Abstract
Functional connectivity (FC) techniques can delineate brain organization as early as infancy, enabling the characterization of early brain characteristics associated with subsequent behavioral outcomes. Previous studies have identified specific functional networks in infant brains that underlie cognitive abilities and pathophysiology subsequently observed in toddlers and preschoolers. However, it is unknown whether and how functional networks emerging within the first 18 months of life contribute to the development of higher order, complex functions of language/literacy at school-age. This 5-year longitudinal imaging project starting in infancy, utilized resting-state functional magnetic resonance imaging and demonstrated prospective associations between FC in infants/toddlers and subsequent language and foundational literacy skills at 6.5 years old. These longitudinal associations were shown independently of key environmental influences and further present in a subsample of infant imaging data (≤12 months), suggesting early emerged functional networks specifically linked to high-order language and preliteracy skills. Moreover, emergent language skills in infancy and toddlerhood contributed to the prospective associations, implicating a role of early linguistic experiences in shaping the FC correlates of long-term oral language skills. The current results highlight the importance of functional organization established in infancy and toddlerhood as a neural scaffold underlying the learning process of complex cognitive functions.
Keywords: functional connectivity, infant, language, longitudinal, literacy
Introduction
Human brains undergo extensive changes within the first years of life, establishing critical foundations of neuroarchitecture that underlie the protracted maturational process of perceptual and higher order cognitive functions. Recent advances in pediatric neuroimaging techniques refine functional mapping of the infant brain, primarily through resting-state functional connectivity (FC) analyses (Gilmore et al. 2018). FC characterizes functional brain organization by examining synchronized fluctuations in the blood oxygen dependent (BOLD) signals among different brain regions (Biswal et al. 1995). For instance, FC analyses conducted in late preterm and full-term neonates have reported adult-like FC patterns in regions underlying motor and primary sensory processing (e.g., primary auditory and visual networks) at birth (Fransson et al. 2007; Doria et al. 2010; Gao et al. 2015a). As maturation progresses, long-distance FC patterns among association cortices develop, coupled with the emergence of higher order cognitive functions such as executive function and language (Fair et al. 2009; Gu et al. 2015; Gao et al. 2015a, 2015b). To date, an increasing number of studies have revealed relationships between FC patterns in infancy and toddlerhood and subsequent cognitive abilities and pathophysiology in toddlers and preschoolers, suggesting the cognitive developmental significance of FC emerged during early developmental stage (Alcauter et al. 2014; Rogers et al. 2017; Graham et al. 2019; Chen et al. 2020). However, it is still unknown whether and how FC within the first 18 months of life can account for individual differences in the long-term trajectory of language and foundational literacy abilities into school-age.
Early language acquisition starts in utero and is significantly shaped by the linguistic environment (Skeide and Friederici 2016). For example, newborns demonstrate selective preferences for human speech compared to other sounds and are able to recognize different aspects of speech, such as phonetic and stress patterns (Sansavini et al. 1997; Kuhl 2004; Vouloumanos and Werker 2007). Infants typically develop an understanding of familiar words as early as the second half of the first year, building a critical foundation for long-term language development into school-age (Fernald et al. 2006; Marchman and Fernald 2008; Swingley 2009). Early linguistic experiences further serve as critical building blocks for the development of emergent literacy skills. These skills include phonological processing, the ability to recognize and manipulate the sound structure of words, one of the key predictors of subsequent reading development (Scarborough et al. 2009; Duff et al. 2015; Pierce et al. 2017). Moreover, this developmental time course is sensitive to environmental characteristics, including language and early literacy exposure in the infant’s environment. For example, it has been shown that the quality and quantity of child-directed speech are highly correlated with vocabulary gain in toddlers (Huttenlocher et al. 1991; Rowe 2012). In addition to language exposure, home literacy practices are also associated with subsequent verbal and literacy skills during preschool and school-age (Hood et al. 2008; Ece Demir-Lira et al. 2019), highlighting the important role of the environment. Moreover, emergent literacy skills are then further deepened through formal instruction once the child starts school and reciprocally influence language development, such as vocabulary (Cunningham and Stanovich 1997; Hatcher et al. 2004; Piasta and Wagner 2010; Nation and Hulme 2011). Overall, the development of language and preliteracy skills starts in infancy and continues through childhood and beyond, constantly shaped by the quantity and quality of input provided in the child’s home, school, and other environmental contexts.
