Abstract
Bordetella pertussis expresses a bvg-regulated 95-kDa protein, Vag8, encoded by vag-8. Southern blot analysis indicates that strains of Bordetella bronchiseptica and Bordetella parapertussis have DNA homologous to vag-8. Antiserum raised to a fusion of maltose binding protein to an N-terminal 60-kDa fragment of Vag8 recognizes the native 95-kDa protein in immunoblots of B. pertussis and B. bronchiseptica but not B. parapertussis. A 95-kDa protein-negative derivative of B. pertussis 18323 containing a deletion of vag-8 colonized mice as efficiently as the parent B. pertussis strain in a mouse aerosol model of pertussis.
Bordetella pertussis is the causative agent of the disease whooping cough. A two-component regulatory locus, the vir, or bvg, locus, has been shown to regulate production of virulence determinants of the organism (26, 28), many of which have been shown to have a role in the infectious process (27). vir-activated genes (vag’s) are expressed under normal laboratory conditions but are not expressed under modulating conditions such as the presence of MgSO4 or nicotinic acid. Recent work has shown that three of these vag-encoded virulence proteins, Tcf (9), BrkA (7), and pertactin (4), belong to a family of proteins that have structural homology at their C termini. The functions of these proteins differ; for example, pertactin has been identified as an adhesin (14) and BrkA is involved in serum resistance (7). However, they share striking structural characteristics: each contains an RGD motif, and the C-terminal 30 kDa of these proteins are homologous.
In addition to the vag products that have been studied in detail, other bvg-regulated proteins have been described but their structural and functional characteristics have not been elucidated. Various authors have described virulence-associated proteins of approximately 90 kDa (6, 19, 21, 22). A B. pertussis surface protein of 91 kDa was described by Armstrong and Parker (2). A 92-kDa B. pertussis outer membrane protein was purified by Hamstra et al. and shown to be a protective antigen in the mouse intracerebral protection assay when nonprotective levels of pertussis toxin were present (11). We have previously described a B. pertussis mutant, SK8, defective in a bvg-regulated 95-kDa protein (8).
In this paper, we describe the cloning and sequencing of the gene encoding this 95-kDa protein and present the initial characterization of this protein. We show that this protein, which we have termed Vag8, is the same as that described by Armstrong and Parker (2) and Hamstra et al. (11). It shows homology with the C-terminal ends of pertactin, BrkA, and Tcf. Thus, Vag8 becomes the fourth member of this Bordetella family of proteins to be identified.
Bacteria, plasmids, enzyme activity assays, and DNA manipulations.
Bacterial strains and plasmids are listed in Table 1. Strains of Bordetella were grown on Bordet-Gengou (BG) medium supplemented with 12% sheep blood or in cyclodextrin solid medium (CSM) (1). Liquid cultures of Bordetella were grown in Stainer-Scholte (SS) medium (24). For modulation studies, BG agar or SS medium was supplemented with MgSO4 (20 mM) and nicotinic acid (5 mM). Escherichia coli strains were grown in Luria-Bertani medium. Antibiotics were used at the following concentrations: ampicillin, 50 μg/ml; tetracycline, 12.5 μg/ml; kanamycin, 50 μg/ml; streptomycin, 100 μg/ml; nalidixic acid, 50 μg/ml; gentamicin, 10 μg/ml; chloramphenicol, 20 μg/ml. Counterselection of E. coli after conjugation was done by spreading BG plates with the appropriate antibiotics; if the recipient in the mating was B. pertussis 18323, colicin B was used to counter-select E. coli. Crude colicin B was isolated from sonicates of E. coli DM1178(pCLB1) (3). Alkaline phosphatase activity and chloramphenicol acetyltransferase (CAT) activity were assayed as previously described (13, 23).