Highlights
-
•
Patients presenting periodontal disease increased the risk of developing gastric adenocarcinoma by 17 %.
-
•
The association remained regardless of the diagnostic method for periodontal disease, i.e., clinical examination and self-report.
-
•
Moreover, Asian patients with periodontal disease had a higher risk of having gastric adenocarcinoma than American and European patients.
Keywords: Gastric neoplasia, Periodontal disease, Periodontitis, Epidemiology
Abstract
Background
The oral cavity is a link between of external environment with gastrointestinal tract. Studies are controversial on the presence of Periodontal Disease (PD) and its association with Gastric Adenocarcinoma (GAC).
Methods
The authors performed a systematic review and meta-analysis to verify the association between PD and GAC. Six electronic databases were evaluated between 1961 and 2022. Titles and abstracts were reviewed independently according to the eligibility criteria, assessing full texts of selected studies. The quality of the included research was verified using the Newcastle-Ottawa Scale for case-control and cohort studies. Statistical analyses were performed based on fixed and/or random effects models to calculate the summarized Relative Risk (RR) and its 95 % Confidence Interval (95 % CI).
Results
There were 639 studies, of which nine articles were included (3 case-controls and 6 cohorts). Overall, the authors identified 1,253 cases of GAC 2,501 controls in case-control studies, and 1,631 patients with GAC enrolled in cohort studies. Patients presenting PD increased the risk of developing GAC by 17 % (RR=1.17; 95 % CI 1.03‒1.32), which remained regardless of the diagnostic method for PD, i.e., clinical examination (RR = 1.19; 95 % CI 1.14‒1.24) and self-report (RR = 1.34; 95 % CI 1.06‒1.69). Moreover, Asian patients (RR=1.17; 95 % CI 1.00‒1.36) with PD had a higher risk of having GAC than American and European patients (RR = 1.18; 95 % CI 0.84‒1.66).
Conclusions
The presence of PD the risk of GAC suggesting that its infectious-inflammatory process of PD may be related to GAC development. Further investigations on the oral-gastric microbiota and its role in the carcinogenesis of gastric cancer should be carried out, and the screening of patients with potential risk for GAC should be considered in the clinical practice of dentists.
Introduction
In 2020, Gastric Cancer (GC) was the malignancy that had more than one million new cases and about 770,000 deaths worldwide. Being it is fifth most frequent cancer and the fourth cause of cancer death in the world.1 Its risk factors already known, in addition to infection by Helicobacter pylori (H. pylori), are obesity, excessive intake of salt and meat, low consumption of fruits and vegetables, smoking, alcoholism, and low socioeconomic status, they are associated with gastric carcinoma and other malignancies.2,3
Periodontal diseases affect up to 50 % of the world's population and rank sixth among the most prevalent pathologies worldwide.4 Alterations in oral dysbioses change the oral microbiome, which may lead to oral pathologies such as Periodontal Disease (PD). Gingivitis and periodontitis are the most common forms of PD. This disease has different clinical signs of inflammation limited to the gum (gingivitis), while periodontitis results in progressive destruction of the periodontal ligament and alveolar bone, forming pouch, gingival retraction, or both5,6 and tooth loss is considered a result a significant increase in periodontal diseases in individuals over 40 years of age.7 Therefore, there is some evidence about that dysbiosis occurring in the oral cavity, such as periodontal disease, is a trigger for cancer, such as gastric adenocarcinoma.8,9
But epidemiological evidence on the association of PD and GAC remains limited and controversial, where some studies suggest positive associations, reporting that the infectious-inflammatory process of PD is capable of initiating inflammation mediators and microorganisms that may initiate the carcinogenesis10, 11, 12, 13, 14, 15 however some studies had null results, mainly due to, lack of pattern about data on exposure.16, 17, 18 This study aimed to conduct a systematic review with meta-analysis to investigate the an association between PD and GAC.
Materials and methods
This project was registered on the International Prospective Register of Systematic Reviews ‒ PROSPERO platform on January 18, 2021, under registration code CRD42021221317.
Literature search
This systematic review was conducted and reported in accordance with PRISMA guidelines.19 Articles were identified through searches limited to the English language on the PubMed, Embase, Web of Science, Scopus, Lilacs and Opengrey databases. The search strategy was based on different terms for each database (Table 1).
Table 1.
Search strategy.
