Key Points
-
•
The majority of US patients receiving haploidentical HCT are unlikely to have an 8/8-matched URD.
-
•
Haploidentical transplant receipients are highly likely to have a 7/8-matched URD, expanding access to HCT in ethnically diverse populations.
Visual Abstract
Abstract
The use of haploidentical related donor (HRD) hematopoietic cell transplants (HCTs) in the United States grew by more than fourfold in the last decade, driven mainly by use of posttransplant cyclophosphamide (PTCy)-based graft-versus-host-disease prophylaxis. However, not all patients have a suitable HRD available. In this study, we explored the existence of unrelated donors (URDs) on the National Marrow Donor Program (NMDP) registry at the 8/8- or 7/8-match level for patients receiving HRD HCT in the United States and reporting to the Center for International Blood and Marrow Transplant Research between 2013 and 2020. The data consist of 9696 HRD HCT recipients. The NMDP search prognosis score and a search simulation were used to estimate counts of URD matches on the registry. NMDP search prognosis varied by patient ancestry, with 27.5% non-Hispanic White having a good score compared with 4.6% of African American HRD HCT recipients. Overall, 34% of recipients had ≥1 8/8-matched URDs and 84% had ≥1 7/8 URDs. Recipients of older HRDs (≥35 years) had a likelihood of between 20%- 65% of having ≥5 existing 7/8-matched URDs who were aged ≤35 years. Donor-selection practices varied among the 10 highest-volume HRD centers: 6 had >20% chance of an existing 8/8-matched URD for their HRD recipients, whereas 4 centers had low likelihood of identifying an 8/8-matched URD. In conclusion, although most US patients undergoing HRD HCT do not have an existing 8/8 URD, the majority have an existing 7/8-matched URD. Studies comparing outcomes in patients receiving either HRD or 7/8-matched URD HCT and PTCy-based graft-versus-host disease prophylaxis may be warranted.
Introduction
Reliance on fully HLA-matched unrelated donors (URDs) creates a gap in access to hematopoietic cell transplantation (HCT) for patients with racially/ethnically diverse (ED) backgrounds. This gap can be filled by successful use of HLA-mismatched donors, such as haploidentical related donors (HRDs), HLA-mismatched umbilical cord blood (UCB) donors, and HLA-mismatched unrelated donors (MMUDs), because the criteria for HLA matching in HCT continues to grow less stringent overall. The number of HRD transplants performed in the United States has grown by more than fourfold over the last decade, mainly because of the use of posttransplant cyclophosphamide (PTCy)-based graft-versus-host disease (GVHD) prophylaxis.1 As most ED patients do not have a readily available 8/8-matched unrelated donor (URD) available in the various global registries, this growth has particularly benefited ED patients.1 This trend is reflected in the National Marrow Donor Program (NMDP) search prognosis score, which predicts the likelihood of identifying an 8/8-matched URD on the NMDP registry based on the haplotype frequency for each patient.2 NMDP search prognosis varies substantially according to patient race and ethnicity.
In exploring therapy options, not all patients have a suitable or healthy HRD, and many patients have donor-specific antibodies (DSAs) directed against mismatched HRD HLA antigens that increase the risk of graft rejection and mortality.3 Lack of adequate cell dose often limits the availability of UCB as an alternative.4 Turning to MMUDs, the use of PTCy has now become a standard of care for GVHD prophylaxis for recipients of both HLA-matched and -mismatched donor allografts, generating new opportunities for successful HCT regardless of patient racial/ethnic background.1 The NMDP-sponsored 15-MMUD study used PTCy-based GVHD prophylaxis and demonstrated encouraging overall survival for recipients of MMUD bone marrow grafts from donors matched at ≤7/8 HLA alleles.5 Notably, 48% of the patients on that study were ED. Recent reports observed similar and, in certain circumstances (eg, reduced intensity conditioning), superior outcomes using 8/8-matched or minimally mismatched (7/8) URDs compared with HRDs when PTCy-based prophylaxis was used for all recipients.6,7 A Blood and Marrow Transplant Clinical Trials Network (BMT CTN) multicenter study (BMT CTN 1702) enrolled patients without an HLA-matched family member and used a donor-selection algorithm based on the NMDP search prognosis score and demonstrated similar rates of achieving HCT regardless of whether the patients had a good or poor NMDP search prognosis.
When a well-matched donor was not available, transplant center (TC) practice for alternative donor selection varied; currently, the situation is in a dynamic state in the United States. Center for International Blood and Marrow Transplant Research (CIBMTR) data from 2021 demonstrate a continued increase in the use of HRDs; continued decline in UCB transplantations; and, after a steady decline, a recent rise in the use of MMUDs.8
In light of this dynamic, we leveraged both the CIBMTR outcomes database and the current NMDP registry to address the following questions: (1) in patients who ultimately received an HRD HCT in the United States, did an 8/8- or 7/8-matched URD exist on the NMDP registry, and, if so, were the existing donors potentially younger than the HRD used? (2) How much did the potential existence of a URD vary as per the NMDP search prognosis and patient race/ethnicity? (3) Did search strategy and HRD selection practice vary based on the TC?
