Abstract
Purpose
Physical activity can provide analgesic benefit but its effect on cancer-related pain is unclear. This review synthesised and appraised the evidence for the effect of physical activity on pain in people living with or beyond cancer.
Methods
A systematic search of Ovid Medline and Embase was performed to identify randomised controlled trials (RCTs), randomised cross-over studies (RXTs), and prospective observational studies that examined physical activity and pain outcomes in adults living with or beyond cancer. Meta-analyses were performed to generate effect estimates. Risk of bias was assessed, and the GRADE system was used to assess evidence quality.
Results
One hundred twenty-one studies (n = 13,806), including 102 RCTs, 6 RXTs, and 13 observational studies, met the criteria for inclusion. Meta-analyses of RCTs identified a decrease in pain intensity (n = 3734; standardised mean difference (SMD) − 0.30; 95% confidence interval (CI) − 0.45, − 0.15) and bodily pain (n = 1170; SMD 0.28; 95% CI 0.01, 0.56) but not pain interference (n = 207; SMD − 0.13, 95% CI − 0.42, 0.15) following physical activity interventions. Individual studies also identified a reduction in pain sensitivity but not analgesic use, although meta-analysis was not possible for these outcomes. High heterogeneity between studies, low certainty in some effect estimates, and possible publication bias meant that evidence quality was graded as very low to low.
Conclusion
Physical activity may decrease pain in people living with and beyond cancer; however, high heterogeneity limits the ability to generalise this finding to all people with cancer or to specific types of cancer-related pain.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00520-024-08343-3.
Keywords: Cancer pain, Exercise, Neoplasm, Review
Introduction
Cancer-related pain includes pain caused by a cancer or its treatments related and is one of the most common symptoms reported by people living with or beyond cancer. Pain is experienced by approximately 60% of all people undergoing treatment for cancer and continues to be reported by 40% of patients after completing their treatment [147, 148]. More than one in four people with cancer describe their pain as severe, and the impact of cancer-related pain can affect every aspect of a person’s life and can be a reason for cessation of treatment [90, 102, 147, 148].
Successful management of cancer-related pain is complex, often reliant on pharmacological intervention, and limitations in the scientific evidence for treatment possibilities compound existing patient, provider, and system barriers [43, 59]. An improved understanding of non-pharmacological treatment strategies for pain was a primary goal identified in the 2019 Australian Strategic Action Plan for Pain Management and has been highlighted by the American Centre for Disease Control as an essential step in improving pain management [44, 54]. This sentiment has been echoed in qualitative studies, which indicate that people with cancer would like more treatment options for pain, including non-pharmacological strategies [90]. Unfortunately, many do not always feel they are supported when seeking non-pharmacological pain treatments [90].
Physical activity, including structured exercise and incidental or leisure time activities, can provide analgesic benefit and is considered an important component of pain management for non-cancer pain. In healthy populations, exercise increases the threshold for experimentally induced pain and prevents the development of several chronic pain conditions [64, 85, 134]. Benefits can be seen after just one session of exercise and have been attributed to the effect of exercise on several central, immune system, and psychological pathways [85, 123]. In several clinical populations (e.g. back pain, arthritic conditions, fibromyalgia), exercise can promote analgesia and reduce pain-related disability [134]. However, an analgesic effect is not evident in all patient populations who experience pain, with some populations (e.g. chronic fatigue syndrome, chronic neck pain) experiencing exacerbations of pain with exercise in some studies [85, 123]. This can present a major barrier to engagement and adherence in physical activity programmes, potentially leading to long-term sedentary behaviours and poor health outcomes.
Although there is some evidence that supports a decrease in cancer-related pain following exercise [79], collectively, the evidence remains sparse. No studies examining the effects of physical activity or exercise on pain sensitivity in people who have cancer-related pain were identified in two recent reviews [14, 123]. Further, recent guidelines addressing the effects of exercise on health outcomes in people with cancer, which were developed by a group of international exercise oncology experts, state that the evidence is insufficient to support prescription of exercise for cancer-related pain [27, 131]. Several international cancer pain management guidelines do not include exercise as a possible treatment [89]. Similarly, many cancer-related pain management documents developed for consumers provide limited information on the potential benefit of physical activity or exercise on cancer pain [28]. A greater understanding of the effects of exercise on cancer-related pain is warranted. This review will examine the evidence for an effect of physical activity on pain outcomes in people with cancer.
Methods
This systematic review and meta-analysis were conducted in accordance with the PRISMA 2020 Statement [108]. The review protocol was uploaded to PROSPERO prior to commencement (CRD42021267826).
Searchstrategy
A systematic search of Ovid Medline and Embase electronic databases was initially performed on the 26th of August 2021. This search was updated on 5th of December 2023. The search strategy included a combination of Medical Subject Headings (MESH) and free text terms, and an example search is presented in Supplementary Table 1. Reference lists from other reviews were manually searched to identify any references that were not otherwise found. No date limits were applied to the search or eligibility criteria.
Eligibility criteria
Eligible studies included peer-reviewed parallel group randomised controlled trials (RCTs), randomised cross-over trials (RXTs), and prospective observational studies. Participants could include adults (> 18 years) who have cancer or survivors of adult cancer. Physical activity could include structured exercise (e.g. moderate intensity continuous exercise) or less structured physical activity (e.g. leisure time physical activity). Comparisons such as usual care, activity quantity (e.g. inactivity or low activity), or activity type (e.g. resistance exercise compared to aerobic exercise) were included. Eligible outcomes included pain prevalence, intensity, impact, quality, sensitivity, or analgesic use. Studies were excluded if they included non-randomised interventions or cross-sectional analyses, participants with childhood cancer or survivors of childhood cancer, and interventions or exposures such as passive movement and manual therapy, highly specific therapy (e.g. jaw strengthening, swallowing exercises), or physical activity combined with a second intervention or exposure (e.g. diet, relaxation therapy). Studies with no comparison condition (e.g. single arm trials) were also excluded. Pain that was clearly not related to cancer (e.g. mechanical low back pain) or broader outcome measures (e.g. ‘cancer symptoms’) were excluded. Only studies published in English were included.
Screening, extraction, and appraisal
Following duplicate removal, all eligible title and abstracts returned via the search were screened independently by two reviewers (MP, CS, DM, or BL). Studies that were clearly not relevant were excluded. Two reviewers (MP, CS) then screened the full texts of all remaining references to determine the final eligibility for inclusion with disagreement resolved via discussion. One investigator performed data extraction and the risk of bias assessment for each study (MP, GB, or DM) using a pre-piloted data extraction form, which was then reviewed by the senior investigator (CS). Extracted data included study details (author, year), population (e.g. cancer type, stage, participant, sample size), intervention or exposure details (e.g. physical activity type) and assessment method (e.g. self-report), comparison or control condition, outcome definitions, confounding factors used for adjustment, and outcome values. Web plot digitiser was used to extract outcome data presented visually [42]. Risk of bias was performed using the Cochrane Collaboration Tool for randomised controlled trials and the Risk of Bias in Studies of Exposures (ROBINS-E) tool for prospective observational studies [73, 100]. The overall quality of evidence for a physical activity—cancer pain relationship—was appraised using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system [66].
Meta-analysis
For all extracted outcomes, data were summarised and presented descriptively. Where study design, exposures, outcomes, and analyses were defined consistently in at least three separate studies, random-effects meta-analysis of final study values was used to generate a standardised mean difference (SMD) with 95% confidence interval (CI). Studies with multiple intervention arms (e.g. two types of exercise) were combined into a single group, as per Cochrane recommendations [74]. Statistical heterogeneity was quantified using the I2 statistic. Where there was more than moderate heterogeneity (I2 > 40%), subgroup analyses were performed to examine the influence of cancer type or physical activity type and setting. Publication bias was assessed by visual inspection of funnel plots. All meta-analyses were performed using Stata version 16 (Stata Corporation, College Station, TX, USA).
Results
Search results
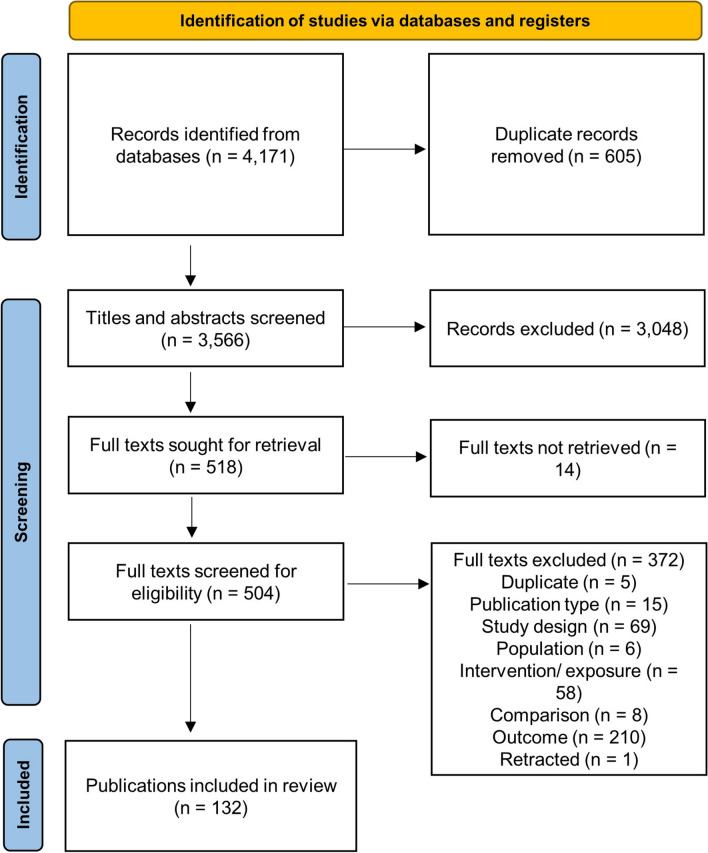
Search results are presented in Fig. 1. From 4171 records first identified, 3566 titles and abstracts and 504 full texts screened, there were 132 publications from 121 studies included in the final review. Studies included 102 RCTs [1, 2, 4, 6–12, 15, 16, 18, 19, 21–24, 26, 29–32, 35, 37, 38, 40, 41, 45–52, 55, 57, 61–63, 65, 67–72, 75–81, 83, 84, 86–88, 91–99, 101, 103–106, 109, 110, 112–114, 116, 118–122, 124–127, 129, 130, 132, 133, 136–140, 143, 145, 146, 149–151, 153–157], six randomised RXTs [17, 34, 36, 39, 142, 152], and 13 observational studies [3, 13, 20, 33, 53, 56, 58, 60, 82, 115, 128, 135, 144, 158]. Overall, these studies included 13,806 participants.
Fig. 1.
