Summary
Background
Psoriasis is a chronic inflammatory skin disease with a Th17-skewed immune phenotype. Although it has been generally accepted that regulatory T cells (Tregs) in lesional psoriatic skin have functional impairment due to the local inflammatory microenvironment, the molecular properties of skin-homing psoriatic Tregs have not been well explored.
Methods
We designed an extensive 39 marker mass cytometry (CyTOF) panel to deeply profile the immune landscape of skin-homing Tregs from 31 people with psoriasis stratified by psoriasis area severity index score as mild (n = 15) to moderate-severe (n = 16) and 32 healthy controls. We further validated the findings with an in-vitro chemokine-mediated Treg migration assay, immunofluorescent imaging of normal and psoriatic lesional skin and analysed public single-cell RNA-sequencing datasets to expand upon our findings into the local tissue microenvironments.
Findings
We discovered an overall decrease in CLAhi Tregs and specifically, CLAhiCCR5+ Tregs in psoriasis. Functional markers CD39 and FoxP3 were elevated in psoriatic Tregs. However, CCR7 expression was significantly increased while CCR4 and CLA expression was reduced in psoriatic Tregs and CLAhi Tregs, which was associated with disease severity. Moreover, psoriatic Tregs revealed increased migratory capacity towards CCR7’s ligands, CCL19/CCL21. Interrogation of public single-cell RNA sequencing data confirmed reduced expression of skin-trafficking markers in lesional-skin Tregs compared to non-lesioned skin, further substantiated by immunofluorescent staining.
Interpretation
Psoriatic circulating Tregs showed an impaired skin-trafficking phenotype thus leading to insufficient suppression of ongoing inflammation in the lesional skin, expanding upon our current understanding of the impairment of Treg-mediated immunosuppression in psoriasis.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science and Information and Communications Technology (2020R1C1C1014513, 2021R1A4A5032185, 2020R1F1A1073692); and the new faculty research seed money grant of Yonsei University College of Medicine for 2021 (2021-32-0033).
Keywords: Chemokine receptors, Mass cytometry, Psoriasis, Regulatory T cells, Skin-trafficking
Research in context.
Evidence before this study
A growing body of evidence has been filed on the potential role of regulatory T cells (Tregs) in the pathogenesis of psoriasis. Tregs play a fundamental role in maintaining immune homeostasis and tolerance. Various factors such as genetic polymorphisms of FoxP3, increased expression of microRNAs, and imbalance in Th17/Treg ratio, have been proposed to contribute to Treg dysfunctionality exhibiting reduced suppressive capacity in psoriasis. Moreover, elevated IL-6, IL-21, and IL-23 seem to promote Treg conversion into Th1/Th17 cells, possibly contributing to the pathogenesis of disease. Although these essential roles of Tregs have been highlighted, high-dimensional, comprehensive profiling of the molecular properties of psoriatic Tregs is limited. In particular, the role of skin-homing Tregs which have the potential to infiltrate into the lesional skin and exert their suppressive capacity against ongoing inflammation within the skin microenvironment has yet to be elucidated.
Added value of this study
High-throughput single-cell targeted proteomics, including mass cytometry, allows for deep phenotyping and molecular characterization of immune cell subsets. Here, we designed a mass cytometry (CyTOF) panel primarily focusing on Tregs in a cohort of 31 people with psoriasis and 32 healthy controls. To our knowledge, this is one of the largest Treg-profiling psoriasis CyTOF datasets reported. We showed an increased frequency in overall circulating Tregs and a decrease in cutaneous lymphocyte-associated antigen (CLA)+ Tregs in the psoriasis cohort. As CLA has been known to play a key role in skin homing, we further analysed circulating CLA+ Tregs. We found CLAhi Tregs showed significant elevation in expression of CCR7, which is involved in migration to secondary lymphoid organs, and marked reduction of skin-homing-related chemokine receptors including CCR4, CCR5, and CXCR3. We additionally analysed public single-cell RNA sequencing data of psoriatic lesional, non-lesional, and normal skin and found increased CCR7 and decreased CCR4, CCR5, CXCR3 and SELPLG (CLA) expression in the psoriatic skin, consistent with our CyTOF findings. This was further confirmed by immunofluorescent imaging showing increased CCR7 expression on Tregs in the lesional skin compared to normal skin. We also showed functional confirmation with an increase in migratory capacity of psoriatic Tregs towards CCR7’s ligands: CCL19 and CCL21 compared to healthy controls. In summary, our results suggest an impaired skin-trafficking phenotype of circulating psoriatic Tregs, leading to insufficient control of inflammation in the skin lesional microenvironment.
Implications of all the available evidence
There has been much evidence supporting the importance of Tregs in psoriasis pathogenesis. However, the change in Treg frequency, function and phenotype are still not clear. We show that despite the increase in overall Tregs in circulation, there is a potential of dysregulated trafficking of Tregs to migrate and persist within the inflammatory lesional skin leading to a suboptimal immune-suppressive environment and uncontrolled inflammation. These findings are in line with analysis of public single-cell RNA sequencing data shedding further light into the previously underreported role of Treg trafficking, advancing our knowledge of the mechanisms of psoriasis pathogenesis.
Introduction
Psoriasis is a chronic, T-cell-mediated inflammatory skin disorder which affects approximately 2% of the global population.1 It is characterised by thickening and scaling of the epidermis due to keratinocyte hyperproliferation.2 Although symptoms vary, the most common signs and symptoms are red plaques of skin covered with silvery scales, dry skin, and itching.2 Different subsets of T-helper (Th) cells are reported to contribute to the pathogenesis of psoriasis.3,4 In particular, Th17 cells and the interleukin (IL)-23/IL-17 axis have an essential role in pathogenesis, and targeting of these signalling molecules has been effective in psoriasis treatment.5, 6, 7
The role of regulatory T cells (Tregs) in the pathogenesis of psoriasis has been proposed in previous studies.8 Tregs are involved in suppressing the immune response and inducing immune homeostasis and self-tolerance.9 They are a subtype of CD4+ T cells expressing the key transcription factor, FoxP3, and are also characterised by high CD25 and low CD127 expression.10 About 1–3% of peripheral blood mononuclear cells (PBMCs) are CD4+ CD25+ FoxP3+ Tregs,11, 12, 13 and over 60% of circulating Tregs express cutaneous lymphocyte-associated antigen (CLA).14,15 CLA is an essential skin-homing receptor that binds to the vascular lectin endothelial cell-leukocyte adhesion molecule 1,16 and has been recently focused on for understanding disease pathogenesis in the context of tissue-homing properties across various disease entities.17,18 CLA+ T cells have been proposed in a recent study as a potential immunologic biomarker for the management of psoriasis.19
Although the precise mechanism of action of Tregs in psoriasis pathogenesis remains to be elucidated, recent studies have observed psoriatic Tregs to be functionally impaired compared to healthy Tregs.8,20, 21, 22 This Treg dysfunctionality may be derived from the pro-inflammatory cytokine milieu, such as IL-6, which downregulates Treg activity and by which Tregs encounter upon infiltrating into the lesional skin.23 Moreover, in the presence of inflammatory signals including IL-1β, IL-6, IL-21, and IL-23 with low levels of transforming growth factor (TGF)-β, Tregs acquire a Th17-like phenotype.24,25 These cytokines also induce phosphorylation of STAT3, resulting in Treg dysfunctionality.23
The concept of insufficient skin homing of Tregs to the lesional skin was also suggested to contribute to the pathogenesis of psoriasis. Soler et al. reported a decrease in the frequency of CCR5+ Tregs and showed its reduced suppressive and migratory capacity in psoriatic PBMCs.26 However, molecular characterization of psoriatic Tregs using high-dimensional approaches has yet to be addressed, particularly in regards to skin-homing propensity into and out of the inflamed lesions.
Cytometry by time of flight (CyTOF) is a powerful approach for high-throughput, high-dimensional single-cell characterization.27 Cells are labelled with metal-conjugated antibodies allowing for detection of over 40 different parameters simultaneously at the single-cell level with minimal signal overlap between detection channels. We designed a CyTOF panel encompassing 39 surface and intracellular markers leveraging lineage markers for broad cell type identification and also specific markers for deep profiling of the Treg compartment.
In the present study, we compared psoriatic PBMCs with healthy control samples, investigating differences in the broad immune landscape and phenotype of Tregs, particularly circulating CLA+ Tregs.