Methods
Data collection
The data in this study include HRD transplants (≥2 antigen mismatches) that were reported to the CIBMTR between the years 2013 and 2020. This time frame was chosen to capture a high proportion of patients who received PTCy as prophylaxis against GVHD. Patients who reported ≥1 transplant in this time frame were reduced to 1 entry. All patients included in the analysis provided consent for participation in the CIBMTR Research Database, and the study was approved by the NMDP Institutional Review Board.
NMDP search prognosis
NMDP search prognosis is a high-level categorical summary statistic that describes how difficult a matched URD search will be based on how common or uncommon an HLA genotype is in the population.2 The algorithm analyzes genotype frequency for the patient’s HLA typing and uses a model to predict whether a search will be “good,” “fair,” or “poor.” Good means that the patient is likely (>90% chance) to have a 10/10-matched donor. Fair means that the patient may (∼27% chance) have a 10/10-matched donor but will likely have a 9/10-matched donor. Poor means that the patient will unlikely (<10% chance) have a 10/10-matched donor and may not have a 9/10-matched donor. This original study was reported using x/10, but when the training data were reviewed for x/8-level matching, there were no significant changes observed in the classification results with >97% of 8/8 matched at 10/10. The patient’s typing was used to calculate the NMDP search prognosis within each broad race group in the model (non-Hispanic White, Hispanic, Asian and Pacific Islander, and African American), and the most optimistic result was assigned.
Search status
To ascertain whether a URD search was performed for a patient in this data set, the list was run through an analysis that compares certain patient demographics in the CIBMTR database with those in the NMDP MatchSource database. The fields that matched included sex, name, date of birth, and TC. This resulted in a subset of patients on the list who had highly probable matches to patients who had MatchSource searches with matching attributes.
Search simulation
Search simulation is a novel technique developed by the CIBMTR Bioinformatics Research Team that mimics the Haplogic search process without the dependency on those systems to create an estimate of possible matches on the NMDP registry. Search Simulation queries a preimputed database that includes full imputation results for all member donors on the NMDP registry. The process uses the imputation probabilities (in which the probability of having a genotype is at least 25%) to calculate the expected count of donors that will match a patient at a particular match level. These counts can be tallied to include any donor or for only young donors, defined as those aged from 18 to 35 years. The summary statistics were calculated for patients of all ages, but tables including only adults can be found in the supplemental Data (supplemental Tables 1-5; supplemental Figure 1).
Results
Study population
The analysis initially included patients who received HRD HCT reported to the CIBMTR from 2013 to 2020 (n = 14 503). For this analysis, high-resolution (2-field), unambiguous HLA typing at HLA-A, HLA-B, HLA-C, and HLA-DRB1 was required. If the available typing for a patient included serology, allele codes, or was missing, their typing was imputed, when possible, to attain the required level of typing. Any patient with HLA-typing ambiguity that could not be resolved was excluded (n = 2297; 15.8%). Patients outside of the United States (n = 2510; 20.6%) were excluded to focus the analysis on US centers only. The resulting analysis included 9696 US recipients with a median age of 50 years (range, 0-87 years). The patients were predominately non-Hispanic White (NHW; 53.3%) followed by African American (AFA; 19.2%), Hispanic (17.1%), and Asian/Pacific Islander (5.5%; Table 1). The majority of patients received HRD HCTs for hematological malignancies.