PRISMA chart
Study characteristics
Study characteristics are presented in Supplementary Table 2–4. Median sample sizes were 60 (range 10 to 577) in RCTs, 19 (range 10 to 21) in RXTs, and 199 (range 42 to 1937) in prospective observational studies. Participants included patients undergoing treatment and survivors of breast (RCT = 48, RXT = 3, observational = 7), prostate (RCT = 10, observational = 1), lung (RCT = 7, observational = 2), colorectal (RCT = 5), head and neck (RCT = 2, observational = 2), haematological (RCT = 2), bladder (RCT = 1), gastrointestinal (RCT = 6), ovarian (RCT = 1), or combined/ non-specific sites (RCT = 15, RXT = 3, observational = 3). Interventions included general physical activity (RCT = 20), aerobic exercise (RCT = 21, RXT = 2), resistance exercise (RCT = 16, RXT = 3), combined aerobic and resistance exercise (RCT = 39, RXT = 1), aquatic exercise (RCT = 4), yoga (RCT = 4), Pilates (RCT = 3), dance (RCT = 3), Tai chi (RCT = 1), or Qigong (RCT = 1, RXT = 1). In observation studies, physical activity was assessed via self-report (n = 9), accelerometry/ smart-phone app (n = 6), or was unclear (n = 1). The comparison condition included usual care or activity (RCT = 81, RXT = 1), type of physical activity or exercise (RCT = 14, RXT = 1), timing of physical activity (RCT = 3), training dose (RCT = 4, RXT = 4), and setting, delivery method, or promotion strategy (RCT = 6). Pain intensity was the most common outcome (RCT = 68, RXT = 5, observation = 10), followed by bodily pain (RCT = 33, RXT = 1, observation = 2), pain interference (RCT = 8, RXT = 1, observation = 1), neuropathic pain (RCT = 4, observational = 1), pain quality (RCT = 1), pain presence (RCT = 1, observation = 2), bone pain (RCT = 1), analgesic use (RCT = 4, observational = 1), pain sensitivity (RCT = 4, RXT = 1), and pain frequency (RCT = 1, observation = 1).
Risk of bias
Risk of bias results are presented in Supplementary Table 5–7. All RCTs scored high for performance bias as it is not possible to blind participants from their exercise intervention status. Other reasons for bias in RCTs included unclear bias arising from randomisation procedures [31, 47–52, 55, 65, 71, 77, 80, 88, 98, 99, 105, 106, 113, 116, 122, 130, 138–140, 151, 156] and unclear or high bias for greater than 10% attrition or less than 90% intervention adherence [1, 2, 6–9, 11, 15–19, 21, 23, 24, 26, 29, 32, 40, 41, 49, 50, 55, 61, 67–71, 75, 76, 79, 80, 84, 86–88, 93, 94, 98, 103, 106, 109, 110, 112–114, 118, 119, 129, 133, 138, 143, 150, 151, 153, 154, 157]. In RXTs, one study was judged to have high bias arising from potential carry over effects [142], and one was judged to have high bias due to deviations from the intended intervention [152]. All observation studies had at least moderate risk of bias due to the likely presence of confounding, self-reported measurement of physical activity, or possible exposure departures (i.e. a change in physical activity). Seven studies had serious risk of bias as they did not adjust for minimally important confounders [33, 56, 58, 82, 128, 135, 144].
Physical activity and pain intensity
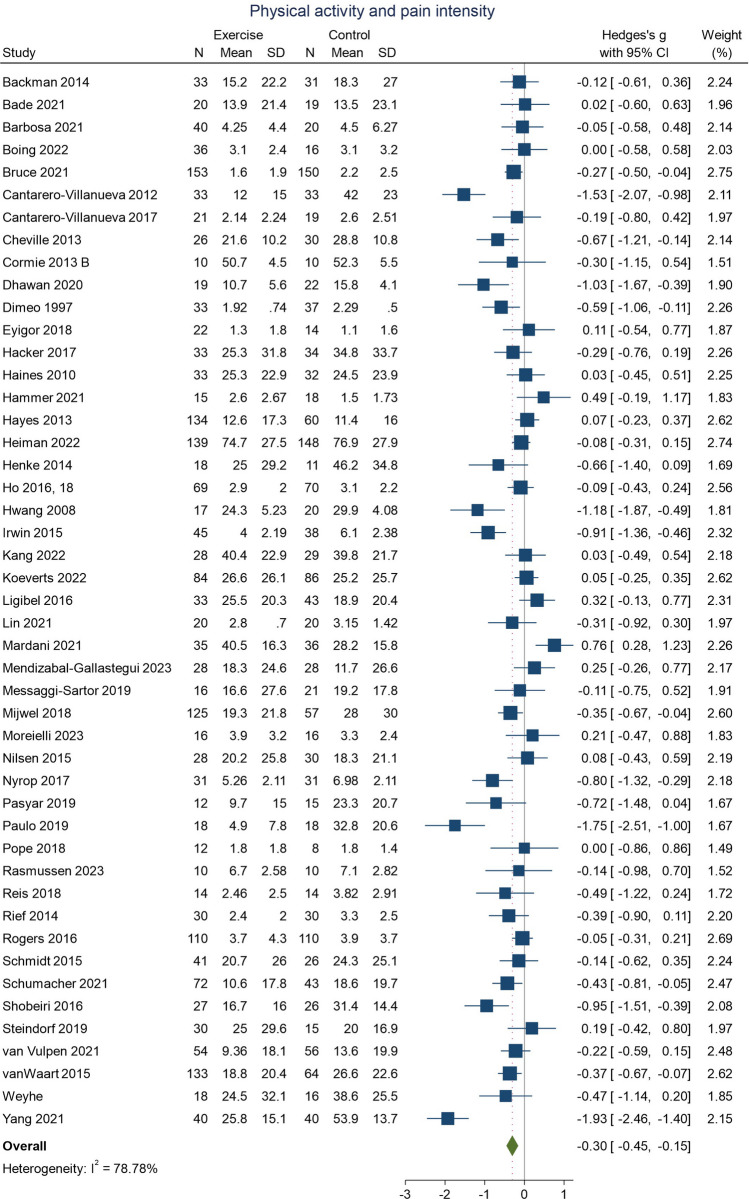
Meta-analysis of RCTs that examined the effect of physical activity compared to a control condition on pain intensity is presented in Fig. 2. Overall, physical activity interventions resulted in a decrease in pain intensity (studies = 47; n = 3734; SMD − 0.30; 95% CI − 0.45, − 0.15; I2 = 79%). There was high heterogeneity and evidence of possible publication bias in the funnel plots (Supplementary Fig. 7).
Fig. 2.
Physical activity and pain intensity in people with cancer. An effect size < 0 indicates a decrease in pain intensity or interference following physical activity intervention
Subgroup analyses (Supplementary Figs. 1–3) demonstrated decreases in pain following physical activity interventions for breast cancer (studies = 24; n = 2340; SMD − 0.34; 95% CI − 0.54, − 0.13; I2 = 81%) and studies that examined other or multiple cancers (studies = 16; n = 1025; SMD − 0.35; 95% CI − 0.62, − 0.08; I2 = 78%) but not for lung (studies = 3; n = 105; SMD − 0.20; 95% CI − 0.58, 0.18; I2 = 0%) or prostate cancer (studies = 4; n = 264; SMD 0.04; 95% CI − 0.51, 0.59; I2 = 78%). Subgroup analyses also identified larger effect estimates for interventions that featured resistance training (studies = 9; n = 697; SMD − 0.35; 95% CI − 0.61, − 0.09; I2 = 57%) or combined modalities (studies = 17; n = 1507; SMD − 0.28; 95% CI − 0.50, − 0.05; I2 = 77%) than for physical activity interventions alone (studies = 6; n = 438; SMD − 0.11; 95% CI − 0.43, 0.21; I2 = 56%). Larger effects for supervised interventions (studies = 27; n = 1945; SMD − 0.35; 95% CI − 0.53, − 0.17; I2 = 70%) compared with unsupervised interventions (studies = 8; n = 603; SMD − 0.27; 95% CI − 0.61, − 0.08; I2 = 72%) were also evident. However, the heterogeneity between studies remained high following subgroup analyses for each cancer site, physical activity type, and supervised or unsupervised intervention.
Individual RCTs not included in the meta-analysis (Supplementary Table 8) had either null findings [50, 65, 78, 150] or small decreases in pain intensity for physical activity interventions compared to a usual care control [2, 5, 91, 109, 110, 114, 143]. One RXT did not identify a difference in pain following exercise or usual care [142]. Most prospective observational studies suggested an association between more physical activity and reduced pain symptoms [20, 58, 82, 115, 128, 135, 144]. Three observational studies found no definitive relationship [33, 56, 158].
Physical activity and bodily pain
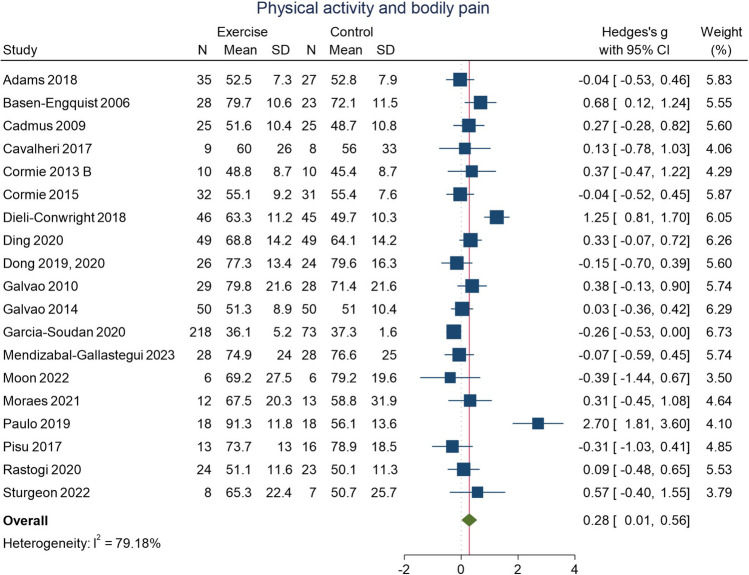
Meta-analysis of RCTs that examined the effect of physical activity on bodily pain is presented in Fig. 3. Overall, physical activity interventions resulted in a decrease (i.e. higher score) in bodily pain (i.e. less pain; studies = 19; n = 1170; SMD 0.28; 95% CI 0.01, 0.56; I2 = 79%). There was high heterogeneity and evidence of possible publication bias in the funnel plots (Supplementary Fig. 8).
Fig. 3.
Physical activity and bodily pain in people with cancer. An effect size > 0 indicates an improvement in bodily pain following physical activity intervention
Heterogeneity remained high following subgroup analyses (Supplementary Figs. 4–7) that stratified for cancer site, physical activity type, and intervention supervision status. Effect estimates may have been higher for breast cancer (studies = 8; n = 609; SMD 0.63; 95% CI 0.00, 1.26; I2 = 90%), for supervised interventions (studies = 10; n = 724; SMD 0.39; 95% CI − 0.14, 0.92; I2 = 92%), and for interventions with combined modalities (studies = 11; n = 823; SMD 0.33; 95% CI − 0.15, 0.81; I2 = 91%). However, wide confidence intervals as well as high heterogeneity limit the certainty of these findings.