A foundational neurobiological scaffold is already present in infant brains for language and literacy acquisition. It has been repeatedly demonstrated that neonates, within the first week after birth, and preverbal babies show greater neural responses specific for human speech as compared to control sounds or speech played backward in language-related areas (Dehaene-Lambertz and Pena 2001; Mahmoudzadeh et al. 2013), largely located in the temporal lobe (Dehaene-Lambertz et al. 2002; Pena et al. 2003; Dehaene-Lambertz et al. 2006; Perani et al. 2011). Electrophysiological responses to speech perception in infancy have been shown to be prospectively associated with language and literacy outcomes at the preschool and school-age, suggesting early brain function has a long-lasting influence on subsequent language and literacy acquisition (Guttorm et al. 2005; Leppänen et al. 2012; van Zuijen et al. 2012). However, it is still unknown how the functional networks emerged early in life relate to the long-term development of language and literacy skills after the start of formal education. While intrinsic FC among regions important for language and reading processing in adults is observable in infants during natural sleep (Li et al. 2020; King et al. 2021), the neural network mechanisms supporting the protracted development of verbal skills is actively developing and evolving postnatally. For example, the developmental characteristics of white matter as early as in infancy have been shown to correlate with concurrent and subsequent oral language skills (Deoni et al. 2016; Zuk et al. 2021), and the developmental changes of large-scale functional topologies within the first 2 years of life are associated with overall developmental progress on language milestones at age 4 in a group of 23 children (Emerson et al. 2016). These results demonstrate the functional relevance of maturational changes in brain networks associated with early language development. Importantly, these neural trajectories of language/reading development are subject to input provided from the environment. For example, it has been demonstrated that the word-specific activation in the left fusiform gyrus of the literate adults, that is, the putative visual word form area (Cohen et al. 2000), emerged in children only after the onset of print exposures (e.g., Brem et al. 2010; Centanni et al. 2017, 2018). Its functional and structural connectivity to the language areas that constitutes the reading network is strengthened over the course of learning to read (Yeatman et al. 2012; Yu et al. 2018). Moreover, the associative effects of the early linguistic environment at home, such as rich language exposure and shared book reading, is evident in the language neural network among preschoolers (Hutton et al. 2015; Powers et al. 2016; Romeo et al. 2018), and have recently been revealed in the FC patterns of infants (King et al. 2021), further highlighting the dynamics and plasticity of the network characteristics relevant for language learning. Therefore, it is still an open question as to what extent functional network characteristics established in infancy and toddlerhood are prospectively associated with individual differences in the long-term development of the complex verbal skills with different cognitive processes, such as oral language and phonological processing, especially in school-age children who have already been exposed to formal instruction. Moreover, it is unknown whether such long-term associations, if observed, are driven by emergent (pre-)language skills and early linguistic environmental variables.
Employing a 5-year longitudinal imaging dataset from infancy to school-age, the current study utilizes resting-state FC MRI methods to investigate prospective associations between functional brain networks observed in a group of infants and toddlers aged 4–18 months old and their subsequent measures of language and emergent literacy skills a mean age of 6.5. The FC approach was applied to characterize region-specific FC patterns given the previously established relationship between the resting-state FC pattern of one region and the functional relevance (i.e., task-based fMRI activation) of this region (Cohen et al. 2008; Gordon et al. 2016; Mars et al. 2018). We first conducted multivariate pattern analysis (MVPA) to identify regional-specific FCs in infancy and toddlerhood that are associated with subsequent school-age performance while controlling for critical environmental factors. Further analyses were performed to assess whether the inclusion of receptive and expressive language performance measured at the time of scanning in infancy/toddlerhood leads to changes in these observed associations. The results of the current study offer the potential to inform our understanding of how early emerging functional network mechanisms can contribute to individual differences in language and reading abilities later in life.
Methods
Participants
All participants were recruited at Boston Children’s Hospital (BCH) as part of an ongoing longitudinal project which aims to characterize brain changes underlying language and reading development from infancy to school-age. A group of 42 participants (21 females) who completed resting-state functional and structural MR imaging in infancy (mean age = 10.2 ± 3.5 months) and were successfully followed over 5 years (5.6 ± 1.0 years) until school-age (mean age = 6.5 ± 0.96 years) were included in the current analyses (Table 1). All children were from English-speaking households and were exposed to the English language since birth. They further demonstrated typical general cognitive ability based on their performance (standard scores (SS) > 85, mean SS = 107 ± 13) on the matrices subtest of the Kaufman Brief Intelligence Test-II at school-age (KBIT-II, Kaufman and Kaufman 2004). Moreover, all participants were born at the gestational age of 37 weeks or above, except one (male) who was born at 36 weeks (Table 1) but with no brain or behavioral atypicalities observed at either time point. None of the subjects had birth complications, neurological trauma, or reported developmental delays, and their MRIs were clinically interpreted as normal by a pediatric neuroradiologist at BCH. The current protocol was reviewed and approved by the Institutional Review Board at BCH. Before participation, informed written consent was obtained from a parent or legal guardian of each infant.
