After an anterior cruciate ligament (ACL) injury, clinicians and athletes face a common challenge of preventing secondary graft rupture or contralateral injury.2 To minimize injury risk, return-to-sport (RTS) testing is often completed to ensure the athlete is physically ready for sport. Standard testing includes muscle strength (eg, quadriceps and hamstring), function (eg, hop tests), and self-reported knee function (eg, International Knee Documentation Committee).2 However, meeting RTS criteria early (before 8-9 months) after ACL reconstruction (ACLR) may only give the appearance of physical readiness for sport, as reinjury risk is higher compared to those who do not restore function as quickly and delay RTS.2 This raises an important question: What factors contributing to reinjury risk are not captured in standard RTS tests? In this Viewpoint, we explore this question and (1) hypothesize that neuroplasticity is the missing factor in RTS testing and (2) share simple principles and new assessments with preliminary data that can be used to enhance RTS testing.
Neural Compensation After ACL Injury
Anterior cruciate ligament reconstruction causes various impairments such as mechanical instability, pain, fear of movement, and muscle inhibition. Those impairments, along with behavioral changes from rehabilitation and movement compensations, elicit central nervous system adaptations (neuroplasticity).7 The neuroplasticity after ACL injury may give the appearance of adequate dynamic knee joint stability at RTS due to compensatory neural processes.6,7 Athletes with ACLR exhibit elevated cognitive and cross-modal neural activity (BOX) to maintain basic knee motor control (ie, neural compensation).7 Neural compensation after injury may result in increased reliance on visual-cognitive abilities to support fundamental elements of function such as knee proprioception and dynamic stability.6
BOX. Key definitions.
Cognitive neural processing refers to activity within regions, typically in the frontal lobe, that are highly associated with cognitive functioning (attention, working memory, decision-making, etc).
Cross-modal neural processing, specific to post-ACL injury neuroplasticity, refers to activity within regions, namely, within the parietal and occipital lobes, that are important for integrating visual and proprioceptive sensory information (eg, maintaining a safe knee position while navigating around environmental stimuli).
Neural Compensation Manifests as Neurocognitive Reliance
In theory, neural compensation after ACLR manifests as elevated attention toward movements that the athlete used to do without thinking (ie, neurocognitive reliance). Neurocognitive reliance is readily observed following ACLR as the quadriceps experiences arthrogenic muscle inhibition, whereby dedicated focus is needed to initiate and regulate muscle function.10 Neurocognitive reliance persists throughout rehabilitation but may require more targeted detection via cognitive-motor dual-task paradigms in later stages. For instance, years after ACLR, postural stability normalizes except when under visual-cognitive challenges.4
Many clinicians will note that after injury, the level of quadriceps inhibition varies among patients, as well as the degree of neurocognition required to engage in coordinated movements. We hypothesize that individual differences in both cognitive and motor reserves mediate the degree of neurocognitive reliance (FIGURE 1). Reserves can be operationally defined as the total cognitive, or motor, resources available to tolerate compensatory neural processes. The theory is exemplified in patients with ACLR having reduced motor reserve (ie, worse quadriceps force control) requiring elevated neural compensation (increased brain activity).12 However, given the inherent difficulty to evaluate brain activity during RTS testing, we propose clinically feasible proxy tests to evaluate neurocognitive reliance.
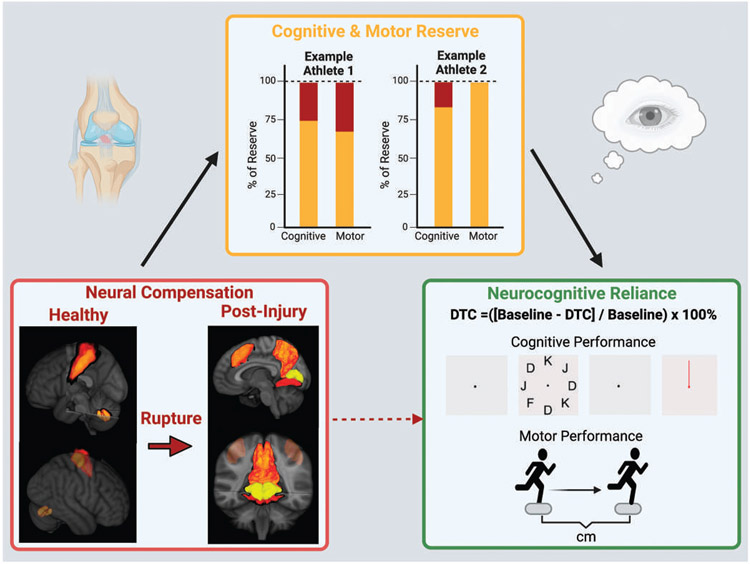
FIGURE 1.
