Summary
Background
Post-acute sequelae after SARS-CoV-2 infection (PASC) remains a concerning long-term complication of COVID-19. Here, we aimed to characterize the epidemiology of PASC in Mexico during 2022 and identify potential associations of covariates with PASC prevalence using nationally representative data.
Methods
We analyzed data from the 2022 Mexican National Health and Nutrition Survey (ENSANUT) from 24,434 participants, representing 85,521,661 adults ≥20 years. PASC was defined using both the National Institute for Health and Care Excellence (NICE) definition and a PASC score ≥12. Estimates of PASC prevalence were stratified by age, sex, rural vs. urban setting, social lag quartiles, number of reinfections, vaccination status and periods of predominance of SARS-CoV-2 circulating variants. Determinants of PASC were assessed using log-binomial regression models adjusted by survey weights.
Findings
Persistent symptoms after SARS-CoV-2 infection were reported by 12.44% (95% CI 11.89–12.99) of adults ≥20 years in Mexico in 2022. The most common persistent symptoms were fatigue, musculoskeletal pain, headache, cough, loss of smell or taste, fever, post-exertional malaise, brain fog, anxiety, and chest pain. PASC was present in 21.21% (95% CI 19.74–22.68) of subjects with previously diagnosed COVID-19. Over 28.6% of patients with PASC reported symptoms persistence ≥6 months and 14.05% reported incapacitating symptoms. Higher PASC prevalence was associated with SARS-CoV-2 reinfections, depressive symptoms and living in states with high social lag. PASC prevalence, particularly its more severe forms, decreased with COVID-19 vaccination and for infections during periods of Omicron variant predominance.
Interpretation
PASC remains a significant public health burden in Mexico as the COVID-19 pandemic transitions into endemic. Promoting SARS-CoV-2 reinfection prevention and booster vaccination may be useful in reducing PASC burden.
Funding
This research was supported by Instituto Nacional de Geriatría in Mexico.
Keywords: Long COVID, PASC, Mexico, COVID-19, Sequelae
Research in context.
Evidence before this study
As SARS-CoV-2 transitions into endemicity, understanding post-acute sequelae after SARS-CoV-2 infection (PACS) has become an utmost priority. PACS is a heterogeneous entity with diverse clinical manifestations which often presents as a debilitating illness; however, inconsistent definitions and the complexity of tracking cases in low- and middle-income countries (LMICs) have obscured our understanding of PASC in regions that were hard-hit during the COVID-19 pandemic, including Mexico. We conducted a PubMed database search without language restriction for studies evaluating the prevalence of PASC in Mexico published between March 1st, 2020, and October 20, 2023. The search terms were (“Long-covid” OR “Post-acute SARS-CoV-2 sequelae” OR “Post-acute COVID-19 condition”) AND “prevalence” AND “Mexico”. We identified a few studies on the topic, most of which were conducted in specific health centers in Mexico, with one study providing estimates of SARS-CoV-2 sequelae using data from ENSANUT 2020 but without using standardized definitions or characterizing symptom heterogeneity in PASC. No other study to date has provided nationally representative estimates of PASC in Mexican adults using the most recent PASC definitions or explored correlates of PASC prevalence.
Added value of this study
Our study is amongst the first to characterize the epidemiology of PASC in Mexico and Latin America using nationally representative data from ENSANUT 2022. We found that 12.44% (95% CI 11.89–12.99) of adults ≥20 in Mexico reported at least one persistent symptom after SARS-CoV-2 infection according to the CDC definition during 2022. The most commonly reported symptoms included fatigue, musculoskeletal pain, headache, cough, loss of smell or taste, fever, post-exertional malaise, brain fog, anxiety, and chest pain. Furthermore, we observed that 21.21% (95% CI 7.71–9.65) of individuals with previously diagnosed COVID-19 had PASC according to the NICE definition. Importantly, a significant proportion of patients with PASC reported symptom persistence for six months or longer (28.6%) and experienced incapacitating symptoms (14.05%). Our study also identified several correlates for increased PASC prevalence, including: increasing number of SARS-CoV-2 reinfections, depressive symptoms, and residing in states with high socio-demographic inequalities. Moreover, we found that PASC prevalence, particularly its more severe forms, decreased among individuals who received ≥1 dose of COVID-19 vaccination and those infected during periods of Omicron variant predominance. Our findings underscore the potential benefits of preventing SARS-CoV-2 reinfections and promoting booster vaccinations to reduce the burden of PASC in LMICs.
Implications of all the available evidence
Our findings highlight a need to continuously monitor the prevalence of PASC to reduce its burden on physical function and disability. By providing nationally representative data on the prevalence and determinants of PASC in Mexico, our study fills an important gap in the literature and highlights an urgent need to promote research to understand the epidemiology of PASC and persistent COVID-19 symptoms in other Latin American countries. Overall, our research may have very relevant implications for public health planning, resource allocation, and the development of clinical guidelines and targeted interventions aimed at mitigating the burden of PASC in similar settings worldwide.
Introduction
Post-acute persistent sequelae after severe acute coronavirus 2 (SARS-CoV-2) infection (PASC) remains a significant challenge to reduce the burden of coronavirus disease (COVID-19) as it progresses towards endemicity.1 Definitions of PASC are still evolving given heterogeneous patient profiles, with other terms also recognizing it as post-acute COVID-19 condition, long COVID, and post-acute COVID-19 syndrome.2 A Delphi consensus by the World Health Organization (WHO) defines PASC as a condition affecting individuals with suspected or confirmed SARS-CoV-2 infection exhibiting persistent symptoms lasting for ≥2 months without an alternative pathophysiological explanation.3 Furthermore, the National Institute for Health and Care Excellence (NICE) defined PASC as an individual with persistent symptoms 12 weeks after acute COVID-19.4 Recently, Thaweethai and colleagues developed a self-reported symptom-based definition to better characterize PASC across cohorts to facilitate diagnosis and characterization of PASC epidemiology.5 Nevertheless, reports on the epidemiology of PASC remain scarce, particularly in Latin American countries, which hinders recognition of its public health impact and delays the development of public policies aimed at reducing PASC burden.6
Mexico experienced one of the highest morbidity and mortality rates related to the COVID-19 pandemic.7 During periods of high community-level transmission of SARS-CoV-2 in Mexico, COVID-19 mortality remained consistently high, yielding one of the highest rates of excess mortality worldwide.8 This scenario was facilitated in the Mexican context by a landscape of high cardio-metabolic burden, characterized by a high prevalence of diabetes, overweight, and obesity, as well as marked socio-demographic inequalities.9, 10, 11 COVID-19 mortality and infection rates in Mexico were reduced significantly by a high vaccination coverage, which achieved 79.8% of the adult population according to ENSANUT 2022.12,13 Despite the well-characterized impact of the COVID-19 pandemic in low- and middle-income settings, information regarding the impact of debilitating chronic sequelae of SARS-CoV-2 infection is lacking. In Mexico, only one report has evaluated the impact of PASC in cases with severe COVID-19.14,15 However, nationally representative estimates of persistent COVID-19 symptoms and particularly PASC as defined by current criteria in Mexico remain unreported. Here, we aimed to estimate the prevalence of persistent COVID-19 symptoms and PASC using a nationally representative sample of Mexican adults for the year 2022. We also aimed to evaluate potential correlates of PASC prevalence including sociodemographic and infection-related determinants to identify potential areas of intervention aimed at ameliorating the long-term burden of endemic COVID-19 in Mexico.
