Summary
One of the major barriers that have restricted successful use of chimeric antigen receptor (CAR) T cells in the treatment of solid tumors is an unfavorable tumor microenvironment (TME). We engineered CAR-T cells targeting carbonic anhydrase IX (CAIX) to secrete anti-PD-L1 monoclonal antibody (mAb), termed immune-restoring (IR) CAR G36-PDL1. We tested CAR-T cells in a humanized clear cell renal cell carcinoma (ccRCC) orthotopic mouse model with reconstituted human leukocyte antigen (HLA) partially matched human leukocytes derived from fetal CD34+ hematopoietic stem cells (HSCs) and bearing human ccRCC skrc-59 cells under the kidney capsule. G36-PDL1 CAR-T cells, haploidentical to the tumor cells, had a potent antitumor effect compared to those without immune-restoring effect. Analysis of the TME revealed that G36-PDL1 CAR-T cells restored active antitumor immunity by promoting tumor-killing cytotoxicity, reducing immunosuppressive cell components such as M2 macrophages and exhausted CD8+ T cells, and enhancing T follicular helper (Tfh)-B cell crosstalk.
Subject areas: Immunology, Cancer, Transcriptomics
Graphical abstract
Highlights
-
•
Developed immune-restoring anti-CAIX CAR-T cells secreting anti-PD-L1 mAb
-
•
A humanized ccRCC orthotopic mouse model recapitulates ccRCC patient TME
-
•
G36-PDL1 IR CAR-T shows superior antitumor efficacy in a humanized mouse model
-
•
G36-PDL1 IR CAR-T promotes tumor killing by reversing immunosuppressive TME
Immunology; Cancer; Transcriptomics
Introduction
Immunotherapy is revolutionizing the practice of medical oncology, and chimeric antigen receptor (CAR) T cell therapy has proven to be effective for hematologic malignancies; however, several barriers have restricted CAR-T in the treatment of solid tumors.1,2,3,4,5,6,7,8,9,10,11,12 While inefficient CAR-T cell homing and metabolic changes have been shown to contribute to treatment inefficiencies in solid tumors, the immunosuppressive tumor microenvironment (TME) is known to be a major barrier to the translation of CAR-T cell therapy into solid tumors due to commandeering of the immune system causing T cell exhaustion and anti-inflammatory polarizations.11,13
Clear cell renal cell carcinoma (ccRCC) is the major histological subtype of RCC and has a paradoxical correlation that CD8+ T cell infiltration is associated with a worse prognosis.14 Thus, understanding the ccRCC TME is a necessary step to thwart disease progression and response to immunotherapy.15 Chevrier et al. observed an association between CD38+ CD204+ CD206- tumor-associated macrophages (TAMs) and immunosuppression within the ccRCC TME.16 Moreover, advanced stage patients with loss of 9p21.3 were found to have increased immune infiltration, and were less responsive to PD-1 blockade, with worse progression-free survival (PFS) and overall survival (OS).17 Thus, we hypothesize that understanding the PD-1 resistance found in ccRCC will provide immunotherapy targets to restore active antitumor immunity.
Single-cell RNA sequencing (scRNA-seq) has emerged as a powerful tool for gaining important insights regarding the TME in ccRCC18,19 and other cancers.20,21,22 Recently, advancing ccRCC disease state was shown to demonstrate not only increased exhausted CD8+ T cells and M2-like macrophages, but also that crosstalk between exhausted CD8+ T and M2 cells forms a dysfunctional immune circuit which promotes further T cell exhaustion and M2-like polarization, leading to a worse prognosis.18
Prior immunotherapy research in ccRCC has been hampered by the limited availability of murine tumor cell lines. Moreover, while genetically engineered models that harbor mutations that lead to the development of kidney cancer closely matching those observed in humans have been generated, they are only useable in immunocompetent syngeneic mice.23,24 We assert that due to inter-species differences in the immune system, a murine model with a humanized immune system is required to accurately reconstitute a TME which more closely matches that of cancer patients. Such a model would certainly allow for a better understanding of the interplay between human immune cells in the ccRCC TME, and provides a powerful tool for immunotherapy development and assessment of agents that might be directly translated to humans.25,26,27 Indeed, CD34+ progenitor hematopoietic stem cells (HSCs)-derived humanized mouse models provide the ability to study antitumor effects, such as response to immune checkpoint inhibitors (ICI)28 in a physiologically relevant immune environment.29
Here, we established a humanized ccRCC orthotopic NSG-SGM3 mouse model (termed hccRCC-NSG-SGM3) using human leukocyte antigen (HLA) partially matched CD34+ HSCs isolated from fetal liver, which recapitulates the ccRCC patient TME, and presents the same pattern of increased exhausted T cells (Tex) and M2-like macrophages as observed in advanced human ccRCC.18 Using this model, we evaluated anti-carbonic anhydrase IX (CAIX) immune-restoring (IR) CAR-T cells which were constructed based on our previous work using 4-1BB costimulatory domain and ICI payload.30,31 The IR CAR-T cells secreting anti-PD-L1 monoclonal antibody (mAb) exhibited superior tumor-killing capacity and the ability to restore active antitumor immunity by overcoming PD-1 resistance of ccRCC. Profiling tumor-infiltrating leukocytes (TILs) via scRNA-seq showed that IR CAR-T cells attenuate the immunosuppression in the TME and maintain a favorable antitumor phenotype in this humanized ccRCC mouse model.
Results
Generation of IR CAR-T cells
CAIX is highly expressed in ccRCC as a downstream gene of the HIF pathway32,33 and has been utilized as a therapeutic target for ccRCC therapy.34,35,36 Previously, we demonstrated that anti-CAIX G36-CD28 CAR-T cells secreting human ICI mAbs locally reduced CAR-T cell exhaustion in the tumor using a ccRCC orthotopic NSG mouse model without a human TME.30 In addition, we demonstrated that CAIX-specific CAR-T with a 4-1BB signaling domain (G36-4-1BB (BBζ)) outperformed both CD28 domain (G36-CD28 (28ζ)) and combined domain (G36-CD28-4-1BB (28BBζ)) and that BBζ with a CD8:CD4 ratio of 1:2 exhibited superior efficacy and persistence in vivo.31 Building on these results, we engineered anti-CAIX G36 BBζ CAR-T cells secreting anti-PD-L1 mAb (G36-PDL1) (Table S1). The PD-L1 mAb was expressed as a scFv with a human IgG4 Fc with mutations in the hinge to stabilize the structure (termed scFv-Fc).37 We assessed the efficacy of these IR CAR-T cells in vitro using the ccRCC skrc-59 cell line and in vivo in the hccRCC-NSG-SGM3 model. To control for CAR specificity and antibody payload, we used anti-B cell maturation antigen (BCMA) A716 CAR-T cells secreting anti-PD-L1 scFv-Fc (A716-PDL1) and anti-CAIX G36 CAR-T cells secreting anti-SARS scFv-Fc (G36-SARS), respectively (Figure 1A; Table S1).
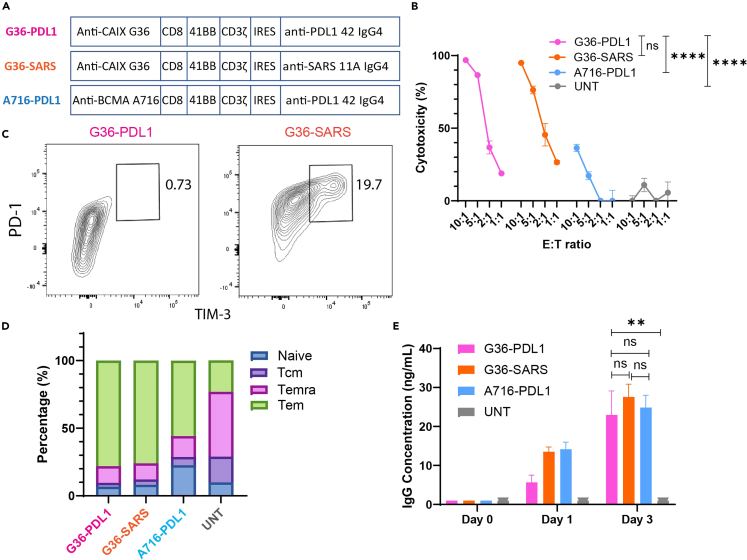
Figure 1.
Anti-CAIX G36-PDL1 CAR-T cells secreting anti-PD-L1 IgG4 payload lowered exhaustion in vitro
(A) The schematic shows G36-PDL1 (pink), G36-SARS (orange), and A716-PDL1 (blue) CAR constructs.
(B) Cytotoxicity of G36-PDL1 (pink), G36-SARS (orange), and A716-PDL1 (blue) CAR-T cells, and untransduced T cells (UNT, gray) with CD8:CD4 ratio of 1:2 under E:T ratio of 10:1, 5:1, 2:1, and 1:1 on CAIX+ ccRCC skrc-59 tumor cells.
(C) Percentage of terminally exhausted T cells (PD1hi, TIM-3+) of G36-PDL1 (pink), and G36-SARS (orange) CAR-T cells.
(D) Percentage of naive T cell (Naive, blue), central memory T cell (Tcm, purple), effector memory T cell re-expressing CD45RA (Temra, magenta), and effector memory T cell (Tem, green) of G36-PDL1 (pink), G36-SARS (orange), A716-PDL1 (blue), and CAR-T cells and UNT cells (gray) after 48 h co-incubation with skrc-59 tumor cells.
(E) Payload secretion quantification of G36-PDL1 (pink), G36-SARS (orange), A716-PDL1 (blue), and CAR-T cells and UNT cells (gray). All data with error bars are presented as mean ± SD. p values are defined by unpaired two-tailed t-tests or two-way ANOVA (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; and ∗∗∗∗p < 0.0001).
Characterization of IR CAR-T cells in vitro
We assessed tumor killing by IR CAR-T cells after 48 h co-incubation with CAIX+ PD-L1+ skrc-59 cells by using an image-based cytotoxicity assay.38 At an effector:target (E:T) ratio of 10:1 and CD8:CD4 ratio of 1:2, both G36-PDL1 and G36-SARS exhibited ∼95% killing capacity in vitro, while A716-PDL1 only exhibited modest cytotoxicity at levels similar to the untransduced T cells (UNT) (Figure 1B). At different E:T ratios, G36-PDL1 showed significantly higher tumor killing compared to A716-PDL1 and UNT but not G36-SARS. After 48 h co-incubation, G36-PDL1 exhibited a lower percentage of terminally exhausted T cells (PD-1hi TIM-3+) (Figure 1C) and a higher percentage of effector memory T cells (Tem) (Figure 1D) compared to G36-SARS CAR-T cells. We conclude that the anti-PD-L1 mAb secreted by G36-PDL1 CAR-T cells contributed to the lower exhaustion, higher Tem percentage, and increased CD8:CD4 ratio (Figure S1A) in G36-PDL1 CAR-T cells compared to G36-SARS CAR-T cells. Quantification of antibody production showed that all IR CAR-T cells secreted mAbs at a rate of ∼0.1 pg/cell/day (Figure 1E).
G36-PDL1 CAR-T cells exhibited superior efficacy in vivo in hccRCC-NSG-SGM3 model
To assess the ability of IR CAR-T cells to control tumor growth in vivo, we established the hccRCC-NSG-SGM3 mouse model (Figure 2A). Human CD34+ HSCs that were partially HLA (HLA-A, HLA-B, HLA-C, DRB1) matched to human ccRCC skrc-59 were isolated from fetal liver and injected intrahepatically into irradiated neonates (1–4 days) (Figures 2B and 2C). Once humanization was mature (greater than 20% circulating human lymphocytes), luciferized CAIX+ PD-L1+ human ccRCC skrc-59 tumor cells were implanted under the kidney capsule to establish ccRCC orthotopic humanized NSG-SGM3 mouse model31 which was further validated. Tumor-bearing humanization matured mice were divided into 5 groups with similar immune cell reconstruction efficiency as indicated by human CD45 positivity (Figure 2B), then a single dose of one million CAR-T cells (CD8:CD4 = 1:2) was injected through the tail vein. CAR-T cells were 6/6 HLA haploidentical (HLA-A, HLA-B, HLA-C, DRB1, DQB1, DPB1) to human ccRCC skrc-59 (Figure 2C). Bioluminescence imaging (BLI) was performed to monitor tumor growth and peripheral blood was analyzed to characterize humanization and peripheral circulating immune cells in vivo. Four weeks later, tumors were collected and digested into single-cell suspensions.