Cognitive and motor reserve modulates neural compensation. Postinjury neuroplasticity and associated neural compensations may not directly cause neurocognitive reliance but be mediated through the athlete’s cognitive and motor reserve. Bottom left figure: Healthy brain activity for knee motor control prior to ACL injury (left side: sensorimotor control regions) and post-ACLR (right side: cognitive and cross-modal neural activity) to represent neural compensation. Top figure: We hypothesize that recovery and observed neurocognitive reliance is contingent on the available cognitive and motor reserves for each athlete (variable due to individual capability). Athlete 1 has less cognitive and motor reserve than Athlete 2 (yellow bar shading). Thus, the influence of neural compensation (red bar shading) would more readily manifest as higher neurocognitive reliance in Athlete 1 relative to Athlete 2 with more reserves and able to accommodate for the neural compensations associated with injury. Bottom right figure: We propose that clinicians evaluate the degree of neurocognitive reliance via integrated neurocognitive and motor assessments (eg, dual-task cost [DTC] with >10% performance decrease). High neurocognitive reliance may indicate lingering effects of neural compensation that may be a liability upon return to sport.
Sport Demand vs RTS Assessment Demand
The transition to sport comes with simultaneous increases in motor coordination complexities and neurocognitive demands. While neurocognitive reliance can preserve elements of function, depending excessively on neurocognitive for motor function in sport may elevate the probability of motor coordination errors.11 Individual differences in cognitive and motor reserves may differentiate athletes who can tolerate neural compensation (avoid injury-risk coordination mechanics). Should the combined cognitive and motor demands exceed reserves, the probability of coordination errors and associated injury increase. Returning to sport brings attentional distractors and unanticipated perturbations (eg, environmental, cognitive, or physical), which may contribute to coordination errors and noncontact ACL injury.8 However, standard RTS tests tend to focus on the injured limb physical performance in isolation, which can be problematic in sports where the athlete needs to pay attention to the environment. To address this issue, tests that better mimic the attention demands of sport could potentially enhance RTS criteria.
Detecting Neurocognitive Reliance
The dual-task paradigm is a classic method used to study how much movement control relies on neurocognition (eg, ). While dual-task neurocognitive challenges such as adding a ball target, static defender, or a quick reaction can expose injury-risk biomechanics,4 RTS tests should evaluate the specific neural compensation associated with ACLR. We suggest that dual tasks are more informative if they target cognitive and cross-modal neural processing during knee motor function. The ideal neurocognitive dual task would distract from or challenge proprioception while also requiring the athlete to make task-relevant decisions (eg, controlling visual attention and occupying working memory). By designing the dual task to occupy compensatory visual cognition, the athlete must depend on implicit, proprioceptive control of movement. In this way, the dual-task cost will reveal the degree the athlete is masking neural compensation via increased neurocognitive reliance. Additive tasks such as isolated working memory challenges (serial 7s) or auditory-based tasks may also be helpful to induce a dual-task cost during a physical task but still may not capture the unique neural compensation strategies seen in athletes after ACLR.
Advancing Return-to-Sport Testing
Many clinically viable testing procedures can test neurocognitive reliance. One method is to augment traditional functional tests such as hop tests with visually mediated response time using a simple light-timing system.13 The neurocognitive hops were designed specifically to target cross-modal neural processing (responding to specific cues [visual] to execute movement [proprioceptive], while avoiding incongruent or distractor cues [cognition]). By completing hops under standard and neurocognitive conditions, a dual-task cost can be calculated to determine the degree of neurocognitive reliance (% hop distance decrease indicating relative reliance on neurocognitive processes for task performance). Using a system to quantify reaction time during functional RTS assessment can not only provide the dual-task condition but also provide visual-motor response time to indicate if the hop distance is maintained at the expense of a slower response. Neurocognitive hop tests can be further modified to include online (during hop) working memory challenges to control attention not only for hop initiation but also throughout the task and particularly during landing (FIGURES 2 and 3).5
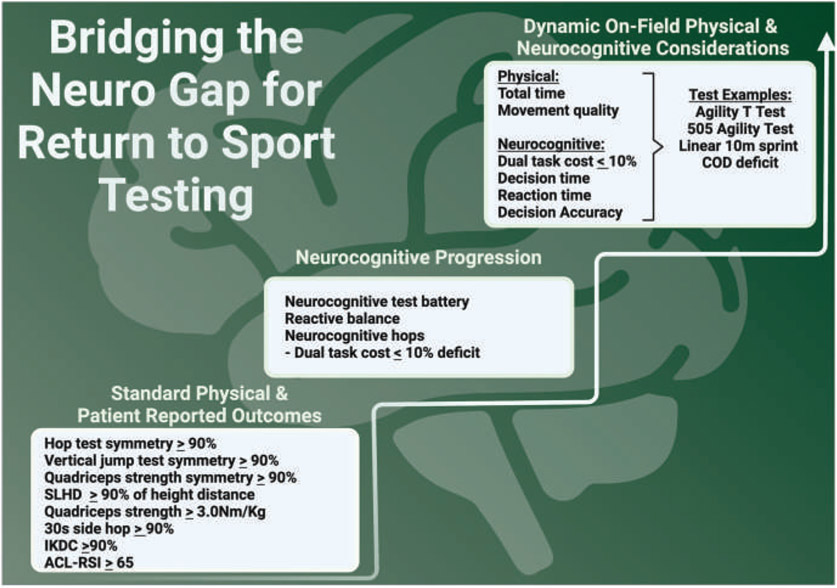
FIGURE 2.