Methods
Study design
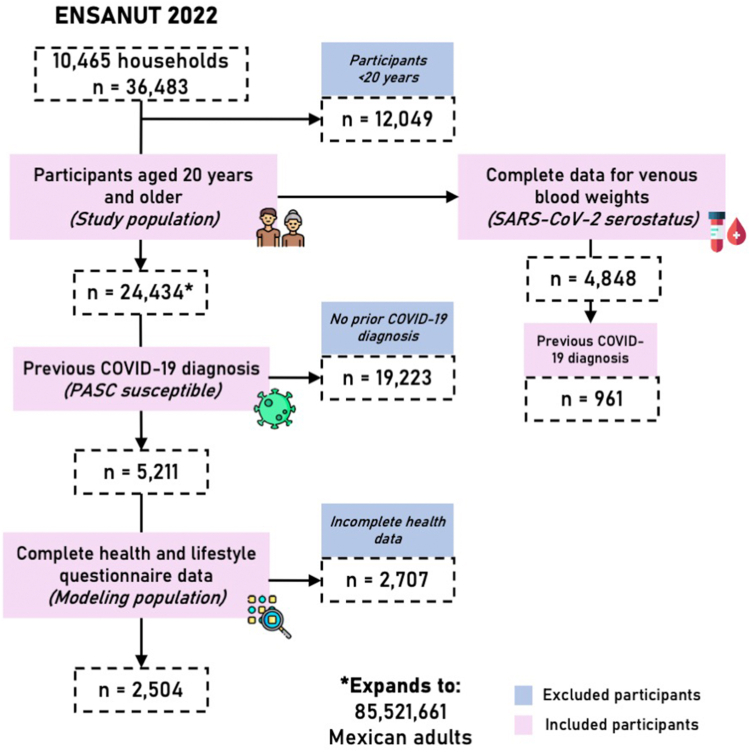
We analyzed data from the Mexican National Health and Nutrition Survey (ENSANUT) for the year 2022. Briefly, ENSANUT is representative at a national and regional level, as it uses a multi-stage probabilistic cluster stratified sampling design based on basic geostatistical areas, localities, households, and individuals. Sample weights are further corrected using the expected response rate in 2021 and calibrated using a post-stratification correction factor to account for over- or underrepresentation of specific subgroups, such that the sample size of ENSANUT expands to the size of the population it represents. This expansion factor is calculated according to household (state, and rural/urban/metropolitan area) and individual (age group) characteristics, for each participant. Participants underwent a comprehensive questionnaire collecting demographic, socioeconomic, and health-related data (available as Supplementary File), and a physical exam including measurement of blood pressure and anthropometry. As outlined in Fig. 1, ENSANUT 2022 was collected from July to December 2022 in 10,160 households (response rate of 73%) which amounts to 11,472 complete interviews for 36,483 participants, amongst which 24,432 were adults ≥20 years.16 This survey additionally aimed to investigate the health and well-being of Mexican adults during the COVID-19 pandemic and a random subsample of 5971 adults ≥20 years old provided a capillary blood sample to detect antibodies for SARS-CoV-2 infection, amongst which 4898 were participants ≥20 years. A subsample of participants with prior COVID-19 diagnosis (n = 2504) had complete health and lifestyle questionnaire data, which were used to model the probability of PASC. A complete flowchart of study participant selection is presented in Fig. 1.
Fig. 1.
Flowchart of participant selection for ENSANUT 2022. We display the total number of participants surveyed per year (first row). The general population of our study is therefore comprised of participants ≥20 years old that completed the health questionnaire (second row), while the laboratory subset includes those with complete weights for venous blood and sampling stratum (third row). A subset of analyses was conducted in individuals with previously diagnosed COVID-19 and complete data (fourth row). This diagram was designed using resources created by Freepik from www.flaticon.com.
SARS-CoV-2 seroprevalence
Seropositivity to SARS-CoV-2 was evaluated using an Elecsys detection assay for IgG against the N-protein (SARS-CoV-2 Nucleocapsid, #09 203,079,190, Roche-N) validated by the Institute for Epidemiological and Diagnostic Reference. Samples were considered positive if quantification was ≥0.72.0 U/ml.17 COVID-19 seroprevalence was estimated using population sample weights with the survey R package and was considered as a proxy of previous infection amongst 4898 participants who provided blood samples in ENSANUT.
Variable definitions
Previously diagnosed COVID-19 and SARS-CoV-2 seropositivity
Previously diagnosed COVID-19 was defined for individuals who self-reported, “at least once” to the question: “Since February 2020, how many times have you been diagnosed with COVID-19 by health personnel?”. Since this ascertainment likely only captured individuals with symptomatic COVID-19 with moderate-to-severe infections who sought medical attention and to consider the possibility of undiagnosed or asymptomatic SARS-CoV-2 infection,18 we used seropositivity to the SARS-CoV-2 N-protein as a proxy of prior infection to evaluate PASC prevalence as a sensitivity analysis.
Persistent COVID-19 symptoms
Amongst individuals with previously diagnosed COVID-19, persistent symptoms after SARS-CoV-2 infection were asked3 using the question: “Regarding the last time you had COVID, did you continue to present any of these symptoms/sequelae one month after your illness began?” and interrogated the presence of the following symptoms: cough, fatigue or tiredness, anxiety, depression, fever, difficulty sleeping, kidney complications, loss of appetite, weight loss, headache, dizziness, muscle or joint pain, difficulty breathing (dyspnea), shortness of breath, chest pain, vomiting or diarrhoea (gastrointestinal symptoms), loss or decrease in smell, loss or decrease in taste, difficulty thinking or concentrating or other symptoms, including post-exertional malaise, which were defined as symptoms of fatigue or brain fog which worsen with activity and impede normal functioning. Participants were also asked to clarify if the symptoms persisted for less than an additional month after COVID-19, from one to three months, from three to six months, more than 6 months, or are still present.
Post-acute COVID-19 sequelae
We used two broad definitions of PASC in our study: 1) A consensus definition by NICE, which defines PASC as the presence of ≥1 persistent symptom lasting ≥3 months after the last COVID-19 diagnosis. Cases in whom persistent symptoms lasted only 4–12 weeks after acute COVID-19 were defined as subacute COVID-19.19 2) We also calculated the PASC score, which defined PASC with a score ≥12 points.5 Symptoms such as palpitations, chronic thirst, decreased sexual desire or capacity, or abnormal movements were not evaluated in ENSANUT 2022 and were thus not included for score calculation. This complementary definition was included as a sensitivity analysis and was considered irrespective of symptom duration. Finally, we used the CDC definition to estimate the frequency of ≥1 SARS-CoV-2 sequelae present ≥4 weeks after acute COVID-19, which were reported for all participants with prior medical diagnosis of COVID-19 evaluated in ENSANUT 2022.20 These definitions are fully summarised in Table 1.
Table 1.
Summary of available definitions of post-acute sequelae after SARS-CoV-2 infection (PASC).
| PASC definition | Description |
|---|---|
| Centers for disease control (20) (PASC-CDC) | Presence of ≥1 persistent symptom lasting ≥4 weeks after onset of the acute phase of COVID-19. |
| National institute for health and care excellence (4) (PASC-NICE) | Presence of ≥1 persistent symptom lasting ≥3 months after the last COVID-19 diagnosis. Cases in whom persistent symptoms lasted only 4–12 weeks after acute COVID-19 were defined as subacute COVID-19 |
| WHO definition (3) | Based on a Delphi consensus, it defines PASC as the presence of ≥1 persistent symptom lasting ≥3 months after the last COVID-19 diagnosis without any alternative explanation for the symptoms. |
| PASC score (5) (PASC positive) | Developed by Thaweethai et al., the score is based on self-reported symptoms using data from the RECOVER adult cohort. PASC is defined based on a score ≥12 points for individuals who met the PASC WHO definition. For our study, we applied the score in individuals meeting the CDC definition and further evaluated it as a proxy of severe PASC. |
The CDC, NICE and PASC score definitions were implemented in our study, as no formal ascertainment of alternative causes was conducted to meet the WHO definition.
Modifying factors
We evaluated age categories per 10-year intervals (20–29, 30–39, 40–49, 50–59, 60–69 or ≥70 years), sex, rural/urban area, smoking status and previous diagnosis of diabetes, hypertension, myocardial infarction, obesity, stable chronic angina, heart failure, stroke and chronic kidney disease as modifying factors of PASC prevalence. The following factors were also considered to evaluate components of SARS-CoV-2 infection:
-
•
SARS-CoV-2 reinfection—Defined as having been diagnosed with COVID-19 more than once by healthcare personnel (self-report).
-
•
Predominant SARS-CoV-2 variants—SARS-CoV-2 infection was assumed to be most likely caused by the predominant variant based on the date of symptom onset. Based on data submitted to GISAID,21 from March 3rd, 2020, until December 30th, 2020, the predominant SARS-CoV-2 variant was the ancestral strain, followed by the predominance of B.1.1.519 until June 6th, 2021, B.1.617.2 (Delta) until December 6th, 2021. B.1.1.529.1 (Omicron) was considered predominant from December 7th, 2022, onwards.22 For modelling, COVID-19 were classified into those likely caused by the Omicron variant or otherwise.
-
•
COVID-19 vaccination—Vaccination was defined by self-report among individuals who answered “yes” to the question: “Have you been vaccinated for COVID-19?” and further questioned if receiving one, two, three or four vaccine doses. Vaccine types for primary vaccination schedules were classified as mRNA (BNT162b2, mRNA-12732), adenovirus vectored (Gam-COVID-Vac, Ad5-nCoV, Ad26.COV2.S, ChAdOx1) and inactivated virus (CoronaVac).
-
•
Social lag—We used data from the National Council for Evaluation of Social Development Policy (CONEVAL), which provides state-level estimates of the 2020 social lag index (SLI), which is a composite measure of access to education, health care, dwelling quality, and basic services in Mexico.23 To assess marginalization independently of urbanization, we extracted mean population density from each state and regressed it onto SLI and obtained the residuals which represent a density-independent social lag index (DISLI).8,11,24 We then categorized states into DISLI quartiles (Q1< −0.676, Q2 −0.676 to −0.131, Q3 −0.131 to 0.110 and Q4 >0.110) and classified individuals into each quartile according to their state of residence. DISLI is a dimensionless measure which is interpreted, when values are higher, as a proxy of higher sociodemographic inequalities independent of population density.