Table 1.
Characteristics of patients receiving haploidentical transplant between 2013 and 2020 at a US TC, reported to the CIBMTR and with sufficient HLA typing for evaluation
| Characteristic | |
|---|---|
| Number of patients | 9696 |
| No. of centers | 180 |
| Patient related | |
| Patient age, y | |
| Median (range) | 50 (0-87) |
| Age group, y (by decade), n (%) | |
| 0-9 | 759 (7.8) |
| 10-19 | 900 (9.3) |
| 20-29 | 1077 (11.1) |
| 30-39 | 877 (9) |
| 40-49 | 1114 (11.5) |
| 50-59 | 1845 (19) |
| 60-69 | 2424 (25) |
| 70-79 | 695 (7.2) |
| ≥80 | 5 (0.1) |
| Broad race, n (%) | |
| African American | 1863 (19.2) |
| Asian and Pacific Islander | 532 (5.5) |
| Non-Hispanic White | 5172 (53.3) |
| Hispanic | 1659 (17.1) |
| Multiple race | 172 (1.8) |
| Native American | 41 (0.4) |
| Unknown | 257 (2.7) |
| Sex, n (%) | |
| Male | 5745 (59.3) |
| Female | 3951 (40.7) |
| Donor related | |
| Age, y | |
| Median (range) | 35 (0-77) |
| Age group, y (by decade), n (%) | |
| 0-9 | 72 (0.7) |
| 10-19 | 800 (8.3) |
| 20-29 | 2430 (25.1) |
| 30-39 | 2880 (29.7) |
| 40-49 | 1996 (20.6) |
| 50-59 | 1027 (10.6) |
| 60-69 | 419 (4.3) |
| ≥70 | 31 (0.3) |
| Missing | 41 (0.4) |
| Sex, n (%) | |
| Male | 5746 (59.3) |
| Female | 3949 (40.7) |
| Missing | 1 (0) |
| Disease related | |
| Primary disease, n (%) | |
| Autoimmune disorder | 7 (0.1) |
| Acute lymphoid leukemia | 1608 (16.6) |
| Acute myeloid leukemia | 3694 (38.1) |
| Chronic myeloid leukemia | 289 (3) |
| Hodgkin lymphoma | 256 (2.6) |
| Histiocytic disorders | 52 (0.5) |
| Hemoglobinopathies | 291 (3) |
| Inherited immune disorders | 193 (2) |
| Inherited disorders of metabolism | 30 (0.3) |
| Inherited platelet abnormalities | 2 (0) |
| Inherited bone marrow failure syndromes | 44 (0.5) |
| Myelodysplastic syndromes | 1281 (13.2) |
| Myeloproliferative neoplasms | 281 (2.9) |
| Non-Hodgkin lymphoma | 851 (8.8) |
| Other disease | 11 (0.1) |
| Other acute leukemia | 119 (1.2) |
| Other leukemia | 156 (1.6) |
| Plasma cell disorders | 159 (1.6) |
| Paroxysmal nocturnal hemoglobinuria | 13 (0.1) |
| Severe aplastic anemia | 310 (3.2) |
| Solid tumor | 49 (0.5) |
| Transplantation related | |
| GVHD prophylaxis, n (%) | |
| PTCy | 8400 (86.6) |
| Other | 796 (8.2) |
| Unknown | 500 (5.2) |
| Transplantation year, n (%) | |
| 2013 | 304 (3.1) |
| 2014 | 653 (6.7) |
| 2015 | 944 (9.7) |
| 2016 | 1178 (12.1) |
| 2017 | 1433 (14.8) |
| 2018 | 1582 (16.3) |
| 2019 | 1787 (18.4) |
| 2020 | 1815 (18.7) |
Total number of patients included, N = 9696.
NMDP search prognosis
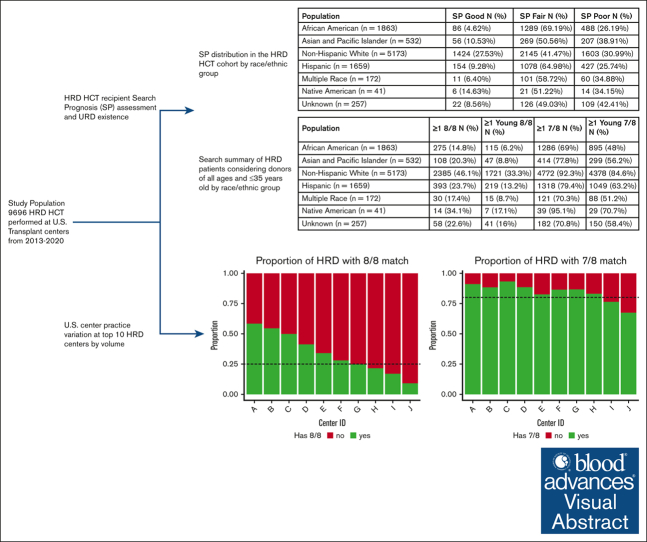
The NMDP search prognosis score for the cohort varied according to patient race/ethnicity. Good NMDP search prognosis scores varied from 4.6% for AFA to 27.5% for NHW patients (Table 2). Most patients had a fair (range, 41.5%-69.2%) or poor (range, 25.7%-38.9%) NMDP search prognosis. Of the 9696 US patients included in the analysis, 7995 (82.4%) were identified who were highly likely to have had a search through the NMDP registry. The NMDP search prognosis score distributions for the subset of patients that searched the NMDP registry were similar to those for the full population (supplemental Table 6).
Table 2.
