Abstract
Objectives
This study aimed to determine the prevalence and factors associated with maternal and neonatal sepsis in sub-Saharan Africa.
Methods
This systematic review and meta-analysis used the PRISMA guideline on sepsis data in sub-Saharan Africa. The bibliographic search was carried out on the following databases: Medline/PubMed, Cochrane Library, African Index Medicus, and Google Scholar. Additionally, the reference lists of the included studies were screened for potentially relevant studies. The last search was conducted on 15 October 2022. The Joanna Briggs Institute quality assessment checklist was applied for critical appraisal. Estimates of the prevalence of maternal and neonatal sepsis were pooled using a random-effects meta-analysis model. Heterogeneity between studies was estimated using the Q statistic and the I2 statistic. The funnel plot and Egger’s regression test were used to assess the publication bias.
Results
A total of 39 studies were included in our review: 32 studies on neonatal sepsis and 7 studies on maternal sepsis. The overall pooled prevalence of maternal and neonatal sepsis in Sub-Saharan Africa was 19.21% (95% CI, 11.46–26.97) and 36.02% (CI: 26.68–45.36), respectively. The meta-analyses revealed that Apgar score < 7 (OR: 2.4, 95% CI: 1.6–3.5), meconium in the amniotic fluid (OR: 2.9, 95% CI: 1.8–4.5), prolonged rupture of membranes >12 h (OR: 2.8, 95% CI: 1.9–4.1), male sex (OR: 1.2, 95% CI: 1.1–1.4), intrapartum fever (OR: 2.4, 95% CI: 1.5–3.7), and history of urinary tract infection in the mother (OR: 2.7, 95% CI: 1.4–5.2) are factors associated with neonatal sepsis. Rural residence (OR: 2.3, 95% CI: 1.01–10.9), parity (OR: 0.5, 95% CI: 0.3–0.7), prolonged labor (OR: 3.4, 95% CI: 1.6–6.9), and multiple digital vaginal examinations (OR: 4.4, 95% CI: 1.3–14.3) were significantly associated with maternal sepsis.
Conclusion
The prevalence of maternal and neonatal sepsis was high in sub-Saharan Africa. Multiple factors associated with neonatal and maternal sepsis were identified. These factors could help in the prevention and development of strategies to combat maternal and neonatal sepsis. Given the high risk of bias and high heterogeneity, further high-quality research is needed in the sub-Saharan African context, including a meta-analysis of individual data.
Systematic review registration: PROSPERO (ID: CRD42022382050).
Keywords: maternal sepsis, neonatal sepsis, prevalence, associated factors, sub-Saharan Africa
Introduction
Maternal and neonatal sepsis is a generalized inflammatory response with systemic manifestations caused by one or more infectious agents (1), which occurs during pregnancy, childbirth, after abortion, or during the postpartum period (42 days) in women or in the first 28 days of life in newborns (2–4). Sepsis is a major cause of maternal and neonatal morbidity and mortality (2, 3, 5–9). It is the third leading cause of death in women and accounts for a quarter of neonatal deaths (1, 10–14). It is an obstacle to achieving the third Sustainable Development Goal (SDG), which aims to reduce maternal and neonatal mortality and morbidity (15). Low- and middle-income countries (LICs) are particularly affected by maternal and neonatal sepsis (11, 16, 17). In sub-Saharan Africa (SSA), it is estimated to be responsible for 130,000 maternal deaths and 300,000 neonatal deaths per year, although this may be an underestimation (2, 18). These deaths reflect a number of challenges, including policy, poverty, health inequalities, and the health system (19). However, few data are available on the prevalence and factors associated with sepsis across the continuum from pregnancy to postpartum or post-abortion, making it difficult to make a real estimation of maternal and neonatal sepsis in these countries (2, 10, 11). In the literature, the factors associated with maternal and neonatal sepsis are diverse, including prolonged labor, failure to perform antenatal consultation (ANC), prolonged rupture of membranes, history of infection in the mother, repeated vaginal examinations, intrapartum fever, gestational age, parity, type of delivery, prematurity, chorioamnionitis, meconium-stained amniotic fluid, Apgar score < 7, low birth weight < 2.5 kg, resuscitation of the newborn, age of the newborn <7 days, and male sex (5–7, 20–23).
Prevention, early recognition of signs, and rapid and appropriate management of cases are the main factors associated with a reduction in the morbidity and mortality associated with maternal and neonatal sepsis (5, 11, 24–26). A recent meta-analysis carried out in SSA in 2022 identified several risk factors for neonatal sepsis (27) but did not explore the magnitude of neonatal and/or maternal sepsis nor the factors associated with maternal sepsis. Other studies have shown that the prevalence of maternal sepsis was 39% in Ethiopia (28), 12.20% in Keyna (29), and 20% in Tanzania (30); for neonatal sepsis, it was 77.9 in Ethiopia (31), 20.5% in South Africa (32), 49.8 in Tanzania (33), 37.6 in Nigeria (34), and 17.5 in Ghana (35). Although these single studies reported data on the prevalence and factors associated with maternal and neonatal sepsis, there are no regionally representative pooled data on the magnitude and factors associated with maternal and neonatal sepsis in SSA. However, a better understanding of the burden and a synthesis of the evidence on the factors associated with maternal and neonatal sepsis are needed to optimize prevention strategies and management guidelines against this scourge. The aim of this systematic review with meta-analysis was to estimate the prevalence and factors associated with maternal and neonatal sepsis in sub-Saharan Africa. To the best of our knowledge, this is the first systematic review and meta-analysis to examine both the prevalence of and factors associated with maternal and neonatal sepsis in SSA. It aimed to answer the following questions:
What is the prevalence of maternal and neonatal sepsis in sub-Saharan Africa?
What are the associated factors with maternal and neonatal sepsis in sub-Saharan Africa?
Materials and methods
This systematic review was reported in accordance with PRISMA (Preferred Reporting Item for Systematic Review and Meta-analysis) guidelines (36). The protocol for this review was developed and registered in the “International prospective register of systematic reviews PROSPERO” (ID: CRD42022382050).
Search strategies
To identify eligible studies, Medline/PubMed, Cochrane Library, African Index Medicus, and Google Scholar databases were searched. We also conducted manual searches of the bibliographic references of included studies and meta-analyses. The adapted PECO format was used for this systematic review. This PECO included population (P), exposure (E), comparison (C), and outcome (O), as shown in Table 1. It consisted of using all the identified keywords and indexing terms to search different databases. All the search terms used and the MeSH terms for the search were added, as well as the Boolean operators, to guarantee the exhaustiveness of the search process. Key terms defining the same concept were introduced using the “OR” operator, and the “AND” operator was used to introduce different concepts.
Table 1.
PECOT framework for the review objective.
| Components | Characteristics |
|---|---|
| Population | Pregnant women, women in labor, postpartum women, newborns, |
| Exposure | Associated factors: prolonged labor, failure to perform ANC, prolonged rupture of membranes, history of infection in the mother, repeated vaginal examinations, intrapartum fever, gestational age, parity, cesarean delivery, prematurity, meconium amniotic fluid, Apgar score < 7, birth weight < 2.5 kg, age of newborn <7 days, prematurity. |
| Comparison | Absence of exposure |
| Results | Maternal sepsis and associated factors |
Selection of studies/eligibility criteria
The result of bibliographic searches carried out on the various search tools was exported to Zotero, where duplicates were identified and removed using the Duplicates command and manually also removed during the screening. The study selection process followed two evaluation stages (37). The first evaluation was based on an examination of the titles and abstracts of the articles. The titles and abstracts that were outside the scope of the study were excluded. The second assessment consisted of examining the full text of eligible studies. The entire process was carried out by two reviewers (FBT and NHD). They independently performed abstract screening and full-text study selection, where both authors had to approve the inclusion of the study in the systematic review. The reference lists of the included studies were screened for potentially relevant studies (38).
Studies reporting on the prevalence and/or at least one factor associated with maternal and/or neonatal sepsis in SSA in pregnant women from 28 weeks of amenorrhea (SA), postpartum women up to 42 days after delivery, post-abortion women, and newborns within 28 days of birth were included. Cross-sectional, cohort, and case–control studies on the prevalence, frequency, and factors associated with maternal and neonatal sepsis in SSA published in French and English between January 2012 and October 2022 were included. Qualitative studies, systematic reviews, and case series were excluded from the analysis, but the reference lists of these were screened.
