Abstract
It is often stated that impaired immune functions in the aged underlie their greater susceptibility to infections. Indeed, in many experimental settings, T-cell responses in aged mice have been shown to be deficient compared with those from young adults. Nonetheless, there are very few examples where a greater susceptibility to infection in aged mice has been demonstrated to result from impaired T-cell function. The clinical importance of understanding the basis for increased susceptibility to infection that accompanies advanced age dictates a need for experimental models with which to study the effect that aging has on immunological resistance to infection. This study was undertaken to investigate whether aged mice were less resistant than young adult control mice to infection with the fungus Cryptococcus neoformans. After a primary intravenous challenge with yeast, aged mice died sooner and developed higher organ burdens of yeast than did young adults. Deficient in vitro responses were observed in T cells from aged mice; however, greater susceptibility to intravenous infection appeared not to result from less effective T-cell-dependent resistance in vivo. In fact, T-cell-replete aged mice were more susceptible to intravenous cryptococcal infection than were T-cell-depleted young adults. Furthermore, aged mice were as resistant to primary pulmonary challenge with Cryptococcus as were young adults. Similarly, vaccinated aged mice were as resistant to rechallenge as were young adult counterparts. Therefore, despite demonstrably deficient in vitro responses of T cells from aged mice, their T-cell-dependent resistance to C. neoformans is as effective as that of young adults.
Immune function is widely reported to decline with age. Defects in humoral immunity (6, 7, 13), especially in the ability to generate particular antibody isotypes (3), in macrophage function (10, 11), and most frequently in T-cell function have been reported. T cells of aged mice are reported to be deficient in production of interleukin-2 (IL-2) (31, 37, 48), IL-3 (7), IL-4 (18), and IL-10 (29). CD4+ T-cell populations in aged mice are reported to be skewed toward a preponderance of cells displaying activation markers, such as CD45RBlo, presumably as the result of in increase with age in the proportion of memory T cells (12, 14, 29, 48).
Cryptococcus neoformans is a ubiquitous opportunistic fungal pathogen which if inhaled typically causes a mild pulmonary infection in immunocompetent individuals. In individuals with deficiencies in cellular immunity, yeast cells disseminate from the primary site of infection and seed distant sites, most notably the brain. Proliferation in the brain causes a potentially life-threatening meningoencephalitis (28, 35, 43). Curiously, although it is well established that resistance to C. neoformans infection is largely mediated by T cells (15, 16, 20, 21, 36–38) and T-cell-derived cytokines (1, 8, 9, 17, 19, 26, 30, 32, 33, 41), and the aging are widely reported to suffer declining T-cell function, the aging population has not been reported to be at greater risk of suffering cryptococcal disease than are young adults. This might be due to lifestyle differences between the elderly and young adults, decreased opportunity of becoming infected, or compensatory increases in innate resistance mechanisms in the elderly, but there are few data to support these hypotheses. It is more likely that the reported T-cell deficiencies of the elderly, which have been the focus of much research, are so subtle as to be without functional consequence in terms of real susceptibility to many infectious agents. The present study was undertaken to investigate whether, absent differences in exposure to infectious agent (C. neoformans) and/or lifestyle differences, deficient T-cell function rendered aged mice less resistant to cryptococcal infection than their young adult counterparts.
In the murine model, it has been shown that both CD4+ and CD8+ T cells are required for control and eventual clearance of yeast from the lungs (15, 16, 20, 21, 36–38), and CD4+ T cells are required to limit both dissemination of yeast cells from the lungs (15) and further proliferation once the cells are established in foci of infection in the brain (16). Additionally, the cytokines tumor necrosis factor (TNF) (1, 8, 9, 30), gamma interferon (1, 8, 17, 26, 32, 41), and granulocyte-macrophage colony-stimulating factor (8, 9) have been implicated in resistance to C. neoformans infection.
We hypothesized that if important functional deficiencies exist in T cells or T-cell-derived cytokines of aged mice, then aged mice should have greater difficulty than identically housed and fed young adult mice in resisting an equivalent dose of C. neoformans. Such a finding would provide an important model system with which to study the effects of age-related impairment in T-cell function in resistance to infection. However, we report here that despite greater susceptibility to intravenous infection with C. neoformans in aged mice, the evidence does not implicate deficient T-cell responses as a cause.
MATERIALS AND METHODS
Mice.
Aged (22 to 24 months old) and young adult (2 to 3 months old) (A/J × C57BL/6J)F1 (abbreviated AB6F1) mice and (C57BL/6J × DBA/2J)F1 (abbreviated B6D2F1) mice were used in this study. The choice of strains was dictated by their availability as aged mice from the Animal Breeding Facility of the Trudeau Institute. Mice were maintained under conventional husbandry conditions and were free of common pathogens, as evidenced by periodic serological testing performed by the Research Animal Diagnostic and Investigative Laboratory, University of Missouri, Columbia. Mice received commercially prepared chow and acidified water ad libitum. Aged mice were checked for a healthy appearance (sleek fur, no evidence of wasting, and normal gait and feeding behavior) before inclusion in experiments. Additionally, all that had visible tumors at necropsy were excluded from experimental results.
C. neoformans.
The mildly virulent serotype A strain 184 (40) was maintained on Sabouraud-dextrose agar (SDA) slants at 26°C. Fresh slants were prepared from one of these slants every 2 weeks. At approximately 6-month intervals, fresh working stocks were initiated from seed stocks maintained in long-term storage at 4°C in distilled water.
Inocula were prepared by seeding yeast cells onto SDA slants. Yeast cells grown for 24 h from slants were inoculated in Sabdex broth and grown at 37°C for 24 h. Log-phase suspensions were pelleted, washed, and resuspended in phosphate-buffered saline (PBS) to the desired concentration. Mice were inoculated intratracheally with 106 strain 184 yeast cells as described previously (15) and intravenously with 2 × 104 cells via the retro-orbital sinus (2). In each experiment, the number of viable organisms inoculated was verified by plating on SDA.
Enumeration of yeast cells from infected mouse organs.