Table 1.
Behavioral characteristics at the time of scanning
| Mean ± Standard deviation | Range | |
|---|---|---|
| Gestational age (weeks) | 38.9 ± 1.1 | 36–41.5 |
| Scanning age (months) | 10.2 ± 3.5 | 4.6–17.7 |
| Head movement during scanning | ||
| # of outlier scans | 8.3 ± 11.9 (5.1% ± 0.07) | 0–44 (0–27.5%) |
| Framewise displacement of the remaining scans (mm) | 0.067 ± 0.016 | 0.037–0.093 |
| Emergent language abilities at the time of scanning (T scores) | ||
| MSEL receptive language | 44.2 ± 8.3 | 27–58 |
| MSEL expressive language | 46.9 ± 7.5 | 38–62 |
MSEL = Mullen scales of early learning assessment.
Psychometric and Environmental Measures
At the infant/toddler stage, emergent receptive and expressive languages were evaluated using the Mullen Scales of Early Learning assessment (MSEL, Mullen 1995) in 29 infants. Furthermore, environmental factors critical for language and literacy development were also collected through parental questionnaires, which included socioeconomic status (SES, i.e., parental education, n = 41) and home literacy environment (HLE, adapted from Denney et al. 2001), n = 38, see SI Methods and Table S1).
At the school-age stage, a comprehensive assessment battery was administered to evaluate each child’s general cognitive ability, language, literacy, reading, and spelling skills. Since most of the child participants had not yet become skilled readers during the second longitudinal timepoint (mean age = 6.5 ± 0.96 years), the present study focused on oral language (OL, the OL subtest of the Woodcock-Johnson-IV assessment (WJ-IV, Schrank and Wendling 2015) and foundational literacy skills commonly recognized as crucial cognitive precursors of reading (Melby-Lervåg et al. 2012; Norton and Wolf 2012; Hulme and Snowling 2013). This included assessments of rapid automatized naming (RAN, Comprehensive Test of Phonological Processing-II (CTOPP-2), Wagner et al. 1999) and phonological processing skills (the phonological processing subtest of WJ-IV, n = 36, see distributions of task performance in Figure 2A and detailed descriptions of assessments in the SI Methods).
Figure 2 .
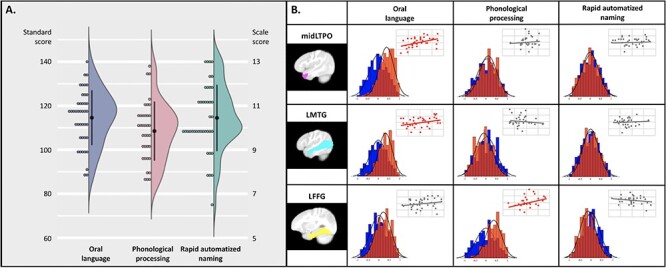
Prospective associations between FC in infancy/toddlerhood and language and foundational literacy development at school-age. (A) Distributions of the oral language (standard scores), phonological processing (standard scores), and rapid automatized naming (scale scores) skills measured at school-age. (B) The association results between school-age performance and each of the infant/toddler FCs of the three identified seed regions, including the left temporal pole (the middle portion, midLTPO), left middle temporal gyrus (LTMG), and left fusiform gyrus (LFFG). The distribution maps for each pair show the performances of the true prediction analyses (red) against the null distribution (blue) based on permutation results. The identified longitudinal associations were further confirmed using leave-one-out (LOOCV) and 4-fold crossvalidation approaches (see details in the SI methods and Table S5). The SVR results obtained based on the LOOCV approach are presented as a scatter plot in the upper right corner of each cell to illustrate the fitness of true performance and predicted outcomes across the entire sample. Significant associations (Bonferroni corrected) with a large effect (Cohen’s d ≥ 0.8) and a 95% bootstrap confidence interval above zeros were highlighted in red.