| DATABASE | WEBSITE | SEARCH STRATEGY | TOTAL |
|---|---|---|---|
| Medline/PUBMED via National Library of Medicine | http://www.ncbi.nlm.nih.gov/pubmed | (((((((Periodontal Diseases [mh]) OR (Periodontal Diseases)) OR (Periodontal Diseases [tiab])) OR (periodontitis)) OR (periodont*)) OR (((((gingivitis [mh]) OR (gingivitis)) OR (gingivitis [tiab])) OR (gingivitis)) OR (gingivitis*))) OR ((((((tooth loss [mh]) OR (tooth loss)) OR (tooth loss [tiab])) OR (tooth loss)) OR (tooth loss*)))) AND (((((((Stomach Neoplasms [mesh]) OR (Stomach Neoplasms)) OR (stomach cancer)) OR (gastric adenocarcinoma)) OR (adenocarcinoma [tiab])) OR (gastric cancer))) | 353 |
| Embase | www.embase.com | ('periodontal disease' OR gingivitis OR periodontitis OR 'tooth loss') AND 'gastric cancer' | 74 |
| Scopus | www.scopus.com | (TITLE-ABS-KEY (periodontal AND disease) OR TITLE-ABS-KEY (periodontitis) OR TITLE-ABS-KEY (gingivitis) OR TITLE-ABS-KEY (tooth AND loss) AND TITLE-ABS-KEY (gastric AND cancer)) | 137 |
| Lilacs | https://lilacs.bvsalud.org/ | "(periodontal diseases OR periodontitis OR gingivitis OR tooth loss) AND (stomach neoplasms OR gastric cancer)" | 0 |
| Web of science | www.webofknowledge.com | TI=(cancer) AND TS=(periodontal* diseases OR gingivitis OR periodontitis OR tooth* loss AND gastric cancer*) AND AB=(stomach cancer OR gastric cancer AND periodontal disease OR gingivitis OR periodontitis OR tooth loss) AND TI=(stomach cancer OR gastric cancer AND periodontal disease OR gingivitis OR periodontitis OR tooth loss) | 75 |
| OpenGrey | http://www.opengrey.eu | "periodontal disease" OR “gingivitis” OR “periodontitis” OR “tooth loss” AND "gastric" | 0 |
Study selection
In this study, the presence of PD was considered whether, at least, one of these clinical characteristics occurs: gingivitis, periodontitis and tooth loss.5,20 This systematic review included case-controls and cohorts studies, and the authors excluded cross-sectional, experimental, animal studies, and case reports. Thus, the authors used the Rayyan software to identify eligible studies and exclude duplicates.21 The researchers (FJNA and MAF) retrieved data from studies independently based on titles and abstracts of the eligible studies according to the question of the systematic review (Are patients with periodontal disease at risk for developing gastric adenocarcinoma?). Moreover, references of the selected articles were reviewed to find relevant studies.
Data extraction
The following variables were collected: (1) First author, year of the publication, and place of the study; (2) Type and period of the study; (3) Sample size, sex, and age; (4) Exposure presence of PD; (5) Diagnosis method for the exposure; (6) Outcome gastric cancer; (7) Diagnosis methods: self-declare and clinical examination; (8) Association measures and 95 % CI (OR, RR, HR); and (9) Adjustment variables according to the articles reviewed: Sex; Smoking; Alcohol; socioeconomic status; Intake of vegetables and fruits; BMI; Regular physical activity among others (Tables 2 and 3). The discrepancies between the two reviewers were solved with the participation of a third evaluator (FSM).
Table 2.
Characteristics of the case-control studies included in the systematic review.
| Author/ Year/ Country | Design/ Study period | Sample/ Sex/ Age | Exposure | Diagnostic Method for Exposure | Outcome | Diagnostic Method for Theutcome | Association measure and 95 % CI | Adjustment variables |
|---|---|---|---|---|---|---|---|---|
| Watabe et al./ 1998/ Japan[37] | case-control 1996 to 1997 | Cases: 242 F = 62 (25. 6 %) M = 180 (74. 4 %) Controls: 484 40 - 79 years | Gingivitis Tooth loss | Self-report | Gastric Cancer | Pathology | Gingivitis OR= 1.2 (0.8–1.8) Tooth loss OR=1.7 (1.2–2.4) | – |
| Hiraki et al./ 2008/ Japan[38] | case-control 2001 to 2005 | Cases: 5240 F = 2541 (48.5 %) M = 2699 (51.5 %) Controls: 10,480 F = 5082 (48.5 %) M = 5398 (51. 5 %) 20 - 79 years | Tooth loss | Self-report | Gastric Cancer N = 702 | Hospital record | 21 teeth left OR=1.0 (reference) 9–20 teeth remaining OR= 1.0 (0.8–1.3) 1–8 teeth remaining OR= 1.1 (0.8–1.5) 0 teeth remaining OR= 0.9 (0.5–1.4) | It acts; Fri; Smoking; Alcohol; Intake of vegetables and fruits; BMI; Regular physical activity. |
| Shakeri et al./ 2013/ Iran[39] | case-control 2004 to 2011 | Cases: 309 F = 83 (26.9 %) M = 226 (73.1 %) Controls: 613 F = 167 (27.2 %) M = 446 (72. 8 %) 40 - 75 years | Tooth loss: Category 1 (≤ 12); Category 2 (13–18); Category 3 (19–24); Category 4 (25–31); Category 5 (32) | Clinical examination | Cardia GC N = 1 61 Non-cardia GC N = 118 | Histology | Tooth loss All gastric adenocarcinomas OR= (reference= Category 1(≤12)) Category 2(13–18) OR=0.5(0.2–1.1) Category 3(19–24) OR =0.9(0.4–1.7) Category 4(25–31) OR =1.6(0.8–3.2) Category 5(32) OR =1.4(0.6–3.0) Cardia GC OR = (reference = Category 1(≤12)) Category 2(13–18) OR = 0.6 (0.2–1.9) Category 3(19–24) OR = 1.6 (0.6–4.35) Category 4(25–31) OR = 3.5 (1.2–9.7) Category 5(32) OR = 1.4 (0.4–4.5) Non-cardia GC OR = (reference= Category 1(≤12)) Category 2(13–18) OR = 0.3 (0.1–1.2) Category 3(19–24) OR = 0.4 (0.1–1.3) Category 4(25–31) OR = 1.7 (0.5–5.6) Category 5(32) OR = 2.1 (0.6–6.9) | It acts; Andthnicity; Education; Consumption of fruits and vegetables, Opium or Tobacco; Denture use. Socioeconomic status |
CI, confidence interval; F, female; M, male; CG, gastric cancer; OR, odds ratio; RR, relative risk; HR, hazard ratio; BMI, body mass index; COPD, chronic obstructive pulmonary disease; ICD, international classification of diseases.