Mice were killed by CO2 asphyxiation. Organs were extensively homogenized by agitating action of a motor-driven sterile chilled Teflon pestle inserted in a large glass test tube containing the whole organ in sterile PBS. All organs of the same type (for example, all brains) were submitted to the same number of strokes of the pestle; that number was chosen because it was sufficient to give a suspension of homogeneous appearance. After serial dilution from neat to 10−5, a sample of each dilution was plated on SDA plates. After 48 h at 28°C, discrete, circular single colonies were enumerated on the appropriate plate quadrant, i.e., a quadrant containing between 20 and 200 colonies.
Flow cytometry.
Splenocytes were suspended to 5 × 107 cells/ml in PBS–1% bovine serum albumin, and 50-μl aliquots were incubated with fluorescein isothiocyanate-conjugated anti-CD4 monoclonal antibody (MAb) (GK1.5; American Type Culture Collection catalog no. TIB 207) and with biotinylated anti-CD45RB (clone 16A; PharMingen, San Diego, Calif.) for 1 h at 4°C. Cells were pelleted, washed once, and then incubated in 50 μl of streptavidin-conjugated phycoerythrin in PBS–1% bovine serum albumin for 1 h at 4°C. Cells were washed and resuspended in sheath buffer for flow cytometric analysis using a FACStar with LYSIS II software (Becton Dickinson, San Jose, Calif.). Samples were gated on lymphocytes by forward scatter/side scatter characteristics. Five thousand events per sample were collected.
Generation of TXB mice.
T-cell-deficient (thymectomized, irradiated [TXB]) mice were prepared by thymectomizing females at 4 weeks of age, lethally irradiating them (1,000 rads) 1 week later, and the following day giving them 107 syngeneic bone marrow cells (24). Mice were rested for 7 weeks and then given 1 mg of MAb GK1.5 and 1 mg of MAb TIB 210 (American Type Culture Collection) intraperitoneally, to deplete CD4+ and CD8+ T cells, respectively. Mice were infected with 2 × 104 C. neoformans 184 cells 24 h after administration of MAbs. T-cell ablation was checked in these mice by flow cytometric analysis of splenocytes of mice killed at 12 days of infection.
IL-2 assay.
Briefly, spleen cells were obtained from mice 12 days after intravenous infection with strain 184 yeast cells and placed in culture with concanavalin A (5 μg/ml) for 48 h. Supernatants were harvested and assayed for IL-2 based on their ability to stimulate the proliferation of NK.3 cells in culture (45). NK.3 cells cultures were supplemented either with dilutions of a known standard of IL-2 (X63-IL2 supernatant; the gift of S. Swain) or sample supernatants for 24 h and then pulsed with [3H]thymidine overnight. Incorporation of thymidine by NK.3 cells supplemented with supernatants from aged spleen cell cultures was compared to incorporation when supernatant from young adult spleen cell culture was used.
Skin grafting.
Skin grafting was performed as described previously (23). Each female mouse received one graft of female syngeneic tail skin and two test grafts of male tail skin. The condition of the grafts was monitored visually with the aid of a 10× magnifying eyepiece. Grafts were scored for the condition of the epithelium, pigmentation, and hair at regular intervals for 40 days. Graft survival time was recorded as the time taken for the test graft to differ irreversibly from that of the syngeneic control grafts on the same group of recipients.
Histology.
Brains were sagittally bisected, and half of each organ was plated to enumerate yeast cells. The other half was fixed in 10% neutral buffered formalin. Tissues were dehydrated and embedded in paraffin. Sections were stained with hematoxylin and eosin. For histopathologic observations, four to five serial sagittal sections of brain taken adjacent to the midline were examined for each animal. The source (young adult or aged) of the particular sections was unknown to the pathologist who assessed the intensity of the mononuclear cell response.
Statistics.
Numbers (log10) of yeast CFU are expressed as the mean ± standard deviation. Data were analyzed by Student’s t test. Data from survival studies were analyzed by the log rank test. In all statistical analyses, significance was defined by a P value of <0.05.
RESULTS
Greater susceptibility of aged mice than of young adult mice to systemic cryptococcal infection.
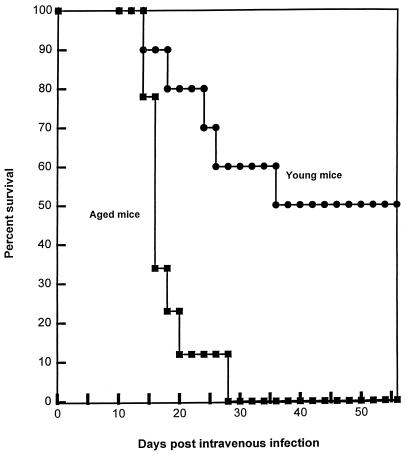
To compare the capabilities of aged and young adult mice to resist cryptococcal infection, the following experiment was performed. Ten 24-month-old male AB6F1 mice and 10 AB6F1 young adults were infected intravenously with 2 × 104 strain 184 yeast cells. Five mice per group were killed at 10 days of infection, and the brain and lung yeast burdens of the two groups were compared. There was no difference in log10 CFU between the two groups (young adult, 4.02 ± 0.51; aged, 4.12 ± 0.46) in the lungs. Additionally, there was no difference in brain yeast burdens (6.79 ± 0.17 [young adult] and 6.85 ± 0.28 [aged] log10 CFU). Similar results were obtained at day 14 of infection. There were no gross differences in the appearance of the aged and young adult mice. Both groups appeared healthy, exhibiting sleek fur, no hydrocephaly, and normal movement. There was no difference in log10 CFU between the two groups (young adult, 3.74 ± 0.33; aged, 4.48 ± 0.84) in the lungs. Brain yeast burdens did not differ between the two groups (6.95 ± 0.13 [young adult] and 6.92 ± 0.21 [aged] log10 CFU). Note that in all cases examined, the numbers of yeast CFU in the lungs were far lower than in brains. Similar results were obtained with females when brains of young adults and aged mice were examined at 7 days of infection. However, in this experiment, an additional 10 AB6F1 mice from each group were allowed to progress to morbidity or mortality (Fig. 1). Aged mice all died by 28 days of infection, while 50% of these hybrid young mice survived until the experiment was terminated at 52 days. (In our experience with several inbred strains of mice, intravenous infection at this dose level results in death in 28 to 35 days, with approximately 108 organisms present in the brain.)
