in the BrkA mutant BPM2041 (Fig. 1, lane F). Three strains of B. bronchiseptica expressed the BrkA protein, while strain RB50 did not (Fig. 1B).
FIG. 1.
Western analysis of BrkA production. Lanes: A, B. bronchiseptica 11OH; B, B. bronchiseptica RB50; C, B. bronchiseptica P-4609; D, B. bronchiseptica 213; E, B. pertussis BP338 (positive control); and F, B. pertussis BPM2041 (negative control). Bars on left indicate migration of the molecular mass markers (104, 89, and 47.7 kDa).
We characterized 36 serum samples from humans, rabbits, guinea pigs, mice, and rats. Most had antibodies to Bordetella by Western blot analysis, which was not unexpected, since Bordetella infections are common in humans and domestic animals. Most samples had bactericidal activity against the BrkA mutant of B. pertussis but less activity against the wild-type strain. The samples were not bactericidal for B. bronchiseptica, except for a commercially available guinea pig serum (complement lot no. 421; Colorado Serum Company) that was bactericidal (but only for strain RB50). This serum reacted with dozens of antigens on all of the B. bronchiseptica strains tested by Western blotting (data not shown). A second commercial source of complement (Sigma guinea pig complement; lot no. 116H9410) had only weak reactivity to a single Bordetella antigen by Western blotting and had no bactericidal activity against the wild-type B. pertussis strain or the BrkA mutant. The Colorado serum with antibodies directed against multiple B. bronchiseptica antigens will be referred to as immune serum, and the Sigma serum devoid of antibodies against B. bronchiseptica will be referred to as the source of complement.
To quantitate bactericidal activity, bacteria from an overnight culture on BGA were suspended to an optical density of approximately 0.2 in Stainer Scholte broth, 2 μl (107 bacteria) was added to 20 μl of serum or a phosphate-buffered saline (PBS) control, and the mixture was incubated for 1 h at 37°C. The reaction was stopped by the addition of PBS containing 10 mM EDTA to chelate the divalent cations necessary for complement activity. Serial dilutions were made, and the bacteria were plated on BGA agar and allowed to grow at 37°C. Percent survival was calculated with the PBS control as the 100% value.
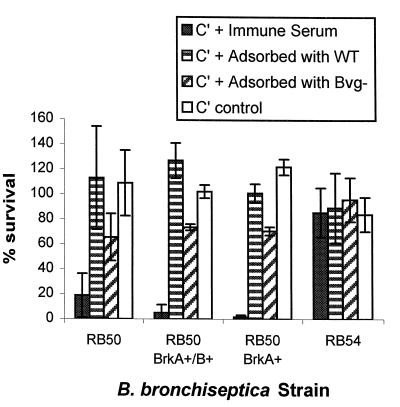
Incubation with serum containing complement but lacking antibodies to B. bronchiseptica did not kill RB50 (Fig. 2 [C′ control]). The immune serum killed RB50 when the complement was intact; however, when this serum was heated to 56°C for 30 min to inactivate the complement, no killing was observed (data not shown). However, when the heat-inactivated immune serum was mixed with the complement lacking antibodies to B. bronchiseptica, killing was restored (Fig. 2 [C′ + immune serum]), suggesting that antibodies as well as intact complement were needed to kill the bacteria.
FIG. 2.
Serum susceptibility of B. bronchiseptica strains. Percent survival was calculated for bacteria incubated in serum. ▪, 50% heat-inactivated immune serum as a source of antibodies (Colorado serum no. 421) plus 50% complement (C′), Sigma guinea pig serum;  , 50% heat-inactivated immune serum preadsorbed with RB50 (serum adsorbed with wild type [WT]) to remove surface antibodies plus 50% complement;
, 50% heat-inactivated immune serum preadsorbed with RB50 (serum adsorbed with wild type [WT]) to remove surface antibodies plus 50% complement;  , 50% heat-inactivated immune serum preadsorbed with RB54 (serum adsorbed with Bvg−) to remove surface antibodies plus 50% complement; □, PBS plus 50% complement. Results from four experiments were averaged. RB50, RB50 BrkA+ BrkB+ (containing plasmid pUW2171), and RB50 BrkA+ (containing plasmid pRF1009) were significantly more sensitive to heat-inactivated serum with complement than complement alone or complement plus serum adsorbed with RB50 (P < 0.02 to 0.04). In addition, RB50 incubated with heat-inactivated serum plus complement was significantly more sensitive than RB54 incubated with heat-inactivated serum plus complement (P < 0.01). The error bars indicate standard deviations. The data were analyzed by the paired t test.
