Abstract
Electrified converting CO2 into valuable fuels and chemicals using a homogeneous electrochemical CO2 reduction (CO2ER) approach simplifies the operation, providing a potential option for decoupling energy harvesting and renewable chemical production. These merits benefit the scenarios where decentralization and intermittent power are key factors. This perspective aims to provide an overview of recent progress in homogeneous CO2ER. We introduce firstly the fundamentals chemistry of the homogeneous CO2ER, followed by a summary of the crucial factors and the important criteria broadly employed for evaluating the performance. We then highlight the recent advances in the most widely explored transition-metal coordinate complexes for the C1 and multicarbon (C2+) products from homogeneous CO2ER. Finally, we summarize the remaining challenges and opportunities for developing homogeneous electrocatalysts for efficient CO2ER. This perspective is expected to favor the rational design of efficient homogeneous electrocatalysts for selective CO2ER toward renewable fuels and feedstocks.
Subject areas: Molecular electrochemistry, Energy materials
Graphical abstract

Molecular electrochemistry; Energy materials
Introduction
During the past few decades, the huge energy demand and the rapid consumption of fossil fuels have caused increasing CO2 emissions, leading to severe environmental impacts and the greenhouse effect. The issues of carbon emissions and global warming have received intensive attention from countries worldwide. Governments worldwide have made imperative and political commitments to achieve carbon neutrality. Notably, the Chinese government has released numerous implementations to realize carbon peaking in 2030 and carbon neutrality goals in 2060. Thus, peaking CO2 emission presses for advanced CO2 capture and transformation technologies. In accordance, developing efficient and durable strategies for the activation, selective conversion, and recovery of CO2 has been receiving tremendous attention in the research community to realize the resource utilization of CO2 toward a sustainable modern society.1,2
The efficient conversion of CO2 into value-added chemicals through the reduction method has long been pursued as one of the most attractive and economical ways for the resource utilization of CO2. Generally, the chemical reduction of CO2 molecules requires using catalysts for activation owing to their inert nature. In the last decades, many catalytic strategies, including chemical catalysis, photocatalysis, biomimetic catalysis, and electrocatalysis have been proposed for CO2 reduction. Chemical catalysis is usually involved with the organic catalyzed reduction of CO2 based on using the functional non-noble metal catalysts and the main group catalysts (e.g., frustrated Lewis pairs, carbene analogs, and so on).3,4,5 The photocatalytic CO2 reduction is often achieved using semiconductors-based photocatalysts such as metal oxides (e.g., TiO2, CeO2, and so on) and metal-organic frameworks.6,7,8,9 As for biomimetic catalysis, metalloenzymes, and some natural products are explored to simulate the process for the biogeochemical cycles of CO2.10,11 In particular, electrocatalytic CO2 reduction, also termed CO2 electroreduction (CO2ER), attracts increasing attention due to the sustainability and safety of electricity and numerous advantages such as mild reaction conditions, easy operability, and good tunability of reactivity and selectivity.12,13,14 In addition, CO2ER is a promising approach to meet the prevailing trends in organic electrosynthesis, which has seen increasingly broad use in the synthetic community over the past several years.15,16,17,18,19,20,21,22,23
The CO2ER is usually carried out in the heterogeneous system and conducted in flow-cell setups to enable industrial-relevant productivity.24 In the past decade, the flow-cell CO2ER devices of a gas-diffusion electrode (Figure 1A) were intensively studied majorly because they resemble industrial water electrolyzers.25 The solid-state catalyst particles or thin film are loaded onto hydrophobic gas-diffusion layers, and the CO2ER occurs at the catalyst-electrolyte-CO2 gas triple-phase boundary,26 i.e., heterogeneous catalysis. As such, most of the studies on the heterogeneous CO2ER focus on developing highly active heterogeneous electrocatalysts and fabricating gas diffusion electrodes.27 The gas diffusion electrode fabrication is complicated and costly, and its poor durability handicaps the industrial implementation of heterogeneous CO2ER. The gas diffusion layer may flood after 10–100 h of operation due to salt formation, electrowetting, or pressure difference.28 The heterogeneous catalyst deactivates due to chemical and mechanical instabilities. Power intermittent deteriorates the efficiency and durability problems. Low-temperature (<80°C) CO2ER-based energy storage for decentralized deployment lies in converting CO2 to liquid fuel molecules, such as ethanol, methanol and propanol.29 To date, Cu-based catalysts predominate the cathodic catalysts for such conversions. Typically, the selectivity of CO2ER in a specific device is determined by the operation current density, and the liquid product selectivity usually reaches the maximum at a narrow current density window (e.g., ±50 mA/cm2).30,31 The input power fluctuation can significantly alternate the selectivity of heterogeneous CO2ER devices and subsequently degrade their efficiency. In addition, the frequent on-off operation challenges Cu-based catalysts' robustness and the gas-diffusion electrode’s stability.32 Besides, replacing degraded catalysts requires the disassembly and reassembly of the electrolyzer, making the decentralized deployment of CO2ER devices challenging and incurring high electrolyzer maintenance costs.
Figure 1.
The flow-cell CO2ER devices for heterogeneous and homogeneous catalysis
The schematic illustration of heterogeneous catalyst (A) and homogeneous catalyst mediated (B) CO2 reduction flow cells (Re stands for reduction, Ox stands for oxidation).
The emerging homogeneous electrocatalytic system offers a potential solution to these challenges.33,34,35,36 We illustrate a typical homogeneous electrocatalytic CO2ER in Figure 1B. A homogeneous electrocatalyst can usually move freely in the electrolyte. It is usually dissolved in the electrolyte, circulating between the electrode and the catholyte reservoir. The homogeneous electrocatalyst is electrochemically charged on the electrode and discharged in the reservoir through catalytic CO2 reduction. The cathodic electrode can be inert, durable materials such as Ti and graphite foil. This installation thus removes the complicated cathodic electrode fabrication. The deactivated catalyst can be replenished by changing the catholyte. Therefore, homogeneous electrocatalytic CO2ER can potentially reduce the maintenance cost of CO2ER devices. Homogeneous CO2ER can decouple energy harvesting and consuming procedures,33,34,35,36 enabling chemicals-in-demand operation, the optimization of each step independently and mitigating the impacts of intermittent power. For instance, we consider a homogeneous CO2ER device coupled with a solar farm. In the daytime, the device works as a flow battery with the redox mediates harvesting the energy from the photovoltaic cells. In the nighttime, the homogeneous CO2ER device works in a discharge mode, using the redox mediates to convert CO2 to desired products under controlled and consistent conditions. Moreover, homogeneous CO2ER devices can use inert materials such as graphite, and the gas diffusion electrode configuration is unnecessary. These promote their robustness to the frequent on-off operation.
Generally, electrocatalysts play the most important critical roles in the overall electrochemical performance of homogeneous CO2ER. The homogeneous electrocatalysts can be roughly categorized into two types based on their different roles in the electron transfer steps. As shown in Figure 2, the Type I homogeneous electrocatalyst works as a redox mediator for shuttling electrons between the electrode and substrate, such as CO2. The electrocatalyst accelerates the redox reactions for homogeneous CO2ER that would otherwise be hindered by slow electron-transfer kinetics with electrode surfaces.38 Since the type I electrocatalyst only serves as the electron shuttler, the electrocatalyst does not form chemical bonds, noncovalent bonds, or polymerized interactions with the CO2 substrate. The type II homogeneous electrocatalyst serves as an electron shuttler to the CO2 substrate and enables the formation of new chemical bonds, noncovalent bonds, or polymerized interactions with the CO2 substrates. As such, the type II homogeneous electrocatalyst can improve both the reaction kinetics and chemical selectivity. Accordingly, type II electrocatalysts are more widely pursued.
Figure 2.
The generally proposed mechanisms for two types of homogeneous electrocatalysts for CO2ER and an example of type I electrocatalyst
(A) The Type I homogeneous electrocatalyst works as a redox mediator for shuttling electrons between the electrode surface and CO2 substrate (the left one).
(B) The type II homogeneous electrocatalyst not only serves as an electron shuttler but also produces new chemical bonds with the CO2 substrates.
(C) The MV2+/MV·+ couple serves as the electron shuttler in the oxygen electroreduction, the electrocatalyst does not form any chemical bonds, noncovalent bonds, or polymerized interactions with the O2 substrate. Reproduced with permission from ref. 37 Copyright, American Chemical Society.
Few type I electrocatalysts has been reported since they generally show less activity than the type II electrocatalysts.37 An typical example is that the MV2+ (MV stands for methyl viologen) electrocatalysts was used for H2O2 production from the O2 electroreduction. The kinetic of catalytic process is similar to the homogeneous electron transfer of type I electrocatalyst. The rate constant of this rate-determining step is the type I electron transfer. The MV2+/MV·+ couple only serves as the electron shuttler.37
To date, many emerging electrocatalysts have been developed for homogeneous CO2ER.39,40,41,42 To the best of our knowledge, although numerous reviews have discussed the recent advances in the design and development of electrocatalysts for heterogeneous CO2ER,43,44,45,46 the current status and challenges for the rational design of electrocatalysts for homogeneous CO2ER are rarely discussed. To follow up on this field, here we provide this tutorial review for the rational design of efficient electrocatalysts for homogeneous CO2ER. We first present the fundamentals of homogeneous CO2ER, followed by a discussion of various critical influencing factors and criteria used to evaluate the performance of electrocatalysts for homogeneous CO2ER. We then highlight the most recent advances in the development of various efficient homogeneous electrocatalysts for selective CO2ER. Finally, we address the remained challenges and opportunities for future efforts in homogeneous CO2ER.
Fundamentals of homogeneous CO2ER
The molecular orbital of CO2
In general, the reduction of CO2 occurs as a result of creating C−H and C−C bonds and cleaving C−O bonds. The molecular structure of CO2 is linear with a very short and strong C−O distance of 1.16 Å. Although CO2 shows nonpolar, it contains polar C−O bonds owing to the difference in electronegativity between C and O atoms. The intrinsic polarity of C−O gives rise to the susceptibility to nucleophilic attack on C and electrophilic attack on O atom. Generally, the reduction of CO2 involves the activation of CO2 through a decrease in the C−O bond.47 This can be usually achieved by the use of functional catalysts owing to their inert nature.