FIG. 1.
Survival of young adult and aged mice after intravenous infection with C. neoformans. Ten AB6F1 female young adult and nine female AB6F1 aged mice were infected with 2 × 104 strain 184 yeast cells given intravenously. The two groups differed significantly in median time to death (log rank test, P < 0.05).
A similar experiment was performed with 14 aged and 15 young adult male B6D2F1 mice. Brain and lung yeast burdens did not differ between the two groups at days 7 and 11 of infection (data not shown). Yet all of the four remaining aged mice died between days 20 and 26 of infection, while all of the five remaining young adult mice survived until the experiment was terminated at 67 days of infection.
Although aged mice do not survive as long as young adults after intravenous infection, they die with lower brain burdens than do young adults.
Inbred young adult mice infected systemically with a lethal inoculum of C. neoformans exhibit a profound meningoencephalitis. Typically, brain yeast burdens are >108, and histological examination of brain tissue shows severe necrosis and edema (16, 39, 43). Moribund C. neoformans-infected mice have marked loss of appetite and weight and exhibit hydrocephaly. It is therefore widely believed that the central nervous system involvement proves lethal for the mouse.
Since brain burdens were identical in aged and young adult AB6F1 and B6D2F1 mice at early time points after infection in the experiments described above, we wondered if there were different yeast burdens in other organs. Due to considerations of availability, the following experiment was performed in B6D2F1 mice. Mice were infected intravenously with 2 × 104 yeast cells, and at day 10 of infection, yeast CFU were compared in liver, lungs, kidney, spleen, and brain (Table 1). No significant differences were found. At day 13, one of five remaining aged mice was found dead in the cage, and the remaining four mice appeared moribund (ruffled fur, hydrocephaly, and ataxia). Young adult controls looked relatively healthy (sleek fur and normal movement). All remaining mice were therefore killed on day 14, and their organs were harvested. Yeast burdens were significantly higher in brains and livers of aged mice than in those of young adult controls (Table 1). Numbers of yeast cells in brains of moribund aged mice were about fourfold lower (7.40 ± 0.03 log10 CFU [n = 4]) than those expected for moribund young adult mice, who typically die with yeast brain burdens greater than 8.00 log10 CFU. Brains of moribund aged mice appeared mushy, with obvious evidence of cerebral hemorrhage and hydrocephaly, as is usual with moribund young adult mice that die with cryptococcal meningoencephalitis. Aged mice experienced ataxia, also typical of moribund young adult mice.
TABLE 1.
C. neoformans 184 yeast burdens in the organs of aged and young adult B6D2F1 mice after intravenous infection
| Mice | Log10 CFU/organ (mean ± SD)a
|
||||
|---|---|---|---|---|---|
| Liver | Lung | Kidney | Spleen | Brain | |
| 10 days | |||||
| Aged | 3.69 ± 0.31 | 2.69 ± 0.76 | 3.08 ± 1.45 | 2.49 ± 0.70 | 5.97 ± 0.92 |
| Young adult | 3.15 ± 0.39 | 1.94 ± 0.46 | 2.36 ± 0.46 | 2.00 ± 0.35 | 5.49 ± 0.61 |
| 14 days | |||||
| Aged | 4.98 ± 1.12 | 3.07 ± 0.78 | 3.97 ± 0.84 | 3.85 ± 1.85 | 7.40 ± 0.03 |
| Young adult | 3.65 ± 0.47* | 2.18 ± 0.69 | 2.43 ± 1.10 | 2.27 ± 0.65 | 6.16 ± 0.76* |
Female mice were given 2 × 104 strain 184 yeast cells intravenously. Groups of mice were killed at 10 and 14 days of infection. For aged mice, n = 4; for young adults, n = 5. ∗, differs significantly from aged group (P < 0.05).
These data suggest that although aged and young adult mice appear to control yeast proliferation equally well at early time points after infection, aged mice die more quickly after infection than do young adult controls. Moreover, only when aged mice were killed very shortly before they would have died of cryptococcal infection were yeast burdens higher in some organs. Additionally, aged mice died with fewer yeast cells in their brains than typically are found in moribund young adult mice.
In a separate experiment, yeast cells were enumerated in the brains of moribund AB6F1 aged mice (7.37 ± 0.45 log10 CFU [n = 3]) and, when they became moribund, in the brains of young adult controls (8.28 ± 0.15 log10 CFU [n = 8]), and tissue samples from the two groups were compared. Histological examination revealed that both sets of mice had space-occupying lesions in the brain. It appeared that young adult mice had a slightly greater degree of inflammatory infiltrate of host defense cells in perivascular areas than did aged mice; however, we did not know whether the magnitude of the difference was significant or whether the greater degree of inflammation would be protective or deleterious.
In vitro responses of T cells of aged, C. neoformans-infected mice.
Hypothesizing that differences in T-cell function might cause the inferior resistance capabilities of intravenously infected aged mice, we undertook the following experiments. Four aged and four young adult AB6F1 mice were given 2 × 104 strain 184 organisms intravenously. At 12 days of infection, mice were killed and splenocyte suspensions were prepared. Aliquots of splenocytes were analyzed by flow cytometry to characterize subpopulations of splenic T cells. There was no significant difference in numbers of CD4+ or CD8+ T cells in aged and young adult mice. However, the fraction of CD4+ T cells that were CD45RBlo was significantly higher for aged mice (0.542 ± 0.063) than for young adult mice (0.255 ± 0.064). This result is consistent with the findings of others (12, 14, 29, 31) who have reported that CD4+ T-cell populations in aged mice are skewed toward a higher proportion of cells bearing markers of previous antigen experience.