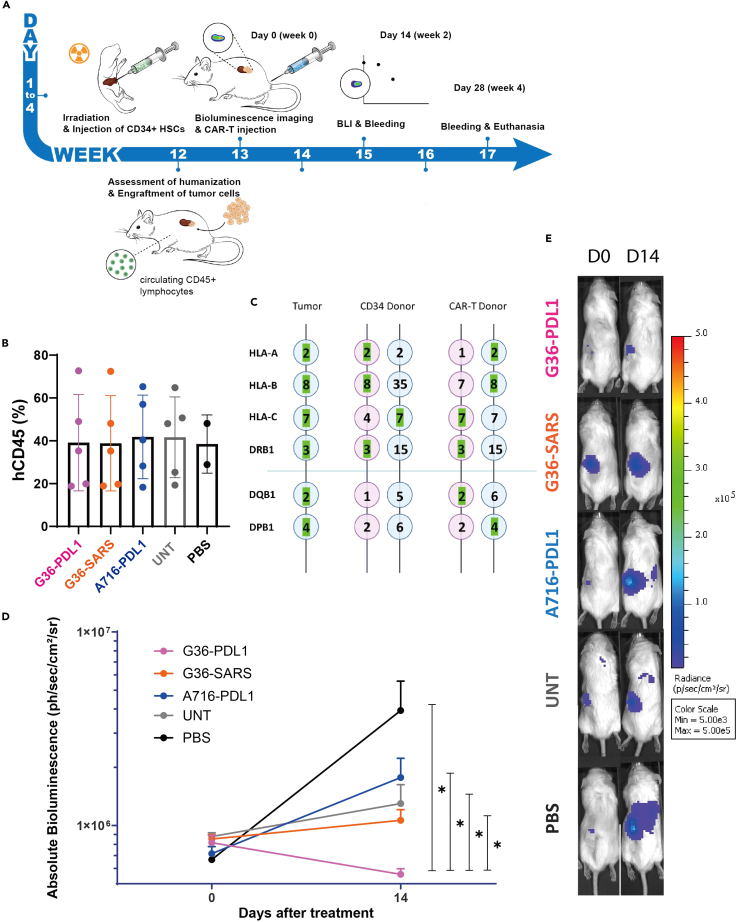
Figure 2.
Anti-CAIX G36-PDL1 CAR-T cells exhibited superior tumor regression compared to G36-SARS and A716-PDL1 CAR-T cells in a humanized ccRCC orthotopic NSG-SGM3 mouse model
(A) The schematic shows the establishment of the hccRCC-NSG-SGM3 mouse model and how it is used to assess different treatments. CD34+ HSCs were injected intrahepatically into 1–4 days old neonates. 12 weeks later, the humanization was assessed. Once the humanization was mature, ccRCC skrc-59 tumor cells were inoculated under the kidney capsule. One week after tumor implantation, one million CAR-T cells, UNT cells, or 100 μl of PBS were injected intravenously. Tumor growth was monitored by BLI at day 0 and 14 and human immunity and circulating CAR-T were phenotyped by weekly bleeding.
(B) Humanization efficiency (the percentage of human CD45+ immune cells out of total live leukocytes in the peripheral blood) in G36-PDL1 (pink), G36-SARS (orange), A716-PDL1 (blue) CAR-T cells, UNT cells (gray), PBS (black) is shown in the bar plot. Peripheral blood was analyzed via flow cytometry. Each dot represents one mouse.
(C) HLA phenotype of ccRCC skrc-59 tumor cells, CD34+ HSC donor and CAR-T donor. HLA-A, HLA-B, HLA-C, DRB1, DQB1, and DPB1 are shown here. Green highlights indicate HLA match.
(D) Tumor growth curve of the mice treated with one million CD8:CD4 = 1:2 G36-PDL1 (pink), G36-SARS (orange), A716-PDL1 (blue) CAR-T cells, UNT cells (gray) or PBS (black).
(E) Representative BLI images of mice treated with one million CD8:CD4 = 1:2 G36-PDL1 (pink), G36-SARS (orange), A716-PDL1 (blue) CAR-T cells, UNT cells (gray) or PBS (black). All data with error bars are presented as mean ± SD. p values are defined by unpaired two-tailed t-tests (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; and ∗∗∗∗p < 0.0001).
Mice treated with G36-PDL1 IR CAR-T cells exhibited superior tumor regression two weeks after a single-dose administration compared to G36-SARS, A716-PDL1, UNT, and PBS as shown in BLI (Figures 2D, 2E, and S2). The kinetics of immune cell expansion were documented and compared to the day of CAR-T cell infusion (day 0) (Figures S1B‒S1E).
The hccRCC-NSG-SGM3 mouse model recapitulated human immunity and treatment broadly altered TME landscape
Tumor-infiltrating human CD45+ cells were sorted and profiled for transcriptome and proteome via scRNA-seq, single-cell T cell receptor sequencing (scTCR-seq), and flow cytometry to study the TME, including population abundances and gene expression among the three CAR-T cell-treated groups (Group 1: G36-PDL1 CAR-T, Group 2: G36-SARS CAR-T, Group 3: A716-PDL1 CAR-T), and two TME baseline groups (Group 4: UNT, and Group 5: PBS) (Figure 3A). After quality control and batch correction (see STAR methods and Figure S3), 14,663 TILs composed of T cells (expressing CD3D, CD3E, and CD3G), B cells (expressing CD19, CD79A, MS4A1/CD20), and myeloid cells (expressing SPP1, TPSAB1, TPSB2, CD14, and CD68) were recovered (Figures 3B and 3C; Tables S2 and S3). Comparison to patient data from prospective clinical trials of advanced ccRCC patients18 revealed that the TILs from our hccRCC-NSG-SGM3 mouse model reconstituted most CD45+ cell types observed in patient ccRCC TME, suggesting similar landscape as human TILs (Figures 3D, 3E, and S4).
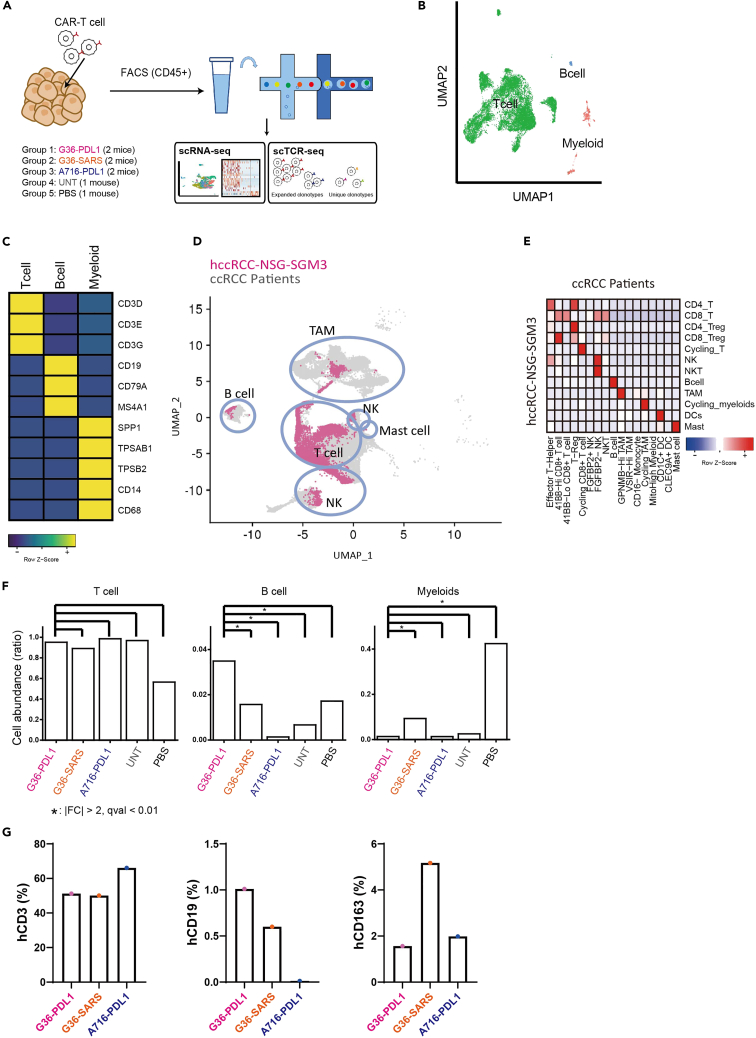
Figure 3.
Overall landscape of tumor microenvironment in the hccRCC-NSG-SGM3 mice treated with different CAR-T cell therapies
(A) Schematic flow of scRNA and scTCR sequencing for tumor samples. Live human CD45+ TILs from five groups were sorted and profiled.
(B) UMAP plot of main cell types (T cell: green, B cell: blue, and Myeloid cells: red).
(C) Heatmap of mean expression of marker genes for each main cell types.
(D) UMAP overlay of all TILs recovered from hccRCC-NSG-SGM3 (in pink) and TILs recovered from ccRCC patients (in gray).
(E) Heatmap of the cell type mapping ratio between hccRCC-NSG-SGM3 (row) and ccRCC patients (column).
(F) Bar plots of relative cell abundance for each main cell type between different groups. ∗ indicates a significant difference (fold change >2, q value <0.01) between two groups.
(G) Bar plots of relative cell abundance for human CD3+ T, human CD19+ B, and human CD163+ M2-like cells between different groups assessed via flow cytometry.
While T cell infiltration was similar in all 5 groups, G36-PDL1 had significantly higher B cell infiltration (Fold change (q value) of group1 vs. group2: 2.22 (3.67E-6); vs. group3: 26.3 (1.47E-36); vs. group4: 5.22 (1.82E-4); vs. group5: 2.03 (9.91E-2)) and lower myeloid infiltration (Fold change (q value) of group1 vs. group2: 0.147 (1.74E-38); vs. group3: 1.04 (8.26E-1); vs. group4: 0.544 (9.00E-2); vs. group5: 0.0326 (1.05E-103)), especially of CD163+ M2-like macrophages (Figures 3F and 3G, and specific cell number and abundance are shown in Table S4). We thus hypothesized that anti-PD-L1 mAb affects the immune response and TME in a way that inhibits M2 macrophage polarization39,40 and enhances B cells infiltration into G36-PDL1 TME. Our data implicate B cells in G36-PDL1 contributed to the antitumor effect. Although B cell influence on RCC TME remains unclear,41,42,43 a role for B cells in the antitumor immune response is emerging.44,45,46,47
G36-PDL1 CAR-T cells maintain a distinct cellular composition and function at the tumor site
Endogenous T cells, CAR-T cells, NK cells, and NKT cells were further clustered into 19 sub-clusters (Figures 4A and 4B), and each cell type was annotated based on differentially expressed genes (DEGs) (Tables S3 and S5). We identified nine CD8+ T subclusters (Figure 4C) and seven CD4+ subclusters (Figure 4D), a CD4− CD8− T subcluster, an NKT subcluster, and an NK subcluster (Figure S5). CAR-T cells were identified by the expression of the G36 CAR sequence and the A716 CAR sequence (Figure 4B; Table S1). Since 242 of 248 G36 CAR+ T cells were from G36-PDL1, we considered the recovered G36 CAR-T cells stemmed from the G36-PDL1 construct.
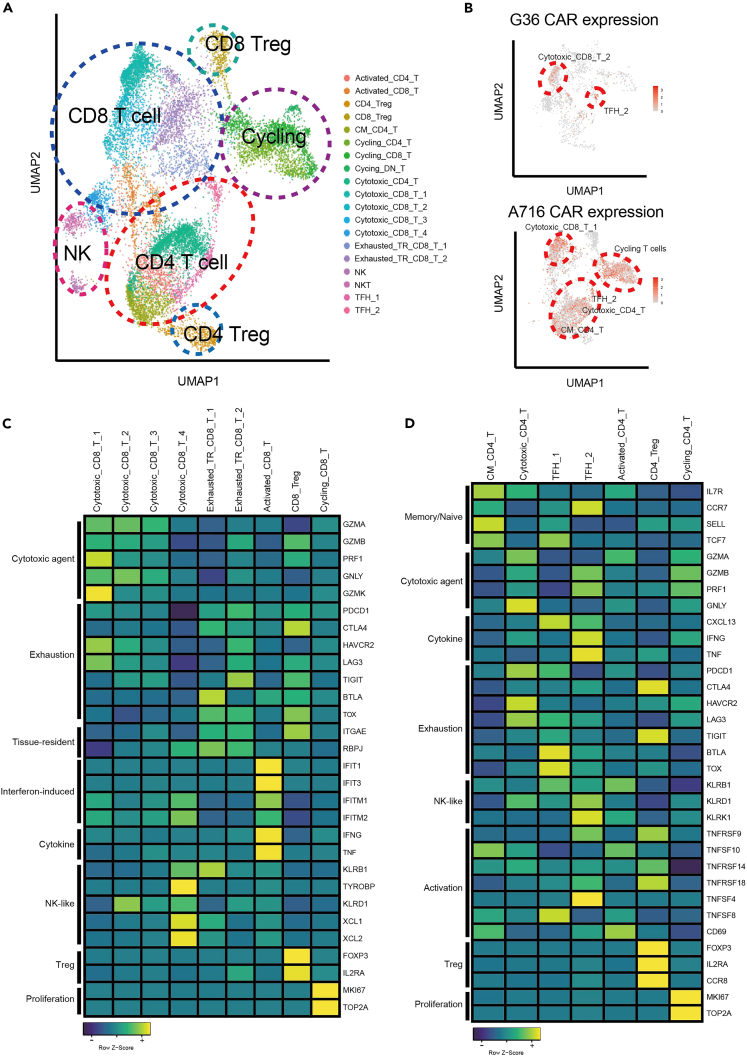
Figure 4.
