Abstract
Background
Recent studies have linked recurrent pregnancy loss (RPL) to abnormalities in the sperm genome, specifically microdeletions in the azoospermia factor (AZF) region. This study investigated the potential association between Y chromosome microdeletions in the AZF region and RPL in Iranian couples.
Methods
The research presents a case–control study of 240 men: 120 whose partners experienced recurrent miscarriage, and 120 who had successful pregnancies without history of miscarriage. The study used semen parameters, hormone analyses, and microdeletion analysis via multiplex PCR and the YChromStrip kit. Thus, the sequence‐tagged site (STS) markers of AZFa (sY84, sY86), AZFb (sY127, sY134), and AZFc (sY254, sY255) regions were examined.
Results
The variations in semen parameters and sex hormone levels between cases and controls are suggest impaired testicular function in men whose partners had recurrent miscarriages (p < 0.05). Furthermore, the study revealed a negative correlation between sperm count and follicle‐stimulating hormone (FSH) level, and a positive one between sperm motility and testosterone concentration. There were no microdeletions in the control group, while the RPL group showed 20 deletions in AZFb (sY134) (16.66%) and 10 deletions each in AZFb (sY127) (8.33%) and AZFc (sY254) (8.33%).
Conclusion
Microdeletions in sY134 (AZFb) were significantly associated with RPL in Iranian men (p = 0.03). AZF microdeletion screening in couples with RPL can provide valuable information for ethnical genetic counseling and management of recurrent miscarriage. Further studies on larger populations or across various ethnic groups, conclusions and the inclusion of other factors like epigenetic changes explain the role of AZF microdeletions in RPL.
Keywords: azoospermia, microdeletions, multiplex PCR, recurrent miscarriage
Male partners of women with RPL showed poorer semen quality and altered hormonal profile. Multiplex‐PCR revealed microdeletions in AZFb (sY134), significantly more prevalent in RPL‐men (16.7%) versus controls (p < 0.001). Y chromosome microdeletions provide valuable biomarkers, complementing semen and hormone analyses for identifying male factor contributions in couples with idiopathic RPL.
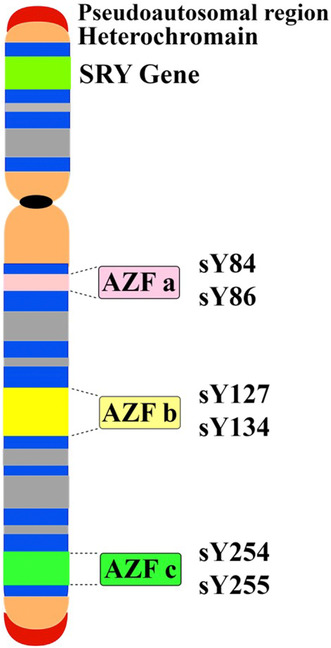
1. INTRODUCTION
Recurrent pregnancy loss (RPL) is a multifactorial problem in which a woman miscarries two or more pregnancies before the 20th week of gestation (Bigdeli et al., 2018; Dimitriadis et al., 2020). Recurrent miscarriage causes are unknown in 30%–40% of cases (Nemati et al., 2020; Shi et al., 2017). Recent studies have discussed the role of abnormalities in men's sperm genomes, such as chromosomal abnormalities, chromatin fragmentation, and microdeletions in the azoospermia factor (AZF) region, on frequent miscarriages in their wives (Pal et al., 2018; Tan et al., 2019). Infertility has been linked to microdeletions of the Y chromosome in numerous studies (Ambulkar & Pande, 2017; Kaminski et al., 2020; Liu et al., 2016; Pylyp et al., 2015), so it is also possible that repeated abortions are related to these microdeletions. The prevalence of AZF region microdeletions in men can vary from 2% to 10%, depending on the society (Zargar et al., 2020).
The AZF region on Yq contains multiple genes involved in male fertility, and is divided into AZFa, AZFb, and AZFc subregions (Torfeh et al., 2012; Yu et al., 2015). These genes encode proteins involved in sperm production, such as transcription factors, RNA‐binding proteins and chromatin remodeling factors involved in regulating gene expression, RNA metabolism, packaging, cytoplasmic transfers, and RNA splicing (Ahmadi Rastegar et al., 2015; Goncalves et al., 2017; Jungwirth et al., 2012). The molecular mechanism behind AZF microdeletions leading to infertility is probably due to intrachromosomal homologous recombination between blocks of repetitive sequences, within palindromic structures. However, it likely involves aberrant gene expression, failure of germ cell differentiation, increased apoptosis, and sperm maturation arrest. Loss of multiple AZF genes due to microdeletions causes severe disruption of transcriptional and post‐transcriptional regulation during spermatogenesis (Asadi & Kiani, 2020; Zhang et al., 2017).
Understanding causes and optimizing diagnosis, treatment, and support is crucial, with genetic factors like AZF microdeletions being studied (Yu et al., 2023). Therefore, the present study was conducted to investigate the relationship between the presence or absence of microdeletions of AZF regions in men and the occurrence of frequent miscarriages in their wives.
There are key knowledge gaps regarding the association between AZF microdeletions and recurrent pregnancy, with varying rates reported across studies. It is unknown which specific AZF region microdeletions (AZFa, AZFb, AZFc) confer the highest risk for contributing to RPL. The genetic mechanisms causing AZF deletions, which can lead to sperm defects and pregnancy loss, are not fully understood, and their role in relation to other factors and unexplained causes of RPL is unclear. This study aims to address some of these gaps by evaluating AZF microdeletions in men with recurrent miscarriage, analyzing semen parameters and hormone levels. It aims to determine if specific deletions increase risk of RPL.
2. METHODS
2.1. Ethical compliance
The study protocol was reviewed and approved by the Research Ethics Committee of Islamic Azad University‐North Tehran Branch (approval number: IR.IAU.TNB.REC.1401.041). The study involved participants signing consent forms that outlined the study's purpose, procedures, risks, benefits, confidentiality measures, and the right to withdraw. Only those who gave voluntary informed consent were recruited, and personal identifiers were removed to maintain confidentiality. Blood samples, medical data, and genetic results were stored securely with restricted access, and any significant findings were disclosed to the participants through their treating physicians. The study followed the Declaration of Helsinki principles for ethical medical research, ensuring proper safeguards and informed consent to protect participants' rights, safety, and privacy in genetic studies.