-
•
Depressive symptoms—Evaluated using the seven-item version of the Center for Epidemiologic Studies Depression Scale (CESD-7). CESD-7 measures the frequency with which depressive symptoms are experienced during the week prior to the interview collected at ENSANUT 2022. Cut-offs to identify the presence of moderate or severe depressive symptoms were ≥9 points for adults aged 20–59, and ≥5 points for adults ≥60 years.25,26
-
•
Incapacitating symptoms—Defined if the person answered “yes” to the question “Do these [persistent COVID-19] symptoms prevent you or did they prevent you from taking care of yourself? For example, causing difficulties to bathe or dress oneself”.
Statistical analyses
Weighted prevalence of PASC and persistent COVID-19 symptoms
Prevalence of PASC and persistent symptoms were estimated using sample weights from ENSANUT for participants ≥20 years, as well as those with SARS-CoV-2 seropositivity and for the population with previously diagnosed COVID-19; all estimations were conducted using the survey R package. To allow for population-level inference, all calculations considered the design of ENSANUT 2022 and the use of survey weights to estimate expansion factors for individuals.16,27 We further performed weighted subgroup estimation for prevalence trends stratified by age category, sex, geographical region, previous reinfection, vaccination status, infection during periods of Omicron variant predominance, indigenous identity, rural or urban area, vaccine types, and DISLI category (high or low/middle). All results presented herein report prevalence which consider survey design and their corresponding 95% confidence intervals.
Clustering of persistent sequelae after SARS-CoV-2 infection
Given the heterogeneity of symptom presentation in patients with SARS-CoV-2 sequelae, we conducted hierarchical cluster analysis in reported persistent sequelae for participants with PASC using the nomclust R package. For clustering we implemented a complete linkage method and used the Goodall 3 similarity measure, which assigns higher weight if the infrequent categories match regardless on frequencies of other categories and has proven to be robust in external validation studies.28 Optimal number of clusters was set at four to identify if symptom clustering was similar in Mexican adults.5
Correlates of PASC prevalence
To identify correlates of PASC positivity we fitted fixed effects log-binomial regression models considering survey weights and robust standard errors amongst individuals with previously diagnosed COVID-19 and complete data including comorbidities to estimate relative risks (RR) of PASC. A sensitivity analysis was conducted exploring correlates for any sequelae present compared to the PASC definition. Complete-case analyses were conducted under the assumption of data missing completely at random; a full report of missing data for analytical models is presented in the Supplementary Material. All statistical analyses were conducted using R version 4.1.2 and p-values thresholds were set for a two-sided significance level of α = 0.05.
Ethics approval and consent to participate
This project was registered and approved by the Research and Ethics Committee at Instituto Nacional de Geriatría, under project number DI-PI-005/2021.
Role of the funding source
This research was supported by Instituto Nacional de Geriatría in Mexico. The funding bodies were not involved in study design; collection, management, analysis and interpretation of data or the decision to submit for publication.
Results
Study population
We included adults aged ≥20 years old who completed the health questionnaire, totaling 24,434 participants (expanded to 85,521,661 adults); among them, 4898 participants were selected for capillary blood sampling to estimate SARS-CoV-2 seropositivity (expanded to 85,098,924 adults). A flowchart diagram detailing the participant selection process is available in Fig. 1. Overall, we identified a prevalence of previously diagnosed COVID-19 amongst adults ≥20 years of 22.0% (95% CI 21.31–22.69), with a SARS-CoV-2 N-protein seroprevalence of 93.66% (95% CI 92.78–94.54). Overall, 86.21% (95% CI 85.64–86.78) of adults received at least one dose of COVID-19 vaccines, with 11.62% (95% CI 11.1–12.14) receiving only one dose, 29.6% (95% CI 28.83–30.37) with two doses, 39.51% (95% CI 38.73–40.29) with three doses and 5.48% (95% CI 5.11–5.85) with four doses.
Prevalence of persistent COVID-19 symptoms
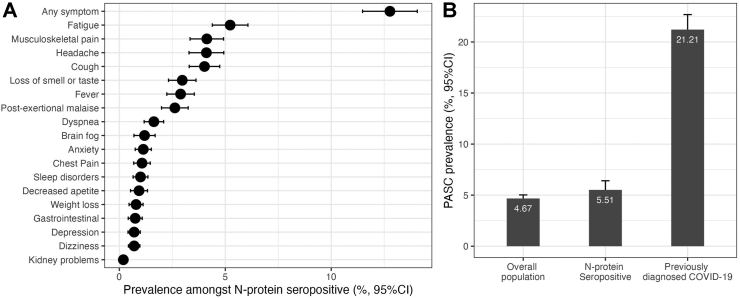
We identified that 12.44% (95% CI 11.89–12.99) of adults ≥20 years had at least one persistent COVID-19 symptom for ≥1 month, expanding to 10,634,663 individuals (95% CI 10,155,424–11,113,902) meeting the PASC-CDC definition. Among them, 45.87% (95% CI 43.49–48.25) lasted <1 additional month, 16.74% (95% CI 14.93–18.55) persisted between 1 and 3 months, 4.54% (95% CI 3.56–5.52) between 3 and 6 months, 4.26% (95% CI 3.28–5.24) for >6 months and 28.6% (95% CI 26.43–30.77) persisting until the date of the interview. The prevalence of PASC using the CDC definition was similar when analyzing N-protein seropositive adults (12.7%, 95% CI 11.48–14.06) and was higher when considering only cases with previously diagnosed COVID-19 by a physician (56.52%, 95% CI 54.75–58.29). The ten most frequent persistent symptoms amongst subjects with SARS-CoV-2 N-protein seropositivity were fatigue, musculoskeletal pain, headache, cough, loss of smell or taste, fever, post-exertional malaise, brain fog, anxiety, and chest pain (Fig. 2A).
Fig. 2.
(A) Prevalence of persistent COVID-19 symptoms amongst SARS-CoV-2 N-protein seropositive adults ≥20 years in ENSANUT 2022; and (B) prevalence of post-acute sequelae of SARS-CoV-2 symptoms (PASC) as identified by a PASC score ≥12 in the overall population, in SARS-CoV-2 N-protein seropositive adults and in individuals with COVID-19 previously diagnosed by a physician in the ENSANUT 2022 sample.
Persistent symptoms in selected subgroups
Amongst SARS-CoV-2 N-protein seropositive individuals the prevalence of subacute COVID-19 was 7.42% (95% CI 6.42–8.42), whilst for PASC-NICE was 5.51% (95% CI 4.61–6.41). The five most common persistent symptoms in subacute COVID-19 were cough, headache, fever, musculoskeletal pain, and loss of smell or taste, whilst for PASC-NICE they were fatigue, musculoskeletal pain, post-exertional malaise, headache, and cough (Supplementary Figure S1). The prevalence of the PASC-CDC definition for any persistent symptom amongst SARS-CoV-2 N-protein seropositive individuals was higher for males (14.12%, 95% CI 12.42–15.85) compared to females (10.50%, 95% CI 8.56–12.44); in males the five most frequent persistent symptoms were fatigue, headache, cough, musculoskeletal pain, and loss of smell or taste, whilst for females they were fatigue, cough, musculoskeletal pain, fever, and headache (Supplementary Figure S2). SARS-CoV-2 primoinfection was associated with lower prevalence of persistent sequelae (55.39%, 95% CI 53.49–57.29) compared to cases with at least one SARS-CoV-2 reinfection (64.41%, 95% CI 59.69–69.13); furthermore, in cases with SARS-CoV-2 primoinfection the most common persistent symptoms were cough, musculoskeletal pain, headache, fever, and loss of smell or taste, whilst for reinfection they were fatigue, cough, musculoskeletal pain, headache, and fever (Supplementary Figure S2).