The stability and chemical reactivity of a molecule is largely determined by the intrinsic molecular orbitals and bond energy. The molecular orbitals (Mos) for the energy diagram of CO2 are illustrated in Figure 3. The doubly occupied nonbonding 1πg MOs mainly account for the HOMO (highest occupied molecular orbital). The 1πg MOs are principally contributed from the terminal oxygen atoms. The empty antibonding 2πu orbitals serving as the lowest unoccupied molecular orbital (LUMO) are mostly contributed by the carbon atom. In this regard, CO2 can be considered an amphoteric molecule. Specifically, the oxygen atoms display a Lewis basic characteristic while the carbon atom behaves as a Lewis acid center. Since CO2 has a slightly negative electron affinity (Ea) of about −0.6 eV48 and a first ionization potential (IP) of about 13.8 eV,49 CO2 tends to accept electrons rather than donor electrons. The intrinsic reactivity of CO2 molecules during reduction is thus usually dominated by the electrophilic character of the C atom rather than the weak nucleophilic properties of O atoms. When one LUMO orbital of CO2 is filled by a transferred electron, the resultant lowest energy state could give rise to a bent geometry of the C−O bond. The C−O bond is thus simultaneously activated. Since one CO2 molecule contains two 2πu, one 3σu, and one 3σg LUMO orbitals, one CO2 molecule can accept up to 8 electrons. Accordingly, the reduction of one CO2 molecule by a different number of electrons could result in different products. A complete reduction of one CO2 molecule with a maximum of 8 electrons can give rise to the formation of CH4.
Figure 3.
Molecular orbital diagram of CO2
The highest occupied molecular orbital (HOMO) of 1πg orbitals is mainly ascribed to the terminal oxygen atoms. The empty antibonding 2πu orbitals of the lowest unoccupied molecular orbital (LUMO) are mostly contributed by the carbon atoms.
Different reaction routes for homogeneous CO2ER
Different products such as CO, HCOOH, HCHO, and CH3CH2OH can be obtained from the homogeneous CO2ER through different reaction pathways. As illustrated in Figure 4, the products of homogeneous CO2ER depend highly on the type of homogeneous electrocatalysts and the experimental conditions. A multiple and cascaded electron transfer process could be also involved under certain conditions, giving rise to the formation of complex products. For example, a specific homogeneous electrocatalyst could enable the generation of CO2·− intermediate through the transfer of one electron to CO2.51 Accordingly, HCOOH can be produced if the radical is subsequently protonated. The HCOO− species can also be further reduced to generate HCHO and CH4.50 In addition, CO can be produced from CO2ER after the reduction by two electrons. If the resultant CO cannot be activated by electrocatalysts, CO tends to be released for producing CH3OH through a hydrogeneration process. Moreover, these intermediates can be further converted into alcohols or other hydrocarbons such as C2H4 and C2H6 through a dimerization pathway.50,52 For heterogeneous CO2ER, the reaction path is a little different. Heterogeneous CO2ER usually involves the adsorption of CO2 on the electrocatalyst surface, activation of the adsorbed CO2 by the catalyst, and protonation of the activated CO2. While homogeneous CO2ER mainly involves the absorption of CO2 in the electrolyte solvents and formation of an intermediate.
Figure 4.
Schematic illustration of homogeneous CO2ER for various products enabled by type II electrocatalysts in aqueous electrolytes, the CAT stands for the homogeneous catalysts dissolved in the electrolyte solution
Reproduced with permission from ref. 50 Copyright, Wiley-VCH.
The homogeneous CO2ER has been reported to be accomplished through one-, two-, four-, six-, and eight-electron reduction pathways.53 Accordingly, the main products such as CO, HCOOH/HCOO−, H2C2O4/C2O42−, HCHO, and CH3OH can be obtained from homogeneous CO2ER.54 Other products such as CH4, CH2CH2, and CH3CH2OH are rarely obtained as the main product but as the byproducts. Notably, the formation of different products from homogeneous CO2ER depends highly on the redox potentials. The standard redox potential for different products from homogeneous CO2ER is listed in Table 1 Generally, homogeneous CO2ER can proceed much more easily under acid conditions, as indicated by the lower required redox potential.55 This could be owing to the higher energy barrier for CO2 activation and more energy consumption in alkaline medium. For example, under alkaline conditions, more than 50% of the energy need to be used to recover CO2 from CO32− during the homogeneous CO2ER.55 As such, homogeneous CO2ER is intentionally carried out in acid electrolytes to improve overall energy efficiency.
Table 1.
Half reactions of homogeneous CO2ER for different routes along with the corresponding standard redox potential (25°C, under 1 atm of gases, in an aqueous electrolyte)
| Reaction | Eθ/V (vs. SHE) |
|---|---|
| −0.530 | |
| −1.347 | |
| −0.913 | |
| −0.610 | |
| −1.491 | |
| −0.480 | |
| −1.311 | |
| −0.380 | |
| −1.225 | |
| −0.240 | |
| −1.072 | |
| −0.340 | |
| −1.177 | |
| −0.330 | |
| −1.157 | |
| −0.270 | |
| −0.320 |
The main factors affecting homogeneous CO2 electroreduction
Since the homogeneous CO2ER is a complicated process owing to many entangled steps during the electrochemical process, many factors can influence the product formation and the final electrocatalytic performance of an electrocatalyst. These factors include but are not limited to the types and concentrations of electrocatalysts, the electrolyte types, the electrolyte additives, and the external polarization potential. In this section, we briefly discuss how these factors impact the homogeneous CO2ER.
Generally, the influence of different factors can largely account for the affected corresponding steps in the CO2ER cycle. As shown in Figure 2, the types of homogeneous electrocatalysts can mainly impact the formation of [CAT-Sub]∗ intermediates owing to the different intrinsic chemical structures and electrochemical kinetics. For example, using a dihydride complex (POCOP)Ir(H)2 (POCOP stands for C6H4(OPPh2)2) as the electrocatalyst for CO2ER, the HCOOH was produced with a Faradaic efficiency of 93% at room temperature.56 However, using homogeneous electrocatalysts based on the bipyridine (bpy) and terpyridine (tpy) complexes such as [Ru(tpy)(bpy)(MeCN)]2+ enables CO production from CO2ER with a Faradaic efficiency of over 85%.57 The reason for producing different products is mainly attributed to the formation of [CAT-Sub]∗ with significantly different structures enabled by the different homogeneous electrocatalysts. The former Ir-based electrocatalyst gives rise to the [CAT-Sub]∗ structure embedded with the formate, while the latter Ru-based electrocatalyst enables the formation of [CAT-Sub]∗ composed of CO coordinate structure. It thus can be expected that the molecular structure of electrocatalysts plays a critical role in the selective homogeneous CO2ER.
Besides, the electrolyte components including the solvent and the additives can also impact the homogeneous CO2ER. Specifically, the formation process of the important intermediates (such as the Int∗ in Figure 2) can be significantly influenced since the electrolyte has a great impact on the dissolved concentration of the CO2 substrate and the stabilization of the transition states or intermediates. For instance, the solubility of CO2 in the MeCN solution is 280 mM under 1.0 atm while this value is much lower in water (33 mM). The different concentrations of CO2 in the electrolyte can significantly affect the reaction rate of CO2ER since the CO2 substrate directly participates in the electrocatalytic reaction process.58 Besides, the nature and the composition of the solvent matter a lot because the solvent can be also involved as the reactant during the CO2ER process. In particular, the protic solvents may be favorable for the formation of hydrogen bonds with the intermediates during homogeneous CO2ER. This can affect the electrocatalytic kinetics and product formation for homogeneous CO2ER. For instance, in the case of a primary amine-substituted Re(bpy)(CO)3Cl electrocatalyst (bpy = bipyridine, Re = Rhenium), the reduction potential for Re−Re dimerization intermediate is −2.11 V (vs. Fc+/0) when the CH3CN was used as the solvent for 0.1 M TBAPF6 electrolyte (TBAPF6 = tetrabutylammonium hexafluorophosphate). However, the intermediate structure of Re−Re dimerization cannot be produced when DMF (N, N-dimethylformamide) was explored as the electrolyte solvent.59 Since CH3CN is a weakly acidic aprotic polar solvent, hydrogen bonds can hardly form. However, DMF is a basic aprotic polar solvent, enabling it to be a good hydrogen bond donor and acceptor. As such, although DMF can form hydrogen bonds with the Re(bpy)(CO)3Cl electrocatalyst, the formation of hydrogen-bonded dimers may be disrupted owing to the competition with intermolecular hydrogen bonds among DMF molecular.60 This is not favorable for hydrogen bonding-facilitated Re−Re dimerization. Therefore, the electrolyte type can also give rise to a noticeable change in the product of the homogeneous CO2ER via mediating the intermediate formation.61
The functional additives are widely employed in advanced electrolytes to promote homogeneous CO2ER since the additive could meditate the structure of the intermediates and affect the electrocatalytic process. For instance, Maurice Brookhart and co-workers reported the use of CH3CN as the favorable additive in the electrolyte to promote the CO2ER when the (POCOP)Ir(OTf) (OTf stands for Trifluoromethanesulfonate) was used as the homogeneous electrocatalyst.56 The CH3CN was revealed to be a key ancillary ligand that can play a critical role in alternating the formation of the intermediate by displacing formate from the insertion product to generate the bis-acetonitrile complex. This is important for the electrocatalytic cycling of HCOOH production from CO2ER.56 The use of water (4.0%) as the additive in a DMF electrolyte was also shown to promote the homogeneous CO2ER by an electrocatalyst based on the cobalt(II) complex with equatorial N4 ligands because the replacement of axial ligand by water ligand results in the no longer interact with the latter intermediates.62
The magnitude of the external polarization potential also significantly affects the homogeneous CO2ER process since the charge accumulation on the electrocatalyst can largely impact the CAT+ formation. Generally, the use of electrocatalysts allows homogeneous CO2ER to occur at much lower potentials lower than the redox potentials of the raw materials (CO2) under the same condition. The different strengths of external potential polarization can result in the formation of different reduced species of CO2ER by the homogeneous electrocatalyst. For instance, the CO2 can be reduced to the radicals CO2·− at a potential of −1.98 V when the DMF was used as the solvent. Such a radical cannot be activated subsequently by the electrocatalyst at such a potential. However, a further decrease in the polarization potential to −2.32 V can result in the CO2ER for HCOOH production with 68% FE.62
Type of homogeneous electrocatalysts for CO2ER
The homogeneous electrocatalyst plays the most critical role in homogeneous CO2ER as the electrocatalyst can promote electron shuttling between the electrode and CO2 for CO2ER.63 To date, the reported types of homogeneous electrocatalysts for CO2ER can be broadly divided into metal-based organic compounds and non-metal organic complexes. Compared with metal-based organic compounds, the non-metal organic complexes are much less explored and are mainly based on ionic liquids composed of alkyl imidazole cations and alkyl amine cations.64 The metal-organic compound-based electrocatalysts are usually coordinated compounds based on transition metals including Cr,65 Re,66 Fe,67 Ru,44 Ir,68,69 and Ni.70 The transition metals are mainly 3days-transition metals with variable valence. The ligands of the metal-coordinated compounds are usually nitrogen- or sulfur-containing compounds.65,67,71,72,73,74 Generally, the lone pair electrons of nitrogen or sulfur ligand can donate electrons. This can stabilize the originally active transition metal complexes to some extent.75 Furthermore, the homogeneous electrocatalysts can donate electrons under a certain voltage polarization,76 causing the saturated transition metal complexes to lose their coordination ligands for providing active coordinating sites.77 As can be seen from Figure 3, the non-bonding electrons are more likely to be localized in the 1πg orbital on the oxygen side. Thus, the electrons at the oxygen end of the CO2 molecule tend to coordinate with the metal center that is not saturated with coordination.