2.2. Study participants
This case–control study was conducted after approval by the Research Ethics Committee of Islamic Azad University‐North Tehran Branch from July 2022 to April 2023 at the Mehragin Medical Diagnostic Laboratory in Tehran. The inclusion criteria for the patient group included 120 healthy men (normospermia) with normal semen parameters with partners with a history of two or more consecutive miscarriages before 20 weeks gestation. Miscarriages were confirmed by ultrasound or clinical examination. Moreover, 120 healthy fertile men with normal semen parameters whose partners had at least one successful pregnancy and no history of miscarriage were included in the study as a control group. Whereas the exclusion criteria for the study encompassed men with abnormal semen parameters, thrombophilic disorders, untreated thyroid disease, diabetes, hyperprolactinemia, positive antiphospholipid antibodies, blood karyotyping showing structural chromosomal abnormalities, active infectious diseases, and other causes of female factors such as endometriosis and luteal phase defect.
Unexplained RPL was determined via the exclusion of these alternate factors. The standardized inclusion/exclusion criteria ensure that the patient and control groups match closely, except for recurrent pregnancy loss. Detailed history, prior medical records, physical examination, hormone tests, blood tests, and imaging studies were utilized to rule out other identifiable causes of RPL based on established diagnostic criteria. To ensure the normality of men's spermograms, sperm were examined in terms of quantity, motility, and sperm shape using the HFT CASA software system version 7.00 xp.
2.2.1. Sample size
Sample size was calculated by using Cochran's formula considering 95% CIs, 5% level of significance, precision level (marginal error) of (d = 0.1), (z = 1.96) and according to the studies conducted in Iran considering the prevalence of recurrent miscarriage (P ≈ 10%) in Iranian couples (Moghbeli, 2019). The formula is .
2.2.2. Semen samples
Semen samples were collected from two groups: male partners of women with recurrent pregnancy loss (RPL) (n = 120) and male partners of women without RPL (control group) (n = 120). Samples were obtained by masturbation after 2–7 days of sexual abstinence.
2.2.3. Semen analysis
After liquefaction, semen samples underwent standard semen analysis according to the WHO guidelines. A spermogram machine and the HFT CASA software version 7.00 xp were used to check semen volume, total sperm count, sperm concentration, sperm movement, percentage of sperm viability, and pH. The Color Sperm Quality Testing System, using the latest image‐processing techniques for clinical and research applications, provides a fast and accurate method for sperm analysis. Results were compared between the RPL and control groups.
2.2.4. Blood sample collection
Blood samples were collected from each participant by venipuncture, and serum was separated.
2.2.5. Hormone analysis
Levels of follicle‐stimulating hormone (FSH), luteinizing hormone (LH), prolactin (PRL), testosterone, and estradiol in serum were measured using an electroquantitative luminescence method with Liaison device (DiaSorin kits, Italy) according to the manufacturer's protocols. Briefly, the kits for each hormone test were placed in the device, and after introducing the serum samples of the subjects studied in the special place for reading the tests, the results were recorded. All assays were run in duplicate and mean reported.
2.3. DNA extraction
Five milliliters of peripheral blood was taken in a tube containing ethylene‐diamine‐tetra‐acetic acid (EDTA) from all the men who entered the study, to comply with the ethical principles, after giving them the necessary explanations and obtaining consent. Then, genomic DNA was extracted from whole blood using the DNA extraction kit (Genall, South Korea) according to the protocol. The concentration and purity of DNA were measured using Nanodrop (Thermoscience 3300‐USA), and then, the isolated DNA was stored at −20°C.
2.3.1. Microdeletion analysis
By multiplex PCR
In this study, multiplex polymerase chain reaction (MPCR) was used to investigate microdeletions of the AZF region. At first, according to the standard guidelines, EAA/EMON (European Academy of Andrology and the European Molecular Genetics Quality Network) (Krausz et al., 2014), primer sequences for six sequence‐tagged sites (STS) markers in three subregions AZFa, AZFb, and AZFc as well as for SRY and ZFX/Y were selected for internal controls and then was ordered to Pishgam company (Iran) for synthesis. The sequences of the primers are given in Table 1. During the evaluation of microdeletion, subregions for AZFa (sY84, sY86), AZFb (sY127, sY134), and AZFc (sY254, sY255) were included in two MPCR A and MPCR B reactions separately. So, six STS loci spanning the long arm of the Y chromosome (Yq) were analyzed.
TABLE 1.
STS markers, primer sequence, and product size for Y chromosome microdeletions.
| AZF region | STS | Primer sequence (5′–3′) | Product size (bp) |
|---|---|---|---|
| AZFa | sY86 |
F:5′‐GTGACACACAGACTATGCTTC‐3′ R:5′‐ACACACAGAGGGACAACCCT‐3′ |
318 |
| AZFa | sY84 |
F:5′‐AGAAGGGTCTGAAAGCAGGT‐3′′ R:5′‐GCCTACTACCTGGAGGCTTC‐3′ |
326 |
| AZFb | sY127 |
F:5′‐GGCTCACAAACGAAAAGAAA‐3′′ R:5′‐CTGCAGGCAGTAATAAGGGA‐3′ |
274 |
| AZFb | sY134 |
F:5′‐GTCTGCCTCACCATAAAACG‐3′′ R:5′‐ACCACTGCCAAAACTTTCAA‐3′ |
310 |
| AZFc | sY254 |
F:5′‐GGGTGTTACCAGAAGGCAAA‐3′ R:5′‐GAACCGTATCTACCAAAGCAGC‐3′ |
380 |
| AZFc | sY255 |
F:5′‐GTTACAGGATTCGGCGTGAT‐3′′ R:5′‐CTCGTCATGAGCAGCCAC‐3′ |
123 |
| Yp | SRY |
F:5′‐GAATATTCCCGCTCTCCGGA‐3′ R:5′‐GCTGGTGCTCCATTCTTGAG‐3′ |
470 |
| AZF region | ZFY |
F:5′‐ACCRTCGTACTGACTGTGATTACAC‐3′ R:5′‐GCACYTCTTTGGTATCYGAGAAAGT‐3′ |
495 |
The total volume of reaction for multiplex PCR was considered 50 μL containing 150 ng of genomic DNA. Each reaction included 25 μL of master mix (1.5 mmol/MgCl2, Genet Bio, South Korea), a concentration of 10 pmol of each primer, 15 μL of sterile distilled water, and 5 μL of DNA. Also, in all reactions, the water sample without the DNA sample was used as the negative control and healthy fertile male DNA was used as positive control.
Polymerase chain reaction was performed on a thermal cycler (Techno, Barloworld Scientific, UK). This reaction consists of 35 cycles including initial denaturation temperature of 95°C for 5 min, denaturation at 95°C for 30 s, annealing at 57°C for 1 min, and extension at 72°C for 1 min. Also, in the end, it was followed by a last extension at 72 for 5 min and a cooling step at 4°C. After the completion of the reaction, to check the quality of the reaction and bands, 5 μL was electrophoresed on a 2% agarose gel using 1XTBE buffer for 50 min with a voltage of 85 amps.