Table 3.
Characteristics of cohort studies included in the systematic review.
| Author/ Year/ Country | Design/ Study period | Sample/ Sex/ Age | Exposure | Diagnostic Method for Exposure | Outcome | Diagnostic Method for Outcome | Association measure and 95 % CI | Adjustment variables |
|---|---|---|---|---|---|---|---|---|
| Michaud et al./ 2008/ USA[31] | Prospective 1986 to 2004 | 48,375 men health professionals 40 - 75 years | Periodontal disease; Tooth loss. | Self-report and imaging | Gastric Cancer N = 106 | Self-report and Medical records | Periodontal disease In HR*=1.0 (reference) Yes HR*= 1.1 (0.7–1.7) tooth loss (reference=25–32 teeth) HR*= 1.0 17–24 teeth HR*=1.1 (0.6–1.8) 0–16 teeth HR*= 1.1 (0.5–2.1) Periodontal disease In HR**= 1.0 (reference) Yes HR**= 1.3 (0.8–2.0) tooth loss (reference=25–32 teeth) HR**= 1.0 17–24 teeth HR**=1.2 (0.7–2.0) 0–16 teeth HR**= 1.3 (0.6–2.5) | *Smoking history (never; former smoker < 10 years; former smoker >10 years; current smoker 1–14 cigarettes/day; 15–24 cigarettes/day; 25+ cigarettes/day) and packets-year (continuous). **Age (continuous), race (white, Asian and black), physical activity, history of diabetes (yes/no), alcohol (quartile), BMI (<22.22–24.9.25–29.9.30+), geographic location (south, east, northeast, midwest) height (quintiles), calcium intake (quintiles), total caloric intake (quintiles), red meat intake (quintiles), fruit and vegetable intake (quintiles), vitamin D (deciles). |
| Arora et al./ 2010/ Sweden[43] | Prospective Cohort 1963 to 2004 | 15. 333 F = 8. 433 (55 %) M = 6. 900 (45 %) 38 - 77 years | Periodontal disease | Self-report | Gastric Cancer N = 193 | National Registry | Periodontal disease HR= 0.8 (0.4–1.5) Less mobility HR= 0.8 (0.5–1.3) in disease HR= 1.00 (reference) | Sex (male/female), age (years), education (no schooling/secondary/vocation/other), employment (yes/no/housewife/pensioner/other), number of siblings (ordinal), smoking status (smoker current > 1 pack/day; smoker 〈1 pack/day; former smoker 〉 1 pack/day; former smoker < 1 pack/day; never smoked), partner's smoking status (smoker/ex-smoker/never smoked), smoking status of alcohol (alcohol drinker/ex drinker/never), diabetes (yes/no) and BMI (<20, 20–24.9, 25–29.9.>30 kg/m2). |
| Wen et al./ 2013/ Taiwan[44] | Retrospective Cohort 1997 to 2010 | 144.896 F = 71,086 (49. 1 %) M = 73,810 (50. 9 %) >20 years | Periodontitis (ICD-9: 523.3 and 523.4) and Gingivitis (ICD-9: 523.0 and 523.1) | Positive diagnosis and treated at least 3 times | Gastric Cancer N = 151 | Histology through national registry | RR= 1.0 (1.0–1.1) Adjusted HR*= 0.9 (0.7–1.1) | *Fri; It acts; Presence of comorbidity. |
| Chung et al./ 2015/ Taiwan[45] | Retrospective Cohort 2002 to 2009 | 40.140 F = 20,190 (50. 3 %) M = 19,950 (49. 7 %) >40 years | chronic periodontitis | Positive diagnosis based on symptoms, medical history and diagnostic test result. | Gastrointestinal cancer (ICD-9: 150–159) N = 1084 | Medical records | Adjusted HR= 1.2 (1.1–1.2) | Monthly income; Geographic region; Diabetes. |
| Nwizu et al./ 2017/ USA[30] | Prospective Cohort 1999 to 2013 | 65,869 post-menopausal women 50 - 79 years | Periodontal disease | Self-report | Stomach | Self-report in biennial questionnaires and medical records | Stomach HR=1.5 (0.9–2.6) | – |
| Chou et al./ 2018/ Taiwan[46] | Retrospective Cohort 2001 to 2010 | 50,970 F = 24,850 (48.7 %) M = 26,120 (51.3 %) 35 - 80 years | Moderate and severe periodontal disease | Database: diagnosed patients who received an additional procedure code for periodontitis. | Stomach (N = 101) | Patients diagnosed with gastrointestinal cancer according to the ICD-O-3 (C-16) | HR=1.0 (0.6–1.4) | It acts; Fri; Comorbidities (diabetes mellitus/colectomy); index of Charlson; Medication (aspirin/NSAIDs); Socioeconomic level (estimated monthly income and educational level). |
CI, confidence interval; F, female; M, male; CG, gastric cancer; OR, odds ratio; RR, relative risk; HR, hazard ratio; BMI, body mass index; COPD, chronic obstructive pulmonary disease; DHF, international classification of diseases; DMFT, decayes, missing and filled teeth.