Assessment of study quality and risk of bias
The quality of the included studies was assessed by two authors (FBT and NHD) using the Joanna Briggs Institute (JBI) quality assessment checklist (39). For cross-sectional studies, the following criteria were used: (1) conformity between target population and source population; (2) appropriate sampling technique; (3) representativeness of the sample; (4) description of the subject and context of the study; (5) data analysis with sufficient sample coverage; (6) valid methods for identifying the condition; (7) standard and reliable way of measuring the condition for all participants; (8) appropriate statistical test; and (9) adequate response rate. When items received a score ≥ 6 out of 9, they were considered to be of high quality. The following were used to assess cohort studies: (1) similarity of groups, (2) similarity of exposure measurement, (3) validity and reliability of measurement, (4) identification of confounders, (5) strategies for dealing with confounders, (6) adequacy of groups/participants at study entry, (7) validity and reliability of measured outcomes, (8) sufficient duration of follow-up, (9) completeness of follow-up or description of reasons for loss to follow-up, (10) strategies for dealing with incomplete follow-up, and (11) adequacy of statistical analysis. The criteria used to evaluate case–control studies are (1) comparable groups, (2) appropriateness of cases and controls, (3) criteria for identifying cases and controls, (4) standard exposure measurement, (5) similarity of exposure measurement for cases and controls, (6) treatment of confounding factors, (7) strategies for treating confounding factors, (8) standard evaluation of results, (9) appropriateness of duration of exposure, and (10) appropriateness of statistical analysis (see Table 2).
Table 2.
Quality assessment results of included studies in sub-Saharan Africa from January 2002–October 2022 using the Joanna Briggs Institute (JBI) quality appraisal checklist.
| Author | Quality assessment questions | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Yes Total | Quality status | |
| Cross-sectional studies | |||||||||||||
| Tsehaynesh G/eyesus | N | Y | Y | UC | Y | Y | UC | Y | UC | 5/9 | Medium risk | ||
| Kumera Bekele | Y | Y | Y | UC | Y | Y | Y | Y | UC | 7/9 | Low risk | ||
| Abebe Sorsa | Y | UC | Y | Y | UC | Y | Y | UC | Y | 6/9 | Low risk | ||
| Abimbola Ellen Akindolire | Y | Y | Y | UC | Y | Y | Y | Y | Y | 8/9 | Low risk | ||
| Fortress Yayra Aku | UC | UC | Y | N | Y | Y | Y | Y | UC | 5/9 | Medium risk | ||
| Alemnew Wale | UC | Y | Y | UC | Y | UC | Y | Y | N | 5/9 | Medium risk | ||
| Bua John, 2015 | N | Y | N | Y | Y | UC | Y | Y | Y | 6/9 | Low risk | ||
| Debora C. Kajegukaa, 2020 | UC | Y | Y | N | UC | Y | Y | Y | UC | 5/9 | Medium risk | ||
| Aytenew Getabelew, 2018 | Y | Y | Y | N | N | N | Y | Y | UC | 5/9 | Medium risk | ||
| Tchouambou SN Clotilde, 2022 | UC | N | Y | UC | N | Y | Y | Y | Y | 5/9 | Medium risk | ||
| Abdulhakeem Abayomi Olorukooba, 2020 | Y | Y | Y | Y | Y | N | UC | Y | Y | 7/9 | Low risk | ||
| Mekitrida L. Kiwone, 2020 | UC | N | Y | Y | UC | Y | Y | Y | UC | 5/9 | Medium risk | ||
| Tilahun Tewabe, 2017 | Y | Y | Y | UC | Y | Y | Y | UC | Y | 7/9 | Low risk | ||
| Endalk Birrie, 2020 | Y | Y | Y | Y | Y | Y | Y | Y | Y | 9/9 | Low risk | ||
| Abdurahman Kedir Roble, 2022 | UC | Y | Y | Y | UC | Y | Y | Y | UC | 7/9 | Low risk | ||
| Zelalem Agnche, 2020 | Y | Y | Y | Y | Y | UC | Y | Y | Y | 8/9 | Low risk | ||
| Daniel Atlaw, 2019 | Y | Y | Y | UC | Y | Y | Y | Y | Y | 8/9 | Low risk | ||
| Alemale Admas, 2020 | Y | Y | Y | U | UV | Y | Y | Y | Y | 8/9 | Low risk | ||
| Yenew Engida Yismaw, 2019 | Y | Y | Y | UC | Y | UC | Y | UC | Y | 6/9 | Low risk | ||
| Tinuade A Ogunlesi, 2010 | Y | Y | Y | Y | UC | Y | Y | Y | Y | 8/9 | Low risk | ||
| Agricola Joachim, 2009 | Y | Y | Y | UC | Y | Y | Y | Y | Y | 8/9 | Low risk | ||
| Neema Kayange, 2010 | Y | Y | Y | UC | Y | UC | UC | Y | Y | 6/9 | Low risk | ||
| Ogundare Ezra Olatunde, 2015 | Y | Y | Y | UC | Y | Y | Y | Y | Y | 8/9 | Low risk | ||
| BA West, 2014 | Y | Y | UC | Y | UC | N | Y | Y | Y | 6/9 | Low risk | ||
| Cohort studies | |||||||||||||
| Violet Okaba Kayom | Y | Y | N | Y | UC | Y | Y | Y | Y | N | Y | 8/11 | Low risk |
| Shatry N. A., 2022 | Y | Y | Y | Y | UC | Y | Y | N | UC | Y | Y | 8/11 | Low risk |
| Case–control study | |||||||||||||
| Mulunesh Alemu | Y | Y | Y | UC | Y | Y | Y | Y | UC | Y | 8/10 | Low risk | |
| Kalkidan Béjituel | Y | Y | Y | UC | Y | Y | Y | Y | Y | Y | 9/10 | Low risk | |
| Getu Alemu Demisse | Y | Y | Y | UC | Y | Y | Y | Y | N | Y | 8/10 | Low risk | |
| Dejene Edosa Dirirsa | Y | Y | Y | UC | Y | Y | UC | Y | Y | Y | 8/10 | Low risk | |
| Gujo Teshome | Y | Y | Y | Y | Y | Y | N | Y | UN | Y | 8/10 | Low risk | |
| Peter Adatara, 2019 | Y | Y | Y | UC | Y | N | UC | Y | Y | Y | 7/10 | Medium risk | |
| Peter Adatara, 2018 | Y | Y | Y | N | UN | Y | Y | UC | UC | Y | 6/10 | Medium risk | |
| Destaalem Gebremedhin | Y | Y | Y | Y | Y | UC | UC | Y | Y | Y | 8/10 | Low risk | |
| Pendo P. Masanja | Y | Y | Y | Y | Y | N | N | Y | UC | Y | 7/10 | Low risk | |
| Atkuregn Alemayehu, 2020 | Y | N | Y | Y | Y | UC | UC | Y | Y | Y | 7/10 | Low risk | |
| Tadesse Yirga AkaluID, 2020 | Y | Y | Y | U | UC | UC | Y | Y | Y | Y | 8/10 | Low risk | |
| Soressa Gemechu Kitessa, 2021 | Y | Y | Y | Y | Y | UC | UC | Y | Y | Y | 8/10 | Low risk | |
| Mate Siakwa, 2014 | Y | Y | Y | UC | UC | N | UC | Y | Y | Y | 6/10 | Medium risk | |
Q, Question; Y, yes; N, no; UC, unclear.
Data extraction
Two authors (FBT and NHD) independently extracted data using a tested form on Microsoft Excel. If discrepancies between data extractors continued, a third reviewer (EMD) was involved. Data extracted included author name and year of publication, country, study period, study setting, study design, study population, sample size, type of sepsis (maternal or neonatal), prevalence of neonatal sepsis, prevalence of maternal sepsis, and risk bias.
For the associated factors, data were extracted on the age of the newborn, Apgar score, gestational age, birth weight, resuscitation of the newborn at birth, sex of the newborn, antenatal consultation (ANC), presence of prolonged rupture of membranes, repeated vaginal examinations, intrapartum fever, gestational age, parity, type of delivery, prematurity, meconium amniotic fluid, history of infection in the mother, maternal fever, type of delivery, and prolonged labor.
Statistical analysis
Data were entered into Microsoft Excel and then exported into Stata version 17 software. We pooled maternal and neonatal sepsis prevalence estimates using a random-effects meta-analysis model because it accounts for variability between studies. We examined the heterogeneity of effect size using the Q statistic and the I2 statistic (40). An I2 value ≥ 50% was considered strong heterogeneity, and a random-effects model was used (41, 42). The random-effect RELM method was mainly used. The funnel plot and Eggers’s regression test were used to check for publication bias (43). To complete the tests for publication bias, we added the Begg and Thompson tests on R software. Forest plots were used to display the results graphically. A subgroup analysis was performed according to study design (prospective or retrospective), sepsis diagnostic criteria (clinic or clinic and biology), risk of bias (low or moderate), and different regions of SSA (East, West, or South Africa).
Results
A total of 2,638 titles were screened (2,033 from PubMed, 58 from the African Index of Medicus, 41 from Cochrane, and 506 from Google Scholar). After removing duplicates, 2,145 studies remained. Evaluation of titles and abstracts led to the exclusion of 2,019 studies. Nine studies were excluded because the full text was not available. The full-text review involved 126 studies. The number of articles retained for inclusion was 39. The reasons for the exclusion of 87 studies were the lack of availability of the variable of interest and the difference in the target population (Figure 1).
Figure 1.
