Abstract
The polysaccharide microcapsule of Staphylococcus aureus has been reported to be differentially expressed depending on growth conditions, with phosphate concentration being the critical environmental component. This study evaluated the effect of growth of a serotype 8 strain of S. aureus in phosphate-replete and phosphate-limiting media on microcapsule production. The presence of the cell wall polymers microcapsule and teichoic acid was measured by both gas chromatography-mass spectrometry and liquid chromatography-mass spectrometry. Production of microcapsule was unaffected by changes in the environmental phosphate concentration. There was, additionally, no evidence for a shift from teichoic acid to teichuronic acid synthesis.
Eleven capsular serotypes for Staphylococcus aureus have been described (13, 23). Capsule production by this bacterium falls into two distinct categories. Types 1 and 2 capsules, exemplified by strains M and Smith, consist of a prominent polysaccharide capsule which gives rise to mucoid colony formation. Production of the mucoid capsule has been associated with increased resistance to phagocytosis and enhanced virulence (19). However, few clinical isolates of S. aureus actually produce this prominent capsule. The mucoid strains of S. aureus, as well as nonmucoid isolates, produce a second type of capsule (27). This polysaccharide capsule is not readily apparent by colony morphology or by typical capsule-staining procedures. It has, therefore, been designated a microcapsule. A serotyping scheme based on the microcapsule has been developed, and the majority of human clinical isolates, approximately 80%, fall into serotypes 5 and 8 (1). Furthermore, preliminary studies indicate that these two serotypes predominate in animal mastitis isolates (20). The chemical compositions and structures of the various serotypes of microcapsules have been elucidated (7, 16–18). The genes encoding the type 1 capsule from strain M, the type 5 microcapsule from strains Newman and Reynolds, and the type 8 microcapsule from strain Becker have been cloned, and their DNA sequences have been determined (14, 22). Antibodies directed against the microcapsule have been shown to be opsonic and to be protective, in certain animals, of infection (6, 12, 21, 25).
Synthesis of microcapsules appears to be a regulated event. It has been reported that the degree of expression of the microcapsules is dependent on the composition of the growth medium, the stage of growth, and whether the organisms are cultured on solid or liquid medium (6, 15, 21, 24, 26). Microcapsule expression has been reported to be enhanced by growth in Columbia broth or agar, and this phenomenon has been attributed to the low-phosphate nature of this medium (5, 6, 15, 21). Giving further credence to this observation is the chemical composition of the microcapsule. The microcapsule contains acidic sugars which are usually associated, in other gram-positive bacteria, with teichuronic acids. In organisms such as Bacillus subtilis, growth under conditions of limiting phosphate is associated with a shift in synthesis of cell wall-associated teichoic acid to production of the acidic sugar-containing teichuronic acid (3, 4, 11, 28). The chemical similarity between teichuronic acid and microcapsules of S. aureus, as well as the enhanced expression of the microcapsule in Columbia medium, has led to the suggestion that microcapsule synthesis may be induced by limitation of environmental phosphate. To investigate this possibility, the expression of microcapsule in a semidefined phosphate-limited growth medium was assessed by gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-mass spectrometry (LC-MS).
PCR-based serotype determination.