Risk of bias
The Newcastle-Ottawa Scale (NOS) was applied to evaluate the quality of selected studies, by two independent, previously trained and approved reviewers. The methodological is divided into three components: group selection (0‒4 points), quality of adjustment for confounding (0‒2 points), and exposure assessment after outcome (0‒3 points). The maximum score can be 9 points, which represents high methodological quality.22 A the funnel plot was carried out to assess the risk of publication bias.23
Statistical analysis
For this meta-analysis, the authors considered the following measures of association: Odds Ratio (OR); Relative Risk (RR): Hazard Ratio (HR) and their respective 95 % Confidence Intervals (95 % CI). Accordingly, the authors carried out fixed and random effects models using the “metan” command.24 The heterogeneity among the studies was assessed by the I2 statistic, where I2 = 0‒25 % indicated low heterogeneity; I2 = 25 %‒50 %, moderate heterogeneity; and I2 > 50 %, high heterogeneity.25 The authors used the random effects model in case of high heterogeneity among studies and the software to perform the meta-analysis was STATA 15.
Results
Study selection
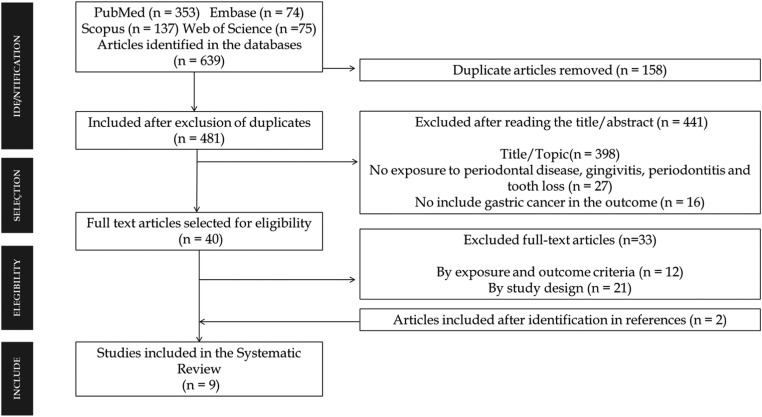
The authors have found 639 articles between 1961 and 2022 (Fig. 1). After reading their title/abstract, the study excluded 441. From that 40 articles were considered eligible for a full reading. Seven studies were shortlisted for the meta-analysis,16,26, 27, 28, 29, 30, 31 two additional articles were included.32,33 Thus, nine articles were included in this systematic review, three case control studies26,27,32 and six cohort studies16,28, 29, 30, 31,33 published between 1998 and 2018. The authors found a population of 2884 cases patients with GAC in the nine studies included.
Fig. 1.
Prisma Selection of eligible studies for the systematic review.
About six studies were carried out in Asia accounting 2280 gastric adenocarcinoma cases and the diagnostic criteria for PD was the clinical examination (Tables 2 and 3).
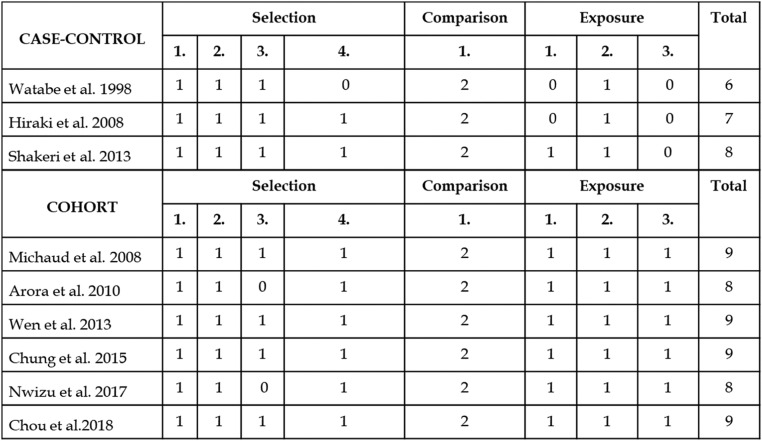
In cohort studies, the NOS ranged from eight points30,33 to nine points,16,28,29,31 and between six and eight points26,27,32 among case-control studies selected therefore selected studies have high scores in quality (Fig. 2).
Fig. 2.
Evaluation of the methodological quality of case-control and cohort studies according to the Newcastle ‒ Ottawa Scale (Wells et al., 2014).
In cohort studies, the NOS ranged from eight points30,33 to nine points.