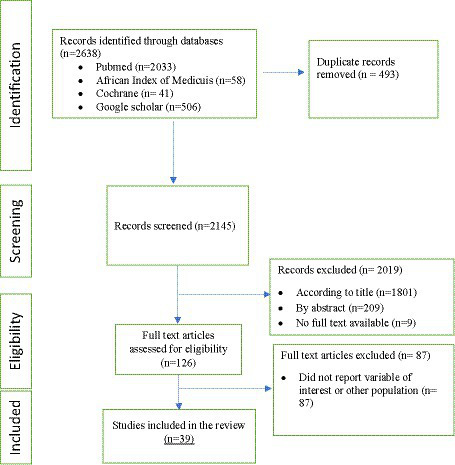
PRISMA flow diagram describing the results of study selection.
Characteristics of the studies included in the meta-analysis
A total of 39 studies were included in our review: 32 studies of neonatal sepsis and 7 studies of maternal sepsis. There were 24 cross-sectional studies (22, 30–34, 44–60), 13 case–control studies (28, 35, 61–69), and 2 cohorts (29, 70).
Twenty-one studies were from Ethiopia (28, 31, 44–46, 49, 51–56, 60–64, 66, 68, 69, 71), five from Tanzania (22, 30, 33, 67, 72), five from Nigeria (34, 47, 59, 73, 74), four from Ghana (35, 48, 65, 75), two from Uganda (50, 70), one from Kenya (29), and one from South Africa (32). The total study population was 12,777, including 10,494 newborns and 2,283 women. After quality assessment, 30 studies had a low risk of bias and 9 had a medium risk of bias (see Table 3).
Table 3.
Characteristics of included studies.
| N | First author, publication year | Country | Study period | Setting | Study design | Study population | Sample size | Type of sepsis | Prevalence | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Tsehaynesh G/eyesus, 2017 | Ethiopia | September 2015 to May 2016 | Hospital | Prospective Cross-sectional | Newborn | 251 | Neonatal | 46.61% | Medium risk |
| 2 | Kumera Bekele, 2022 | Ethiopia | January 2021 to March 2021 | Hospitals | Transversale prospective | Newborn | 378 | Neonatal | 52.27%. | Low risk |
| 3 | Mulunesh Alemu, 2019 | Ethiopia | 1 February to 30 March 2018 | Hospitals | Case-control | Newborn | 246 | Neonatal | - | Low risk |
| 4 | Kalkidan Béjituel, 2022 | Ethiopia | 1 August to 30 September 2020 | Hospitals | Case-control | Newborn | 331 | Neonatal | - | Low risk |
| 5 | Getu Alemu Demisse, 2019 | Ethiopia | 1 February to 30 April 2018 | Hospitals | Case-control | Mother | 280 | Maternal | - | Low risk |
| 6 | Dejene Edosa Dirirsa, 2021 | Ethiopia | May 2018 to August 2018 | Hospitals | Case-control | Newborn | 220 | Neonatal | Low risk | |
| 7 | Abebe Sorsa, 2019 | Ethiopia | April 2016 to May 2017 | Hospital | Prospective Cross-sectional | Newborn | 303 | Neonatal | 34% | Low risk |
| 8 | Abimbola Ellen Akindolire, 2016 | Nigeria | November 2013 and February 2014 | Hospitals | Prospective Cross-sectional | Newborn | 202 | Neonatal | 12.37 | Low risk |
| 9 | Fortress Yayra Aku, 2020 | Ghana | January and May 2016 | Hospitals | Prospective Cross-sectional | Newborn | 150 | Neonatal | 17.3% | Medium risk |
| 10 | Alemnew Wale, 2021 | Ethiopia | May to November 2019 | Hospital | Prospective Cross-sectional | Newborn | 193 | Neonatal | 26.1% | Medium risk |
| 11 | Gujo Teshome, 2022 | Ethiopia | 1 October to 10 November 2021 | Hospitals | Case-control | Newborn | 293 | Néonatal | - | Low risk |
| 12 | Peter Adatara, 2019 | Ghana | January and December 2017 | Hospital | Case-control | Newborn | 900 | Neonatal | - | Low risk |
| 13 | Peter Adatara, 2018 | Ghana | 4 weeks | Hospital | Case-control | Newborn | 383 | Neonatal | 17.50% | Medium risk |
| 14 | Destaalem Gebremedhin, 2016 | Ethiopia | December 2014 to June 2015 | Hospitals | Case-control | Newborn | 234 | Neonatal | - | Low risk |
| 15 | Bua John, 2015 | Uganda | January and August 2013 | Health Center | Prospective Cross-sectional | Mother and newborn | 174 | Neonatal | 21.80% | Low risk |
| 16 | Debora C. Kajegukaa, 2020 | Tanzania | January 2015 to December 2015 | Hospital | Retrospective Cross-sectional | Mother | 183 | Maternal | 11.5% | Medium risk |
| 17 | Violet Okaba Kayom, 2018 | Uganda | March to May 2012 | Community | Prospective Cohort | Mother and newborn | 335 | Neonatal | Low risk | |
| 18 | Pendo P. Masanja, 2019 | Tanzania | May to July 2017 | Hospitals | Case–control | Mother and newborn | 322 | Neonatal | Low risk | |
| 19 | Atkuregn Alemayehu, 2020 | Ethiopia | April to July 2019 | Hospitals | Case–control | Newborn | 385 | Neonatal | Low risk | |
| 20 | Tadesse Yirga AkaluID, 2020 | Ethiopia | March 2018 to April 2018 | Hospitals | Case–control | Newborn | 231 | Neonatal | Low risk | |
| 21 | Aytenew Getabelew, 2018 | Ethiopia | 1 February 2016 to 1 February 2017 | Hospitals | Retrospective Cross-sectional | Newborn | 224 | Neonatal | 77.9% | Medium risk |
| 22 | Tchouambou SN Clotilde, 2022 | South Africa | 1 January and 30 June 2018 | Hospitals | Prospective Cross-sectional | Newborn | 210 | Neonatal | 20.5% | Medium risk |
| 23 | Abdulhakeem Abayomi Olorukooba, 2020 | Nigeria | May 2017 to May 2018 | Hospital | Retrospective Cross-sectional | Newborn | 409 | Neonatal | 37.6%. | Low risk |
| 24 | Mekitrida L. Kiwone, 2020 | Tanzania | August to October 2018 | Hospital | Retrospective Cross-sectional | Newborn | 263 | Neonatal | 49.8% | Medium risk |
| 25 | Tilahun Tewabe, 2017 | Ethiopia | 30 April to 30 May 2016 | Hospital | Retrospective Cross-sectional | Newborn | 225 | Neonatal | Low risk | |
| 26 | Endalk Birrie, 2020 | Ethiopia | 1 January to 30 July 2021 | Hospital | Prospective Cross-sectional | Newborn | 344 | Neonatal | 79.4% | Low risk |
| 27 | Abdurahman Kedir Roble, 2022 | Ethiopia | 1 January 2019 to 31 December 2019 | Hospitals | Prospective Cross-sectional | Newborn | 361 | Neonatal | 45.80% | Low risk |
| 28 | Zelalem Agnche, 2020 | Ethiopia | March to April 2019 | Hospitals | Prospective Cross-sectional | Mother and newborn | 352 | Neonatal | 64.8% | Low risk |
| 29 | Soressa Gemechu Kitessa, 2021 | Ethiopia | May to October 2020 | Hospitals | Case–control | Mother | 428 | Maternal | −39% | Low risk |
| 30 | Shatry N. A., 2022 | Kenya | March to November 2015 | Hospital | Prospective Cohort | Mother | 566 | Maternal | 12.20% | Low risk |
| 31 | Daniel Atlaw, 2019 | Ethiopia | 1 September to 30 December 2017 | Hospital | Prospective Cross-sectional | Mother | 219 | Maternal | 17.2%, | Low risk |
| 32 | Alemale Admas, 2020 | Ethiopia | January to May 2017 | Hospital | Prospective Cross-sectional | Mother | 166 | Maternal | 33.70% | Low risk |
| 33 | Ayenew Engida Yismaw, 2019 | Ethiopia | 1st September to 30th November 2017 | Hospital | Prospective Cross-sectional | Newborn | 423 | Neonatal | 11.70% | Low risk |
| 34 | Tinuade A Ogunlesi, 2010 | Nigeria | January 2006 to December 2008 | Hospital | Retrospective Cross-sectional | Newborn | 1,050 | Neonatal | 16.5 | Low risk |
| 35 | Agricola Joachim, 2009 | Tanzania | October 2008 to March 2009 | Hospital | Prospective Cross-sectional | Mother | 300 | Maternal | 20% | Low risk |
| 36 | Neema Kayange, 2010 | Tanzania | March to November 2009 | Hospital | Prospective Cross-sectional | Newborn | 770 | Neonatal | 39% | Low risk |
| 37 | Mate Siakwa, 2014 | Ghana | January 2011 and December 2013 | Hospital | Case-control | Newborn | 196 | Neonatal | Medium risk | |
| 38 | Ogundare Ezra Olatunde, 2015 | Nigeria | September 2008 to March 2009 | Hospital | Prospective Cross-sectional | Newborn | 360 | Neonatal | 16% | Low risk |
| 39 | BA West, 2012 | Nigeria | July to December 2007 | Hospital | Prospective Cross-sectional | Newborn | 406 | Neonatal | 41.6% | Low risk |
Prevalence of maternal sepsis
The pooled prevalence of maternal sepsis in SSA was 19.21% (95% CI, 11.46–26.97). Significant heterogeneity was observed between studies (I2 = 93.26%, p < 0.000). A random effects model was used to measure pooled prevalence. The highest prevalence was reported by Alemale et al. (54) (33.7%) and the lowest by Debora et al. (22) (11.5%); Figure 2 shows the details.