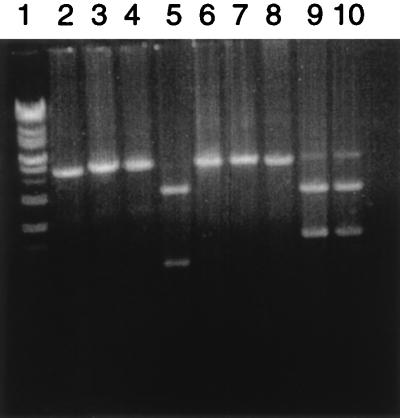
Approximately 80% of S. aureus clinical isolates belong to serotype 5 or 8. The operons encoding these microcapsules have been cloned and sequenced (22). Analysis of the sequence has indicated that 4 determinants located in the central region of each operon, capH to capK, are sequence distinct, whereas the 12 determinants which flank these 4 are essentially identical in the two serotypes. The two serotypes can thus be distinguished based upon the four-gene unique regions. PCR primers have been designed to allow exact annealing to sequences flanking the unique sequences and thus to allow amplification of the serotype-unique sequences. The primer sequences are as follows: cap585, ATACTTGGAGGAAATGACGATG; cap583, CACTCATCTAATCGACGTCCT. PCR was performed under the following conditions. Initial denaturation of the template was done at 94°C for 3 min, followed by 35 cycles of amplification including denaturation of the template at 94°C for 30 s, primer annealing at 59°C for 45 s and then at 67°C for 30 s, and chain extension at 72°C for 5 min. A final extension at 72°C for 4 min was included. The resulting PCR products were digested with the restriction endonucleases SmaI (American Allied Biochemical) and Tth111I (Promega) to determine if the organism was serotype 5 or serotype 8. Results of such amplifications with strains 8325-4 (serotype 5), Becker (serotype 8), and DAW (a 1978 methicillin-resistant clinical isolate) are illustrated in Fig. 1. The expected PCR products are DNA fragments of 4,145 bp (serotype 5) and 4,327 bp (serotype 8). Furthermore, the serotype 5 fragment has a single Tth111I site which, after digestion, yields fragments of 2,921 and 1,225 bp. The serotype 8 fragment lacks a Tth111I cleavage site. The serotype 8 fragment possesses a single SmaI site, and cleavage with this endonuclease produces fragments of 2,740 and 1,587 bp. The serotype 5 fragment lacks a SmaI cleavage site. The PCR amplifications gave the expected patterns for the type 5 (8325-4) and type 8 (Becker) strains, and the DAW clinical isolate yielded a serotype 8-specific pattern.
FIG. 1.
PCR-based serotyping of strain DAW. capH- to capK-containing DNA fragments from serotype 5 strain 8325-4 (lanes 2, 5, and 8), serotype 8 strain Becker (lanes 4, 7, and 10), and the methicillin-resistant clinical isolate DAW (lanes 3, 6, and 9) were electrophoresed in a 0.8% agarose gel. The PCR fragments were digested with the restriction endonuclease Tth111I (lanes 5 to 7) or SmaI (lanes 8 to 10) or were not treated with a restriction endonuclease (lanes 2 to 4). Lane 1 contains the Supermarker (Bioworks) DNA size standard.
Carbohydrate analysis of strain DAW.
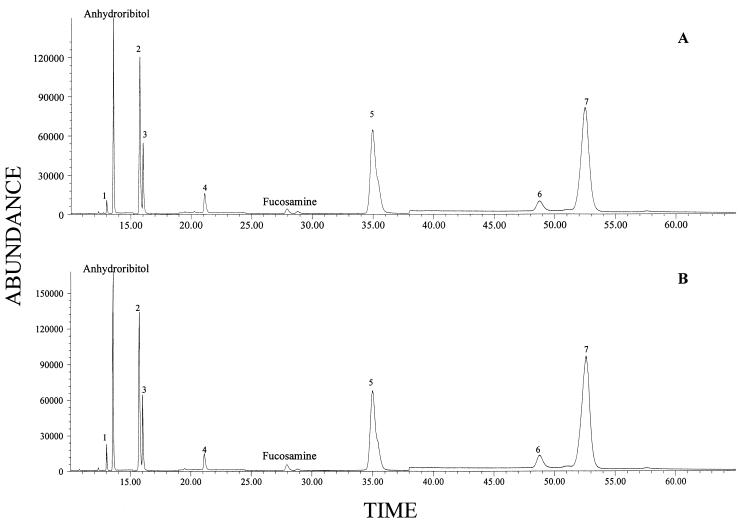
Carbohydrate analysis was carried out on S. aureus DAW cells grown in phosphate-replete (10.5 mM total phosphate) and phosphate-deficient (0.023 mM total phosphate) semidefined media (28). B. subtilis W23 cells were grown in the same media to serve as controls for environmental phosphate levels. Under phosphate-limiting conditions, this organism shifts from synthesis of a ribitol-containing teichoic acid to a teichuronic acid (11, 28). Neutral and aminosugar profiles were determined by the alditol acetate method, as described previously (8, 9). Analysis of neutral and acidic sugars by LC-MS was performed by the method of Fox et al. (11). The major sugars observed for S. aureus DAW by GC-MS analysis were anhydroribitol (derived from ribitol by dehydration on hydrolysis), ribose, fucosamine, glucose, muramic acid, and glucosamine (Fig. 2). Glucosamine and glucose were found in high concentrations. Although these sugars can be present in teichoic acid, they are also found in other cellular constituents and thus were not quantified. For example, glucosamine (along with muramic acid) is present in peptidoglycan.