Summary of meta-analysis
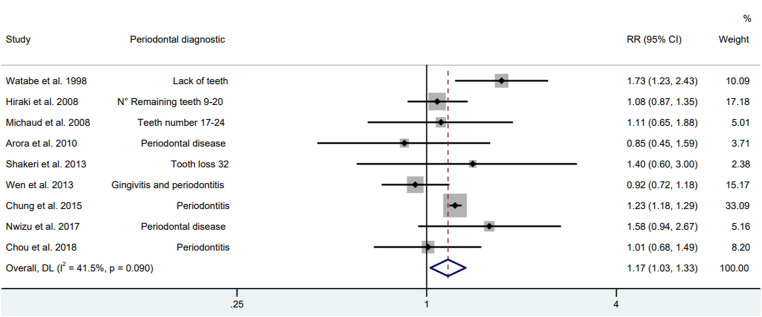
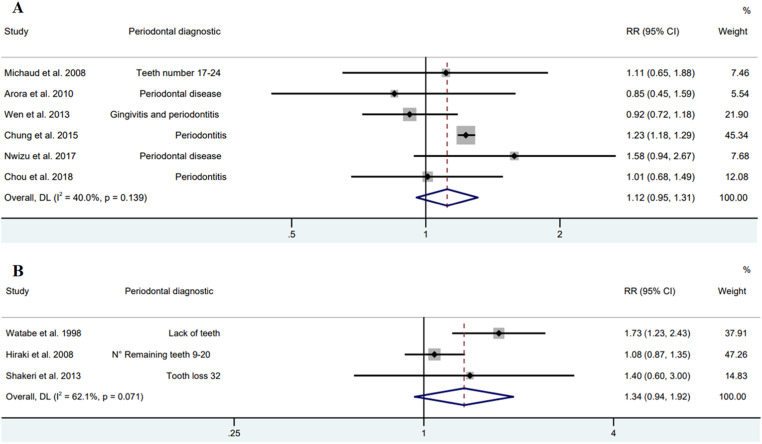
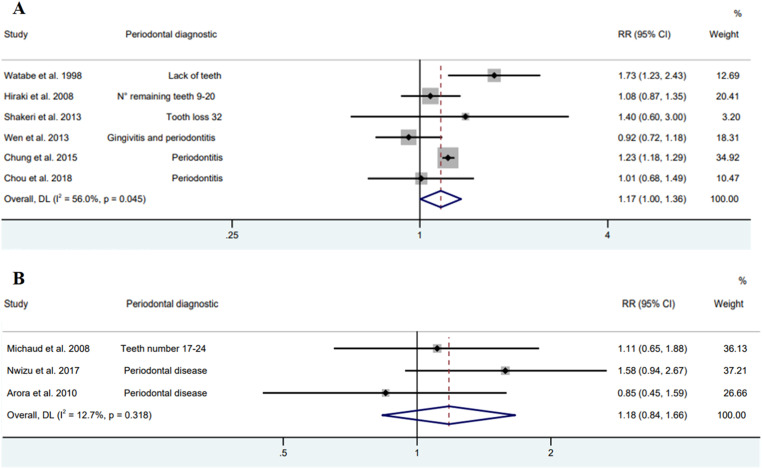
In the meta-analysis of nine observational studies, the presence of PD was associated to an increase in the risk of GAC by 17 % (RR = 1.17; 95 % CI 1.03‒1.32), with a heterogeneity of 39.9 % (Fig. 3). While in the subgroup analysis, cohort and case-control studies had no association with PD and GAC (Fig. 4).
Fig. 3.
Forest plot of cohort and case-control studies between periodontal disease and gastric adenocarcinoma (Random model).
Fig. 4.
Forest plot of cohort (A) and case-control (B) studies between periodontal diseases and gastric adenocarcinoma (Random model).
An association between GAC and PD was found to be a risk in the Asian population (RR = 1.17; 95 % CI 1.00‒1.36); however, in American and European studies there was no risk (RR = 1.18; 95 % CI 0.84‒1.66) (Fig. 5).
Fig. 5.
Forest plot of Asian (A) and American and European (B) studies between periodontal diseases and gastric adenocarcinoma (Random model).
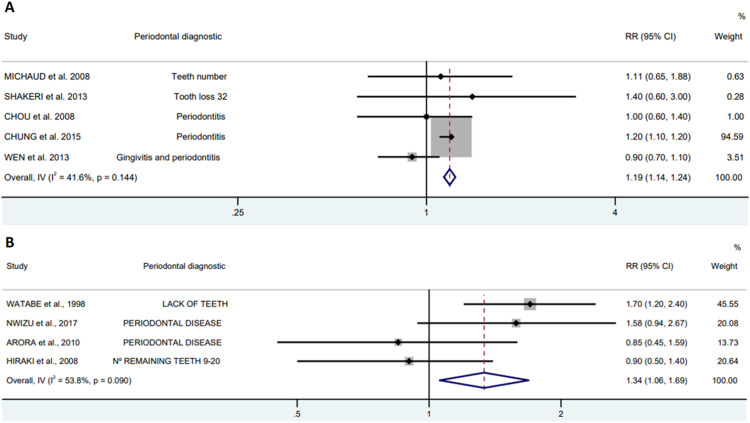
To evaluate the presence of PD In epidemiological studies, it can be assessed by patient self-report and by clinical examination. The authors observed an increased risk of patients with PD presenting GAC, that remained regardless of the diagnostic method for PD, 19 % (RR=1.19; 95 % CI 1.14‒1.24) to 34 % (RR = 1.34; 95 % CI 1.06‒1.69) for clinical examination and self-report, respectively (Fig. 6).