Figure 2.
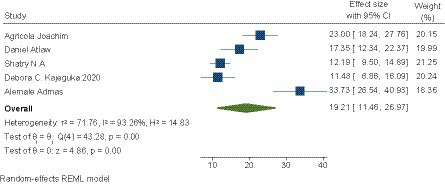
Pooled prevalence of maternal sepsis in SSA.
Subgroup analysis of the prevalence of maternal sepsis
A subgroup analysis of the prevalence of maternal sepsis was carried out according to the diagnostic criteria for sepsis (clinical or clinical and biological). It was 25.33 (9.28–41.38) for sepsis based on clinical signs and 15.43 (8.28–22.58) for sepsis based on clinical signs and biology (22, 29, 30). See Table 4 for details.
Table 4.
Sub-group analysis of the prevalence of maternal sepsis in sub-Saharan Africa.
| Variable | Characteristics | Pooled prevalence (95% CI) | I2 (value of p) |
|---|---|---|---|
| Diagnostic criteria | Clinical | 25.33 (9.28–41.38) | 92.54% (<0.000) |
| Clinical and biological | 15.43 (8.28–22.58) | 89.72% (<0.000) |
Prevalence of neonatal sepsis
The pooled prevalence of neonatal sepsis in SSA was 36.02% (95% CI, 26.68–49.36). A significant heterogeneity between the included studies was observed (I2 = 99.1%, p < 0.000). Therefore, a random effects model was used to estimate the pooled prevalence. The prevalence ranged from 11.1 (56) up to 77.9% (31) (see Figure 3).
Figure 3.
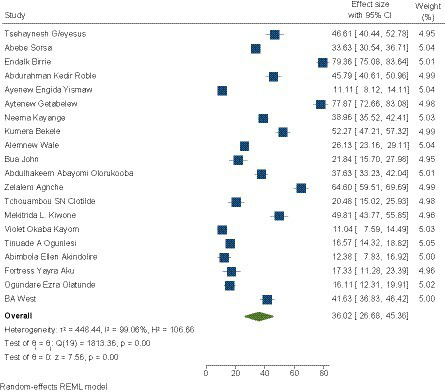
Pooled prevalence of neonatal sepsis in SSA.
Subgroup analysis of the prevalence of neonatal sepsis
A subgroup analysis of prevalence was performed according to the design (prospective and retrospective), subdivision of SSA (East, West, or South Africa), definition of sepsis (clinical when diagnostic based on clinical signs or clinical and biological when diagnostic based on clinical signs and confirmed by biological tests), and risk of bias (low or moderate).
Depending on the design, the prevalence of neonatal sepsis was 32.88 (22.35–43.41) for prospective studies and 45.46 (25.97–64.94) for retrospective studies. According to the diagnostic criteria for sepsis, it was 44.11 (27.33–60.90) for sepsis based on clinical signs and 31.25 (22.24–40.26) for sepsis based on clinical signs and biology. According to the subdivision, the prevalence of neonatal sepsis was 42.96 (30.75–55.16) in East Africa, 23.59 (15.53–33.65) in West Africa, and 20.48 (15.0–25.93) in South Africa. The prevalence of neonatal sepsis was 34.45 (23.93–44.98) for studies with a low risk of bias and 39.69 (21.58–57.80) for studies with a medium risk of bias (see Table 5).
Table 5.
Sub-group analysis of the prevalence of neonatal sepsis in sub-Saharan Africa.
| Variables | Characteristics | Pooled prevalence (95% CI) | I2 (value of p) |
|---|---|---|---|
| Countries | East Africa | 42.96 (30.75–55.16) | 97.41% (<0.000) |
| West Africa | 23.59 (15.53–33.65) | 98.98% (<0.000) | |
| South Africa | 20.48 (15.0–25.93) | 0% | |
| Study design | Foresight | 32.88 (22.35–43.41) | 98.98% (<0.000) |
| Retrospective | 45.46 (25.97–64.94) | 99.05% (<0.000) | |
| Diagnostic criteria | Clinical | 44.11 (27.33–60.90) | 99.37% (<0.000) |
| Clinical and biological | 31.25 (22.24–40.26) | 97.64% (<0.000) | |
| Risk of bias | Low | 34.45 (23.93–44.98) | 99.06% (<0.000) |
| Medium | 39.69 (21.58–57.80) | 98.95% (<0.000) |
Publication bias
We assessed publication bias using the funnel plot and Egger’s regression test (76). Compared with the maternal sepsis studies, a visual asymmetrical distribution was observed, and Egger’s regression test (value of p = 0.01) indicated the presence of a publication bias (see Figure 4). We did not use the Begg and Thompson tests due to the number of studies less than 10.
Figure 4.
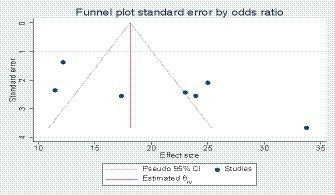
Funnel plot showing publication bias in maternal sepsis studies.
For the study concerning neonatal sepsis, we assessed publication bias using the funnel plot, Egger’s regression test, Begg’s test, and Thompson test. The diagrams showed asymmetry, and the result of Egger’s test showed the presence of bias (p = 0.04; see Figure 5). However, Begg’s test (value of p = 0.06) and the Thompson test (value of p = 0.14) did not reject the city Ho. Therefore, the asymmetry on the funnel plot is reflected much more by a small study effect than by a publication bias.
Figure 5.

Funnel plot showing publication bias d in neonatal sepsis studies.
Meta-analysis of factors associated with maternal sepsis
A total of eight risk factors were included in the meta-analysis: place of residence for 3 studies (22, 28, 63), parity for 3 studies (22, 30, 54), mode of delivery for 5 studies (22, 28, 54, 60, 63), prolonged labor for 5 studies (22, 28, 29, 54, 63), multiple vaginal examinations for 3 studies (28, 29, 63), performance of ANC for 4 studies (28, 54, 60, 63), history of urinary tract infection for 3 studies (22, 29, 30), and level of education for 4 studies (28, 29, 60, 63). Table 6 shows the details.
Table 6.
Risk factors included in the meta-analysis for maternal sepsis.
| Result | Comparison | Number studies | Effect size | Pooled estimate | Q | Heterogeneity I2, value of p |
|---|---|---|---|---|---|---|
| Residence | Rural, urban | 3 | Odds ratio | 3.32 (1.1–10.96) | 20.64 | 90%, 0.048 |
| Parity | Primiparous, multiparous | 3 | Odds ratio | 0.50 (0.32–0.78) | 0.42 | 0.0%, 0.003 |
| Delivery method | CS/ VD | 5 | Odds ratio | 1.47 (0.54–4.01) | 35.70 | 88.8%, 0.448 |
| Prolonged labor | >12 h,<12 h | 5 | Odds ratio | 3.37 (1.64–6.95) | 21.60 | 81.5%, 0.001 |
| Multiple vaginal examinations | >5, <5 | 3 | Odds ratio | 4.33 (1.31–14.36) | 20.73 | 90.4%, 0.016 |
| ANC | <4, ≥4 | 4 | Odds ratio | 1.849 (0.77–4.42) | 15.65 | 80.8%, 0.168 |
| History of urinary tract infections | Yes or No | 3 | Odds ratio | 1.672 (0.71–3.90) | 5.02 | 60.2%, 0.234 |
| Level of education | No education, education | 4 | Odds ratio | 1.32 (0.93–1.86) | 0.99 | 0.0%, 0.112 |
CS, Cesarean section; VD, Vaginal delivery.
Meta-analysis of factors associated with neonatal sepsis
A meta-analysis was carried out for 15 risk factors classified into maternal and neonatal factors. The maternal factors were history of urinary tract infection in the mother (13 studies) (31, 45, 53, 55, 56, 61, 62, 65, 66, 68–71), parity (10 studies) (31, 45, 48, 53, 56, 61, 62, 65, 67, 68), prolonged labor (5 studies) (31, 53, 62, 69, 73), intrapartum fever (13 studies) (31, 45, 48, 52, 55, 56, 61, 64, 66, 68, 70, 72, 73), multiple vaginal examinations (7 studies) (45, 53, 61, 64, 66–68), performance of ANC (13 studies) (45, 48, 50, 55, 61, 64, 65, 67–71), prolonged rupture of membranes (18 studies) (31, 33, 45, 46, 52, 53, 56, 61, 62, 65–73), and mode of delivery (14 studies) (31, 32, 35, 44, 46, 48, 52, 61, 62, 64, 66–68, 72). The neonatal factors reported were Apgar (18 studies) (31, 33–35, 44–46, 48, 52, 56, 61, 62, 64–66, 68, 69, 71), prematurity (17 studies) (31, 33–35, 44–46, 50, 52, 53, 61, 62, 64–67, 71), meconium amniotic fluid (12 studies) (31, 45, 46, 52, 61, 64–69, 72), birth weight (17 studies) (31–35, 44–48, 52, 53, 61, 62, 64–69, 72), neonatal resuscitation (09 studies) (33–35, 52, 53, 62, 65, 66, 71), neonatal age < 7 days (11 studies) (36, 38, 48, 53, 57, 65, 66, 69, 72, 75), and neonatal sex (14 studies) (32, 34, 35, 45, 46, 48, 53, 55, 61, 62, 65, 66, 72, 73) (see Table 7).