Progressive neurocognitive return-to-sport criteria. Progress RTS testing to capture physical capacity, patient-reported function, and neurocognitive reliance. Once a patient achieves symmetry or sufficient performance on standard physical measures, they are progressed to similar tests under a neurocognitive challenge (calculating dual-task cost). The same approach to adding neurocognitive demand applies for on-the-field and sport-specific testing once an athlete is in later stages of rehabilitation. Athletes who perform poorly during the added neurocognitive challenges may be sufficiently recovered and ready to return to sport. Abbreviations: ACL-RSI, Anterior Cruciate Ligament Return to Sport Index; COD, change of direction; IKDC, International Knee Documentation Committee patient-reported outcome; RSI, reactive strength index; SLHD, single-leg hop distance.
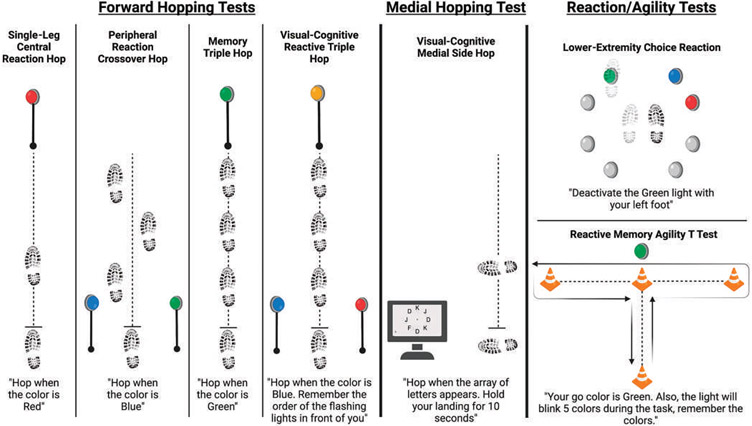
FIGURE 3.
Neurocognitive testing battery. Exemplar tests with evidence supporting reliability and psychometric properties.3,9,13,15 While research is ongoing to validate for RTS assessment to reduce secondary injury risk or improve patient outcomes, their addition carries little additional risk and provides additional sport readiness information. Tests build on previously established functional performance tests to provide a meaningful dual-task cost in addition to metrics of neurocognitive ability (reaction or response time, online working memory, gaze control, visual-spatial attention).
Adding artificial (lights, colors, numbers, etc) cognitive challenges may appear to lack relevance to sport, yet the lights do provide a reliable proxy for detecting neurocognitive reliance. Just as traditional hop or quadriceps strength tests are not sport-specific but are reproducible tests that quantify the foundational physical capacity required for sport performance. In a similar way, non–sports-specific stimuli can assay foundational cognitive-motor reserve. However, just as physical function testing should progress to incorporate more sport-related complexity, cognitive-motor testing should too. Completing established sports performance assessments of linear speed (eg, 10-m sprint time) and change of direction (eg, 5-0-5 agility and Agility T tests) under dual-task conditions can provide additional information about readiness to RTS.1 Clinicians should note that while sport-specific perceptual challenges (avoid defender, track ball, maintain field positions in working memory, plan action relative to changing environment) may have greater ecological validity, they can be limited by a lack of standardization and reliability.
Creative, Low-Tech Solutions
Even if minimal technology is available, detecting neurocognitive reliance and calculating a dual-task cost are possible. Using PowerPoint slides, flashcards (various colors), or even clinician hand signals (eg, even numbers indicate do not jump and an odd number indicates to jump) can induce reactive responses and challenge cognition during motor performance. While these approaches have limited ecological validity, detecting neurocognitive reliance in any form still provides valuable RTS information.