An aliquot of the splenocyte suspension from each mouse was also placed in culture, and concanavalin A-stimulated IL-2 production was measured. Consistent with reports on other experimental systems (47, 48), splenocytes from young adult mice produced significantly more IL-2 upon stimulation than did splenocytes from aged mice (data not shown).
T-cell-depleted young adult mice are more resistant to intravenous C. neoformans infection than are T-cell-intact aged mice.
Having demonstrated differences in aged and young adult mice in resistance to intravenous cryptococcal infection and correlative differences in vitro in T-cell phenotype and function, we sought to establish a causal link between the observations. If the defective resistance of the aged mice was caused by their T-cell deficiency, ablating the T-cell compartment of young adult mice ought to render them as susceptible as or more susceptible than T-cell-intact aged mice to intravenous C. neoformans infection. The following experiment was performed with B6D2F1 mice, due to considerations of availability. Twenty young adult B6D2F1 female mice were thymectomized, irradiated, and treated with MAbs to deplete CD4+ and CD8+ T cells. Fifteen nonirradiated, thymus-intact young adult controls received isotype-matched MAbs, as did 15 nonirradiated, thymus-intact aged mice. The efficacy of the T-cell ablation in the MAb-treated young adult TXB mice was checked by flow cytometry. On average, spleens of treated young adult mice had (9.62 ± 2.80) × 104 CD4+ T cells and (2.00 ± 2.85) × 104 CD8+ T cells, while spleens of young adult controls had (1.22 ± 1.21) × 107 CD4+ T cells and (7.60 ± 0.86) × 106 CD8+ T cells. Therefore, CD4+ T-cell depletion was >99% complete in the treated mice, and CD8+ T-cell depletion was >97% complete. T-cell ablation was also checked by comparing the abilities to reject male skin grafts in five of the young adult TXB females (no grafts rejected within 40 days) and in five untreated young female adult controls (grafts rejected by days 22, 22, 26, 26, and 32). This result confirmed that T-cell function of the TXB mice was significantly impaired, as ability to reject allografts is T-cell dependent (4).
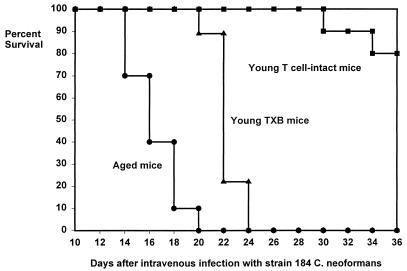
The 15 remaining T-cell-ablated young adult mice, along with the 15 young adult controls and 15 aged controls, were infected intravenously with 2 × 104 C. neoformans 184 cells. Twelve days after infection, five mice from each treatment group were killed and yeast cells were enumerated in lungs, kidneys, brains, and livers (Table 2). T-cell-ablated young adult mice had significantly higher kidney yeast burdens than did aged mice. No other differences in organ burden were found between aged and T-cell-ablated young adult mice. T-cell-intact young adult mice had significantly fewer yeast cells in their brains than did aged or T-cell-ablated young adult mice. The remaining 10 mice from each treatment group were allowed to progress to morbidity or mortality. Both T-cell-ablated young adult mice and T-cell-intact young adult controls lived significantly longer than did aged mice (Fig. 2). The fact that young adult mice depleted of T cells are more resistant to intravenous C. neoformans infection than are T-cell-intact aged mice argues against the hypothesis that the decreased resistance observed in aged mice is caused by diminished or defective T-cell function.
TABLE 2.
Yeast burdens in the organs of young adult TXB, young adult, and aged B6D2F1 mice after intravenous C. neoformans infection
| Groupa | Log10 CFUs/organ (mean ± SD)b
|
|||
|---|---|---|---|---|
| Lung | Liver | Kidney | Brain | |
| A | 2.85 ± 1.34 | 3.68 ± 1.55 | 4.26 ± 0.56‡ | 7.24 ± 0.25‡ |
| B | 2.33 ± 0.78 | 3.38 ± 0.66 | 3.22 ± 0.43* | 6.28 ± 0.33* |
| C | 3.60 ± 0.81 | 4.20 ± 0.67‡ | 5.14 ± 0.28*‡ | 7.23 ± 0.16‡ |
Group A, aged female B6D2F1 mice given 2 mg of control MAb LTF-2 intraperitoneally 24 h prior to intravenous infection with 2 × 104 strain 184 yeast cells; group B, young adult female B6D2F1 mice given control antibody and infected as above; group C, young adult female B6D2F1 TXB mice given 1 mg of anti-CD4 MAb and 1 mg of anti-CD8 MAb intraperitoneally 24 h prior to intravenous infection as above. Mice were killed at 12 days of infection.
n = 5 mice/group. ∗, differs significantly from group A (P < 0.05); ‡, differs significantly from group B (P < 0.05).
FIG. 2.
Survival of young adult, aged, and young adult TXB mice after intravenous infection with C. neoformans. Female B6D2F1 mice were infected as for Fig. 1. Young TXB mice survived significantly longer (log rank test, P < 0.05) than aged T-cell-intact mice (n = 10 for aged and young adult mice; n = 9 for young TXB mice).
Aged mice and young adult mice have comparable numbers of yeast cells in their lungs and brains after pulmonary inoculation with C. neoformans.