Findings from RCTs, RXTs, and observational studies that examined changes in bodily pain following physical activity intervention compared to control that were not included in the meta-analyses had mostly null findings [3, 6, 23, 31, 34, 38, 136, 146, 157]. Some individual studies did suggest possible decrease in bodily pain following physical activity [13, 22, 119].
Physical activity and pain interference
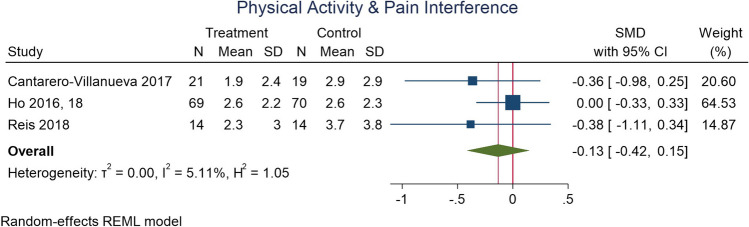
Results for an effect of physical activity on pain interference were unclear. Meta-analysis (Fig. 4) noted no definitive change in interference following interventions (studies = 3; n = 207; SMD − 0.13; 95% CI − 0.42, 0.15; I2 = 5%). In studies not included in the meta-analysis (Supplementary Table 8), two RCTs identified no change [57, 78] and two identified small to moderate decreases in pain interference [38, 79]. In a RXT, there were small decreases in pain interference 24 and 72 days following resistance training [36].
Fig. 4.
Physical activity and pain interference in people with cancer. Although an effect size < 0 suggests a decrease in pain interference after physical activity intervention, as the confidence intervals cross the null, the certainty of this finding is limited
Physical activity and pain sensitivity
Five studies, including four parallel group RCTs and one RXT, examined the relationship between physical activity and pain sensitivity in people with cancer (Supplementary Table 8). Each study documented a decrease in pain sensitivity at some affected sites following physical activity. In women with breast cancer undergoing chemotherapy, high-intensity interval training combined with resistance or aerobic training decreased pain sensitivity when compared to usual care, with stronger effects with resistance training [96]. In survivors of any cancer, a single bout of low or high intensity exercise decreased pain sensitivity in exercising but not non exercising muscles, with greater effects for higher intensity exercise [34]. A 2-week period of low or high intensity exercise decreased post-exercise pain sensitivity at both sites, with exercise eliciting a medium effect in exercising muscles and a small effect in non-exercising muscles [34]. In breast cancer survivors, both aquatic therapy and resistance training reduced pain sensitivity at select sites [30, 120]. In colon cancer survivors, lumbopelvic exercises and light aerobic exercise decreased pain sensitivity at lumbar and some, but not all abdominal sites, and not at the second metacarpal [29].
Physical activity and analgesic use
Four RCTs reported on the use of pain medication, with no suggestion that physical activity interventions decrease analgesic use more than control conditions [11, 38, 79, 138]. One RCT reported that analgesic use in people with lung cancer scheduled to receive surgery was higher in the exercise intervention group compared to the control group after 3 months of activity [138].
Physical activity type and pain outcomes
Twelve RCTs and one RXT compared types of exercise (Supplementary Table 8). Results of individual studies cannot support any optimal type of exercise. Some studies showed no difference in effect for type [80, 88, 106, 152, 153, 156], while others showed larger effects of aerobic exercise compared to resistance [40, 63, 129], or for resistance and high intensity interval training compared to aerobic exercise combined with high intensity interval training [19, 113]. In other studies, aquatic exercises may have been more effective than land exercises [4], and Pilates more effective than circuit exercises [11].
Physical activity dose and pain outcomes
Five RCTs, four RXTs, and three observational studies examined the relationship between intervention or physical activity dose and pain outcomes (Supplementary Table 8—9). Results either favoured a higher dosage (i.e. a higher dose led to a greater decrease in pain) [16, 22, 34, 40, 58], a low-moderate dosage/ intensity [8], or did not identify differences by dose [36, 38, 39, 104].
Timing of physical activity intervention
Three RCTs compared the timing of physical activity delivery, including comparison of physical activity during treatment and after treatment (Supplementary Table 8). There were no clear differences in pain outcomes by intervention timing in these studies [15, 49, 50].
Physical activity setting or delivery method and pain outcomes
Eight RCTs compared physical activity delivery settings or approaches (Supplementary Table 8). For supervised compared to home-based or unsupervised activity, two studies found greater effects for supervised interventions [16, 21] while four studies found no difference [137, 140, 150, 156]. There were no clear differences for telehealth supported compared to self-directed interventions or for an oncologist exercise recommendation with or without a motivational package [109, 145].
GRADE
GRADE appraisal results are presented in Table 1. The quality of the evidence for an effect of physical activity on pain intensity, bodily pain, or pain interference in adults with cancer was initially graded as high, as results were based on meta-analysis of RCTs. However, these were then graded down to low, owing to high heterogeneity in effects between studies, possible publication bias, and wide confidence limits. The evidence for an effect of physical activity on pain sensitivity or analgesic use was graded as very low, owing to the low number of studies that prevented meta-analysis, as well as variation within these studies.
Table 1.
GRADE evidence appraisal for physical activity and cancer pain outcomes
| Outcome | Meta-analysis study N (participant N) | Meta-analysis effect estimate (SMD (95% CI)) | Quality of evidence |
|---|---|---|---|
| Pain intensity | 47 (3734) | − 0.30 (− 0.45, − 0.15) | Lowaa |
| Bodily pain | 19 (1170) | 0.28 (0.01, 0.56) | Lowaa |
| Pain interference | 3 (207) | − 0.13 (− 0.42, 0.15) | Lowb |
| Pain sensitivity | NA | NA | Very lowc |
| Analgesic use | NA | NA | Very lowc |
aGraded down due to heterogeneity and publication bias
bGraded down due to low certainty and study sample size
cGraded very low as meta-analysis not possible
Discussion
This review examined the effect of physical activity on pain outcomes in people who have or have had cancer. The review found that physical activity can reduce pain intensity and pain sensitivity in people with cancer. However, high heterogeneity does limit certainty of these findings.
The primary strength of this review is the inclusion of RCTs, RXTs, and prospective observational studies, thus synthesising evidence from multiple study designs. The review also synthesises evidence for numerous cancer types, physical activity types, and pain outcomes. It addresses clinically relevant questions that are central to the lived experience of most people with cancer, as well as many cancer survivors. This review also has several limitations that should be considered when interpreting the findings. First, high heterogeneity limits the certainty of the review findings. To an extent, high heterogeneity should be expected, given the range of cancer types and stages, as well as the range in timing, dose, and delivery setting of physical activity. Nonetheless, our ability to generalise this finding to all persons with cancer-related pain is limited. The review did not include children or adolescents or survivors of childhood cancers, and therefore, the findings only apply to adults. Few of the included studies assessed pain as a primary outcome, with most assessing it as a part of a broader quality of life assessment. This meant that while pain intensity was well measured, additional outcome domains recommended for pain trials, such as pain quality and affect, or analgesic use, were not widely captured. It also meant that few studies considered baseline pain levels in their inclusion criteria, and therefore, we cannot guarantee all participants included in the review were those that most need pain interventions. Further, as pain was not a primary outcome for many of the included studies, it is likely that the interventions delivered were not optimally designed for pain reduction. This may limit the potential benefit of observed effects relative to pain. Although the inclusion of prospective observational studies is a strength, as it facilitates triangulation of evidence and may provide a real-world perspective not offered by tightly controlled efficacy studies, several of these studies contained serious risk of bias and therefore provided only limited contribution to the overall findings.
That physical activity can reduce pain intensity as well as pain sensitivity in people who have or have had cancer is consistent with findings for other populations that experience pain. For example, healthy populations who are more active typically report less pain and have reduced sensitivity to painful stimuli than those who are less active [64, 141]. Further, physical activity has been shown to reduce pain and pain-related disability for people with conditions like back pain or arthritis [107, 134]. Physical activity has been proposed to decrease pain via central, biological, and psychosocial pathways [85, 134]. For people with cancer, additional factors that may underlie benefit include an improved ability to tolerate treatment or recovery from surgery, reduced cancer fatigue that could increase susceptibility to pain, or an increased perception of control, which can also improve perception of pain [85, 117].
Exercise type and delivery setting appeared to influence effect estimates. Subgroup meta-analyses showed stronger effects for resistance and/ or aerobic exercise than general physical activity and for supervised than unsupervised exercise. This may indicate increased benefit of exercise, which refers to more planned, structured, and intentional movement like lifting weights or doing continuous cardiorespiratory exercise at a set intensity, compared to broader physical activity, which refers to any type of movement performed by the muscles like working in a garden or walking for transport. It may also reflect that supervised exercise interventions are often delivered by exercise professionals, like physiotherapists or exercise physiologists, who may be more deliberate with their exercise prescription than people completing self-directed, unsupervised activity. This was not a finding in all individual studies and does not imply that self-directed physical activity is not beneficial or should not be recommended, as these interventions also saw a decrease in pain, and they may represent a more accessible or sustainable behaviour. However, it does suggest that an ideal physical activity intervention might include a supervised exercise component [25].
The evidence was stronger for breast cancer than other cancer types. This may be due to the larger number of studies that included women with breast cancer. It may also reflect the benefits of physical activity to the types of pain often experienced by women with breast cancer, such as post-surgery pain, lymphoedema pain, or arthralgia. Importantly, subgroup meta-analysis suggested likely pain improvement for multiple cancer types, and few individual studies documented increases in pain after physical activity. While further studies may be required to untangle the benefits of physical activity on pain-related to different types of cancer or treatments, our results support potential benefit for many people that experience cancer.
Future research should assess the recommended outcome domains for pain trials, which include pain intensity, pain quality, and pain impact on physical and mental function, as well as participant satisfaction [111]. Pending research questions to be investigated in future research could include how physical activity affects different types of pain experienced by people with cancer, what specific mechanisms underlie benefit, reasons for variation between patients, and how physical activity programmes should be delivered to people impacted by cancer-related pain. Clinically, these results support that physical activity can decrease pain in people with cancer and should be considered as a non-pharmacological option in cancer-related pain management guidelines. The findings do not have sufficient detail to recommend physical activity for many specific types of cancer-related pain; however, the findings do support the inclusion of physical activity in broader guidelines or patient facing materials [28].
Conclusion
This review provides an overview for the evidence of an effect of physical activity on cancer pain. Physical activity interventions may decrease pain intensity and pain sensitivity in people living with and beyond cancer; however, high heterogeneity suggests that not all persons will respond the same way to all interventions. More research is needed to understand the specific effects of physical activity on different types of cancer-related pain.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
MP, GB, BL, LD, and CS contributed to study concept. MP and CS conducted the systematic searches. MP, CS, BL, and DM contributed to title and abstract as well as full text screening. MP, GB, DM, and CS performed data extraction and risk of bias appraisal. CS conducted the meta-analysis. MP drafted an initial manuscript, which was reviewed and edited by GB, DM, BL, LD, and CS. MP, GB, DM, BL, LD, and CS read and approved the final manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions B. M. Lynch was supported by the Victorian Cancer Agency (MCRF18005).