Methods
Subjects
We have recruited and collected samples from a total of 63 subjects: 31 people with psoriasis between 2017 and 2021 and 32 healthy donors. All participants did not receive any systemic treatment for at least 1 month except topical agents such as topical corticosteroids or vitamin D analogue and did not have any other concurrent systemic comorbidities. Within the psoriatic cohort, 16 people with psoriasis had moderate-to-severe chronic plaque psoriasis defined by the psoriasis area severity index (PASI) score >10 and 15 people had mild psoriasis (PASI score 5–10, Table 1).
Table 1.
Demographic and clinical characteristics of included participants.
| Value (count [%] or mean ± SD) |
||||
|---|---|---|---|---|
| Healthy | Psoriasis | p-value | ||
| No. of subjects | 32 | 31 | ||
| Age, years | 40.25 ± 13.02 | 41.68 ± 14.97 | 0.707 | |
| Sex | 0.020 | |||
| Female | 15 (46.88) | 12 (38.71) | ||
| Male | 17 (53.12) | 19 (61.29) | ||
| Severity, n (PASI score) | NA | Mild Moderate-to-Severe |
31 (12.37 ± 5.50) 15 (8.43 ± 0.85) 16 (16.06 ± 5.45) |
|
PASI, psoriasis area severity index.
Ethics
All participants provided written informed consent. The study was approved by the Institutional Review Board (IRB) of Severance Hospital (No. 4-2017-0782) for people with psoriasis and of Seoul National University Hospital (No. 2108-123-1246) for healthy donors.
Sample preparation
Whole blood samples were collected in sodium heparin vacutainer CPT cell preparation tubes (BD Bioscience, 362753) and processed within 2 h. Collected blood was stored at room temperature (20 °C) until isolation of PBMCs by density gradient centrifugation. Samples were centrifuged at 2,000 g for 20 min and PBMCs were collected and washed twice with RPMI-1640 medium containing 10% fetal bovine serum (FBS). PBMC counts of each sample were estimated to be approximately 8 × 106 cells from 8 ml of whole blood. Samples were then cryopreserved in cell banker 1 (Zenoaq, BLC-1) in liquid nitrogen until CyTOF analysis.
Mass cytometry staining, acquisition and data pre-processing
Samples were processed in two batches and were randomised to ensure an equal number of psoriasis and healthy donor samples within each batch. PBMC samples were first thawed in a water bath at 37 °C, transferred into cell media (RPMI + 10% FBS), washed and centrifuged at 300 g for 5 min. Cells were then incubated with Cell-ID Rh103 Intercalator (Fluidigm, 201103A) for 20 min in a 37 °C water bath to label dead cells. Cells were blocked with Fc receptor blocking solution (Biolegend, 422302) and stained with a cocktail of surface antibodies on ice for 30 min. All antibodies were either commercially purchased from Fluidigm or conjugated in-house using Fluidigm’s Maxpar ×8 antibody labelling kit. Next, samples were barcoded using Fluidigm’s 20-Plex Pd barcoding kit (201060), pooled and then fixed and permeabilized using the FoxP3/Transcription Factor Staining Buffer Kit (eBioscience, 00-5523-00). The cells were then heparin blocked at a concentration of 100 U/mL to prevent nonspecific antibody binding by eosinophils28 and stained with a cocktail of intracellular antibodies on ice for 30 min. Antibody information is shown in Supplementary Table S1. Cells were then fixed with fresh 2.4% paraformaldehyde in PBS containing 0.02% saponin and Cell-ID Intercalator-Ir (Fluidigm, 201192A) to label nucleated cells. The samples were stored in PBS until acquisition.
For CyTOF acquisition, the pooled sample was first washed with Cell Staining Buffer and then with Cell Acquisition Solution (Fluidigm, 201240) and resuspended in Cell Acquisition Solution at a concentration of 1 million cells per ml. EQ normalization beads (Fluidigm, 201078) were spiked in at a 1:20 dilution for post-acquisition normalisation. The sample was acquired on the Fluidigm Helios Mass Cytometer using the wide bore injector configuration at a speed of <400 cells/sec.29 Fluidigm’s CyTOF software v7.0 was used to normalise and concatenate the resulting FCS files. Data processing was performed to remove EQ beads, low DNA debris and gaussian multiplets and de-multiplexed using the Human Immune Monitoring Center’s (HIMC) in-house debarcoding pipeline.
CyTOF data analysis
Analysis of the entire cohort of 31 psoriasis and 32 healthy donor samples was performed by using computational pipelines developed from open-source algorithms in Python. First, each sample was individually K-means clustered into 500 clusters using Mini-Batch-K Means clustering. K-means clusters across all of the samples were aggregated together and visualised using the Clustergrammer heatmap.30 Dead-cell clusters were excluded based on high Rh-103 expression and immune cell clusters were defined based on CD45 and Ir-193 DNA intensity. Next, K-means immune clusters were manually annotated to identify broad immune cell types and the annotations were mapped back to the single-cell level for visualisation using the Uniform Manifold Approximation and Projection (UMAP) dimensionality reduction method and plotted using the plotnine package. Cell type annotations were further evaluated for accuracy using a marker expression heatmap and by parallel manual gating using Cytobank. Heatmap-based annotations were highly concordant with manual gating (Supplementary Fig. S1).
In order to further phenotype the Treg compartment, Tregs across all samples were aggregated and unbiasedly clustered using the Scanpy31 implementation of the Leiden community detection clustering algorithm32 to generate Treg meta-clusters, which were annotated based on expression of the major markers driving clustering. Treg meta-cluster annotation was visualised using UMAP along with the corresponding marker expression of the annotated Tregs.
The cell counts, frequencies, median and mean marker expression of each annotated cell population along with the annotated Treg subclusters were exported for downstream correlative analyses. Cell frequencies of broad immune cell types were exported as a percent of total CD45+ immune cells excluding cell debris and multiplets and frequencies of Treg subsets were exported as a percent of total Tregs. One sample from the mild psoriasis cohort was excluded due to a low cell count of <1000 defined singlet immune cells for a total of 31 psoriasis samples (15 mild and 16 moderate-to-severe) and 32 healthy control samples.
Analysis of public single-cell RNA sequencing dataset derived from healthy and psoriatic skin
To assess the overall tendency and correlation in our PBMC findings, we additionally evaluated two distinct single-cell RNA sequencing (scRNA-seq) datasets from the public repository. The Reynolds et al. dataset compared psoriatic lesional and non-lesional skin biopsy specimens33 (Zenodo https://doi.org/10.5281/zenodo.4288748) and the Liu et al. dataset compared skin biopsies between individuals with psoriasis and healthy donors34 (Zenodo https://doi.org/10.5281/zenodo.6529821). We used the original cell annotations provided by the authors of the study. The Seurat package (4.1.1) was used in R (4.2.1) to perform dimensionality reduction of Treg scRNA data. A total of 6 samples (3 lesional vs. 3 non-lesional) from the Reynolds et al. dataset and 15 samples (8 psoriasis vulgaris vs. 7 normal) from the Liu et al. dataset were integrated with batch correction using the R package Harmony (0.1.1). Cell counts were matched between all 3 groups by random subsampling from the psoriasis lesional and healthy donor groups to match cell counts from the non-lesional skin group, minimising potential bias from variations in cell counts between groups (n = 11,816 cells per group). We implemented the R package CellChat (1.4.0) for thorough identification of cell-to-cell interactions. We conducted gene set enrichment analysis GSEA35,36 for functional enrichment of signature genes and used the Bioconductor package fgsea (1.22.0) for identifying pathway alterations. The normalised enrichment score was calculated using the fgsea package to represent gene enrichment in the hallmark GSEA gene set. The pathway resource came from the MSigDB hallmark gene set and the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. All analyses were performed according to the standard workflow.
Isolation of Tregs from PBMCs
Cryopreserved PBMCs were thawed and rested overnight in RPMI +10% FBS in a 5% CO2 incubator at 36 °C. PBMCs were washed in EasySep Buffer (Stemcell, 20144) and resuspended at a concentration of 5 × 107 cells/ml. Tregs were then isolated using the EasyStep Human CD4+ CD127low CD25+ Regulatory T Cell Isolation Kit (Stemcell, 18063). Isolation purity was verified by flow cytometry. Cells were stained with Zombie Aqua fixable viability dye (Biolegend, 423102), CD3-FITC (BD Bioscience, 555332), CD4-BV605 (BD Bioscience, 566908), CD25-BV421 (BD Bioscience, 567485), CD127-PE-cy7 (eBioscience, 25127842), and FoxP3-PE (BD Bioscience, 560852).