Table 7.
Risk factors included in the meta-analysis for neonatal sepsis.
| Results | Comparison | Number studies | Effect size | Pooled estimate | Q | Heterogeneity I2, value of p |
|---|---|---|---|---|---|---|
| Apgar | <7 or >7 | 18 | Odds ratio | 2.38 (1.61–3.53) | 151.21 | 88.8%, 0.000 |
| Preterm | <37 or >37 | 17 | Odds ratio | 1.36 (0.81–2.26) | 221.03 | 92.8%, 0.235 |
| Birth weight | <2.5 kg >2.5 kg | 17 | Odds ratio | 1.28 (0.85–1.93) | 210.79 | 90.5%, 0.228 |
| Mode of delivery | CS/VD | 14 | Odds ratio | 1.05 (0.67–1.65) | 93.07 | 86.0%, 0.807 |
| Amios | Yes or No | 12 | Odds ratio | 2.9 (1.83–4.58) | 51.37 | 78.6%, 0.000 |
| PROM | >12H, <12H | 18 | Odds ratio | 2.8 (1.96–4.18) | 99.27 | 82.9%, 0.000 |
| Sex | Male/Female | 14 | Odds ratio | 1.2 (1.1–1.42) | 11.92 | 0.0%, 0.001 |
| CPN | <4, ≥4 | 13 | Odds ratio | 1.4 (0.82–2.18) | 67.69 | 82.3%, 0.170 |
| Vaginal examination | >5, <5 | 7 | Odds ratio | 1.2 (0.51–3.09) | 126.14 | 95.2%, 0.616 |
| Intrapartum fever | Yes or No | 13 | Odds ratio | 2.4 (1.52–3.75) | 76.14 | 84.2%, 0.000 |
| Age of newborn | <7 days, >7 days | 11 | Odds ratio | 0.9 (0.52–1.89) | 108.72 | 90.8%, 0.995 |
| Newborn resuscitation | Yes, No | 9 | Odds ratio | 1,7 (0.94–3.30) | 94.67 | 91.5%, 0.076 |
| Parity | Primiparous, multiparous | 10 | Odds ratio | 1.2 (0.83–1.69) | 36.48 | 75.3%, 0.324 |
| History of maternal UTI | Yes, No | 13 | Odds ratio | 2.76 (1.38–5.21) | 114.73 | 89.5%, 0.003 |
| Prolonged labor | >12H, <12H | 5 | Odds ratio | 1.8 (1.47–1.90) | 40.15 | 90.0%, 0.128 |
Associated factors with maternal and neonatal sepsis
Factors associated with maternal sepsis
Rural residence (OR: 2.32, IC 95%: 1.01–10.9, I2 = 90.3%), parity (OR: 0.5, IC95%: 0.3–0.7, I2 = 0%), prolonged labor (OR: 3.4, IC95%: 1.6–6.9, I2 = 81.5%), and multiple vaginal examinations (OR: 4.4, IC95%: 1.3–14.3, I2 = 90.4%) were independently associated with maternal sepsis.
Factors associated with neonatal sepsis
The meta-analysis identified 6 factors as being significantly associated with neonatal sepsis, including Apgar score < 7 (OR: 2.4, CI95%: 1.6–3.5, I2 = 88.8%), the presence of meconium in the amniotic fluid (OR: 2.9, CI95%: 1.8–4.5, I2 = 78.6%), prolonged rupture of membranes >12 h (OR: 2.8, IC95%: 1.9–4.1, I2 = 82.9%), male sex (OR: 1.2, IC95%: 1.1–1.4, I2 = 0%), intrapartum fever (OR: 2.4, IC95%: 1.5–3.7, I2 = 84.2%), and history of urinary tract infection in the mother (OR: 2.76, IC95%: 1.4–5.2, I2 = 89.5%).
Discussion
Knowledge of the burden and risk factors of maternal and neonatal sepsis is crucial for developing preventive measures and reducing maternal and neonatal mortality. This systematic review with meta-analysis filled the gaps in the literature on the prevalence of maternal and neonatal sepsis and associated factors in SSA. Based on 39 studies included in the final analysis, we found a pooled prevalence of 19.21% for maternal sepsis and 36.02% for neonatal sepsis. However, considerable heterogeneity was observed.
Factors such as rural residence, parity, prolonged labor, and multiple vaginal examinations significantly increased the risk of maternal sepsis in our study. The factors associated with neonatal sepsis in this review are classified into maternal and neonatal factors. Maternal factors such as prolonged rupture of membranes > 12 h, intrapartum fever, and history of maternal urinary tract infection and neonatal factors such as Apgar score < 7, presence of meconium in amniotic fluid, and male sex were significantly associated with neonatal sepsis. Our estimate confirms that maternal and neonatal sepsis is a major public health problem in SSA. The pooled prevalence of maternal sepsis in our review is in line with other reviews conducted in 2009 (77) and 2021 (20). This high prevalence can be explained by various factors, such as the coverage of childbirth in health facilities, the asepsis and hygiene of surfaces and materials used, the personal hygiene of pregnant women, and certain harmful practices. Our study is the first to examine both the extent and the factors associated with maternal and neonatal sepsis, which sets it apart from other reviews. It identified other factors, such as rural residence and parity, which were not identified in the 2022 review or the WHO guidelines (78). Our results could serve as a broader database for the development of interventions for the prevention and management of maternal and neonatal sepsis.
All the maternal sepsis studies included were from East Africa. The subgroup analysis of the prevalence of maternal sepsis ranged from 15.43% based on clinical signs and biology to 25.33% based on clinical signs.
The subgroup analysis of the prevalence of neonatal sepsis varied from one subdivision to another. East Africa recorded the highest prevalence, 42.96%, which was higher than the pooled prevalence. This could be explained by the weakness of the health system, the quality of services offered, and socio-cultural factors. The under-analysis of sepsis in other parts of Africa, due to the small number of studies carried out there, masks a probable high prevalence in these countries, as areas of high prevalence in these countries have probably not been covered by the small number of studies published. The prevalence of neonatal sepsis was 44.11% for the diagnostic criterion based on clinical signs and 31.25% for clinical signs and biology. Despite the various subgroup analyses, they did not make it possible to explore the source of the heterogeneity. This could be explained by the heterogeneous definition of sepsis in the different studies. The clinical signs differed from one study to another, as did the laboratory tests, which is why a meta-analysis of the individual data was necessary to identify a single algorithm.
The factors associated with maternal sepsis in our review are partly in accordance with a review of the literature in other systematic reviews in 2009 and 2022 (27, 77), where the risk factors identified were intrapartum maternal fever, multiple vaginal examinations, foul-smelling vaginal discharge, prematurity, prolonged rupture of membranes, prolonged labor, and multiple vaginal touches.
Living in a rural area was significantly associated with maternal sepsis. Women living in rural areas were 2.3 times more likely to develop sepsis than those living in urban areas. The possible explanation could be poor hygiene levels, lack of water sources, poor cord care, overcrowding in homes, and low education level of mothers in rural areas (73, 75, 79). Another possible explanation could be the fact that mothers living in urban areas are close to health facilities and have various means of transport to get to these facilities, and the availability of qualified staff and adequate technical platforms in urban areas. A vaginal examination ≥ 5 times was significantly associated with maternal sepsis. The probability of maternal sepsis was 4.4 times higher in women who had undergone a vaginal examination ≥ 5 times compared with those who had not. During vaginal examinations, there is a high likelihood of microorganisms ascending from the lower to the upper genital tract, which can lead to sepsis. Simple interventions accepted to reduce the incidence of maternal sepsis are using sterile and aseptic technical equipment by providers (hand washing, sterile drapes and instruments, and sterile gloves).
The factors associated with neonatal sepsis in our review are in line with other reviews in Pakistan (78), East Africa (21), SSA (27), and India (80), which reported maternal factors such as intrapartum maternal fever, prolonged rupture of water membranes, gestational age < 37 weeks, prolonged labor, multiple vaginal touches, history of maternal urinary tract infection and low socioeconomic status, neonatal factors such as resuscitation at birth, low birth weight < 2.5 kg, Apgar score < 7, absence of crying immediately after birth, meconium-stained amniotic fluid, and male sex.
Intrapartum fever was significantly associated with maternal sepsis. The probability of maternal sepsis was 2.4 times higher in women with a fever during pregnancy than in those without a fever. Intrapartum fever is suggestive of a maternal infection that is transmitted to the baby in utero or during passage through the genital tract, leading to sepsis.