By Operon kit
In this study, the YChromStrip kit (Operon, Spain) was used to examine microtargets confirming multiplex result in the research. The procedure included DNA extraction, PCR amplification, and hybridization and development. After PCR, hybridization was performed on the available strips in the kit according to the instructions. Then, the available bands for the studied STS (ZFY/XFX, SRY, sY84, sY86, sY127, sY134, sY254, and sY255) were checked according to the strip reading guide.
2.4. Statistical analysis
Semen and hormone levels were analyzed using unpaired t‐tests, comparing mean semen volume, sperm count, motility, FSH, LH, testosterone, and estradiol levels between RPL and control groups. The frequency of AZF deletions was compared between groups using Pearson's chi‐square test or Fisher's exact test. The association of Specific deletions with increased RPL risk was determined by calculating the odds ratio and 95% confidence interval. The correlation between sperm parameters and hormone levels was assessed using Pearson's correlation coefficient. The association between AZF deletions and sperm/hormone abnormalities was evaluated using logistic regression analysis. Data analysis was done using SPSS version 20 software. This study considered a p < 0.05 statistically significant.
3. RESULTS
3.1. Sample size
The calculated sample size was: , n = 1.962 × 0.35 × 0.65/0.12 = 34.56 ≈ 35. The sample size was calculated to be at least 35 in each group, considering the significance level of 5%, the power of 95%, and the error of 0.1. However, we considered a total of 120 samples for this study.
3.2. Semen analysis results
Semen analysis was performed in the examined groups and compared with standard values, summarized in Table 2. The reports were generated by the HFTCASA computer‐aided semen analysis system (Figure 1). The RPL group showed poorer semen parameters across all measures compared to controls, with statistically significant differences in sperm count (p = 0.012), motility (p = 0.002), and velocity (p < 0.001). The morphology and viability were also lower in the RPL group but did not differ significantly from controls. Overall, the RPL group had abnormal semen analysis results.
TABLE 2.
Semen and sex hormone analysis in investigated groups compared with standard values (mean ± SD).
| Parameters | Men with RPL wives (n = 120) | Men without RPL wives (n = 120) | Standard values mean (min–max) | p value |
|---|---|---|---|---|
| Semen analysis | ||||
| Semen volume (mL) | 1.4 ± 0.02 | 1.6 ± 0.05 | 1.5 (1.4–1.7) | 0.23NS |
| Sperm count (×106/ejaculated semen) | 30.24 ± 1.3 | 41.81 ± 2.6 | 39 (33–46) | 0.012S |
| Sperm concentration (×106/mL) | 13.9 ± 1.8 | 14.2 ± 2.1 | 15 (12–16) | 0.81NS |
| Sperm motility (%) | 33.1 ± 0.5 | 41 ± 1.9 | 40 (38–42) | 0.002S |
| Sperm velocity (μm/s) | 24.6 ± 0.4 | 29.8 ± 0.2 | 55 (20–70) | <0.001S |
| Sperm viability (%) | 49.1 ± 1.2 | 60.9 ± 2.0 | 58 (55–63) | 0.11NS |
| Sperm morphology (% of normal appearing) | 2.5 ± 0.01 | 3.5 ± 0.4 | 4 (3–4) | 0.15NS |
| PH | 7.2 | 7.2 | 7.2–7.8 | 0.13NS |
| Sex hormones analysis | ||||
| FSH (mIU/mL) | 25.9 ± 2.1 | 12.6 ± 1.1 | 1–14 | <0.001S |
| LH (mIU/mL) | 12.2 ± 3.5 | 5.3 ± 2 | 0.7–7.4 | <0.001S |
| PRL (ng/mL) | 11.9 ± 0.87 | 8.5 ± 4.4 | 1.8–17 | 0.012S |
| Testosterone (ng/mL) | 1 ± 0.5 | 4.9 ± 3.4 | 2–6.9 | <0.001S |
| Estradiol (pg/mL) | 101.9 ± 5.3 | 46.8 ± 7.9 | 4–94 | <0.001S |
Note: RPL, recurrent pregnancy loss, Chi‐square tests. Differences were statistically significant when p < 0.05.
Abbreviations: NS, nonsignificant; S, significant.
FIGURE 1.
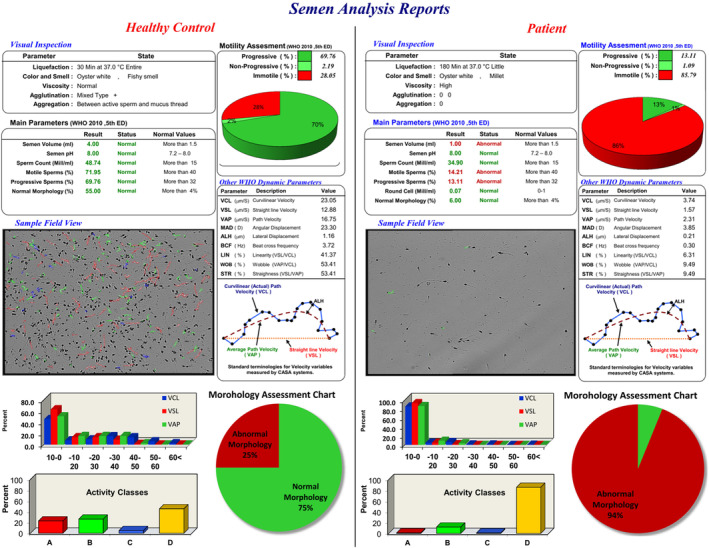
Semen analysis reports were generated by the HFTCASA Computer Aided System. Left panel: Semen analysis of a healthy control; Right panel: Semen analysis of a man with two recurrent pregnancy losses in his wife.
3.3. Hormone analysis results
The sex hormone levels evaluated in different groups are depicted in Table 2. There were significant alterations in sex hormone levels in the RPL group. In summary, the hormone profile of the RPL group suggests impaired testicular function marked by elevated FSH, LH, and estradiol and low testosterone (p < 0.001). The p‐values indicate that these differences are statistically significant.
Pearson's correlation coefficient (r) was used to assess the correlation between sperm parameters and hormone levels. There was a strong negative correlation between sperm count and FSH level (r = −0.85, p < 0.001). Sperm motility was positively correlated with testosterone concentration (r = 0.67, p < 0.01). The morphology of spermatozoa was strongly negatively correlated with LH (r = −0.72, p < 0.001). No significant correlation was observed between semen volume and estradiol level (r = −0.23, p = 0.09). FSH levels had a significant inverse correlation with sperm count and motility (r = −0.79 and r = −0.62, respectively, p < 0.05). Pearson's correlation analysis revealed a poor relationship between sperm viability and prolactin concentration (r = 0.28, p = 0.13).