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant feature(s) | Source or reference |
|---|---|---|
| B. pertussis strains | ||
| 18323 | Wild type | ATCC 9797 |
| SK8 | 18323::TnphoA; lacking 95-kDa protein | 13 |
| Tohama I | Wild type | Laboratory of Pertussis collection |
| SK8vag-8::pSKCAT | Lacking 95-kDa protein; PhoA− | This study |
| 18323Δvag-8 | 18323; Knr; lacking 95-kDa protein | This study |
| B. bronchiseptica strains | ||
| 110H | 17; Laboratory of Pertussis collection | |
| 207 | Laboratory of Pertussis collection | |
| 058 | Laboratory of Pertussis collection | |
| B. parapertussis strains | ||
| 500 | Laboratory of Pertussis collection | |
| 482 | Laboratory of Pertussis collection | |
| 23054 | 17; Laboratory of Pertussis collection | |
| 13367 | Clinical isolate | C. Mink (UCLA) |
| 9807 | Clinical isolate | C. Mink (UCLA) |
| E. coli strains | ||
| SM10λpir | thi thr leu tonA lacY supE recA::RP4-2-Tc::Mu λpir R6K | 16 |
| DM1187(pCLB1) | Source of colicinB | 3; A. Weiss (University of Cincinnati) |
| Plasmids | ||
| pSS1129 | Suicide vector | 25 |
| pSKCAT | pJM703.1 with ′phoA′-CAT insert; Apr Cmr | 13 |
| pTF470 | 2-kb SalI fragment containing the fusion junction between pSKCAT and vag-8 in pUC18 | This study |
| pDA676 | pMal-c2 expressing maltose binding protein–Vag8 N-terminal fusion protein | This study |
| p4A10 | pLAFR2 cosmid derivative containing vag-8 | 13; this study |
| pDA626 | vag-8 in pBluescriptSK+ | This study |
| pDA669 | Derivative of pDA626 with a 1.3-kb Knr cassette replacing a 0.6-kb internal fragment of vag-8 (Δvag-8) | This study |
| pDA672 | 4.1-kb SstI-KpnI fragment from pDA669 containing Δvag-8 in pUC19 | This study |
| pDA681 | 4.1-kb XbaI fragment from pDA672 containing Δvag-8 in pSS1129 | This study |
Standard DNA manipulation methods were used (15). Single-stranded sequencing was performed using M13 subclones as templates by the dideoxy method with Sequenase (USB, Cleveland, Ohio). Double-stranded sequencing was performed by Lark Sequencing Technologies (Houston, Tex.) with pBluescript subclones as templates.
Analysis of SK8 chromosomal DNA.
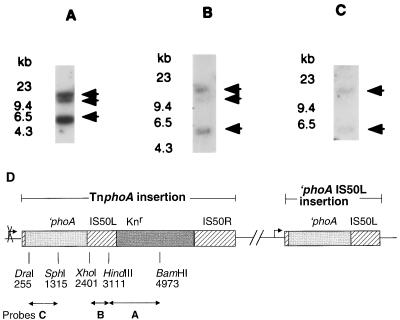
To determine the number of copies of TnphoA in SK8, a Southern blot of BamHI-digested chromosomal DNA was analyzed with the following probes: a 1.8-kb HindIII-BamHI fragment derived from TnphoA (corresponding to nucleotides 3111 to 4973 of TnphoA) encoding the kanamycin resistance determinant and a small portion of IS50L, a 710-bp XhoI-HindIII fragment (corresponding to nucleotides 2401 to 3111 of TnphoA) which hybridizes to the IS50 sequences, and a 1,060-bp DraI-SphI fragment which hybridizes to ′phoA (corresponding to nucleotides 255 to 1315 of TnphoA). This analysis revealed that SK8 has two ′phoA insertions, one contained on a complete copy of TnphoA and the other on an incomplete copy of TnphoA, IS50L (Fig. 1).
FIG. 1.