Data Availability
Data are available upon request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Adams SC, DeLorey DS, Davenport MH, Fairey AS, North S, Courneya KS. Effects of high-intensity interval training on fatigue and quality of life in testicular cancer survivors. Br J Cancer. 2018;118:1313–1321. doi: 10.1038/s41416-018-0044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adeline F, Hugo PR, Rene M, Tamas F, Eleonor R, Michel P. Effects of a mixed exercise program on cancer related-fatigue and health-related quality of life in oncogeriatric patients: a feasibility study J Geriatr. Oncol. 2021;12:915–921. doi: 10.1016/j.jgo.2021.02.025. [DOI] [PubMed] [Google Scholar]
- 3.Alfano CM, Smith AW, Irwin ML, Bowen DJ, Sorensen B, Reeve BB, Meeske KA, Bernstein L, Baumgartner KB, Ballard-Barbash R, Malone KE, McTiernan A. Physical activity, long-term symptoms, and physical health-related quality of life among breast cancer survivors: a prospective analysis. J Cancer Surviv. 2007;1:116–128. doi: 10.1007/s11764-007-0014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali KM, El Gammal ER, Eladl HM. Effect of aqua therapy exercises on postmastectomy lymphedema: a prospective randomized controlled trial Ann Rehabil Med. 2021;45:131–140. doi: 10.5535/arm.20127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ammitzboll G, Andersen KG, Bidstrup PE, Johansen C, Lanng C, Kroman N, Zerahn B, Hyldegaard O, Andersen EW, Dalton SO. Effect of progressive resistance training on persistent pain after axillary dissection in breast cancer: a randomized controlled trial. Breast Cancer Res Treat. 2020;179:173–183. doi: 10.1007/s10549-019-05461-z. [DOI] [PubMed] [Google Scholar]
- 6.Arbane G, Douiri A, Hart N, Hopkinson NS, Singh S, Speed C, Valladares B, Garrod R. Effect of postoperative physical training on activity after curative surgery for non-small cell lung cancer: a multicentre randomised controlled trial. Physiotherapy. 2014;100:100–107. doi: 10.1016/j.physio.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Arthuso FZ, Morielli AR, Usmani N, Joseph K, Nijjar T, Tankel K, Fairchild A, Severin D, Boule NG, Courneya KS. Effects of exercise on motivational outcomes in rectal cancer patients during and after neoadjuvant chemoradiation: a phase ii randomized controlled trial. Semin Oncol Nurs. 2023;39:151419. doi: 10.1016/j.soncn.2023.151419. [DOI] [PubMed] [Google Scholar]
- 8.Ax AK, Johansson B, Lyth J, Nordin K, Borjeson S. Short- and long-term effect of high versus low-to-moderate intensity exercise to optimise health-related quality of life after oncological treatment-results from the Phys-Can project. Support Care Cancer. 2022;30:5949–5963. doi: 10.1007/s00520-022-07016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Backman M, Wengstrom Y, Johansson B, Skoldengen I, Borjesson S, Tarnbro S, Berglund A. A randomized pilot study with daily walking during adjuvant chemotherapy for patients with breast and colorectal cancer. Acta Oncol. 2014;53:510–520. doi: 10.3109/0284186X.2013.873820. [DOI] [PubMed] [Google Scholar]
- 10.Bade BC, Gan G, Li F, Lu L, Tanoue L, Silvestri GA, Irwin ML. Randomized trial of physical activity on quality of life and lung cancer biomarkers in patients with advanced stage lung cancer: a pilot study. BMC Cancer. 2021;21:352. doi: 10.1186/s12885-021-08084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbosa KP, da Silva LGT, Garcia PA, Freitas CA, da Silva ECF, Pereira TV, Alves AT, Matheus LBG. Effectiveness of Pilates and circuit-based exercise in reducing arthralgia in women during hormone therapy for breast cancer: a randomized, controlled trial. Support Care Cancer. 2021;29:6051–6059. doi: 10.1007/s00520-021-06180-2. [DOI] [PubMed] [Google Scholar]
- 12.Basen-Engquist K, Taylor CL, Rosenblum C, Smith MA, Shinn EH, Greisinger A, Gregg X, Massey P, Valero V, Rivera E. Randomized pilot test of a lifestyle physical activity intervention for breast cancer survivors. Patient Educ Couns. 2006;64:225–234. doi: 10.1016/j.pec.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Belanger LJ, Plotnikoff RC, Clark A, Courneya KS. Physical activity and health-related quality of life in young adult cancer survivors: a Canadian provincial survey. J Cancer Surviv. 2011;5:44–53. doi: 10.1007/s11764-010-0146-6. [DOI] [PubMed] [Google Scholar]
- 14.Belavy DL, Van Oosterwijck J, Clarkson M, Dhondt E, Mundell NL, Miller CT, Owen PJ. Pain sensitivity is reduced by exercise training: evidence from a systematic review and meta-analysis. Neurosci Biobehav Rev. 2021;120:100–108. doi: 10.1016/j.neubiorev.2020.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Bland KA, Kirkham AA, Bovard J, Shenkier T, Zucker D, McKenzie DC, Davis MK, Gelmon KA, Campbell KL. Effect of exercise on taxane chemotherapy-induced peripheral neuropathy in women with breast cancer: a randomized controlled trial. Clin Breast Cancer. 2019;19:411–422. doi: 10.1016/j.clbc.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Bloomquist K, Adamsen L, Hayes SC, Lillelund C, Andersen C, Christensen KB, Oturai P, Ejlertsen B, Tuxen MK, Moller T. Heavy-load resistance exercise during chemotherapy in physically inactive breast cancer survivors at risk for lymphedema: a randomized trial. Acta Oncol. 2019;58:1667–1675. doi: 10.1080/0284186X.2019.1643916. [DOI] [PubMed] [Google Scholar]
- 17.Bloomquist K, Oturai P, Steele ML, Adamsen L, Moller T, Christensen KB, Ejlertsen B, Hayes SC. Heavy-load lifting: acute response in breast cancer survivors at risk for lymphedema. Med Sci Sports Exerc. 2018;50:187–195. doi: 10.1249/MSS.0000000000001443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boing L, Fretta TB, Lynch BM, Dias M, Rosa LMD, Baptista F, Bergmann A, Fausto DY, Bocchi Martins JB, Guimaraes ACA. Mat Pilates and belly dance: effects on patient-reported outcomes among breast cancer survivors receiving hormone therapy and adherence to exercise Complement Ther Clin Pract. 2023;50:101683. doi: 10.1016/j.ctcp.2022.101683. [DOI] [PubMed] [Google Scholar]
- 19.Bolam KA, Mijwel S, Rundqvist H, Wengstrom Y. Two-year follow-up of the OptiTrain randomised controlled exercise trial. Breast Cancer Res Treat. 2019;175:637–648. doi: 10.1007/s10549-019-05204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Branstrom R, Petersson LM, Saboonchi F, Wennman-Larsen A, Alexanderson K. Physical activity following a breast cancer diagnosis: implications for self-rated health and cancer-related symptoms. Eur J Oncol Nurs. 2015;19:680–685. doi: 10.1016/j.ejon.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Brocki BC, Andreasen J, Nielsen LR, Nekrasas V, Gorst-Rasmussen A, Westerdahl E. Short and long-term effects of supervised versus unsupervised exercise training on health-related quality of life and functional outcomes following lung cancer surgery - a randomized controlled trial. Lung Cancer. 2014;83:102–108. doi: 10.1016/j.lungcan.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 22.Brown JC, Damjanov N, Courneya KS, Troxel AB, Zemel BS, Rickels MR, Ky B, Rhim AD, Rustgi AK, Schmitz KH. A randomized dose-response trial of aerobic exercise and health-related quality of life in colon cancer survivors. Psychooncology. 2018;27:1221–1228. doi: 10.1002/pon.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown JC, Sarwer DB, Troxel AB, Sturgeon K, DeMichele AM, Denlinger CS, Schmitz KH. A randomized trial of exercise and diet on health-related quality of life in survivors of breast cancer with overweight or obesity. Cancer. 2021;127:3856–3864. doi: 10.1002/cncr.33752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruce J, Mazuquin B, Canaway A, Hossain A, Williamson E, Mistry P, Lall R, Petrou S, Lamb SE, Rees S, Padfield E, Vidya R, Thompson AM, Prevention of Shoulder Problems Trial Study G Exercise versus usual care after non-reconstructive breast cancer surgery (UK PROSPER): multicentre randomised controlled trial and economic evaluation. BMJ. 2021;375:e066542. doi: 10.1136/bmj-2021-066542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buffart LM, Kalter J, Sweegers MG, Courneya KS, Newton RU, Aaronson NK, Jacobsen PB, May AM, Galvao DA, Chinapaw MJ, Steindorf K, Irwin ML, Stuiver MM, Hayes S, Griffith KA, Lucia A, Mesters I, van Weert E, Knoop H, Goedendorp MM, Mutrie N, Daley AJ, McConnachie A, Bohus M, Thorsen L, Schulz KH, Short CE, James EL, Plotnikoff RC, Arbane G, Schmidt ME, Potthoff K, van Beurden M, Oldenburg HS, Sonke GS, van Harten WH, Garrod R, Schmitz KH, Winters-Stone KM, Velthuis MJ, Taaffe DR, van Mechelen W, Kersten MJ, Nollet F, Wenzel J, Wiskemann J, Verdonck-de Leeuw IM, Brug J (2017) Effects and moderators of exercise on quality of life and physical function in patients with cancer: an individual patient data meta-analysis of 34 RCTs Cancer Treat Rev. 52:91–104 [DOI] [PubMed]
- 26.Cadmus LA, Salovey P, Yu H, Chung G, Kasl S, Irwin ML. Exercise and quality of life during and after treatment for breast cancer: results of two randomized controlled trials. Psychooncology. 2009;18:343–352. doi: 10.1002/pon.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, Zucker DS, Matthews CE, Ligibel JA, Gerber LH, Morris GS, Patel AV, Hue TF, Perna FM, Schmitz KH. Exercise guidelines for cancer survivors: consensus statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc. 2019;51:2375–2390. doi: 10.1249/MSS.0000000000002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cancer Council Australia (2021) Understanding cancer pain. Cancer Council Australia, Sydney.