3.4. DNA extraction results
The concentration of DNA samples measured by nanodrop was 77–247 ng/μL. The optical density (OD) 260/280 ratios of extracted DNAs were 1.78–1.89, which shows their purities.
3.5. Microdeletion analysis results
3.5.1. By multiplex PCR
The subjects studied in this case–control study were all male, and their average age was 31.42 ± 5.78 (the minimum age of the subject was 25 years old, and the maximum was 36 years old). A study of microdeletion in all three subregions, including AZFa, AZFb, and AZFc, in a group of healthy men without any history of abortion in their wives showed that there were no microdeletions in any of the 120 subjects. In 120 men of the second group, whose wives had at least two repeated abortions, the investigations showed that there were deletions in 40 cases, including 20 deletions in the AZFb region (sY134) and 10 deletions in each of the AZFb region (sY127) and AZFc region (sY254). The analysis of the statistical results showed that the occurrence of microdeletions in the sY134 (AZFb) region has a significant relationship with the occurrence of abortion, with a p < 0.05 (p = 0.03). Figure 2a,b demonstrates successful multiplex PCR detection of AZF microdeletions in men whose wives had recurrent miscarriages, particularly showing the common deletion of sY134 in the AZFb region.
FIGURE 2.
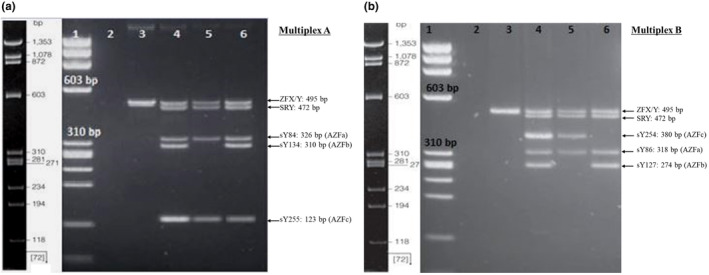
The result of the multiplex PCR on a 2% agarose gel. (a) Multiplex A. Lane 1: Molecular weight marker (72–1353 bp) phi X‐HeaIII; Lane 2: Negative control (water); Lane 3: The female DNA control, Lanes 4 and 6: Healthy men DNA samples without microdeletion; Lane 5: Men with couples experiencing RPL (microdeletion for sY134‐AZFb). (b) Multiplex B. Lane 1: Molecular weight marker (72–1353 bp) phi X‐HeaIII; Lane 2: Negative control (Water); Lane 3: The female DNA control; Lane 4: Healthy men DNA sample (without microdeletion); Lane 5: Men with couples experiencing RPL (microdeletion for sY127‐AZFb); Lane 6: Men with couples experiencing RPL (microdeletion for sY254‐AZFc).
No deletions were detected in AZFa (sY84, sY86) or AZFc (sY255) in either group. Deletions in AZFb (sY134) were significantly higher in the RPL group (16.66%) versus control (0%) with p = 0.03. Deletions in AZFb (sY127, 8.33%) and AZFc (sY254, 8.33%) were only detected in the RPL group but were not statistically significant. Therefore, microdeletions in the AZFb region, particularly sY134, were significantly associated with recurrent pregnancy loss in partners. No associations were found for AZFa or AZFc deletions. Table 3 provides evidence that AZFb microdeletions may contribute to recurrent miscarriage risk.
TABLE 3.
The results of microdeletions in different regions of AZF in the two studied groups.
| Regions | STS | Male partners of women with RPL n (%) | Male partners of women without RPL n (%) | χ 2 | OR | p‐value |
|---|---|---|---|---|---|---|
| AZFa | sY86 | 0 (0%) | 0 (0%) | – | – | 0.511NS |
| AZFa | sY84 | 0 (0%) | 0 (0%) | – | – | 0.819NS |
| AZFb | sY127 | 10 (8.33%) | 0 (0%) | 10.24 | 21 | 0.230NS |
| AZFb | sY134 | 20 (16.66%) | 0 (0%) | 20.48 | 31.6 | 0.030S |
| AZFc | sY254 | 10 (8.33%) | 0 (0%) | 10.24 | 21 | 0.679NS |
| AZFc | sY255 | 0 (0%) | 0 (0%) | – | – | 0.702NS |
Note: RPL, recurrent pregnancy loss, Chi‐square tests. Differences were statistically significant when p < 0.05.
Abbreviations: NS, nonsignificant; S, significant.
3.5.2. By Operon kit
The Operon YChromStrip contains probes for the detection of eight sequence‐tagged sites: sY84, sY86, sY127, sY134, sY254, and sY255, as well as SRY and ZFX/ZFY controls on the Y chromosome in blue bands. The results of the microdeletions analysis of DNA samples from two men with a history of miscarriage in their wives are illustrated in Figure 3a. The Operon strips provide rapid and reliable identification of the sY134 AZFb and sY254 AZFc microdeletions with the lack of blue bands on assigned locations. Figure 3b maps six studied STS loci spanning the long arm of the Y chromosome. The visualized results and diagram help interpret the findings. This suggests that the sY134 AZFb and sY254 AZFc microdeletions may have been associated with the increased risk of recurrent miscarriage, likely due to their impact on spermatogenesis.
FIGURE 3.
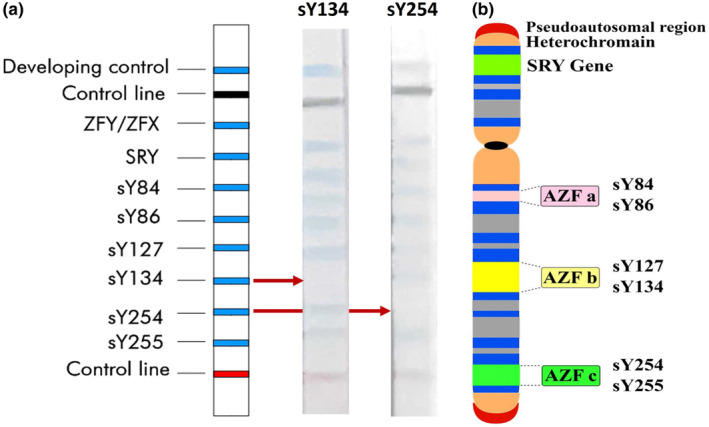
The result of the microdeletion analysis by the Operon kit. (a) Two sample strips from two men with a history of miscarriage in their wives, with microdeletion in the sY134 (AZFb) and sY254 (AZFc) regions. (b) Diagrammatic representation of the Y chromosome, the three azoospermia factor regions, and the location of the six sequence‐tagged site loci (sY84, sY86, sY127, sY134, sY254, sY255) used for analyzing Y chromosome microdeletions.