Landscape of tumor-infiltrating T/NK/NKT cells
(A) UMAP plot of tumor infiltrating T cell subsets for both endogenous T cells and CAR-T cells, and NK and NKT cell subsets, including 19 subclusters.
(B) The distribution of CAR-seq in T cell subsets. G36 CAR contains two major clusters Cytotoxic_CD8_2 and TFH_2 (upper panel) and A716 CAR contains Cytotoxic_CD8_T_1, Cycling T cells, TFH_2, Cytotoxic_CD4_T, CM_CD4_T (lower panel).
(C) Heatmap of mean expression of signature genes for characterizing CD8+ T cell subsets.
(D) Heatmap of mean expression of signature genes for characterizing CD4+ T cell subsets.
G36 CAR-T cells were predominantly found in the Cytotoxic_CD8_T_2 (G36 CD8+ CAR-T) and TFH_2 (G36 CD4+ CAR-T) clusters. A716 CAR-T cells were also contained in the TFH_2 cluster but in addition, in other clusters composed of cycling T cells, Cytotoxic_CD8_T_1, Cytotoxic_CD4_T, CM_CD4_T, and TFH_2 (Figure 4B). These data indicate that A716 CD4+ CAR-T cells share similar diverse status to the endogenous CD4+ T cells that are derived from reconstituted human bone marrow and do not express CAR, while G36 CD4+ CAR-T cells are skewed toward a phenotype which is more favorable for immune response as shown in Figure 2.
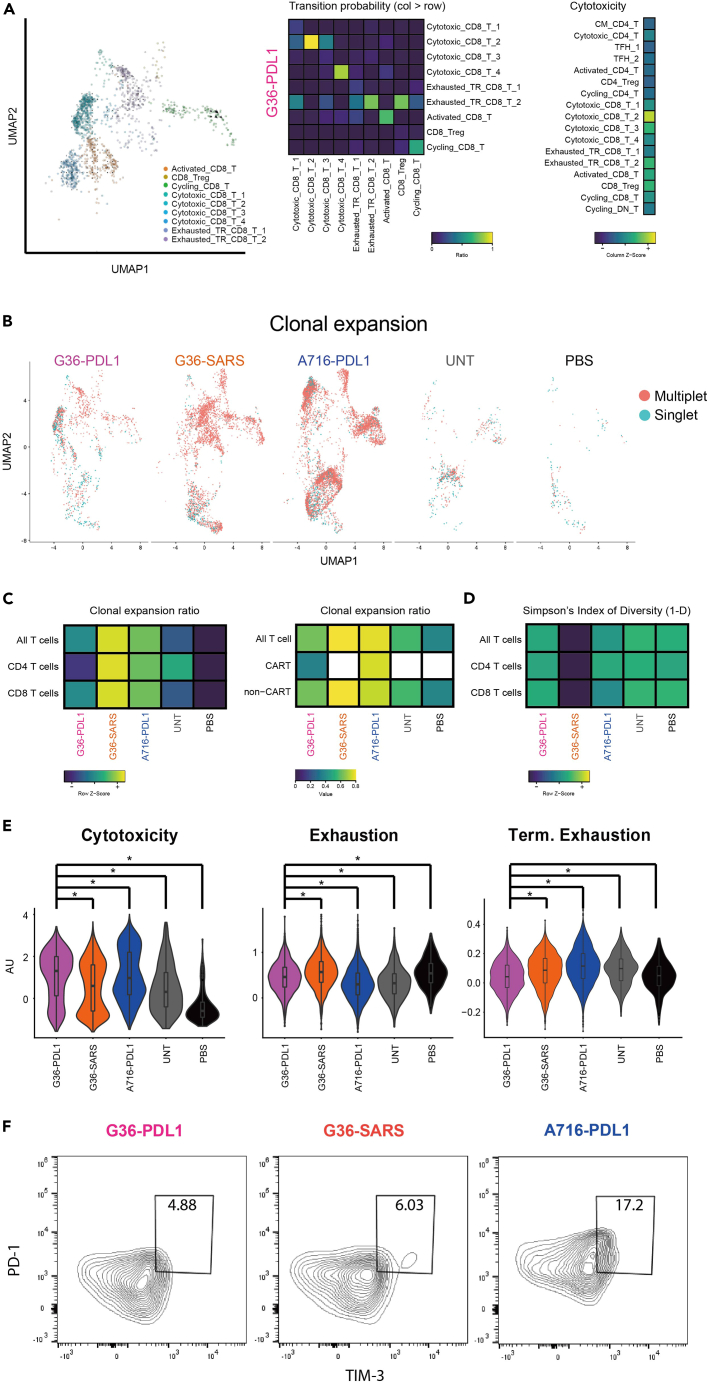
Cytotoxic_CD8_T_1 and Cytotoxic_CD8_T_2 showed the highest expression of cytotoxicity-related genes compared to other CD8+ T cell subsets (Figure 4C; Table S5) along with high expression of MHC class II molecules, indicating these CAR-T cells are highly activated31 (Figure S6B). However, Cytotoxic_CD8_T_1 showed high GZMK expression, indicating CD8+ A716-PDL1 CAR-T dysregulation at the tumor site.48,49 Moreover, by using RNA velocity50 analysis to estimate transition probabilities between the different T cell subsets, we found that Cytotoxic_CD8_T_2, one of the most cytotoxic subsets found in G36-PDL1 CAR-T cells, was more likely to retain its state rather than to differentiate into other states (Figures 5A, S8, and S9; Table S6).
Figure 5.
Reshaping of T cells to a favorable state for anti-cancer immunity in G36-PDL1
(A) UMAP plot of RNA velocity for CD8+ T cells in G36-PDL1 (left panel). Heatmap of transition ratio from cell types in column to cell types in row (middle panel). Heatmap of mean signature score of cytotoxicity in G36-PDL1 (right panel).
(B) UMAP plot of clonal expansion in each group (multiplet in red, singlet in cyan).
(C) Heatmap of multiplet ratio in all the T cells, CD4+ T cells, and CD8+ T cells from each group (left). Heatmap of multiplet ratio in all the T cells, CAR-seq expressing T cells, or non-CAR-seq expressing T cells from each group (right). White color indicates no value.
(D) Heatmap of Simpson’s Index of Diversity (1-D) in all the T cells, CD4+ T cells, and CD8+ T cells from each group.
(E) Violin plot of a given signature between groups. ∗ indicates p value <0.05.
(F) Percentage of terminally exhausted T cells (PD1hi TIM-3+) in tumor-infiltrating T cells of G36-PDL1 (pink), G36-SARS (orange), A716-PDL1 (blue) groups.
G36-PDL1 rewired endogenous TME with high cytotoxic CD8+ T and low Treg infiltration
We identified two other cytotoxic endogenous T cell subsets (Cytotoxic_CD8_T_3 and Cytotoxic_CD8_T_4) with high cytotoxicity and high NK signature gene expression, as well as one Activated_CD8_T cell subset with interferon-induced factors and IFNG51,52 (Figure 4C). Comparing the distribution of T cell subsets in each group, G36-PDL1 showed enhanced functional cytotoxic CD8+ CAR-T and endogenous T cell infiltration (Cytotoxic_CD8_T_2, Cytotoxic_CD8_T_4, Activated_CD8_T) (Figure S6A), which was likely due to the anti-PD-L1 payload promoting favorable antitumor immunity.53
In addition to the different subclusters composition described previously, we observed a lower abundance of CD8_Treg (Fold change of group1 vs. group2: 0.32) and CD4_Treg (Fold change of group1 vs. group2: 0.24) (FOXP3, IL2RA/CD25, TNFRSF9/4-1BB, CCR8, and immune checkpoint molecules) (Table S4)45,54 in the G36-PDL1 TME compared to G36-SARS (Figure S6A). Furthermore, comparison of the clonotypes found in CD4+ Treg and the other CD4+ T cell subsets using Jaccard coefficient revealed a lower similarity score in the G36-PDL1 group (Figure S7C). Since Treg can differentiate from conventional T cells,55 and anti-PD-L1 mAb inhibits differentiation of induced Treg,56 the lower Jaccard coefficient observed in G36-PDL1 indicates that the ICI payload changes TME conditions so they maintain active antitumor immunity but are unfavorable to support Treg differentiation.
The highest clonal expansion and lowest clonal diversity were observed in the G36-SARS group
T cell receptor (TCR) clonal diversity of peripheral circulating T cells is positively associated with cancer patient prognosis and ICI response.57,58,59 However, the correlation between TCR clonal diversity of tumor-infiltrating T cells and prognosis and response remains unclear. We analyzed the clonal expansion and clonal diversity of tumor-infiltrating CAR-T and endogenous T cells (Figures 5B–5D). G36-SARS showed the highest clonal expansion (Fold change of group2 vs. group1: 1.27, vs. group3: 1.04, vs. group4: 1.43, vs. group5: 2.08) and the lowest clonal diversity (T: 0.96, 0.54, 0.91, 0.99, 0.98; CD8 T: 0.94, 0.36, 0.8, 0.98, 0.96) compared to other groups (Figures 5C and 5D). This finding indicates that CAIX targeted CAR-T cells alone (without ICI payload), while showing antitumor activity in vivo, remain susceptible to extrinsic factors in the TME that lead to TIL exhaustion. The data are also consistent with observations in tumor-infiltrating exhausted T cells in advanced stage RCC patients that high clonal expansion was associated with advanced disease stage.19
Clonal expansion was also reflected by the change in CD8:CD4 ratio from the 1:2 used for CAR-T administration, which we have previously shown results in better expansion, efficacy, and persistence in vivo.31 Four weeks after administration, we observed higher infiltration of CD8+ T cells than CD4+ T cells at the tumor site (Figure S1F) (group1: 2.61, group2: 0.89, group3: 0.72). Comparing the CD8/CD4 ratio, both CAR-T (group1: 3.08, group3: 0.86) and endogenous T cells (group1: 3.54, group2: 2.06, group3: 0.85, group4: 0.30, group5: 0.73) in G36-PDL1 group showed the highest CD8/CD4 T cell ratio, indicating specific CD8 clonal expansion upon CAR or TCR activation (Figures S7A and S7B). In CD8+ T cells from hccRCC-NSG-SGM3, we also observed high expression of CD39 (ENTPD1), a biomarker of tumor-specific CD8+ TILs,60 showing the tumor-specific response within the TME (Figure S7D).
Tumor-infiltrating T cells in G36-PDL1 TME have high cytotoxic, low exhausted, low terminally exhausted phenotype
Profiling T cell signatures among the five groups, we found that G36-PDL1 tumor-infiltrating T cells showed greater cytotoxicity, as well as lower exhaustion, terminal exhaustion, and percentage of PD1hi TIM-3+ T cell population compared to G36-SARS (Figures 5E and 5F) suggesting that G36-PDL1 CAR-T cells have reshaped the TME and enabled the TILs to maintain their antitumor effect. Interestingly, tumor-infiltrating A716-PDL1 CAR-T cells showed the highest cytotoxicity score and the lowest exhaustion score (Figure 5E; Table S7) but also the highest terminal exhaustion signature18 and the most abundant PD1hi TIM-3+ T cell population (Figures 5E and 5F). This suggests an irreversible exhaustion state61,62 in A716-PDL1, reflecting their poor therapeutic efficacy.
G36-PDL1 CAR-T reshaped TME into an active antitumor environment with decreased Tex-M2 crosstalk and enhanced Tfh-B cell crosstalk
Myeloid cells were further categorized into macrophages (expressing CD14, CD68), dendritic cells (expressing CLEC10A/CLC10, CD1C), and mast cells (TPSAB1/TRYB1, CPA3, TPSB2) (Figure 6A and S10; Table S8). Furthermore, we could distinguish M1- and M2-like macrophages by known marker genes (Figure 6B; Table S2). M2 macrophages express anti-inflammatory molecules and restrict the antitumor effect,63 and a high abundance of M2-like macrophages leads to an unfavorable outcome in cancer immunotherapy in ccRCC patients.17,18 The anti-PD-L1 mAb is known to inhibit M2 polarization,39,40 and we observed the same effect in the mouse model with low abundance of M2-like macrophages in G36-PDL1 compared to G36-SARS (Figures 6C and 3G).