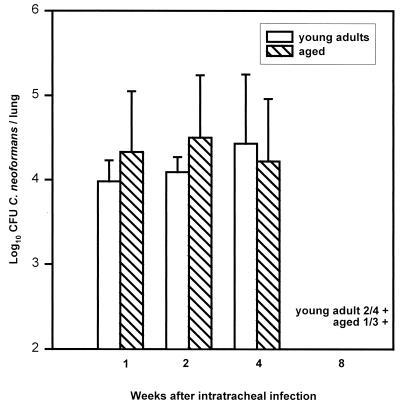
To further examine T-cell function in aged mice, we investigated whether clearance of C. neoformans from the lungs of intratracheally infected mice, which is dependent on intact CD8+ and CD4+ T-cell function (15, 16, 20, 21, 36–38), was impaired in aged mice. Aged and young adult AB6F1 mice were inoculated with 106 strain 184 yeast cells, and lung and brain yeast burdens were monitored for 8 weeks (Fig. 3). No significant differences were found in lung yeast burdens at 1, 2, or 4 weeks of infection. By 8 weeks, two of four young adult mice had cleared yeast from their lungs, as had two of three remaining aged mice. Low numbers of yeast cells were detected in the lungs of two young adult mice (1.95 and 1.95 log10 CFU) and one aged mouse (3.15 log10 CFU). In brains, no yeast cells were detected in either group at 1 or 2 weeks of infection (data not shown). By 4 weeks, one of five mice in each group had detectable yeast cells in the brain (young adult, 3.56; aged, 5.12). Similar results were found at 8 weeks. From these data, we conclude that aged mice are as efficient as young adult mice at controlling yeast proliferation in the lungs and containing yeast cells in the lungs after a primary intratracheal infection, functions reported to be dependent on CD8+ and CD4+ T cells (15, 16, 20, 21, 36–38).
FIG. 3.
Lung yeast burdens in young adult and aged mice after intratracheal instillation of C. neoformans. Young adult and aged AB6F1 female mice received 106 strain 184 yeast cells intratracheally. Mice were killed at 1, 2, 4, and 8 weeks of infection, and yeast cells were enumerated. Data are the means ± standard deviations (n = 5 mice/group except for 8 weeks, where n = 4 for young adult mice and n = 3 for aged mice). There were no significant differences between the two groups (Student’s t test, P < 0.05).
Aged mice express effective acquired resistance to C. neoformans in their brains and lungs.
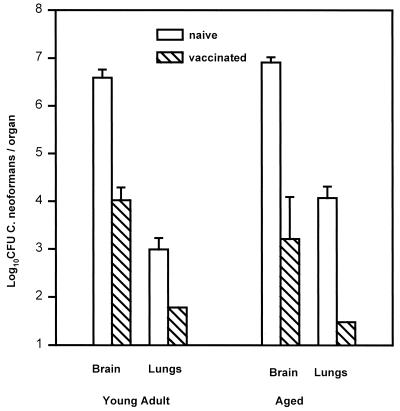
Experiments from this laboratory have demonstrated that mice vaccinated by an intratracheal instillation of 106 strain 184 yeast cells exhibit an acquired resistance to intravenous cryptococcal challenge that allows them to survive an ordinarily lethal inoculation and sterilize their brains by 12 to 16 weeks after intravenous challenge. Resistance is measurable by 7 days of infection, when vaccinated mice have approximately 10- to 100-fold fewer yeast in their brains. Moreover, this resistance is CD4+ T-cell dependent (16). To investigate whether aged mice could express effective acquired resistance to C. neoformans, we vaccinated young adult and aged B6D2F1 mice as described above. Ten weeks later, 7 of 10 surviving aged vaccinated mice and 10 young adult vaccinated mice, along with age-matched naive controls, were administered an intravenous challenge of 2 × 104 strain 184 yeast cells. Four aged vaccinated mice and five mice from each of the other groups were killed 10 days after the challenge infection, and brain and lung yeast CFU were enumerated (Fig. 4). Note that unvaccinated aged mice had higher lung burdens of yeast cells than unvaccinated young adult mice in this experiment. However, the resistance expressed by vaccinated aged mice was, if anything, more effective than the resistance expressed by vaccinated young adult mice.
FIG. 4.
Acquired immunity to C. neoformans in young adult and aged mice. Female B6D2F1 young adult and aged mice were vaccinated by intratracheal instillation of 106 strain 184 yeast cells. After 10 weeks, five young adult mice and four aged mice, along with age-matched naive controls, received 2 × 104 yeast cells intravenously. Mice were killed at 10 days of infection, and yeast cells were enumerated in brains and lungs. Data are the means ± standard deviations for five mice except for aged, vaccinated mice (n = 4). Differences in yeast burdens between naive and immune brains and lungs are significant (P < 0.05) both for the young adult and aged mice.
The remaining aged and young adult mice were killed 52 days after intravenous infections, and brain yeast cells were enumerated. A single CFU was found in one young adult mouse; no yeast cells were detected in other young adult mice or in aged mice.
The long-term efficacy of vaccination was retested in AB6F1 mice, by recording survival of vaccinated and unvaccinated young adult and aged mice after challenge. Again, vaccination offered effective long-term protection of both aged and young adult mice against an ordinarily lethal inoculum of C. neoformans. Four of four aged mice survived for 50 days after intravenous challenge. Of these mice, three had no detectable yeast cells in their brains and one had a single CFU. These data suggest that T-cell-mediated immunity against C. neoformans is as effective in aged mice as in young adults.
DISCUSSION
We have shown that young adult F1 hybrid mice survive significantly longer than aged counterparts after systemic C. neoformans infection and in some cases even survive an inoculum that kills the aged mice. Burdens of yeast in livers and brains were higher in moribund aged B6D2F1 mice than in simultaneously killed young adult mice that were not moribund, but there were no significant differences observed between the groups in any organ examined at earlier time points in either B6D2F1 or AB6F1 mice. Although the kinetics of infection in several experiments differed considerably, with aged mice dying between 13 and 28 days of infection and young adult mice surviving significantly longer in every case, higher organ yeast burdens were observed in aged mice only when the organs sampled were harvested from moribund mice. These data suggest that aged and young adult mice did not significantly differ in innate resistance mechanisms that would operate prior to the induction of specific immunity.