FIG. 2.
Carbohydrate profiles of S. aureus determined by GC-MS. (A) Cells grown in phosphate-rich medium. (B) Cells grown in phosphate-limited medium. Note that the levels of anhydroribitol and fucosamine (markers for teichoic acid and capsule, respectively) are unchanged between the two growth conditions. Other peaks: 1, glycerol; 2, ribose plus ribitol; 3, arabinitol (internal standard); 4, glucose; 5, muramic acid; 6, methylglucamine (internal standard); 7, glucosamine.
Fucosamine and anhydroribitol served as markers for the serotype 8 microcapsule and teichoic acid, respectively, for S. aureus. Residual ribitol (as well as ribose derived from RNA) produces ribitol pentaacetate on conversion to alditol acetates for GC-MS analysis. Thus the “ribitol-ribose” peak does not accurately represent teichoic acid content. Anhydroribitol and fucosamine were present at high concentrations under all growth conditions for S. aureus. Fucosamine was produced equally well under conditions of phosphate excess and limitation (0.2 and 0.3% [dry weight], respectively). This behavior is inconsistent with microcapsule expression being induced by phosphate limitation.
Staphylococci cultured under conditions of phosphate excess produced large amounts of ribitol (0.42% [dry weight]), as determined by LC-MS analysis. Ribitol production was essentially unchanged in cells grown under phosphate-limiting conditions (0.49%). Glucuronic acid (a marker for teichuronic acid) has been reported to be synthesized by certain strains of S. aureus. Trace levels of a peak at the retention time for glucuronic acid was observed under excess phosphate conditions. There was only a slight increase in the amount of glucuronic acid under phosphate-limiting conditions (total levels of 0.06%). Because there was no decrease in teichoic acid production and no significant synthesis of glucuronic acid, there is no evidence for a shift from teichoic acid to teichuronic acid synthesis. Similarly, no reduction in the teichoic acid marker was detected when staphylococcal strains 8325-4 (serotype 5) and Becker (serotype 8) were cultured under the various phosphate concentrations utilized in this study (10). Therefore, the results obtained with DAW appear to be more universally applicable to S. aureus. These findings are in contrast to the results obtained by Ellwood and Tempest but are consistent with the conclusions of Dobson and Archibald (2, 4).