Figure 6.
TME in G36-PDL1 has been reshaped into a preferable environment for anti-cancer effect
(A) UMAP plot of myeloid subsets.
(B) Heatmap of M1 and M2 macrophage signature gene in M1_like_TAM and M2_like_TAM.
(C) Heatmap of relative cell abundance of myeloid subsets out of total TILs between groups. ∗ indicates a significant difference (fold change >2, q value <0.01) comparing to G36-PDL1.
(D) The difference of cell-cell interaction counts between two groups from CellChat package. The positive (red) sign indicates a higher value from G36-PDL1 comparing to G36-SARS (left panel) and to A716-PDL1 (right panel).
To explore cell-cell interactions within the TME, we used CellChat64 and found a substantial decrease in the number of interactions between M2_like_TAM and most T subtypes in the G36-PDL1 TME compared to the G36-SARS TME (Figures 6D and S11; Table S9). DEGs analysis showed downregulation of myeloid regulators (MIF,65 CSF166) in Exhausted_TR_CD8_T_2 in the G36-PDL1 TME compared to the G36-SARS TME. This suggests an attenuated circuit between exhausted CD8 T cells and M2 cells due to the anti-PD-L1 payload (Table S9). The interactions between TFH_1, TFH_2, and B cells were enhanced in G36-PDL1 compared to the ones in other groups (Figures 6D and S11), which is consistent with reports that the interaction between T follicular helper cell (Tfh) and B cell has a favorable effect in the TME due to the formation of tertiary lymphoid structures.43,45,46,47 Taken together, these results suggest that G36-PDL1 supports a more favorable TME that leads to increased tumor cell killing, decreased communication among exhausted T cells and M2 macrophages (Tex-M2), and enhanced Tfh-B cell crosstalk.
Discussion
We have established a humanized ccRCC mouse model which can recapitulate cardinal features of the ccRCC human TME and serves as a powerful tool for immunotherapy assessment. The efficacy and ability to restore active immunity of IR CAR-T cells were assessed in this hccRCC-NSG-SGM3 mouse model. CAR-T cells with target recognition and PD-L1 mAb were superior to either alone, i.e., G36-PDL1 CAR-T cells that secreted PD-L1 mAb exhibited superior tumor inhibition compared to G36-SARS or A716-PDL1. This increase in G36-PDL1 CAR-T cell therapeutic effect in vivo is likely due to multiple factors including their increased cytotoxicity, resistance to exhaustion, and their ability to change the TME by decreasing communication among exhausted T cells and M2-like macrophages and enhancing Tfh-B cell crosstalk.
Previous studies have demonstrated that the absence of human immunity in mouse models compromises their value in translational research and the development of novel immuno-oncology therapies.67,68 The construction of humanized animal models has allowed for the development of a rudimentary level of innate and adaptive human immunity in mice.69 The NSG-SGM3 strain expressing human stem cell factor, GM-CSF, and IL-3 transgenes is a superior mouse model for humanization to assess immunotherapies and to study the TME.70,71 NSG-SGM3 strain shows the most efficient engraftment of HSCs to date as well as marked improvement in development of myeloid cells following HSC engraftment in vivo72,73,74,75 thereby serving as an ideal tool for studying myeloid cell crosstalk. Here, to achieve high humanization efficiency, we humanized NSG-SGM3 mouse with fetal tissue as it has been reported that transferring fetal liver or cord blood-derived HSCs results in a higher engraftment of human CD45+ cells compared to engrafting the bone marrow or mobilized peripheral blood derived HSCs.76,77 In addition, we engrafted newborns as they exhibited a better reconstitution of human CD45+ cells compared to adult recipients.75,78,79 To avoid graft-versus-host disease and nonspecific tumor killing, we used HSCs that were partially HLA matched to the tumor. The humanization and the corresponding immune response are dynamic, with high B cell reconstitution and low T cell reconstitution at the early stage (less than 10 weeks after reconstitution), and then significant T cell expansion at the later stage (around week 30).80 In our hccRCC-NSG-SGM3 mouse model, we observed a similar trend and started the experiment at circa 70% T cell percentage (Figure S1C) which is similar to the percentage of circulating T cells in human peripheral leukocytes.81
The lack of clinically relevant models for testing human immunotherapy hinders the development of cancer therapies. This is also true for ccRCC, where existing in vivo models poorly recapitulate the TME.82 For example, traditional mouse models of renal cancer poorly represent human immune cells since some members of the B7 immune checkpoint family, including HHLA2 which is highly expressed in kidney cancer,83,84 have no mouse ortholog.85
Previous studies in tumor-bearing HuNSG mice have shown that both CD4+ and CD8+ T cell clones recovered from tumor-bearing mice demonstrated HLA-restricted tumor cell killing75 and antitumor responses with pembrolizumab treatment.28 In addition, human thymopoiesis within the mouse thymus has been shown to occur,28 and education could be further improved by rectifying restrictions in cross-species HLA-dependent antigen-specific immune responses.86 Here, we further advanced the humanized mouse models by profiling the TME of treatment naive at baseline (Figure S4) and in all treatment groups (Figures 3D and 3E), demonstrating that hccRCC-NSG-SGM3 is able to reconstitute most types of human ccRCC patient TILs, thus, providing a promising tool for preclinical evaluation of immunotherapies and studying TME. To study the human antigen-specific response, we studied CD39 (ENTPD1)-positive tumor-infiltrating T cells as CD39 is highly expressed in tumor-specific CD8+ TILs but not bystander TILs.60 We found CD39 expression in all CD8+ T cell subsets, indicating a general tumor-specific response in hccRCC-NSG-SGM3 mouse model (Figure S7D).
This IR CAR-T strategy showed that a secreted anti-PD-L1 mAb can work in trans to reverse exhaustion of TILs, a significant advantage over CAR-T cells that use CRISPR to knock out PD-1 expression, which only prevents this checkpoint activity in the CAR-T cells,87,88 but cannot reverse exhaustion of endogenous T cells or myeloid cells.89 Moreover, deletion of PDCD1 has been reported to impair T cell survival, memory development, and function.90 This cell-based combination immunotherapy is formulated to be administered as one single agent which should also restrict effects of the ICI mainly to the tumor site and mitigate against the side effects caused by systematically administrated ICIs.91,92
In the ccRCC TME, M2 macrophages and exhausted CD8+ T cells form a bidirectional circuit where exhausted T cells express high inhibitory checkpoint molecules (PD-1, CTLA-4, TIGIT, LAG-3) and produce M2 modulators (CSF1, MIF) to promote M2 polarization, while M2-like macrophages highly express the corresponding ligands and receptors (PD-L1/2, CD86, PVR, CSF1R, CD74) to facilitate T cell exhaustion.18,19 Our data support that G36-PDL1 CAR-T cells were able to interrupt this dysfunctional regulation and rewire TME with higher cytotoxic CD8+ T infiltration and lower M2 infiltration by downregulating the crosstalk between CD8+ T and M2 as well as downregulating the negative regulators (Figures 3F, 3G, and 6D; Table S10).
In summary, the hccRCC-NSG-SGM3 is able to model advanced ccRCC TME and provides a powerful tool for ccRCC TME study and immunotherapy assessment. Anti-CAIX G36-PDL1 IR CAR-T cells secreting anti-PD-L1 mAb exhibited superior tumor control and successfully restored active antitumor immunity in the hccRCC-NSG-SGM3 model. This treatment holds promise to achieve ccRCC cures by rewiring local TME.
Limitations of the study
Potential limitations of our study include limited animal numbers, lack of analysis of neutrophils, and other immune components which are sensitive to degradation and have relatively low RNA content.93,94 The lack of human HLA class I and II in the thymus of the humanized mouse model can be improved using Thy/HSC,95 BLT,96 and human HLA gene-expressing86 models to generate HLA-restricted antigen-specific human T cell reactions. To further study the dynamics of humanization, scRNA-seq of samples collected at different time points should be performed. Mouse PD-L1 was reported to negatively signal human PD-1,97 thus the cross reactivity needs to be further investigated in this humanized mouse model. Previous study has shown that in a xenograft mouse model of metastatic ovarian tumor, mice treated with CAR-T cells secreting anti-PD-1 scFv had enhanced survival benefit over mice treated with CAR-T cells and systemic administration of anti-PD-1 mAb, so that we did not include systemic anti-PD-L1 mAb as a control in our experimental design.94 Additionally in the future, due to the high conservation between human and murine CXCR4 and CXCL12, we will include mouse fibroblasts to comprehensively study the TME.98 Finally, additional animal models and experimental efforts are needed to have a better understanding of the enhanced Tfh-B crosstalk that was observed in the G36-PDL1 TME.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Goat-anti human IgG-Fc | Bethyl Laboratories | A80-104A |
| MSD SULFO-TAG Labeled Anti-Human Antibody (Goat) | MSD | R32AJ-1 |
| Anti-human CD45 PerCP/Cy5.5 | BioLegend | 304028 |
| Anti-mouse CD45 Dazzle594 | BioLegend | 103146 |
| Anti-human CD3 BV650 | BioLegend | 317324 |
| Anti-human CD4 AF700 | BioLegend | 357418 |
| Anti-human CD8 BV785 | BioLegend | 344740 |
| Anti-human CD19 BV421 | BioLegend | 302234 |
| Anti-human CD27 BV711 | BioLegend | 356430 |
| Anti-human CD62L BV510 | BioLegend | 304844 |
| Anti-human PD1 PE | BD | 557946 |
| Anti-human TIM-3 APC/Cyanine7 | BioLegend | 345026 |
| Anti-human CD45RA PerCP/Cyanine5.5 | BioLegend | 304122 |
| Anti-human CD45RO PE/Cyanine7 | BioLegend | 304230 |
| Bacterial and virus strains | ||
| pHAGE-Ef1a-IRES-WPRE | This paper | N/A |
| Biological samples | ||
| Leukopak | StemCell | 70500 |
| Apheresis leukoreduction collars | obtained from the blood bank of the Brigham and Women's Hospital under DFCI approved IRB protocol #14-343 | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Polyethylenimine, PEI | ||
| Lenti-X™ Concentrator | Clontech | 631231 |
| Ficoll-Paque-PLUS | GE Healthcare | 45001750 |
| Gentamicin | Gibco | 15750060 |
| IL-21 | Miltenyi Biotec | 130-095-784 |
| IL-7 | Miltenyi Biotec | 130-095-363 |
| IL-15 | Miltenyi Biotec | 130-095-765 |
| D-luciferin potassium salt | Promega | E1605 |
| Critical commercial assays | ||
| Zombie Yellow™ Fixable Viability Kit | BioLegend | 423104 |
| Tumor Dissociation Kit | Miltenyi Biotec | 130-095-929 |
| CD34 microbead kit | Miltenyi Biotec | 130-100-453 |
| CD4 microbeads | Miltenyi Biotec | 130-097-048 |
| CD8 microbeads | Miltenyi Biotec | 130-097-057 |
| TransAct kit | Miltenyi Biotec | 130-111-160 |
| Deposited data | ||
| scRNAseq data | This paper | GSE210664 |
| Kevin Bi et al. | Single Cell Portal | Single Cell Portal: https://singlecell.broadinstitute.org/single_cell/study/SCP1288/tumor-and-immune-reprogramming-during-immunotherapy-in-advanced-renal-cell-carcinoma#study-summary |
| Codes | This paper | https://doi.org/10.5281/zenodo.7274663 |
| Supplementary tables | This paper; Zenodo Data | Table S2∼10; https://doi.org/10.5281/zenodo.10456704 |
| Software and algorithms | ||
| Cellranger | 10x genomics | https://www.10xgenomics.com/support/software/cell-ranger |
| Seurat | Hao et al.99 | https://satijalab.org/seurat/ |
| harmony | Korsunsky et al.100 | https://portals.broadinstitute.org/harmony/index.html |
| CellChat | Jin et al.64 | https://github.com/sqjin/CellChat |
| Velocyto | La Manno et al.50 | https://velocyto.org/ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Wayne Marasco (wayne_marasco@dfci.harvard.edu).
Materials availability
All plasmids and cell lines used in this paper will be shared by the lead contact upon request.