Presence of insertion element (IS) and phoA sequences in SK8 chromosomal DNA. Shown are Southern blots of BamHI-digested chromosomal DNA from SK8 probed with a probe which hybridizes to the TnphoA IS sequences and the gene encoding the kanamycin resistance determinant (A), a probe to the IS sequence (B), and a probe to the phoA sequence of TnphoA (C). The arrows indicate bands hybridizing to each probe. (D) Schematic representation of SK8 chromosomal DNA with insertion of TnphoA and IS50L containing ′phoA. The production of alkaline phosphatase activity from the latter is indicated by the arrow; the insertion which does not produce alkaline phosphatase activity is indicated by the crossed-out arrow. The probes derived from TnphoA are highlighted, as double-headed arrows, below the diagram. The letters under the probes refer to the Southern blots in panels A, B, and C.
SK8 chromosomal DNA was digested with BamHI and ligated to BamHI-digested pUC19. However, sequencing of plasmids isolated from Knr colonies did not yield an open reading frame next to the fusion junction. In addition, these plasmids did not express alkaline phosphatase activity (data not shown). Thus, it appears that the incomplete copy of TnphoA was inserted into vag-8.
Construction of SK8vag-8::pSKCAT.
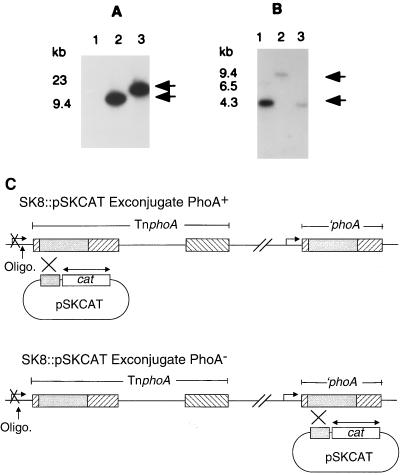
To distinguish between the two copies of ′phoA in SK8, regulated PhoA activity was converted to regulated CAT activity. A derivative of pJM703.1 containing 368 bp of phoA and a promoterless CAT gene, pSKCAT (13), was transferred into SK8 with SM10λpir. With this vector, the vag-8::′phoA fusion is converted to a CAT transcriptional fusion by insertion of the plasmid through homologous recombination between the ′phoA′ sequence of pSKCAT and those of vag-8::′phoA; these exconjugants, SK8vag-8::pSKCAT, are PhoA−. Homologous recombination between this vector and the nonproductive ′phoA results in an exconjugant which remains PhoA+. Apr and Smr exconjugants were selected and scored for loss of alkaline phosphatase activity. To distinguish between the two copies of ′phoA in these SK8::pSKCAT exconjugants, a CAT gene probe (a gift of G. Pogue, Center for Biologics Evaluation and Research, Food and Drug Administration) and an oligonucleotide probe, 5′-TTCCCAACTCCCCATTGG-3′, derived from the sequence 164 bp upstream of the insertion of the nonproductive ′phoA were used. This analysis confirmed that pSKCAT had been inserted into two locations on the chromosome of SK8 (Fig. 2). An exconjugant which was PhoA−, SK8vag-8::pSKCAT, produced bvg-regulated CAT activity (data not shown).
FIG. 2.
Insertion of pSKCAT into two chromosomal locations in SK8. (A) SmaI-digested chromosomal DNA probed with a CAT gene probe. Lane 1, SK8; lane 2, SK8::pSKCAT exconjugate PhoA+; lane 3, SK8::pSKCAT exconjugate PhoA−. (B) SmaI-digested DNA probed with an oligonucleotide derived from sequence upstream of ′phoA, which did not produce PhoA. Lane 1, SK8; lane 2, SK8::pSKCAT exconjugate PhoA+; lane 3, SK8::pSKCAT exconjugate PhoA−. (C) Schematic representation of homologous recombination between the ′phoA′ sequence of pSKCAT and either of the two chromosomal copies of ′phoA in SK8. The exconjugate resulting from the single crossover event is either PhoA+ or PhoA−. The shaded boxes represent ′phoA sequences. The area of hybridization of the CAT probe used in panel A is indicated by the double-headed arrow; the point of hybridization of the oligonucleotide probe used in panel B is indicated by the arrow.