Image Acquisition
All images were acquired during natural sleep without sedation on a Siemens 3 T Trio MRI scanner with a 32-channel adult head coil (see Raschle et al. 2012 for a detailed infant imaging procedure). A motion-compensated T1 weighted multiecho MPRAGE sequence was applied to collect high-quality structural images for each infant with the following imaging parameters: number of slices = 176, TR = 2270 ms, TE1,2,3,4 = [1.66, 3.48, 5,3, 7.12] ms, flip angle = 7°, TI = 1450 ms, field of view = 220 mm2, voxel size =1.1 × 1.1 × 1.0 mm3. Prospective motion correction was achieved by acquiring low resolution EPI-based volumetric navigators each TR (Alhamud et al. 2012). The signals from the individual echoes were combined using root mean square averaging, yielding improved contrast. Moreover, an 8-min EPI-BOLD sequence was applied to collect resting-state fMRI data for every infant (TR = 3000 ms, TE = 30 ms, flip angle = 60°, voxel size =3 × 3 × 3 mm3).
Image Analysis
Resting-State fMRI Preprocessing
Functional MRI images were first corrected for slice timing using FSL/SLICETIMER. Rigid body alignment for motion correction and estimation of motion regressors was performed using FSL/MCFLIRT. Anatomical and functional MRI volumes were spatially normalized to the UNC 1-year-old infant template (Shi et al. 2011) using affine transformations (FSL/FLIRT). After spatial normalization, linear regression was performed using the following nuisance regressors: (1) six motion regressors from realignment estimates (accounting for translations and rotations in three directions), (2) mean cerebral spinal fluid (CSF) and white matter (WM) signals extracted from subject-specific anatomical masks (computed with FSL/FAST), and (3) spikes regressors based on motion outliers (see section Artifact Detection). Temporal band-pass filtering (0.01–0.1 Hz) and spatial smoothing (Gaussian filter, FWHM = 6 mm) were applied after nuisance regression.
Artifact Detection
An in-house script was used to identify artifacts in the fMRI data, including motion, and spiking (https://github.com/xiyu-bnu/infant_restingstate_prediction). Framewise displacement (FD) was estimated from translations and rotations obtained from rigid-body alignment after motion correction (Power et al. 2012). Volumes with FD > 0.3 mm were considered outliers. In order to minimize potential artifacts in neighboring volumes due to temporal filtering or spin history, one preceding and two subsequent frames were also marked as outliers. A binary vector indexing all the outliers was built for each subject and used as motion regressors to remove contaminated frames from the time series prior to filtering. On average, 5.1% of outlier scans were removed from each participant, and all subjects included in the current analyses had more than 5-min usable volumes (Table 1).
Seed-Based Connectivity Analysis
Seed regions were defined as 78 cortical regions of interest (ROIs) based on the 1-year-old Infant Brain Atlases (Shi et al. 2011) derived from the Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al. 2002). Seed-based time series were extracted by averaging the BOLD signal across all the voxels in each ROI. The FC pattern associated with each seed ROI was then generated by correlating the BOLD time series of the ROI with that of all other ROIs using the Pearson product–moment correlation formula (Fig. 1A and B). Correlation coefficients were normalized to z-scores using Fisher’s transformation. In the current analyses, we focused on the FCs of 16 seed ROIs located in the bilateral temporal lobes, given the important role of temporal lobe regions in speech and language perception tasks in infants (Dehaene-Lambertz et al. 2002, 2006; Telkemeyer et al. 2009; Sato et al. 2012; Shultz et al. 2014).
Figure 1 .
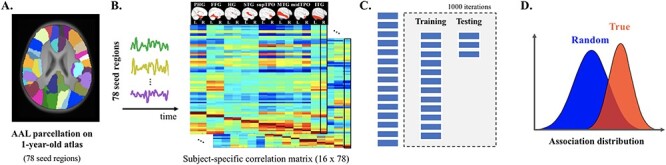
General pipeline of the association analyses between regional-specific FC in infancy and toddlerhood and school-age performance of language and preliteracy skills. (A) Seed regions were defined as 78 cortical parcellations derived from the 1-year-old infant AAL atlas (Shi et al. 2011). (B) Time series were extracted for every participant by averaging the BOLD signal across all the voxels within each seed region. The FC pattern was then obtained by correlating the BOLD time series of the ROI with that of all other ROIs. The current analyses focused on 16 seed regions residing in the bilateral temporal lobes given the previously reported engagement of these regions in speech and language processing during early developmental stage. (C) Each FC pattern was entered into a SVR model as features for the prediction analyses on every school-age performance. To objectively evaluate the prediction performance, the entire sample was randomly divided into training (80%) and testing (20%) datasets. The SVR model obtained based on the training dataset was then applied to the FC patterns of the testing datasets, which generated the predicted task performance. The predicted values were then correlated with the true performance, and the correlation coefficient was considered as performance of the SVR model. This procedure was repeated 1000 times to produce a distribution of prediction performance for each pair of seed region and task performance. (D) The mean of the distribution of prediction performance based on the true language and literacy outcomes was compared against that of a null distribution generated by the same procedure, but with the assignment of random scores of school-age performance (permutation tests) using a t-test. An effect size and a 95% bootstrap confidence interval were further calculated for the prediction performance of each seed region-behavioral assessment pair.