When the transition metal-organic complex-based electrocatalysts are used for homogeneous CO2ER, the coordinated transition metal usually works as the active center for electrocatalysis. However, not all such transition metal-based coordinates are active for homogeneous CO2ER. We deem that the following characteristics can be favorable for being a good homogeneous electrocatalyst based on the transition-metal complexes: (1) showing high intrinsic activity for CO2ER; (2) working stably under normal electrolysis conditions (e.g., aqueous electrolytes, room temperature, etc.); (3) containing unsaturated coordination and valence electrons less than 18 electrons; (4) showing good stability under air and moisture environment; and (5) achieving stable electrochemical performance at a close thermodynamic potential of CO2ER reaction.
A metal-organic compound-based electrocatalyst for homogeneous CO2ER can be rationally designed and adjusted according to the desired electrocatalytic conditions and performance by choosing a suitable metal and tuning the ligand type.78,79,80 In the recent two decades, tremendous progress has been made in the development of homogeneous electrocatalysts and optimizing the electrocatalytic performance as well as the mechanistic understanding.81,82,83 As summarized in Table 2, we present some recently developed typical metal-organic compound-based electrocatalysts for homogeneous CO2ER. We can find that most of these metal-organic compound-based electrocatalysts show typical coordinated structures. The main products obtained from the homogeneous CO2ER are still the C1 compounds such as CO and HCOOH.
Table 2.
Typical electrocatalysts recently developed for homogeneous CO2ER
| Types | Molecular structure | Main product | Potential | Faradic efficiency | Reference |
|---|---|---|---|---|---|
| Ir-based complexes84 | 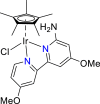 |
HCOO− | −1.80 V vs. (Ag/AgCl) | 38% | Sypaseuth et al.84 |
| Ni-based complexes85 | 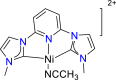 |
CO | −1.77 V vs. (SHE) | 34% | Sheng et al.85 |
| Pd-based complex86 | 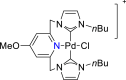 |
CO | −1.60 V vs. (Ag/AgNO3) | 85% | Therrien and Wolf86 |
| Re-based complex87 | 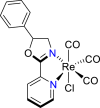 |
CO | −2.80 V vs. (Fc/Fc+) | 30-55% | Nganga et al.87 |
| Co-based complex62 | 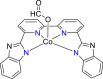 |
HCOO− | −2.32 V vs. (Fc/Fc+) | 68% | Tsubonouchi et al.62 |
| Mn-based complex88 | 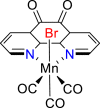 |
CO | −2.24 V vs. (Fc/Fc+) | 30-60% | Stanbury et al.88 |
| Ru-based complex89 | 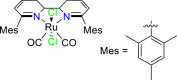 |
CO | −2.20 V vs. (Fc/Fc+) | 95% | Machan et al.89 |
| Non-metal ionic liquid complex90 | 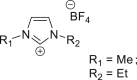 |
C2O42− | −2.60 V vs. (Fc/Fc+) | 39.3% | Rosen et al.90 |
The main products, potential, and Faradic efficiency for the corresponding electrocatalyst are also listed.
Key criterion for evaluating the performance of homogeneous CO2ER
To better quantify and evaluate the electrochemical performance of an electrocatalyst for homogeneous CO2ER, some key criteria need to be established and obtained in experiments. Several important metrics have been explored to evaluate the activity and performance of a homogeneous electrocatalyst for CO2ER. As such, we here summarized these widely used metrics including the overpotential, current density, faradic efficiency (FE), turnover frequency (TOF), turnover number, and product yield in detail.
The overpotential refers to the potential difference (voltage) between the thermodynamically determined reduction potential of a half-reaction and the potential at which the electrochemical redox reaction is observed experimentally. This value also indicates that the CO2ER process (with electricity) requires how much electrical energy than the thermodynamic process (without electricity). The lost energy could be heat and other reactions. As such, the overpotential η is an important parameter to evaluate the catalytic activity of the electrocatalyst. The overpotential is usually measured experimentally by linear sweep voltammetry (LSV) and cyclic voltammetry (CV) tests. The overpotential η can be calculated by the following equation:
Where the Et is the theoretical potential, and the Ei refers to the experimental potential in a CV measurement. When comparing the overpotentials of different catalysts in the same type of reaction, a specific current density needs to be specified (usually the current density is 10 mA cm−2). The overpotential thus is widely used to compare the intrinsic activity of an electrocatalyst. The lower the overpotential of the electrocatalyst, the generally higher the electrocatalytic activity for the target product. For instance, a homogeneous electrocatalyst Co(II) complex for CO2ER shows a good catalytic activity with an overpotential of 0.62 V for producing formic acid (at −2.07 V vs. Fc/Fc+). Without the electrocatalyst, a much larger overpotential of 0.87 V (at −2.32 V vs. Fc/Fc +) is required to proceed under the same condition.62
The current density is normally referred to as the total amount of current flowing through one-unit value of the cross-sectional area, featuring the rate of the overall electrochemical reaction. The current density can be calculated as follows:
Where the I stands for the whole current and the S is the area of the electrode. Generally, it is difficult to develop a highly active homogeneous electrocatalyst for CO2ER with high selectivity (high Faraday efficiency).91 The origin of activity and selectivity of electrocatalysts can be understood by an interplay between ensemble (or geometrical) effects and ligand (or electronic) effects.91 Under the same condition, a larger current density usually indicates the higher activity of the electrocatalysts. While the current density is widely used to compare the activity of an electrocatalyst, the intrinsic size and surface area of the electrode could also show an obvious impact on the current density for homogeneous CO2ER.92
The Faradic efficiency (FE) is another important criterion for evaluating the performance of an electrocatalyst. The FE for homogeneous CO2ER is usually defined as the ratio of the experimentally detected amount of objective products to the amount of theoretically generated products. The theoretical product production can be obtained by theoretical calculation, and the actual amount of product can be determined in practice. In a certain reaction period, the average Faraday rate can be calculated according to the following equation:
Where Qt is the electron consumption for the corresponding product, and Qi stands for the total electron consumption of the total products in this period. Because of the secondary reactions occurring during CO2ER, the FE is usually lower than 100%. Accordingly, the FE can be also used to describe electrocatalytic selectivity and energy efficiency.93
The turnover frequency (TOF) is widely a key parameter used to evaluate electrocatalytic efficiency. The TOF is derived from the turnover number, referring to the turnover per unit of time. It is defined as the number of catalytic reactions occurring on the active site per unit of time. The TOF can be calculated as follows:
Where the I(A) is the value of the catalytic current (directly given by the electrochemical workstation), α is the number of transferred electrons corresponding to the half-reaction that generates a molecule of a target product or consumes a molecule of target reactant (the reaction equation Coefficient of electrons), N (mol) is the number of catalytically active sites,94 and F (C/mol) is the Faraday constant.95
The maximal turnover number of an electrocatalyst is the maximal number of substrate molecules converted into the product in a unit of time when the electrocatalyst is fully saturated with substrate.96 This criterion can be also usually used to evaluate the stability of homogeneous electrocatalysts.97 The TON can be calculated by the following equation:
Where t is the time (the period before the catalyst completely deactivates).
Another key criterion of the CO2ER process is the yield of the target product, and the overall cost of the process can make a big difference in industrial practice. Whether in an electrochemical process or a non-electrochemical process, the calculation of the product yield is all the same. The Yield can be calculated as follows.98
Where na stands for the mole amount of the product yielded, and ne is the mole amount of the product expected.
Recent development of electrocatalysts for homogeneous CO2ER
Homogeneous CO2ER for C1 products
Theoretically, a single CO2 molecular can accept a different number of electrons to form C1 products, including CO or HCOOH (2e−), HCHO (4e−), CH3OH (6e−), and CH4 (8e−). However, the C1 products from the homogeneous CO2ER are usually CO and HCOOH. In particular, CO is the most broadly reported C1 product. Normally, a carbon dioxide-free radical (CO2·−) is produced when a single CO2 molecule accepts an electron. Afterward, the CO2·− will combine with the homogeneous electrocatalyst to produce the C1 product after reduction by two electrons. Although CO, HCOOH, and CH4 have been reported as the C1 product, producing CH4 as the main product is still a challenging task for homogeneous CO2ER. So far, CH4 is usually reported to be produced as a byproduct. As such, we focus on discussing the homogeneous CO2ER for producing CO and HCOOH.
The homogeneous CO2ER for producing CO
As shown in Figure 5A, the main process in homogeneous CO2ER to produce CO involves the following steps. The transient state CAT∗ can be first obtained after the activation of the electrocatalyst. Afterward, [CAT–CO2]∗ will be produced when the CO2 interacts with the reduced CAT∗. The attack of a proton on the [CAT–CO2]∗ gives rise to the release of an H2O molecular and the formation of [CAT–CO]∗ intermediate. Finally, the CO can be released from the catalytic cycle.
Figure 5.
Scheme for CO production from the homogeneous CO2ER
(A) A general mechanism of CO production from homogeneous CO2ER.
(B) A specific example of CO production from CO2ER by Mn(I)-Tricarbonyl species. Reproduced with permission from ref. 99 Copyright, American Chemical Society.
As a typical example, Bocarsly and coworkers revealed the detailed mechanism of CO production through CO2ER by using the Mn(I)-Tricarbonyl species as the homogeneous electrocatalyst and the 0.1 M tetrabutylammonium perchlorate (Bu4NClO4) in CH3CN as the electrolyte.99 The pathways of these carbonyl-metal complexes for the electroreduction of CO2 to CO are illustrated in Figure 5B. Specifically, the Mn(I)-Tricarbonyl component of molecular electrocatalyst can get two electrons to produce the [Mn]− compound composed of the Mn (0) species. Then the CO2 molecular can be inserted into the [Mn]− complex to form the [Mn–CO2]− intermediate with Mn (I) species. The following protonation of the [Mn–CO2]− gives rise to the formation of [Mn–CO2H]0 complexes with Mn (I) species. Afterward, the [Mn–CO]+ complexes were obtained without altering the valence of Mn (I) after the dehydration of [Mn–CO2H]0. Finally, the CO was obtained after getting two electrons. As such, the [Mn]− complex is produced again, enabling the electrocatalytic cycling process for homogeneous CO2ER.