Cloning of vag-8.
Chromosomal DNA from SK8vag-8::pSKCAT was digested with SalI and ligated into pUC18 (29) digested with SalI. DH5α (Gibco BRL, Gaithersburg, Md.) transformants were selected on media containing ampicillin and chloramphenicol. pTF470, which contains the fusion junction between pSKCAT and vag-8 on a 2-kb SalI fragment, was isolated. M13 subclones of this plasmid were sequenced, and the reading frame and point of insertion of pSKCAT in vag-8 were deduced. The 2-kb SalI fragment from pTF470 was used as a probe to isolate vag-8 from a cosmid library of strain 18323 in the vector pLAFR2. Cosmid p4A10 was isolated. From cosmid p4A10, pBluescript subclones were derived and vag-8 was sequenced.
DNA and protein sequence analysis.
Vag8 is encoded in an open reading frame of 2,744 bp. Use of FASTA in the Genetics Computer Group (GCG) package (version 8 [5]) to search the GenBank database showed that the sequence had similarity to the Bordetella pertactin DNA sequences. This similarity extended from the ends of the sequences encoding the signal sequences to the termination codons. The highest similarity seen (54.4% identity in a 2,783-bp overlap) was to the B. pertussis pertactin gene (4). This search also showed that vag-8 has similarity to B. pertussis brkA (53.8% identity in a 2,794-bp overlap [7]) and tcfA (55.8% identity in a 1,771-bp overlap [9]).
The deduced amino acid sequence predicts a protein of 94.8 kDa. The N terminus contains a signal sequence of 37 amino acids terminating in the sequence Ala-Gly-Ala. Cleavage here would generate a 91.1-kDa protein, Vag8. The derived amino acid sequence of Vag8 was used to search the SwissProt database with FASTA in the GCG package (version 8). This search indicated a high degree of homology to the amino acid sequences of the Bordetella pertactin sequences. Vag8 had 24 to 30% identity over 881 to 890 amino acids to the pertactin proteins of B. pertussis, Bordetella parapertussis, and Bordetella bronchiseptica. Most of this similarity was found in the C-terminal 279 amino acids. Analysis by the GAP program within the GCG package showed that Vag8 has 43.7% identity over the C-terminal 279 amino acids of B. pertussis pertactin, 43% identity to the C terminus of B. bronchiseptica P.68, and 42.7% identity to the C terminus of B. parapertussis P.70. Two other B. pertussis sequences having similarity to the Bordetella pertactin sequences have recently been published, those of BrkA (7) and Tcf (9). Vag8 has 32% and 42% identity, respectively, to these proteins. The identity is greatest over the C-terminal 279 amino acids. By using GAP within the GCG package, 33% identity to the Tcf C-terminal 279 amino acids and 44% to the BrkA C-terminal 279 amino acids were seen. Like the pertactin proteins, BrkA and Tcf, an Arg-Gly-Asp sequence exists at positions 151 to 153 of the Vag8 sequence.
Presence of vag-8 in other Bordetella species.
Chromosomal DNA from strains of B. pertussis, B. bronchiseptica, and B. parapertussis was cut with EcoRI and probed with a SalI-SmaI fragment encoding a portion of the Vag8 protein (nucleotides 1234 to 1528). This probe does not contain any DNA encoding the region of Vag8 that shows the greatest homology with the pertactin C terminus. The DNA from all Bordetella strains examined, including B. pertussis Tohama I, three B. bronchiseptica strains, and three B. parapertussis strains, contained a 4-kb fragment that bound to the probe (data not shown). Thus, it appears that the other species of Bordetella have sequences homologous to vag-8.
pMAL-c2 fusion protein and generation of antiserum to Vag8.