Abbreviations: PHG = ParaHippocampal gyrus; FFG = Fusiform gyrus; HG = Heschl’s gyrus; STG = Superior temporal gyrus; supTPO = Temporal pole (the superior portion); MTG = Middle temporal gyrus; midTPO = Temporal pole (the middle portion); ITG = Inferior temporal gyrus.
Prediction
To identify functional networks in infancy and toddlerhood that were prospectively associated with the school-age performance of language and preliteracy skills, support vector regression (SVR) analyses were performed for each pair of the regional-specific FCs and every measure of language and preliteracy skill using the LIBSVM package (Chang and Lin 2011). To do this, the FC pattern associated with a particular seed region was entered into the SVR models as the input feature with performance on each of the three assessments (OL, phonological processing, RAN) as the outcome measures in order to identify the early emerged functional networks important for the subsequent development in each specific cognitive domain. Moreover, to probe the functional networks specifically associated with long-term language and literacy development, special consideration was taken to minimize the impact of other factors known to influence children’s verbal skills, including key environmental factors of SES and HLE, as well as the infant age at the scan. General cognitive abilities measured at school-age were further included to partial out the individual variance in general cognitive maturation.
The performance of the SVR analyses was evaluated using a hold-out random resampling procedure (similar to Rudolph et al. 2018), in which 20% of the datasets were held out (testing dataset) while the remaining datasets were used to train an SVR model. Correction for covariates was first performed in the training dataset only, where each of the input features (i.e., regional-specific FCs) and the outcome measures (i.e., task performance in terms of standard scores) was submitted to a linear regression model with SES, HLE, age of scan, as well as general cognitive skill at school-age as independent regressors. The residuals of these models thus represented the individual differences in the FC pattern and verbal skills after controlling for environmental and general cognitive influences. These residuals were then entered into the SVR analyses. The obtained regression models were further applied to the testing dataset to obtain the residual values corresponding to the regional-specific FC and task performance for the testing participants. The trained SVR model was then applied to the testing sample to produce the predicted values, which were further correlated (Spearman) with true performance (residual values) as the SVR performance. This process was repeated 1000 times, generating a (real) distribution of the SVR performance. Permutation tests were subsequently performed following the same procedure except that each of the task performance was randomly assigned to every participant in order to generate a random/null distribution (Fig. 1C and D). The t-tests were applied to examine the potential differences in the mean of the two distributions obtained by the true and shuffled performance. The effect size was calculated using Cohen’s d (Cohen 2013). Moreover, a 95% bootstrap confidence interval (BCI) was further estimated for the mean association strength for each FCF-behavioral measure pair based on the real distribution of the SVR performance. Here, we focused only on strong associations with: (a) an effect size values of 0.80 or higher, signaling a large effect based on Cohen (2013), (b) a 95% BCI excluding zero mean, indicating that a null correlation was not plausible, and (c) significantly outperformed the respective randomized distribution, that is, p < 0.05 after correcting for multiple comparisons (n = 48).
Lastly, correlation analyses were further performed between motion parameters (number of outliers and mean FD) and language/literacy assessments at school-age, in order to evaluate whether any observed associations between early developed FCs and subsequent language skills could be explained by head movement during image acquisition.
Follow-Up Analysis 1: Re-Evaluation of the Obtained Prospective Associations Based on Infant FCs Established within the First Year of Life
The main analyses were performed to identify prospective associations of school-age performance in the FC patterns of participants between the ages of 4 and 18 months old. However, since functional neural networks develop rapidly during the first 2 years of life (Gilmore et al. 2018), the age range of the imaging collection time point might lead to the possibility that the observed relationship could be mainly driven by the more established functional networks among the older infants (>12 months old). Therefore, to further evaluate the importance of the early emerged FC patterns in the observed associations, a subset of 29 infants who completed the imaging session before 1 year of age (mean age = 8.6 ± 1.8 months) was selected. The prospective associations identified in the main analyses were thus re-evaluated based on data of these 29 infants following the same procedure as described above. The same thresholding criteria were also applied here.