Fig. 6.
Forest plot of periodontal diagnostic, clinical examination (A) self-report (B) between periodontal diseases and gastric adenocarcinoma.
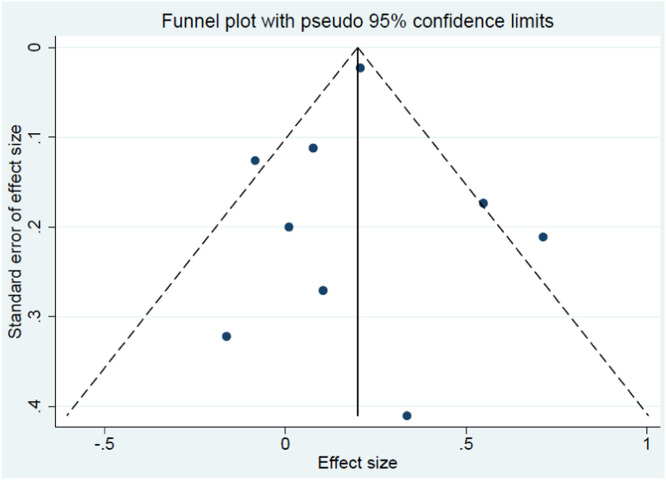
No publication bias was observed in the selected studies according to the Egger test (p = 0.860) (Fig. 7).
Fig. 7.
Funnel plot of all studies included in the meta-analysis.
Discussion
To our knowledge, this is the first meta-analysis to explore the association of PD with the risk of GAC. In this study, patients with PD were at risk of developing GAC, which validates the hypothesis that PD is proposed as a potential carcinogenic factor.16 The authors also observed that the risk for GAC continues regardless of the diagnostic method for PD. However, there were differences between populations; Asian patients were at risk of developing GAC associated with PD than Americans and Europeans. It is necessary to point out that the studies had several adjustment variables: Sex; Smoking; Alcohol; socioeconomic status; Intake of vegetables and fruits; BMI; Regular physical activity. Therefore, the present results highlight that periodontal diseases have a significant effect on GAC, further studies are needed to assess how this mechanism occurs and which other microorganisms may be linked to oral-gastric dysbiosis.
Gastric cancer is the leading cause of death among men in South Asian countries.1 This study found an association between periodontal disease and gastric adenocarcinoma in Asian studies, as opposed to American and European studies. Asian populations have polymorphisms of the interleukin genes (IL-17 and IL-10) that increase the risk of gastric cancer, due to their interaction with H. pylori and the habit of smoking.34 These same genetic polymorphisms can cause phenotypic differences in the inflammatory responses in PD, which are important in the individual's sensitivity to the disease, in the progression of the disease or in the response to treatment.35 The prevalence of PD varies between 16 % (Western Pacific region) and 23 % (Africa region), while case numbers reflect the demographic share of the respective regions, with Southeast Asia and Western Pacific regions having the highest number of cases and the Eastern Mediterranean region with the lowest number of PD cases.4
The gold standard for evaluating PD is probing all teeth and radiographic interpretation.36,37 However, in studies conducted with large populations, they are less feasible since they require a big number of trained examiners, high-costly dental equipment, and infection control protocols that demand unpractical execution time.37, 38, 39 Self-report and clinical examination are an accessible, reliable, and cost-saving method.39, 40, 41 In this meta-analysis, the authors observed an increased risk of PD patients developing GAC, regardless of the diagnostic method used for PD.
This meta-analysis presents limitations. There is a missing information among the studies, such as sex16,26,27,30, 31, 32, 33 topography (cardia and non-cardia),16,26,30, 31, 32, 33 and different age groups.16,26,30, 31, 32, 33 In addition to what studies use as a proxy for PD (gingivitis, periodontitis and tooth loss), therefore, the authors included it in the systematic review. However, it has strength as many participants providing accurate risk estimates, and a high methodological quality of the selected studies. Furthermore, the sensitivity analysis identified that patients with PD may be associated with the development of GAC, regardless of the diagnostic method for PD.
Since there is not enough evidence demonstrating how this association between PD and risk of GAC occurs. Additional studies with more detailed PD data and assessment of the oral microbiome may provide more clarity.
Conclusion
The presence of PD increased the risk of GAC. Several studies suggest that the infectious-inflammatory process of PD can initiate complex reactions involving inflammation mediators and microorganisms that may link the risk of tumor development, therefore there are biological bases to support a relationship between PD and GAC, but more studies are needed to assess the depth of this connection. In addition to considering the screening of patients at potential risk for GAC in the clinical practice of dentists.
PRISMA 2009 checklist statement
The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to it.
Conflicts of Interest
The authors declare no conflicts of interest.