Prevalence of PASC-NICE in Mexico
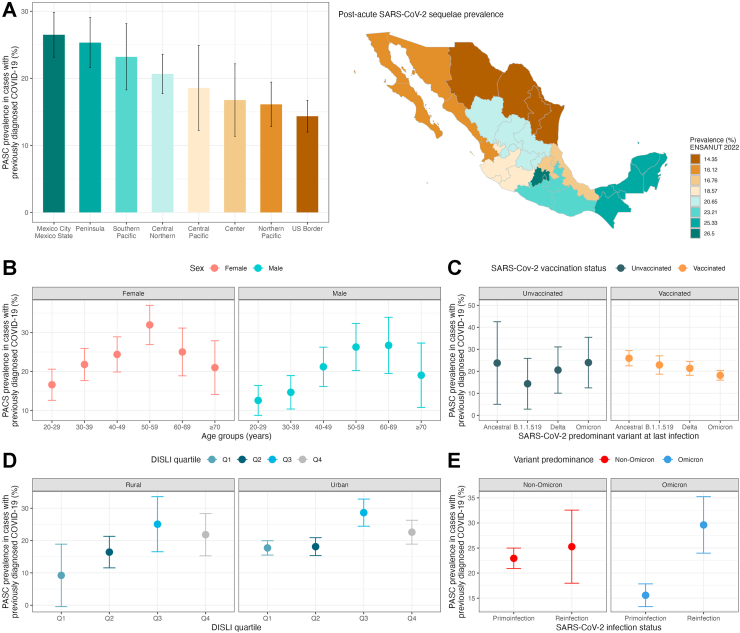
Using the NICE definition, we identified an overall PASC-NICE prevalence of 4.67% (95% CI 4.32–5.02), 5.51% (95% CI 4.61–6.41) amongst SARS-CoV-2 N-protein seropositive, and 21.21% (95% CI 19.74–22.68) in subjects with previously diagnosed COVID-19, expanding to 3,990,011 affected adults (95% CI 3,685,878–4,294,144; Fig. 2B). The highest prevalence of PASC-NICE was observed for individuals living in Mexico City/Mexico State and the Peninsula region, whilst the lowest prevalence was observed in the US Border region (Fig. 3A). Individuals with PASC-NICE clustered most persistent symptoms, even those not included in the PASC score; furthermore, individuals with PASC-NICE had higher rates of reinfections, diabetes, myocardial infarction, stable angina, heart failure and hypertension (Table 2); in contrast, individuals with PASC-CDC had higher rates of reinfection, lived in states with higher social lag, higher CES-D scores and higher prevalence of diabetes and hypertension, but lower vaccination coverage (Supplementary Table S1). Overall, 15.13% (95% CI 13.50–16.76) of individuals affected by persistent symptoms reported incapacity to perform everyday tasks including take care of oneself; this rate was 14.06% (95% CI 11.37–16.75) amongst PASC-NICE compared to 7.13% (95% CI 6.17–8.09) for cases with persisting sequelae that did not meet the PASC-NICE definition. We observed a prevalence of PASC Score ≥12 of 1.91% (95% CI 1.69–2.13) in the general population, 2.02% (95% CI 1.48–2.56) in SARS-CoV-2 N-protein seropositive individuals and 8.68% (95% CI 7.72–9.65) in individuals with previously diagnosed COVID-19. Notably, incapacitating persistent symptoms were observed in 45.38% (95% CI 39.65–51.11) of subjects with PASC Score ≥12, indicating a more severe phenotype. When comparing PASC positive (score ≥12) vs. PASC indeterminate subjects (score <12) reinfection rates and symptom clustering occurred most frequently in PASC positive subjects; interestingly, lower vaccination and infections detected during periods of Omicron variant predominance were observed in PASC positive vs. PASC indeterminate individuals (Supplementary Table S2);. This indicated that the PASC score likely detected individuals with a more severe PASC phenotype.
Fig. 3.
(A) Prevalence of post-acute sequelae of SARS-CoV-2 symptoms (PASC) as identified by a PASC score ≥12 stratified by geographical regions in Mexico within the ENSANUT 2022 sample of adults ≥20 years. The figure also shows PASC prevalence stratified by age and sex categories (B), by predominant circulating variant at the time of last SARS-CoV-2 infection and vaccination status (C), by density-independent social lag index (DISLI) quartiles in rural vs. urban setting (D), and in subjects with primoinfection compared to at least one SARS-CoV-2 reinfection during periods of non-Omicron and Omicron variant predominance (E).
Table 2.
Sociodemographic and clinical characteristics of individuals with post-acute sequelae of SARS-CoV-2 infection (PASC) as defined by the National Institute for Health and Care Excellence compared individuals without PASC and previously diagnosed with COVID-19 in the ENSANUT 2022 sample.
| Characteristic | Overall sample |
p-valueb | ||
|---|---|---|---|---|
| Overall, N = 5211a | Without PACS, N = 4161a | PACS, N = 1050a | ||
| Male sex (%) | 2322 (45%) | 1934 (46%) | 388 (37%) | <0.001 |
| Age (years) | 43 (31, 55) | 42 (30, 54) | 47 (35, 57) | <0.001 |
| Reinfection (%) | 638 (12%) | 465 (11%) | 173 (16%) | <0.001 |
| Omicron variant (%) | 2087 (40%) | 1716 (41%) | 371 (35%) | <0.001 |
| COVID-19 vaccine (%) | 4830 (93%) | 3843 (92%) | 987 (94%) | 0.068 |
| Symptom duration | <0.001 | |||
| No persistent symptoms | 2320 (45%) | 2320 (56%) | 0 (0%) | |
| <1 month | 1363 (26%) | 1363 (33%) | 0 (0%) | |
| 1–3 months | 478 (9.2%) | 478 (11%) | 0 (0%) | |
| 3–6 months | 124 (2.4%) | 0 (0%) | 124 (12%) | |
| >6 months | 126 (2.4%) | 0 (0%) | 126 (12%) | |
| Still persistent | 800 (15%) | 0 (0%) | 800 (76%) | |
| Any symptom (%) | 2839 (54%) | 1793 (43%) | 1046 (100%) | <0.001 |
| Loss of smell/taste (%) | 692 (13%) | 501 (12%) | 191 (18%) | <0.001 |
| Brain fog (%) | 166 (3.2%) | 74 (1.8%) | 92 (8.8%) | <0.001 |
| Gastrointestinal symptoms (%) | 160 (3.1%) | 119 (2.9%) | 41 (3.9%) | 0.079 |
| Chest pain (%) | 285 (5.5%) | 170 (4.1%) | 115 (11%) | <0.001 |
| Breathlessness (%) | 358 (6.9%) | 198 (4.8%) | 160 (15%) | <0.001 |
| Dyspnea (%) | 513 (9.8%) | 283 (6.8%) | 230 (22%) | <0.001 |
| Musculoskeletal pain (%) | 913 (18%) | 617 (15%) | 296 (28%) | <0.001 |
| Dizziness (%) | 213 (4.1%) | 130 (3.1%) | 83 (7.9%) | <0.001 |
| Headache (%) | 817 (16%) | 575 (14%) | 242 (23%) | <0.001 |
| Weight loss (%) | 203 (3.9%) | 149 (3.6%) | 54 (5.1%) | 0.019 |
| Loss of appetite (%) | 210 (4.0%) | 169 (4.1%) | 41 (3.9%) | 0.8 |
| Kidney problems (%) | 55 (1.1%) | 30 (0.7%) | 25 (2.4%) | <0.001 |
| Sleep disturbances (%) | 288 (5.5%) | 169 (4.1%) | 119 (11%) | <0.001 |
| Fever (%) | 670 (13%) | 608 (15%) | 62 (5.9%) | <0.001 |
| Depression (%) | 187 (3.6%) | 117 (2.8%) | 70 (6.7%) | <0.001 |
| Anxiety (%) | 256 (4.9%) | 145 (3.5%) | 111 (11%) | <0.001 |
| Fatigue (%) | 1250 (24%) | 783 (19%) | 467 (44%) | <0.001 |
| Chronic cough (%) | 978 (19%) | 737 (18%) | 241 (23%) | <0.001 |
| Post-exertional malaise (%) | 541 (10%) | 342 (8.2%) | 199 (19%) | <0.001 |
| PASC score | 0.0 (0.0, 5.0) | 0.0 (0.0, 4.0) | 3.0 (1.0, 8.0) | <0.001 |
| DISLI quartile | <0.001 | |||
| Q1 | 1707 (33%) | 1430 (34%) | 277 (26%) | |
| Q2 | 1630 (31%) | 1304 (31%) | 326 (31%) | |
| Q3 | 965 (19%) | 721 (17%) | 244 (23%) | |
| Q4 | 909 (17%) | 706 (17%) | 203 (19%) | |
| CES-D score | 5.0 (3.0, 8.0) | 5.0 (3.0, 7.0) | 6.0 (4.0, 9.0) | <0.001 |
| Diabetes (%) | 358 (14%) | 258 (13%) | 100 (17%) | 0.015 |
| Hypertension (%) | 505 (20%) | 357 (18%) | 148 (26%) | <0.001 |
| Daily smoking (%) | 178 (7.1%) | 140 (7.3%) | 38 (6.6%) | 0.6 |
| Previous myocardial infarction (%) | 61 (1.2%) | 41 (1.0%) | 20 (1.9%) | 0.013 |
| Chronic angina (%) | 53 (1.0%) | 34 (0.8%) | 19 (1.8%) | 0.004 |
| Heart failure (%) | 53 (1.0%) | 36 (0.9%) | 17 (1.6%) | 0.030 |
| Previous stroke (%) | 24 (1a.0%) | 20 (1.0%) | 4 (0.7%) | 0.6 |
| Chronic kidney disease (%) | 45 (1.8%) | 32 (1.7%) | 13 (2.3%) | 0.3 |
| BMI categories | 0.10 | |||
| Low BMI | 15 (0.9%) | 14 (1.1%) | 1 (0.2%) | |
| Normal-range BMI | 293 (17%) | 232 (18%) | 61 (14%) | |
| Overweight | 622 (36%) | 467 (36%) | 155 (36%) | |
| Obesity | 796 (46%) | 585 (45%) | 211 (49%) | |
| Vaccine type | 0.091 | |||
| Unvaccinated | 381 (7.3%) | 318 (7.6%) | 63 (6.0%) | |
| mRNA | 1607 (31%) | 1291 (31%) | 316 (30%) | |
| Adenovirus vector | 2629 (50%) | 2075 (50%) | 554 (53%) | |
| Inactivated virus | 438 (8.4%) | 344 (8.3%) | 94 (9.0%) | |
| Other | 156 (3.0%) | 133 (3.2%) | 23 (2.2%) | |
| Incapacitating symptoms | 457 (8.8%) | 310 (7.5%) | 147 (14%) | <0.001 |
n (%); Median (IQR).