Membrane rupture > 12 h significantly increased the risk of neonatal sepsis, and a history of urinary tract infection in the mother was significantly associated with neonatal sepsis. The water membrane protects the upper genital tract; when it is ruptured, bacteria can proliferate through the dilated cervix into the upper internal genital tract, causing infection. These pathogens also colonize the birth canal, which could contaminate the newborn during passage through the canal. Intrapartum antibiotic prophylaxis has been recommended as an effective practice for at-risk mothers to reduce sepsis worldwide (81).
Having an Apgar score < 7 significantly increased the risk of neonatal sepsis. As a result, the probability of neonatal sepsis was 2.4 times higher in neonates with an Apgar score < 7 compared with those with an Apgar score > 7. This finding is consistent with studies from Iraq (82) and Indonesia (83). Neonates with low Apgar scores tend to have a poor adaptation to extrauterine life due to the stress experienced during labor and therefore are more prone to infection. In addition, resuscitation procedures following birth asphyxia tend to expose newborns to pathogenic microbes. In our study, however, we did not assess birth asphyxia as a risk factor for neonatal sepsis.
Male sex has been identified as a factor associated with neonatal sepsis, and this finding has been made in the journals in India and SSA. However, we suggest further research into this factor to give a more rational explanation.
Strengths and limitations
Our review used an appropriate search strategy, with the combination of global and regional databases reducing the risk of missing relevant regional studies. Duplicate screening and data extraction, as well as rigorous quality assessment of included data and subgroup analysis, was also performed. The relatively high number of included studies for neonatal sepsis is a strength. The number of articles included was small for maternal sepsis (7), which may limit the generalizability of the results. Most of the studies were cross-sectional, which could be a limitation. The majority of studies included in this systematic review were from East Africa, which may affect the generalizability of our results to sub-Saharan Africa. There were also publication biases between studies and a high degree of heterogeneity. We believe that this is an important subject that has not been sufficiently explored and that a meta-analysis of individual data will be necessary.
Conclusion
In our review, the prevalence of maternal and neonatal sepsis was high. Several factors were significantly associated with this prevalence, which could help prevent maternal and neonatal sepsis by developing appropriate standard infection prevention techniques, reducing certain harmful practices, and reducing susceptibility to infection by improving maternal health through nutritional supplementation and treating infections during pregnancy. However, there is a need for evidence on other important risk factors for maternal and neonatal sepsis, including in the community. Given the high risk of bias and high heterogeneity, further high-quality research is needed in the sub-Saharan African context.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
FT: Conceptualization, Formal analysis, Investigation, Methodology, Software, Supervision, Writing – original draft, Writing – review & editing. CS: Conceptualization, Methodology, Writing – review & editing. ED: Conceptualization, Writing – review & editing, Investigation. BC: Investigation, Methodology, Writing – review & editing. SS: Software, Writing – review & editing, Formal analysis. ADi: Formal analysis, Validation, Writing – review & editing. ND: Conceptualization, Investigation, Writing – review & editing. BL: Methodology, Writing – review & editing. MA: Conceptualization, Writing – review & editing. KK: Formal analysis, Software, Supervision, Writing – review & editing. AT: Supervision, Writing – review & editing. AC: Writing – review & editing. ADe: Supervision, Validation, Writing – review & editing. HS: Supervision, Validation, Writing – review & editing. IT: Formal analysis, Writing – review & editing.
Acknowledgments
The authors thank the West African Network of African Center of Excellence on Infectious Diseases (WANIDA) for support. We thank colleagues of the National Institute of Public Health and those of the African Center of Excellence for Prevention and Control of Communicable Diseases for their support during this study.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the West African Network of African Center of Excellence on Infectious Diseases (WANIDA); however, the institution played no role in the study design, data collection, data analysis, or writing of the article. The corresponding author was responsible for the data and submission of the article for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declared no conflicts of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Galvão A, Braga AC, Gonçalves DR, Guimarães JM, Braga J. Sepsis during pregnancy or the postpartum period. J Obstet Gynaecol. (2016) 36:735–43. doi: 10.3109/01443615.2016.1148679 [DOI] [PubMed] [Google Scholar]
- 2.Otu A, Nsutebu EF, Hirst JE, Thompson K, Walker K, Yaya S. How to close the maternal and neonatal sepsis gap in sub-Saharan Africa. BMJ Glob Health. (2020) 5:e002348. doi: 10.1136/bmjgh-2020-002348, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sands K, Spiller OB, Thomson K, Portal EAR, Iregbu KC, Walsh TR. Early-onset neonatal Sepsis in low- and middle-income countries: current challenges and future opportunities. Infect Drug Resist. (2022) 15:933–46. doi: 10.2147/IDR.S294156, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assemie MA, Alene M, Yismaw L, Ketema DB, Lamore Y, Petrucka P, et al. Prevalence of neonatal Sepsis in Ethiopia: a systematic review and Meta-analysis. Int J Pediatr. (2020) 2020:e6468492: 1–9. doi: 10.1155/2020/6468492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kendle AM, Salemi JL, Tanner JP, Louis JM. Delivery-associated sepsis: trends in prevalence and mortality. Am J Obstet Gynecol. (2019) 220:391.e1–391.e16. doi: 10.1016/j.ajog.2019.02.002, PMID: [DOI] [PubMed] [Google Scholar]
- 6.Beck C, Gallagher K, Taylor LA, Goldstein JA, Mithal LB, Gernand AD. Chorioamnionitis and risk for maternal and neonatal Sepsis: a systematic review and Meta-analysis. Obstet Gynecol. (2021) 137:1007–22. doi: 10.1097/AOG.0000000000004377, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tesfaye T, Samuel S, Lera T. Determinants of puerperal sepsis among postpartum women at public hospitals of Hawassa city, southern Ethiopia: institution-based unmatched case-control study. Heliyon. (2023) 9:e14809. doi: 10.1016/j.heliyon.2023.e14809, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, Schlapbach LJ, Reinhart K, Kissoon N. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med. (2018) 6:223–30. doi: 10.1016/S2213-2600(18)30063-8, PMID: [DOI] [PubMed] [Google Scholar]
- 9.Reinhart K, Daniels R, Kissoon N, Machado FR, Schachter RD, Finfer S. Recognizing Sepsis as a Global Health priority — a WHO resolution. N Engl J Med. (2017) 377:414–7. doi: 10.1056/NEJMp1707170, PMID: [DOI] [PubMed] [Google Scholar]
- 10.HAA AL-K, Hameed RH. Sepsis during pregnancy in the postpartum duration. J Crit Rev. (2020) 7:2435–9. [Google Scholar]
- 11.Bonet M, Brizuela V, Abalos E, Cuesta C, Baguiya A, Chamillard M, et al. Frequency and management of maternal infection in health facilities in 52 countries (GLOSS): a 1-week inception cohort study. Lancet Glob Health. (2020) 8:e661–71. doi: 10.1016/S2214-109X(20)30109-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brizuela V, Bonet M, Romero CLT, Abalos E, Baguiya A, Fawole B, et al. Early evaluation of the ‘STOP SEPSIS!‘WHO global maternal Sepsis awareness campaign implemented for healthcare providers in 46 low, middle and high-income countries. BMJ Open. (2020) 10:e036338. doi: 10.1136/bmjopen-2019-036338, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brizuela V, Cuesta C, Bartolelli G, Abdosh AA, Abou Malham S, Assarag B, et al. Availability of facility resources and services and infection-related maternal outcomes in the WHO global maternal Sepsis study: a cross-sectional study. Lancet Glob Health. (2021) 9:e1252–61. doi: 10.1016/S2214-109X(21)00248-5, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. (2014) 2:e323–33. doi: 10.1016/S2214-109X(14)70227-X [DOI] [PubMed] [Google Scholar]
- 15.Ebener S, Stenberg K, Brun M, Monet JP, Ray N, Sobel HL, et al. Proposing standardised geographical indicators of physical access to emergency obstetric and newborn care in low-income and middle-income countries. BMJ Glob Health. (2019) 4:e000778. doi: 10.1136/bmjgh-2018-000778, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hug L, Alexander M, You D, Alkema L. National, regional, and global levels and trends in neonatal mortality between 1990 and 2017, with scenario-based projections to 2030: a systematic analysis. Lancet Glob Health. (2019) 7:e710–20. doi: 10.1016/S2214-109X(19)30163-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nyenga AM. Trends in neonatal mortality in Lubumbashi (Democratic Republic of Congo) from 2011 to 2018. Pediatr Surg. (2019) 2:5. doi: 10.4236/oje.2021.115029 [DOI] [Google Scholar]