In the majority of previous studies testing T-cell function in aged mice, immune function was measured by various in vitro or ex vivo assays; these have not generally been correlated with resistance to experimental infection in vivo in mice of different ages. A notable exception is a study showing that aged mice are markedly inferior to young adults in controlling proliferation of the parasite Trypanosoma musculi and in clearing infection with that organism (3). However, a later study showed that the T-cell defects identified in vitro were more severe in aged C57BL/6 mice, which handle T. musculari infection more efficiently than aged animals of another strain with less severe T-cell deficiencies (48). The authors hypothesize that a more robust natural (nonimmune) resistance to T. musculari infection in C57BL/6 mice is responsible for this discrepancy. Similarly, although both the ability to generate circulating antibody and delayed-type hypersensitivity responses were found to be depressed in aged mice sensitized by exposure to mouse thyroglobulin or thyroid extract, aged mice developed autoimmune disease as intense as that observed in young adult counterparts (44). The authors suggest these paradoxical results might be explained by the observation that the aged thyroid tissue itself may be more susceptible to tissue damage.
Similarly, we describe three lines of evidence that indicate that although concanavalin A-stimulated production of IL-2 was deficient and proportions of antigen-experienced T cells were higher in the spleens of aged mice than in those of young adults, these differences were not causally related to the increased susceptibility of aged mice to intravenous cryptococcal infection. First, young adult mice that lacked T cells were more resistant to intravenous cryptococcal infection than T-cell-intact aged mice, as measured by longer survival time of the T-cell-ablated young adult mice than of aged mice. Second, intratracheally vaccinated aged mice were shown to be as capable of controlling, sequestering, and eventually eradicating yeast from their lungs as young adult counterparts. These events are widely believed to be mediated by CD8+ and CD4+ T cells (1, 16, 20, 21, 36–38). Third, aged and young adult mice that had been vaccinated by a sublethal intratracheal infection were equally resistant to a subsequently delivered intravenous challenge, and acquired resistance to cryptococcal infection is CD4+ T-cell dependent (16).
These data suggest that the increased vulnerability of the aged mice to severe systemic involvement may reflect age-related physiological changes that are not related to cell-mediated immunity per se. Cryptococcus-specific T-cell-mediated immunity is apparently quite robust in aged mice, since vaccinated aged mice express impressive resistance to intravenous cryptococcal infection by 10 days after the challenge and eventually eradicate yeast from their brains.
It is striking that despite the extensive evidence from in vitro studies that T-cell functions in aged mice are impaired, it is difficult to find conclusive evidence that impairments are responsible for reduced resistance to infection in vivo. For example, aged mice were found to have effective acquired immunity to Listeria monocytogenes as well as a normal capacity for generating T-cell-mediated resistance to primary infection (34). Despite a greater susceptibility to a primary Toxoplasma gondii infection, aged mice evinced no impaired resistance to rechallenge with T. gondii once vaccinated (11), nor could evidence be found that aged mice suffered from deficiencies in innate immunological mechanisms of resistance to T. gondii (25) that would explain their greater susceptibility. In the present study, we find that aged mice are as resistant to primary pulmonary challenge with C. neoformans as are young adults and, once vaccinated by intratracheal inoculation of yeast, display excellent acquired immune resistance to an intravenous challenge infection. Only if mice were given a primary intravenous challenge with yeast was it revealed that aged mice were more susceptible to infection. Nonetheless, aged mice died with brain yeast burdens considerably lower than brain yeast burdens in moribund young adult mice, although these differences were not statistically significant. It would appear that differences in T-cell function between aged and young adult individuals measured in vitro do not correspond to functional deficiency in host response to Cryptococcus infection in vivo.
It is intriguing to speculate as to the cause of the accelerated death of aged mice after the rather unphysiological primary intravenous instillation of yeast cells. In the course of performing this study, we experienced significant difficulty in predicting the time of death of aged mice, finding cages of dead mice that had not appeared so gravely ill upon inspection 24 h previously. This swift onset of fatality may be an important clue, along with the relatively low brain yeast burdens of moribund aged mice, to important physiological differences between aged and young adult mice that result in the earlier death of the aged mice. Differences in the fibrinolytic systems of aging rats have been documented (22). Also, aged rats are more susceptible to endotoxic shock than young adult rats (27) and have elevated levels of IL-6 in response to bacterial lipopolysaccharide (44) and elevated expression of TNF in some tissues (5). Studies are planned to investigate the potential role of differences in IL-6 and TNF responses between aged and young adult mice. It is quite possible, however, that the shortened survival time of the aged mice after intravenous infection is not causally related to any immunological parameter but rather lies in some nonimmunological difference between aged and young adult animals.
These results, along with those of other laboratories (44, 48), indicate the need for a combination of in vitro and in vivo approaches by investigators in immunology and other biomedical disciplines if we are to understand the basis for the increased vulnerability of the aged to grave infections.
ACKNOWLEDGMENTS
This work was supported by funds provided by the Trudeau Institute.
We thank Paula Lanthier, Shannon Miller, and Bryan Wolfe for technical assistance.