Pearson's Chi-squared test; Wilcoxon rank sum test; Fisher's exact test.
PASC prevalence in selected subgroups
PASC-NICE prevalence increased with age and was higher in females compared to males (Fig. 3B); furthermore, PASC-NICE prevalence was higher for individuals living in states with increasing DISLI values, particularly in urban settings (Fig. 3D). For infection-related variables the prevalence of PASC-NICE was lower if the last infection occurred during periods of high Omicron circulation compared to other SARS-CoV-2 variants and was generally lower in vaccinated subjects irrespective of variant predominance (Fig. 3C). Similarly, PASC-NICE prevalence was higher in subjects with at least one reinfection compared to primoinfection, but this was more evident in cases for COVID-19 cases acquired during periods of Omicron variant predominance (Fig. 3E). There was no apparent trend on the prevalence of PASC-NICE related to vaccine type; however, PASC-NICE prevalence was consistently higher for vaccinated participants with reinfection compared to primoinfection, particularly during periods of Omicron predominance, particularly for mRNA and adenovirus vectored vaccines (Supplementary Figure S3). Finally, PASC-NICE prevalence changed from 25.82% (95% CI 22.40–29.24) for cases infected in 2020, to 21.96% for cases infected in 2021 (95% CI 19.65–24.27), and to 17.60% (95% CI 15.36–19.84) for cases infected in 2022 (Supplementary Figure S4).
Modifiers of PASC prevalence
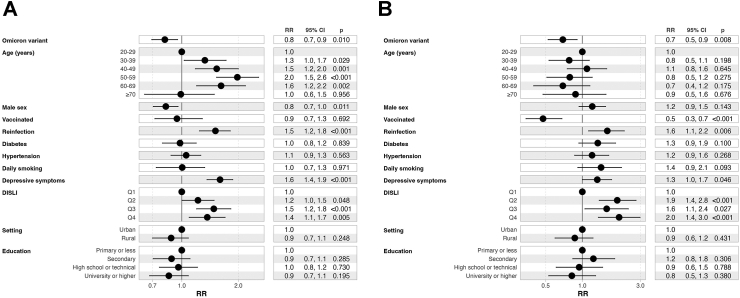
We fitted fixed effects log-binomial regression models considering survey weights to identify correlates of PASC-NICE amongst individuals with previously diagnosed COVID-19 and complete data including comorbidities (n = 2504). We identified that infection during periods of Omicron variant predominance (RR 0.81, 95% CI 0.69–0.21) and male sex (RR 0.82, 95% CI 0.71–0.96) were associated with lower probability of PASC-NICE. In addition, having had at least one SARS-CoV-2 reinfection (RR 1.51, 95% CI 1.25–1.83), having moderate to severe depressive symptoms as identified by CES-D (RR 1.60, 95% CI 1.37–1.87), older age, and living in states with higher social lag (RR 1.37, 95% CI 1.10–1.70 for Q4 vs. Q1) were associated with higher probability of PASC-NICE (Fig. 4A). When evaluating the same predictors but using the definition of PASC score ≥12 points, which likely indicates a more severe PASC phenotype, infection during periods of Omicron variant predominance, COVID-19 vaccination, were associated with lower probability of the outcome, whilst having had at least one SARS-CoV-2 reinfection, living in states with higher social lag, and moderate to severe depressive symptoms by CES-D were associated with higher probability of the outcome (Fig. 4B); further disaggregating by the number of vaccine doses compared to unvaccinated individuals showed a similar protective effect with increasing doses and irrespective of vaccine platform for primary vaccination schedule (Supplementary Figures S6–S8).
Fig. 4.
(A) Fixed effects log-binomial regression model with robust standard errors adjusted by survey weights for prediction of post-acute sequelae of SARS-CoV-2 symptoms (PASC) identified by a PASC score ≥12, and (B) the presence of any persistent COVID-19 symptom amongst Mexican adults enrolled in ENSANUT 2022. All results are presented as Relative Risks (RR) with their corresponding 95% confidence intervals. Abbreviations: DISLI, Density-independent social lag-index; Depressive symptoms: Moderate to severe depressive symptoms identified by the Center for Epidemiologic Studies Depression Scale.
Correlates of incapacitating PASC symptoms
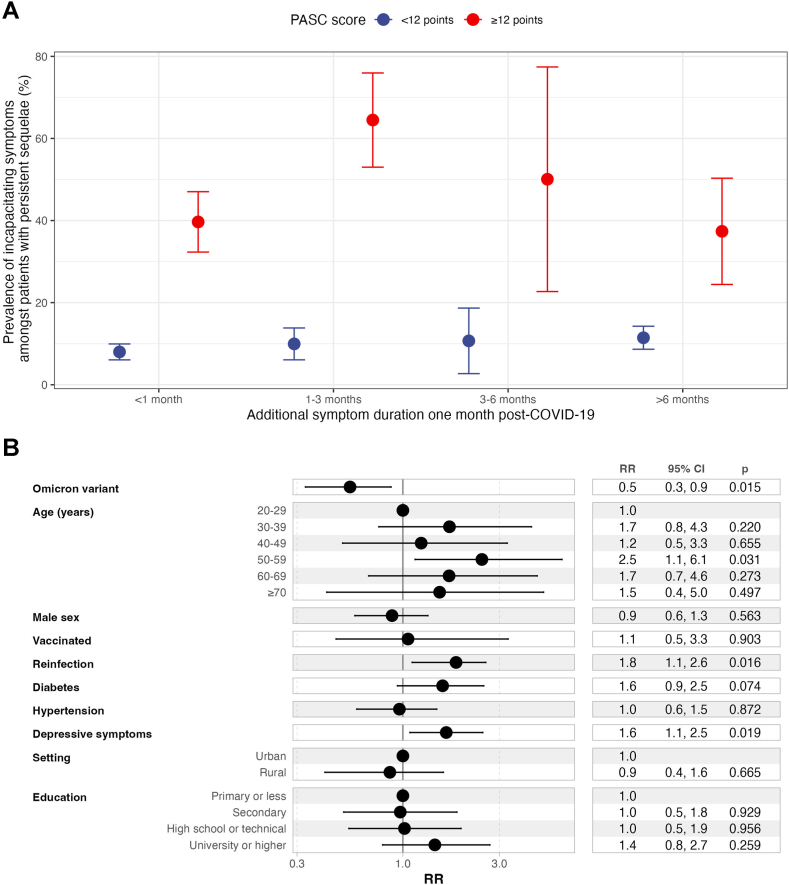
Amongst individuals with persistent symptoms, a PASC score ≥12 points resulted in higher rates of incapacitating symptoms at all time points and independent of the PASC definition (Fig. 5A). Correlates of incapacitating symptoms included moderate to severe depressive symptoms (RR 1.64, 95% CI 1.09–2.47), age 50–59 years compared to younger individuals (RR 2.46, 95% CI 1.08–5.56), having had at least one SARS-CoV-2 reinfection (RR 1.83, 95% CI 1.12–2.99) and a lower risk observed in individuals infected during periods of Omicron variant predominance (RR 0.55, 95% CI 0.34–0.89, Fig. 5B). When further adjusting for PASC score, the most significant predictor of incapacitating PASC symptoms was a PASC score ≥12 points (RR 3.16, 95% CI 2.09–4.79, not shown).
Fig. 5.
(A) Prevalence of incapacitating symptoms amongst patients with persistent post-COVID-19 sequelae disaggregated by PASC scores using a 12-point threshold. (B) Fixed effects log-binomial regression model adjusted by survey weights for prediction of debilitating PASC symptoms amongst Mexican adults enrolled in ENSANUT 2022. Abbreviations: PASC, Post-acute sequelae of SARS-CoV-2 infection; Depressive symptoms: Moderate to severe depressive symptoms identified by the Center for Epidemiologic Studies Depression Scale.