Figure 6 shows an example of a Ru-based complex that catalyzes the reductive disproportionation of CO2 to CO32− and CO through a new mechanistic pathway. The binding of CO2 with the reduced Ru-based electrocatalyst is followed by catalytic turnover at the same potential. Electron transfer from the initially reduced tpy ligand to the coordinated CO2 enables the subsequent electron uptake at the same potential, resulting in a lower of the reduction potentials and a decrease in the overpotential for catalysis from 0.87 V to 0.47 V100,101
Figure 6.
Scheme for CO production from the homogeneous CO2ER
A specific example of CO production from CO2ER by [Ru(tpy)(bpy)(MeCN)]2+ species. Reproduced with permission from ref. 100 Copyright, John Wiley and Sons.
The homogeneous CO2ER for producing HCOOH
HCOOH is another main C1 product obtained widely from homogeneous CO2ER. The main process for the homogeneous CO2ER into HCOOH involves the following steps. As shown in Figure 7A, CAT∗ intermediate can be first produced from the reduction of electrocatalyst after the elimination of the ligand. Then the hydrogen coordination with the central metal of molecular electrocatalysts can give rise to the hydride species in the presence of H+. Afterward, CO2 and H2O can simultaneously interact with the [CAT–H]∗ species to produce the [CAT–CO2–H2O]∗. Due to the strong interaction of hydrogen bonds, the aggregation of [CAT–CO2–H2O]∗ can occur as a result of producing an eight-ring intermediate. Finally, an acceptance of an H+ can result in the release of HCOOH and the completion of the electrocatalytic cycling process.
Figure 7.
Scheme for HCOOH production from homogeneous CO2ER
(A) A general mechanism of HCOOH production from homogeneous CO2ER.
(B) A specific example of HCOOH production from CO2ER by a Co (III) species-based electrocatalyst. Reproduced with permission from ref. 102 Copyright, American Chemical Society.
For example, Vincent Artero and coworkers reported the highly efficient and selective cobalt-based electrocatalysts for HCOOH production from the homogeneous CO2ER.102 These electrocatalysts exhibited impressive electrocatalytic performance with a low overpotential and fewer byproducts such as H2 and CO.102 The revealed electrocatalytic mechanism for the HCOOH formation is illustrated in Figure 7B. They carried out the homogeneous CO2ER with a glassy carbon electrode in 0.1 M Bu4NBF4 (DMF) electrolyte. The Co (III) species of the electrocatalyst (I) can be reduced into Co (II) species (II) after receiving one electron from the glassy electrode. After getting one more electron, the Co (I) species (III) was produced. The protonated Co (I) species (VI) can be further reduced to produce a Co (II)-hydride species (V) for chelating CO2. The resultant Co (II)–H intermediate (V) can be attacked by CO2 and H2O for generating the [H2O–Co(II)–H–CO2] (VI) intermediates. The internal hydride transfer from Co(II)–H to CO2 can give rise to the release of HCOOH, enabling the completion of the electrocatalytic cycles. Since the Co (III)-based electrocatalyst (I) is air-stable and can be easily handled, it shows great potential for future practical applications.
Meyer and coworkers reported Iridium dihydride complexes supported by PCP-type pincer ligands as the electrocatalyst in Figure 8. In acetonitrile/water mixtures, these complexes (POCOP)Ir(H)2 become efficient and selective catalysts for the electrocatalytic reduction of CO2 to formate.103 Besides, another earth-abundant molecular electrocatalyst based on carbonyl cluster [Fe4N(CO)12]− was reported for CO2ER to produce HCOOH.104 When a mixed solvent of MeCN and water (5%) was used, the HCCOH was obtained in a current density of 0.7 mA/cm2 with an FE of 94%. One of the main processes for the homogeneous CO2ER to produce HCOOH by a carbonyl cluster [Fe4N(CO)12]− is the formation of a Fe–H–Fe intermediate. Then the internal hydride transfer to CO2 can give rise to the release of HCOOH, similar to that for the above-mentioned Co (III) species.104
Figure 8.
Scheme for HCOOH production from homogeneous CO2ER
A specific example of HCOOH production from CO2ER by a (POCOP)Ir(H)2 electrocatalyst. Reproduced with permission from ref. 103 Copyright, American Chemical Society.
The homogeneous CO2ER for multicarbon (C2) products
The product with two carbon (C2) products can be also obtained from the homogeneous CO2ER. Among various C2+ products, oxalic acid (H2C2O4) is the most widely obtained and valuable since it is an important chemical for pharmaceutical, rare-earth extraction, and metal processing. Typically, the ionic liquid-based electrocatalysts for CO2ER generally involve the following process to produce H2C2O4. First, the aromatic ester anion functionalized ionic liquids can be reduced to produce the CAT∗ intermediate containing active sites for CO2·− binding. The resultant CAT∗ complex can further interact with CO2 and H+ to generate the [CAT-COOH]∗ intermediate (Figure 9A). The H2C2O4 can be finally produced after a C-O bond breaking and dimerization of COO−.105
Figure 9.
Scheme for H2C2O4 (oxalic acid) production from homogeneous CO2ER
(A) A general mechanism of H2C2O4 formation from homogeneous CO2ER.
(B) A specific example of the H2C2O4 production from homogeneous CO2ER by ionic liquids-based electrocatalysts. Reproduced with permission from ref. 105 Copyright, John Wiley and Sons.
For instance, Gennaro et al. reported the use of aromatic ester or aromatic nitrile-based electrocatalysts for efficient CO2ER into oxalate product of an FE over 99% by carrying out in the 0.1 M n-Bu4NClO4-DMF aprotic electrolyte.106 However, the current density was only 1.6 mA cm−2. The oxalate formation rate was smaller than 30 μmol cm−2 h−1. In another case, Zhang et al. developed aromatic ester anion functionalized ionic liquids as homogeneous electrocatalysts.105 A high current density of 9.03 mA cm−2 was obtained with an oxalic acid formation rate of 168.4 μmol cm−2 h−1. As shown in Figure 9B, the proposed mechanism for high-efficiency CO2ER by the aromatic ester anion functionalized ionic liquids. Specifically, the ionic liquid with phenoxy and ester double active sites can effectively activate the stable linear CO2 molecular into CO2·−. Besides, CO2 can be activated to form a new C−O single bond with the O atom. The newly formed C−O bond can be successively broken as a result of generating the parent aromatic ester ionic liquid and −COOH intermediate. The resultant −COOH can be eventually dimerized to produce oxalic acid and complete the catalytic cycles.
Homogeneous CO2ER for other C2+ value-added products
The above discussion mainly focuses on the C1 and C2+ products widely obtained from homogeneous CO2ER. Furthermore, other value-added products can be also obtained from the homogeneous CO2ER owing to the coupling of CO2 molecules. For instance, CH3CH2OH is one of the most important chemical feedstocks and fuel for conventional combustion engines or fuel cells. CH3CH2OH can be also produced from homogeneous CO2ER through the following steps. One electron transfer to CO2 can lead to the generation of CO2·− intermediate with the specific homogeneous electrocatalysts (CAT). The resultant [CAT–CO]∗ intermediate can give rise to CO production. When the CO is not captured by the electrocatalyst, CH3OH can be produced through a hydrogeneration process.52
For instance, Nakajima et al. reported the first catalytic formation of CH3COCH3 by the double methylation of the carbonyl moiety with FE 16%. The reduction in the presence of Me4NBF4 enables the formation of CH3COCH3 in CH3CN/DMSO (1:1, v/v). The final product results from the reductive disproportionation of CO2 followed by the subsequent carboxylation in the electrochemical CO2 reduction.107 As shown in Figure 10B, the proposed mechanism for the CH3COCH3 formation. Specifically, the Ru(0) complex can effectively bind the CO2 to form [Ru−CO2]0. Besides, [Ru−CO2]0 can further promote the formation of the species of CO32− and [Ru−CO]2+. Through two electrons transfer, the [Ru−CO]0 forms and the (CH3)4N+ functions as the methylation agent of the carbonyl moiety. The reductive disproportionation of CO2 can subsequently undergo a carboxylation in the electrochemical CO2 reduction. This enables the formation of CH3COCH3 product.
Figure 10.
Scheme for CH3COCH3 (acetone) production from homogeneous CO2ER
(A) A general mechanism of CH3COCH3 formation from homogeneous CO2ER.
(B) A specific example of the CH3COCH3 production from homogeneous CO2ER by Ru-based electrocatalysts.107 Reproduced with permission from ref. 107 Copyright, American Chemical Society.
In addition, other C2+ products such as hydrocarbons (C2H4, and C2H6) are highly sought after as potential sustainable fuels, especially methane, and ethylene due to their high energy densities and extensive application. The formation of C2+ products is usually produced through the transfer of two or more electrons to the [CAT–COOH]∗ intermediates. The further dimerization of the [CAT–COOH]∗ intermediates can give rise to the hydrocarbons such as C2H4 (Cu porphyrin complex as electrocatalyst, faradic efficiency 44%, current density 8.4 mA/cm2) and C2H6 (Cu nanowires as electrocatalyst, faradic efficiency 20.3%, current density 4–5 mA/cm2).50 Although the above-mentioned value-added products have been reported from heterogeneous CO2ER methods, achieving high selectivity for value-added products is still challenging. The typical homogeneous CO2ER electrocatalysts are summarized in Table 3.
Table 3.
The summarized typical homogeneous electrocatalysts for CO2ER
| Electrocatalyst | TOF | Main product | Potential | Electrolyte | Reference |
|---|---|---|---|---|---|
| Mn(I)-Tricarbonyl complex99 | 119 s−1 | CO | −1.30 V vs. SCE | 1.1 M Bu4NClO4 (CH3CN) |
Agarwal et al.99 |
| [Ru(tpy)(bpy)(MeCN)]2+ species100,101 | Not mentioned | CO | −1.92 V vs. Fc+/0 | 0.1 M Bu4NPF6 (CH3CN) | Johnson et al.,100 Johnson et al.101 |
| Co (III) species102 | 150 s−1 | HCOOH | −2.15 V vs. Fc+/0 | 1.1 M Bu4NBF4 (DMF) |
Roy et al.102 |
| (POCOP)Ir(H)2 complex103 | Not mentioned | HCOO− | −1.2 V vs. NHE | 5% H2O/THF | Kang et al.103 |
| Ionic liquids species105 | Not mentioned | H2C2O4 | −2.60 V vs. (Ag/AgNO3) | 1.1 M Bu4NClO4 (DMF) |
Yang et al.105 |
| Ru-based species107 | Not mentioned | CH3C(O)CH3 | −1.60 V vs. (Ag/AgNO3) | 0.1 M Bu4NPF6 (CH3CN) | Nakajima et al.107 |
Conclusions and perspectives
To conclude, we here present the fundamentals of homogeneous CO2ER and highlight the recent advances in the development of efficient homogeneous electrocatalysts. So far, the transition metal coordinate complexes and non-metal ionic liquids are the most widely explored homogeneous electrocatalysts for CO2ER. Numerous factors can significantly influence the homogeneous CO2ER process and product formation. In particular, the type and concentration of a homogeneous electrocatalyst can affect the chemical structures and electrochemical kinetics of the [CAT-Sub]∗ intermediates. The electrolytes can also impact the concentration of the dissolved CO2 and the stabilization of the transition states or intermediates. The external polarization potential can largely impact the important intermediate CAT+ formation by changing the charge accumulation on the electrocatalyst. The C1 compound such as the CO or HCOOH is generally the main product when using transition metal coordinates-based electrocatalysts while H2C2O4 is usually the main C2+ product when using non-metal ionic liquids-based electrocatalysts. Although numerous homogeneous electrocatalysts have been developed for efficient CO2ER, the following challenges are expected to be addressed in future studies for promoting this promising sustainable technology.