The results validated the microdeletions that were detected by multiplex PCR. The AZFa, AZFb, and AZFc regions were examined using STS markers (sY84, sY86, sY127, sY134, sY254, and sY255). There were no microdeletions in the control group. The RPL group showed 20 deletions in AZFb (sY134) and 10 deletions each in AZFb (sY127) and AZFc (sY254). Microdeletions in sY134 (AZFb) were significantly associated with RPL (p < 0.05). Therefore, the prevalence of Y chromosome microdeletions in the RPL group was 17% for AZFb (sY134), 8% for AZFb (sY127), and 8% for AZFc (sY254).
Pearson's chi‐square test and Fisher's exact test were used to compare the frequency of AZF deletions between the RPL and control groups. No significant difference was found in the frequency of AZFa sY84 or AZFc sY255 deletions between groups (p > 0.05). However, AZFb sY134 deletions were significantly more prevalent in the RPL group compared to controls based on Pearson's chi‐square test (16.7% vs. 0%, χ 2 = 20.48, p < 0.001). Similarly, AZFb sY127 and AZFc sY254 deletions were more common in the RPL group compared to controls by Fisher's exact test (8.3% vs. 0%, χ 2 = 10.24, p = 0.002). Men with an AZFb sY134 deletion had 31.6 times higher odds of having a partner with recurrent pregnancy loss compared to men without this deletion (OR: 31.6, 95% CI: 1.9–535.9, p = 0.02). The odds of recurrent pregnancy loss were 21 times higher for men with AZFb sY127 and AZFc sY254 deletions relative to those without this deletion (OR: 21.0, 95% CI: 1.2–363.3, p = 0.03). No significant difference in the odds of recurrent pregnancy loss was found for deletions in the AZFa sY84, AZFa sY86, or AZFc sY255 markers. Wide confidence intervals reflected the limited sample size. In summary, AZFb and AZFc microdeletions were associated with a higher risk of recurrent pregnancy loss compared to men without Y chromosome deletions.
The association between AZF deletions and sperm/hormone abnormalities was evaluated by logistic regression analysis. AZFc deletions were associated with a significantly increased risk of reduced sperm motility after adjusting for potential confounders in the logistic regression model (OR: 3.6, 95% CI: 1.1–12.1, p = 0.04). The odds of elevated FSH levels were four times greater for men with the AZFc deletions versus those without, based on the logistic regression analysis (OR: 4.1, 95% CI: 1.4–14.2, p = 0.01). Logistic regression revealed AZFb deletions as an independent predictor of low testosterone levels in men after controlling for covariates (OR: 2.8, 95% CI: 1.2–6.9, p = 0.02). Sperm abnormalities and altered hormone levels were more likely in men with AZF deletions compared to those without deletions, according to multivariate logistic regression analysis.
4. DISCUSSION
Recurrent pregnancy loss (RPL) is actually a type of infertility in which a woman suffers two or more consecutive miscarriages. Based on several studies, different genetic and chromosomal factors, such as chromosomal abnormalities, both in terms of structure and number, in each of the parents can be effective in reducing the occurrence of repeated abortions (Ewington et al., 2019; Pazoki & Naseri, 2019). There are also reports based on the role of male factors on the development process of the fetus or increasing the probability of miscarriage (Niederberger, 2011; Pina‐Aguilar et al., 2012).
Sperm integrity is required for fertilization, sperm–ovule interactions, and early embryonic development. Sperm quality affects the embryo's ability to reach the blastocyst phase and develop into implantation. Many investigations have failed to explain the cause of pregnancy loss in approximately 50% of women with RPL. A study on microdeletions of AZF regions due to their inheritance is very important, especially in cases of assisted reproductive methods (ART) (Rabinowitz et al., 2021; Sultana et al., 2020). Based on the analysis of clinical genetic articles, the examination of these microdeletions in candidates for intrauterine sperm injection (IUI), artificial insemination (IVF), and intracytoplasmic sperm injection (ICSI), and even couples whose women have frequent miscarriages for no reason is very beneficial.
Semen analysis can improve outcomes, as Y chromosome microdeletions are often linked to sperm defects (Yuen et al., 2021). The semen analysis results indicate poorer sperm parameters like count, motility, and velocity in male partners of women with RPL compared to the control group. This suggests that abnormal semen quality may be associated with RPL. In particular, the significantly lower sperm velocity in the RPL group is notable as it may impact sperm function and fertilization. Morphology and viability were also lower in the RPL group, though not significantly. Overall, comprehensive semen analysis seems valuable in RPL workups. The sex hormone analysis highlights differences in FSH, LH, and testosterone levels between the RPL group and control. The hormonal profile provisions testicular function and spermatogenesis in men with impaired sperms, which can guide treatment plans.
Three AZF regions in the long arm of the Y chromosome are essential for normal spermatogenesis. As a result, microdeletion in any of the AZF subregions can negatively affect sperm quality and function. Microdeletion of AZFb and AZFc may increase pairing failure of chromosomal abnormalities, potentially leading to recurrent pregnancy loss. The Y chromosome contains repetitive sequences, with most deleted genes in infertile men located in Yq's palindromic regions (Hossein & Farhat, 2006). Because AZF microdeletions usually involve more than one gene, the role of one AZF gene alone cannot be determined and requires more extensive studies. This article also mentioned that the main effect of microdeletion in different regions of AZF is on the quantity and quality of sperms. This can be a more correct hypothesis on the relationship between microdeletion of AZF and male infertility or low fertility. Men with complete deletions in the AZFa, b, bc, and abc regions cannot recover sperm from testicular tissue. Men with AZFa typically have very few germ cells in a testicular biopsy. Men with AZFb usually lack sperm‐producing cells, which medication can sometimes rectify. When deletions occur only in the AZFc region, testicular sperm extraction (TESE) succeeds in 75% of cases. For men with an azoospermia pattern with an AZFc deletion, sperm can be located for artificial insemination in 60% of cases (Krausz et al., 2014).