Search prognosis distribution in the HRD HCT cohort based on race/ethnic group
| Population | Good search prognosis, n (%) | Fair search prognosis, n (%) | Poor search prognosis, n (%) |
|---|---|---|---|
| AFA (n = 1863) | 86 (4.62%) | 1289 (69.19%) | 488 (26.19%) |
| Asian and Pacific Islander (n = 532) | 56 (10.53%) | 269 (50.56%) | 207 (38.91%) |
| NHW (n = 5173) | 1424 (27.53%) | 2145 (41.47%) | 1603 (30.99%) |
| Hispanic (n = 1659) | 154 (9.28%) | 1078 (64.98%) | 427 (25.74%) |
| Multiple race (n = 172) | 11 (6.40%) | 101 (58.72%) | 60 (34.88%) |
| Native American (n = 41) | 6 (14.63%) | 21 (51.22%) | 14 (34.15%) |
| Unknown (n = 257) | 22 (8.56%) | 126 (49.03%) | 109 (42.41%) |
Matched URD and MMUD existence on the NMDP registry
The existence of an 8/8-matched donor of any age varied based on patient race/ethnicity, ranging from 14.8% for AFA to 46.1% for NHW patients. When considering only young donors (aged ≤35 years), the existence dropped to 6.2% for AFA and 33.3% for NHW. However, donor existence, regardless of recipient ancestry, increased substantially when 7/8-matched donors were included in the evaluation, ranging from 69% to 95.1% and 48% to 84.6% when considering donors of all ages and donors restricted to those aged ≤35 years, respectively (Table 3). To adjust for donor availability, we also considered the existence of ≥5 8/8- or 7/8-matched donors with a notable decrease in existence rates (Table 4). Next, we evaluated the existence of younger 8/8- and 7/8-matched donors for those patients receiving HRD HCT from an older related donor (aged ≥35 years). Among those patients who received an HRD HCT from an older donor, between 6.2% and 33.3% had an existing younger 8/8-matched donor, depending on race/ethnicity. When considering a mismatch, 50.4% to 84.2% had an existing younger 7/8-matched donor (Table 5). The Search Simulation process has the capacity to query as low as a 4/8-matched level. Data for 5/8 and 6/8 matches are included in the supplemental Results (supplemental Table 7). In the supplemental Data, we also include numbers for restricting the location of the 7/8 mismatch at either class 1 or class 2 (supplemental Table 8) and cytomegalovirus infection status (supplemental Table 9).
Table 3.
Percentage of recipients of HRD HCT who had at least 1 8/8 or 7/8-matched URD on the NMDP registry, considering donors of all ages and of age 35 years or less according to race/ethnic group
| Population | At least 1 8/8 matched, n (%) | At least 1 young 8/8 matched, n (%) | At least 1 7/8 matched, n (%) | At least 1 young 7/8 matched, n (%) |
|---|---|---|---|---|
| AFA (n = 1863) | 275 (14.8%) | 115 (6.2%) | 1286 (69%) | 895 (48%) |
| Asian and Pacific Islander (n = 532) | 108 (20.3%) | 47 (8.8%) | 414 (77.8%) | 299 (56.2%) |
| NHW (n = 5173) | 2385 (46.1%) | 1721 (33.3%) | 4772 (92.3%) | 4378 (84.6%) |
| Hispanic (n = 1659) | 393 (23.7%) | 219 (13.2%) | 1318 (79.4%) | 1049 (63.2%) |
| Multiple Race (n = 172) | 30 (17.4%) | 15 (8.7%) | 121 (70.3%) | 88 (51.2%) |
| Native American (n = 41) | 14 (34.1%) | 7 (17.1%) | 39 (95.1%) | 29 (70.7%) |
| Unknown (n = 257) | 58 (22.6%) | 41 (16%) | 182 (70.8%) | 150 (58.4%) |
Table 4.
Percentage of recipients of HRD HCT who had at least 5 8/8- or 7/8-matched URDs on the NMDP registry, considering donors of all ages and of age 35 years or less according to race/ethnic group
| Population | At least 5 8/8 matched, n (%) | At least 5 young 8/8-matched, n (%) | At least 5 7/8 matched, n (%) | At least 5 young 7/8 matched, n (%) |
|---|---|---|---|---|
| AFA (n = 1863) | 44 (2.4%) | 15 (0.8%) | 786 (42.2%) | 323 (17.3%) |
| Asian and Pacific Islander (n = 532) | 27 (5.1%) | 8 (1.5%) | 254 (47.7%) | 116 (21.8%) |
| NHW (n = 5173) | 1455 (28.1%) | 992 (19.2%) | 4251 (82.2%) | 3455 (66.8%) |
| Hispanic (n = 1659) | 113 (6.8%) | 42 (2.5%) | 944 (56.9%) | 542 (32.7%) |
| Multiple race (n = 172) | 6 (3.5%) | 4 (2.3%) | 72 (41.9%) | 37 (21.5%) |
| Native American (n = 41) | 5 (12.2%) | 1 (2.4%) | 27 (65.9%) | 19 (46.3%) |
| Unknown (n = 257) | 23 (8.9%) | 12 (4.7%) | 147 (57.2%) | 89 (34.6%) |
Table 5.