- 29.Cantarero-Villanueva I, Cuesta-Vargas AI, Lozano-Lozano M, Fernandez-Lao C, Fernandez-Perez A, Galiano-Castillo N. Changes in pain and muscle architecture in colon cancer survivors after a lumbopelvic exercise program: a secondary analysis of a randomized controlled trial Pain Medicine (United States) 2020;18:1366–1376. doi: 10.1093/pm/pnx026. [DOI] [PubMed] [Google Scholar]
- 30.Cantarero-Villanueva I, Fernandez-Lao C, Fernandez-de-Las-Penas C, Lopez-Barajas IB, Del-Moral-Avila R, de la-Llave-Rincon AI, Arroyo-Morales M (2012) Effectiveness of water physical therapy on pain, pressure pain sensitivity, and myofascial trigger points in breast cancer survivors: a randomized, controlled clinical trial Pain Med 13:1509-1519 [DOI] [PubMed]
- 31.Chan H, Van Loon K, Kenfield SA, Chan JM, Mitchell E, Zhang L, Paciorek A, Joseph G, Laffan A, Atreya C, Fukuoka Y, Miaskowski C, Meyerhardt JA, Venook AP, Van Blarigan EL. Quality of life of colorectal cancer survivors participating in a pilot randomized controlled trial of physical activity trackers and daily text messages. Support Care Cancer. 2022;30:4557–4564. doi: 10.1007/s00520-022-06870-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheville AL, Kollasch J, Vandenberg J, Shen T, Grothey A, Gamble G, Basford JR. A home-based exercise program to improve function, fatigue, and sleep quality in patients with Stage IV lung and colorectal cancer: a randomized controlled trial. J Pain Symptom Manage. 2013;45:811–821. doi: 10.1016/j.jpainsymman.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho MH, Dodd MJ, Cooper BA, Miaskowski C. Comparisons of exercise dose and symptom severity between exercisers and nonexercisers in women during and after cancer treatment. J Pain Symptom Manage. 2012;43:842–854. doi: 10.1016/j.jpainsymman.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clifford BK, Jones MD, Simar D, Barry BK, Goldstein D. The effect of exercise intensity on exercise-induced hypoalgesia in cancer survivors: a randomized crossover trial Physiol Rep. 2021;9:e15047. doi: 10.14814/phy2.15047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cormie P, Galvao DA, Spry N, Joseph D, Chee R, Taaffe DR, Chambers SK, Newton RU. Can supervised exercise prevent treatment toxicity in patients with prostate cancer initiating androgen-deprivation therapy: a randomised controlled trial. BJU Int. 2015;115:256–266. doi: 10.1111/bju.12646. [DOI] [PubMed] [Google Scholar]
- 36.Cormie P, Galvao DA, Spry N, Newton RU. Neither heavy nor light load resistance exercise acutely exacerbates lymphedema in breast cancer survivor. Integr Cancer Ther. 2013;12:423–432. doi: 10.1177/1534735413477194. [DOI] [PubMed] [Google Scholar]
- 37.Cormie P, Newton RU, Spry N, Joseph D, Taaffe DR, Galvao DA. Safety and efficacy of resistance exercise in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 2013;16:328–335. doi: 10.1038/pcan.2013.22. [DOI] [PubMed] [Google Scholar]
- 38.Cormie P, Pumpa K, Galvao DA, Turner E, Spry N, Saunders C, Zissiadis Y, Newton RU. Is it safe and efficacious for women with lymphedema secondary to breast cancer to lift heavy weights during exercise: a randomised controlled trial. J Cancer Surviv. 2013;7:413–424. doi: 10.1007/s11764-013-0284-8. [DOI] [PubMed] [Google Scholar]
- 39.Cormie P, Singh B, Hayes S, Peake JM, Galvao DA, Taaffe DR, Spry N, Nosaka K, Cornish B, Schmitz KH, Newton RU. Acute inflammatory response to low-, moderate-, and high-load resistance exercise in women with breast cancer-related lymphedema. Integr Cancer Ther. 2016;15:308–317. doi: 10.1177/1534735415617283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Courneya KS, McKenzie DC, Mackey JR, Gelmon K, Friedenreich CM, Yasui Y, Reid RD, Cook D, Jespersen D, Proulx C, Dolan LB, Forbes CC, Wooding E, Trinh L, Segal RJ. Effects of exercise dose and type during breast cancer chemotherapy: multicenter randomized trial. J Natl Cancer Inst. 2013;105:1821–1832. doi: 10.1093/jnci/djt297. [DOI] [PubMed] [Google Scholar]
- 41.Courneya KS, McKenzie DC, Mackey JR, Gelmon K, Friedenreich CM, Yasui Y, Reid RD, Vallerand JR, Adams SC, Proulx C, Dolan LB, Wooding E, Segal RJ. Subgroup effects in a randomised trial of different types and doses of exercise during breast cancer chemotherapy. Br J Cancer. 2014;111:1718–1725. doi: 10.1038/bjc.2014.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cramond F, O'Mara-Eves A, Doran-Constant L, Rice AS, Macleod M, Thomas J. The development and evaluation of an online application to assist in the extraction of data from graphs for use in systematic reviews Wellcome Open Res. 2018;3:157. doi: 10.12688/wellcomeopenres.14738.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deandrea S, Montanari M, Moja L, Apolone G. Prevalence of undertreatment in cancer pain. A Rev Published Lit Ann Oncol. 2008;19:1985–1991. doi: 10.1093/annonc/mdn419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Department of Health CoA (2019) National strategic action plan for pain management. Australian Government Department of Health, Australia, Canberra.
- 45.Dhawan S, Andrews R, Kumar L, Wadhwa S, Shukla G. A randomized controlled trial to assess the effectiveness of muscle strengthening and balancing exercises on chemotherapy-induced peripheral neuropathic pain and quality of life among cancer patients. Cancer Nurs. 2020;43:269–280. doi: 10.1097/NCC.0000000000000693. [DOI] [PubMed] [Google Scholar]
- 46.Dieli-Conwright CM, Courneya KS, Demark-Wahnefried W, Sami N, Lee K, Sweeney FC, Stewart C, Buchanan TA, Spicer D, Tripathy D, Bernstein L, Mortimer JE. Aerobic and resistance exercise improves physical fitness, bone health, and quality of life in overweight and obese breast cancer survivors: a randomized controlled trial. Breast Cancer Res. 2018;20:124. doi: 10.1186/s13058-018-1051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dimeo F, Fetscher S, Lange W, Mertelsmann R, Keul J. Effects of aerobic exercise on the physical performance and incidence of treatment-related complications after high-dose chemotherapy. Blood. 1997;90:3390–3394. doi: 10.1182/blood.V90.9.3390. [DOI] [PubMed] [Google Scholar]
- 48.Ding Y, Ji L, Hu Y. Effects of tai chi on catheter management and quality of life in tumor patients with PICC at the intermission of chemotherapy: a non-inferiority randomized controlled trial Ann. Palliat Med. 2020;9:3293–3303. doi: 10.21037/apm-20-1456. [DOI] [PubMed] [Google Scholar]
- 49.Do J, Cho Y, Jeon J. Effects of a 4-week multimodal rehabilitation program on quality of life, cardiopulmonary function, and fatigue in breast cancer patients. J Breast Cancer. 2015;18:87–96. doi: 10.4048/jbc.2015.18.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dodd MJ, Cho MH, Miaskowski C, Painter PL, Paul SM, Cooper BA, Duda J, Krasnoff J, Bank KA. A randomized controlled trial of home-based exercise for cancer-related fatigue in women during and after chemotherapy with or without radiation therapy. Cancer Nurs. 2010;33:245–257. doi: 10.1097/NCC.0b013e3181ddc58c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dong X, Yi X, Ding M, Gao Z, McDonough DJ, Yi N, Qiao W. A longitudinal study of a multicomponent exercise intervention with remote guidance among breast cancer patients. Int J Environ Res Public Health. 2020;17:14. doi: 10.3390/ijerph17103425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dong X, Yi X, Gao D, Gao Z, Huang S, Chao M, Chen W, Ding M. The effects of the combined exercise intervention based on internet and social media software (CEIBISMS) on quality of life, muscle strength and cardiorespiratory capacity in Chinese postoperative breast cancer patients:a randomized controlled trial. Health Qual Life Outcomes. 2019;17:109. doi: 10.1186/s12955-019-1183-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dore I, Plante A, Peck SS, Bedrossian N, Sabiston CM. Physical activity and sedentary time: associations with fatigue, pain, and depressive symptoms over 4 years post-treatment among breast cancer survivors. Support Care Cancer. 2022;30:785–792. doi: 10.1007/s00520-021-06469-2. [DOI] [PubMed] [Google Scholar]
- 54.Dowell D, Haegerich TM. Chou R (2016) CDC guideline for prescribing opioids for chronic pain–United States. JAMA. 2016;315:1624–1645. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eyigor S, Uslu R, Apaydin S, Caramat I, Yesil H. Can yoga have any effect on shoulder and arm pain and quality of life in patients with breast cancer? A Random Controlled Single-bBind trial Complement Ther Clin Pract. 2018;32:40–45. doi: 10.1016/j.ctcp.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 56.Feng G, Parthipan M, Breunis H, Timilshina N, Soto-Perez-de-Celis E, Mina DS, Emmenegger U, Finelli A, Krzyzanowska MK, Clarke H, Puts M, Alibhai SMH. Daily physical activity monitoring in older adults with metastatic prostate cancer on active treatment: feasibility and associations with toxicity J Geriatr. Oncol. 2023;14:101576. doi: 10.1016/j.jgo.2023.101576. [DOI] [PubMed] [Google Scholar]
- 57.Fields J, Richardson A, Hopkinson J, Fenlon D. Nordic walking as an exercise intervention to reduce pain in women with aromatase inhibitor-associated arthralgia: a feasibility study. J Pain Symptom Manage. 2016;52:548–559. doi: 10.1016/j.jpainsymman.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 58.Flowers KM, Beck M, Colebaugh C, Haroutounian S, Edwards RR, Schreiber KL. Pain, numbness, or both? Distinguishing the longitudinal course and predictors of positive, painful neuropathic features vs numbness after breast cancer surgery Pain Rep. 2021;6:e976. doi: 10.1097/PR9.0000000000000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Foley KM. How well is cancer pain treated? Palliat Med. 2011;25:398–401. doi: 10.1177/0269216311400480. [DOI] [PubMed] [Google Scholar]
- 60.Forsythe LP, Alfano CM, George SM, McTiernan A, Baumgartner KB, Bernstein L, Ballard-Barbash R. Pain in long-term breast cancer survivors: the role of body mass index, physical activity, and sedentary behavior. Breast Cancer Res Treat. 2013;137:617–630. doi: 10.1007/s10549-012-2335-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Galvao DA, Spry N, Denham J, Taaffe DR, Cormie P, Joseph D, Lamb DS, Chambers SK, Newton RU. A multicentre year-long randomised controlled trial of exercise training targeting physical functioning in men with prostate cancer previously treated with androgen suppression and radiation from TROG 03.04 RADAR. Eur Urol. 2014;65:856–864. doi: 10.1016/j.eururo.2013.09.041. [DOI] [PubMed] [Google Scholar]