REFERENCES
- 1.Aguirre K, Havell E A, Gibson G W, Johnson L L. Role of tumor necrosis factor and gamma interferon in acquired resistance to Cryptococcus neoformans in the central nervous system of mice. Infect Immun. 1995;63:1725–1731. doi: 10.1128/iai.63.5.1725-1731.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguirre K M, Sayles P C, Gibson G W, Johnson L L. Resistance to Cryptococcus neoformans is associated with an inflammatory response to Toxoplasma gondii in the central nervous system of mice. Infect Immun. 1996;64:77–82. doi: 10.1128/iai.64.1.77-82.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albright J W, Matusewicz N M, Albright J F. Aging of the murine immune system is reflected by declining ability to generate antibodies that promote elimination of Trypanosoma musculi. J Immunol. 1988;141:1318–1325. [PubMed] [Google Scholar]
- 4.Auchincloss H, Sachs D H. Transplantation and graft rejection. In: Paul W E, editor. Fundamental immunology. 3rd ed. New York, N.Y: Raven Press, Ltd.; 1993. pp. 1099–1141. [Google Scholar]
- 5.Belmin J, Bernard C, Corman B, Merval R, Esposito B, Tedgui A. Increased production of tumor necrosis factor and IL-6 by arterial wall of aged rats. Am J Physiol. 1995;268:H2288–H2293. doi: 10.1152/ajpheart.1995.268.6.H2288. [DOI] [PubMed] [Google Scholar]
- 6.Callard R E, Basten A, Waters L K. Immune function in aged mice. II. B-cell function. Cell Immunol. 1977;31:26–36. doi: 10.1016/0008-8749(77)90003-x. [DOI] [PubMed] [Google Scholar]
- 7.Chang M P, Utsuyama M, Hirokawa K, Makinodan T. Decline in the production of interleukin-3 with age in mice. Cell Immunol. 1988;115:1–12. doi: 10.1016/0008-8749(88)90157-8. [DOI] [PubMed] [Google Scholar]
- 8.Chen G-H, Curtis J L, Mody C H, Christensen P J, Armstrong L R, Toews G B. Effect of granulocyte-macrophage colony-stimulating factor on rat alveolar macrophage anticryptococcal activity in vitro. J Immunol. 1994;152:725–734. [PubMed] [Google Scholar]
- 9.Collins H L, Bancroft G J. Cytokine enhancement of complement-dependent phagocytosis by macrophages: synergy of TNFα and GM-CSF for phagocytosis of Cryptococcus neoformans. Eur J Immunol. 1992;22:1447–1454. doi: 10.1002/eji.1830220617. [DOI] [PubMed] [Google Scholar]
- 10.Ding A, Hwang S, Schwab R. Effect of aging on murine macrophages. Diminished response to IFN-gamma for enhanced oxidative metabolism. J Immunol. 1994;153:2146–2152. [PubMed] [Google Scholar]
- 11.Emmerling P, Hof H, Finger H. Age-related defense against infection with intracellular pathogens. Gerontology. 1979;25:327–336. doi: 10.1159/000212361. [DOI] [PubMed] [Google Scholar]
- 12.Ernst D N, Hobbs M V, Torbett B E, Glasebrook A L, Rehse M A, Bottomly K, Hayakawa K, Hardy R R, Weigle W O. Differences in the expression profiles of CD45RB, Pgp-1, and 3G11 membrane antigens and in the patterns of lymphokine secretion by splenic CD4+ T cells from young and aged mice. J Immunol. 1990;145:1295–1302. [PubMed] [Google Scholar]
- 13.Gahring L C, Weigle W O. The effect of aging on the induction of humoral and cellular immunity and tolerance in two long-lived mouse strains. Cell Immunol. 1990;138:142–151. doi: 10.1016/0008-8749(90)90013-h. [DOI] [PubMed] [Google Scholar]
- 14.Haynes L, Linton P J, Swain S L. Age-related changes in CD4+ T cells of T cell receptor transgenic mice. Mech Ageing Dev. 1997;93:95–105. doi: 10.1016/s0047-6374(96)01826-x. [DOI] [PubMed] [Google Scholar]
- 15.Hill J O, Harmsen A G. Intrapulmonary growth and dissemination of an avirulent strain of Cryptococcus neoformans in mice depleted of CD4+ or CD8+ T cells. J Exp Med. 1991;173:755–758. doi: 10.1084/jem.173.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill J O, Aguirre K M. CD4+ T cell-dependent acquired state of immunity that protects the brain against Cryptococcus neoformans. J Immunol. 1994;152:2344–2350. [PubMed] [Google Scholar]
- 17.Hoag K A, Street N E, Huffnagle G B, Lipscomb M F. Early cytokine production in pulmonary Cryptococcus neoformans infections distinguishes susceptible and resistant mice. Am J Respir Cell Mol Biol. 1995;13:487–495. doi: 10.1165/ajrcmb.13.4.7546779. [DOI] [PubMed] [Google Scholar]
- 18.Hobbs M V, Weigle W O, Ernst D N. Interleukin-10 production by splenic CD4+ cells and cell subsets from young and old mice. Cell Immunol. 1994;154:264–272. doi: 10.1006/cimm.1994.1076. [DOI] [PubMed] [Google Scholar]
- 19.Huffnagle G B, Toews G B, Burdick M D, Boyd M B, McAllister K S, McDonald R A, Kunkel S L, Streiter R M. Afferent phase production of TNFα is required for the development of protective T cell immunity to Cryptococcus neoformans. J Immunol. 1996;157:4529–4536. [PubMed] [Google Scholar]
- 20.Huffnagle G B, Yates J L, Lipscomb M F. T cell-mediated immunity in the lung: a Cryptococcus neoformans pulmonary infection model using SCID and athymic nude mice. Infect Immun. 1991;59:1423–1433. doi: 10.1128/iai.59.4.1423-1433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huffnagle G B, Lipscomb M F, Lovchik J A, Hoag K A, Street N E. The role of CD4+ and CD8+ T cells in the protective inflammatory response to a pulmonary cryptococcal infection. J Leukocyte Biol. 1994;55:35–42. doi: 10.1002/jlb.55.1.35. [DOI] [PubMed] [Google Scholar]
- 22.Iacoviello L, D’Adamo M C, DeCurtis A, Buczko W, Donati M B. Enhanced vascular plasminogen activator (t-PA) release by epinephrine in aged rats. Thromb Haemostasis. 1995;73:841–844. [PubMed] [Google Scholar]
- 23.Johnson L L. Prolonged minor allograft survival in intravenously primed mice—a test of the veto hypothesis. Transplantation. 1987;44:92–97. doi: 10.1097/00007890-198707000-00020. [DOI] [PubMed] [Google Scholar]
- 24.Johnson L L. Apparent lack of H-Y-specific suppressor cells in female mice given male spleen cells intravenously. Transplantation. 1990;49:152–155. doi: 10.1097/00007890-199001000-00034. [DOI] [PubMed] [Google Scholar]