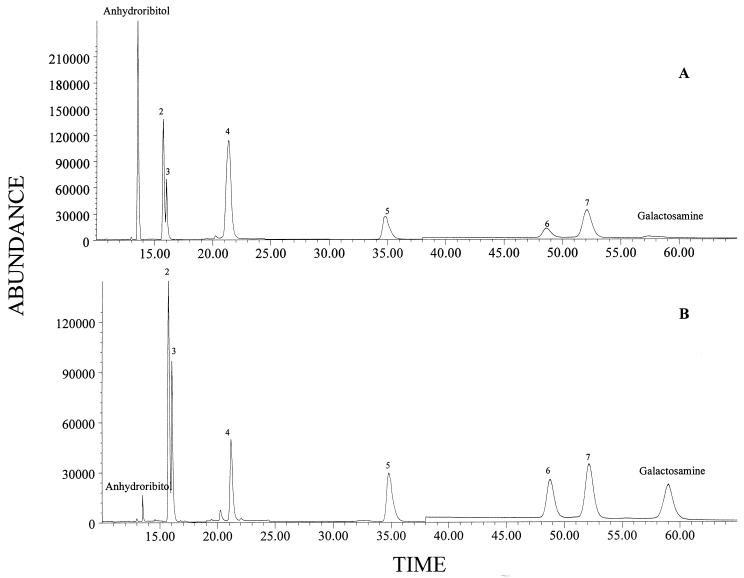
The behavior of B. subtilis cells in phosphate-limiting medium was in agreement with a shift from teichoic acid to teichuronic acid synthesis. B. subtilis W23 synthesizes a ribitol-containing teichoic acid in the presence of excess phosphate and a glucuronic acid-containing teichuronic acid under phosphate limitation. The amount of anhydroribitol was seen to decrease during growth at the lower phosphate concentration (0.6 versus 0.04% [dry weight]) (Fig. 3). Galactosamine has been previously reported to be a major component of teichuronic acid but also has been reported to be a component of a “secondary” teichoic acid present in small amounts compared to the primary teichoic acids (3). Galactosamine was present at trace levels in cells grown in 10.5 mM phosphate but at high levels (0.9% [dry weight]) in cells grown in medium with 0.023 mM phosphate supplementation.
FIG. 3.
Carbohydrate profiles of B. subtilis determined by GC-MS. (A) Cells grown in phosphate-rich medium. (B) Cells grown in phosphate-limited medium. Note that the level of anhydroribitol (marker for teichoic acid) is markedly reduced under phosphate-limited growth conditions. Other peaks: 2, ribose plus ribitol; 3, arabinitol (internal standard); 4, glucose; 5, muramic acid; 6, methylglucamine (internal standard); 7, glucosamine.
By LC-MS analyses, ribitol was readily detected in B. subtilis W23 cells grown under conditions of phosphate excess (0.59% on a dry weight basis) while the peak for glucuronic acid was too low to quantify. For cells grown under phosphate-limiting conditions, ribitol production was decreased 20-fold (0.03%) and glucuronic acid became a major component of chromatograms (0.31%).
The microcapsule of S. aureus is chemically similar to the teichuronic acid of B. subtilis. The wall-attached polymer contains uronic acid sugars and was reported to be expressed at elevated levels under conditions of phosphate limitation. The presence of the microcapsule does not confer a mucoid phenotype on the cells, again consistent with a teichuronic acid. However, the classical model of teichoic acid-teichuronic acid expression would suggest that teichoic acid markers (anhydroribitol and/or ribitol) should disappear from cells grown under low-phosphate conditions whereas a teichuronic acid marker (e.g., glucuronic acid) should increase significantly under low-phosphate conditions. For S. aureus DAW, anhydroribitol and ribitol were present at similar levels under all growth conditions, suggesting continuing production of a teichoic acid. Almost no glucuronic acid was observed under any of the growth conditions tested. These results are inconsistent with a switch from teichoic acid to teichuronic acid synthesis. The level of fucosamine, a component of the type 8 capsule, did not increase under phosphate limitation. These results are consistent with production of a microcapsule under conditions of both phosphate excess and limitation. Thus the reported differences in the amount of microcapsule expressed in vitro must be due to environmental cues unrelated to phosphate availability. Consistent with this is the observation that microcapsule production can be enhanced by growth under conditions unrelated to environmental phosphate concentrations, such as in the presence of milk whey (26).
Acknowledgments
This research was supported by the U.S. Department of Agriculture (94-37204-1088).