PBMC activation and FACS staining
Cryopreserved PBMCs were thawed and washed in pre-warmed RPMI +10% FBS and resuspended at a stimulation cocktail containing 50 ng/ml of PMA (Sigma Aldrich, P8139), 2 μg/ml of ionomycin (Sigma Aldrich, I9657), and 10 μg/ml of Brefeldin A (eBioscience, 450651). Cells were incubated in a 5% CO2 incubator at 36 °C for 4 h. After incubation, cells were stained with Fixable viability stain 700 (BD Bioscience, 564997) at room temperature for 15 min, washed, and Fc blocked with Human TruStain FcX (Biolegend, 422301) for 10 min on ice. Then following the instructions of Foxp3/Transcription Factor Staining Buffer Set (eBioscience, 00-5523-00), cells were stained at room temperature using the following antibodies: CD3-BV786 (BD Bioscience, 565491), CD4-FITC (eBioscience, 11–0048042), CD25-BV421 (BD Bioscience, 567485), CD127-PE-cy7 (eBioscience, 25127842), CCR7-APC-cy7 (Biolegend, 353212), CCR4-BV605 (BD Bioscience, 562906), FoxP3-PE (BD Bioscience, 560852), RORγt-APC (Invitrogen, 17-6988-82), IL-17A-BV510 (BD Bioscience, 563295), and IL-17F-PerCP-eFluor710 (Invitrogen, 46-7169-42).
Treg transwell migration assay
The transwell migration assay was performed in vitro using 24-well transwell plates with a 5.0 μm pore size polycarbonate membrane insert (Corning, 3421). Chemo-attractant media was made with 0, 10 or 100 μg/ml of human recombinant CCL19 (Peprotech, 300-29 B) and CCL21 (Peprotech, 300-35 A) in 600 μL of culture media (RPMI + 10% FBS) and placed in the lower chamber. Tregs isolated from PBMCs (2 × 104 cells in 100 μl culture media) were loaded into the upper insert. After a 4-h incubation at 36 °C in a 5% CO2 incubator, migrated Tregs were counted using a haemocytometer. Migration was expressed as the ratio between treated-media vs. control (referred to as relative migration index).
Immunofluorescence
Skin biopsies were first fixed in 10% formalin, paraffin-embedded and sectioned with a Leica microtome at 7 μm. Sections were blocked in 5% normal goat serum (Vector Laboratories, Burlingame, CA, USA) and were subsequently incubated with primary antibodies including anti-FoxP3 (Santa Cruz, sc-28705), and anti-CCR7 (BD Bioscience, 565868) overnight at 4 °C. After washing, skin sections were incubated with fluorochrome-conjugated secondary antibodies (Invitrogen, A11006) and mounted with VECTASHIELD mounting medium containing DAPI (Vector Laboratories). Images were taken using a Ziess LSM780 Confocal Microscope and Ziess Zen software for image analysis.
Statistics
CyTOF statistical analyses were performed using the Python library Scipy and Prism v9.5.1. Pairwise statistical analyses on the cell frequencies and median/mean marker expression comparing the psoriasis and healthy donor cohorts were evaluated for non-normality using F-tests and violin plots. Pairwise statistical analyses were performed using the 2-sided Mann–Whitney U test and results were adjusted for multiple comparisons using the Benjamini-Hochberg method with an adjusted p < 0.05 considered statistically significant (referred to as adj. p). Biological sex-based sub-analyses were performed to verify that the observed results were not influenced by the imbalanced proportion of sex between the two cohorts by comparing cell type frequencies and differential marker expression on Tregs. This was conducted using the Python library scikit-learn for principal component analysis (PCA) and the R statistical software (3.4.1) stats package for multiple linear regression.
Role of funders
The funders had no role in study design, data collection, data analyses, interpretation or writing of report.
Results
Distinct Treg and monocyte compartments in psoriatic PBMCs compared to healthy controls
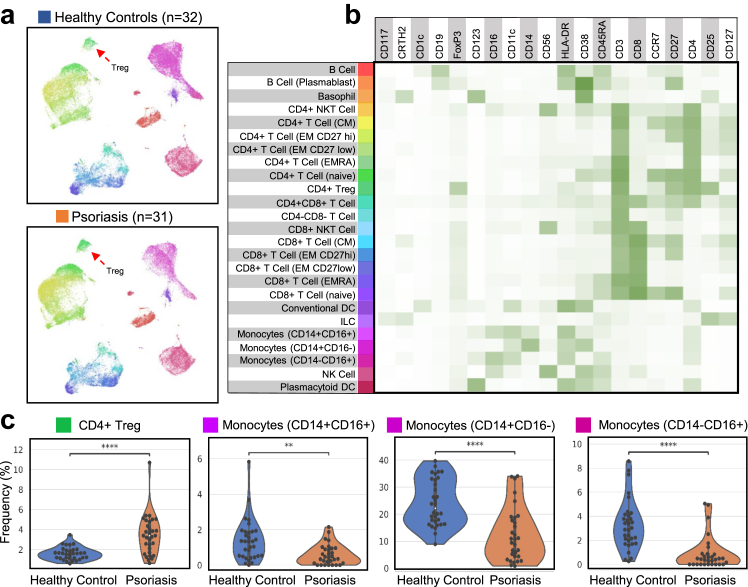
Annotation of the immune cell types unbiasedly across all of the samples gave insights into the broad immune landscape of psoriasis PBMCs compared to healthy donors. UMAPs of the cell populations are shown in Fig. 1a with the corresponding cell-type by marker expression heatmap shown in Fig. 1b. Notably, psoriasis samples showed a significant increase in CD4+ Tregs (CD25+ CD127- FoxP3+, adj. p = 7.70e-5) compared to healthy donors (Fig. 1c). We also found changes in the myeloid compartment with a significant decrease in all three types of monocytes (CD14+ CD16-, adj. p = 7.70e-5; CD14- CD16+, adj. p = 9.84e-6 and CD14+ CD16+, adj. p = 2.49e-3) in the psoriasis group (Fig. 1c).
Fig. 1.
Phenotypic characterization of broad immune cell types of psoriasis PBMCs (n = 31) vs. healthy donors (n = 32). (a) UMAP plots of PBMCs (2000 subsampled cells from each sample) aggregated across all healthy donor and psoriasis samples (total of 32,000 cells in each cohort) with the colour corresponding to the annotated cell population shown in panel B. (b) Heatmap of the median marker expression for each annotated cell type. (c) Overlaid violin and swarm plots of significantly differing immune cell population frequencies (% of total live CD45+ cells) between healthy control and psoriasis samples. An asterisk indicates a significantly differing cell population frequency with an adjusted p < 0.05. ∗adj. p < 0.05, ∗∗adj. p < 0.01, ∗∗∗adj. p < 0.001, ∗∗∗∗adj. p < 0.0001.