Data and code availability
All generated scRNAseq data are available at the Gene Expression Omnibus (GEO) with accession code GSE210664. Supplementary Tables from 2 to 10 can be found in https://doi.org/10.5281/zenodo.10456704. Original codes can be accessed via https://doi.org/10.5281/zenodo.7274663. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and study participant details
Cell lines and culture
Human clear cell renal cell carcinoma cell line skrc-59 (obtained from Dr. Gerd Ritter, Memorial Sloan Kettering Cancer Center, New York, USA) was engineered to express high levels of human CAIX (skrc-59 CAIX+)30 and mCardinal fluorescent protein. These cells were grown in RPMI-1640 medium (Gibco) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS, Gibco) at 37°C with 5% CO2.
Ethical approval
Apheresis leukoreduction collars were obtained from the blood bank of the Brigham and Women's Hospital under DFCI approved IRB protocol #14–343. Humanized mouse model experiments were performed under DFCI approved protocol #05–035.
Methods details
Production of lentivirus particles
For lentivirus production, polyethylenimine (PEI), DNA of the helper plasmids VSVG, TAT, GAG and REV (10 μg per 15 cm dish of 293T cells) and 20 μg of the respective CAR DNA were added to Opti-MEM medium (Gibco). This mixture was incubated for 20 min at RT and was afterward added drop by drop to a 15 cm dish of LentiX-293T cells (Clontech). After 48 h of incubation, the supernatant was collected, debris was removed and lentiviral concentrator (Clontech) was added in a 1:3 (v/v) ratio. This mixture was incubated overnight at 4°C, the next day the tubes were centrifuged for 45 min at 1,500 g and the supernatant was discarded. The pelleted lentivirus was resuspended in RPMI-1640 medium and stored at −80°C.
Generation of CAR-T cells
A leukopak (StemCell) and apheresis leukoreduction collars (blood bank of the Brigham and Women's Hospital) were obtained under DFCI approved IRB protocol #14–343. Human peripheral blood mononuclear cells (PBMCs) were separated using Ficoll-Paque-PLUS (GE Healthcare). CD4 and CD8 T cells were isolated by positive selection using CD8 MicroBeads and CD4 MicroBeads (Miltenyi Biotec) respectively. T cells were cultured in RPMI-1640 medium supplemented with 10% FBS, IL-21 (30 ng/mL, Miltenyi Biotec) and activated by T cell TransAct (Miltenyi Biotec). CAR-T cells were generated by lentiviral transduction (MOI 20) with 10 μg/mL Diethylaminoethyl (DEAE). The CAR positive T cells were sorted by SONY sorter MA900. After sorting, the T cells were cultured in RPMI-1640 medium supplemented with 10% FBS, IL-7 (5 ng/mL, Miltenyi Biotec), IL-15 (2.5 ng/mL, Miltenyi Biotec) and Gentamicin (50 μg/mL) (Gibco).
Payload quantification
Standard MSD High Bind Plates were coated with 25 μL of goat-anti human IgG-Fc fragment (affinity purified: A80-104A) diluted to 1 μg/mL in PBS. After centrifugation at 500xg for 1 min, plates were incubated overnight at 4C. The following day, plates were washed with 50 μL PBS per well blocked with 25 μL 1% BSA in PBS per well, centrifuged at 500xg for 1 min, and incubated overnight at 4C. The following day, plates were washed 3 times with 50 μL PBS per well before adding 25 μL of samples diluted 4-fold in 1% BSA in PBS. Samples were centrifuged at 500xg for 1 min, and incubated on a shaker for 2 h at room temperature. Plates were then washed 3 times with 50 μL PBS per well before adding 25 μL of detection antibody (MSD SULFO-TAG Labeled Anti-Human Antibody (Goat), catalog #R32AJ-1) at 1 μL/mL diluted in PBS per well, followed by incubation for 1 h at room temperature, and 5 washes with 50 μL PBS. 35 μL per well 1X MSD read buffer with surfactant diluted in water was added and the plates were read using MESO SECTOR S 600.
HLA typing
HLA typing was performed by American Red Cross HLA Laboratory (Dedham, MA) using molecular SSO (Luminex).101
In vitro killing assay
Celigo in vitro killing assay was performed as described before.31,38 Approximately 3000 skrc-59 tumor cells (target cells) were seeded in a 96-well plate (Greiner 655090). After 12 h of incubation, the plate was scanned and analyzed in bright field and far red channel for mCardinal as the 0 h time point. CAR-T cells were added and co-incubated with the target cells. Additional control wells were prepared with target cells only (negative control) and target cells with 1% Triton X-(positive control). Subsequently, the plate was scanned and analyzed at the 48 h time point with the equation, .
Flow cytometry
All samples were analyzed with an LSR Fortessa (BD Bioscience) and data were analyzed using FlowJo software (FlowJo LLC). T cell phenotype was evaluated via:Zombie Yellow Fixable Viability Kit (BioLegend), human CD45 (clone HI30, BioLegend), mouse CD45 (clone 30F11, BioLegend), CD3 (clone OKT3, BioLegend), CD4 (clone A161A1, BioLegend), CD8 (clone SK1, BioLegend), CD19 (clone HIB19, BioLegend), CD27 (clone M-T271, BioLegend), CD62L (DREG-56, BioLegend), PD-1 (clone MIH4, BD Bioscience), TIM-3 (clone F38-2E2, BioLegend), CD45RO (clone UCHL1, BioLegend), CD45RA (HI100, BioLegend).
In vivo orthotopic humanized ccRCC model
Humanized mouse model was established based on publication reported before25 and performed under DFCI approved protocol #05–035. CD34 HSCs were isolated from an 18-week-old fetal liver tissue using CD34 microbeads (Miltenyi Biotec), which was 4/6 HLA matched to the skrc-59 tumor cell line. One hundred thousand CD34+ HSCs were injected intrahepatically in 1–4 days old neonates irradiated with a single sublethal dose of 1 Gy whole body irradiation. After 12 weeks, humanized mice were bled to assess the humanization efficiency. Once the humanization was matured (human CD45 > 20% is considered successfully humanized), 50,000 skrc-59 CAIX+ luciferase+ cells were resuspended in 10 μL of RPMI-1640 medium and diluted 1: 1 in Matrigel (Corning). This cell mixture was injected under the left kidney capsule of NSG-SGM3 mice (Jackson Laboratories). One week after, tumor engraftment was confirmed with bioluminescence (BLI) imaging and 1 million CAR-T cells or untransduced T cells were injected through the tail vein of the mice (Day 0, N = 5 mice per group). The tumor BLI was determined 14 days post CAR-T cell injection. On Day 28, the mice were sacrificed by CO2 inhalation, final blood was drawn and organs and tumors were harvested for analysis.
Bioluminescence imaging (BLI)
Tumor growth was monitored at Day 0 and 14 using the IVIS Spectrum In Vivo Imaging System (PerkinElmer). Briefly, mice were injected subcutaneously with 75 mg/kg D-luciferin potassium salt (Promega E1605) in sterile PBS and anesthetized with 2% isoflurane in medical air. Serial bioluminescence images were acquired using the automated exposure set-up. The peak bioluminescence signal intensity within selected regions of interest (ROIs) was quantified using the Living Image Software (PerkinElmer), and expressed as photon flux (p/sec/cm2/sr). Representative planar bioluminescence images were displayed with indicated adjusted minimal and maximal thresholds.
Tumor dissociation and tumor infiltrating CAR-T cell isolation
Renal tumor samples were dissociated into single cells by a semi-automated combined mechanical/enzymatic process. The tumor tissue was cut into pieces of (2-3mm) in size and transferred to C Tubes (Miltenyi Biotech) containing a mix of Enzymes H, R and A (Tumor Dissociation Kit, human; Miltenyi Biotech). Mechanical dissociation was accomplished by performing a program (37C_h_TDK_1) on the gentleMAC Octo Dissociator with Heaters (Miltenyi Biotech). Single cell suspensions generated from ccRCC tumor samples were FACS sorted on a SONY MA900 sorter (SONY) for viable human TILs (Zombie-mCD45-hCD45+, BioLegend).
RNA-seq 10X genomics library preparation, hash tag, and single cell 5′ sequencing
Single-cell RNA-seq experiments were performed by the Brigham and Women’s Hospital Center for Cellular Profiling. Sorted cells were stained with a distinct barcoded antibody (Cell-Hashing antibody, TotalSeq-C, Biolegend) as previously described.102 After washing, the stained cells were resuspended in 0.4% BSA in PBS at a concentration of 2,000 cells per μL, then loaded onto a single lane (Chromium chip K, 10X Genomics) followed by encapsulation in a lipid droplet (Single Cell 5′kit V2, 10X Genomics) followed by cDNA and library generation according to the manufacturer’s protocol. 5′ mRNA library was sequenced to an average of 50,000 reads per cell, V(D)J library and protein (hashtags) library sequenced to an average of 15,000 reads per cell, all using Illumina Novaseq.
Data processing
Since our sample contained an artificial gene construct (CAR sequence), we added the 5-prime part of the CAR sequences by using mkref function in the Cell Ranger software (v6.1.2) (Table S1). Using the modified genome, we obtained a gene-count matrix with a hashtag for each group from the first experiment (group 1, 2, 3, 4, 5) from raw fastq files of scRNAseq and CITEseq data. We also used Cell Ranger (v4.0.0) to obtain a clonotype for each cell from raw fastq files of VDJ-seq data with only the productive TCR sequence. First, we filtered out a potential doublet by scDblfinder103 (v1.2.0) with default parameters. Second, we used the Seurat package99 (v 4.0.6) for preprocessing and downstream analysis of the data. We filtered cells by discarding the cells that had high contamination of mitochondrial genes or those which were outliers in nfeatures_RNA and nCount_RNA from the “FeatureScatter” plot. Third, we distinguished each group by HTODemux function with default parameters. We only retained the unique hashtag for each group. Finally, we merged scRNAseq data with VDJ-seq data by saving only the cells that had paired data for scRNAseq and VDJ-seq in T cells. We performed the same procedure for the second experiment except de-multiplexing since scRNAseq for groups 1, 2, and 3 was conducted separately.
Merging and batch correction of main celltypes
After merging the Seurat objects together, we applied “NormalizeData” as the recommended setting for each patient (Log-normalization, scale factor:10,000). We used 2,000 highly variable genes for downstream analysis, such as dimension reduction and clustering. “ScaleData,” “RunPCA,” and “RunUMAP” functions were used with default settings. We ran Harmony100 (version 0.1.0) with 1∼50 PCs to correct the batch effect between each sample from each main cell type, respectively. After batch correction, “RunUMAP” functions in Seurat v4 were utilized for dimension reduction and visualization using default settings, except for giving the reduction parameter as “harmony”. Unsupervised clustering was performed by “FindNeighbors,” which conducts shared-nearest-neighbor-based clustering with highly variable genes, and “FindClusters” which conducts Louvain clustering with the resolution 0.3. During the procedure, we further removed cancer cells or bad cells. Finally, T cells, B cells, and Myeloid cells were identified by their marker genes (Table S2) from differentially expressed gene (DEG) by “FindAllMarkers”. DEGs were defined by |avgLog2FC| > 0.25, min.pct <0.25, and adjusted p value <0.01 (Table S3).
Subclustering of myeloid and T cells
We further subclustered the myeloid and T cells with another round of processing as above. For myeloids, we used 1–30 PCs for the analysis with 0.4 resolution for the clustering parameter. We found 5 subclusters from myeloid cells and annotated with a marker gene list (Table S2) from DEG as above (Table S8).
For T cells, after excluding TCR genes from the variable gene list, we used Seurat integration method (PC: 1–40) to merge the T cells from different samples for subclustering, we split the data into CD4 T cells, CD8 T cells, NK lineage, and cycling T cells based on the distribution of CD4, CD8A, cycling markers (MKI67 and TOP2A), NK markers (TYROBP and NCAM1), and T cell markers (CD3D, CD3E, CD3G). For each compartment, we conducted another round of clustering for fine clustering. Annotation of cell type was defined by DEG using all the T cells, CD4 T cells only, and CD8 T cells only (Tables S2 and S5).
Data transferring to human ccRCC data
We downloaded processed scRNAseq data18 from Single Cell Portal (https://singlecell.broadinstitute.org/single_cell/study/SCP1288/tumor-and-immune-reprogramming-during-immunotherapy-in-advanced-renal-cell-carcinoma#study-summary). We obtained clinical information and meta data from the supplementary material. We followed the original cell type annotation for our analysis. We performed data-transferring by Seurat package with “FindTransferAnchors” and “MapQuery” functions.
T cell clonality analysis
We obtained clonal type of T cells from the “clonotypes.csv” output of cellranger pipeline. We assigned a cell as “multiplet” if a given T cell shared the same clonotype with other T cells in each sample. Otherwise, we assigned it as “singlet”. If the TCR sequence was not available for a given T cell, we did not consider it for the clonotype analysis.