- 18.Hall J, Adams NH, Bartlett L, Seale AC, Lamagni T, Bianchi-Jassir F, et al. Maternal disease with group B Streptococcus and serotype distribution worldwide: systematic review and Meta-analyses. Clin Infect Dis. (2017) 65:S112–24. doi: 10.1093/cid/cix660, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yaya S, Bishwajit G, Okonofua F, Uthman OA. Under five mortality patterns and associated maternal risk factors in sub-Saharan Africa: a multi-country analysis. PLoS One. (2018) 13:e0205977. doi: 10.1371/journal.pone.0205977, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melkie A, Dagnew E. Burden of puerperal sepsis and its associated factors in Ethiopia: a systematic review and meta-analysis. Arch Public Health. (2021) 79:216. doi: 10.1186/s13690-021-00732-y, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abate BB, Kasie AM, Reta MA, Kassaw MW. Neonatal sepsis and its associated factors in East Africa: a systematic review and meta-analysis. Int J Public Health. (2020) 65:1623–33. doi: 10.1007/s00038-020-01489-x, PMID: [DOI] [PubMed] [Google Scholar]
- 22.Kajeguka DC, Mrema NR, Mawazo A, Malya R, Mgabo MR. Factors and causes of puerperal Sepsis in Kilimanjaro, Tanzania: a descriptive study among postnatal women who attended Kilimanjaro Christian medical Centre. East Afr Health Res J. (2020) 4:158–62. doi: 10.24248/eahrj.v4i2.639, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chepchirchir MV, Nyamari J, Keraka M. Associated factors with puerperal Sepsis among reproductive age women in Nandi County, Kenya. JMRH. (2017) 5:9348. doi: 10.22038/jmrh.2017.9348 [DOI] [Google Scholar]
- 24.Edwards W, Dore S, van Schalkwyk J, Armson BA. Prioritizing maternal Sepsis: National Adoption of an obstetric early warning system to prevent morbidity and mortality. J Obstet Gynaecol Can. (2020) 42:640–3. doi: 10.1016/j.jogc.2019.11.072, PMID: [DOI] [PubMed] [Google Scholar]
- 25.Cheshire J, Jones L, Munthali L, Kamphinga C, Liyaya H, Phiri T, et al. The FAST-M complex intervention for the detection and management of maternal sepsis in low-resource settings: a multi-site evaluation. BJOG Int J Obstet Gynaecol. (2021) 128:1324–33. doi: 10.1111/1471-0528.16658 [DOI] [PubMed] [Google Scholar]
- 26.Shifera N, Dejenie F, Mesafint G, Yosef T. Risk factors for neonatal sepsis among neonates in the neonatal intensive care unit at Hawassa university comprehensive specialized hospital and Adare general Hospital in Hawassa City, Ethiopia. Front Pediatr. (2023) 11:671. doi: 10.3389/fped.2023.1092671, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bech CM, Stensgaard CN, Lund S, Holm-Hansen C, Brok JS, Nygaard U, et al. Risk factors for neonatal sepsis in sub-Saharan Africa: a systematic review with meta-analysis. BMJ Open. (2022) 12:e054491. doi: 10.1136/bmjopen-2021-054491, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitessa SG, Teferi Bala E, Makuria M, Senbeta Deriba B. Determinants of puerperal sepsis at public hospitals in West Ethiopia: a case-control study. Front Womens Health. (2021) 6. doi: 10.15761/FWH.1000207 [DOI] [Google Scholar]
- 29.Naima S. Magnitude and risk factors for puerperal Sepsis at the Pumwani maternity hospital. [Thesis]. University of Nairobi; (2017). Available at:http://erepository.uonbi.ac.ke/handle/11295/101747
- 30.Joachim A, Matee MI, Massawe FA, Lyamuya EF. Maternal and neonatal colonisation of group B streptococcus at Muhimbili National Hospital in Dar Es Salaam, Tanzania: prevalence, risk factors and antimicrobial resistance. BMC Public Health. (2009) 9:437. doi: 10.1186/1471-2458-9-437, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Getabelew A, Aman M, Fantaye E, Yeheyis T. Prevalence of neonatal Sepsis and associated factors among neonates in neonatal intensive care unit at selected governmental hospitals in Shashemene town, Oromia regional State, Ethiopia, 2017. Int J Pediatr. (2018) 2018:1–7. doi: 10.1155/2018/7801272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clotilde TS, Motara F, Laher AE. Prevalence and presentation of neonatal sepsis at a paediatric emergency department in Johannesburg, South Africa. Afr J Emerg Med. (2022) 12:362–5. doi: 10.1016/j.afjem.2022.07.013, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiwone ML, Chotta NS, Byamungu D, Mghanga FP. Prevalence and factors associated with neonatal sepsis among hospitalized newborns at Ruvuma, southern Tanzania. South Sudan Med J. (2020) 13:86–9. [Google Scholar]
- 34.Olorukooba AA, Ifusemu WR, Ibrahim MS, Jibril MB, Amadu L, Lawal BB. Prevalence and factors associated with neonatal Sepsis in a tertiary hospital, north West Nigeria. Niger Med J. (2020) 61:60–6. doi: 10.4103/nmj.NMJ_31_19, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adatara P, Afaya A, Salia SM, Afaya RA, Kuug AK, Agbinku E, et al. Risk factors for neonatal Sepsis: a retrospective case-control study among neonates who were delivered by caesarean section at the trauma and specialist hospital, Winneba, Ghana. Biomed Res Int. (2018) 2018:1–7. doi: 10.1155/2018/6153501, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and Meta-analyses: the PRISMA statement. Ann Intern Med. (2009) 151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 37.Vu-Ngoc H, Elawady SS, Mehyar GM, Abdelhamid AH, Mattar OM, Halhouli O, et al. Quality of flow diagram in systematic review and/or meta-analysis. PLoS One. (2018) 13:e0195955. doi: 10.1371/journal.pone.0195955, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madrid L, Seale AC, Kohli-Lynch M, Edmond KM, Lawn JE, Heath PT, et al. Infant group B streptococcal disease incidence and serotypes worldwide: systematic review and Meta-analyses. Clin Infect Dis. (2017) 65:S160–72. doi: 10.1093/cid/cix656, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munn Z, Moola S, Riitano D, Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag. (2014) 3:123–8. doi: 10.15171/ijhpm.2014.71, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186, PMID: [DOI] [PubMed] [Google Scholar]
- 41.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang X, Chen M, Fu R, He W, He Y, Shentu H, et al. Efficacy of kangaroo mother care combined with neonatal phototherapy in newborns with non-pathological jaundice: a meta-analysis. Front Pediatr. (2023) 11:1098143. doi: 10.3389/fped.2023.1098143, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.G/eyesus T, Moges F, Eshetie S, Yeshitela B, Abate E. Bacterial etiologic agents causing neonatal sepsis and associated risk factors in Gondar, Northwest Ethiopia. BMC Pediatr. (2017) 17:137. doi: 10.1186/s12887-017-0892-y, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bekele K, Bekele F, Edosa D, Mekonnen M, Benayew M. Magnitude and associated factors of neonatal sepsis among neonates admitted to neonatal intensive care unit of northern Oromia hospitals, Ethiopia: a multicenter cross-sectional study. Ann Med Surg. (2022) 78:103782. doi: 10.1016/j.amsu.2022.103782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sorsa A. Epidemiology of neonatal Sepsis and associated factors implicated: observational study at neonatal intensive care unit of Arsi university teaching and referral hospital, south East Ethiopia. Ethiop J Health Sci. (2019) 29:333–42. doi: 10.4314/ejhs.v29i3.5, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akindolire AE, Tongo O, Dada-Adegbola H, Akinyinka O. Etiology of early onset septicemia among neonates at the university college hospital, Ibadan, Nigeria. J Infect Dev Ctries. (2016) 10:1338–44. doi: 10.3855/jidc.7830, PMID: [DOI] [PubMed] [Google Scholar]