REFERENCES
- 1.Arbeit R, Karakawa W W, Vann W F, Robbins J B. Predominance of two newly described capsular polysaccharide types among clinical isolates of Staphylococcus aureus. Diagn Microbiol Infect Dis. 1984;2:85–91. doi: 10.1016/0732-8893(84)90002-6. [DOI] [PubMed] [Google Scholar]
- 2.Dobson B C, Archibald A R. Effect of specific growth limitations on cell wall composition of Staphylococcus aureus H. Arch Microbiol. 1978;119:295–301. doi: 10.1007/BF00405409. [DOI] [PubMed] [Google Scholar]
- 3.Duckworth M, Archibald A R, Baddiley J. The location of N-acetylgalactosamine in the walls of Bacillus subtilis 168. Biochem J. 1972;130:691–696. doi: 10.1042/bj1300691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellwood D C, Tempest D W. Effects of environment on bacterial wall content and composition. Adv Microb Physiol. 1972;7:83–117. [Google Scholar]
- 5.Fattom A, Schneerson R, Szu S C, Vann W F, Shiloach J, Karakawa W W, Robbins J B. Synthesis and immunologic properties in mice of vaccines composed of Staphylococcus aureus type 5 and type 8 capsular polysaccharides conjugated to Pseudomonas aeruginosa exotoxin A. Infect Immun. 1990;58:2367–2374. doi: 10.1128/iai.58.7.2367-2374.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fattom A I, Sarwar J, Ortiz A, Naso R. A Staphylococcus aureus capsular polysaccharide (CP) vaccine and CP-specific antibodies protect mice against bacterial challenge. Infect Immun. 1996;64:1659–1665. doi: 10.1128/iai.64.5.1659-1665.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fournier J-M, Vann W F, Karakawa W W. Purification and characterization of Staphylococcus aureus type 8 capsular polysaccharide. Infect Immun. 1984;45:87–93. doi: 10.1128/iai.45.1.87-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox A, Black G. Identification and detection of carbohydrate markers for bacteria: derivatization and gas chromatography-mass spectrometry. In: Fenselau C, editor. Mass spectrometry for the characterization of bacteria. Washington, D.C: American Chemical Society; 1994. pp. 107–131. [Google Scholar]
- 9.Fox A, Morgan S L, Gilbart J. Preparation of alditol acetates and their analysis by gas chromatography (GC) and mass spectrometry (MS) In: Bierman C, McGinnis G, editors. Analysis of carbohydrates by GLC and MS. Boca Raton, Fla: CRC Press; 1989. pp. 87–117. [Google Scholar]
- 10.Fox, K. F., G. Stewart, and A. Fox. 1998. Unpublished data.
- 11.Fox, K. F., D. S. Wunschel, A. Fox, and G. Stewart. Complementarity of GC-MS and LC-MS analyses for determination of carbohydrate profiles of vegetative cells and spores of bacilli. J. Microbiol. Methods, in press.