Cell abundance analysis
The relative abundance of each cell type was measured by normalizing the total number of cell counts in each group. We performed a Fisher’s exact test between the two different groups to obtain a p value. Benjamini-Hochberg correction was conducted to obtain adjusted p values. We considered adjusted |FC| > 2 and p value <0.01 as a significant difference (Table S4).
Signature analysis
We measured the signature score by “AddModuleScore” function in Seurat package. The list of genes for each Table S7. We used the same size of a given genes for “ctrl” parameter. We used the average score from the results of “AddModuleScore” for each cell. While comparing between two groups, we performed Wilcoxon rank-sum test to obtain p values.
DEG analysis between groups
The DEG analysis to compare between two groups was conducted by using “FindMarkers” function in Seurat package. DEGs were defined by |avgLog2FC| > 0.25, min.pct <0.25, and adjusted p value <0.01 (Table S10).
Cell-cell interaction analysis
We used CellChat64 (v1.4.0) to infer the interaction between celltypes. We followed the tutorial pipeline. In detail, we used the receptor-ligand database provided by cellchat. “subsetData”, “identifyOverExpressedGenes”, “identigyOverExpressedInteractions”, “computeCommunProb”, “filterCommunication”, and “computeCommunProbPathway” functions were used (Table S9).
RNA velocity analysis
We used velocyto50 (v0.17.16) to infer the RNA velocity with default pipeline. But we used previous results of variable genes, UMAP coordinate from Seurat package. We obtained the transition probability from the “transition_prob.T” variable (Table S6).
Quantification and statistical analysis
The data are presented as mean ± s.d. as indicated in the figure legends. For comparisons between groups, the data were analyzed by one-way ANOVA analysis. All statistical analyses were performed using GraphPad Prism software. The calculated p values are reported using the symbols: ‘n.s.’ means not significant, ‘∗’ means p < 0.05, ‘∗∗’ represents p < 0.01, and ‘∗∗∗’ stands for p < 0.001.
Acknowledgments
This work was supported by the Assistant Secretary of Defense for Health Affairs endorsed by the Department of Defense, through the FY21 Translational Research Partnership Award (W81XWH-21-1-0442) and FY21 Idea Development Award (W81XWH-21-1-0482) to W.A.M. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense. This work was partially supported by NCI P50CA101942 to G.J.F. This work was also supported by the Wong Family Award and KCA Trailblazer Award to Y.W. In addition, J.W.C. and M.H. were funded by the Evergrande Center and the Helmsley Foundation (2008-04050). K.W. is supported by a Burroughs Wellcome Fund Career Awards for Medical Scientists, a Doris Duke Charitable Foundation Clinical Scientist Development Award, and a Rheumatology Research Foundation Innovative Research Award. D.A.B. is in part supported by the DOD CDMRP Academy of Kidney Cancer Investigators (KC190128). T.K.C. is supported in part by the Dana-Farber/Harvard Cancer Center Kidney SPORE (2P50CA101942-16) and Program 5P30CA006516-56, the Kohlberg Chair at Harvard Medical School and the Trust Family, Michael Brigham, Pan Mass Challenge, and Loker Pinard Funds for Kidney Cancer Research at DFCI.
Author contributions
Y.W. and W.A.M. designed the study. Y.W., J.-W.C., G.K., A.B., C.R., A.C.C., J.S., K.J., Q.-D.N., and Z.Z. performed the experiments. Y.W., J.-W.C., G.K., A.C.C., and M.H. analyzed the data. Y.W., J.-W.C., G.K., M.H., G.J.F., and W.A.M. wrote the manuscript. Y.W., J.-W.C., G.K., A.B., C.R., A.C.C., J.S., D.A.B., T.K.C., C.J.W., K.J., Q.-D.N., Z.Z., K.W., Q.Z., S.S., G.J.F., M.H., and W.A.M. edited and approved the manuscript.
Declaration of interests
W.A.M. has patents in the PD-1/PDL1 field. G.J.F. has patents/pending royalties on the PD-1/PD-L1 pathway from Roche, Merck MSD, Bristol-Myers-Squibb, Merck KGA, Boehringer-Ingelheim, AstraZeneca, Dako, Leica, Mayo Clinic, Eli Lilly, and Novartis. G.J.F. has served on advisory boards for Roche, Bristol-Myers-Squibb, Xios, Origimed, Triursus, iTeos, NextPoint, IgM, Jubilant, Trillium, IOME, GV20, Invaria, and Geode. G.J.F. has equity in NextPoint, Triursus, Xios, iTeos, IgM, GV20, Invaria, and Geode. M.H. is a co-founder of Neomer Diagnostics where he is also on the advisory board. K.W. received a sponsored-research agreement from Gilead Sciences. K.W. served as a consultant for Gilead Sciences and Horizon Therapeutics. D.A.B. reports nonfinancial support from Bristol Myers Squibb, honoraria from LM Education/Exchange Services, advisory board fees from Exelixis and AVEO, personal fees from Charles River Associates, Schlesinger Associates, Imprint Science, Insight Strategy, Trinity Group, Cancer Expert Now, Adnovate Strategies, MDedge, CancerNetwork, Catenion, OncLive, Cello Health BioConsulting, PWW Consulting, Haymarket Medical Network, Aptitude Health, ASCO Post/Harborside, Targeted Oncology, and AbbVie, and research support from Exelixis and AstraZeneca, outside of the submitted work. C.J.W. holds equity in BioNTech, Inc., and receives research funding from Pharmacyclics. S.S. reports receiving commercial research grants from Bristol-Myers Squibb, AstraZeneca, Exelixis, and Novartis; is a consultant/advisory board member for Merck, AstraZeneca, Bristol-Myers Squibb, CRISPR Therapeutics AG, AACR, and NCI; receives royalties from Biogenex; and mentored several non-US citizens on research projects with potential funding (in part) from non-US sources/Foreign Components. T.K.C. reports institutional and personal, paid and unpaid support for research, advisory boards, consultancy, and honoraria from AstraZeneca, Aravive, Aveo, Bayer, Bristol Myers-Squibb, Calithera, Circle Pharma, Eisai, EMD Serono, Exelixis, GlaxoSmithKline, IQVA, Infinity, Ipsen, Jansen, Kanaph, Lilly, Merck, Nikang, Nuscan, Novartis, Pfizer, Roche, Sanofi/Aventis, Surface Oncology, Takeda, Tempest, Up-To-Date, and CME events (Peerview, OncLive, MJH, and others), outside the submitted work. T.K.C. has institutional patents filed on molecular alterations and immunotherapy response/toxicity, and ctDNA. T.K.C. has equity in Tempest, Pionyr, Osel, and Precede Bio. T.K.C. has served on committees for NCCN, GU Steering Committee, ASCO/ESMO, ACCRU, and KidneyCan. T.K.C. has medical writing and editorial assistance support may have been funded by Communications companies in part. T.K.C. has mentored several non-US citizens on research projects with potential funding (in part) from non-US sources/Foreign Components. The institution (Dana-Farber Cancer Institute) may have received additional independent funding of drug companies or/and royalties potentially involved in research around the subject matter.
Published: January 15, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.108879.
Supplemental information
References
- 1.Maude S.L., Laetsch T.W., Buechner J., Rives S., Boyer M., Bittencourt H., Bader P., Verneris M.R., Stefanski H.E., Myers G.D., et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuster S.J., Bishop M.R., Tam C.S., Waller E.K., Borchmann P., McGuirk J.P., Jäger U., Jaglowski S., Andreadis C., Westin J.R., et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019;380:45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 3.Wang M., Munoz J., Goy A., Locke F.L., Jacobson C.A., Hill B.T., Timmerman J.M., Holmes H., Jaglowski S., Flinn I.W., et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N. Engl. J. Med. 2020;382:1331–1342. doi: 10.1056/NEJMoa1914347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abramson J.S., Palomba M.L., Gordon L.I., Lunning M.A., Wang M., Arnason J., Mehta A., Purev E., Maloney D.G., Andreadis C., et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839–852. doi: 10.1016/S0140-6736(20)31366-0. [DOI] [PubMed] [Google Scholar]
- 5.Neelapu S.S., Locke F.L., Bartlett N.L., Lekakis L.J., Miklos D.B., Jacobson C.A., Braunschweig I., Oluwole O.O., Siddiqi T., Lin Y., et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Locke F.L., Ghobadi A., Jacobson C.A., Miklos D.B., Lekakis L.J., Oluwole O.O., Lin Y., Braunschweig I., Hill B.T., Timmerman J.M., et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20:31–42. doi: 10.1016/S1470-2045(18)30864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.FDA Approves First Cell-Based Gene Therapy for Adult Patients with Multiple Myeloma. https://www.fda.gov/news-events/press-announcements/fda-approves-first-cell-based-gene-therapy-adult-patients-multiple-myeloma
- 8.Berdeja J.G., Madduri D., Usmani S.Z., Jakubowiak A., Agha M., Cohen A.D., Stewart A.K., Hari P., Htut M., Lesokhin A., et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398:314–324. doi: 10.1016/S0140-6736(21)00933-8. [DOI] [PubMed] [Google Scholar]
- 9.Tahmasebi S., Elahi R., Esmaeilzadeh A. Solid Tumors Challenges and New Insights of CAR T Cell Engineering. Stem Cell Rev. Rep. 2019;15:619–636. doi: 10.1007/s12015-019-09901-7. [DOI] [PubMed] [Google Scholar]
- 10.Leko V., Rosenberg S.A. Identifying and Targeting Human Tumor Antigens for T Cell-Based Immunotherapy of Solid Tumors. Cancer Cell. 2020;38:454–472. doi: 10.1016/j.ccell.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rafiq S., Hackett C.S., Brentjens R.J. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 2020;17:147–167. doi: 10.1038/s41571-019-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y., Suarez E.R., Kastrunes G., de Campos N.S.P., Abbas R., Pivetta R.S., Murugan N., Chalbatani G.M., D'Andrea V., Marasco W.A. Evolution of cell therapy for renal cell carcinoma. Mol. Cancer. 2024;23:8. doi: 10.1186/s12943-023-01911-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kakarla S., Gottschalk S. CAR T cells for solid tumors: armed and ready to go? Cancer J. 2014;20:151–155. doi: 10.1097/PPO.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fridman W.H., Zitvogel L., Sautès-Fridman C., Kroemer G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017;14:717–734. doi: 10.1038/nrclinonc.2017.101. [DOI] [PubMed] [Google Scholar]
- 15.Binnewies M., Roberts E.W., Kersten K., Chan V., Fearon D.F., Merad M., Coussens L.M., Gabrilovich D.I., Ostrand-Rosenberg S., Hedrick C.C., et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chevrier S., Levine J.H., Zanotelli V.R.T., Silina K., Schulz D., Bacac M., Ries C.H., Ailles L., Jewett M.A.S., Moch H., et al. An Immune Atlas of Clear Cell Renal Cell Carcinoma. Cell. 2017;169:736–749.e18. doi: 10.1016/j.cell.2017.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braun D.A., Hou Y., Bakouny Z., Ficial M., Sant’ Angelo M., Forman J., Ross-Macdonald P., Berger A.C., Jegede O.A., Elagina L., et al. Interplay of somatic alterations and immune infiltration modulates response to PD-1 blockade in advanced clear cell renal cell carcinoma. Nat. Med. 2020;26:909–918. doi: 10.1038/s41591-020-0839-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bi K., He M.X., Bakouny Z., Kanodia A., Napolitano S., Wu J., Grimaldi G., Braun D.A., Cuoco M.S., Mayorga A., et al. Tumor and immune reprogramming during immunotherapy in advanced renal cell carcinoma. Cancer Cell. 2021;39:649–661.e5. doi: 10.1016/j.ccell.2021.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braun D.A., Street K., Burke K.P., Cookmeyer D.L., Denize T., Pedersen C.B., Gohil S.H., Schindler N., Pomerance L., Hirsch L., et al. Progressive immune dysfunction with advancing disease stage in renal cell carcinoma. Cancer Cell. 2021;39:632–648.e8. doi: 10.1016/j.ccell.2021.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng C., Zheng L., Yoo J.K., Guo H., Zhang Y., Guo X., Kang B., Hu R., Huang J.Y., Zhang Q., et al. Landscape of Infiltrating T Cells in Liver Cancer Revealed by Single-Cell Sequencing. Cell. 2017;169:1342–1356.e16. doi: 10.1016/j.cell.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y., Chen H., Mo H., Hu X., Gao R., Zhao Y., Liu B., Niu L., Sun X., Yu X., et al. Single-cell analyses reveal key immune cell subsets associated with response to PD-L1 blockade in triple-negative breast cancer. Cancer Cell. 2021;39:1578–1593.e8. doi: 10.1016/j.ccell.2021.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L., Li Z., Skrzypczynska K.M., Fang Q., Zhang W., O'Brien S.A., He Y., Wang L., Zhang Q., Kim A., et al. Single-Cell Analyses Inform Mechanisms of Myeloid-Targeted Therapies in Colon Cancer. Cell. 2020;181:442–459.e29. doi: 10.1016/j.cell.2020.03.048. [DOI] [PubMed] [Google Scholar]