Clustering of PASC phenotypes
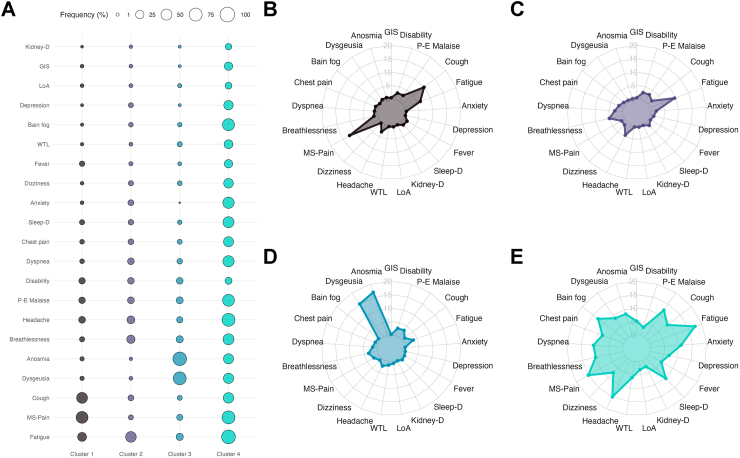
Finally, we performed hierarchical cluster analysis to identify clustering of participants with PASC according to symptom patterns and to identify any predominant PASC phenotypes in Mexico. We identified four main clusters with an overall agglomerative score of 0.31: Cluster 1 (n = 286), characterized by musculoskeletal pain and cough; Cluster 2 (n = 563), characterized by fatigue, headache, and breathlessness; Cluster 3 (n = 111), characterized by dysgeusia, anosmia, and fatigue; and Cluster 4 (n = 90), characterized by fatigue, headache, musculoskeletal pain, brain fog, and sleep disorders (Fig. 6). Notably, rates of disability were similar between groups (Supplementary Table S3). When comparing characteristics according to symptom clusters, we identified that individuals in cluster 3 were younger, less likely to live in states in the upper DISLI quartile, had higher rates of current smoking and lower rates of SARS-CoV-2 reinfections compared to other clusters and, along with cluster 2, had a higher percentage of affected female participants. Individuals in cluster 4 had higher CES-D and PASC scores, along with higher rates of reinfections. Individuals in Cluster 1 had the highest rates of diabetes and hypertension, along with the lowest number of participants living in states within the lowest DISLI quartile (Supplementary Table S3).
Fig. 6.
(A) Bubble plot of symptom cluster frequency derived from hierarchical clustering of persistent SARS-CoV-2 symptoms in patients with PASC, and (B–E) radar plots displaying the prevalence of persistent symptoms and disability across all four clusters. Abbreviations: WTL: Weight loss; LoA: Loss of Appetite; Kidney-D: Kidney disease; Sleep-D: Sleep disorders; GIS: Gastrointestinal symptoms; P-E malaise: Post-exertional malaise; M-S: Musculoskeletal pain.
Discussion
Our understanding of the epidemiology and impact of debilitating chronic sequelae of SARS-CoV-2 infection remain scarce. To our knowledge, this is among the first comprehensive reports on the prevalence of PASC in Mexico during the year 2022, surveying a total of 24,434 adults. Persistent COVID-19 symptoms meeting the CDC definition were reported by 12.44% of adults ≥20 years in Mexico during 2022. The most commonly reported persistent COVID-19 symptoms amongst SARS-CoV-2 N-protein seropositive adults include fatigue, musculoskeletal pain, followed by headache, cough, loss of smell or taste, fever, post-exertional malaise, brain fog, anxiety, and chest pain. By implementing hierarchical clustering, we identified that PASC phenotypes with predominance of fatigue, headache, and breathlessness are the most common in Mexico; notably, sociodemographic characteristics differ amongst symptom clusters, warranting further evaluations in future studies. Using the NICE definition4 we identified a PASC prevalence of 5.51% amongst SARS-CoV-2 N-protein seropositive Mexican adults and 21.21% amongst cases with previously diagnosed COVID-19. PASC-NICE was associated with increasing number of SARS-CoV-2 reinfections, moderate to severe depressive symptoms and living in states burdened by sociodemographic inequalities. Despite slightly higher PASC-NICE prevalence for females in our study, no significant association between sex and PASC-NICE was confirmed in multivariate models. We also confirm previous reports that COVID-19 vaccination and infection during periods of Omicron variant predominance decreases the risk of severe forms of PASC.2,29,30 Notably, over 28.6% patients with PASC reported persistence of symptoms ≥6 months and over 14.06% reported incapacitating symptoms, a subset which will likely require a multidisciplinary approach to reduce disability.31 Incapacitating PASC symptoms were associated moderate to severe depressive symptoms, SARS-CoV-2 reinfections and socio-demographic inequalities as proxied by DISLI. Notably, our study also showed that PASC scores ≥12 may detect individuals with more severe PASC phenotypes, and those who have a higher burden of PASC related disability.
A previous systematic review identified that approximately 72.5% of individuals previously infected by SARS-CoV-2 experienced post-acute sequelae.32 This is higher than the observed 56.52% prevalence amongst individuals with previously diagnosed COVID-19 reported in our study and with the even lower estimate amongst SARS-CoV-2 seropositive adults. A previous study using data from ENSANUT 2020 reported that amongst SARS-CoV-2 seropositive individuals who recovered from COVID-19, 15.7% reported unspecified sequelae.15 According to estimates from a multi-country meta-regression study, persistent fatigue and bodily pains were the most common PASC presentations, followed by ongoing respiratory problems; this agrees with our findings from cluster analysis, albeit with different symptom combinations which may be due to differences between studies.33 In the US, reports of PASC prevalence for cases with previously diagnosed COVID-19 were 18.9% in 2022, which were similar to estimates from our study. Interestingly, although PASC prevalence been reported to decline in recent months in the US, rates of disability remain alarmingly high.34 A previous survey of Latin American countries reported a PASC prevalence of 48% amongst previous COVID-19 survivors with over a third reporting debilitating symptoms.35 And a study of survivors of severe COVID-19 in Brazil reported up to 61% of cases developing persistent sequelae after SARS-CoV-2 infection.36 As now shown in the Mexican context by our results, there is a need to gather population-based data which allows to unravel the complex dynamics of PASC in low-and-middle income countries, particularly with the association between socio-demographic inequalities with PASC and debilitating PASC symptoms observed in our study.
Similar to our findings, previous reports from the UK showed that individuals with PASC clustered in areas of higher socioeconomic deprivation,37 indicating that structural determinants of health may influence the incidence and natural history of chronic debilitating diseases including chronic fatigue syndrome, myalgic encephalitis and PASC.38,39 Our findings are also in agreement with previous data supporting that SARS-CoV-2 reinfections increased the risk of PASC, irrespective of predominant variant; nevertheless, lower risk of PASC has been consistently associated with infections caused by the Omicron compared to previous circulating variants.40,41 These findings have also been confirmed in children, in whom infections with the Omicron variant less commonly associated with PASC.42,43 Finally, the higher rate of moderate to severe depressive symptoms, brain fog and self-reported anxiety in PASC corroborate with reports that viral persistence in neural tissue, neuroinflammation and blood–brain barrier dysfunction may underlie the long-term neuropsychiatric sequelae reported in SARS-CoV-2 convalescent individuals after recovery from acute infection.5,19,44, 45, 46 Furthermore, decreased resilience and impaired homeostatic reserve might be underlying mechanisms linking anxiety and depression with the development of PASC and subacute COVID-19.47,48 It is speculated that increased inflammatory responses and an inadequate adaptation of the hypothalamic–pituitary–adrenal axis to hypocortisolemia after acute SARS-CoV-2 infection may underlie the occurrence of symptoms related to brain fog, post-exertional malaise, and neuropsychiatric symptoms, including anxiety and depression in PASC,2,21,49,50 which often last the longest in patients with persistent COVID-19 symptoms.14,51
Strengths and limitations
Our study had several strengths including a nationally representative sample of individuals with assessment of SARS-CoV-2 N-protein seropositivity, previously diagnosed COVID-19 and reports of long-term sequelae. Furthermore, we were able to assess risk factors related to state-level sociodemographic inequalities, vaccination, reinfection, and predominant variant at the time of infection to explore correlates of PASC in a representative sample of Mexican adults. However, some limitations must be acknowledged to adequately interpret our results. Since the definition of PASC is still evolving, we applied two main definitions based on the NICE consensus and a recent definition based on a symptom score3,5; however, we were unable to consider many symptoms and post-COVID-19 conditions reported in other studies including palpitations, chronic thirst, decreased sexual desire or capacity, abnormal movements, new-onset diabetes, and cardiovascular events, some of which have previously been reported for cases recovered from severe COVID-19 in Mexico.14 We should also acknowledge that no comparison group was implemented to discern symptoms not directly related to PASC as it has been done in previous studies52; notably, this prevented us from implementing the WHO definition based on Delphi consensus outlined in Table 1, as there was no formal ascertainment to exclude alternative causes of persistent symptoms after COVID-19. Furthermore, since previously diagnosed COVID-19 required diagnosis by a physician, it is likely that using this as the only proxy of previous infection may lead to prioritize moderate to severe COVID-19 over asymptomatic SARS-CoV-2 infection, leading to an overestimation of PASC prevalence for cases with previous diagnosis of COVID-19. To overcome this, we used SARS-CoV-2 N-protein seropositivity as a proxy of previous SARS-CoV-2 infection given the high rates of undetected asymptomatic SARS-CoV-2 infections in Mexico; however, persistent symptoms were not interrogated for cases without prior diagnosis of SARS-CoV-2, thus leading to a potential underestimation of symptom prevalence in this population.18,53 Notably, a subset of participants who received the CoronaVac inactivated virus vaccine may display seropositivity despite not having been previously infected, this represents 9.44% (95% CI 10.63–11.97) of the total SARS-CoV-2 N-protein seropositive population.54,55 To address this, we also conducted an analysis excluding individuals who received with CoronaVac and individuals who had COVID-19 previously diagnosed by a physician, obtaining largely similar results with slightly lower prevalence estimates (Supplementary Figure S9). Finally, although the observed associations are consistent with data from previous studies, its observational nature precludes us from making temporal associations with the onset of PASC, which require further longitudinal studies to further support these findings in Mexican population.