-
(1)
The exact mechanism of homogeneous CO2ER for producing a specific product is still waiting to be explored in-depth. In particular, the capture and identification of some key intermediates are still challenging for both theoretical simulation and experiments owing to the complicated and interplayed multistep processes of homogeneous CO2ER. As such, more multiscale simulations and advanced Operando experimental techniques need to be further developed to reveal the exact mechanism in detail. While previous mechanistic studies through experiments largely relied entirely on electroanalytical techniques, more emerging advanced techniques such as spectro-electrochemistry (such as surface-enhanced infrared spectroscopy,108 and surface-enhanced Raman scattering,109 have been intensively used to probe the structure and composition of intermediates during CO2ER.110 As for the theoretical study, the combined use of multiscale simulation including the density functional theory,111,112 finite element simulations,113 and machine learning114 will be highly expected for future efforts to reveal the exact reaction mechanism at different scales. As such, it is practicable to rationally design efficient homogeneous electrocatalysts for selective CO2ER toward on-demand products.
-
(2)
The precise design and scalable preparation of efficient homogeneous electrocatalysts for the on-demand products from CO2ER are still challenging tasks. As discussed above, although many valuable products can be obtained from homogeneous CO2ER, these products are usually produced simultaneously with low selectivity. As such, tremendous efforts are expected to develop efficient electrocatalysts with high selectivity. This requires insightful knowledge of the mechanism for homogeneous CO2ER as well as the establishment of the exact structure-function relationship of homogeneous electrocatalysts. Although homogeneous CO2ER shows the advantages of controlling the reaction process by a rational design of the molecular structure of an electrocatalyst, efficient separation of electrocatalysts and various products from the homogeneous system also needs to be extensively explored. Accordingly, considerable efforts need to be paid to the predictive design of selective electrocatalysts that can be easily separated from the electrolyte for recycling use.115
-
(3)
The criterion for evaluating the specific electrochemical performance under the standard experimental operations are also needed to be strictly established and implemented for studying homogeneous CO2ER. Many factors like the nature of the electrocatalyst, the composition of the electrolyte, the size and hydrodynamics of the electrolytic cell, and the purity of the reagent, show an impact on the electrochemical performance of homogeneous electrocatalysts for CO2ER. Therefore, it is crucial to establish standard experimental methods and protocols that can be explored to exactly evaluated the electrocatalytic performance of a homogeneous electrocatalyst. Designing and carrying out the benchmark electrocatalytic tests to exclusively study the influence of the electrocatalyst composition and structure will be preferred for future study. Before evaluating the electrocatalytic performance of new catalysts, using unified test devices and experimental conditions to assess the capability of conducting accurate and repeatable activity measurements is of great importance to compare the overall electrochemical performance. For example, thoroughly mixing the electrolyte to lessen the impact of mass transfer on the intrinsic activity can reduce the experimental error to some degree.116 The activity and selectivity of the electrocatalysts should be also accurately determined without surface contamination. Therefore, future efforts are expected to develop better electrocatalytic systems and investigate the factors that can improve the accuracy of electrocatalytic activity evaluation parameters.
-
(4)
Last but not least, the scale-up for the preparation of electrocatalysts and scalable implementation of homogeneous CO2ER is an also formidable task but highly desired for future industrial applications. The large-scale preparation of electrocatalysts with low-cost and high efficiency is essential for the scaling up of homogeneous CO2ER for industrial applications in the future.117 Although many studies have reported the preparation of efficient electrocatalysts on the lab scale,118 more efforts are expected to develop large setups and automatic processes to achieve industrialized production.119 Besides, it is necessary to improve the product selectivity of various homogeneous electrocatalysts by optimizing the experimental conditions. Another important but challenging aspect is scaling up the implementation of homogeneous CO2ER for mass production because the multi-step product separation of homogeneous electrocatalysis complicates the integration with electrolytic technologies. In this regard, more efforts will be needed to develop advanced systems capable of continuous production and separation for future industrial applications.120
In summary, the rational design of efficient electrocatalysts for homogeneous CO2ER is of significance to advance this exciting and burgeoning technology. The importance of the second coordination sphere has been increasingly recognized and has pushed ligand design in new directions. Metal–ligand cooperativity, ligand-based proton relays, and functional ionic groups that enhance catalysis through Coulombic interactions. These advancements have helped to establish new benchmarks and will continue to drive further progress.
With respect to the metal center, the focus has shifted from precious elements of the second and third d-block series, such as Ru, Pd, and Re, to more abundant 3d metals such as Mn, Fe, Co, and Ni. It has been demonstrated that these 3d metals can be highly competitive when appropriate ligands are used. The development of new ligands that are tailored to specific applications has opened up new possibilities for ligands that previously had not been considered for complexation with 3d metals.
The integration of computational methods and experimental approaches has allowed for a better understanding of reaction mechanisms and has facilitated the rational design of new catalysts. The future of coordination chemistry lies in the continued exploration of new ligands and metal centers, as well as in the development of more efficient and sustainable catalytic systems.
Although the study on homogeneous CO2ER is still much behind the research on heterogeneous CO2ER, significant advances will be expected over the coming years due to the above-mentioned numerous opportunities. We hope this perspective will provide favorable guidance for the research community in the rational design of more efficient homogeneous electrocatalysts for selective CO2ER in the future.
Acknowledgments
Q. Liang thanks the financial support from the Research Projects of Ganjiang Innovation Academy (No. E355F003), Chinese Academy of Sciences (CAS).
Declaration of interests
The authors declare no competing interests.
Contributor Information
Qinghua Liang, Email: qhliang@gia.cas.cn.
Ke Xie, Email: ke-xie@northwestern.edu.
References
- 1.Zhang S., Chen W. Assessing the energy transition in China towards carbon neutrality with a probabilistic framework. Nat. Commun. 2022;13:87. doi: 10.1038/s41467-021-27671-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mallapaty S. How China could be carbon neutral by mid-century. Nature. 2020;586:482–483. doi: 10.1038/d41586-020-02927-9. [DOI] [PubMed] [Google Scholar]
- 3.Su Y., Kinjo R. small molecule activation by boron-containing heterocycles. Chem. Soc. Rev. 2019;48:3613–3659. doi: 10.1039/c9cs00072k. [DOI] [PubMed] [Google Scholar]
- 4.Pal A., Vanka K. Small molecule activation by constrained phosphorus compounds: insights from theory. Inorg. Chem. 2016;55:558–565. doi: 10.1021/acs.inorgchem.5b01074. [DOI] [PubMed] [Google Scholar]
- 5.Chapman A.M., Haddow M.F., Wass D.F. Frustrated Lewis pairs beyond the main group: synthesis, reactivity, and small molecule activation with cationic zirconocene–phosphinoaryloxide complexes. J. Am. Chem. Soc. 2011;133:18463–18478. doi: 10.1021/ja207936p. [DOI] [PubMed] [Google Scholar]
- 6.Zanardo D., Forghieri G., Tieuli S., Ghedini E., Menegazzo F., Di Michele A., Cruciani G., Signoretto M. Effects of SiO2-based scaffolds in TiO2 photocatalyzed CO2 reduction. Catal. Today. 2022;387:54–60. [Google Scholar]
- 7.Cheng L., Yue X., Fan J., Xiang Q. Site-specific electron-driving observations of CO2-to-CH4 photoreduction on Co-doped CeO2/crystalline carbon nitride S-scheme heterojunctions. Adv. Mater. 2022;34 doi: 10.1002/adma.202200929. [DOI] [PubMed] [Google Scholar]
- 8.Wang X.-Z., Meng S.-L., Chen J.-Y., Wang H.-X., Wang Y., Zhou S., Li X.-B., Liao R.-Z., Tung C.-H., Wu L.-Z. Mechanistic insights into iron(II) bis(pyridyl)amine-bipyridine skeleton for selective CO2 photoreduction. Angew. Chem. Int. Ed. 2021;60:26072–26079. doi: 10.1002/anie.202107386. [DOI] [PubMed] [Google Scholar]