Percentage of patients receiving HCT from an older HRD (aged more than 35 years) who had at least 1 young (aged at least 35 years) 8/8- or 7/8-matched URD on the NMDP registry according to race/ethnic group
| Population | At least 1 young 8/8 matched, n (%) | At least 5 young 8/8 matched, n (%) | At least 1 young 7/8 matched, n (%) | At least 5 young 7/8 matched, n (%) |
|---|---|---|---|---|
| AFA (n = 940) | 68 (7.2%) | 8 (0.9%) | 474 (50.4%) | 188 (20%) |
| Asian and Pacific Islander (n = 236) | 21 (8.9%) | 5 (2.1%) | 138 (58.5%) | 51 (21.6%) |
| NHW (n = 2785) | 927 (33.3%) | 554 (19.9%) | 2344 (84.2%) | 1832 (65.8%) |
| Hispanic (n = 752) | 106 (14.1%) | 19 (2.5%) | 474 (63%) | 247 (32.8%) |
| Multiple race (n = 69) | 5 (7.2%) | 1 (1.4%) | 39 (56.5%) | 19 (27.5%) |
| Native American (n = 16) | 1 (6.2%) | 0 (0%) | 10 (62.5%) | 6 (37.5%) |
| Unknown (n = 126) | 15 (11.9%) | 4 (3.2%) | 75 (59.5%) | 43 (34.1%) |
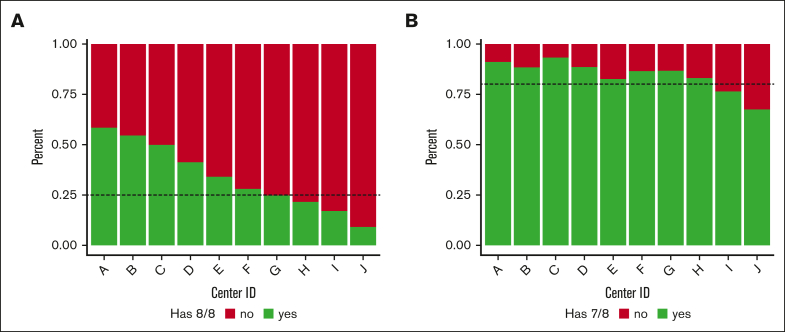
US TC practice variation
To determine the degree of practice variation among US TCs that use HRD HCT, we focused on the 10 TCs performing >150 HRD HCTs in the US during the study period. There was a clear delineation among centers considering HRD usage and existence of an 8/8-matched URD on the NMDP registry. At 6 of these TCs, search simulations identified at least 1 existing 8/8-matched URD in >25% of the patients ultimately receiving HRD HCT (range, 27.9%-58.4%), whereas at the remaining 4 TCs, <25% (range, 9.1%-24.7%) of the simulations identified an existing 8/8-matched URD. At all but 2 of these 10 TCs, >80% of the recipient simulations yielded at least 1 existing 7/8-matched URD (Figures 1A-B). These results align with the search prognosis distribution for each of the individual centers (Table 6). Six of these TCs performed searches for at least 90% of the patients who received HRD HCT at their sites, whereas at the remaining 4 TCs, this value ranged from 48.9% to 84.9% (Table 6).
Figure 1.
URD existence for HRD HCT recipients treated at the 10 highest volume US Centers. (A) Percent of 8/8-match existence for HRD HCT recipients treated at the highest-volume HRD centers, highlighting a potential center preference/prioritization of HRD donors. The portion shown in green represents the percent of patients who received HRD HCT at that center who had an existing 8/8-matched URD, whereas the portion in red represents the patients who did not have an existing 8/8-matched URD. The dotted line illustrates the observation that 6 of 10 centers had existing 8/8-matched URDs for at least 25% of these patients. (B) Percent of 7/8–matched existence for recipients of HRD HCT treated at the highest-volume HRD centers, highlighting a potential center preference/prioritization of HRD donors. The portion shown in green represents the percent of patients who received an HRD HCT at that center who had an existing 7/8-matched URD, whereas the portion in red represents the patients who did not have an existing 7/8-matched URD. The dotted line illustrates the observation that 8t of 10 centers had existing 7/8-matched URDs for at least 80% of these patients.
Table 6.
Percent of patients with a probable search ID at NMDP and NMDP search prognosis score distributions at the highest-volume HRD TCs
| Center ID | TCs that ran a search (%) | Good search prognosis (%) | Fair search prognosis (%) | Poor search prognosis (%) |
|---|---|---|---|---|
| A | 48.9 | 38.5 | 37.6 | 23.9 |
| B | 84.9 | 29.0 | 53.4 | 17.6 |
| C | 95.7 | 33.7 | 39.6 | 26.7 |
| D | 96.6 | 25.4 | 40.1 | 34.5 |
| E | 90.7 | 21.6 | 48.1 | 30.2 |
| F | 72.1 | 11.5 | 60.6 | 27.9 |
| G | 94.8 | 10.3 | 62.6 | 27.0 |
| H | 95.2 | 6.6 | 61.4 | 32.0 |
| I | 64.3 | 3.5 | 56.1 | 40.4 |
| J | 97.3 | 2.1 | 58.5 | 39.3 |
Discussion
Herein, we explored URD existence on the NMDP registry at the 8/8- or 7/8-match level in >9600 patients in the United States receiving HRD HCT from 2013 to 2020. We found that the majority of recipients were unlikely to have an existing 8/8-matched URD but that >80% of patients were likely to have at least 1 7/8-matched donor, and, in many cases, >5 7/8-matched donors aged <35 years had been identified. As hypothesized, URD existence differed based on NMDP search prognosis score, which, in most instances, correlated to patient race/ethnicity. Interestingly, among the TCs performing the most HRD transplants, we identified an apparent dichotomy in donor-selection practices. In 6 of the top 10 US TCs, URD searches were either not undertaken or at least 25% of their HRD HCT recipients had an available 8/8-matched URD. This was particularly the case for their NHW patients. In contrast, an 8/8-matched URD for patients receiving HRD HCT at 4 of the high-volume TCs were generally very unlikely to be identified. However, at all 10 TCs, young 7/8-matched donors (aged 18-35 years) could have been identified for most of the recipients of HRD HCT. The recent growth in the number of MMUD transplantations performed in the United States suggests there may be a shift in TC donor-selection practice over time, and it will be interesting to follow trends within the CIBMTR database.8 These shifts may be further accelerated by adoption of PTCy-based GVHD prophylaxis as a standard of care for all donor types at an increasing number of US TCs, but the answer to this requires additional observational and prospective studies.9