Follow-Up Analysis 2: Examining the Influences of Early Language Milestones on the Observed Associations between Early Emerged FCs and School-Age Language Performance
Foundational language skills have been demonstrated to emerge early in infancy, which lay a critical foundation for (and are often associated with) the development of more complex language functions (see Cristia et al. 2014 for a review). It is, therefore, critical to address the question as to whether the emergent language skills at the time of scanning might contribute to the observed longitudinal links between the FCs in infancy and toddlerhood and school-age performance. To answer this question, a subset of 24 infants were selected from the original sample, whose receptive and expressive language skills were assessed at the time of scanning (Table 1). We then examined whether the inclusion of these emergent language skills changed the observed associations between infant/toddler functional networks and school-age performance identified in previous analyses. Specifically, two SVR models were run for each identified association. While both models included regular covariates similar to the ones included in the main analyses, only the second model further considered the receptive and expressive language skills measured at the time of scanning. The means of the association distributions derived from the two models were compared using t-tests. If the identified prospective associations are at least partially mediated by these emergent language skills, we should expect significant decreases in association performances of the model controlling for the language skills compared to the model that does not.
Results
Functional Connectivity in Infancy and Toddlerhood Can Predict School-Age Language and Foundational Literacy Skills
The association analyses between FC in infancy and toddlerhood and the school-age performance of language and foundational literacy skills revealed strong prospective associations of oral language skills in the early emerged FC patterns of the middle portion of the left temporal pole (midLTP, r = 0.40 ± 0.34, 95% BCI = [0.38, 0.42], pcorrected < 0.001, Cohen’s d = 1.0, Fig. 2B) and the left middle temporal gyrus (LMTG, r = 0.34 ± 0.32, 95% BCI = [0.31, 0.36], pcorrected < 0.001, Cohen’s d = 0.95, Fig. 2B). Moreover, a strong association between the FC pattern of the left fusiform gyrus (LFFG, r = 0.36 ± 0.36, 95% BCI = [0.33, 0.38], pcorrected < 0.001, Cohen’s d = 0.90) and phonological processing skills at the school-age was further identified (Fig. 2B, see the full results in Supplementary Table S3). Similar result patterns were also obtained based on 30% and 40% test datasets (see Supplementary Table S4), as well as adopting the leave-one-out and 4-fold crossvalidation approaches (SI Methods and Table S5). No region in the temporal lobe showed strong associations between the early emerged FCs and subsequent RAN abilities. Lastly, correlation analyses between motion parameters and language/literacy assessments at school-age revealed no significant results for either number of outliers or mean FD (all puncorrected > 0.1, see Supplementary Table S6).
Early Functional Network Correlates of Long-Term Verbal Development are Established within the First Year of Life
The SVR analyses based on imaging data acquired before 1 year of age further demonstrated that strong associations were still present between the infant FC of midLTP and oral language skills at school-age (r = 0.51 ± 0.35, Cohen’s d = 1.3, 95% BCI = [0.49, 0.53], pcorrected < 0.001) as well as between the FC of the left fusiform gyri and the phonological skills (r = 0.47 ± 0.39, Cohen’s d = 1.0, 95% BCI = [0.45, 0.50], pcorrected < 0.001) measured at age 6.5. However, the association between the infant FC of LMTG and the subsequent oral language abilities (r = 0.22 ± 0.39, Cohen’s d = 0.47, 95% BCI = [0.20, 0.25], pcorrected < 0.001) failed to outperform permutation results by large effect (Cohen’s d > 0.8) and therefore was not considered in the subsequent analyses.
Early Language Milestones Contribute to the Prospective Associations between Infant/Toddler FCs and School-Age Language and Phonological Performance
Compared to the association results based on models with regular covariates similar to those included in the main analyses, the SVR analyses additionally considering the emergent language skills as covariates showed significant reductions in the association strength between the identified FCs and school-age oral language (midLTP: t1998 = 2.3, pcorrected = 0.048) and phonological skills (LFFG: t1998 = 7.7, pcorrected < 0.001). Lastly, correlation analyses were also computed between each of the school-age language and preliteracy scores and their infancy MSEL scores for the receptive and expressive language skills, which did not reveal any significant associations, even before correction for multiple comparisons (all puncorrected > 0.1, see Supplementary Table S6).