- 62.Galvao DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol. 2010;28:340–347. doi: 10.1200/JCO.2009.23.2488. [DOI] [PubMed] [Google Scholar]
- 63.Garcia-Soidan JL, Perez-Ribao I, Leiros-Rodriguez R, Soto-Rodriguez A. Long-term influence of the practice of physical activity on the self-perceived quality of life of women with breast cancer: a randomized controlled trial. Int J Environ Res Public Health. 2020;17:10. doi: 10.3390/ijerph17144986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Geva N, Defrin R. Enhanced pain modulation among triathletes: a possible explanation for their exceptional capabilities. Pain. 2013;154:2317–2323. doi: 10.1016/j.pain.2013.06.031. [DOI] [PubMed] [Google Scholar]
- 65.Griffith K, Wenzel J, Shang J, Thompson C, Stewart K, Mock V. Impact of a walking intervention on cardiorespiratory fitness, self-reported physical function, and pain in patients undergoing treatment for solid tumors. Cancer. 2009;115:4874–4884. doi: 10.1002/cncr.24551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schunemann HJ. What is “quality of evidence” and why is it important to clinicians? BMJ. 2008;336:995–998. doi: 10.1136/bmj.39490.551019.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hacker ED, Collins E, Park C, Peters T, Patel P, Rondelli D. Strength training to enhance early recovery after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2017;23:659–669. doi: 10.1016/j.bbmt.2016.12.637. [DOI] [PubMed] [Google Scholar]
- 68.Haines TP, Sinnamon P, Wetzig NG, Lehman M, Walpole E, Pratt T, Smith A. Multimodal exercise improves quality of life of women being treated for breast cancer, but at what cost? Randomized trial with Econ Eval Breast Cancer Res Treat. 2010;124:163–175. doi: 10.1007/s10549-010-1126-2. [DOI] [PubMed] [Google Scholar]
- 69.Hammer MJ, Eckardt P, Cartwright F, Miaskowski C. Prescribed walking for glycemic control and symptom management in patients without diabetes undergoing chemotherapy. Nurs Res. 2021;70:6–14. doi: 10.1097/NNR.0000000000000468. [DOI] [PubMed] [Google Scholar]
- 70.Hayes SC, Rye S, Disipio T, Yates P, Bashford J, Pyke C, Saunders C, Battistutta D, Eakin E. Exercise for health: a randomized, controlled trial evaluating the impact of a pragmatic, translational exercise intervention on the quality of life, function and treatment-related side effects following breast cancer. Breast Cancer Res Treat. 2013;137:175–186. doi: 10.1007/s10549-012-2331-y. [DOI] [PubMed] [Google Scholar]
- 71.Heiman J, Onerup A, Bock D, Haglind E, OlofssonBagge R. The effect of nonsupervised physical activity before and after breast cancer surgery on quality of life: results from a randomized controlled trial (PhysSURG-B) Scand J Surg. 2022;111:75–82. doi: 10.1177/14574969221123389. [DOI] [PubMed] [Google Scholar]
- 72.Henke CC, Cabri J, Fricke L, Pankow W, Kandilakis G, Feyer PC, de Wit M. Strength and endurance training in the treatment of lung cancer patients in stages IIIA/IIIB/IV. Support Care Cancer. 2014;22:95–101. doi: 10.1007/s00520-013-1925-1. [DOI] [PubMed] [Google Scholar]
- 73.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods G, Cochrane Statistical Methods G The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ho RT, Fong TC, Cheung IK, Yip PS, Luk MY. Effects of a short-term dance movement therapy program on symptoms and stress in patients with breast cancer undergoing radiotherapy: a randomized, controlled, single-blind trial. J Pain Symptom Manage. 2016;51:824–831. doi: 10.1016/j.jpainsymman.2015.12.332. [DOI] [PubMed] [Google Scholar]
- 76.Ho RTH, Fong TCT, Yip PSF. Perceived stress moderates the effects of a randomized trial of dance movement therapy on diurnal cortisol slopes in breast cancer patients. Psychoneuroendocrinology. 2018;87:119–126. doi: 10.1016/j.psyneuen.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 77.Hwang JH, Chang HJ, Shim YH, Park WH, Park W, Huh SJ, Yang JH. Effects of supervised exercise therapy in patients receiving radiotherapy for breast cancer. Yonsei Med J. 2008;49:443–450. doi: 10.3349/ymj.2008.49.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ibrahim M, Muanza T, Smirnow N, Sateren W, Fournier B, Kavan P, Palumbo M, Dalfen R, Dalzell MA. The long-term effects of posttreatment exercise on pain in young women with breast cancer Journal of Community and Supportive. Oncology. 2018;16:e145–e151. [Google Scholar]
- 79.Irwin ML, Cartmel B, Gross CP, Ercolano E, Li F, Yao X, Fiellin M, Capozza S, Rothbard M, Zhou Y, Harrigan M, Sanft T, Schmitz K, Neogi T, Hershman D, Ligibel J. Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors. J Clin Oncol. 2015;33:1104–1111. doi: 10.1200/JCO.2014.57.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jensen W, Baumann FT, Stein A, Bloch W, Bokemeyer C, de Wit M, Oechsle K. Exercise training in patients with advanced gastrointestinal cancer undergoing palliative chemotherapy: a pilot study. Support Care Cancer. 2014;22:1797–1806. doi: 10.1007/s00520-014-2139-x. [DOI] [PubMed] [Google Scholar]
- 81.Kang JJ, Lee H, Park BH, Song YK, Park SE, Kim R, Lee KA (2022) Efficacy of a 4-week nurse-led exercise rehabilitation program in improving the quality of life in women receiving a post-mastectomy reconstruction using the Motiva ErgonomixTM Round SilkSurface Int J Environ Res Public Health 20(1):16 [DOI] [PMC free article] [PubMed]
- 82.Klein I, Kalichman L, Chen N, Susmallian S. Effect of physical activity levels on oncological breast surgery recovery: a prospective cohort study Sci Rep. 2021;11:10432. doi: 10.1038/s41598-021-89908-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koevoets EW, Schagen SB, de Ruiter MB, Geerlings MI, Witlox L, van der Wall E, Stuiver MM, Sonke GS, Velthuis MJ, Jobsen JJ, Menke-Pluijmers MBE, Goker E, van der Pol CC, Bos M, Tick LW, van Holsteijn NA, van der Palen J, May AM, Monninkhof EM, group PAMs, Effect of physical exercise on cognitive function after chemotherapy in patients with breast cancer: a randomized controlled trial (PAM study) Breast Cancer Res. 2022;24:36. doi: 10.1186/s13058-022-01530-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ligibel JA, Giobbie-Hurder A, Shockro L, Campbell N, Partridge AH, Tolaney SM, Lin NU, Winer EP. Randomized trial of a physical activity intervention in women with metastatic breast cancer. Cancer. 2016;122:1169–1177. doi: 10.1002/cncr.29899. [DOI] [PubMed] [Google Scholar]
- 85.Lima LV, Abner TSS, Sluka KA. Does exercise increase or decrease pain? Central Mechan Underlying these two Phenomena J Physiol. 2017;595:4141–4150. doi: 10.1113/JP273355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lin KY, Cheng HC, Yen CJ, Hung CH, Huang YT, Yang HL, Cheng WT, Tsai KL. Effects of exercise in patients undergoing chemotherapy for head and neck cancer: a pilot randomized controlled trial. Int J Environ Res Public Health. 2021;18:01. doi: 10.3390/ijerph18031291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin Y, Wu C, He C, Yan J, Chen Y, Gao L, Liu R, Cao B. Effectiveness of three exercise programs and intensive follow-up in improving quality of life, pain, and lymphedema among breast cancer survivors: a randomized, controlled 6-month trial. Support Care Cancer. 2022;31:9. doi: 10.1007/s00520-022-07494-5. [DOI] [PubMed] [Google Scholar]
- 88.Litterini AJ, Fieler VK, Cavanaugh JT, Lee JQ. Differential effects of cardiovascular and resistance exercise on functional mobility in individuals with advanced cancer: a randomized trial. Arch Phys Med Rehabil. 2013;94:2329–2335. doi: 10.1016/j.apmr.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 89.Lovell M, Luckett T, Boyle F, Stubbs J, Phillips J, Davidson PM, Olver I, von Dincklage J, Agar M. Adaptation of international guidelines on assessment and management of cancer pain for the Australian context Asia Pac. J Clin Oncol. 2015;11:170–177. doi: 10.1111/ajco.12352. [DOI] [PubMed] [Google Scholar]
- 90.Luckett T, Davidson PM, Green A, Boyle F, Stubbs J, Lovell M. Assessment and management of adult cancer pain: a systematic review and synthesis of recent qualitative studies aimed at developing insights for managing barriers and optimizing facilitators within a comprehensive framework of patient care. J Pain Symptom Manage. 2013;46:229–253. doi: 10.1016/j.jpainsymman.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 91.Machado P, Pimenta S, Garcia AL, Nogueira T, Silva S, Dos Santos CL, Martins MV, Canha A, Oliveiros B, Martins RA, Cruz J. Effect of preoperative home-based exercise training on quality of life after lung cancer surgery: a multicenter randomized controlled trial. Ann Surg Oncol. 2024;31:847–859. doi: 10.1245/s10434-023-14503-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mardani A, Pedram Razi S, Mazaheri R, Haghani S, Vaismoradi M. Effect of the exercise programme on the quality of life of prostate cancer survivors: a randomized controlled trial. Int J Nurs Pract. 2021;27:e12883. doi: 10.1111/ijn.12883. [DOI] [PubMed] [Google Scholar]
- 93.Mendizabal-Gallastegui N, Arietaleanizbeaskoa MS, Latorre PM, Garcia-Alvarez A, Sancho A, Iruarrizaga E, Lopez-Vivanco G, Grandes G. Nurse-supervised exercise for people with stage IV cancer: the EFICANCER randomized clinical trial. Semin Oncol Nurs. 2023;39:151448. doi: 10.1016/j.soncn.2023.151448. [DOI] [PubMed] [Google Scholar]
- 94.Messaggi-Sartor M, Marco E, Martinez-Tellez E, Rodriguez-Fuster A, Palomares C, Chiarella S, Muniesa JM, Orozco-Levi M, Barreiro E, Guell MR. Combined aerobic exercise and high-intensity respiratory muscle training in patients surgically treated for non-small cell lung cancer: a pilot randomized clinical trial. Eur J Phys Rehabil Med. 2019;55:113–122. doi: 10.23736/S1973-9087.18.05156-0. [DOI] [PubMed] [Google Scholar]