Meta-cluster analysis of Tregs in psoriasis reveals a reduction in CLAhi Tregs, specifically activated Tregs, CCR5hi Tregs and CXCR3hi Tregs
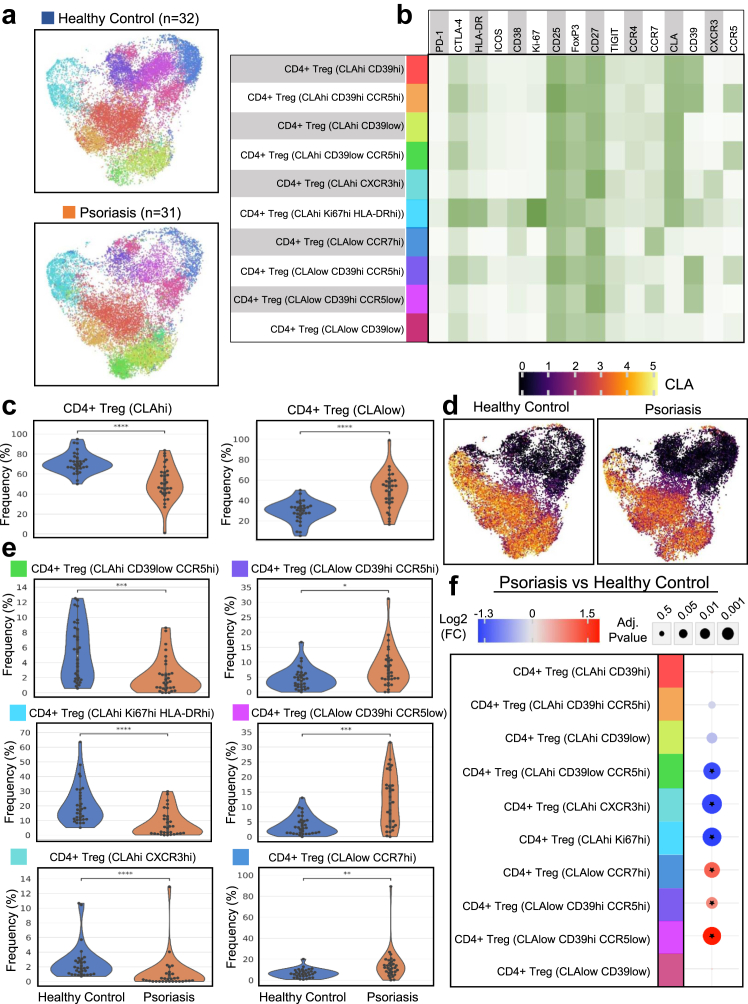
Upon our findings of increased Tregs in the psoriatic PBMCs, we decided to further investigate the Treg compartment by applying the Leiden community detection algorithm to meta-cluster across all the psoriasis and healthy donor samples to characterise the consensus Treg clusters in an unbiased fashion. Visualization by UMAP and median-marker expression of the consensus Treg clusters are shown in Fig. 2a and b. Treg subsets were largely split by CLA expression (Fig. 2b), and we observed a profound decrease in the frequencies of CLAhi Tregs in the psoriasis cohort compared to the healthy donors (adj. p = 3.98e-6, Fig. 2c and d). When analysing Treg meta-clusters within the CLAhi compartment, we found that the CD39low CCR5hi Tregs were significantly reduced (adj. p = 1.79e-4) in the psoriasis samples (Fig. 2e and f). A previous study reported CCR5+ Tregs to be more potent in regulatory function and showed that frequencies, migratory capacity, and suppressive function of the CCR5+ Tregs were markedly decreased in psoriatic PBMCs.25 In our study, we show further findings of reduced CLAhi CD39low CCR5hi Tregs, indicating a potentially decreased propensity for homing of psoriatic Tregs to the inflamed skin.
Fig. 2.
Phenotypic characterization of CD4+ regulatory T cells from healthy and psoriasis PBMCs. CD4+ Tregs were aggregated across all samples and clustered using the Leiden community detection algorithm. Treg Leiden meta-clusters were further grouped and annotated based on their marker expression profile. (a) UMAP plots of CD4+ Tregs (19,401 subsampled Treg cells in each cohort) aggregated across all healthy donor and psoriasis samples with the colour corresponding to the annotated Treg subset shown in panel B. (b) Heatmap of the median marker expression for each annotated Treg subset. (c) Overlaid violin and swarm plots of Treg subsets aggregated into either CLAhi Tregs or CLAlow Tregs. (d) UMAP plots of Tregs coloured by CLA expression (arcsinh-transformed intensity). (e) Overlaid violin and swarm plots of significantly differing Treg subset frequencies (% of total Tregs) between healthy control and psoriasis samples. (f) Integrated dot plot showing the log2 fold change and adjusted p-values of cell population frequencies between psoriasis and healthy controls. An asterisk indicates a significantly differing Treg subset frequency with an adjusted p < 0.05. ∗adj. p < 0.05, ∗∗adj. p < 0.01, ∗∗∗adj. p < 0.001, ∗∗∗∗adj. p < 0.0001.
We also found a decreased frequency of CLAhi Ki-67hi HLA-DRhi Tregs (adj. p = 9.24e-5, Fig. 2e and f) displaying an activated phenotype and further characterised by high expression of CTLA-4 and ICOS (Fig. 2b) in psoriasis PBMCs compared to those of healthy donors. Treg meta-clustering also revealed a subset of CLAhi Tregs expressing the Th1-associated chemokine receptor CXCR3 that was significantly reduced in the psoriasis cohort (adj. p = 2.72e-5). Within the CLAlow Treg compartment, we observed a significant increase in CD39hi CCR5hi, CD39hi CCR5low and CCR7hi Tregs (adj. p = 0.04, 1.72e-4 and 1.71e-3 respectively, Fig. 2e and f).
Supervised analysis of Tregs shows chemokine receptor and functional marker dynamics between psoriasis and healthy cohorts
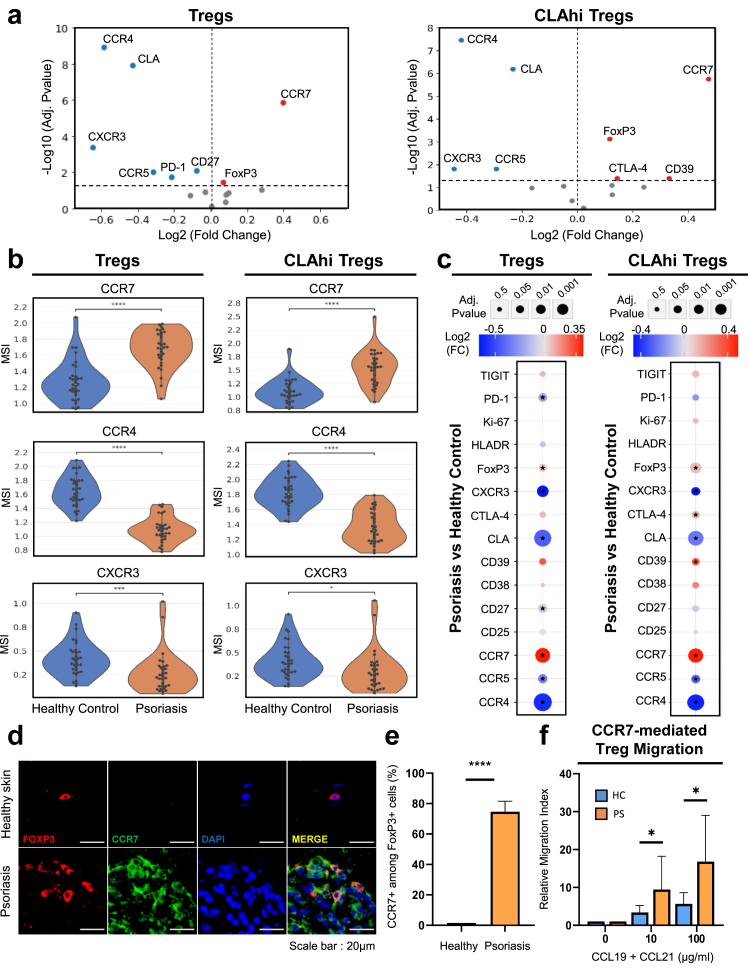
To further interrogate the Treg landscape, we evaluated differential expression of Treg functional markers and chemokine receptors between the psoriasis and healthy donor samples on both total Tregs and CLAhi Tregs. Differential marker analysis on total Tregs revealed that recirculating marker CCR7 expression is significantly elevated (adj. p = 1.41e-6) within the psoriasis group compared to healthy donor group. In contrast, there was a significant reduction in expression of the skin-homing-related makers including CLA, CCR4, CCR5, and CXCR3 (adj. p = 1.24e-8, 1.24e-9, 9.93e-3, and 4.32e-4 respectively; Fig. 3a and b).
Fig. 3.
Differential expression analysis of Treg functional markers and chemokine receptors between psoriatic and healthy Tregs. (a) Volcano plots of differentially expressed Treg markers between healthy control and psoriatic Tregs on total Tregs and CLAhi Tregs. Red dots represent markers with increased expression in psoriasis samples while blue dots represent markers with increased expression in healthy control samples. The Y-axis denotes –log10 adjusted p-values and the X-axis denotes log2 fold change values based on arcsinh-transformed median signal intensity values (MSI). (b) Overlaid violin and swarm plots of significantly differentially expressed markers (arcsinh-transformed) between psoriasis and healthy controls. (c) Integrated dot plot showing log2 fold change and adjusted p-values of Treg markers between psoriasis and healthy Tregs. An asterisk indicates a significantly differentially expressed marker with an adjusted p < 0.05. ∗adj. p < 0.05, ∗∗adj. p < 0.01, ∗∗∗adj. p < 0.001, ∗∗∗∗adj. p < 0.0001. (d) Representative immunofluorescence imaging of Tregs indicated by FoxP3 and CCR7 staining between healthy and psoriatic skin. (e) Percentages of CCR7+ cells among FoxP3+ cells in healthy (n = 3) and psoriatic skin (n = 3). (f) Migration of Tregs isolated from PBMCs in response to CCL19 and CCL21 at concentrations of 0, 10, and 100 μg/ml, shown as relative migration index, in both healthy (n = 5) and psoriasis samples (n = 7). ∗p < 0.05, ∗∗∗∗p < 0.0001.