Clinical Targets and Recommendations
Depending on the athlete’s capability and degree of complexity added to the task, a dual-task cost of greater than 10% could indicate excessive neurocognitive reliance.13,14 This recommendation is based on aging and neurological literature, albeit with limited preliminary data in those after ACLR. We acknowledge that more work is needed to calibrate proposed recommendations related to quantifying neurocognitive reliance. Under the clinician’s supervision, we suggest that there is minimal risk to augmenting RTS assessments with a neurocognitive challenge to calculate dual-task cost. Almost any performance measure of movement quality (hop distance, completion time, or mechanics) can be augmented to capture dual-task cost by retesting with a neurocognitive challenge. When possible, capturing neurocognitive performance (reaction time, errors in response accuracy) can indicate the degree each task is prioritized and give additional insight into neurocognitive reliance. Quantifying both motor and cognitive performance can highlight whether an athlete slowed down or made cognitive errors despite the appearance of restored motor function.
To better detect neurocognitive reliance in RTS after ACLR, consider the following.
Conducting RTS functional tests under standard and neurocognitive conditions
- Integrating neurocognitive challenges with physical performance to target cross-modal processing.
- Replicating the demands of sport is ideal, but incorporating cognition in the motor task is preferred relative to adding separate parallel tasks.
- Using neurocognitive augmented RTS tests with demonstrated high reliability and synchronized with task performance (FIGURE 3).
Summary
Detecting neurocognitive reliance in RTS assessments may enhance clinical decision-making for RTS after ACLR. Recovery of isolated musculoskeletal impairments is the minimum prerequisite for RTS but does not ensure the athlete is ready across all the domains of physiology that are affected by injury and competition demands. By integrating neurocognitive challenges during RTS testing, clinicians can detect the degree of neurocognitive reliance and better prepare athletes to RTS. We look forward to the ongoing and future research that aims to better understand the neurocognitive contributors to performance and help athletes safely RTS.
SYNOPSIS:
Neuroplasticity after anterior cruciate ligament (ACL) injury alters how the nervous system generates movement and maintains dynamic joint stability. The postinjury neuroplasticity can cause neural compensations that increase reliance on neurocognition. Return-to-sport testing quantifies physical function but fails to detect important neural compensations. To assess for neural compensations in a clinical setting, we recommend evaluating athletes’ neurocognitive reliance by augmenting return-to-sport testing with combined neurocognitive and motor dual-task challenges. In this Viewpoint, we (1) share the latest evidence related to ACL injury neuroplasticity and (2) share simple principles and new assessments with preliminary data to improve return-to-sport decisions following ACL reconstruction.
Key Points.
Current physical performance-based return-to-sport testing after anterior cruciate ligament reconstruction is not sufficient to prevent new injury.
Nervous system differences for the control of knee movements remain despite anterior cruciate ligament reconstruction, rehabilitation, and return to sport.
Neural compensations to preserve function after injury may be an under-recognized factor contributing to second-injury risk upon return to sport.
Clinicians can detect neural compensation by assessing the degree of neurocognitive reliance for functional performance via dual-task return to sport tests.
Acknowledgments
This study was supported in part by the US Department of Defense Congressionally Directed Medical Research Program Peer Reviewed Orthopaedic Research Program (research award W81XWH-18-1-0707) and National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR076153, R01AR077248). Dustin R. Grooms: consultant for Kinesport Physiotherapy & Inventors share contribution to biofeedback technology licensed between Cincinnati Children’s Hospital Medical Center and IncludeHealth Inc. Gregory D. Myer consults with commercial entities to support commercialization strategies and applications to the US Food and Drug Administration but has no direct financial interest in the products. Dr. Myer’s institution receives current and ongoing grant funding from National Institutes of Health/NIAMS Grants U01AR067997, R01 AR070474, R01AR055563, R01AR076153, R01 AR077248. His addition Dr. Myer has received industry sponsored research funding to his institutions related to injury prevention, sport performance and has current ongoing funding from Arthrex, Inc. to evaluate ACL surgical treatment optimization strategies. Dr. Myer receives author royalties from Human Kinetics and Wolters Kluwer. Dr. Myer is an inventor of biofeedback technologies (Patent No: US11350854B2, Augmented and Virtual reality for Sport Performance and Injury Prevention Application, Approval Date: 06/07/2022, Software Copyrighted) designed to enhance rehabilitation and prevent injuries that receives licensing royalties.
Footnotes
PATIENT AND PUBLIC INVOLVEMENT: No patients were involved in this work. No patient data are reported.