In this study, an attempt has been made to investigate the relationship between repeated miscarriages in women and the presence of deletions in each of the AZF regions, including AZFa, AZFb, and AZFc in men and its possible role in the incidence of miscarriage in their wives. Based on the EAA/EMON agenda, six suggested sequences were selected for STS, which cover 95% of all deletions (Krausz et al., 2014). These sequences include (sY84, sY86) AZFa, (sY127, sY134) AZFb, and (sY254.sY255) AZFc. In this research, 120 healthy (neurospermic) men with a history of two or more consecutive abortions in their wives and 120 healthy fertile men with no history of repeated abortions in their wives with at least one child were studied as a control group. The results of two multiplex PCR reactions (A and B) detected Y chromosome microdeletions in different sample groups. Multiplex A amplifies markers for AZF regions of sY84, sY134, and sY255. All samples show amplification, indicating AZFa is intact. Multiplex B amplifies AZF markers of sY86, sY127, and sY254. Based on the missing bands, there was a deletion in the AZFb (sY134) region. The clinical value is linking specific Y chromosome microdeletions to male infertility phenotypes. Probably, AZFb deletions may be associated with severe oligozoospermia (Rabinowitz et al., 2021; Soares et al., 2012). AZFc deletions may result in phenotypes ranging from azoospermia to residual sperm production and oligozoospermia (Krausz et al., 2014; Yu et al., 2015).
This study found significant associations between specific AZF microdeletions and recurrent pregnancy loss. Pearson's chi‐square analysis revealed AZFb sY134 and sY127 deletions were significantly more prevalent in the RPL group compared to controls (16.7% vs. 0%, p < 0.001 and 8.3% vs. 0%, p = 0.002, respectively). Similarly, AZFc sY254 deletions were more common in the RPL group (8.3% vs. 0%, p = 0.002). Importantly, the odds of recurrent pregnancy loss were 21–31 times higher for men with AZFb sY134, sY127, and AZFc sY254 deletions compared to those without. This quantifies the substantial increase in RPL risk conferred by these AZF aberrations. However, wide confidence intervals reflect the limited sample size. This aligns with previous studies reporting higher rates of AZFc deletions in men with RPL partners (Dewan et al., 2006; Golin et al., 2021; Li et al., 2016).
According to the obtained results, none of the men in the control group had any microdeletions in the AZF area. In the patient group, there were 20 microdeletions in the sY134 (AZFb) region and 10 deletions in each of the sY127 (AZFb) and sY254 (AZFc) regions, which were statistically significant with the risk of repeated miscarriage. Based on the results of several other studies, there is a possibility of a significant relationship between microdeletions in AZF regions and the occurrence of miscarriage in women (RPL). Agarwal et al. (2015) revealed that 13 of the 40 RPL cases showed Y chromosome microdeletions at three azoospermia factor loci. Therefore, AZF microdeletions were significantly associated with the incidence of miscarriage (Agarwal et al., 2015). As the same as multiplex PCR results, the use of PCR and a genetic test kit to identify Y microdeletions associated with infertility. The clinical value is identifying deletions like in AZFb that may cause sperm abnormalities and pregnancy loss. Testing for Y microdeletions can complement semen analysis and hormone profile in assessing male factor infertility.
Other studies like Peng (2023) in 2023 found no evidence that AZF deletions were associated with RPL. According to Liang et al. (2019), infertile patients with normal Y chromosomes and oligozoospermic patients with Y chromosome AZF microdeletion have similar clinical outcomes from ICSI. Ultimately, it appears that Y chromosomal microdeletions are not linked to RPL, and further study is required (Liang et al., 2019). Also, Pina‐Aguilar et al. (2012) showed that, in 66 male subjects, there were no microdeletions in the triple AZF regions. Of course, in Piña‐Aguilar's study, in addition to the sY254 and sY255 markers for the AZFc subregion, the sY150 and sY155 markers were also investigated in the same subregion, which could be one of the reasons for the difference in the results with the present study. AZF microdeletions and frequent miscarriages have not been studied extensively in Iran. Soleimanian et al. (2013) reported a significant relationship between microdeletion in the sY67 (DYS262) region and RPL by studying 30 men with a history of miscarriage in their wives (Soleimanian et al., 2013). The location of this marker is close to the desired region, AZF, and lies in the short arm of the chromosome (Yp). Therefore, the report of this microdeletion cannot be attributed to the proposed relationship with AZF microdeletions.
The contradictory findings across studies likely stem from several key factors. Ethnic and genetic differences in study populations lead to variable Y microdeletion frequencies geographically. Many studies utilize small sample sizes lacking sufficient power to detect true associations. Heterogeneity in recurrent pregnancy loss diagnostic criteria also contributes to inconsistent results. Variability in AZF screening methodology including the number and selection of markers analyzed and techniques used makes comparing findings across studies difficult. In addition, some borderline statistically significant results likely represent false‐positive chance findings.
This study has several key limitations warranting acknowledgement. The modest sample size of 120 men per group, restricted ethnic population, and recruitment from a single center may reduce generalizability and introduce selection bias. Testing only six AZF markers overlooks less common deletions. Most importantly, this study failed to explore potential gene–gene/gene–environment interactions or control for confounders that could modulate recurrent pregnancy loss risk. While these data provide preliminary evidence linking AZF microdeletions to recurrent pregnancy loss, the limitations underscore the need for more rigorous research.
Examining and reflecting on the results reported in various articles, as well as the present study, highlights the fact that there is a possibility of a significant relationship between microdeletions of AZF regions in men and repeated abortions in their wives. However, conducting a more extensive study on a large statistical population with a larger sample size is suggested to make a more definitive decision. Since the genetic changes in this area were examined in this study, it is suggested that epigenetic changes should also be examined for a more detailed investigation and possible investigation of the existence of a relationship. Also, to improve the quality of the results, this study can be conducted with different ethnicities in Iran. To better understand the performance of microdeletions, it is suggested to study their relationship with the occurrence of azoospermia and oligospermia in men and their effect on fertility quality.
5. CONCLUSION
Male partners of couples with RPL showed significant abnormalities in seminal parameters compared to a control group, including lower sperm count, motility, and velocity. Hormonal profiling revealed altered FSH, LH, testosterone, and estrogen levels in the RPL group men versus controls, indicating impaired testicular function. Y chromosome microdeletion analysis identified specific AZFb and AZFc deletions in some of the men in the RPL group using multiplex PCR and genetic testing kits. In summary, thorough laboratory evaluation of semen quality, sex hormones, and genetic tests for Y microdeletions provides evidence of the association between male factor infertility and causes of recurrent pregnancy loss.
AUTHOR CONTRIBUTIONS
The first author (principal researcher) was involved in drafting of the proposal, information collection, and writing of the article (30%); the second author (principal researcher) was involved in project design, statistical analysis of the design, and scientific editing of the article (25%); the third author (principal researcher) was responsible for correspondence, participation in compiling different parts of the article (25%); the fourth author (co‐researcher) was involved in clinical diagnoses and scientific consultation (20%).
FUNDING INFORMATION
This project has not received any financial support.
CONFLICT OF INTEREST STATEMENT
There is no conflict of interest among the authors in this study.