Discussion
In this study, we provide the first evidence for prospective associations between functional network characteristics in 4–18-month-old infants and toddlers and their development of language and foundational literacy skills at school-age. Utilizing a 5-year longitudinal imaging dataset, we identify two temporal regions whose FC patterns in infancy and toddlerhood could reliably account for individual differences in oral language (left temporal pole) or phonological skills (left fusiform gyrus) measured at school-age in the same group of children. These associations are present when the same analyses are confined to the imaging data acquired before 1 year of age, suggesting the early emergence of the neural networks, possibly in infancy, for long-term language and phonological development. Moreover, these strong associations are observed when the critical environmental factors of socio-economic status (SES) and the home literacy environment (HLE) in infancy, as well as general cognitive abilities at school-age, were controlled for, minimizing potential environmental influences on the observed relationship. Finally, significant reductions in the association strength were observed in the SVR models with the emergent language skills measured at the time of scanning as covariates compared to those without, indicating that the early FC correlates of school-age language and preliteracy skills might, at least partially, reflect the foundational role of early emergent language skills for this protracted learning process. Altogether, these findings may uncover a critical role of FC patterns established in infancy and toddlerhood on the protracted development of high-order cognitive functions of language and literacy.
The current study utilizes FC analyses to identify brain regions associated with subsequent cognitive development, highlighting the importance of inter-regional interactions in regional functional specialization through development. It has been long hypothesized that the functional development of individual regions is facilitated and shaped by earlier developing connectivity patterns (Johnson 2001; Mars et al. 2018). In line with this account, it has been shown that the FC pattern of one region at rest is associated with task-based fMRI activation of this region (Cole et al. 2016), and changes in the FC patterns parallel the anatomical boundaries between functionally distinct neighbors (Johansen-Berg et al. 2004). Moreover, it has been recently demonstrated that the (structural) connectivity at age 5 before children learn to read is predictive of word-specific functional activation after 3 years of reading instruction (Saygin et al. 2016), providing direct evidence for the developmental significance of early network mechanisms for neural functional specialization. Following the same line of research, our current study demonstrates that the FC pattern established in infancy and toddlerhood for a given region is associated with the cognitive skill this region subsequently supports. This suggests that functional topologies that emerge within the first years of life lay a critical neural foundation for the functional specialization of neural regions supporting the long-term cognitive development. However, future studies are needed to further examine the role of early developed FC in shaping the neural correlates of cognitive functions.
The FC pattern of the left temporal pole in infancy and toddlerhood is associated with oral language performance at school-age, shedding light on an early functional network mechanism that could underlie the acquisition of complex oral language skills. Previous fMRI studies have revealed neural activation in the left temporal pole during speech perception in infants (Dehaene-Lambertz et al. 2002, 2006; Shultz et al. 2014), suggesting the early enrollment of this region for language-related processing. Meanwhile, the role of the temporal pole in lexico-semantic processing is well established in adult literature (Patterson et al. 2007; Schwartz et al. 2009; Ralph et al. 2017; Hung et al. 2020). This region is typically activated in semantic tasks with various types of stimuli, such as spoken/written words and pictures, revealing an amodal neural representation of the semantic system (Visser et al. 2010). Moreover, the associations between (virtual) lesions in the temporal pole and impaired semantic recognition are further demonstrated through transcranial magnetic stimulation studies in a typical population (Pobric et al. 2010) and neuropsychological investigations of semantic dementia with atrophies in the temporal pole (Lambon Ralph et al. 2010), indicating a causal link. Interestingly, it has been recently shown that this region is involved in color knowledge representation of both typical and congenitally blind participants, who presumably acquire such knowledge through different approaches (i.e., vision and language vs. language only), suggesting a preregistered function of semantic representation in the temporal pole independently of sensory-dependent experiences (Wang et al. 2020).
Aligning with the functional implications in adult studies, we hypothesize that the temporal pole in infant brains might serve as a core component of a wide-spread neural substrate that enables the learning of lexico-semantic representation via early language and speech learning/processing. Consistent with this conjecture, we further observe significant contribution of the emergent language skills to the identified associations between infant FC and later oral language skills, highlighting a close link between early language experiences and the development of the semantic neural network. Moreover, these prospective associations are obtained when key environmental factors of socioeconomic and home literacy characteristics were controlled for, and they are still present when only the FC patterns of a subsample of infants (≤12 months old) were investigated. This further suggests the inherent nature of the neural network of the left temporal pole linking to the lexico-semantic function. Our hypothesis is also consistent with Dehaene-Lambertz et al. (2020)'s recent proposal of an early symbolic system associated with speech/language learning observed in infants from the first months of life onward. In summary, the current finding provides empirical evidence of the far-reaching developmental significance of this early developing functional network on the trajectory of language acquisition. Future work is warranted to further investigate the network characteristics and specific roles of their constituent brain regions in supporting this complex learning process.