For patients lacking an HLA-matched related donor, an approach based on NMDP search prognosis score should be very useful in guiding donor selection and may facilitate early alternative donor use in patients with a poor NMDP search prognosis. This was recently demonstrated in the secondary analysis of the BMT CTN 1702 study.10 In that study, alternative donors were defined as any donor other than an HLA-matched or 1 antigen-mismatched related donor. The primary comparison for the interventional study was the overall survival based on biologic assignment and was analyzed on an intention-to-treat basis: in arm 1 of the study, patients with a good NMDP search prognosis score who were very likely to find an 8/8-matched URD were assigned to pursue the fully matched URD; in arm 2, patients who were very unlikely to find an 8/8-matched URD, defined as having a <10% chance, were assigned to pursue an alternative donor. In a secondary analysis of the study recently presented, the cumulative incidence of receiving HCT according to the search prognosis was similar among the 2 arms, and despite the large, multicenter design, there was a high degree of compliance with the intended search/selection strategy.10 This approach promises to assure timely and equitable access to the most appropriate donor, regardless of patient ancestry. The primary comparison of overall survival between the 2 arms awaits further patient follow-up.
The rationale for selection of an HRD over an 8/8-matched URD is based on a number of factors, both real and theoretical.11 HRD may be more readily available than URD. The NMDP has developed tools to improve its ability to predict donor availability based on a recently developed machine-learning tool called the donor readiness score, or DRS.12 The DRS is based on several factors easily obtained from potential donors and predicts the likelihood that a particular donor will agree to donation when asked. The recently developed NMDP search summary score may be a further enhancement over the NMDP search prognosis score because it combines Haplogic match probabilities and the DRS to generate a score between 0 and 1 that predicts both donor existence and donor availability.13 The NMDP search summary score awaits further validation for utility in predicting URD availability. Notwithstanding, HRD may be more readily available than URD.
Another factor presumed to favor the selection of an HRD over a URD is accelerated time to donor work-up and graft procurement. For patients in urgent need of HCT, this has been posited as a reason to select an HRD over a URD.11 Indeed, the data from this study suggest that patients with acute indications for HCT (eg, acute leukemia and myelodysplastic syndrome) were more likely to undergo transplantation using an HRD than patients having a so-called “nonacute” indication (eg, hemoglobinopathy or immune deficiency). Although urgency is often cited as a reason for HRD preference, evidence in support of this premise within the published literature is limited. Data on time from initial diagnosis to HCT in most studies comparing outcomes of HRD with those of URD have not shown clear differences6,7; however, this may not be a good reference point. Here, again, the BMT CTN 1702 study can shed some light. The vast majority of HCT cancellations and postponements reported by the TCs on that study were because of patient-related reasons and not because of donor issues.10 Therefore, donor-selection practice based on predicted outcome of HCT considering individual patient characteristics, available donor characteristics, and intended therapeutic approach (eg, planned intensity of conditioning and choice of GVHD prophylaxis) may yield better aggregate patient outcomes for TCs. The availability of high-quality prospective clinical trials should also strongly influence donor selection.
In the absence of an available 8/8-matched URD, limited high-quality data are available to guide the choice between selecting an HRD or a 7/8-matched URD. Recent nonrandomized data suggest that with the use of PTCy or abatacept for GVHD prophylaxis, no substantial differences in overall survival is likely between the 2 donor options.5,14,15 In a recent observational study by the European Society for Blood and Marrow Transplantation, outcomes favored the recipients of HCT from 9/10-matched URDs over those undergoing HRD HCT, but patient numbers were limited.16 Given the shrinking family sizes in recent years, the availability of HRD in the future may become more limited. In addition, greater options may exist among 8/8- and 7/8-matched URDs to select for other favorable characteristics, including donor age, cytomegalovirus serostatus, and ABO blood group. In addition, URDs may lack heritable genetic traits that can be carried by related donors. Finally, DSAs are well known to complicate HRD selection, so in many circumstances, the sheer volume of potentially available 7/8-matched URD facilitates selection of a donor lacking a mismatched allele against which the DSAs are directed.