- 9.Ouyang T., Wang H.J., Huang H.H., Wang J.W., Guo S., Liu W.J., Zhong D.C., Lu T.B. Dinuclear Metal Synergistic Catalysis Boosts Photochemical CO2 to CO Conversion. Angew. Chem. Int. Ed. 2018;57:16480–16485. doi: 10.1002/anie.201811010. [DOI] [PubMed] [Google Scholar]
- 10.Luo Z., Hou Y., Zhang J., Wang S., Wang X. Bioinspired cobalt cubanes with tunable redox potentials for photocatalytic water oxidation and CO2 reduction. Beilstein J. Org. Chem. 2018;14:2331–2339. doi: 10.3762/bjoc.14.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le J.M., Bren K.L. Engineered enzymes and bioinspired catalysts for energy conversion. ACS Energy Lett. 2019;4:2168–2180. [Google Scholar]
- 12.Möhle S., Zirbes M., Rodrigo E., Gieshoff T., Wiebe A., Waldvogel S.R. Modern electrochemical aspects for the synthesis of value-added organic products. Angew. Chem. Int. Ed. 2018;57:6018–6041. doi: 10.1002/anie.201712732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan M., Kawamata Y., Baran P.S. Synthetic organic electrochemistry: calling all engineers. Angew. Chem. Int. Ed. 2018;57:4149–4155. doi: 10.1002/anie.201707584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollok D., Waldvogel S.R. Electro-organic synthesis – a 21st century technique. Chem. Sci. 2020;11:12386–12400. doi: 10.1039/d0sc01848a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu N., Sauer G.S., Saha A., Loo A., Lin S. Metal-catalyzed electrochemical diazidation of alkenes. Science. 2017;357:575–579. doi: 10.1126/science.aan6206. [DOI] [PubMed] [Google Scholar]
- 16.Peters B.K., Rodriguez K.X., Reisberg S.H., Beil S.B., Hickey D.P., Kawamata Y., Collins M., Starr J., Chen L., Udyavara S., et al. Scalable and safe synthetic organic electroreduction inspired by Li-ion battery chemistry. Science. 2019;363:838–845. doi: 10.1126/science.aav5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horn E.J., Rosen B.R., Chen Y., Tang J., Chen K., Eastgate M.D., Baran P.S. Scalable and sustainable electrochemical allylic C–H oxidation. Nature. 2016;533:77–81. doi: 10.1038/nature17431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdinejad M., Hossain M.N., Kraatz H.-B. Homogeneous and heterogeneous molecular catalysts for electrochemical reduction of carbon dioxide. RSC Adv. 2020;10:38013–38023. doi: 10.1039/d0ra07973a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdinejad M., Seifitokaldani A., Dao C., Sargent E.H., Zhang X.A., Kraatz H.B. Enhanced electrochemical reduction of CO2 catalyzed by cobalt and iron amino porphyrin complexes. ACS Appl. Energy Mater. 2019;2:1330–1335. [Google Scholar]
- 20.Ait Ahsaine H., Zbair M., BaQais A., Arab M. CO2 electroreduction over metallic oxide, carbon-based, and molecular catalysts: a mini-review of the current advances. Catalysts. 2022;12:450. [Google Scholar]
- 21.Das B., Jia C., Ching K., Bhadbhade M., Chen X., Ball G.E., Colbran S.B., Zhao C. Ruthenium Complexes in Homogeneous and Heterogeneous Catalysis for Electroreduction of CO2. ChemCatChem. 2020;12:1292–1296. [Google Scholar]
- 22.Saha P., Amanullah S., Dey A. Selectivity in electrochemical CO2 reduction. Acc. Chem. Res. 2022;55:134–144. doi: 10.1021/acs.accounts.1c00678. [DOI] [PubMed] [Google Scholar]
- 23.Zheng H., Yang Z., Kong X., Geng Z., Zeng J. Progresses on carbon dioxide electroreduction into methane. Chin. J. Catal. 2022;43:1634–1641. [Google Scholar]
- 24.Ozden A., Li J., Kandambeth S., Li X.-Y., Liu S., Shekhah O., Ou P., Zou Finfrock Y., Wang Y.-K., Alkayyali T., et al. Energy- and carbon-efficient CO2/CO electrolysis to multicarbon products via asymmetric ion migration–adsorption. Nat. Energy. 2023;8:179–190. [Google Scholar]
- 25.Dinh C.-T., Burdyny T., Kibria M.G., Seifitokaldani A., Gabardo C.M., García de Arquer F.P., Kiani A., Edwards J.P., De Luna P., Bushuyev O.S., et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science. 2018;360:783–787. doi: 10.1126/science.aas9100. [DOI] [PubMed] [Google Scholar]
- 26.García de Arquer F.P., Dinh C.-T., Ozden A., Wicks J., McCallum C., Kirmani A.R., Nam D.-H., Gabardo C., Seifitokaldani A., Wang X., et al. CO2 electrolysis to multicarbon products at activities greater than 1 A cm-2. Science. 2020;367:661–666. doi: 10.1126/science.aay4217. [DOI] [PubMed] [Google Scholar]
- 27.Ding P., Zhao H., Li T., Luo Y., Fan G., Chen G., Gao S., Shi X., Lu S., Sun X. Metal-based electrocatalytic conversion of CO2 to formic acid/formate. J. Mater. Chem. A. 2020;8:21947–21960. [Google Scholar]
- 28.Yang K., Kas R., Smith W.A., Burdyny T. Role of the Carbon-Based Gas Diffusion Layer on Flooding in a Gas Diffusion Electrode Cell for Electrochemical CO2 Reduction. ACS Energy Lett. 2021;6:33–40. [Google Scholar]
- 29.Spurgeon J.M., Kumar B. A comparative technoeconomic analysis of pathways for commercial electrochemical CO2 reduction to liquid products. Energy Environ. Sci. 2018;11:1536–1551. [Google Scholar]
- 30.Miao R.K., Xu Y., Ozden A., Robb A., O’Brien C.P., Gabardo C.M., Lee G., Edwards J.P., Huang J.E., Fan M., et al. Electroosmotic flow steers neutral products and enables concentrated ethanol electroproduction from CO2. Joule. 2021;5:2742–2753. [Google Scholar]
- 31.Xu A., Hung S.-F., Cao A., Wang Z., Karmodak N., Huang J.E., Yan Y., Sedighian Rasouli A., Ozden A., Wu F.-Y., et al. Copper/alkaline earth metal oxide interfaces for electrochemical CO2-to-alcohol conversion by selective hydrogenation. Nat. Catal. 2022;5:1081–1088. [Google Scholar]
- 32.Samu A.A., Kormányos A., Kecsenovity E., Szilágyi N., Endrődi B., Janáky C. Intermittent Operation of CO2 Electrolyzers at Industrially Relevant Current Densities. ACS Energy Lett. 2022;7:1859–1861. doi: 10.1021/acsenergylett.2c00923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang F., Wang Q. Redox-Mediated Water Splitting for Decoupled H2 Production. ACS Mater. Lett. 2021;3:641–651. [Google Scholar]
- 34.Zhang F., Zhang H., Salla M., Qin N., Gao M., Ji Y., Huang S., Wu S., Zhang R., Lu Z., Wang Q. Decoupled Redox Catalytic Hydrogen Production with a Robust Electrolyte-Borne Electron and Proton Carrier. J. Am. Chem. Soc. 2021;143:223–231. doi: 10.1021/jacs.0c09510. [DOI] [PubMed] [Google Scholar]
- 35.Zhang F., Wang Q. Redox-mediated electrocatalytic and photocatalytic hydrogen production. Curr. Opin. Electrochem. 2022;35 [Google Scholar]
- 36.Rausch B., Symes M.D., Chisholm G., Cronin L. Decoupled catalytic hydrogen evolution from a molecular metal oxide redox mediator in water splitting. Science. 2014;345:1326–1330. doi: 10.1126/science.1257443. [DOI] [PubMed] [Google Scholar]
- 37.Savéant J.M. Molecular Catalysis of Electrochemical Reactions. Mechanistic Aspects. Chem. Rev. 2008;108:2348–2378. doi: 10.1021/cr068079z. [DOI] [PubMed] [Google Scholar]
- 38.Francke R., Little R.D. Redox catalysis in organic electrosynthesis: basic principles and recent developments. Chem. Soc. Rev. 2014;43:2492–2521. doi: 10.1039/c3cs60464k. [DOI] [PubMed] [Google Scholar]
- 39.Zhang S., Fan Q., Xia R., Meyer T.J. CO2 reduction: from homogeneous to heterogeneous electrocatalysis. Acc. Chem. Res. 2020;53:255–264. doi: 10.1021/acs.accounts.9b00496. [DOI] [PubMed] [Google Scholar]
- 40.Li X., Yang B., Wu Y., Lin S., Zhang L. Homogeneous Co3O4 film electrode with enhanced oxygen evolution electrocatalysis via surface reduction. Chin. J. Chem. Eng. 2021;29:221–227. [Google Scholar]
- 41.Liu M., Li N., Cao S., Wang X., Lu X., Kong L., Xu Y., Bu X.H. A "pre-constrained metal twins" strategy to prepare efficient dual-metal-atom catalysts for cooperative oxygen electrocatalysis. Adv. Mater. 2022;34 doi: 10.1002/adma.202107421. [DOI] [PubMed] [Google Scholar]
- 42.Wang L., Fan F., Liu J., Cheng M. Homogeneous electrocatalytic CO2 reduction by hexacarbonyl diiron dithiolate complex bearing hydroquinone. J. Organomet. Chem. 2021;954–955:122094. [Google Scholar]
- 43.Li J., Chen G., Zhu Y., Liang Z., Pei A., Wu C.-L., Wang H., Lee H.R., Liu K., Chu S., Cui Y. Efficient electrocatalytic CO2 reduction on a three-phase interface. Nat. Catal. 2018;1:592–600. [Google Scholar]
- 44.Li T.T., Shan B., Xu W., Meyer T.J. Electrocatalytic CO2 reduction with a ruthenium catalyst in solution and on nanocrystalline TiO2. ChemSusChem. 2019;12:2402–2408. doi: 10.1002/cssc.201900730. [DOI] [PubMed] [Google Scholar]
- 45.Su X., Yang X.F., Huang Y., Liu B., Zhang T. Single-Atom Catalysis toward Efficient CO2 Conversion to CO and Formate Products. Acc. Chem. Res. 2019;52:656–664. doi: 10.1021/acs.accounts.8b00478. [DOI] [PubMed] [Google Scholar]
- 46.Jin S., Hao Z., Zhang K., Yan Z., Chen J. Advances and Challenges for the Electrochemical Reduction of CO2 to CO: From Fundamentals to Industrialization. Angew. Chem. Int. Ed. 2021;60:20627–20648. doi: 10.1002/anie.202101818. [DOI] [PubMed] [Google Scholar]
- 47.D'Alessandro D.M., Smit B., Long J.R. Carbon dioxide capture: prospects for new materials. Angew. Chem. Int. Ed. 2010;49:6058–6082. doi: 10.1002/anie.201000431. [DOI] [PubMed] [Google Scholar]
- 48.Gutsev G.L., Bartlett R.J., Compton R.N. Electron affinities of CO2, OCS, and CS2. J. Chem. Phys. 1998;108:6756–6762. [Google Scholar]