- 25.Johnson L L, Gibson G W, Sayles P C. Preimmune resistance to Toxoplasma gondii in aged and young adult mice. J Parasitol. 1995;81:894–899. [PubMed] [Google Scholar]
- 26.Kawakami K, Tohyama M, Teruya K, Kudeken N, Xie Q, Saito A. Contribution of gamma-IFN in protecting mice during pulmonary and disseminated infection with Cryptococcus neoformans. FEMS Immunol Med Microbiol. 1996;13:123–130. doi: 10.1016/0928-8244(95)00093-3. [DOI] [PubMed] [Google Scholar]
- 27.Knook D L, Brouwer A. Kupffer cells and the acute phase response: the effect of aging. Immunol Invest. 1989;18:339–350. doi: 10.3109/08820138909112247. [DOI] [PubMed] [Google Scholar]
- 28.Kozel T R. Cryptococcosis. In: Murphy J W, Friedman H, Bendinelli M, editors. Fungal infections and immune responses. New York, N.Y: Plenum Press; 1993. pp. 277–302. [Google Scholar]
- 29.Lerner A, Yamada T, Miller R A. Pgp-1hi T lymphocytes accumulate with age in mice and respond poorly to concanavalinA. Eur J Immunol. 1989;19:977–982. doi: 10.1002/eji.1830190604. [DOI] [PubMed] [Google Scholar]
- 30.Levitz S M, Tabuni A, Kornfeld H, Reardon C C, Golenbock D T. Production of tumor necrosis factor alpha in human leukocytes stimulated by Cryptococcus neoformans. Infect Immun. 1994;62:1975–1981. doi: 10.1128/iai.62.5.1975-1981.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linton P J, Haynes L, Klinman N R, Swain S L. Antigen-independent changes in naïve CD4+ T cells with aging. J Exp Med. 1996;184:1891–1900. doi: 10.1084/jem.184.5.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lovchik J A, Lyons C R, Lipscomb M F. A role for IFNγ-induced nitric oxide in pulmonary clearance of Cryptococcus neoformans. Am J Respir Cell Mol Biol. 1995;13:116–124. doi: 10.1165/ajrcmb.13.1.7598935. [DOI] [PubMed] [Google Scholar]
- 33.Lovchik J, Lipscomb M F, Lyons C R. Expression of lung-inducible NOS does not correlate withnitric oxide production in vivo in a pulmonary immune response against Cryptococcus neoformans. J Immunol. 1997;158:1772–1778. [PubMed] [Google Scholar]
- 34.Lovik M, North R J. Effect of aging on antimicrobial immunity: old mice display a normal capacity for generating protective T cells and immunologic memory in response to infection with Listeria monocytogenes. J Immunol. 1985;135:3479–3486. [PubMed] [Google Scholar]
- 35.Mitchell T G, Perfect J R. Cryptococcosis in the era of AIDS: 100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev. 1995;8:515–548. doi: 10.1128/cmr.8.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mody C H, Chen G-H, Jackson C, Curtis J L, Toews G B. Depletion of murine CD8+ T cells in vivo decreases pulmonary clearance of a moderately virulent strain of Cryptococcus neoformans. J Lab Clin Med. 1993;121:765–773. [PubMed] [Google Scholar]
- 37.Mody C H, Chen G-H, Jackson C, Curtis J L, Toews G B. In vivo depletion of murine CD8 positive T cells impairs survival during infection with a highly virulent strain of Cryptococcus neoformans. Mycopathologia. 1994;125:7–17. doi: 10.1007/BF01103969. [DOI] [PubMed] [Google Scholar]
- 38.Mody C H, Lipscomb M F, Street N E, Toews G B. Depletion of CD4+ (L3T4+) lymphocytes in vivo impairs murine host defense to Cryptococcus neoformans. J Immunol. 1990;144:1472–1477. [PubMed] [Google Scholar]
- 39.Moser S A, Lyon F, Domer J E, Williams J E. Immunization of mice by intracutaneous inoculation with viable virulent Cryptococcus neoformans: immunological and histopathological parameters. Infect Immun. 1982;35:685–696. doi: 10.1128/iai.35.2.685-696.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy J W, Cozad G C. Immunological unresponsiveness induced by cryptococcal polysaccharide assayed by the hemolytic plaque technique. Infect Immun. 1972;5:896–901. doi: 10.1128/iai.5.6.896-901.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy J W. Cytokine profiles associated with induction of the anticryptococcal cell-mediated immune response. Infect Immun. 1993;61:4750–4759. doi: 10.1128/iai.61.11.4750-4759.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nicoletti C, Cerny J. The repertoire diversity and magnitude of antibody responses to bacterial antigens in aged mice. I. Age-associated changes in antibody responses differ according to the mouse strain. Cell Immunol. 1991;133:72–83. doi: 10.1016/0008-8749(91)90180-j. [DOI] [PubMed] [Google Scholar]
- 43.Powderly W G. Cryptococcal meningitis and AIDS. Clin Infect Dis. 1993;17:837–842. doi: 10.1093/clinids/17.5.837. [DOI] [PubMed] [Google Scholar]
- 44.Romball C G, Weigle W O. The effect of aging on the induction of experimental autoimmune thyroiditis. J Immunol. 1987;139:1490–1495. [PubMed] [Google Scholar]
- 45.Swain S L, Weinberg A D, English M. CD4+ T cell subsets: lymphokine secretion of memory cells and of effector cells that develop from precursors in vitro. J Immunol. 1990;144:1788–1799. [PubMed] [Google Scholar]
- 46.Terrazzino S, Perego C, DeLuigi A, DeSimoni M G. Interleukin-6, tumor necrosis factor and corticosterone induction by central nervous system infection of lipopolysaccharide in aged rats. Life Sci. 1997;61:695–701. doi: 10.1016/s0024-3205(97)00534-1. [DOI] [PubMed] [Google Scholar]
- 47.Thoman M L, Weigle W O. Lymphokines and aging: interleukin-2 production and activity in aged animals. J Immunol. 1981;127:2102–2106. [PubMed] [Google Scholar]
- 48.Utsuyama M, Albright J W, Holmes K L, Hirokawa K, Albriytokines J F. Changes in the subsets of CD4+ T cells in Trypanosoma musculi infection: delay of immunological cure in young mice and the weak ability of aged mice to control the infection. Int Immunol. 1994;6:1107–1115. doi: 10.1093/intimm/6.8.1107. [DOI] [PubMed] [Google Scholar]