- 23.Cho H., Du X., Rizzi J.P., Liberzon E., Chakraborty A.A., Gao W., Carvo I., Signoretti S., Bruick R.K., Josey J.A., et al. On-target efficacy of a HIF-2α antagonist in preclinical kidney cancer models. Nature. 2016;539:107–111. doi: 10.1038/nature19795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu Y.F., Cohn S., Christie A., McKenzie T., Wolff N., Do Q.N., Madhuranthakam A.J., Pedrosa I., Wang T., Dey A., et al. Modeling Renal Cell Carcinoma in Mice: Bap1 and Pbrm1 Inactivation Drive Tumor Grade. Cancer Discov. 2017;7:900–917. doi: 10.1158/2159-8290.CD-17-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez L., Strbo N., Podack E.R. Humanized mice: novel model for studying mechanisms of human immune-based therapies. Immunol. Res. 2013;57:326–334. doi: 10.1007/s12026-013-8471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chuprin J., Buettner H., Seedhom M.O., Greiner D.L., Keck J.G., Ishikawa F., Shultz L.D., Brehm M.A. Humanized mouse models for immuno-oncology research. Nat. Rev. Clin. Oncol. 2023;20:192–206. doi: 10.1038/s41571-022-00721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y., Shelton S.E., Kastrunes G., Barbie D.A., Freeman G.J., Marasco W.A. Preclinical models for development of immune–oncology therapies. Immuno-oncol. Insights. 2022;3:379–398. doi: 10.18609/ioi.2022.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang M., Yao L.C., Cheng M., Cai D., Martinek J., Pan C.X., Shi W., Ma A.H., De Vere White R.W., Airhart S., et al. Humanized mice in studying efficacy and mechanisms of PD-1-targeted cancer immunotherapy. Faseb j. 2018;32:1537–1549. doi: 10.1096/fj.201700740R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Y., Shuen T.W.H., Toh T.B., Chan X.Y., Liu M., Tan S.Y., Fan Y., Yang H., Lyer S.G., Bonney G.K., et al. Development of a new patient-derived xenograft humanised mouse model to study human-specific tumour microenvironment and immunotherapy. Gut. 2018;67:1845–1854. doi: 10.1136/gutjnl-2017-315201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suarez E.R., Chang D.K., Sun J., Sui J., Freeman G.J., Signoretti S., Zhu Q., Marasco W.A. Chimeric antigen receptor T cells secreting anti-PD-L1 antibodies more effectively regress renal cell carcinoma in a humanized mouse model. Oncotarget. 2016;7:34341–34355. doi: 10.18632/oncotarget.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y., Buck A., Grimaud M., Culhane A.C., Kodangattil S., Razimbaud C., Bonal D.M., Nguyen Q.D., Zhu Z., Wei K., et al. Anti-CAIX BBζ CAR4/8 T cells exhibit superior efficacy in a ccRCC mouse model. Mol. Ther. Oncolytics. 2022;24:385–399. doi: 10.1016/j.omto.2021.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen C., Kaelin W.G., Jr. The VHL/HIF axis in clear cell renal carcinoma. Semin. Cancer Biol. 2013;23:18–25. doi: 10.1016/j.semcancer.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruf M., Mittmann C., Nowicka A.M., Hartmann A., Hermanns T., Poyet C., van den Broek M., Sulser T., Moch H., Schraml P. pVHL/HIF-regulated CD70 expression is associated with infiltration of CD27+ lymphocytes and increased serum levels of soluble CD27 in clear cell renal cell carcinoma. Clin. Cancer Res. 2015;21:889–898. doi: 10.1158/1078-0432.CCR-14-1425. [DOI] [PubMed] [Google Scholar]
- 34.Xu C., Lo A., Yammanuru A., Tallarico A.S.C., Brady K., Murakami A., Barteneva N., Zhu Q., Marasco W.A. Unique biological properties of catalytic domain directed human anti-CAIX antibodies discovered through phage-display technology. PLoS One. 2010;5 doi: 10.1371/journal.pone.0009625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang D.K., Moniz R.J., Xu Z., Sun J., Signoretti S., Zhu Q., Marasco W.A. Human anti-CAIX antibodies mediate immune cell inhibition of renal cell carcinoma in vitro and in a humanized mouse model in vivo. Mol. Cancer. 2015;14:119. doi: 10.1186/s12943-015-0384-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lo A.S.Y., Xu C., Murakami A., Marasco W.A. Regression of established renal cell carcinoma in nude mice using lentivirus-transduced human T cells expressing a human anti-CAIX chimeric antigen receptor. Mol. Ther. Oncolytics. 2014;1 doi: 10.1038/mto.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silva J.P., Vetterlein O., Jose J., Peters S., Kirby H. The S228P mutation prevents in vivo and in vitro IgG4 Fab-arm exchange as demonstrated using a combination of novel quantitative immunoassays and physiological matrix preparation. J. Biol. Chem. 2015;290:5462–5469. doi: 10.1074/jbc.M114.600973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y., Chan L.L., Grimaud M., Fayed A., Zhu Q., Marasco W.A. High-Throughput Image Cytometry Detection Method for CAR-T Transduction, Cell Proliferation, and Cytotoxicity Assays. Cytometry. 2020;99:689–697. doi: 10.1002/cyto.a.24267. [DOI] [PubMed] [Google Scholar]
- 39.Dhupkar P., Gordon N., Stewart J., Kleinerman E.S. Anti-PD-1 therapy redirects macrophages from an M2 to an M1 phenotype inducing regression of OS lung metastases. Cancer Med. 2018;7:2654–2664. doi: 10.1002/cam4.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamaguchi Y., Gibson J., Ou K., Lopez L.S., Ng R.H., Leggett N., Jonsson V.D., Zarif J.C., Lee P.P., Wang X., et al. PD-L1 blockade restores CAR T cell activity through IFN-gamma-regulation of CD163+ M2 macrophages. J. Immunother. Cancer. 2022;10 doi: 10.1136/jitc-2021-004400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang F., Zhao J., Luo X., Li T., Wang Z., Wei Q., Lu H., Meng Y., Cai K., Lu L., et al. Transcriptome Profiling Reveals B-Lineage Cells Contribute to the Poor Prognosis and Metastasis of Clear Cell Renal Cell Carcinoma. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.731896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li S., Huang C., Hu G., Ma J., Chen Y., Zhang J., Huang Y., Zheng J., Xue W., Xu Y., Zhai W. Tumor-educated B cells promote renal cancer metastasis via inducing the IL-1β/HIF-2α/Notch1 signals. Cell Death Dis. 2020;11:163. doi: 10.1038/s41419-020-2355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meylan M., Petitprez F., Becht E., Bougoüin A., Pupier G., Calvez A., Giglioli I., Verkarre V., Lacroix G., Verneau J., et al. Tertiary lymphoid structures generate and propagate anti-tumor antibody-producing plasma cells in renal cell cancer. Immunity. 2022;55:527–541.e5. doi: 10.1016/j.immuni.2022.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Italiano A., Bessede A., Pulido M., Bompas E., Piperno-Neumann S., Chevreau C., Penel N., Bertucci F., Toulmonde M., Bellera C., et al. Pembrolizumab in soft-tissue sarcomas with tertiary lymphoid structures: a phase 2 PEMBROSARC trial cohort. Nat. Med. 2022;28:1199–1206. doi: 10.1038/s41591-022-01821-3. [DOI] [PubMed] [Google Scholar]
- 45.Cho J.W., Park S., Kim G., Han H., Shim H.S., Shin S., Bae Y.S., Park S.Y., Ha S.J., Lee I., Kim H.R. Dysregulation of TFH-B-TRM lymphocyte cooperation is associated with unfavorable anti-PD-1 responses in EGFR-mutant lung cancer. Nat. Commun. 2021;12:6068. doi: 10.1038/s41467-021-26362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crotty S. Follicular helper CD4 T cells (TFH) Annu. Rev. Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 47.Gu-Trantien C., Migliori E., Buisseret L., de Wind A., Brohée S., Garaud S., Noël G., Dang Chi V.L., Lodewyckx J.N., Naveaux C., et al. CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI Insight. 2017;2 doi: 10.1172/jci.insight.91487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flemming A. GZMK(+) T cells a hallmark of immune ageing. Nat. Rev. Immunol. 2021;21:1. doi: 10.1038/s41577-020-00486-8. [DOI] [PubMed] [Google Scholar]
- 49.Mogilenko D.A., Shpynov O., Andhey P.S., Arthur L., Swain A., Esaulova E., Brioschi S., Shchukina I., Kerndl M., Bambouskova M., et al. Comprehensive Profiling of an Aging Immune System Reveals Clonal GZMK(+) CD8(+) T Cells as Conserved Hallmark of Inflammaging. Immunity. 2021;54:99–115.e12. doi: 10.1016/j.immuni.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 50.La Manno G., Soldatov R., Zeisel A., Braun E., Hochgerner H., Petukhov V., Lidschreiber K., Kastriti M.E., Lönnerberg P., Furlan A., et al. RNA velocity of single cells. Nature. 2018;560:494–498. doi: 10.1038/s41586-018-0414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mishra S., Srinivasan S., Ma C., Zhang N. CD8(+) Regulatory T Cell - A Mystery to Be Revealed. Front. Immunol. 2021;12:708874. doi: 10.3389/fimmu.2021.708874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Churlaud G., Pitoiset F., Jebbawi F., Lorenzon R., Bellier B., Rosenzwajg M., Klatzmann D. Human and Mouse CD8(+)CD25(+)FOXP3(+) Regulatory T Cells at Steady State and during Interleukin-2 Therapy. Front. Immunol. 2015;6:171. doi: 10.3389/fimmu.2015.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhulai G., Oleinik E. Targeting regulatory T cells in anti-PD-1/PD-L1 cancer immunotherapy. Scand. J. Immunol. 2022;95 doi: 10.1111/sji.13129. [DOI] [PubMed] [Google Scholar]
- 54.Plitas G., Konopacki C., Wu K., Bos P.D., Morrow M., Putintseva E.V., Chudakov D.M., Rudensky A.Y. Regulatory T Cells Exhibit Distinct Features in Human Breast Cancer. Immunity. 2016;45:1122–1134. doi: 10.1016/j.immuni.2016.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tong A.A., Forestell B., Murphy D.V., Nair A., Allen F., Myers J., Klauschen F., Shen C., Gopal A.A., Huang A.Y., Mandl J.N. Regulatory T cells differ from conventional CD4(+) T cells in their recirculatory behavior and lymph node transit times. Immunol. Cell Biol. 2019;97:787–798. doi: 10.1111/imcb.12276. [DOI] [PubMed] [Google Scholar]
- 56.Francisco L.M., Salinas V.H., Brown K.E., Vanguri V.K., Freeman G.J., Kuchroo V.K., Sharpe A.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Valpione S., Mundra P.A., Galvani E., Campana L.G., Lorigan P., De Rosa F., Gupta A., Weightman J., Mills S., Dhomen N., Marais R. The T cell receptor repertoire of tumor infiltrating T cells is predictive and prognostic for cancer survival. Nat. Commun. 2021;12:4098. doi: 10.1038/s41467-021-24343-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Valpione S., Galvani E., Tweedy J., Mundra P.A., Banyard A., Middlehurst P., Barry J., Mills S., Salih Z., Weightman J., et al. Immune awakening revealed by peripheral T cell dynamics after one cycle of immunotherapy. Nat. Cancer. 2020;1:210–221. doi: 10.1038/s43018-019-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kato T., Kiyotani K., Tomiyama E., Koh Y., Matsushita M., Hayashi Y., Nakano K., Ishizuya Y., Wang C., Hatano K., et al. Peripheral T cell receptor repertoire features predict durable responses to anti-PD-1 inhibitor monotherapy in advanced renal cell carcinoma. OncoImmunology. 2021;10 doi: 10.1080/2162402X.2020.1862948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simoni Y., Becht E., Fehlings M., Loh C.Y., Koo S.-L., Teng K.W.W., Yeong J.P.S., Nahar R., Zhang T., Kared H., et al. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature. 2018;557:575–579. doi: 10.1038/s41586-018-0130-2. [DOI] [PubMed] [Google Scholar]