- 49.Wang L.-S., Reutt J.E., Lee Y.T., Shirley D.A. High resolution UV photoelectron spectroscopy of CO+2, COS+ and CS+2 using supersonic molecular beams. J. Electron Spectros. Relat. Phenomena. 1988;47:167–186. [Google Scholar]
- 50.Zhang W., Hu Y., Ma L., Zhu G., Wang Y., Xue X., Chen R., Yang S., Jin Z. Progress and perspective of electrocatalytic CO2 reduction for renewable carbonaceous fuels and chemicals. Adv. Sci. 2018;5 doi: 10.1002/advs.201700275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Birdja Y.Y., Pérez-Gallent E., Figueiredo M.C., Göttle A.J., Calle-Vallejo F., Koper M.T.M. Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels. Nat. Energy. 2019;4:732–745. [Google Scholar]
- 52.Ding P., Zhao H., Li T., Luo Y., Fan G., Chen G., Gao S., Shi X., Lu S., Sun X. Metal-based electrocatalytic conversion of CO2 to formic acid/formate. J. Mater. Chem. A. 2020;8:21947–21960. [Google Scholar]
- 53.Sun Z., Ma T., Tao H., Fan Q., Han B. Fundamentals and challenges of electrochemical CO2 reduction using two-dimensional materials. Chem. 2017;3:560–587. [Google Scholar]
- 54.Qiao J., Liu Y., Hong F., Zhang J. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem. Soc. Rev. 2014;43:631–675. doi: 10.1039/c3cs60323g. [DOI] [PubMed] [Google Scholar]
- 55.Huang J.E., Li F., Ozden A., Sedighian Rasouli A., García de Arquer F.P., Liu S., Zhang S., Luo M., Wang X., Lum Y., et al. CO2 electrolysis to multicarbon products in strong acid. Science. 2021;372:1074–1078. doi: 10.1126/science.abg6582. [DOI] [PubMed] [Google Scholar]
- 56.Kang P., Meyer T.J., Brookhart M. Selective electrocatalytic reduction of carbon dioxide to formate by a water-soluble iridium pincer catalyst. Chem. Sci. 2013;4:3497–3502. doi: 10.1002/anie.201310722. [DOI] [PubMed] [Google Scholar]
- 57.Chen Z., Concepcion J.J., Brennaman M.K., Kang P., Norris M.R., Hoertz P.G., Meyer T.J. Splitting CO2 into CO and O2 by a single catalyst. Proc. Natl. Acad. Sci. USA. 2012;109:15606–15611. doi: 10.1073/pnas.1203122109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Creutz C., Chou M.H. Rapid transfer of hydride ion from a ruthenium complex to C1 species in water. J. Am. Chem. Soc. 2007;129:10108–10109. doi: 10.1021/ja074158w. [DOI] [PubMed] [Google Scholar]
- 59.Hellman A.N., Haiges R., Marinescu S.C. Rhenium bipyridine catalysts with hydrogen bonding pendant amines for CO2 reduction. Dalton Trans. 2019;48:14251–14255. doi: 10.1039/c9dt02689d. [DOI] [PubMed] [Google Scholar]
- 60.Machan C.W., Chabolla S.A., Yin J., Gilson M.K., Tezcan F.A., Kubiak C.P. Supramolecular Assembly Promotes the Electrocatalytic Reduction of Carbon Dioxide by Re(I) Bipyridine Catalysts at a Lower Overpotential. J. Am. Chem. Soc. 2014;136:14598–14607. doi: 10.1021/ja5085282. [DOI] [PubMed] [Google Scholar]
- 61.Hellman A.N., Haiges R., Marinescu S.C. Influence of Intermolecular hydrogen bonding interactions on the electrocatalytic reduction of CO2 to CO by 6,6 '-amine substituted rhenium bipyridine complexes. Chemelectrochem. 2021;8:1864–1872. [Google Scholar]
- 62.Tsubonouchi Y., Takahashi D., Berber M.R., Mohamed E.A., Zahran Z.N., Alenad A.M., Althubiti N.A., Yagi M. Highly selective electrocatalysis for carbon dioxide reduction to formic acid by a Co(II) complex with an equatorial N4 ligand. Electrochim. Acta. 2021;387 [Google Scholar]
- 63.Francke R., Schille B., Roemelt M. Homogeneously catalyzed electroreduction of carbon dioxide-methods, mechanisms, and catalysts. Chem. Rev. 2018;118:4631–4701. doi: 10.1021/acs.chemrev.7b00459. [DOI] [PubMed] [Google Scholar]
- 64.Mena S., Guirado G. Electrochemical tuning of CO2 reactivity in ionic liquids using different cathodes: from oxalate to carboxylation products. Chimia. 2020;6:34. [Google Scholar]
- 65.Lei K., Yu Xia B. Electrocatalytic CO2 reduction: from discrete molecular catalysts to their integrated catalytic materials. Chem. Eur J. 2022;28 doi: 10.1002/chem.202200141. [DOI] [PubMed] [Google Scholar]
- 66.Rotundo L., Azzi E., Deagostino A., Garino C., Nencini L., Priola E., Quagliotto P., Rocca R., Gobetto R., Nervi C. Electronic effects of substituents on fac-M(bpy-R)(CO)3 (M = Mn, Re) complexes for homogeneous CO2 electroreduction. Front. Chem. 2019;7:417. doi: 10.3389/fchem.2019.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guo K., Lei H., Li X., Zhang Z., Wang Y., Guo H., Zhang W., Cao R. Alkali metal cation effects on electrocatalytic CO2 reduction with iron porphyrins. Chin. J. Catal. 2021;42:1439–1444. [Google Scholar]
- 68.Kanega R. In: Iridium catalysts for organic reactions. Oro L.A., Claver C., editors. SpringerLink; 2021. Electroreduction of carbon dioxide by homogeneous iridium catalysts; pp. 325–339. [Google Scholar]
- 69.Sheehan, S.W. , Hintermair, U. , Thomsen, J.M. , Brudvig, W., and Crabtree, R.H. (2017) Iridium complexes for electrocatalysis. patent US20150021194.
- 70.Gerschel P., Battistella B., Siegmund D., Ray K., Apfel U.P. Electrochemical CO2 reduction-the effect of chalcogenide exchange in Ni-isocyclam complexes. Organometallics. 2020;39:1497–1510. [Google Scholar]
- 71.Huang Y., He H., Liu J., Thummel R.P., Tong L. Electrocatalytic CO2 reduction by molecular ruthenium complexes with polypyridyl ligands. Chem. Asian J. 2022;17 doi: 10.1002/asia.202200217. [DOI] [PubMed] [Google Scholar]
- 72.Dou S., Sun L., Xi S., Li X., Su T., Fan H.J., Wang X. Enlarging the pi-Conjugation of Cobalt Porphyrin for Highly Active and Selective CO2 Electroreduction. ChemSusChem. 2021;14:2126–2132. doi: 10.1002/cssc.202100176. [DOI] [PubMed] [Google Scholar]
- 73.Huang M., Gong S., Wang C., Yang Y., Jiang P., Wang P., Hu L., Chen Q. Lewis-basic EDTA as a highly active molecular electrocatalyst for CO2 reduction to CH4. Angew. Chem. Int. Ed. 2021;60:23002–23009. doi: 10.1002/anie.202110594. [DOI] [PubMed] [Google Scholar]
- 74.Okumura A., Ghana P., Fink F., Schmidt R., Hoffmann A., Spaniol T.P., Herres-Pawlis S., Okuda J. Formate complexes of tri- and tetravalent titanium supported by a tris(phenolato)amine ligand. Dalton Trans. 2022;51:14345–14351. doi: 10.1039/d2dt01739c. [DOI] [PubMed] [Google Scholar]
- 75.Yue Z., Ou C., Ding N., Tao L., Zhao J., Chen J. Advances in metal phthalocyanine based carbon composites for electrocatalytic CO2 reduction. ChemCatChem. 2020;12:6103–6130. [Google Scholar]
- 76.Lu S., Zhang Y., Mady M.F., Egwu Eleri O., Mekonnen Tucho W., Mazur M., Li A., Lou F., Gu M., Yu Z. Sulfur-decorated Ni-N-C catalyst for electrocatalytic CO2 reduction with near 100 % CO selectivity. ChemSusChem. 2022;15 doi: 10.1002/cssc.202200870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mukherjee J., Siewert I. Manganese and rhenium tricarbonyl complexes equipped with proton relays in the electrochemical CO2 reduction reaction. Eur. J. Inorg. Chem. 2020;2020:4319–4333. [Google Scholar]
- 78.Li Q., Wang Y.-C., Zeng J., Zhao X., Chen C., Wu Q.-M., Chen L.-M., Chen Z.-Y., Lei Y.-P. Bimetallic chalcogenides for electrocatalytic CO2 reduction. Rare Met. 2021;40:3442–3453. [Google Scholar]
- 79.Blasczak V., McKinnon M., Suntrup L., Aminudin N.A., Reed B., Groysman S., Ertem M.Z., Grills D.C., Rochford J. Steric and lewis basicity influence of the second coordination sphere on electrocatalytic CO2 reduction by manganese bipyridyl complexes. Inorg. Chem. 2022;61:15784–15800. doi: 10.1021/acs.inorgchem.2c02586. [DOI] [PubMed] [Google Scholar]
- 80.Guo K., Li X., Lei H., Guo H., Jin X., Zhang X.-P., Zhang W., Apfel U.-P., Cao R. Role-specialized division of labor in CO2 reduction with doubly-functionalized iron porphyrin atropisomers. Angew. Chem. Int. Ed. 2022;61 doi: 10.1002/anie.202209602. [DOI] [PubMed] [Google Scholar]
- 81.Yang Z.-W., Chen J.-M., Qiu L.-Q., Xie W.-J., He L.-N. Molecular engineering of metal complexes for electrocatalytic carbon dioxide reduction: from adjustment of Intrinsic activity to molecular immobilization. Angew. Chem. Int. Ed. 2022;61 doi: 10.1002/anie.202205301. [DOI] [PubMed] [Google Scholar]
- 82.Chandy S.K., Bowers S.A., Yin M., Liu L., Raghavachari K., Li L.-s. Proton-coupled, low-energy pathway for electrocatalytic CO2 reduction at Re(diimine) complexes with a conjugated pyrazinyl moiety. Inorg. Chem. 2022;61:17505–17514. doi: 10.1021/acs.inorgchem.2c02400. [DOI] [PubMed] [Google Scholar]
- 83.Gonell S., Lloret-Fillol J., Miller A.J.M. An iron pyridyl-carbene electrocatalyst for low overpotential CO2 reduction to CO. ACS Catal. 2021;11:615–626. [Google Scholar]
- 84.Sypaseuth F.D., Matlachowski C., Weber M., Schwalbe M., Tzschucke C.C. Electrocatalytic carbon dioxide reduction by using cationic pentamethylcyclopentadienyl–iridium complexes with unsymmetrically substituted bipyridine ligands. Chem. Eur J. 2015;21:6564–6571. doi: 10.1002/chem.201404367. [DOI] [PubMed] [Google Scholar]
- 85.Sheng M., Jiang N., Gustafson S., You B., Ess D.H., Sun Y. A nickel complex with a biscarbene pincer-type ligand shows high electrocatalytic reduction of CO2 over H2O. Dalton Trans. 2015;44:16247–16250. doi: 10.1039/c5dt02916c. [DOI] [PubMed] [Google Scholar]