Conclusion
We report a prevalence of PASC-NICE of 4.67% in the general population, affecting 21.21% of individuals with previously diagnosed COVID-19. In general, over 12.44% of Mexican adults report at least one persistent COVID-19 symptom meeting the PASC-CDC definition, with the most common being fatigue, musculoskeletal pain, headache, cough, loss of smell or taste, and fever. PASC prevalence increased with age and was more common in females; severe PASC was less frequent for cases infected during periods of Omicron variant predominance and in vaccinated individuals in a dose-dependent manner but was more common in individuals with at least one detected SARS-CoV-2 reinfection. Moreover, prevalence of PASC increased in states with higher marginalization, particularly in urban settings. Of note, over 14.05% of participants affected by PASC reported trouble in everyday physical functioning, highlighting the disabling nature of persistent symptoms and indicating a need to implement multidisciplinary teams for patient treatment in Mexico. Further studies are required to characterize the epidemiology of PASC in Mexico using standardized definitions and with longer follow-up in order to identify strategies to mitigate its long-term impact in physical functioning and quality of life as SARS-CoV-2 transitions into endemicity.
Contributors
Research idea and study design: OYBC, CAFM; data acquisition and processing: CAFM, NEAV, OYBC; statistical analysis: OYBC; analysis/interpretation: CAFM, OYBC, NEAV, AVV, LFC, DRG; manuscript drafting: OYBC, CAFM, DRG, LFC, AVV, MRBA, PSC, ANL, NEAV; supervision or mentorship: OYBC, NEAV. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Data sharing statement
All code, datasets and materials are available for reproducibility of results at http://github.com/oyaxbell/pasc_ensanut/. ENSANUT 2022 data is openly available at: https://ensanut.insp.mx/encuestas/ensanutcontinua2022/descargas.php.
Editor's note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
Nothing to disclose.
Acknowledgements
CAFM is enrolled at the PECEM Program of the Faculty of Medicine at UNAM. CAFM and DRG are supported by CONACyT. This research was supported by Instituto Nacional de Geriatría in Mexico.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2024.100688.
Appendix A. Supplementary data
References
- 1.Davis H.E., McCorkell L., Vogel J.M., Topol E.J. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21(3):133–146. doi: 10.1038/s41579-022-00846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sudre C.H., Murray B., Varsavsky T., et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soriano J.B., Murthy S., Marshall J.C., Relan P., Diaz J.V. WHO clinical case definition working group on post-COVID-19 condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22(4):e102–e107. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Venkatesan P. NICE guideline on long COVID. Lancet Respir Med. 2021;9(2):129. doi: 10.1016/S2213-2600(21)00031-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thaweethai T., Jolley S.E., Karlson E.W., et al. Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA. 2023;329(22):1934–1946. doi: 10.1001/jama.2023.8823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakhamuri S.M., Jankie S., Pereira L.M.P. Calling on Latin America and the Caribbean countries to recognise the disability from long COVID. Lancet Reg Health Am. 2022;15:100362. doi: 10.1016/j.lana.2022.100362. https://www.thelancet.com/journals/lanam/article/PIIS2667-193X(22)00179-X/fulltext [cited 2023 Jul 4] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathieu E., Ritchie H., Rodés-Guirao L., et al. Our World in Data; 2020. Coronavirus Pandemic (COVID-19)https://ourworldindata.org/coronavirus/country/mexico [cited 2023 Jul 4]; Available from: [Google Scholar]
- 8.Antonio-Villa N.E., Bello-Chavolla O.Y., Fermín-Martínez C.A., et al. Socio-demographic inequalities and excess non-COVID-19 mortality during the COVID-19 pandemic: a data-driven analysis of 1 069 174 death certificates in Mexico. Int J Epidemiol. 2022;51(6):1711–1721. doi: 10.1093/ije/dyac184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bello-Chavolla O.Y., Bahena-López J.P., Antonio-Villa N.E., et al. Predicting mortality due to SARS-CoV-2: a mechanistic score relating obesity and diabetes to COVID-19 outcomes in Mexico. J Clin Endocrinol Metabolism. 2020;105(8):2752–2761. doi: 10.1210/clinem/dgaa346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vargas-Vázquez A., Bello-Chavolla O.Y., Ortiz-Brizuela E., et al. Impact of undiagnosed type 2 diabetes and pre-diabetes on severity and mortality for SARS-CoV-2 infection. BMJ Open Diabetes Res Care. 2021;9(1) doi: 10.1136/bmjdrc-2020-002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antonio-Villa N.E., Fernandez-Chirino L., Pisanty-Alatorre J., et al. Comprehensive evaluation of the impact of sociodemographic inequalities on adverse outcomes and excess mortality during the COVID-19 pandemic in Mexico City. Clin Infect Dis. 2021;74(5):785–792. doi: 10.1093/cid/ciab577. [DOI] [PubMed] [Google Scholar]
- 12.Carnalla M., Basto-Abreu A., Stern D., et al. Prevalencia de anticuerpos y vacunación contra SARS-CoV-2 en 2022 en México. Salud Publica Mex. 2023;65:s135–s145. doi: 10.21149/14834. [DOI] [PubMed] [Google Scholar]
- 13.Bello-Chavolla O.Y., Antonio-Villa N.E., Valdés-Ferrer S.I., et al. Effectiveness of a nationwide COVID-19 vaccination program in Mexico against symptomatic COVID-19, hospitalizations, and death: a retrospective analysis of national surveillance data. Int J Infect Dis. 2023;129:188–196. doi: 10.1016/j.ijid.2023.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Núñez I., Gillard J., Fragoso-Saavedra S., et al. 2022. Longitudinal clinical phenotyping of post COVID condition in Mexican patients recovering from severe COVID-19: a prospective cohort study.https://papers.ssrn.com/abstract=4238650 Rochester, NY. [cited 2023 Jul 4]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vidaña-Pérez D., López-Olmedo N., González-Morales R., Shamah-Levy T., Barrientos-Gutiérrez T. Prevalence of covid-19 sequelae in the national health and nutrition survey 2020. Salud Publica Mex. 2021;63(6, Nov-Dic):799–802. doi: 10.21149/13269. [DOI] [PubMed] [Google Scholar]
- 16.Romero-Martínez M., Barrientos-Gutiérrez T., Cuevas-Nasu L., et al. Metodología de la Encuesta nacional de Salud y nutrición 2022 y planeación y diseño de la Ensanut continua 2020-2024. Salud Publica Mex. 2022;64(5, sept-oct):522–529. doi: 10.21149/14186. [DOI] [PubMed] [Google Scholar]
- 17.Sánchez-Pájaro A., Ferrer C.P., Basto-Abreu A., et al. Seroprevalencia de SARS-CoV-2 en adultos y adultos mayores en México y su asociación con enfermedades crónicas. Ensanut 2020 Covid-19. Salud Publica Mex. 2021;63(6, Nov-Dic):705–712. doi: 10.21149/13163. [DOI] [PubMed] [Google Scholar]
- 18.Bello-Chavolla O.Y., Antonio-Villa N.E., Vargas-Vázquez A., Fermín-Martínez C.A., Márquez-Salinas A., Bahena-López J.P. Profiling cases with nonrespiratory symptoms and asymptomatic severe acute respiratory syndrome coronavirus 2 infections in Mexico city. Clin Infect Dis. 2021;72(10):e655–e658. doi: 10.1093/cid/ciaa1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nalbandian A., Sehgal K., Gupta A., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CDC . Healthcare Workers; 2020. Centers for disease control and prevention.https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html [cited 2023 Dec 26] Available from: [Google Scholar]
- 21.Klein J., Wood J., Jaycox J., et al. Distinguishing features of Long COVID identified through immune profiling. medRxiv. 2022 doi: 10.1038/s41586-023-06651-y. https://www.medrxiv.org/content/10.1101/2022.08.09.22278592v1 p. 2022.08.09.22278592. [cited 2023 Jul 4] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montes-González J.A., Zaragoza-Jiménez C.A., Antonio-Villa N.E., et al. Protection of hybrid immunity against SARS-CoV-2 reinfection and severe COVID-19 during periods of Omicron variant predominance in Mexico. Front Public Health. 2023;11 doi: 10.3389/fpubh.2023.1146059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Índice Rezago Social 2020. https://www.coneval.org.mx/Medicion/IRS/Paginas/Indice_Rezago_Social_2020.aspx [cited 2023 Jul 1]. Available from:
- 24.Bello-Chavolla O.Y., Antonio-Villa N.E., Fermín-Martínez C.A., et al. Diabetes-related excess mortality in Mexico: a comparative analysis of national death registries between 2017–2019 and 2020. Diabetes Care. 2022;45(12):2957–2966. doi: 10.2337/dc22-0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salinas-Rodríguez A., Manrique-Espinoza B., Acosta-Castillo G.I., et al. [Validation of a cutoff point for the short version of the Depression Scale of the Center for Epidemiologic Studies in older Mexican adults] Salud Publica Mex. 2014;56(3):279–285. [PubMed] [Google Scholar]
- 26.Salinas-Rodríguez A., Manrique-Espinoza B., Acosta-Castillo I., et al. [Validation of a cutoff for the depression Scale of the center for epidemiologic studies, brief version (CESD-7)] Salud Publica Mex. 2013;55(3):267–274. [PubMed] [Google Scholar]
- 27.survey.pdf. https://cran.r-project.org/web/packages/survey/survey.pdf [cited 2023 Apr 21]. Available from:
- 28.Goodall D.W. A new similarity index based on probability. Biometrics. 1966;22(4):882–907. [Google Scholar]
- 29.Tran V.T., Perrodeau E., Saldanha J., Pane I., Ravaud P. Social Science Research Network; Rochester, NY: 2021. Efficacy of COVID-19 vaccination on the symptoms of patients with long COVID: a target trial emulation using data from the ComPaRe e-cohort in France.https://papers.ssrn.com/abstract=3932953 Report No.: ID 3932953. [cited 2021 Oct 6] Available from: [Google Scholar]
- 30.Brannock M.D., Chew R.F., Preiss A.J., et al. Long COVID risk and pre-COVID vaccination in an EHR-based cohort study from the RECOVER program. Nat Commun. 2023;14(1):2914. doi: 10.1038/s41467-023-38388-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ballouz T., Menges D., Anagnostopoulos A., et al. Recovery and symptom trajectories up to two years after SARS-CoV-2 infection: population based, longitudinal cohort study. BMJ. 2023;381 doi: 10.1136/bmj-2022-074425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nasserie T., Hittle M., Goodman S.N. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open. 2021;4(5) doi: 10.1001/jamanetworkopen.2021.11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Global Burden of Disease Long COVID Collaborators Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA. 2022;328(16):1604–1615. doi: 10.1001/jama.2022.18931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ford N.D., Slaughter D., Edwards D., et al. Long COVID and significant activity limitation among adults, by age - United States, June 1-13, 2022, to June 7-19, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(32):866–870. doi: 10.15585/mmwr.mm7232a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Angarita-Fonseca A., Torres-Castro R., Benavides-Cordoba V., et al. Exploring long COVID condition in Latin America: its impact on patients' activities and associated healthcare use. Front Med. 2023;10 doi: 10.3389/fmed.2023.1168628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lapa J., Rosa D., Mendes J.P.L., Deusdará R., Romero G.A.S. Prevalence and associated factors of post-COVID-19 syndrome in a Brazilian cohort after 3 and 6 Months of hospital discharge. Int J Environ Res Public Health. 2023;20(1):848. doi: 10.3390/ijerph20010848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shabnam S., Razieh C., Dambha-Miller H., et al. Socioeconomic inequalities of long COVID: a retrospective population-based cohort study in the United Kingdom. J R Soc Med. 2023;116(8):263–273. doi: 10.1177/01410768231168377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collin S.M., Bakken I.J., Nazareth I., Crawley E., White P.D. Trends in the incidence of chronic fatigue syndrome and fibromyalgia in the UK, 2001-2013: a Clinical Practice Research Datalink study. J R Soc Med. 2017;110(6):231–244. doi: 10.1177/0141076817702530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berger Z., Altiery de Jesus V., Assoumou S.A., Greenhalgh T. Long COVID and health inequities: the role of primary care. Milbank Q. 2021;99(2):519–541. doi: 10.1111/1468-0009.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bowe B., Xie Y., Al-Aly Z. Acute and postacute sequelae associated with SARS-CoV-2 reinfection. Nat Med. 2022;28(11):2398–2405. doi: 10.1038/s41591-022-02051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doll M.K., Waghmare A., Heit A., et al. Acute and postacute COVID-19 outcomes among immunologically naive adults during delta vs Omicron waves. JAMA Netw Open. 2023;6(2) doi: 10.1001/jamanetworkopen.2023.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buonsenso D., Morello R., Mariani F., et al. Risk of long Covid in children infected with Omicron or pre-Omicron SARS-CoV-2 variants. Acta Paediatr. 2023;112(6):1284–1286. doi: 10.1111/apa.16764. [DOI] [PubMed] [Google Scholar]
- 43.Morello R., Mariani F., Mastrantoni L., et al. Risk factors for post-COVID-19 condition (Long Covid) in children: a prospective cohort study. eClinicalMedicine. 2023;59 doi: 10.1016/j.eclinm.2023.101961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mazza M.G., De Lorenzo R., Conte C., et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Postolache T.T., Benros M.E., Brenner L.A. Targetable biological mechanisms implicated in emergent psychiatric conditions associated with SARS-CoV-2 infection. JAMA Psychiatry. 2021;78(4):353–354. doi: 10.1001/jamapsychiatry.2020.2795. [DOI] [PubMed] [Google Scholar]
- 46.Villalpando J.M.G., Forcelledo H.A., Castillo J.L.B., et al. COVID-19, long COVID syndrome, and mental health sequelae in a Mexican population. Int J Environ Res Public Health. 2022;19(12):6970. doi: 10.3390/ijerph19126970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lyra E Silva N.M., Barros-Aragão F.G.Q., De Felice F.G., Ferreira S.T. Inflammation at the crossroads of COVID-19, cognitive deficits and depression. Neuropharmacology. 2022;209 doi: 10.1016/j.neuropharm.2022.109023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taube M. Depression and brain fog as long-COVID mental health consequences: difficult, complex and partially successful treatment of a 72-year-old patient—a case report. Front Psychiatry. 2023;14 doi: 10.3389/fpsyt.2023.1153512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.PHOSP-COVID Collaborative Group Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: a prospective observational study. Lancet Respir Med. 2022;10(8):761–775. doi: 10.1016/S2213-2600(22)00127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yavropoulou M.P., Tsokos G.C., Chrousos G.P., Sfikakis P.P. Protracted stress-induced hypocortisolemia may account for the clinical and immune manifestations of Long COVID. Clin Immunol. 2022;245 doi: 10.1016/j.clim.2022.109133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hastie C.E., Lowe D.J., McAuley A., et al. Natural history of long-COVID in a nationwide, population cohort study. Nat Commun. 2023;14(1):3504. doi: 10.1038/s41467-023-39193-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whittemore R., Vilar-Compte M., De La Cerda S., et al. Challenges to diabetes self-management for adults with type 2 diabetes in low-resource settings in Mexico City: a qualitative descriptive study. Int J Equity Health. 2019;18(1):133. doi: 10.1186/s12939-019-1035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bello-Chavolla O.Y., Antonio-Villa N.E., Fernández-Chirino L., et al. Diagnostic performance and clinical implications of rapid SARS-CoV-2 antigen testing in Mexico using real-world nationwide COVID-19 registry data. PLoS One. 2021;16(8) doi: 10.1371/journal.pone.0256447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosa Duque J.S., Wang X., Leung D., et al. Immunogenicity and reactogenicity of SARS-CoV-2 vaccines BNT162b2 and CoronaVac in healthy adolescents. Nat Commun. 2022;13(1):3700. doi: 10.1038/s41467-022-31485-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fonseca M.H.G., Pinto A.C.M.D., Silva M.F.S., et al. Dynamics of SARS-CoV-2 antibody response to CoronaVac followed by booster dose of BNT162b2 vaccine. Emerg Infect Dis. 2022;28(6):1237–1240. doi: 10.3201/eid2806.220061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.