ETHICS STATEMENT
This study has been approved by the Research Ethics Committee of Islamic Azad University‐North Tehran Branch with ID IR.IAU.TNB.REC.1401.041. In none of the stages of conducting the study and recording the data, the personal information of the patients was not disclosed and was not provided to any natural or legal person. In addition, written informed consent was received from the control and patient groups.
ACKNOWLEDGMENTS
This article is taken from the thesis of the specialized doctoral course in molecular genetics approved by Islamic Azad University, North Tehran branch. The authors hereby appreciate the efforts and cooperation of Mehragin Medical Diagnostic Laboratory staff and respected patients in carrying out this project.
Pazoki, N. , Salehi, M. , Angaji, S. A. , & Abdollahpour‐Alitappeh, M. (2024). Association of Y chromosome AZF region microdeletions with recurrent miscarriage in Iranian couples: A case–control study. Molecular Genetics & Genomic Medicine, 12, e2392. 10.1002/mgg3.2392
Contributor Information
Mitra Salehi, Email: mitra.salehi.microbiology046@gmail.com, Email: m_salehi@iau-tnb.ac.ir.
Seyed Abdolhamid Angaji, Email: angaji@khu.ac.ir.
Meghdad Abdollahpour‐Alitappeh, Email: abdollahpour1983@yahoo.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Agarwal, S. , Agarwal, A. , Khanna, A. , & Singh, K. (2015). Microdeletion of Y chromosome as a cause of recurrent pregnancy loss. Journal of Human Reproductive Sciences, 8(3), 159–164. 10.4103/0974-1208.165145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi Rastegar, D. , Sharifi Tabar, M. , Alikhani, M. , Parsamatin, P. , Sahraneshin Samani, F. , Sabbaghian, M. , Sadighi Gilani, M. A. , Mohammad Ahadi, A. , Mohseni Meybodi, A. , Piryaei, A. , Ansari‐Pour, N. , Gourabi, H. , Baharvand, H. , & Salekdeh, G. H. (2015). Isoform‐level gene expression profiles of human Y chromosome azoospermia factor genes and their X chromosome paralogs in the testicular tissue of non‐obstructive azoospermia patients. Journal of Proteome Research, 14(9), 3595–3605. 10.1021/acs.jproteome.5b00520 [DOI] [PubMed] [Google Scholar]
- Ambulkar, P. S. , & Pande, S. S. (2017). Male Infertility: Screening of Azoospermia Factor (AZF) microdeletion in idiopathic infertile men. Journal of Experimental Biology and Agricultural Sciences, 5, 7–13. [Google Scholar]
- Asadi, S. , & Kiani, A. (2020). The role of genetic mutations in Y chromosome Infertility syndrome. Ann Inter Cli Med CaRe: AICMCR‐108, 11, 3. 10.46715/aicmcr2020 [DOI] [Google Scholar]
- Bigdeli, R. , Younesi, M. R. , Panahnejad, E. , Asgary, V. , Heidarzadeh, S. , Mazaheri, H. , & Aligoudarzi, S. L. (2018). Association between thrombophilia gene polymorphisms and recurrent pregnancy loss risk in the Iranian population. Systems Biology in Reproductive Medicine, 64(4), 274–282. [DOI] [PubMed] [Google Scholar]
- Dewan, S. , Puscheck, E. E. , Coulam, C. B. , Wilcox, A. J. , & Jeyendran, R. S. (2006). Y‐chromosome microdeletions and recurrent pregnancy loss. Fertility and Sterility, 85(2), 441–445. 10.1016/j.fertnstert.2005.08.035 [DOI] [PubMed] [Google Scholar]
- Dimitriadis, E. , Menkhorst, E. , Saito, S. , Kutteh, W. H. , & Brosens, J. J. (2020). Recurrent pregnancy loss. Nature Reviews. Disease Primers, 6(1), 98. 10.1038/s41572-020-00228-z [DOI] [PubMed] [Google Scholar]
- Ewington, L. J. , Tewary, S. , & Brosens, J. J. (2019). New insights into the mechanisms underlying recurrent pregnancy loss. Journal of Obstetrics and Gynaecology Research, 45(2), 258–265. [DOI] [PubMed] [Google Scholar]
- Golin, A. P. , Yuen, W. , & Flannigan, R. (2021). The effects of Y chromosome microdeletions on in vitro fertilization outcomes, health abnormalities in offspring and recurrent pregnancy loss. Translational Andrology and Urology, 10(3), 1457–1466. 10.21037/tau-19-672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves, C. , Cunha, M. , Rocha, E. , Fernandes, S. , Silva, J. , Ferraz, L. , Oliveira, C. , Barros, A. , & Sousa, M. (2017). Y‐chromosome microdeletions in nonobstructive azoospermia and severe oligozoospermia. Asian Journal of Andrology, 19(3), 338–345. 10.4103/1008-682X.172827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossein, S. N. , & Farhat, F. (2006). Genetics of azoospermia: Current knowledge, clinical implications, and future directions part I. The Journal of urology, 3(4), 193–203. [PubMed] [Google Scholar]
- Jungwirth, A. , Giwercman, A. , Tournaye, H. , Diemer, T. , Kopa, Z. , Dohle, G. , Krausz, C. , & EAU Working Group on Male Infertility . (2012). European Association of Urology guidelines on male Infertility: The 2012 update. European Urology, 62(2), 324–332. [DOI] [PubMed] [Google Scholar]
- Kaminski, P. , Baszynski, J. , Jerzak, I. , Kavanagh, B. P. , Nowacka‐Chiari, E. , Polanin, M. , Szymański, M. , Woźniak, A. , & Kozera, W. (2020). External and genetic conditions determining male infertility. International Journal of Molecular Sciences, 21(15), 5274. 10.3390/ijms21155274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krausz, C. , Hoefsloot, L. , Simoni, M. , Tuttelmann, F. , & European Academy of Andrology; European Molecular Genetics Quality Network . (2014). EAA/EMQN best practice guidelines for molecular diagnosis of Y‐chromosomal microdeletions: State‐of‐the‐art 2013. Andrology, 2(1), 5–19. 10.1111/j.2047-2927.2013.00173.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q. , Song, N. H. , Cao, W. Z. , Shao, Q. , Xie, J. J. , Liu, C. , Wang, Y. M. , & Shen, H. (2016). Relationship between AZFc deletions and testicular histology in infertile south Chinese men with azoospermia and severe oligospermia. Springerplus, 5(1), 1805. 10.1186/s40064-016-3512-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, W.‐N. , Xue, S. , & Shang, X.‐J. (2019). Role of sperm genetic abnormality in recurrent pregnancy loss: An update. Zhonghua nan ke xue = National Journal of Andrology, 25(12), 1126–1130. [PubMed] [Google Scholar]