Due to the critical role of CLA in recruitment of T cells to the skin, we analysed the CLAhi compartment separately. We observed similar trends with increased CCR7 (adj. p = 2.46e-6) and decreased CLA, CCR4, CCR5, and CXCR3 expression (adj. p = 6.42e-8, 3.25e-8, 0.017, and 0.017 respectively; Fig. 3a and b). These findings were consistent with Treg subset frequencies found by Leiden meta-clustering showing a decrease in CLAhi CXCR3hi and CLAhi CD39low CCR5hi Treg subsets in psoriasis compared to healthy controls.
We also found multiple Treg functional markers that were elevated in the psoriasis group. Within total Tregs, FoxP3 expression was significantly increased in the psoriasis cohort (adj. p = 0.037). Within the CLAhi Treg compartment, we found a significant increase in FoxP3, CTLA-4, and CD39 expression (adj. p = 9.34e-4, 0.043, and 0.043, respectively) in the psoriasis cohort (Fig. 3c). As the increase in functional markers was surprising, we further investigated this phenotype. CD39 has been shown to be important in Treg function and stability and CD39low Tregs can lose FoxP3 expression and trans-differentiate into Th1 or Th17 cells in inflammatory conditions.37 Similarly, in our dataset, we found that CD39low Tregs had a significant reduction in FoxP3 expression compared to CD39hi Tregs (p = 1.4e-19, Supplementary Fig. S2a). When examining these more stable and functional CD39hi FoxP3hi Tregs and CLAhi CD39hi FoxP3hi Tregs, we found that CCR7 expression was significantly increased in psoriasis samples (p = 1.50e-5 and 2.58e-6 respectively, Supplementary Fig. S2b).
Considering this consistently upregulated expression of CCR7 in circulating psoriatic Tregs, we sought to examine this phenotype within the psoriatic skin microenvironment. Indeed, we found an overall increase in CCR7 expression in Tregs in the psoriatic skin lesion compared to healthy skin biopsies by immunofluorescence suggesting a tendency for psoriatic Tregs to leave the skin and re-enter circulation (p = 2.47e-5, Fig. 3d and e). To validate this finding, we conducted an in vitro migration assay on Tregs isolated from psoriatic and healthy donor PBMCs and found significantly enhanced, concentration-dependent migration of psoriatic Tregs towards CCR7’s ligands CCL19 and CCL21 (10 μg/ml and 100 μg/ml; p = 0.02 and 0.01 respectively) compared to healthy donor Tregs (Fig. 3f).
Additionally, to verify and minimize potential sex-based differences, we conducted PCA and multiple linear regression to compare cell type frequencies and differential marker expression on Tregs. PCA analysis did not show any noticeable differences in Treg frequencies or differential marker expression on Tregs between sexes (Supplementary Fig. S3). Furthermore, multiple linear regression adjusting for age and sex (Supplementary Table S2) showed that the observed differences between the psoriasis and healthy control cohorts persisted after these adjustments.
Severity of psoriasis relates to dynamics in Treg chemokine receptor and functional marker expression
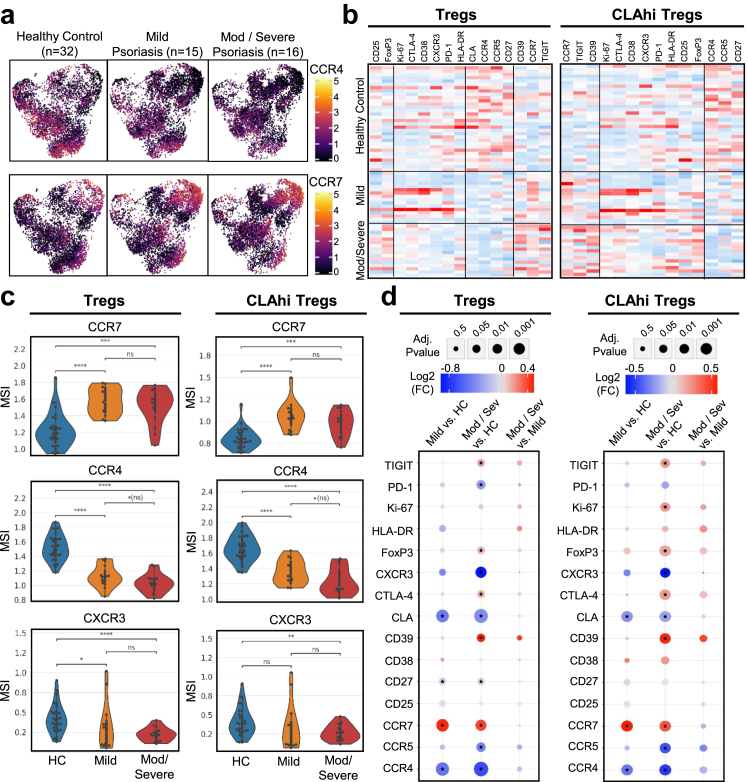
We subsequently sub-grouped the psoriatic PBMCs by severity (mild: PASI 5–10; moderate to severe: PASI >10) and performed pairwise comparisons between severity groups and healthy donors to analyse changes in chemokine receptor and functional marker expression on total Tregs and CLAhi Tregs in relation to severity. In the Treg compartment, we found a significant reduction of CCR4 (adj. p = 6.4e-6) in the mild cohort compared to the healthy control (Fig. 4a and b). This reduction was further exacerbated by disease severity with the most dramatic and significant decrease observed in the moderate-severe psoriasis cohort (adj. p = 6.43e-7). We also noted a reduction, albeit more limited (p = 0.02, adj. p = 0.14), in CCR4 expression on Tregs between mild and moderate-severe psoriasis groups. We observed similar findings of exacerbated CCR4 reduction in the CLAhi Treg compartment. CCR7, CXCR3, and CLA expression was also found to be increased in both mild and moderate-severe psoriasis compared to healthy controls (adj. p = 3.28e-5, 4.64e-4, and 8.70e-5 respectively), but with no marked differences between the mild and moderate-severe psoriasis cohorts (Fig. 4c and d).
Fig. 4.
Differential expression analysis of Treg functional markers and chemokine receptors comparing healthy controls (n = 32), people with mild (n = 15) and moderate-severe psoriasis (n = 16). (a) UMAP plots of CD4+ Tregs aggregated across all samples showing marker expression (arcsinh-transformed intensity) of CCR4 and CCR7 within each cohort. (b) Heatmap of z-scored normalized expression across all samples of markers on Tregs and CLAhi Tregs with markers organized by hierarchical cosine distance clustering. Red heatmap values represent relatively higher marker expression and blue heatmap values represent relatively lower marker expression. (c) Overlaid violin and swarm plots of CCR4, CCR7 and FoxP3 on Tregs and CLAhi Tregs comparing healthy control, people with mild and moderate-severe psoriasis. (d) Integrated heatmap dot plot showing pairwise comparisons of cohorts with the log2 fold change and adjusted p-values of Treg markers on Tregs and CLAhi Tregs. An asterisk indicates a significantly differentially expressed marker with an adjusted p < 0.05. ∗adj. p < 0.05, ∗∗adj. p < 0.01, ∗∗∗adj. p < 0.001, ∗∗∗∗adj. p < 0.0001.
Within the Treg functional markers, there was no significant increase in FoxP3, CD39, TIGIT and CTLA-4 on Tregs in the mild psoriasis cohort compared to healthy controls. However, we observed a significant increase in expression in the moderate-severe psoriasis cohort compared to healthy controls indicating that disease severity is associated with upregulation of FoxP3, CD39, TIGIT and CTLA-4 (adj. p = 0.023, 0.019, 0.038, and 0.375 respectively) with the most severe disease showing the highest expression on Tregs (Fig. 4d). Additionally, due to the dynamic balance of Treg and Th17 cells, we examined the production of IL-17A by Th17 cells and Tregs in a separate, smaller cohort by flow cytometry and found a slight positive correlation between PASI score and production of IL-17A by Th17 cells and also by CCR7+ Tregs contributing to the Th17-skewed phenotype (Supplementary Fig. S4a and b).