DATA SHARING:
There are no data in this manuscript.
REFERENCES
- 1.Allen T, Wilson S, Cohen DD, Taberner M. Drill design using the ‘control-chaos continuum’: blending science and art during return to sport following knee injury in elite football. Phys Ther Sport. 2021;50:22–35. 10.1016/j.ptsp.2021.02.011 [DOI] [PubMed] [Google Scholar]
- 2.Bodkin SG, Hertel J, Diduch DR, et al. Predicting anterior cruciate ligament reinjury from return-to-activity assessments at 6 months postsurgery: a prospective cohort study. J Athl Train. 2022;57:325–333. 10.4085/1062-6050-0407.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinkman C, Baez SE, Quintana C, et al. The reliability of an upper- and lower-extremity visuomotor reaction time task. J Sport Rehabil. 2021;30:828–831. 10.1123/jsr.2020-0146 [DOI] [PubMed] [Google Scholar]
- 4.Burcal CJ, Needle AR, Custer L, Rosen AB. The effects of cognitive loading on motor behavior in injured individuals: a systematic review. Sports Med Auckl NZ. 2019;49:1233–1253. 10.1007/s40279-019-01116-7 [DOI] [PubMed] [Google Scholar]
- 5.Chaput M, Farraye B, Simon J, Kim H, Monfort S, Grooms D. American Academy of Sports Physical Therapy Platform Presentation Abstracts (SPL1-SPL75). J Orthop Sports Phys Ther. 2022;52:CSM25–CSM54. 10.2519/jospt.2022.52.1.CSM25 [DOI] [Google Scholar]
- 6.Chaput M, Onate JA, Simon JE, et al. Visual cognition associated with knee proprioception, time to stability, and sensory integration neural activity after ACL reconstruction. J Orthop Res. 2022;40:95–104. 10.1002/jor.25014 [DOI] [PubMed] [Google Scholar]
- 7.Criss CR, Melton MS, Ulloa SA, et al. Rupture, reconstruction, and rehabilitation: a multi-disciplinary review of mechanisms for central nervous system adaptations following anterior cruciate ligament injury. The Knee. 2021;30:78–89. 10.1016/j.knee.2021.03.009 [DOI] [PubMed] [Google Scholar]
- 8.Della Villa F, Tosarelli F, Ferrari R, et al. Systematic video analysis of anterior cruciate ligament injuries in professional male rugby players: pattern, injury mechanism, and biomechanics in 57 consecutive cases. Orthop J Sports Med. 2021;9:23259671211048182. 10.1177/23259671211048182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farraye BT, Chaput M, Simon JE, Kim H, Grooms DR, Monfort SM. Development and reliability of a visual-cognitive medial side hop for return to sport testing. Phys Ther Sport. 2022;57:40–45. 10.1016/j.ptsp.2022.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lepley AS, Lepley LK. Mechanisms of arthrogenic muscle inhibition. J Sport Rehabil. 2021;31:1–10. 10.1123/jsr.2020-0479 [DOI] [PubMed] [Google Scholar]
- 11.Sherman DA, Baumeister J, Stock MS, Murray AM, Bazett-Jones DM, Norte GE. Inhibition of motor planning and response selection following ACL reconstruction. Med Sci Sports Exerc. 2023;55:440–449. 10.1249/MSS.0000000000003072 [DOI] [PubMed] [Google Scholar]
- 12.Sherman DA, Baumeister J, Stock MS, Murray AM, Bazett-Jones DM, Norte GE. Weaker quadriceps corticomuscular coherence in individuals following ACL reconstruction during force tracing. Med Sci Sports Exerc. 2023;55:625–632. 10.1249/MSS.0000000000003080 [DOI] [PubMed] [Google Scholar]
- 13.Simon JE, Millikan N, Yom J, Grooms DR. Neurocognitive challenged hops reduced functional performance relative to traditional hop testing. Phys Ther Sport. 2020;41:97–102. 10.1016/j.ptsp.2019.12.002 [DOI] [PubMed] [Google Scholar]
- 14.Venema DM, Hansen H, High R, Goetsch T, Siu KC. Minimal detectable change in dual-task cost for older adults with and without cognitive impairment. J Geriatr Phys Ther. 2019;42:E32–E38. 10.1519/JPT.0000000000000194 [DOI] [PubMed] [Google Scholar]
- 15.Wilke J, Vogel O, Ungricht S. Can we measure perceptual-cognitive function during athletic movement? A framework for and reliability of a sports-related testing battery. Phys Ther Sport. 2020;43:120–126. 10.1016/j.ptsp.2020.02.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no data in this manuscript.