- Liu, X. , Hu, H. , Guo, Y. , & Sun, Y. (2016). Correlation between Y chromosome microdeletion and male infertility. Genetics and Molecular Research, 15(2), 1–6. [DOI] [PubMed] [Google Scholar]
- Moghbeli, M. (2019). Genetics of recurrent pregnancy loss among Iranian population. Molecular Genetics & Genomic Medicine, 7(9), e891. 10.1002/mgg3.891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemati, V. S. , Kheirkhah, B. , & Malekirad, A. A. (2020). Association between polymorphism of has‐miR‐125 (rs12976445) and susceptibility to idiopathic recurrent pregnancy loss in Iranian women. The Iranian Journal of Obstetrics, Gynecology and Infertility, 23(8), 37–48. [Google Scholar]
- Niederberger, C. (2011). Re: Y chromosome microdeletions, sperm DNA fragmentation and sperm oxidative stress as causes of recurrent spontaneous abortion of unknown etiology. The Journal of Urology, 185(4), 1404–1405. [DOI] [PubMed] [Google Scholar]
- Pal, A. K. , Ambulkar, P. S. , Waghmare, J. E. , Wankhede, V. , Shende, M. R. , & Tarnekar, A. M. (2018). Chromosomal aberrations in couples with pregnancy loss: A retrospective study. Journal of Human Reproductive Sciences, 11(3), 247–253. 10.4103/jhrs.JHRS_124_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazoki, N. , & Naseri, F. (2019). The prevalence of common mutations in thrombophilic patients in Iranian population with recurrent miscarriage. International Journal of Medical Laboratory, 6(2), 115–123. [Google Scholar]
- Peng, L.‐F. (2023). Sperm genetic abnormality testing in recurrent pregnancy loss cases: A narrative review. Middle East Fertility Society Journal, 28(1), 23. [Google Scholar]
- Pina‐Aguilar, R. E. , Martinez‐Garza, S. G. , Kohls, G. , Vargas‐Maciel, M. A. , Vazquez de Lara, L. G. , Gonzalez‐Ortega, C. , Cancino‐Villarreal, P. , & Gutierrez‐Gutierrez, A. M. (2012). Y chromosome microdeletions in Mexican males of couples with idiopathic recurrent pregnancy loss. The Journal of Obstetrics and Gynaecology Research, 38(6), 912–917. 10.1111/j.1447-0756.2011.01809.x [DOI] [PubMed] [Google Scholar]
- Pylyp, L. Y. , Spinenko, L. O. , Verhoglyad, N. V. , Kashevarova, O. O. , & Zukin, V. D. (2015). Chromosomal abnormalities in patients with infertility. Tsitologiia i Genetika, 49(3), 33–39. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26214903 [PubMed] [Google Scholar]
- Rabinowitz, M. J. , Huffman, P. J. , Haney, N. M. , & Kohn, T. P. (2021). Y‐chromosome microdeletions: A review of prevalence, screening, and clinical considerations. The Application of Clinical Genetics, 14, 51–59. 10.2147/TACG.S267421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, X. , Xie, X. , Jia, Y. , & Li, S. (2017). Maternal genetic polymorphisms and unexplained recurrent miscarriage: A systematic review and meta‐analysis. Clinical Genetics, 91(2), 265–284. 10.1111/cge.12910 [DOI] [PubMed] [Google Scholar]
- Soares, A. R. , Costa, P. , Silva, J. , Sousa, M. , Barros, A. , & Fernandes, S. (2012). AZFb microdeletions and oligozoospermia‐‐which mechanisms? Fertility and Sterility, 97(4), 858–863. 10.1016/j.fertnstert.2012.01.099 [DOI] [PubMed] [Google Scholar]
- Soleimanian, S. , Kalantar, S. M. , Sheikhha, M. H. , Zaimy, M. A. , Rasti, A. , & Fazli, H. (2013). Association between Y‐chromosome AZFc region micro‐deletions with recurrent miscarriage. Iranian Journal of Reproductive Medicine, 11(5), 431–434. [PMC free article] [PubMed] [Google Scholar]
- Sultana, S. , Nallari, P. , & Ananthapur, V. (2020). Recurrent pregnancy loss (RPL): An overview. Journal of Womens Health and Development, 3(3), 302–315. [Google Scholar]
- Tan, J. , Taskin, O. , Albert, A. , & Bedaiwy, M. A. (2019). Association between sperm DNA fragmentation and idiopathic recurrent pregnancy loss: A systematic review and meta‐analysis. Reproductive Biomedicine Online, 38(6), 951–960. [DOI] [PubMed] [Google Scholar]
- Torfeh, M. , Sakhinia, E. , Hasani, H. , Ahmadi Asre Badr, Y. , Nourizadeh, D. , Heshmat, Y. , & Rohani, M. (2012). Molecular analysis and comparison of y chromosome microdeletions in tabriz and kashan infertile men with azoospermia and severe oligospermia. The Iranian Journal of Obstetrics, Gynecology and Infertility, 15(2), 22–28. [Google Scholar]
- Yu, N. , Kwak‐Kim, J. , & Bao, S. (2023). Unexplained recurrent pregnancy loss: Novel causes and advanced treatment. Journal of Reproductive Immunology, 155, 103785. 10.1016/j.jri.2022.103785 [DOI] [PubMed] [Google Scholar]
- Yu, X. W. , Wei, Z. T. , Jiang, Y. T. , & Zhang, S. L. (2015). Y chromosome azoospermia factor region microdeletions and transmission characteristics in azoospermic and severe oligozoospermic patients. International Journal of Clinical and Experimental Medicine, 8(9), 14634–14646. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26628946 [PMC free article] [PubMed] [Google Scholar]
- Yuen, W. , Golin, A. P. , Flannigan, R. , & Schlegel, P. N. (2021). Histology and sperm retrieval among men with Y chromosome microdeletions. Translational Andrology and Urology, 10(3), 1442–1456. 10.21037/tau.2020.03.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zargar, M. , Ghafourian, M. , Nikbakht, R. , Mir Hosseini, V. , & Moradi Choghakabodi, P. (2020). Evaluating chronic endometritis in women with recurrent implantation failure and recurrent pregnancy loss by hysteroscopy and immunohistochemistry. Journal of Minimally Invasive Gynecology, 27(1), 116–121. 10.1016/j.jmig.2019.02.016 [DOI] [PubMed] [Google Scholar]
- Zhang, Y. S. , Li, L. L. , Xue, L. T. , Zhang, H. , Zhu, Y. Y. , & Liu, R. Z. (2017). Complete azoospermia factor b deletion of Y chromosome in an infertile male with severe oligoasthenozoospermia: Case report and literature review. Urology, 102, 111–115. 10.1016/j.urology.2016.07.022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