Tregs in the lesional psoriatic skin show decreased skin-trafficking phenotypes
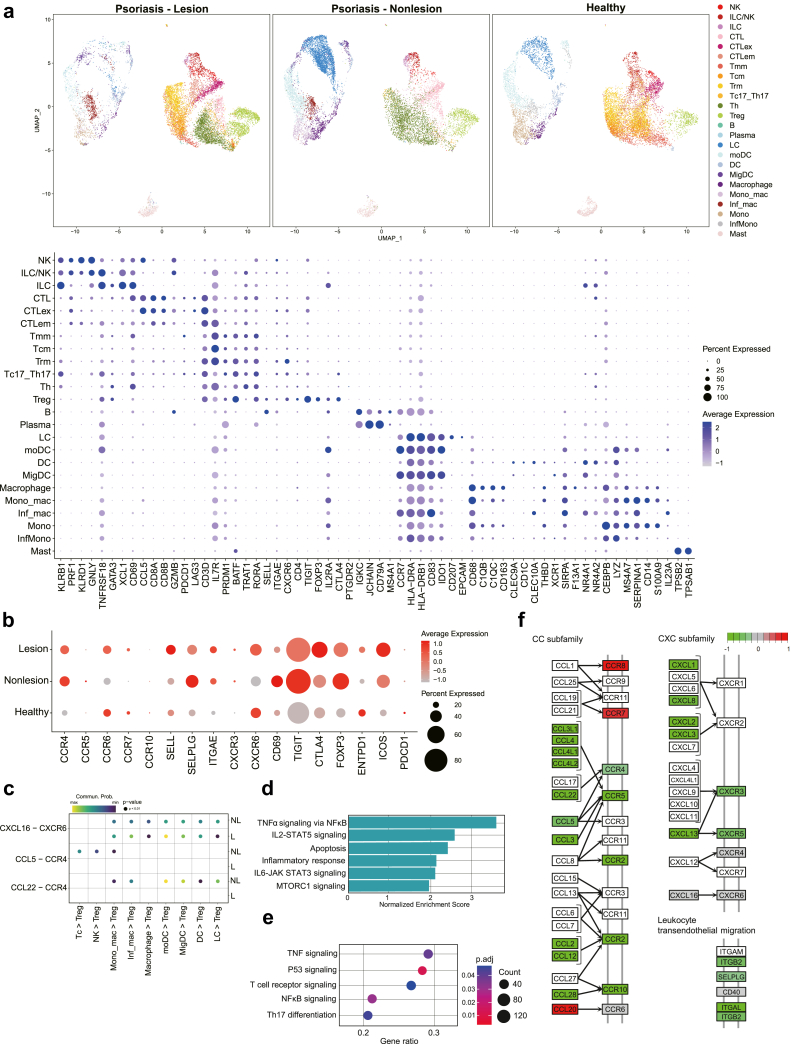
Based on insights of an impaired skin-homing property of psoriatic circulating Tregs from our CyTOF results, we compared the immune cell profiles of lesional and non-lesional skin biopsies from people with psoriasis and normal skin utilising scRNA-seq datasets from the public repository to more relevantly reflect the psoriatic local microenvironments.33 The dot plot (Fig. 5a) shows gene expression of the diverse immune cell types found in the skin datasets. Compared to normal skin Tregs, both lesional and non-lesional psoriatic skin Tregs exhibited higher expression of CCR4 and SELPLG (Fig. 5b). However, when specifically comparing the psoriatic lesional and non-lesional skin, lesional Tregs showed decreased CCR4 and SELPLG expression compared to non-lesional Tregs and increased expression of CCR7, highly consistent with our findings from the circulating Treg CyTOF data.
Fig. 5.
Single-cell RNA sequencing analyses of skin from people with psoriasis (lesion vs. non-lesion) and healthy donors, extracted from the public datasets. (a) UMAP plots and dot plots of marker expressions of single cells aggregated across psoriatic lesional, non-lesional, and healthy skin. (b) A subpanel of Tregs showing relative gene expression. (c) Bubble plot of significant specific ligand–receptor interactions within CCL-CCR and CXCL-CXCR signalling in lesional and non-lesional skin. (d) Hallmark pathways in lesional Tregs where the normalized enrichment score is significantly increased, compared to non-lesional Tregs, based on the MSigDB-GO hallmark gene set database. (e) Dot plot of activated signalling in lesional Tregs compared to non-lesional Tregs, based on the MSigDB-KEGG database. (f) KEGG pathway analysis showing overall alteration in expression of skin-trafficking genes.
Next, we analysed significant ligand–receptor interactions between cell types to investigate cell-to-cell signalling within psoriatic lesional and non-lesional skin. Among diverse cell–cell signalling associated with skin-homing, the specific ligand–receptor interactions of CCL5-CCR4 and CCL22-CCR4 were significantly enriched in the Tregs of non-lesional skin, whereas those interactions were not significant in lesional Tregs (Fig. 5c). GSEA-GO and -KEGG pathway analyses (Fig. 5d and e respectively) based on the MSigDB database show that prototypical phenotypes such as TNF-α and Th17 signalling pathways were upregulated in lesional Tregs. Consistent with gene expression profiles, KEGG pathway analysis showed increased CCR7 and decreased CCR4 and SELPLG in lesional Tregs (Fig. 5f). It also revealed a reduction in cell adhesion-related genes, ITGB2, SELPLG and ITGAL in lesional Tregs compared to non-lesional Tregs. Collectively, these results strongly support that psoriatic Tregs are characterised by an impaired skin-trafficking property, which may result in suboptimal immune-suppressive effects of Tregs in psoriatic inflammation.
Discussion
Our study reveals the immune landscape dynamics and phenotypic characteristics of circulating Tregs and CLAhi Tregs in the peripheral blood of people with psoriasis compared to healthy controls.
There have been conflicting reports over the differences in frequency and count of Tregs in the peripheral blood of people with psoriasis compared to healthy individuals.20,38,39 Even with such variabilities, most of the studies have shown impaired suppressive function or hallmarks of anergy in circulating Tregs.20,22,39 Our data shows an increased frequency of total circulating Tregs in the psoriatic cohort, but a decrease in overall CLAhi Tregs and specifically a decrease in CLAhi Ki-67hi HLA-DRhi Tregs and CLAhi CCR5hi Tregs, which is consistent with previous findings of reduced CCR5+ Tregs.26 This is further shown by differential marker analysis showing a significant reduction in CCR5 expression, indicating a potentially reduced skin-homing propensity of psoriatic Tregs.
Our data demonstrated that the expression of another key chemokine receptor for skin-homing, CCR4, was markedly decreased in Tregs and CLAhi Tregs. CCR4 is involved in trafficking to its ligands, CCL17 and CCL22, which are expressed by skin epithelial cells and myeloid cells, respectively.40,41 Reduced CCR4 expression implies decreased propensity of skin-homing and a reduced tendency of infiltration into the inflamed tissue.42, 43, 44 A previous report showed loss of CCR4 in Tregs leads to impaired accumulation in the skin and complete loss of CCR4 on Tregs in mice results in severe inflammatory skin disease.41 In addition, a recent study reported the expansion of circulating CD8+ central memory T cells (TCM) display CLA+ CCR4+, a skin-tropic phenotype, in people with psoriasis,45 and this skin-homing TCM could potentially have a role in disease recurrences of psoriasis. Previous reports have also shown the importance of CXCR3 in the trafficking of Tregs to sites of inflammation to suppress excessive Th1 responses46, 47, 48 and here we also report a decrease in the frequency of CLAhi CXCR3hi Tregs in the psoriatic PBMCs and a decrease in CXCR3 expression in psoriatic Tregs compared to healthy controls. When subdividing by disease severity, the aforementioned results were consistent and showed correlation with disease severity.
In contrast to the skin-homing phenotypes, we observed higher expression of CCR7 in both circulating whole Tregs and CLAhi Tregs compartments in the psoriasis cohort. While Tregs are continuously recruited to the inflamed skin from circulation, upregulated CCR7 expression in circulating Tregs may hamper persistent skin trafficking. Among T cells, CCR7 is mainly expressed on naïve and central memory T cells and is involved in the migration from the peripheral tissues into secondary lymphoid organs.49,50 Findings of increased CCR7 on psoriatic Tregs were confirmed in the lesional skin by immunofluorescent imaging where over 75% of Tregs showed positive CCR7 staining. In addition, our in vitro Treg migration assay showed increased migration of psoriatic Tregs towards CCR7’s ligands CCL19 and CCL21 in a concentration-dependent manner. Therefore, while Tregs can migrate to the psoriatic lesional inflammatory environment they may be more sensitive to recirculation and are less capable of persisting and maintaining immune homeostasis in the psoriatic skin lesion, which may result in disease progression. Significantly increased levels of CCR7 were also seen consistently across Treg compartments and particularly of note in the more stable and suppressive CD39hi Foxp3hi Treg subset. The initial findings of increased expression of functional markers CD39, FoxP3 and CTLA-4 in CLAhi psoriatic Tregs that correlated with severity seemed somewhat contradictory. Yet, taken in context with the findings of upregulated CCR7 even in these more highly suppressive Treg subsets offered further insight into the potential trafficking defects of psoriatic Tregs.