- 95.Mijwel S, Backman M, Bolam KA, Jervaeus A, Sundberg CJ, Margolin S, Browall M, Rundqvist H, Wengstrom Y. Adding high-intensity interval training to conventional training modalities: optimizing health-related outcomes during chemotherapy for breast cancer: the OptiTrain randomized controlled trial. Breast Cancer Res Treat. 2018;168:79–93. doi: 10.1007/s10549-017-4571-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mijwel S, Backman M, Bolam KA, Olofsson E, Norrbom J, Bergh J, Sundberg CJ, Wengstrom Y, Rundqvist H. Highly favorable physiological responses to concurrent resistance and high-intensity interval training during chemotherapy: the OptiTrain breast cancer trial. Breast Cancer Res Treat. 2018;169:93–103. doi: 10.1007/s10549-018-4663-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mijwel S, Jervaeus A, Bolam KA, Norrbom J, Bergh J, Rundqvist H, Wengstrom Y. High-intensity exercise during chemotherapy induces beneficial effects 12 months into breast cancer survivorship. J Cancer Surviv. 2019;13:244–256. doi: 10.1007/s11764-019-00747-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moon C, Gallegos AM, Sheikh B, Kumar P, Liss M, Patel DI. Pilot study on the impact of a home-based exercise program on inflammatory cytokines and quality of life in men with prostate cancer under active surveillance. Cancer Control. 2022;29:10732748221130964. doi: 10.1177/10732748221130964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moraes RF, Ferreira-Junior JB, Marques VA, Vieira A, Lira CAB, Campos MH, Freitas-Junior R, Rahal RMS, Gentil P, Vieira CA. Resistance training, fatigue, quality of life, anxiety in breast cancer survivors. J Strength Cond Res. 2021;35:1350–1356. doi: 10.1519/JSC.0000000000003817. [DOI] [PubMed] [Google Scholar]
- 100.Morgan RL, Thayer KA, Santesso N, Holloway AC, Blain R, Eftim SE, Goldstone AE, Ross P, Ansari M, Akl EA, Filippini T, Hansell A, Meerpohl JJ, Mustafa RA, Verbeek J, Vinceti M, Whaley P, Schunemann HJ, Group GW A risk of bias instrument for non-randomized studies of exposures: a users’ guide to its application in the context of GRADE. Environ Int. 2019;122:168–184. doi: 10.1016/j.envint.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morielli AR, Boule NG, Usmani N, Tankel K, Joseph K, Severin D, Fairchild A, Nijjar T, Courneya KS. Effects of exercise during and after neoadjuvant chemoradiation on symptom burden and quality of life in rectal cancer patients: a phase II randomized controlled trial. J Cancer Surviv. 2023;17:1171–1183. doi: 10.1007/s11764-021-01149-w. [DOI] [PubMed] [Google Scholar]
- 102.Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat. 2012;134:459–478. doi: 10.1007/s10549-012-2114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nilsen TS, Raastad T, Skovlund E, Courneya KS, Langberg CW, Lilleby W, Fossa SD, Thorsen L. Effects of strength training on body composition, physical functioning, and quality of life in prostate cancer patients during androgen deprivation therapy. Acta Oncol. 2015;54:1805–1813. doi: 10.3109/0284186X.2015.1037008. [DOI] [PubMed] [Google Scholar]
- 104.Norris MK, Bell GJ, North S, Courneya KS. Effects of resistance training frequency on physical functioning and quality of life in prostate cancer survivors: a pilot randomized controlled trial. Prostate Cancer Prostatic Dis. 2015;18:281–287. doi: 10.1038/pcan.2015.28. [DOI] [PubMed] [Google Scholar]
- 105.Nyrop KA, Callahan LF, Cleveland RJ, Arbeeva LL, Hackney BS, Muss HB. Randomized controlled trial of a home-based walking program to reduce moderate to severe aromatase inhibitor-associated arthralgia in breast cancer survivors. Oncologist. 2017;22:1238–1249. doi: 10.1634/theoncologist.2017-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Odynets T, Briskin Y, Sydorko O, Tyshchenko V, Putrov S. Effectiveness of individualized physical rehabilitation programs on post-mastectomy pain in breast cancer survivors Physiotherapy Quarterly. 2018;26:1–5. [Google Scholar]
- 107.Owen PJ, Miller CT, Mundell NL, Verswijveren S, Tagliaferri SD, Brisby H, Bowe SJ, Belavy DL. Which specific modes of exercise training are most effective for treating low back pain? Network meta-analysis Br J Sports Med. 2020;54:1279–1287. doi: 10.1136/bjsports-2019-100886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hrobjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Park JH, Lee J, Oh M, Park H, Chae J, Kim DI, Lee MK, Yoon YJ, Lee CW, Park S, Jones LW, Kim NK, Kim SI, Jeon JY. The effect of oncologists’ exercise recommendations on the level of exercise and quality of life in survivors of breast and colorectal cancer: a randomized controlled trial. Cancer. 2015;121:2740–2748. doi: 10.1002/cncr.29400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pasyar N, Barshan Tashnizi N, Mansouri P, Tahmasebi S. Effect of yoga exercise on the quality of life and upper extremity volume among women with breast cancer related lymphedema: a pilot study. Eur J Oncol Nurs. 2019;42:103–109. doi: 10.1016/j.ejon.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 111.Patel KV, Amtmann D, Jensen MP, Smith SM, Veasley C, Turk DC. Clinical outcome assessment in clinical trials of chronic pain treatments Pain Rep. 2021;6:e784. doi: 10.1097/PR9.0000000000000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Paulo TRS, Rossi FE, Viezel J, Tosello GT, Seidinger SC, Simoes RR, de Freitas R, Jr., Freitas IF, Jr. The impact of an exercise program on quality of life in older breast cancer survivors undergoing aromatase inhibitor therapy: a randomized controlled trial. Health Qual Life Outcomes. 2019;17:17. doi: 10.1186/s12955-019-1090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pelzer F, Leisge K, Schluter K, Schneider J, Wiskemann J, Rosenberger F. Effects of exercise mode and intensity on patient-reported outcomes in cancer survivors: a four-arm intervention trial. Support Care Cancer. 2023;31:315. doi: 10.1007/s00520-023-07757-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Peppone LJ, Janelsins MC, Kamen C, Mohile SG, Sprod LK, Gewandter JS, Kirshner JJ, Gaur R, Ruzich J, Esparaz BT, Mustian KM (2015) The effect of YOCAS©<sup></sup> yoga for musculoskeletal symptoms among breast cancer survivors on hormonal therapy Breast Cancer Research and Treatment 150:597–604 [DOI] [PMC free article] [PubMed]
- 115.Phillips SM, Welch WA, Fanning J, Santa-Maria CA, Gavin KL, Auster-Gussman LA, Solk P, Lu M, Cullather E, Khan SA, Kulkarni SA, Gradishar W, Siddique J. Daily physical activity and symptom reporting in breast cancer patients undergoing chemotherapy: an intensive longitudinal examination. Cancer Epidemiol Biomarkers Prev. 2020;29:2608–2616. doi: 10.1158/1055-9965.EPI-20-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pisu M, Demark-Wahnefried W, Kenzik KM, Oster RA, Lin CP, Manne S, Alvarez R, Martin MY. A dance intervention for cancer survivors and their partners (RHYTHM) J Cancer Surviv. 2017;11:350–359. doi: 10.1007/s11764-016-0593-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pitcher MH, Tarum F, Rauf IZ, Low LA, Bushnell C. Modest amounts of voluntary exercise reduce pain- and stress-related outcomes in a rat model of persistent hind limb inflammation. J Pain. 2017;18:687–701. doi: 10.1016/j.jpain.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pope ZC, Zeng N, Zhang R, Lee HY, Gao Z (2018) Effectiveness of combined smartwatch and social media intervention on breast cancer survivor health outcomes: a 10-week pilot randomized trial J Clin Med 7 [DOI] [PMC free article] [PubMed]
- 119.Porserud A, Sherif A, Tollback A. The effects of a physical exercise programme after radical cystectomy for urinary bladder cancer. A Pilot Randomized Controlled Trial Clin Rehabil. 2014;28:451–459. doi: 10.1177/0269215513506230. [DOI] [PubMed] [Google Scholar]
- 120.Rasmussen GHF, Kristiansen M, Arroyo-Morales M, Voigt M, Madeleine P. The analgesic effect of resistance training after breast cancer (ANTRAC): a randomized controlled trial. Med Sci Sports Exerc. 2023;55:167–176. doi: 10.1249/MSS.0000000000003034. [DOI] [PubMed] [Google Scholar]
- 121.Rastogi S, Tevaarwerk AJ, Sesto M, Van Remortel B, Date P, Gangnon R, Thraen-Borowski K, Cadmus-Bertram L. Effect of a technology-supported physical activity intervention on health-related quality of life, sleep, and processes of behavior change in cancer survivors: a randomized controlled trial. Psychooncology. 2020;29:1917–1926. doi: 10.1002/pon.5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Reis AD, Pereira P, Diniz RR, de Castro Filha JGL, Dos Santos AM, Ramallo BT, Filho FAA, Navarro F, Garcia JBS. Effect of exercise on pain and functional capacity in breast cancer patients. Health Qual Life Outcomes. 2018;16:58. doi: 10.1186/s12955-018-0882-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rice D, Nijs J, Kosek E, Wideman T, Hasenbring MI, Koltyn K, Graven-Nielsen T, Polli A. Exercise-induced hypoalgesia in pain-free and chronic pain populations: state of the art and future directions. J Pain. 2019;20:1249–1266. doi: 10.1016/j.jpain.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 124.Rief H, Omlor G, Akbar M, Welzel T, Bruckner T, Rieken S, Haefner MF, Schlampp I, Gioules A, Habermehl D, von Nettelbladt F, Debus J. Feasibility of isometric spinal muscle training in patients with bone metastases under radiation therapy - first results of a randomized pilot trial. BMC Cancer. 2014;14:67. doi: 10.1186/1471-2407-14-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rogers LQ, Courneya KS, Carter SJ, Anton PM, Verhulst S, Vicari SK, Robbs RS, McAuley E. Effects of a multicomponent physical activity behavior change intervention on breast cancer survivor health status outcomes in a randomized controlled trial. Breast Cancer Res Treat. 2016;159:283–291. doi: 10.1007/s10549-016-3945-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rogers LQ, Courneya KS, Oster RA, Anton PM, Phillips S, Ehlers DK, McAuley E. Physical activity intervention benefits persist months post-intervention: randomized trial in breast cancer survivors. J Cancer Surviv. 2023;17:1834–1846. doi: 10.1007/s11764-022-01329-2. [DOI] [PubMed] [Google Scholar]
- 127.Rogers LQ, Hopkins-Price P, Vicari S, Pamenter R, Courneya KS, Markwell S, Verhulst S, Hoelzer K, Naritoku C, Jones L, Dunnington G, Lanzotti V, Wynstra J, Shah L, Edson B, Graff A, Lowy M. A randomized trial to increase physical activity in breast cancer survivors. Med Sci Sports Exerc. 2009;41:935–946. doi: 10.1249/MSS.0b013e31818e0e1b. [DOI] [PubMed] [Google Scholar]