The DNA encoding the N terminus of Vag8, minus the signal sequence, was amplified by PCR. The forward primer was 5′-TAGAATTCGCCGCTGTCACGGCA-3′ (bases 202 to 216), and the reverse primer was 5′-GCTCTAGACTACACGTCCATTTCGGCC-3′ (bases 1983 to 1998) (an amber stop codon was also included in this primer). The 1.8-kb amplified fragment was ligated to EcoRI–XbaI-digested pMAL-c2 (New England Biolabs, Beverly, Mass.) to generate pDA676. E. coli DH5α containing the plasmid and expressing the fusion protein was induced with 0.3 mM IPTG (isopropyl-β-d-thiogalactopyranoside) (Gibco BRL) for 2 h prior to freezing overnight at −20°C. After thawing and sonication, the fusion protein was purified with an amylose column, as recommended by New England Biolabs. The purified 103-kDa fusion protein was recognized by monoclonal antibody P8E7 (Fig. 3), a gift from C. Parker (2, 10). Adult female BALB/c mice were injected with 50 μg of purified fusion protein in 0.5 ml of phosphate-buffered saline containing a 1:100 dilution of Alhydrogel (Superfos a/B, Vedbaek, Denmark) as adjuvant; mice were boosted with 60 μg 2 weeks later. Three weeks after boosting, the mice were exsanguinated and serum was collected. This polyclonal antiserum recognized the 95-kDa protein in whole-cell lysates of B. pertussis; no protein was recognized in whole-cell lysates of SK8 (Fig. 3).
FIG. 3.
Immunoblots of the maltose binding protein–Vag8 fusion and reactivity of antiserum raised to this fusion protein. (A) Fusion protein (5 μg) was subjected to SDS-PAGE, transferred to nitrocellulose, and probed with monoclonal antibody P8E7. (B) Fusion protein and whole-cell lysates from Tohama I, 18323, and SK8 were subjected to SDS-PAGE, transferred to nitrocellulose, and probed with antiserum raised in mice to the maltose binding protein–Vag8 fusion. Lane 1, Tohama I; lane 2, 18323; lane 3, SK8; lane 4, 5 μg of maltose binding protein fusion to an N-terminal fragment of Vag8. The large arrow indicates Vag8; the smaller arrows indicate the 103-kDa fusion protein.
Presence of Vag8 in other Bordetella species.
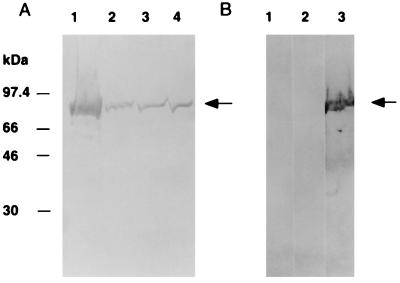
The polyclonal antiserum generated to the maltose binding protein–Vag8 fusion recognized a 95-kDa protein in whole-cell lysates from strains of B. pertussis and B. bronchiseptica; however, no protein was detected in whole-cell lysates of strains of B. parapertussis. The amount of Vag8 expressed by B. pertussis appeared to be higher than that expressed by B. bronchiseptica (Fig. 4). Acetone- and trichloroacetic acid-concentrated supernatant fractions of Bordetella strains grown in SS medium that were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose did not show any protein which hybridized to the antiserum (data not shown).
FIG. 4.
Presence of Vag8 in other Bordetella species. Antiserum which reacted with the maltose binding protein–Vag8 fusion was raised in mice and used in immunoblots of whole-cell lysates of Bordetella species grown on BG plates. Each lane represents approximately 2 × 108 cells. (A) Lane 1, B. pertussis 18323; lane 2, B. bronchiseptica 058; lane 3, B. bronchiseptica 110H; lane 4, B. bronchiseptica 207. (B) Lane 1, B. parapertussis 13367; lane 2, B. parapertussis 9807; lane 3, B. pertussis 18323. The arrows indicate Vag8. The migrations of the molecular size markers are indicated.
Construction of a vag-8 deletion strain, 18323Δvag-8.