Interrogating the skin microenvironment by analysing scRNA-seq data provided a further detailed landscape of psoriatic lesional and non-lesional Tregs. GSEA-GO and -KEGG pathway analyses showed increased activation of TNF-α/NF-κB, IL-6/STAT3, and Th17 differentiation pathways within lesional Tregs suggesting a skewed gene expression program towards inflammatory-associated Th17 cells due to the psoriatic inflammatory milieu.51,52 Considering the essential role of CD39 in retaining Treg suppressive function in inflammatory conditions and our findings of increased expression in the circulating Tregs, we found, interestingly, that the CD39 encoding gene (ENTPD1) was not prominently expressed in skin-infiltrated psoriatic Tregs and was rather decreased in both lesional and non-lesional Tregs compared to healthy individuals in the scRNA-seq data.
Consistently, CCR4 gene expression and CCR4-mediated chemokine interactions (CCL5/CCL22-CCL4), which are involved in Treg skin migration, were significantly reduced in lesional Tregs compared to those in non-lesional skin. Similarly, we observed a significant downregulation in the expression of SELPLG, CXCR3, and CCR5 in Tregs within the lesional skin. Moreover, CCR7 was upregulated, although not significantly, in the lesional Tregs compared to non-lesional Tregs. Of note, gene expression of cell adhesion molecules that are involved in immune cell migration and retention within tissue sites, specifically SELPLG, CD69 and ITGB2 were also found to be reduced in Tregs within the lesional skin. Taken all together, this suggests a potential alteration in the trafficking properties of psoriatic Tregs with a reduced propensity to migrate and persist within the psoriatic lesional skin to exert its immunosuppressive function.
Despite being one of the largest mass cytometry studies conducted on deeply profiling Tregs in people with psoriasis, the study has limitations in generalizability due to its sample collection from a single ethnic cohort. The study is also limited due to its lack of functional assessments in determining the underlying mechanisms involved in the alteration of Treg migratory properties and its role in the pathogenesis of psoriasis. Also, there are potential confounders such as donor characteristics and genetics. Additionally, the use of topical agents for the treatment of psoriasis could be a confounding factor affecting systemic circulation, although its impact is considered to be minimal. Finally, the scRNA-seq datasets were not derived from the same donors as those analysed in the CyTOF data, which introduces potential variations and limits direct comparisons between the two results. Further dissection of a larger cohort is needed to confirm and generalize this study’s findings. Nonetheless, our study possesses notable strengths, consistently demonstrating an altered migratory property of psoriatic Tregs in circulation utilizing an extensive mass cytometry analysis focused on profiling Tregs. These findings are further corroborated by in-vitro investigations of Treg migratory propensities and also within the skin microenvironment using scRNA-seq which were validated by immunofluorescence staining. This multi-faceted approach enhances the robustness of our findings and offers valuable insights into the role of Tregs in psoriasis pathogenesis.
In conclusion, we identified differences in the immune phenotypes of circulating Tregs and CLA+ Tregs in people with psoriasis compared to healthy controls by high-dimensional profiling. Our integrated results of CyTOF and scRNA-seq data demonstrated an inefficient skin-trafficking property of psoriatic Tregs leading to a suboptimal immune-suppressive environment in controlling ongoing psoriatic inflammation. Further investigation is required to comprehend the underlying mechanisms for impaired skin-trafficking of psoriatic Tregs and for developing therapeutic strategies to promote Treg recruitment and retention within the local inflamed tissues to optimally exert their immunosuppressive function and control the inflammatory microenvironment of psoriasis.
Contributors
Conceptualisation: H.J.K. and T.G.K.; Sample collection: Y.J.B. and S.H.K.; Data curation: B.H.L.; Data analysis: B.H.L., S.H.L. and S.J.K.; Data interpretation and Investigation: B.H.L., Y.J.B. and S.H.L.; Data visualization: B.H.L., Y.J.B., S.H.L. and S.J.K.; Resources: S.K.S.; Funding acquisition: T.G.K.; Writing - Original draft: B.H.L., Y.J.B. and S.H.L.; Writing - Review and editing: B.H.L., Y.J.B., S.H.L, S.K.S., C.G.P, H.J.K. and T.G.K.; Supervision: S.K.S., H.J.K., C.G.P. and T.G.K.
All authors read, revised, and approved the final manuscript. B.H.L., Y.J.B. and S.H.L. contributed equally.
All authors had complete access to the study's data and had final responsibility for the decision to submit the paper for publication. B.H.L., Y.J.B. and S.H.L. accessed and verified the data.
Data sharing statement
The authors declare that all the data supporting the findings in this study are available from the corresponding author upon reasonable request.
Declaration of interests
The authors declare that they have no conflict of interest.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science and Information and Communications Technology and the new faculty research seed money grant of Yonsei University College of Medicine for 2021.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2024.104985.
Contributor Information
Chung-Gyu Park, Email: chgpark@snu.ac.kr.
Hyun Je Kim, Email: tte9801@snu.ac.kr.
Tae-Gyun Kim, Email: TGMED83@yuhs.ac.
Appendix A. Supplementary data
References
- 1.Parisi R., Symmons D.P., Griffiths C.E., Ashcroft D.M. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377–385. doi: 10.1038/jid.2012.339. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong A.W., Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1945–1960. doi: 10.1001/jama.2020.4006. [DOI] [PubMed] [Google Scholar]
- 3.Diani M., Altomare G., Reali E. T helper cell subsets in clinical manifestations of psoriasis. J Immunol Res. 2016;2016 doi: 10.1155/2016/7692024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elder J.T., Bruce A.T., Gudjonsson J.E., et al. Molecular dissection of psoriasis: integrating genetics and biology. J Invest Dermatol. 2010;130(5):1213–1226. doi: 10.1038/jid.2009.319. [DOI] [PubMed] [Google Scholar]
- 5.Di Cesare A., Di Meglio P., Nestle F.O. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129(6):1339–1350. doi: 10.1038/jid.2009.59. [DOI] [PubMed] [Google Scholar]
- 6.Phan C., Beneton N., Delaunay J., et al. Effectiveness and safety of anti-interleukin-17 therapies in elderly patients with psoriasis. Acta Derm Venereol. 2020;100(18) doi: 10.2340/00015555-3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruggiero A., Fabbrocini G., Cinelli E., Ocampo Garza S.S., Camela E., Megna M. Anti-interleukin-23 for psoriasis in elderly patients: guselkumab, risankizumab and tildrakizumab in real-world practice. Clin Exp Dermatol. 2022;47(3):561–567. doi: 10.1111/ced.14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nussbaum L., Chen Y.L., Ogg G.S. Role of regulatory T cells in psoriasis pathogenesis and treatment. Br J Dermatol. 2021;184(1):14–24. doi: 10.1111/bjd.19380. [DOI] [PubMed] [Google Scholar]
- 9.Sakaguchi S., Yamaguchi T., Nomura T., Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Haddadi M.H., Negahdari B. Clinical and diagnostic potential of regulatory T cell markers: from bench to bedside. Transpl Immunol. 2022;70 doi: 10.1016/j.trim.2021.101518. [DOI] [PubMed] [Google Scholar]
- 11.Levings M.K., Sangregorio R., Roncarolo M.G. Human cd25(+)cd4(+) t regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med. 2001;193(11):1295–1302. doi: 10.1084/jem.193.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taams L.S., Smith J., Rustin M.H., Salmon M., Poulter L.W., Akbar A.N. Human anergic/suppressive CD4(+)CD25(+) T cells: a highly differentiated and apoptosis-prone population. Eur J Immunol. 2001;31(4):1122–1131. doi: 10.1002/1521-4141(200104)31:4<1122::aid-immu1122>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 13.Bahador A., Hadjati J., Hassannejad N., et al. Frequencies of CD4+ T regulatory cells and their CD25(high) and FoxP3(high) subsets augment in peripheral blood of patients with acute and chronic brucellosis. Osong Public Health Res Perspect. 2014;5(3):161–168. doi: 10.1016/j.phrp.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuhlbrigge R.C., Kieffer J.D., Armerding D., Kupper T.S. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature. 1997;389(6654):978–981. doi: 10.1038/40166. [DOI] [PubMed] [Google Scholar]