Finally, we noted substantial practice variation among the US TCs. Although most TCs performing large numbers of HRD transplants during the study period appeared to be following an algorithm similar to that of the BMT CTN 1702 study, a minority of the large TCs seem to have a clear preference for HRDs. This may be reasonable based on their substantial experience and the favorable outcomes observed. Whether there may also be financial considerations (eg, cost of donor procurement) influencing these decisions remain purely speculative. However, we found that the vast majority of recipients of HRD in the United States were unlikely to have an existing 8/8-matched URD. Another point is that practice varies based on age of the recipient. In adults, most of the growth in the use of HRD HCT has been driven by use of PTCy-based GVHD prophylaxis, whereas in children, many of the HRD transplantations are performed using some form of T-cell depletion, such as, α/β T-cell depletion, given the favorable results with that approach in the pediatric setting.1
There are several limitations to this analysis. A substantial fraction of patients were excluded based on the available HLA-typing resolution, generating a potential source of bias. The analysis only considered US patients, and practices vary globally. The search simulation method is less stringent than a standard clinical search in that it adds a probability cutoff of 25%. This specifically biases the search against donors with low-resolution typing although this is offset somewhat by the age constraint. It is also important to note that these simulations are carried out using the current state of the registry and not the registry at the time of the original search. There is also a difference between the existence of a donor and the existence of an available donor. New methods for providing individualized predictions of donor availability could be applied to future analyses to more accurately determine the existence of an available donor who will be able to proceed with donation.12 The study period included patients who mostly underwent HCT before the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic. During the SARS-CoV-2 pandemic, logistical challenges caused a temporary decline in the use of URDs in the United States as well as an increase in the use of cryopreserved donor products. The use of HRDs continued to increase during this pandemic because related donors were generally more readily available. It is not yet clear how lessons learned during the SARS-CoV-2 pandemic may affect selection of related donors vs URDs, although recent data from the CIBMTR demonstrate rising demand for both matched and mismatched URDs.8
In conclusion, these data demonstrate that most patients in the United States undergoing HRD HCT are unlikely to have an available 8/8-matched URD. However, we show the existence of a 7/8-matched URD for most candidates of HRD HCT and predict that options will further increase when considering additional levels of HLA mismatch (eg, 5/8 and 6/8).17 A prospective randomized comparative study of outcomes in patients receiving HCT from HRDs vs from URDs was deemed to be high priority during the recent state of the science symposium conducted by the Blood and Marrow Transplant Clinical Trials Network.18 Our analysis supports the feasibility of such a study. This will generate greater opportunities for patients to benefit from HCT regardless of racial or ethnic background and will support the growth of prospective clinical research in HCT involving a more diverse patient demographic than ever thought possible.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Acknowledgments
The CIBMTR is supported primarily by the US Public Health Service U24CA076518 from the National Cancer Institute, the National Heart, Lung, and Blood Institute, and the National Institute of Allergy and Infectious Diseases; 75R60222C00011 from the Health Resources and Services Administration; N00014-21-1-2954 and N00014-23-1-2057 from the Office of Naval Research. Support was also provided by Be The Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, Gateway for Cancer Research, and Pediatric Transplantation and Cellular Therapy Consortium and from the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc; Adaptimmune; Adaptive Biotechnologies Corporation; ADC Therapeutics; Adienne SA; Allogene; Allovir, Inc; Amgen, Inc; Angiocrine; Astellas Pharma US; Atara Biotherapeutics; BeiGene; bluebird bio, Inc; Bristol Myers Squibb Co; CareDx Inc; CSL Behring; CytoSen Therapeutics, Inc; Elevance Health; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Gamida-Cell, Ltd; GlaxoSmithKline; HistoGenetics; Incyte Corporation; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc; Karius; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Mallinckrodt Pharmaceuticals; Merck & Co; Mesoblast; Millennium, the Takeda Oncology Co; Miltenyi Biotec, Inc; MorphoSys; Novartis Pharmaceuticals Corporation; Omeros Corporation; OptumHealth; Orca Biosystems, Inc; Ossium Health, Inc; Pfizer, Inc; Pharmacyclics, LLC, an AbbVie Company; PPD Development, LP; Regimmune; Sanofi; Sarah Cannon; Sobi, Inc; Stemcyte; Takeda Pharmaceuticals; Talaris Therapeutics; Vertex Pharmaceuticals; Vor Biopharma Inc; and Xenikos BV.
Authorship
Contribution: S.F. designed and performed the analysis and wrote the manuscript; S.R.S. and S.M.D. advised in analysis design and wrote the manuscript; Y.-T.B. and M.M. advised in analysis design and revised the manuscript; and all authors approved the final manuscript.