, 50% heat-inactivated immune serum preadsorbed with RB54 (serum adsorbed with Bvg−) to remove surface antibodies plus 50% complement; □, PBS plus 50% complement. Results from four experiments were averaged. RB50, RB50 BrkA+ BrkB+ (containing plasmid pUW2171), and RB50 BrkA+ (containing plasmid pRF1009) were significantly more sensitive to heat-inactivated serum with complement than complement alone or complement plus serum adsorbed with RB50 (P < 0.02 to 0.04). In addition, RB50 incubated with heat-inactivated serum plus complement was significantly more sensitive than RB54 incubated with heat-inactivated serum plus complement (P < 0.01). The error bars indicate standard deviations. The data were analyzed by the paired t test.
An unexpected observation was that several of the colonies that survived the complement killing were nonhemolytic. Nonhemolytic variants due to mutations in the bvg locus arise in B. bronchiseptica at a very high rate. Bvg is a global regulator required for the expression of virulence factors and hemolysis. A well-characterized bvg mutant, RB54 (7), was characterized for susceptibility to complement. RB54 was not killed (Fig. 2 [RB54]), suggesting that the nonhemolytic survivors from RB50 were spontaneous bvg mutants in the population.
To assess the contribution of antibodies to killing, adsorption was used to remove the antibodies to surface determinants. Bacterial growth from a 24-h BGA plate was harvested in PBS, divided into aliquots, and pelleted by centrifugation. The bacterial pellet was suspended in heat-inactivated serum and incubated on ice for several hours. The serum was separated from the bacteria by centrifugation, the process was repeated twice, and the sample was filter sterilized. Adsorption with RB50 should remove antibodies to all surface determinants of RB50 and protect it from killing. That appeared to be the case (Fig. 2 [C′ + serum adsorbed with WT]). However, RB54 would remove only antibodies to shared determinants, not antibodies to antigens expressed only by RB50, allowing one to assess their contribution to bactericidal activity. Although adsorption with RB54 (Fig. 2 [C′ + serum adsorbed with Bvg−]) produced a result that was not quite statistically significant (P < 0.055), it appeared to confer some protection to RB50, suggesting that bactericidal antibodies recognize both virulent-phase and shared antigens.
To assess the role of BrkA in serum resistance, cloned copies of the brkA gene on plasmid pRF1009 (8) or the entire brkAB operon on pUW2171 (9) were introduced into RB50 by homologous recombination of a suicide plasmid as previously described for B. pertussis (8, 9). Gentamicin-resistant transconjugants were characterized for BrkA expression by Western analysis. Both constructs conferred BrkA expression on RB50 (Fig. 3), but BrkA expression did not confer serum resistance to RB50 (Fig. 2 [RB50 BrkA+/B+ and RB50 BrkA+]). However, it is theoretically possible that in both cases the recombination event that allowed RB50 to express BrkA generated a chimeric protein that lacked the domains needed to confer resistance to complement.
FIG. 3.
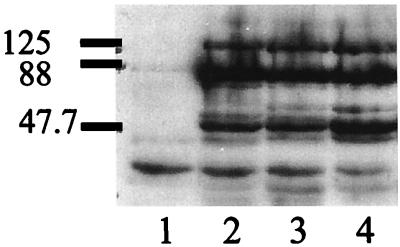
Western analysis to detect restoration of BrkA expression in B. bronchiseptica. Lanes: 1, B. bronchiseptica RB50 control; 2, B. bronchiseptica 213; 3, B. bronchiseptica 11OH; 4, RB50 gentamicin-resistant pUW2171 transconjugant. Approximate molecular masses are indicated in kilodaltons.