A derivative of 18323 harboring a deletion in vag-8 was constructed by allelic exchange between the chromosomal copy of vag-8 and a plasmid copy containing an internal deletion in vag-8 and an insertion of a Knr cassette. This allelic exchange plasmid, pDA681, was constructed as follows. A 3.4-kb ClaI-PstI fragment from p4A10 containing vag-8 was cloned into pBluescriptSK+ (Stratagene, La Jolla, Calif.) to generate pDA626. An internal fragment of vag-8 was deleted by digesting pDA626 with StuI and NruI, which have sites at positions 1001 and 1607 of vag-8, respectively. A Knr cassette (27-4897; Pharmacia Biotech) was inserted at these sites to generate pDA669. To generate restriction enzyme sites suitable for cloning into the gene replacement vector pSS1129, pDA669 was digested with SstI-KpnI and the 4.1-kb fragment was subcloned into pUC19 (29). This plasmid, pDA672, was digested with XbaI, and the 4.1-kb fragment was subcloned into pSS1129 to generate pDA681. This plasmid was transformed into SM10λpir prior to transfer into 18323 by conjugation. B. pertussis exconjugants which were Apr, Gmr, Knr and colicin B resistant were selected. One of these exconjugants was grown in SS medium overnight before dilutions were plated onto CSM media (containing kanamycin) to isolate single colonies. These single colonies were replica plated onto CSM medium containing gentamicin and ampicillin to screen for the second crossover event and loss of the vector. A Knr, Aps, Gms colony, 18323Δvag-8, was isolated. Southern blot analysis of chromosomal DNA from this strain using a 0.8-kb NruI-EcoRV probe (bases 1607 to 2429) for vag-8 confirmed that 18323Δvag-8 contained a copy of vag-8 with the Knr cassette (data not shown). When an immunoblot of a whole-cell lysate of this strain was probed with antiserum raised to the maltose binding protein–Vag8 fusion, no 95-kDa protein was detected in the deletion strain (data not shown).
Aerosol challenge of mice with 18323, SK8, and 18323Δvag-8.
Groups of 20 to 22 17-day-old BALB/cAnNcR mice were challenged with 109 CFU of B. pertussis 18323, SK8, or 18323Δvag-8 in saline as an aerosol for 30 min, as previously described (18). A group of mice were sacrificed upon removal from the chamber to determine the number of viable bacteria in the lungs. To determine bacterial persistence, 8 to 10 mice were sacrificed after 14 days and the numbers of viable bacteria in the lungs and tracheas were determined. Another group of 10 to 12 mice were bled periodically from the orbital sinus to determine leukocyte counts. Recovery of 18323 from the lungs of mice was log10 8.65 (standard deviation [SD], ±0.38), that of SK8 was log10 5.31 (SD, ±0.46), and that of 18323Δvag-8 was log10 8.45 (SD, ±0.24). Recovery of 18323 from the trachea was log10 6.09 (SD, ±0.29), that of SK8 was log10 2.48 (SD, ±1.2), and that of SK8Δvag-8 was log10 6.18 (SD, ±0.18). Thus, the 95-kDa protein-deficient strain 18323Δvag-8 did not differ significantly from 18323 in the number of organisms isolated from the lungs and tracheas of mice. SK8 was significantly less able to colonize and persist in the lungs and tracheas of mice than was 18323 (P < 0.0001). Animals infected with 18323 and animals infected with 18323Δvag-8 exhibited leukocytosis; those infected with SK8 did not show leukocytosis (results not shown).
Summary.
The bvg-regulated 95-kDa protein we have cloned and characterized is the fourth member of a family of Bordetella proteins defined on the basis of their conserved C termini. The other members of this family, pertactin (4), Tcf (9), and BrkA (7), appear to be expressed as large precursor proteins, and cleavage of a C-terminal 30-kDa fragment results in the mature protein. In the case of Vag8, the cleavage event may not occur since the predicted size corresponds to the size seen on SDS-PAGE. In addition, supernatant fractions of strains of B. pertussis do not contain a protein which reacts with the polyclonal antiserum raised to Vag8. In common with the other members of this protein family, Vag8 contains an RGD motif. Southern blot and immunoblot analyses indicate that Vag8 is differentially expressed within the Bordetella species. The N-terminal sequence of the 92-kDa protein described by Hamstra et al. (11) identifies it as Vag8. The 91-kDa virulence-associated protein described by Armstrong and Parker which reacted with monoclonal antibody P8E7 (2) can also now be identified as Vag8.