- 61.Jin H.T., Anderson A.C., Tan W.G., West E.E., Ha S.J., Araki K., Freeman G.J., Kuchroo V.K., Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. USA. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sen D.R., Kaminski J., Barnitz R.A., Kurachi M., Gerdemann U., Yates K.B., Tsao H.W., Godec J., LaFleur M.W., Brown F.D., et al. The epigenetic landscape of T cell exhaustion. Science. 2016;354:1165–1169. doi: 10.1126/science.aae0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duan Z., Luo Y. Targeting macrophages in cancer immunotherapy. Signal Transduct. Target. Ther. 2021;6:127. doi: 10.1038/s41392-021-00506-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jin S., Guerrero-Juarez C.F., Zhang L., Chang I., Ramos R., Kuan C.H., Myung P., Plikus M.V., Nie Q. Inference and analysis of cell-cell communication using CellChat. Nat. Commun. 2021;12:1088. doi: 10.1038/s41467-021-21246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roger T., David J., Glauser M.P., Calandra T. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature. 2001;414:920–924. doi: 10.1038/414920a. [DOI] [PubMed] [Google Scholar]
- 66.Stanley E.R., Chitu V. CSF-1 receptor signaling in myeloid cells. Cold Spring Harb. Perspect. Biol. 2014;6 doi: 10.1101/cshperspect.a021857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aparicio S., Hidalgo M., Kung A.L. Examining the utility of patient-derived xenograft mouse models. Nat. Rev. Cancer. 2015;15:311–316. doi: 10.1038/nrc3944. [DOI] [PubMed] [Google Scholar]
- 68.Tian H., Lyu Y., Yang Y.G., Hu Z. Humanized Rodent Models for Cancer Research. Front. Oncol. 2020;10:1696. doi: 10.3389/fonc.2020.01696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brehm M.A., Cuthbert A., Yang C., Miller D.M., DiIorio P., Laning J., Burzenski L., Gott B., Foreman O., Kavirayani A., et al. Parameters for establishing humanized mouse models to study human immunity: analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rgamma(null) mutation. Clin. Immunol. 2010;135:84–98. doi: 10.1016/j.clim.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shultz L.D., Brehm M.A., Garcia-Martinez J.V., Greiner D.L. Humanized mice for immune system investigation: progress, promise and challenges. Nat. Rev. Immunol. 2012;12:786–798. doi: 10.1038/nri3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morton J.J., Bird G., Refaeli Y., Jimeno A. Humanized Mouse Xenograft Models: Narrowing the Tumor-Microenvironment Gap. Cancer Res. 2016;76:6153–6158. doi: 10.1158/0008-5472.CAN-16-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nicolini F.E., Cashman J.D., Hogge D.E., Humphries R.K., Eaves C.J. NOD/SCID mice engineered to express human IL-3, GM-CSF and Steel factor constitutively mobilize engrafted human progenitors and compromise human stem cell regeneration. Leukemia. 2004;18:341–347. doi: 10.1038/sj.leu.2403222. [DOI] [PubMed] [Google Scholar]
- 73.Wunderlich M., Chou F.S., Link K.A., Mizukawa B., Perry R.L., Carroll M., Mulloy J.C. AML xenograft efficiency is significantly improved in NOD/SCID-IL2RG mice constitutively expressing human SCF, GM-CSF and IL-3. Leukemia. 2010;24:1785–1788. doi: 10.1038/leu.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jangalwe S., Shultz L.D., Mathew A., Brehm M.A. Improved B cell development in humanized NOD-scid IL2Rγnull mice transgenically expressing human stem cell factor, granulocyte-macrophage colony-stimulating factor and interleukin-3. Immun. Inflamm. Dis. 2016;4:427–440. doi: 10.1002/iid3.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ishikawa F., Yasukawa M., Lyons B., Yoshida S., Miyamoto T., Yoshimoto G., Watanabe T., Akashi K., Shultz L.D., Harada M. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood. 2005;106:1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lepus C.M., Gibson T.F., Gerber S.A., Kawikova I., Szczepanik M., Hossain J., Ablamunits V., Kirkiles-Smith N., Herold K.C., Donis R.O., et al. Comparison of human fetal liver, umbilical cord blood, and adult blood hematopoietic stem cell engraftment in NOD-scid/gammac-/-Balb/c-Rag1-/-gammac-/-, and C.B-17-scid/bg immunodeficient mice. Hum. Immunol. 2009;70:790–802. doi: 10.1016/j.humimm.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matsumura T., Kametani Y., Ando K., Hirano Y., Katano I., Ito R., Shiina M., Tsukamoto H., Saito Y., Tokuda Y., et al. Functional CD5+ B cells develop predominantly in the spleen of NOD/SCID/gammac(null) (NOG) mice transplanted either with human umbilical cord blood, bone marrow, or mobilized peripheral blood CD34+ cells. Exp. Hematol. 2003;31:789–797. doi: 10.1016/s0301-472x(03)00193-0. [DOI] [PubMed] [Google Scholar]
- 78.Katano I., Ito R., Kamisako T., Eto T., Ogura T., Kawai K., Suemizu H., Takahashi T., Kawakami Y., Ito M. NOD-Rag2null IL-2Rγnull mice: an alternative to NOG mice for generation of humanized mice. Exp. Anim. 2014;63:321–330. doi: 10.1538/expanim.63.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Traggiai E., Chicha L., Mazzucchelli L., Bronz L., Piffaretti J.C., Lanzavecchia A., Manz M.G. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–107. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 80.Comprehensive Evaluation of Human Immune System Reconstitution in NSG and NSG-SGM3 Mouse Models. https://resources.jax.org/white-papers/whitepaper-onco-hu-2
- 81.Lepone L.M., Donahue R.N., Grenga I., Metenou S., Richards J., Heery C.R., Madan R.A., Gulley J.L., Schlom J. Analyses of 123 Peripheral Human Immune Cell Subsets: Defining Differences with Age and between Healthy Donors and Cancer Patients Not Detected in Analysis of Standard Immune Cell Types. J. Circ. Biomark. 2016;5:5. doi: 10.5772/62322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brodaczewska K.K., Szczylik C., Fiedorowicz M., Porta C., Czarnecka A.M. Choosing the right cell line for renal cell cancer research. Mol. Cancer. 2016;15:83. doi: 10.1186/s12943-016-0565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang Z., Liu J., Zhang C., Li F., Li L., Wang D., Chand D., Guan F., Zang X., Zhang Y. Over-Expression and Prognostic Significance of HHLA2, a New Immune Checkpoint Molecule, in Human Clear Cell Renal Cell Carcinoma. Front. Cell Dev. Biol. 2020;8:280. doi: 10.3389/fcell.2020.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bhatt R.S., Berjis A., Konge J.C., Mahoney K.M., Klee A.N., Freeman S.S., Chen C.H., Jegede O.A., Catalano P.J., Pignon J.C., et al. KIR3DL3 Is an Inhibitory Receptor for HHLA2 that Mediates an Alternative Immunoinhibitory Pathway to PD1. Cancer Immunol. Res. 2021;9:156–169. doi: 10.1158/2326-6066.CIR-20-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sobczuk P., Brodziak A., Khan M.I., Chhabra S., Fiedorowicz M., Wełniak-Kamińska M., Synoradzki K., Bartnik E., Cudnoch-Jędrzejewska A., Czarnecka A.M. Choosing The Right Animal Model for Renal Cancer Research. Transl. Oncol. 2020;13 doi: 10.1016/j.tranon.2020.100745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shultz L.D., Saito Y., Najima Y., Tanaka S., Ochi T., Tomizawa M., Doi T., Sone A., Suzuki N., Fujiwara H., et al. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2r gamma(null) humanized mice. Proc. Natl. Acad. Sci. USA. 2010;107:13022–13027. doi: 10.1073/pnas.1000475107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stadtmauer E.A., Fraietta J.A., Davis M.M., Cohen A.D., Weber K.L., Lancaster E., Mangan P.A., Kulikovskaya I., Gupta M., Chen F., et al. CRISPR-engineered T cells in patients with refractory cancer. Science. 2020;367 doi: 10.1126/science.aba7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rupp L.J., Schumann K., Roybal K.T., Gate R.E., Ye C.J., Lim W.A., Marson A. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci. Rep. 2017;7:737. doi: 10.1038/s41598-017-00462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Strauss L., Mahmoud M.A.A., Weaver J.D., Tijaro-Ovalle N.M., Christofides A., Wang Q., Pal R., Yuan M., Asara J., Patsoukis N., Boussiotis V.A. Targeted deletion of PD-1 in myeloid cells induces antitumor immunity. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.aay1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Odorizzi P.M., Pauken K.E., Paley M.A., Sharpe A., Wherry E.J. Genetic absence of PD-1 promotes accumulation of terminally differentiated exhausted CD8+ T cells. J. Exp. Med. 2015;212:1125–1137. doi: 10.1084/jem.20142237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Riley R.S., June C.H., Langer R., Mitchell M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019;18:175–196. doi: 10.1038/s41573-018-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rafiq S., Yeku O.O., Jackson H.J., Purdon T.J., van Leeuwen D.G., Drakes D.J., Song M., Miele M.M., Li Z., Wang P., et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat. Biotechnol. 2018;36:847–856. doi: 10.1038/nbt.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Monaco G., Lee B., Xu W., Mustafah S., Hwang Y.Y., Carré C., Burdin N., Visan L., Ceccarelli M., Poidinger M., et al. RNA-Seq Signatures Normalized by mRNA Abundance Allow Absolute Deconvolution of Human Immune Cell Types. Cell Rep. 2019;26:1627–1640.e7. doi: 10.1016/j.celrep.2019.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grieshaber-Bouyer R., Radtke F.A., Cunin P., Stifano G., Levescot A., Vijaykumar B., Nelson-Maney N., Blaustein R.B., Monach P.A., Nigrovic P.A., ImmGen Consortium The neutrotime transcriptional signature defines a single continuum of neutrophils across biological compartments. Nat. Commun. 2021;12:2856. doi: 10.1038/s41467-021-22973-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lan P., Wang L., Diouf B., Eguchi H., Su H., Bronson R., Sachs D.H., Sykes M., Yang Y.G. Induction of human T-cell tolerance to porcine xenoantigens through mixed hematopoietic chimerism. Blood. 2004;103:3964–3969. doi: 10.1182/blood-2003-10-3697. [DOI] [PubMed] [Google Scholar]
- 96.Melkus M.W., Estes J.D., Padgett-Thomas A., Gatlin J., Denton P.W., Othieno F.A., Wege A.K., Haase A.T., Garcia J.V. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat. Med. 2006;12:1316–1322. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
- 97.Magiera-Mularz K., Kocik J., Musielak B., Plewka J., Sala D., Machula M., Grudnik P., Hajduk M., Czepiel M., Siedlar M., et al. Human and mouse PD-L1: similar molecular structure, but different druggability profiles. iScience. 2021;24 doi: 10.1016/j.isci.2020.101960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Costa M.J., Kudaravalli J., Liu W.H., Stock J., Kong S., Liu S.H. A mouse model for evaluation of efficacy and concomitant toxicity of anti-human CXCR4 therapeutics. PLoS One. 2018;13 doi: 10.1371/journal.pone.0194688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hao Y., Hao S., Andersen-Nissen E., Mauck W.M., 3rd, Zheng S., Butler A., Lee M.J., Wilk A.J., Darby C., Zager M., et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184:3573–3587.e29. doi: 10.1016/j.cell.2021.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Korsunsky I., Millard N., Fan J., Slowikowski K., Zhang F., Wei K., Baglaenko Y., Brenner M., Loh P.R., Raychaudhuri S. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods. 2019;16:1289–1296. doi: 10.1038/s41592-019-0619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Testi M., Andreani M. Luminex-Based Methods in High-Resolution HLA Typing. Methods Mol. Biol. 2015;1310:231–245. doi: 10.1007/978-1-4939-2690-9_19. [DOI] [PubMed] [Google Scholar]
- 102.Stoeckius M., Zheng S., Houck-Loomis B., Hao S., Yeung B.Z., Mauck W.M., Smibert P., Satija R. Cell Hashing with barcoded antibodies enables multiplexing and doublet detection for single cell genomics. Genome Biol. 2018;19:224. doi: 10.1186/s13059-018-1603-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Germain P.L., Lun A., Garcia Meixide C., Macnair W., Robinson M.D. Doublet identification in single-cell sequencing data using scDblFinder. F1000Res. 2021;10:1–23. doi: 10.12688/f1000research.73600.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All generated scRNAseq data are available at the Gene Expression Omnibus (GEO) with accession code GSE210664. Supplementary Tables from 2 to 10 can be found in https://doi.org/10.5281/zenodo.10456704. Original codes can be accessed via https://doi.org/10.5281/zenodo.7274663. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.