- 15.Hirahara K., Liu L., Clark R.A., Yamanaka K., Fuhlbrigge R.C., Kupper T.S. The majority of human peripheral blood CD4+CD25highFoxp3+ regulatory T cells bear functional skin-homing receptors. J Immunol. 2006;177(7):4488–4494. doi: 10.4049/jimmunol.177.7.4488. [DOI] [PubMed] [Google Scholar]
- 16.Berg E.L., Yoshino T., Rott L.S., et al. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991;174(6):1461–1466. doi: 10.1084/jem.174.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klarquist J., Denman C.J., Hernandez C., et al. Reduced skin homing by functional Treg in vitiligo. Pigment Cell Melanoma Res. 2010;23(2):276–286. doi: 10.1111/j.1755-148X.2010.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sernicola A., Russo I., Silic-Benussi M., Ciminale V., Alaibac M. Targeting the cutaneous lymphocyte antigen (CLA) in inflammatory and neoplastic skin conditions. Expert Opin Biol Ther. 2020;20(3):275–282. doi: 10.1080/14712598.2020.1715937. [DOI] [PubMed] [Google Scholar]
- 19.Cordiali-Fei P., Bianchi L., Bonifati C., et al. Immunologic biomarkers for clinical and therapeutic management of psoriasis. Mediators Inflamm. 2014;2014 doi: 10.1155/2014/236060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugiyama H., Gyulai R., Toichi E., et al. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J Immunol. 2005;174(1):164–173. doi: 10.4049/jimmunol.174.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang K., Li X., Yin G., Liu Y., Niu X., Hou R. Functional characterization of CD4+CD25+ regulatory T cells differentiated in vitro from bone marrow-derived haematopoietic cells of psoriasis patients with a family history of the disorder. Br J Dermatol. 2008;158(2):298–305. doi: 10.1111/j.1365-2133.2007.08359.x. [DOI] [PubMed] [Google Scholar]
- 22.Yan K., Xu W., Huang Y., et al. Methotrexate restores the function of peripheral blood regulatory T cells in psoriasis vulgaris via the CD73/AMPK/mTOR pathway. Br J Dermatol. 2018;179(4):896–905. doi: 10.1111/bjd.16560. [DOI] [PubMed] [Google Scholar]
- 23.Goodman W.A., Levine A.D., Massari J.V., Sugiyama H., McCormick T.S., Cooper K.D. IL-6 signaling in psoriasis prevents immune suppression by regulatory T cells. J Immunol. 2009;183(5):3170–3176. doi: 10.4049/jimmunol.0803721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lochner M., Wang Z., Sparwasser T. The special relationship in the development and function of T helper 17 and regulatory T cells. Prog Mol Biol Transl Sci. 2015;136:99–129. doi: 10.1016/bs.pmbts.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 25.Zhou L., Lopes J.E., Chong M.M., et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453(7192):236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soler D.C., Sugiyama H., Young A.B., Massari J.V., McCormick T.S., Cooper K.D. Psoriasis patients exhibit impairment of the high potency CCR5(+) T regulatory cell subset. Clin Immunol. 2013;149(1):111–118. doi: 10.1016/j.clim.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spitzer M.H., Nolan G.P. Mass cytometry: single cells, many features. Cell. 2016;165(4):780–791. doi: 10.1016/j.cell.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahman A.H., Tordesillas L., Berin M.C. Heparin reduces nonspecific eosinophil staining artifacts in mass cytometry experiments. Cytometry A. 2016;89(6):601–607. doi: 10.1002/cyto.a.22826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee B.H., Kelly G., Bradford S., et al. A modified injector and sample acquisition protocol can improve data quality and reduce inter-instrument variability of the Helios mass cytometer. Cytometry A. 2019;95(9):1019–1030. doi: 10.1002/cyto.a.23866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernandez N.F., Gundersen G.W., Rahman A., et al. Clustergrammer, a web-based heatmap visualization and analysis tool for high-dimensional biological data. Sci Data. 2017;4 doi: 10.1038/sdata.2017.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolf F.A., Angerer P., Theis F.J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 2018;19(1):15. doi: 10.1186/s13059-017-1382-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Traag V.A., Waltman L., van Eck N.J. From Louvain to Leiden: guaranteeing well-connected communities. Sci Rep. 2019;9(1):5233. doi: 10.1038/s41598-019-41695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reynolds G., Vegh P., Fletcher J., et al. Developmental cell programs are co-opted in inflammatory skin disease. Science. 2021;371(6527) doi: 10.1126/science.aba6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y., Wang H., Cook C., et al. Defining patient-level molecular heterogeneity in psoriasis vulgaris based on single-cell transcriptomics. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.842651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mootha V.K., Lindgren C.M., Eriksson K.F., et al. PGC-1 alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34(3):267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 36.Subramanian A., Tamayo P., Mootha V.K., et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gu J., Ni X., Pan X., et al. Human CD39(hi) regulatory T cells present stronger stability and function under inflammatory conditions. Cell Mol Immunol. 2017;14(6):521–528. doi: 10.1038/cmi.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richetta A.G., Mattozzi C., Salvi M., et al. CD4+ CD25+ T-regulatory cells in psoriasis. Correlation between their numbers and biologics-induced clinical improvement. Eur J Dermatol. 2011;21(3):344–348. doi: 10.1684/ejd.2011.1362. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L., Li Y., Yang X., et al. Characterization of Th17 and FoxP3(+) Treg cells in paediatric psoriasis patients. Scand J Immunol. 2016;83(3):174–180. doi: 10.1111/sji.12404. [DOI] [PubMed] [Google Scholar]
- 40.Kalekar L.A., Rosenblum M.D. Regulatory T cells in inflammatory skin disease: from mice to humans. Int Immunol. 2019;31(7):457–463. doi: 10.1093/intimm/dxz020. [DOI] [PubMed] [Google Scholar]
- 41.Sather B.D., Treuting P., Perdue N., et al. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J Exp Med. 2007;204(6):1335–1347. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanchez Rodriguez R., Pauli M.L., Neuhaus I.M., et al. Memory regulatory T cells reside in human skin. J Clin Invest. 2014;124(3):1027–1036. doi: 10.1172/JCI72932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang L., Yang X.Q., Cheng J., Hui R.S., Gao T.W. Increased Th17 cells are accompanied by FoxP3(+) Treg cell accumulation and correlated with psoriasis disease severity. Clin Immunol. 2010;135(1):108–117. doi: 10.1016/j.clim.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 44.Bovenschen H.J., van Vlijmen-Willems I.M., van de Kerkhof P.C., van Erp P.E. Identification of lesional CD4+ CD25+ Foxp3+ regulatory T cells in psoriasis. Dermatology. 2006;213(2):111–117. doi: 10.1159/000093849. [DOI] [PubMed] [Google Scholar]
- 45.Casciano F., Diani M., Altomare A., et al. CCR4(+) skin-tropic phenotype as a feature of central memory CD8(+) T cells in healthy subjects and psoriasis patients. Front Immunol. 2020;11:529. doi: 10.3389/fimmu.2020.00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paust H.J., Riedel J.H., Krebs C.F., et al. CXCR3+ regulatory T cells control TH1 responses in crescentic GN. J Am Soc Nephrol. 2016;27(7):1933–1942. doi: 10.1681/ASN.2015020203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoerning A., Koss K., Datta D., et al. Subsets of human CD4(+) regulatory T cells express the peripheral homing receptor CXCR3. Eur J Immunol. 2011;41(8):2291–2302. doi: 10.1002/eji.201041095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Groom J.R., Luster A.D. CXCR3 in T cell function. Exp Cell Res. 2011;317(5):620–631. doi: 10.1016/j.yexcr.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.von Andrian U.H., Mackay C.R. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343(14):1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 50.Bromley S.K., Thomas S.Y., Luster A.D. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol. 2005;6(9):895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- 51.Bovenschen H.J., van de Kerkhof P.C., van Erp P.E., Woestenenk R., Joosten I., Koenen H.J. Foxp3+ regulatory T cells of psoriasis patients easily differentiate into IL-17A-producing cells and are found in lesional skin. J Invest Dermatol. 2011;131(9):1853–1860. doi: 10.1038/jid.2011.139. [DOI] [PubMed] [Google Scholar]
- 52.Komatsu N., Okamoto K., Sawa S., et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med. 2014;20(1):62–68. doi: 10.1038/nm.3432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.