- 86.Therrien J.A., Wolf M.O. The influence of para substituents in bis(N-heterocyclic carbene) palladium pincer complexes for electrocatalytic CO2 reduction. Inorg. Chem. 2017;56:1161–1172. doi: 10.1021/acs.inorgchem.6b02213. [DOI] [PubMed] [Google Scholar]
- 87.Nganga J.K., Samanamu C.R., Tanski J.M., Pacheco C., Saucedo C., Batista V.S., Grice K.A., Ertem M.Z., Angeles-Boza A.M. Electrochemical Reduction of CO2 Catalyzed by Re(pyridine-oxazoline)(CO)3Cl Complexes. Inorg. Chem. 2017;56:3214–3226. doi: 10.1021/acs.inorgchem.6b02384. [DOI] [PubMed] [Google Scholar]
- 88.Stanbury M., Compain J.D., Trejo M., Smith P., Gouré E., Chardon-Noblat S. Mn-carbonyl molecular catalysts containing a redox-active phenanthroline-5,6-dione for selective electro- and photoreduction of CO2 to CO or HCOOH. Electrochim. Acta. 2017;240:288–299. [Google Scholar]
- 89.Machan C.W., Sampson M.D., Kubiak C.P. A molecular ruthenium electrocatalyst for the reduction of carbon dioxide to CO and formate. J. Am. Chem. Soc. 2015;137:8564–8571. doi: 10.1021/jacs.5b03913. [DOI] [PubMed] [Google Scholar]
- 90.Rosen B.A., Salehi-Khojin A., Thorson M.R., Zhu W., Whipple D.T., Kenis P.J.A., Masel R.I. Ionic liquid-mediated selective conversion of CO to CO at low overpotentials. Science. 2011;334:643–644. doi: 10.1126/science.1209786. [DOI] [PubMed] [Google Scholar]
- 91.Guo X., Lin S., Gu J., Zhang S., Chen Z., Huang S. Simultaneously achieving high activity and selectivity toward two-electron O2 electroreduction: the power of single-atom catalysts. ACS Catal. 2019;9:11042–11054. [Google Scholar]
- 92.Dutta G., Siddiqui S., Zeng H., Carlisle J.A., Arumugam P.U. The effect of electrode size and surface heterogeneity on electrochemical properties of ultrananocrystalline diamond microelectrode. J. Electroanal. Chem. 2015;756:61–68. doi: 10.1016/j.jelechem.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shi Y., Zhang B. Recent advances in transition metal phosphide nanomaterials: synthesis and applications in hydrogen evolution reaction. Chem. Soc. Rev. 2016;45:1529–1541. doi: 10.1039/c5cs00434a. [DOI] [PubMed] [Google Scholar]
- 94.Martins F.L., Pordea A., Jäger C.M. Computationally driven design of an artificial metalloenzyme using supramolecular anchoring strategies of iridium complexes to alcohol dehydrogenase. Faraday Discuss. 2022;234:315–335. doi: 10.1039/d1fd00070e. [DOI] [PubMed] [Google Scholar]
- 95.Costentin C., Savéant J.M. Towards an intelligent design of molecular electrocatalysts. Nat. Rev. Chem. 2017;1 0087. [Google Scholar]
- 96.Canton P., Menegazzo F., Signoretto M., Pinna F., RieIlo P., Bencdetti A., Pernicone N. In: Stud. Surf. Sci. Catal. Gaigneaux E., De Vos D.E., Grange P., Jacobs P.A., Martens J.A., Ruiz P., Poncelet G., editors. Elsevier; 2000. Influence of preparation procedure on physical and catalytic properties of carbon supported Pd-Au catalysts; pp. 1011–1018. [Google Scholar]
- 97.Ye S., Ding C., Li C. In: Adv. Inorg. Chem. van Eldik R., Hubbard C.D., editors. Academic Press; 2019. Chapter one - artificial photosynthesis systems for catalytic water oxidation; pp. 3–59. [Google Scholar]
- 98.Ni Y., Lin L., Shang Y., Luo L., Wang L., Lu Y., Li Y., Yan Z., Zhang K., Cheng F., Chen J. Regulating electrocatalytic oxygen reduction activity of a metal coordination polymer via d–π conjugation. Angew. Chem. Int. Ed. 2021;60:16937–16941. doi: 10.1002/anie.202104494. [DOI] [PubMed] [Google Scholar]
- 99.Agarwal J., Shaw T.W., Schaefer H.F., Bocarsly A.B. Design of a catalytic active site for electrochemical CO2 reduction with Mn(I)-tricarbonyl species. Inorg. Chem. 2015;54:5285–5294. doi: 10.1021/acs.inorgchem.5b00233. [DOI] [PubMed] [Google Scholar]
- 100.Johnson B.A., Maji S., Agarwala H., White T.A., Mijangos E., Ott S. Activating a Low Overpotential CO2 Reduction Mechanism by a Strategic Ligand Modification on a Ruthenium Polypyridyl Catalyst. Angew. Chem. Int. Ed. 2016;55:1825–1829. doi: 10.1002/anie.201508490. [DOI] [PubMed] [Google Scholar]
- 101.Johnson B.A., Agarwala H., White T.A., Mijangos E., Maji S., Ott S. Judicious Ligand Design in Ruthenium Polypyridyl CO2 Reduction Catalysts to Enhance Reactivity by Steric and Electronic Effects. Chemistry (Basel). 2016;22:14870–14880. doi: 10.1002/chem.201601612. [DOI] [PubMed] [Google Scholar]
- 102.Roy S., Sharma B., Pécaut J., Simon P., Fontecave M., Tran P.D., Derat E., Artero V. Molecular cobalt complexes with pendant amines for selective electrocatalytic reduction of carbon dioxide to formic acid. J. Am. Chem. Soc. 2017;139:3685–3696. doi: 10.1021/jacs.6b11474. [DOI] [PubMed] [Google Scholar]
- 103.Kang P., Cheng C., Chen Z., Schauer C.K., Meyer T.J., Brookhart M. Selective Electrocatalytic Reduction of CO2 to Formate by Water-Stable Iridium Dihydride Pincer Complexes. J. Am. Chem. Soc. 2012;134:5500–5503. doi: 10.1021/ja300543s. [DOI] [PubMed] [Google Scholar]
- 104.Taheri A., Thompson E.J., Fettinger J.C., Berben L.A. An iron electrocatalyst for selective reduction of CO2 to formate in water: including thermochemical insights. ACS Catal. 2015;5:7140–7151. [Google Scholar]
- 105.Yang Y., Gao H., Feng J., Zeng S., Liu L., Liu L., Ren B., Li T., Zhang S., Zhang X. Aromatic ester-functionalized ionic liquid for highly efficient CO2 electrochemical reduction to oxalic acid. ChemSusChem. 2020;13:4900–4905. doi: 10.1002/cssc.202001194. [DOI] [PubMed] [Google Scholar]
- 106.Gennaro A., Isse A.A., Severin M.-G., Vianello E., Bhugun I., Savéant J.M. Mechanism of the electrochemical reduction of carbon dioxide at inert electrodes in media of low proton availability. J. Chem. Soc., Faraday Trans. 1996;92:3963–3968. [Google Scholar]
- 107.Nakajima H., Kushi Y., Nagao H., Tanaka K. Multistep CO2 Reduction Catalyzed by [Ru(bpy)2(qu)(CO)]2+ (bpy = 2,2'-Bipyridine, qu = Quinoline). Double Methylation of the Carbonyl Moiety Resulting from Reductive Disproportionation of CO2. Organometallics. 1995;14:5093–5098. [Google Scholar]
- 108.Wang J.Y., Zhang H.X., Jiang K., Cai W.B. From HCOOH to CO at Pd electrodes: a surface-enhanced infrared spectroscopy study. J. Am. Chem. Soc. 2011;133:14876–14879. doi: 10.1021/ja205747j. [DOI] [PubMed] [Google Scholar]
- 109.Wang G., Wang Y., Wang G., Xiao L., Zhuang L. In situ surface enhanced Raman spectroscopy study of electrode–polyelectrolyte interfaces. Faraday Discuss. 2022;233:100–111. doi: 10.1039/d1fd00051a. [DOI] [PubMed] [Google Scholar]
- 110.Han X.X., Rodriguez R.S., Haynes C.L., Ozaki Y., Zhao B. Surface-enhanced Raman spectroscopy. Nat. Rev. Methods Primers. 2022;1:87. [Google Scholar]
- 111.Wu Y., Chen C., Yan X., Wu R., Liu S., Ma J., Zhang J., Liu Z., Xing X., Wu Z., Han B. Enhancing CO2 electroreduction to CH4 over Cu nanoparticles supported on N-doped carbon. Chem. Sci. 2022;13:8388–8394. doi: 10.1039/d2sc02222b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Armstrong C.G., Potter M., Malcomson T., Hogue R.W., Armstrong S.M., Kerridge A., Toghill K.E. Exploring the electrochemistry of iron dithiolene and its potential for electrochemical homogeneous carbon dioxide reduction. Chemelectrochem. 2022;9 doi: 10.1002/celc.202200610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Song P., Li Y., Yin S. Mechanistic insights into homogeneous electrocatalytic reaction for energy storage using finite element simulation. Chin. J. Chem. Eng. 2022;42:285–296. [Google Scholar]
- 114.Liu X., Zhang Y., Wang W., Chen Y., Xiao W., Liu T., Zhong Z., Luo Z., Ding Z., Zhang Z. Transition metal and N doping on AlP monolayers for bifunctional oxygen electrocatalysts: density functional theory study assisted by machine learning description. ACS Appl. Mater. Interfaces. 2022;14:1249–1259. doi: 10.1021/acsami.1c22309. [DOI] [PubMed] [Google Scholar]
- 115.Tan X., Yu C., Ren Y., Cui S., Li W., Qiu J. Recent advances in innovative strategies for the CO2 electroreduction reaction. Energy Environ. Sci. 2021;14:765–780. [Google Scholar]
- 116.Wang G., Chen J., Ding Y., Cai P., Yi L., Li Y., Tu C., Hou Y., Wen Z., Dai L. Electrocatalysis for CO2 conversion: from fundamentals to value-added products. Chem. Soc. Rev. 2021;50:4993–5061. doi: 10.1039/d0cs00071j. [DOI] [PubMed] [Google Scholar]
- 117.Li G., Liu Y., Zhang Q., Hu Q., Guo W., Cao X., Dou Y., Cheng L., Song Y., Su J., et al. Development of catalysts and electrolyzers toward industrial-scale CO2 electroreduction. J. Mater. Chem. A. 2022;10:19254–19277. [Google Scholar]
- 118.Su J., Zhang J.-J., Chen J., Song Y., Huang L., Zhu M., Yakobson B.I., Tang B.Z., Ye R. Building a stable cationic molecule/electrode interface for highly efficient and durable CO2 reduction at an industrially relevant current. Energy Environ. Sci. 2021;14:483–492. [Google Scholar]
- 119.Xu D., Li K., Jia B., Sun W., Zhang W., Liu X., Ma T. Electrocatalytic CO2 reduction towards industrial applications. Carbon Energy. 2022;5:e230. [Google Scholar]
- 120.Zhao X., Du L., You B., Sun Y. Integrated design for electrocatalytic carbon dioxide reduction. Catal. Sci. Technol. 2020;10:2711–2720. [Google Scholar]