Footnotes
Data are available on request from the corresponding author, Stephen R. Spellman (sspellma@nmdp.org).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Auletta JJ, Kou J, Chen M, et al. Real-world data showing trends and outcomes by race and ethnicity in allogeneic hematopoietic cell transplantation: a report from the Center for International Blood and Marrow Transplant Research. Transplant Cell Ther. 2023;29(6):346.e1–346.e10. doi: 10.1016/j.jtct.2023.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wadsworth K, Albrecht M, Fonstad R, Spellman S, Maiers M, Dehn J. Unrelated donor search prognostic score to support early HLA consultation and clinical decisions. Bone Marrow Transplant. 2016;51(11):1476–1481. doi: 10.1038/bmt.2016.162. [DOI] [PubMed] [Google Scholar]
- 3.Ciurea SO, Cao K, Fernandez-Vina M, et al. The European Society for Blood and Marrow Transplantation (EBMT) Consensus Guidelines for the detection and treatment of donor-specific anti-hla antibodies (DSA) in haploidentical hematopoietic cell transplantation. Bone Marrow Transplant. 2018;53(5):521–534. doi: 10.1038/s41409-017-0062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Politikos I, Davis E, Nhaissi M, et al. Guidelines for cord blood unit selection. Biol Blood Marrow Transplant. 2020;26(12):2190–2196. doi: 10.1016/j.bbmt.2020.07.030. [DOI] [PubMed] [Google Scholar]
- 5.Shaw BE, Jimenez-Jimenez AM, Burns LJ, et al. National Marrow Donor Program-sponsored multicenter, phase ii trial of HLA-mismatched unrelated donor bone marrow transplantation using post-transplant cyclophosphamide. J Clin Oncol. 2021;39(18):1971–1982. doi: 10.1200/JCO.20.03502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gooptu M, Romee R, St Martin A, et al. HLA-haploidentical vs matched unrelated donor transplants with posttransplant cyclophosphamide-based prophylaxis. Blood. 2021;138(3):273–282. doi: 10.1182/blood.2021011281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mussetti A, Kanate AS, Wang T, et al. haploidentical versus matched unrelated donor transplants using post-transplantation cyclophosphamide for lymphomas. Transplant Cell Ther. 2023;29(3):184.e1–184.e9. doi: 10.1016/j.jtct.2022.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolon YT AR, Allbee-Johnson M, Estrada-Merly N, Lee SJ. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR summary slides. 2022. https://cibmtr.org/CIBMTR/Resources/Summary-Slides-Reports
- 9.Bolaños-Meade J, Hamadani M, Wu J, et al. Post-transplantation cyclophosphamide-based graft-versus-host disease prophylaxis. N Engl J Med. 2023;388(25):2338–2348. doi: 10.1056/NEJMoa2215943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dehn J LB, He N, Shaw BE, et al. A novel donor search and selection algorithm facilitates a comparable incidence of transplant for patients regardless of baseline search prognosis: report from the BMTCTN 1702 trial [abstract] Bone Marrow Transplant. 2023;58(58):150–152. [Google Scholar]
- 11.McCurdy SR, Luznik L. How we perform haploidentical stem cell transplantation with posttransplant cyclophosphamide. Blood. 2019;134(21):1802–1810. doi: 10.1182/blood.2019001323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sivasankaran A, Williams E, Albrecht M, Switzer GE, Cherkassky V, Maiers M. Machine learning approach to predicting stem cell donor availability. Biol Blood Marrow Transplant. 2018;24(12):2425–2432. doi: 10.1016/j.bbmt.2018.07.035. [DOI] [PubMed] [Google Scholar]
- 13.Fingerson S BY, Garrett N, Wadsworth K, Maiers M. Search summary: the next generation of rapid assessment for unrelated bone marrow donor searches. Hum Immunol. 2021;82(suppl):179. [Google Scholar]
- 14.Al Malki MM, Tsai NC, Palmer J, et al. Posttransplant cyclophosphamide as GVHD prophylaxis for peripheral blood stem cell HLA-mismatched unrelated donor transplant. Blood Adv. 2021;5(12):2650–2659. doi: 10.1182/bloodadvances.2021004192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watkins B, Qayed M, McCracken C, et al. Phase II trial of costimulation blockade with abatacept for prevention of acute GVHD. J Clin Oncol. 2021;39(17):1865–1877. doi: 10.1200/JCO.20.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Battipaglia G, Galimard JE, Labopin M, et al. Post-transplant cyclophosphamide in one-antigen mismatched unrelated donor transplantation versus haploidentical transplantation in acute myeloid leukemia: a study from the Acute Leukemia Working Party of the EBMT. Bone Marrow Transplant. 2022;57(4):562–571. doi: 10.1038/s41409-022-01577-x. [DOI] [PubMed] [Google Scholar]
- 17.Chowdhury AS, Maiers M, Spellman SR, Deshpande T, Bolon YT, Devine SM. Existence of HLA-mismatched unrelated donors closes the gap in donor availability regardless of recipient ancestry. Transplant Cell Ther. 2023;29(11):686.e1-686.e8 doi: 10.1016/j.jtct.2023.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heslop HE, Stadtmauer EA, Levine JE, et al. Blood and Marrow Transplant Clinical Trials Network State of the Science Symposium 2021: looking forward as the network celebrates its 20th year. Transplant Cell Ther. 2021;27(11):885–907. doi: 10.1016/j.jtct.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.