Footnotes
Supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior ‒ Brasil (CAPES) ‒ student fellowship by Finance Code 001.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Zali H., Rezaei-Tavirani M., Azodi M. Gastric cancer: prevention, risk factors and treatment. Gastroenterol Hepatol Bed Bench. 2011;4(4):175–185. [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrari F., Reis M.A.M. Study of risk factors for gastric cancer by populational databases analysis. World J Gastroenterol. 2013;19(48):9383–9391. doi: 10.3748/wjg.v19.i48.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernabe E., Marcenes W., Hernandez C.R., Bailey J., Abreu L.G., Alipour V., et al. Global, regional, and national levels and trends in burden of oral conditions from 1990 to 2017: a systematic analysis for the global burden of disease 2017 study. J Dent Res. 2020;99(4):362–373. doi: 10.1177/0022034520908533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caton J.G., Armitage G., Berglundh T., Chapple I.L.C., Jepsen S., Kornman K.S., et al. A new classification scheme for periodontal and peri-implant diseases and conditions – Introduction and key changes from the 1999 classification. J Clin Periodontol. 2018;45 Suppl 20:S1–S8. doi: 10.1111/jcpe.12935. [DOI] [PubMed] [Google Scholar]
- 6.Kornman K.S. Interleukin 1 genetics, inflammatory mechanisms, and nutrigenetic opportunities to modulate diseases of aging. Am J Clin Nutr. 2006;83(2):475S–483S. doi: 10.1093/ajcn/83.2.475S. [DOI] [PubMed] [Google Scholar]
- 7.Chapple I.L.C., van der Weijden F., Doerfer C., Herrera D., Shapira L., Polak D., et al. Primary prevention of periodontitis: managing gingivitis. J Clin Periodontol. 2015;42(16):S71–S76. doi: 10.1111/jcpe.12366. Suppl. [DOI] [PubMed] [Google Scholar]
- 8.Nicolae F.M., Didilescu A.C., Șurlin P., Ungureanu B.S., Șurlin V.M., Pătrașcu Ș., et al. Subgingival periopathogens assessment and clinical periodontal evaluation of gastric cancer patients ‒ A cross sectional pilot study. Pathogens. 2022;11(3):360. doi: 10.3390/pathogens11030360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li N., Wang Z. Integrative analysis of deregulated mirnas reveals candidate molecular mechanisms linking H. pylori infected peptic ulcer disease with periodontitis. Dis Markers. 2022;2022 doi: 10.1155/2022/1498525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schenkein H.A. Host responses in maintaining periodontal health and determining periodontal disease. Periodontol. 2000;40:77–93. doi: 10.1111/j.1600-0757.2005.00144.x. 2006. [DOI] [PubMed] [Google Scholar]
- 11.Salazar C.R., Francois F., Li Y., Corby P., Hays R., Leung C., et al. Association between oral health and gastric precancerous lesions. Carcinogenesis. 2012;33(2):399–403. doi: 10.1093/carcin/bgr284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al Sayed A., Anand P.S., Kamath K.P., Patil S., Preethanath R.S., Anil S. Oral cavity as an extragastric reservoir of helicobacter pylori. ISRN Gastroenterol. 2014;2014 doi: 10.1155/2014/261369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tonetti M.S., Greenwell H., Kornman K.S. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Clin Periodontol. 2018;89(1):S159–S172. doi: 10.1002/JPER.18-0006. Suppl. [DOI] [PubMed] [Google Scholar]
- 14.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15(1):30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nisha K.J., Nandakumar K., Shenoy K.T., Janam P. Periodontal disease, and Helicobacter pylori infection: a community-based study using serology and rapid urease test. J Investig Clin Dent. 2016;7(1):37–45. doi: 10.1111/jicd.12122. [DOI] [PubMed] [Google Scholar]
- 16.Michaud D.S., Liu Y., Meyer M., Giovannucci E., Joshipura K. Periodontal disease, tooth loss, and cancer risk in male health professionals: a prospective cohort study. Lancet Oncol. 2008;9(6):550–558. doi: 10.1016/S1470-2045(08)70106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lo C.H., Kwon S., Wang L., Polychronidis G., Knudsen M.D., Zhong R., et al. Periodontal disease, tooth loss, and risk of oesophageal and gastric adenocarcinoma: a prospective study. Gut. 2021;70(3):620–621. doi: 10.1136/gutjnl-2020-321949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun J., Zhou M., Salazar C.R., Hays R., Bedi S., Chen Y., et al. Chronic periodontal disease, periodontal pathogen colonization, and increased risk of precancerous gastric lesions. J Periodontol. 2017;88(11):1124–1134. doi: 10.1902/jop.2017.160829. [DOI] [PubMed] [Google Scholar]
- 19.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peres M.A., Macpherson L.M.D., Weyant R.J., Daly B., Venturelli R., Mathur M.R., et al. Oral diseases: a global public health challenge. Lancet. 2020;395(10219):185–186. doi: 10.1016/S0140-6736(19)32997-6. [DOI] [PubMed] [Google Scholar]
- 21.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wells G.A., Shea B., O'Connell D., Peterson J., Welch V., Losos M. The Newcastle-Ottawa scale. J Hell Stud. 113 (2914) 198–199.