The mutant strain of B. pertussis 18323 defective in Vag8, SK8, had been shown to be defective in the mouse aerosol model of pertussis. However, when a strain containing an internal deletion in the vag-8 structural gene, 18323Δvag-8, was used to infect mice, the strain colonized mice as well as the parent, 18323. The lack of effect in vivo was somewhat disappointing but perhaps not unexpected, since previous experiments to evaluate the effect of adhesin-defective B. pertussis strains in the aerosol model have shown anomalous results (8, 12, 20). Such results may indicate that B. pertussis expresses an array of virulence determinants, some of which may have similar functions such that loss of one may be compensated for by the others.
Nucleotide sequence accession number.
The nucleotide sequence of vag-8 and the predicted amino acid sequence encoded therein appear in the GenBank sequence data library under accession no. U90124.
Acknowledgments
We thank Drusilla Burns, Karen Elkins, and Scott Stibitz for critical comments. We are grateful to Mary Leef for animal immunizations and help with the aerosol challenge assay.
REFERENCES
- 1.Aoyama T, Murase Y, Iwata T, Imaizumi A, Suzuki Y, Sato Y. Comparison of blood-free medium (cyclodextrin solid medium) with Bordet-Gengou medium for clinical isolation of Bordetella pertussis. J Clin Microbiol. 1986;23:1046–1048. doi: 10.1128/jcm.23.6.1046-1048.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong S K, Parker C D. Heat-modifiable envelope proteins of Bordetella pertussis. Infect Immun. 1986;54:109–117. doi: 10.1128/iai.54.1.109-117.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bullock J, Armstrong S K, Shear J L, Lies D P, McIntosh M A. Formation of ion channels by colicin B in planar lipid bilayers. J Membr Biol. 1990;114:79–95. doi: 10.1007/BF01869387. [DOI] [PubMed] [Google Scholar]
- 4.Charles I G, Dougan G, Pickard D, Chatfield S, Smith M, Novotny P, Morrisey P, Fairweather N F. Molecular cloning and characterization of protective outer membrane protein P.69 from Bordetella pertussis. Proc Natl Acad Sci USA. 1989;86:3554–3558. doi: 10.1073/pnas.86.10.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devereux J, Haberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ezzell J, Dobrogosz W J, Kloos W E, Manclark C R. Phase-shift markers in Bordetella: alterations in envelope proteins. J Infect Dis. 1981;143:562–569. doi: 10.1093/infdis/143.4.562. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez R C, Weiss A A. Cloning and sequencing of a Bordetella pertussis serum resistance locus. Infect Immun. 1994;62:4727–4738. doi: 10.1128/iai.62.11.4727-4738.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finn T M, Shahin R, Mekalanos J J. Characterization of vir-activated TnphoA gene fusions in Bordetella pertussis. Infect Immun. 1991;59:3273–3279. doi: 10.1128/iai.59.9.3273-3279.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finn T M, Stevens L A. Tracheal colonization factor: a Bordetella pertussis secreted virulence determinant. Mol Microbiol. 1995;16:625–634. doi: 10.1111/j.1365-2958.1995.tb02425.x. [DOI] [PubMed] [Google Scholar]
- 10.Frank D W, Parker C D. Isolation and characterization of monoclonal antibodies to Bordetella pertussis. J Biol Stand. 1984;12:353–365. doi: 10.1016/s0092-1157(84)80060-8. [DOI] [PubMed] [Google Scholar]
- 11.Hamstra H-J, Kuipers B, Schijf-Evers D, Loggen H G, Poolman J T. The purification and protective capacity of Bordetella pertussis outer membrane proteins. Vaccine. 1995;13:747–752. doi: 10.1016/0264-410x(94)00040-t. [DOI] [PubMed] [Google Scholar]