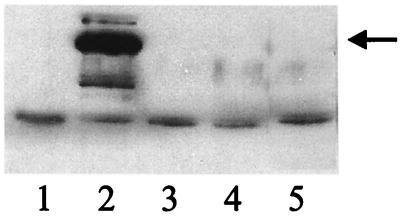
Similarly, a partial copy of the brkA gene in plasmid pRF1022 (8) was used to generate BrkA mutants in strains 213 and 110H (Fig. 4). In experiments performed in triplicate, survival for the BrkA mutant of 110H containing pRF1022 was 65.7% ± 16.9%, which was not statistically different from the value of 77.2% ± 17.8% observed for the parental 11OH. In a single experiment, survival for the gentamicin-resistant BrkA mutant of strain 213 containing pRF1022 was 100% (versus 87% for the parental strain).
FIG. 4.
Western analysis to characterize BrkA mutants in B. bronchiseptica. Lanes: 1, B. bronchiseptica RB50 (negative control); 2, B. bronchiseptica 213 (positive control); 3, B. bronchiseptica RB50 gentamicin-resistant pRF1022 transconjugant; 4, B. bronchiseptica 11OH gentamicin-resistant pRF1022 transconjugant; 5, B. bronchiseptica 213 gentamicin-resistant pRF1022 transconjugant. Arrow, mature 70-kDa BrkA protein.
BrkA was identified in B. pertussis and was shown to mediate both adherence and complement resistance (8). The B. bronchiseptica isolates characterized in the present study all possessed the brkA gene; however, not all expressed BrkA. Only RB50, the strain that was unable to express BrkA, was susceptible to complement. However, when BrkA expression was restored to this strain, it did not become resistant. Three strains that expressed BrkA were completely resistant to complement and remained resistant when the brkA gene was mutated. We cannot entirely rule out a role for BrkA in complement resistance in B. bronchiseptica because, as for B. pertussis, it appears that antibodies are necessary for killing. The immune serum used in this study recognizes dozens of antigens on all of the B. bronchiseptica strains tested as determined by Western blotting. However it is well known that not all antibodies are bactericidal. This can be due either to the inability of the constant region to activate complement or to the inability of the antibody to recognize an antigen that will allow the membrane attack complex to form correctly on the bacterial surface (11, 13). When the serum lacks bactericidal antibodies against a specific strain, the contribution of a complement resistance mechanism, if present, cannot be assessed.
Our results are consistent with reports that the species B. bronchiseptica is more resistant to complement than B. pertussis (6). A major difference that could account for this observation is the structure of their LPS. B. pertussis has an abbreviated LPS structure. Its O chain is composed of a single trisaccharide that is not repeated (3). In contrast, B. bronchiseptica produces long LPS molecules with many O-chain repeats, similar to the LPS of Escherichia coli. Long LPS molecules of the enterics are thought to confer resistance to complement by blocking the membrane attack complex from binding to the bacterial membrane (11, 13). In agreement with this, a strain of B. bronchiseptica lacking O-chain repeats was shown to be extremely sensitive to complement (6). In addition, the core structure of B. bronchiseptica LPS varies according to the phase of the microorganism (15). Whether this accounts for the resistance of the avirulent-phase bvg mutant RB54 remains to be determined. While it appears that BrkA does not mediate serum resistance in B. bronchiseptica, it is clear that these pathogens do have a mechanism of serum resistance. This is consistent with our hypothesis that all mucosal pathogens must have a mechanism to avoid killing by complement. The discovery that under certain conditions these bacteria can be killed by complement allows one to study this process and to optimize killing as a possible therapeutic strategy.
Acknowledgments
This work was supported by grant R01 AI38415 to A.A.W.