- 128.Sande TA, Scott AC, Laird BJ, Wan HI, Fleetwood-Walker SM, Kaasa S, Klepstad P, Mitchell R, Murray GD, Colvin LA, Fallon MT. The characteristics of physical activity and gait in patients receiving radiotherapy in cancer induced bone pain. Radiother Oncol. 2014;111:18–24. doi: 10.1016/j.radonc.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 129.Schmidt T, Weisser B, Durkop J, Jonat W, Van Mackelenbergh M, Rocken C, Mundhenke C. Comparing endurance and resistance training with standard care during chemotherapy for patients with primary breast cancer. Anticancer Res. 2015;35:5623–5629. [PubMed] [Google Scholar]
- 130.Schmitt J, Lindner N, Reuss-Borst M, Holmberg HC, Sperlich B (2016) A 3-week multimodal intervention involving high-intensity interval training in female cancer survivors: a randomized controlled trial Physiol Rep 4:e12693 [DOI] [PMC free article] [PubMed]
- 131.Schmitz KH, Campbell AM, Stuiver MM, Pinto BM, Schwartz AL, Morris GS, Ligibel JA, Cheville A, Galvao DA, Alfano CM, Patel AV, Hue T, Gerber LH, Sallis R, Gusani NJ, Stout NL, Chan L, Flowers F, Doyle C, Helmrich S, Bain W, Sokolof J, Winters-Stone KM, Campbell KL, Matthews CE. Exercise is medicine in oncology: engaging clinicians to help patients move through cancer. CA Cancer J Clin. 2019;69:468–484. doi: 10.3322/caac.21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schumacher O, Galvao DA, Taaffe DR, Spry N, Joseph D, Tang C, Chee R, Newton RU. Effect of exercise adjunct to radiation and androgen deprivation therapy on patient-reported treatment toxicity in men with prostate cancer: a secondary analysis of 2 randomized controlled trials. Pract Radiat Oncol. 2021;11:215–225. doi: 10.1016/j.prro.2021.01.005. [DOI] [PubMed] [Google Scholar]
- 133.Shobeiri F, Masoumi SZ, Nikravesh A, Heidari Moghadam R, Karami M. The impact of aerobic exercise on quality of life in women with breast cancer: a randomized controlled trial J Res Health Sci. 2016;16:127–132. [PMC free article] [PubMed] [Google Scholar]
- 134.Sluka KA, O'Donnell JM, Danielson J, Rasmussen LA (2013) Regular physical activity prevents development of chronic pain and activation of central neurons J Appl Physiol (1985) 114: 725–733 [DOI] [PMC free article] [PubMed]
- 135.Solberg Nes L, Liu H, Patten CA, Rausch SM, Sloan JA, Garces YI, Cheville AL, Yang P, Clark MM. Physical activity level and quality of life in long term lung cancer survivors. Lung Cancer. 2012;77:611–616. doi: 10.1016/j.lungcan.2012.05.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Steffens D, Solomon MJ, Beckenkamp PR, Koh CE, Yeo D, Sandroussi C, Fit-4-Home C, Hancock MJ (2022) Individualised, targeted step count intervention following gastrointestinal cancer surgery: the Fit-4-Home randomised clinical trial ANZ J Surg 92: 703-711 [DOI] [PubMed]
- 137.Steindorf K, Clauss D, Tjaden C, Hackert T, Herbolsheimer F, Bruckner T, Schneider L, Ulrich CM, Wiskemann J. Quality of life, fatigue, and sleep problems in pancreatic cancer patients-a randomized trial on the effects of exercise. Dtsch Arztebl Int. 2019;116:471–478. doi: 10.3238/arztebl.2019.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stigt JA, Uil SM, van Riesen SJ, Simons FJ, Denekamp M, Shahin GM, Groen HJ. A randomized controlled trial of postthoracotomy pulmonary rehabilitation in patients with resectable lung cancer. J Thorac Oncol. 2013;8:214–221. doi: 10.1097/JTO.0b013e318279d52a. [DOI] [PubMed] [Google Scholar]
- 139.Sturgeon KM, Smith AM, Federici EH, Kodali N, Kessler R, Wyluda E, Cream LV, Ky B, Schmitz KH. Feasibility of a tailored home-based exercise intervention during neoadjuvant chemotherapy in breast cancer patients BMC Sports Sci Med Rehabil. 2022;14:31. doi: 10.1186/s13102-022-00420-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Su TL, Chen AN, Leong CP, Huang YC, Chiang CW, Chen IH, Lee YY. The effect of home-based program and outpatient physical therapy in patients with head and neck cancer: a randomized, controlled trial. Oral Oncol. 2017;74:130–134. doi: 10.1016/j.oraloncology.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 141.Swain CTV, Bassett JK, Hodge AM, Bruinsma FJ, Mahmood S, Jayasekara H, Macinnis RJ, Giles GG, Milne RL, English DR, Lynch BM. Domain-specific physical activity, pain interference, and muscle pain after activity. Med Sci Sports Exerc. 2020;52:2145–2151. doi: 10.1249/MSS.0000000000002358. [DOI] [PubMed] [Google Scholar]
- 142.Thomas VJ, Seet-Lee C, Marthick M, Cheema BS, Boyer M, Edwards KM. Aerobic exercise during chemotherapy infusion for cancer treatment: a novel randomised crossover safety and feasibility trial. Support Care Cancer. 2020;28:625–632. doi: 10.1007/s00520-019-04871-5. [DOI] [PubMed] [Google Scholar]
- 143.Travier N, Velthuis MJ, Steins Bisschop CN, van den Buijs B, Monninkhof EM, Backx F, Los M, Erdkamp F, Bloemendal HJ, Rodenhuis C, de Roos MA, Verhaar M, ten Bokkel Huinink D, van der Wall E, Peeters PH, May AM. Effects of an 18-week exercise programme started early during breast cancer treatment: a randomised controlled trial. BMC Med. 2015;13:121. doi: 10.1186/s12916-015-0362-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tuomi L, Magnusson-Sandkvist J, Fridolfsson J, Arvidsson D, Borjesson M, Finizia C. A pilot study using pre-treatment physical activity level to predict long-term health-related quality of life in patients with head and neck cancer. Head Neck. 2023;45:1288–1298. doi: 10.1002/hed.27345. [DOI] [PubMed] [Google Scholar]
- 145.Vallerand JR, Rhodes RE, Walker GJ, Courneya KS. Feasibility and preliminary efficacy of an exercise telephone counseling intervention for hematologic cancer survivors: a phase II randomized controlled trial. J Cancer Surviv. 2018;12:357–370. doi: 10.1007/s11764-018-0675-y. [DOI] [PubMed] [Google Scholar]
- 146.Van Blarigan EL, Kenfield SA, Olshen A, Panchal N, Encabo K, Tenggara I, Graff RE, Bang AS, Shinohara K, Cooperberg MR, Carroll PR, Jones LW, Winters-Stone K, Luke A, Chan JM (2023) Effect of a home-based walking intervention on cardiopulmonary fitness and quality of life among men with prostate cancer on active surveillance: the active surveillance exercise randomized controlled trial Eur Urol Oncol 23:00228–6 [DOI] [PubMed]
- 147.van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18:1437–1449. doi: 10.1093/annonc/mdm056. [DOI] [PubMed] [Google Scholar]
- 148.van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage. 2016;51(1070–1090):e1079. doi: 10.1016/j.jpainsymman.2015.12.340. [DOI] [PubMed] [Google Scholar]
- 149.van Vulpen JK, Hiensch AE, van Hillegersberg R, Ruurda JP, Backx FJG, Nieuwenhuijzen GAP, Kouwenhoven EA, Groenendijk RPR, van der Peet DL, Hazebroek EJ, Rosman C, Wijnhoven BPL, van Berge Henegouwen MI, van Laarhoven HWM, Siersema PD, May AM. Supervised exercise after oesophageal cancer surgery: the PERFECT multicentre randomized clinical trial. Br J Surg. 2021;108:786–796. doi: 10.1093/bjs/znab078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Van Vulpen JK, Velthuis MJ, Steins Bisschop CN, Travier N, Van Den Buijs BJ, Backx FJ, Los M, Erdkamp FL, Bloemendal HJ, Koopman M, De Roos MA, Verhaar MJ, Ten Bokkel-Huinink D, Van Der Wall E, Peeters PH, May AM. Effects of an exercise program in colon cancer patients undergoing chemotherapy. Med Sci Sports Exerc. 2016;48:767–775. doi: 10.1249/MSS.0000000000000855. [DOI] [PubMed] [Google Scholar]
- 151.van Waart H, Stuiver MM, van Harten WH, Geleijn E, Kieffer JM, Buffart LM, de Maaker-Berkhof M, Boven E, Schrama J, Geenen MM, MeerumTerwogt JM, van Bochove A, Lustig V, van den Heiligenberg SM, Smorenburg CH, Hellendoorn-van Vreeswijk JA, Sonke GS, Aaronson NK. Effect of low-intensity physical activity and moderate- to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. J Clin Oncol. 2015;33:1918–1927. doi: 10.1200/JCO.2014.59.1081. [DOI] [PubMed] [Google Scholar]
- 152.Vanderbyl BL, Mayer MJ, Nash C, Tran AT, Windholz T, Swanson T, Kasymjanova G, Jagoe RT. A comparison of the effects of medical Qigong and standard exercise therapy on symptoms and quality of life in patients with advanced cancer. Support Care Cancer. 2017;25:1749–1758. doi: 10.1007/s00520-017-3579-x. [DOI] [PubMed] [Google Scholar]
- 153.Vardar Yagli N, Sener G, Arikan H, Saglam M, Inal Ince D, Savci S, Calik Kutukcu E, Altundag K, Kaya EB, Kutluk T, Ozisik Y. Do yoga and aerobic exercise training have impact on functional capacity, fatigue, peripheral muscle strength, and quality of life in breast cancer survivors? Integr Cancer Ther. 2015;14:125–132. doi: 10.1177/1534735414565699. [DOI] [PubMed] [Google Scholar]
- 154.Weyhe D, Obonyo D, Uslar V, Tabriz N. Effects of intensive physiotherapy on quality of life (QoL) after pancreatic cancer resection: a randomized controlled trial. BMC Cancer. 2022;22:520. doi: 10.1186/s12885-022-09586-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yang LH, Duan PB, Hou QM, Wang XQ. Qigong exercise for patients with gastrointestinal cancer undergoing chemotherapy and at high risk for depression: a randomized clinical trial. J Altern Complement Med. 2021;27:750–759. doi: 10.1089/acm.2020.0531. [DOI] [PubMed] [Google Scholar]
- 156.Zengin Alpozgen A, Razak Ozdincler A, Karanlik H, Yaman Agaoglu F, Narin AN (2017) Effectiveness of Pilates-based exercises on upper extremity disorders related with breast cancer treatment Eur J Cancer Care (Engl) 26:1–8 [DOI] [PubMed]
- 157.Zhou Y, Cartmel B, Gottlieb L, Ercolano EA, Li F, Harrigan M, McCorkle R, Ligibel JA, von Gruenigen VE, Gogoi R, Schwartz PE, Risch HA, Irwin ML (2017) Randomized trial of exercise on quality of life in women with ovarian cancer: women’s activity and lifestyle study in Connecticut (WALC) J Natl Cancer Inst 109:01 [DOI] [PMC free article] [PubMed]
- 158.Zhuang J, Liu Y, Xu X, Cai Y, Liu M, Chen Z, Yang S, Lin J, Hu Z, Kang M, Lin M, He F. Association between physical activity and health-related quality of life: time to deterioration model analysis in lung adenocarcinoma. J Cancer Surviv. 2023;17:1769–1779. doi: 10.1007/s11764-022-01259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request.