- 48.Aku FY, Akweongo P, Nyarko KM, Mensah LG, Amegan-Aho K, Kumi L, et al. Factors associated with culture proven neonatal sepsis in the ho municipality 2016. Pan Afr Med J. (2020) 36:281. doi: 10.11604/pamj.2020.36.281.20408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wale A, Chelkeba L, Wobie Y, Abebe A. Treatment outcome and associated factors of neonatal Sepsis at Mizan Tepi university teaching hospital, south West Ethiopia: a prospective observational study. Pediatric Health Med Ther. (2021) 12:467–79. doi: 10.2147/PHMT.S322069, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.John B, David M, Mathias L, Elizabeth N. Risk factors and practices contributing to newborn sepsis in a rural district of eastern Uganda, august 2013: a cross sectional study. BMC Res Notes. (2015) 8:339. doi: 10.1186/s13104-015-1308-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tewabe T, Mohammed S, Tilahun Y, Melaku B, Fenta M, Dagnaw T, et al. Clinical outcome and risk factors of neonatal sepsis among neonates in Felege Hiwot referral hospital, Bahir Dar, Amhara regional State, north West Ethiopia 2016: a retrospective chart review. BMC Res Notes. (2017) 10:265. doi: 10.1186/s13104-017-2573-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roble AK, Ayehubizu LM, Olad HM. Neonatal Sepsis and associated factors among neonates admitted to neonatal intensive care unit in general hospitals, eastern Ethiopia 2020. Clin Med Insights Pediatr. (2022) 16:117955652210983. doi: 10.1177/11795565221098346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agnche Z, Yenus Yeshita H, Abdela GK. Neonatal Sepsis and its associated factors among neonates admitted to neonatal intensive care units in primary hospitals in Central Gondar zone, Northwest Ethiopia, 2019. Infect Drug Resist. (2020) 13:3957–67. doi: 10.2147/IDR.S276678, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Admas A, Gelaw B, BelayTessema WA, Melese A. Proportion of bacterial isolates, their antimicrobial susceptibility profile and factors associated with puerperal sepsis among post-partum/aborted women at a referral Hospital in Bahir Dar, Northwest Ethiopia. Antimicrob Resist Infect Control. (2020) 9:14. doi: 10.1186/s13756-019-0676-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Birrie E, Sisay E, Tibebu NS, Tefera BD, Zeleke M, Tefera Z. Neonatal Sepsis and associated factors among newborns in Woldia and Dessie comprehensive specialized hospitals, north-East Ethiopia, 2021. Infect Drug Resist. (2022) 15:4169–79. doi: 10.2147/IDR.S374835, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yismaw AE, Abebil TY, Biweta MA, Araya BM. Proportion of neonatal sepsis and determinant factors among neonates admitted in University of Gondar comprehensive specialized hospital neonatal intensive care unit Northwest Ethiopia 2017. BMC Res Notes. (2019) 12:542. doi: 10.1186/s13104-019-4587-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ogunlesi TA, Ogunfowora OB. Predictors of mortality in neonatal septicemia in an underresourced setting. J Natl Med Assoc. (2010) 102:915–22. doi: 10.1016/S0027-9684(15)30710-0, PMID: [DOI] [PubMed] [Google Scholar]
- 58.Ogundare E, Akintayo A, Aladekomo T, Adeyemi L, Ogunlesi T, Oyelami O. Presentation and outcomes of early and late onset neonatal sepsis in a Nigerian hospital. Afr Health Sci. (2019) 19:2390–9. doi: 10.4314/ahs.v19i3.12, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.West BA, Peterside O. Sensitivity pattern among bacterial isolates in neonatal septicaemia in port Harcourt. Ann Clin Microbiol Antimicrob. (2012) 11:7. doi: 10.1186/1476-0711-11-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Atlaw D, Seyoum K, Handiso D, Berta M. Puerperal sepsis and associated factors among women attending postnatal care service at University of Gondar Referral Hospital. Int J Pregnancy Child Birth. (2019) 5:190–5. doi: 10.15406/ipcb.2019.05.00175 [DOI] [Google Scholar]
- 61.Alemayehu A, Alemayehu M, Arba A, Abebe H, Goa A, Paulos K, et al. Predictors of neonatal Sepsis in hospitals at Wolaita Sodo town, southern Ethiopia: institution-based unmatched case-control study, 2019. Int J Pediatr. (2020) 2020:1–10. doi: 10.1155/2020/3709672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bejitual K, Fikre R, Ashegu T, Zenebe A. Determinants of neonatal sepsis among neonates admitted to the neonatal intensive care unit of public hospitals in Hawassa City administration, Sidama region, Ethiopia, 2020: an unmatched, case-control study. BMJ Open. (2022) 12:e056669. doi: 10.1136/bmjopen-2021-056669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Demisse GA, Sifer SD, Kedir B, Fekene DB, Bulto GA. Determinants of puerperal sepsis among post partum women at public hospitals in west SHOA zone Oromia regional STATE, Ethiopia (institution BASEDCASE control study). BMC Pregnancy Childbirth. (2019) 19:95. doi: 10.1186/s12884-019-2230-x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Teshome G, Hussen R, Abebe M, Melaku G, Wudneh A, Molla W, et al. Factors associated with early onset neonatal sepsis among neonates in public hospitals of Sidama region, southern Ethiopia, 2021: unmatched case control study. Ann Med Surg. (2022) 81:104559. doi: 10.1016/j.amsu.2022.104559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Adatara P, Afaya A, Salia SM, Afaya RA, Konlan KD, Agyabeng-Fandoh E, et al. Risk factors associated with neonatal Sepsis: a case study at a specialist Hospital in Ghana. Sci World J. (2019) 2019:1–8. doi: 10.1155/2019/9369051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gebremedhin D, Berhe H, Gebrekirstos K. Risk factors for neonatal Sepsis in public hospitals of Mekelle City, North Ethiopia, 2015: unmatched case control study. PLoS One. (2016) 11:e0154798. doi: 10.1371/journal.pone.0154798, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Masanja PP, Kibusi SM, Mkhoi ML. Predictors of early onset neonatal Sepsis among neonates in Dodoma, Tanzania: a case control study. J Trop Pediatr. (2020) 66:257–66. doi: 10.1093/tropej/fmz062, PMID: [DOI] [PubMed] [Google Scholar]
- 68.Akalu TY, Gebremichael B, Desta KW, Aynalem YA, Shiferaw WS, Alamneh YM. Predictors of neonatal sepsis in public referral hospitals, Northwest Ethiopia: a case control study. PLoS One. (2020) 15:e0234472. doi: 10.1371/journal.pone.0234472, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dirirsa DE, Dibaba Degefa B, Gonfa AD. Determinants of neonatal sepsis among neonates delivered in Southwest Ethiopia 2018: a case-control study. SAGE Open Med. (2021) 9:205031212110270. doi: 10.1177/20503121211027044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kayom VO, Mugalu J, Kakuru A, Kiguli S, Karamagi C. Burden and factors associated with clinical neonatal sepsis in urban Uganda: a community cohort study. BMC Pediatr. (2018) 18:355. doi: 10.1186/s12887-018-1323-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alemu M, Ayana M, Abiy H, Minuye B, Alebachew W, Endalamaw A. Determinants of neonatal sepsis among neonates in the northwest part of Ethiopia: case-control study. Ital J Pediatr. (2019) 45:150. doi: 10.1186/s13052-019-0739-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kayange N, Kamugisha E, Mwizamholya DL, Jeremiah S, Mshana SE. Predictors of positive blood culture and deaths among neonates with suspected neonatal sepsis in a tertiary hospital, Mwanza-Tanzania. BMC Pediatr. (2010) 10:39. doi: 10.1186/1471-2431-10-39, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ogunlesi TA, Ogunfowora OB, Osinupebi O, Olanrewaju DM. Changing trends in newborn sepsis in Sagamu, Nigeria: bacterial aetiology, risk factors and antibiotic susceptibility. J Paediatr Child Health. (2011) 47:5–11. doi: 10.1111/j.1440-1754.2010.01882.x, PMID: [DOI] [PubMed] [Google Scholar]
- 74.Ogundare E, Akintayo A, Florence D, Okeniyi J, Adeyemi A, Ogunlesi T, et al. Neonatal Septicaemia in a rural Nigerian hospital: Aetiology, presentation and antibiotic sensitivity pattern. Br J Med Med Res. (2016) 12:1–11. doi: 10.9734/BJMMR/2016/22325 [DOI] [Google Scholar]
- 75.Siakwa M, Kpikpitse D, Mupepi S, Semuatu M. Neonatal Sepsis in rural Ghana: a case control study of risk factors in a birth cohort. Peer Reviewed Articles. (2014); Available at:https://scholarworks.gvsu.edu/kcon_articles/43
- 76.Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. (2011) 343:d4002. doi: 10.1136/bmj.d4002, PMID: [DOI] [PubMed] [Google Scholar]
- 77.Seale AC, Mwaniki M, Newton CRJC, Berkley JA. Maternal and early onset neonatal bacterial sepsis: burden and strategies for prevention in sub-Saharan Africa. Lancet Infect Dis. (2009) 9:428–38. doi: 10.1016/S1473-3099(09)70172-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alam MM, Saleem AF, Shaikh AS, Munir O, Qadir M. Neonatal sepsis following prolonged rupture of membranes in a tertiary care hospital in Karachi, Pakistan. J Infect Dev Ctries. (2014) 8:067–73. doi: 10.3855/jidc.3136 [DOI] [PubMed] [Google Scholar]
- 79.Onyedibe KI, Utoh-Nedosa AU, Okolo M, Onyedibe KIO, Ita OI, Udoh UA, et al. Impact of socioeconomic factors on neonatal Sepsis in Jos. Nigeria Jos J Med. (2012) 6:54–8. [Google Scholar]
- 80.Murthy S, Godinho MA, Guddattu V, Lewis LES, Nair NS. Risk factors of neonatal sepsis in India: a systematic review and meta-analysis. PLoS One. (2019) 14:e0215683. doi: 10.1371/journal.pone.0215683, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tewari V. Current evidence on prevention and Management of Early Onset Neonatal Sepsis. J Infect Dis Ther. (2016) 4:2–5. [Google Scholar]
- 82.Mohamed DA, Ibrahim SA, Suleman SK. Risk factors associated with early and late-onset of neonatal Sepsis in Duhok City. Erbil J Nurs Midwifery. (2020) 3:1–10. doi: 10.15218/ejnm.2020.01 [DOI] [Google Scholar]
- 83.Hayun M, Alasiry E, Daud D, Febriani DB, Madjid D. The risk factors of early onset neonatal sepsis. Am J Clin Exp Med. (2015) 3:78–82. doi: 10.11648/j.ajcem.20150303.11 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.