REFERENCES
- 1.Ackermann M R, Rimler R B, Thurston J R. Experimental model of atrophic rhinitis in gnotobiotic pigs. Infect Immun. 1991;59:3626–3629. doi: 10.1128/iai.59.10.3626-3629.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akerley B J, Miller J F. Understanding signal transduction during bacterial infection. Trends Microbiol. 1996;4:141–146. doi: 10.1016/0966-842x(96)10024-x. [DOI] [PubMed] [Google Scholar]
- 3.Allen A, Maskell D. The identification, cloning and mutagenesis of a genetic locus required for lipopolysaccharide biosynthesis in Bordetella pertussis. Mol Microbiol. 1996;19:37–52. doi: 10.1046/j.1365-2958.1996.354877.x. [DOI] [PubMed] [Google Scholar]
- 4.Arico B, Rappuoli R. Bordetella parapertussis and Bordetella bronchiseptica contain transcriptionally silent pertussis toxin genes. J Bacteriol. 1987;169:2847–2853. doi: 10.1128/jb.169.6.2847-2853.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barry E M, Weiss A A, Ehrmann I E, Gray M C, Hewlett E L, Goodwin M S M. Bordetella pertussis adenylate cyclase toxin and hemolytic activities require a second gene, cycC, for activation. J Bacteriol. 1991;173:720–726. doi: 10.1128/jb.173.2.720-726.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrd D W, Roop R M, Veit H P, Schurig G G. Serum sensitivity and lipopolysaccharide characteristics in Bordetella bronchiseptica, B. pertussis, and B. parapertussis. J Med Microbiol. 1991;34:159–165. doi: 10.1099/00222615-34-3-159. [DOI] [PubMed] [Google Scholar]
- 7.Cotter P A, Miller J F. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect Immun. 1994;62:3381–3390. doi: 10.1128/iai.62.8.3381-3390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez R C, Weiss A A. Cloning and sequencing of a Bordetella pertussis serum resistance locus. Infect Immun. 1994;62:4727–4738. doi: 10.1128/iai.62.11.4727-4738.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez R C, Weiss A A. Serum resistance in bvg-regulated mutants of Bordetella pertussis. FEMS Microbiol Lett. 1998;163:57–63. doi: 10.1111/j.1574-6968.1998.tb13026.x. [DOI] [PubMed] [Google Scholar]
- 10.Finn T M, Stevens L A. Tracheal colonization factor: a Bordetella pertussis secreted virulence determinant. Mol Microbiol. 1995;16:625–634. doi: 10.1111/j.1365-2958.1995.tb02425.x. [DOI] [PubMed] [Google Scholar]
- 11.Frank M M. The mechanism by which microorganisms avoid complement attack. Curr Opin Immunol. 1992;4:14–19. doi: 10.1016/s0952-7915(92)90117-w. [DOI] [PubMed] [Google Scholar]
- 12.Leininger E, Roberts M, Kenimer J, Charles I, Fairweather N, Novotny P, Brennan M. Pertactin, an Arg-Gly-Asp-containing Bordetella pertussis surface protein that promotes adherence of mammalian cells. Proc Natl Acad Sci USA. 1991;88:345–349. doi: 10.1073/pnas.88.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moffitt M C, Frank M M. Complement resistance in microbes. Springer Semin Immunopathol. 1994;15:327–344. doi: 10.1007/BF01837364. [DOI] [PubMed] [Google Scholar]
- 14.Musser J M, Hewlett E L, Peppler M S, Selander R K. Genetic diversity and relationships in populations of Bordetella spp. J Bacteriol. 1986;166:230–237. doi: 10.1128/jb.166.1.230-237.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peppler M S, Schrumpf M E. Phenotypic variation and modulation in Bordetella bronchiseptica. Infect Immun. 1984;44:681–687. doi: 10.1128/iai.44.3.681-687.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Persson C G A, Erjefalt I, Alkner U, Baumgarten C, Grief L, Gustafsson B, Luts A, Pipkorn U, Sundler F, Svensson C, Wollmer P. Plasma exudation as a first line respiratory mucosal defense. Clin Exp Allergy. 1991;21:17–24. doi: 10.1111/j.1365-2222.1991.tb00799.x. [DOI] [PubMed] [Google Scholar]
- 17.Weiss A A. The genus Bordetella. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. 2nd ed. III. New York, N.Y: Springer-Verlag; 1991. pp. 2530–2543. [Google Scholar]
- 18.Weiss A A, Goodwin M S. Lethal infection by Bordetella pertussis mutants in the infant mouse model. Infect Immun. 1989;57:3757–3764. doi: 10.1128/iai.57.12.3757-3764.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]