- 12.Karakawa W W, Sutton A, Schneerson R, Karpas A, Vann W F. Capsular antibodies induce type-specific phagocytosis of capsulated Staphylococcus aureus by human polymorphonuclear leukocytes. Infect Immun. 1988;56:1090–1095. doi: 10.1128/iai.56.5.1090-1095.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karakawa W W, Vann W F. Capsular polysaccharides of Staphylococcus aureus. Semin Infect Dis. 1982;4:285–293. [Google Scholar]
- 14.Lee C Y. Cloning of genes affecting capsule expression in Staphylococcus aureus strain M. Mol Microbiol. 1992;6:1515–1522. doi: 10.1111/j.1365-2958.1992.tb00872.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee J C, Takeda S, Livolsi P J, Paoletti L C. Effects of in vitro and in vivo growth conditions on expression of type 8 capsular polysaccharide by Staphylococcus aureus. Infect Immun. 1993;61:1853–1858. doi: 10.1128/iai.61.5.1853-1858.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liau D-F, Hash J H. Structural analysis of the surface polysaccharide of Staphylococcus aureus M. J Bacteriol. 1977;131:194–200. doi: 10.1128/jb.131.1.194-200.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreau M, Richard J C, Fournier J-M, Byrd R A, Karakawa W W, Vann W F. Structure of the type 5 capsular polysaccharide of Staphylococcus aureus. Carbohydr Res. 1990;201:285–297. doi: 10.1016/0008-6215(90)84244-o. [DOI] [PubMed] [Google Scholar]
- 18.Murthy S V K N, Melly M A, Harris T M, Hellerqvist C G, Hash J H. The repeating sequence of the capsular polysaccharide of Staphylococcus aureus M. Carbohydr Res. 1983;117:113–123. doi: 10.1016/0008-6215(83)88080-x. [DOI] [PubMed] [Google Scholar]
- 19.Peterson P K, Wilkinson B J, Kim Y, Schmeling D, Quie P G. Influence of encapsulation on staphylococcal opsonization and phagocytosis by human polymorphonuclear leukocytes. Infect Immun. 1978;19:943–949. doi: 10.1128/iai.19.3.943-949.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poutrel B, Boutonnier A, Sutra L, Fournier J-M. Prevalence of capsular polysaccharide types 5 and 8 among Staphylococcus aureus isolates from cow, goat, and ewe milk. J Clin Microbiol. 1988;26:38–40. doi: 10.1128/jcm.26.1.38-40.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robbins J B, Schneerson R, Vann W F, Bryla D A, Fattom A. Prevention of systemic infections caused by group B streptococcus and Staphylococcus aureus by multivalent polysaccharide-protein conjugate vaccines. Ann N Y Acad Sci. 1995;754:68–82. doi: 10.1111/j.1749-6632.1995.tb44439.x. [DOI] [PubMed] [Google Scholar]
- 22.Sau S, Bhasin N, Wann E R, Lee J C, Foster T J, Lee C Y. The Staphylococcus aureus allelic genetic loci for serotype 5 and 8 capsule expression contain the type-specific genes flanked by common genes. Microbiology. 1997;143:2395–2405. doi: 10.1099/00221287-143-7-2395. [DOI] [PubMed] [Google Scholar]
- 23.Sompolinski E, Samra Z, Karakawa W W, Vann W F, Schneerson R, Malik Z. Encapsulation and capsular types in isolates of Staphylococcus aureus from different sources and relationship to phage types. J Clin Microbiol. 1985;22:828–834. doi: 10.1128/jcm.22.5.828-834.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stringfellow W T, Dassy D, Lieb M, Fournier J-M. Staphylococcus aureus growth and type 5 capsular polysaccharide production in synthetic media. Appl Environ Microbiol. 1991;57:618–621. doi: 10.1128/aem.57.2.618-621.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verbrugh H A, Peterson P K, Nguyen B T, Sisson S P, Kim Y. Opsonization of encapsulated Staphylococcus aureus: the role of specific antibody and complement. J Immunol. 1982;129:1681–1687. [PubMed] [Google Scholar]
- 26.Watson D L. Expression of a pseudocapsule by Staphylococcus aureus: influence of cultural conditions and relevance to mastitis. Res Vet Sci. 1989;47:152–157. [PubMed] [Google Scholar]
- 27.Wilkinson B J. Staphylococcal capsules and slime. In: Easmon C S G, Adlam C, editors. Staphylococci and staphylococcal infections. New York, N.Y: Academic Press; 1983. pp. 481–523. [Google Scholar]
- 28.Wunschel D S, Fox K F, Nagpal M L, Kim K K, Stewart G C, Shagholi M. Quantitative analysis of neutral and acidic sugars in whole bacterial cell hydrolysates using high performance anion exchange liquid chromatography electrospray ionization tandem mass spectrometry. J Chromatogr A. 1997;776:205–219. doi: 10.1016/s0021-9673(97)00356-7. [DOI] [PubMed] [Google Scholar]