- 23.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris R.J., Deeks J.J., Altman D.G., Bradburn M.J. Metan: fixed- and random-effects meta-analysis. Stata J. 2008;8(1):3–28. [Google Scholar]
- 25.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. Br Med J. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiraki A., Matsuo K., Suzuki T., Kawase T., Tajima K. Teeth loss and risk of cancer at 14 common sites in Japanese. Cancer Epidemiol Biomarkers Prev. 2008;17(5):1222–1228. doi: 10.1158/1055-9965.EPI-07-2761. [DOI] [PubMed] [Google Scholar]
- 27.Shakeri R., Malekzadeh R., Etemadi A., Nasrollahzadeh D., Abedi-Ardekani B., Khoshnia M., et al. Association of tooth loss and oral hygiene with risk of gastric adenocarcinoma. Cancer Prev Res. 2013;6(5):477–482. doi: 10.1158/1940-6207.CAPR-12-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wen B., Tsai C., Lin C., Chang Y., Lee C., Hsu C. Cancer risk among gingivitis and periodontitis patients: a nationwide cohort study. QJM. 2014;107(4):283–290. doi: 10.1093/qjmed/hct248. [DOI] [PubMed] [Google Scholar]
- 29.Chou S.-H., Tung Y.-C., Wu L-S, Chang C.-J., Kung S., Chu P.-H. Severity of chronic periodontitis and risk of gastrointestinal cancers: a population-based follow-up study from Taiwan. Medicine (Baltimore) 2018;97(27):e11386. doi: 10.1097/MD.0000000000011386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nwizu N.N., Marshall J.R., Moysich K., Genco R.J., Hovey K.M., Mai X., et al. Periodontal disease and incident cancer risk among postmenopausal women: results from the women's health initiative (WHI) observational cohort. Cancer Epidemiol Biomarkers Prev. 2017;26(8):1255–1265. doi: 10.1158/1055-9965.EPI-17-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung S.-D., Tsai M.-C., Huang C.-C., Kao L-T, Chen C.-H. A population based study on the associations between chronic periodontitis and the risk of cancer. Int J Clin Oncol. 2016;21:219–223. doi: 10.1007/s10147-015-0884-6. [DOI] [PubMed] [Google Scholar]
- 32.Watabe K., Nishi M., Miyake H., Hirata K. Lifestyle and gastric cancer: a case-control study. Oncol Rep. 1998;5(5):1191–1194. doi: 10.3892/or.5.5.1191. [DOI] [PubMed] [Google Scholar]
- 33.Arora M., Weuve J., Fall K., Pedersen N.L., Mucci L.A. An exploration of shared genetic risk factors between periodontal disease and cancers: a prospective co-twin study. Am J Epidemiol. 2010;171(2):253–259. doi: 10.1093/aje/kwp340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGuire S. International agency for research on cancer. World Health Organizarion. World cancer report 2014. World cancer Report 2014. Adv Nutr. 2016;7(2):418–419. doi: 10.3945/an.116.012211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linhartova P.B., Danek Z., Deissova T., Hromcik F., Lipovy B., Szaraz D., et al. Interleukin gene variability and periodontal bacteria in patients with generalized aggressive form of periodontitis. Int J Mol Sci. 2020;21(13):4728. doi: 10.3390/ijms21134728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eke P.I., Dye B.A., Wei L., Slade G.D., Thornton-Evans G.O., Beck J.D., et al. Self-reported measures for surveillance of periodontitis. J Dent Res. 2013;92(11):1041–1047. doi: 10.1177/0022034513505621. [DOI] [PubMed] [Google Scholar]
- 37.Taylor G.W., Borgnakke W.S. Self-reported periodontal disease: validation in an epidemiological survey. J Periodontol. 2007;78(7S):1407–1420. doi: 10.1902/jop.2007.060481. Suppl. [DOI] [PubMed] [Google Scholar]
- 38.Abbood H.M., Hinz J., Cherukara G., Macfarlane T.V. Validity of self-reported periodontal disease: a systematic review and meta-analysis. J Periodontol. 2016;87(12):1474–1483. doi: 10.1902/jop.2016.160196. [DOI] [PubMed] [Google Scholar]
- 39.Carra M.C., Gueguen A., Thomas F., Pannier B., Caligiuri G., Steg P.G., et al. Self-report assessment of severe periodontitis: periodontal screening score development. J Clin Periodontol. 2018;45(7):818–831. doi: 10.1111/jcpe.12899. [DOI] [PubMed] [Google Scholar]
- 40.Blicher B., Joshipura K., Eke P. Validation of self-reported periodontal disease: a systematic review. J Dent Res. 2005;84(10):881–890. doi: 10.1177/154405910508401003. [DOI] [PubMed] [Google Scholar]
- 41.Chatzopoulos G.S., Cisneros A., Sanchez M., Lunos S., Wolff L.F. Validity of self-reported periodontal measures, demographic characteristics, and systemic medical conditions. J Periodontol. 2018;89(8):924–932. doi: 10.1002/JPER.17-0586. [DOI] [PubMed] [Google Scholar]