- 12.Kimura A, Mountzouros K T, Relman D A, Falkow S, Cowell J L. Bordetella pertussis filamentous hemagglutinin: evaluation as a protective antigen and colonization factor in a mouse respiratory infection model. Infect Immun. 1990;58:7–16. doi: 10.1128/iai.58.1.7-16.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knapp S, Mekalanos J J. Two trans-acting regulatory genes (vir and mod) control antigenic modulation in Bordetella pertussis. Infect Immun. 1988;170:5059–5066. doi: 10.1128/jb.170.11.5059-5066.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leininger E, Roberts M, Kenimer J G, Charles I G, Fairweather N, Novotny P, Brennan M J. Pertactin, an Arg-Gly-Asp-containing Bordetella pertussis surface protein that promotes adherence of mammalian cells. Proc Natl Acad Sci USA. 1991;88:345–349. doi: 10.1073/pnas.88.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 16.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musser J M, Hewlett E L, Peppler M S, Selander R K. Genetic diversity and relationships in populations of Bordetella spp. J Bacteriol. 1986;166:230–237. doi: 10.1128/jb.166.1.230-237.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oda M, Cowell J L, Burstyn D G, Manclark C R. Protective activities of the filamentous hemagglutinin and the lymphocytosis promoting factor of Bordetella pertussis in mice. J Infect Dis. 1984;150:823–833. doi: 10.1093/infdis/150.6.823. [DOI] [PubMed] [Google Scholar]
- 19.Peppler M S. Isolation and characterization of isogenic pairs of domed hemolytic and flat nonhemolytic colony types of Bordetella pertussis. Infect Immun. 1982;35:840–851. doi: 10.1128/iai.35.3.840-851.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts M, Fairweather N F, Leininger E, Pickard D, Hewlett E L, Robinson A, Hayward C, Dougan G, Charles I G. Construction and characterization of Bordetella pertussis mutants lacking the vir-regulated P.69 outer membrane protein. Mol Microbiol. 1991;5:1393–1404. doi: 10.1111/j.1365-2958.1991.tb00786.x. [DOI] [PubMed] [Google Scholar]
- 21.Robinson A, Hawkins D C. Structure and biological properties of solubilized envelope proteins of Bordetella pertussis. Infect Immun. 1983;39:590–598. doi: 10.1128/iai.39.2.590-598.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider D R, Parker C D. Effect of pyridines on phenotypic properties of Bordetella pertussis. Infect Immun. 1982;38:548–553. doi: 10.1128/iai.38.2.548-553.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw W V. Chloramphenicol acetyltransferase from chloramphenicol resistant bacteria. Methods Enzymol. 1975;43:735–755. doi: 10.1016/0076-6879(75)43141-x. [DOI] [PubMed] [Google Scholar]
- 24.Stainer D W, Scholte M J. A simple chemically defined medium for the production of Phase I Bordetella pertussis. J Gen Microbiol. 1970;63:211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- 25.Stibitz S. Use of conditionally counterselectable suicide vectors for allelic exchange. Methods Enzymol. 1994;235:458–465. doi: 10.1016/0076-6879(94)35161-9. [DOI] [PubMed] [Google Scholar]
- 26.Stibitz S, Aaronson W, Monack D, Falkow S. Phase variation in Bordetella pertussis by frameshift mutation in a gene for a novel two-component system. Nature. 1989;338:266–269. doi: 10.1038/338266a0. [DOI] [PubMed] [Google Scholar]
- 27.Weiss A A, Hewlett E L. Virulence factors of Bordetella pertussis. Annu Rev Microbiol. 1986;40:661–686. doi: 10.1146/annurev.mi.40.100186.003305. [DOI] [PubMed] [Google Scholar]
- 28.Weiss A A, Hewlett E L, Myers G A, Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983;42:33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]