We further identify prospective associations between the preliteracy skill, that is, phonological processing at school-age, and the FC patterns of the left fusiform gyrus observed in infants and toddlers. The fusiform gyrus is a critical competent of the ventral visual pathway along the occipital–temporal cortex of adult brains and contains functionally specialized regions for different visual categories, such as faces and animals (e.g., Kanwisher et al. 1997; Grill-Spector and Weiner 2014). Among them, one region located in the left middle fusiform gyrus invariantly responds to orthographic processing of words in literate adults regardless of the written scripts, languages of instruction, or experimental tasks. This region has been termed the visual word form area (VWFA, Dehaene and Cohen 2011). Importantly, the functional tuning to prints in the VWFA, that is, greater activation for words/letters compared to false fonts, emerges in children only after the start of reading acquisition (Centanni et al. 2018; Dehaene-Lambertz et al. 2018; Fraga-González et al. 2021) and is strongly correlated with their reading abilities (Centanni et al. 2018; Chyl et al. 2018), suggesting an experience/environment-dependent specialization. It is therefore hypothesized that the formation of the VWFA reflects a functional reorganization process occurring over the course of learning to read, during which the print-specific processing emerges in a subregion of the ventral visual cortex in proximity to neural regions encoding oral language information (e.g., semantics and phonology) for the efficient integration of orthographic information with speech abilities developed earlier in life (Price and Devlin 2003; Behrmann and Plaut 2015; Dehaene-Lambertz et al. 2018).
Here, we demonstrate that FC patterns of the left fusiform gyrus established with the first 18 months of life are longitudinally associated with the development of phonological skills in school-age children, providing novel evidence for an early placement of the network characteristics of the fusiform gyrus as the initial neural scaffolding for the experience-driven functional development of this region in linking oral language and print processing. This interpretation is in line with the recent findings of the intrinsic FC between the putative VWFA and language areas in neonates (Li et al. 2020). Moreover, we demonstrated that early language skills contributed significantly to this longitudinal associations, further indicating a neural network mechanism underlying the facilitative role of early language/speech processing in subsequent literacy development (Puolakanaho et al. 2004; Flax et al. 2009; Torppa et al. 2010). Together, these findings might suggest an early role of the fusiform area in language and literacy development through its rich connections with perisylvian language areas. This may then drive the emergence of the functional tuning to the print stimuli in the VWFA once children start to learn to read. Indeed, the VWFA has been found to activate during the phonological processing tasks (Dietz et al. 2005), and its neural activation pattern encodes both phonological and orthographic information of words (Zhao et al. 2017). Moreover, this conjecture is in accordance with our earlier findings that white matter connectivity patterns of the ventral visual word pathway (including the fusiform) in prereaders precede and predict its functional tuning to words after reading onset (Saygin et al. 2016).
Lastly, it is also interesting to note that rapid automatized naming (RAN) abilities measured at school-age failed to be reliably associated with FCF of any temporal region. The RAN assessment measures the efficiency of integrating multiple language-related (e.g., access and retrieval of phonological and semantic information) and general cognitive (e.g., attention, vision, and motor planning) processes (Norton and Wolf 2012). Therefore, the null results might indicate that the RAN skills reflecting the integration abilities might be minimally associated with early emerged functional networks in infancy.
Broadly speaking, our study illustrates the importance of combining developmental psychology with infant imaging techniques to characterize the initial “blueprints” of the brain’s functional organization that support the protracted development of complex cognitive function. High-order cognitive functions, such as language and reading, are complex systems that involve orchestration of multiple distinct yet interactive cognitive processes (Rayner and Reichle 2010). These components are supported by specific neural mechanisms in adults (e.g., Taylor et al. 2013; Fedorenko and Thompson-Schill 2014), and often exhibit characteristic trajectories of neural functional specialization. For example, while neural responses of speech perception are evident in newborns (Dehaene-Lambertz et al. 2002), functional tuning of visual letter recognition is demonstrated only after the onset of reading instruction (Brem et al. 2010; Dehaene-Lambertz et al. 2018). Therefore, surveying the relationship between infant brain characteristics and the subsequent development of high-order cognitive abilities within a cognitive framework enables the depiction of structured cortical scaffolding, upon which cognitive abilities emerge. In line with this, our current study extends the previous observation of the associations between infant functional network and overall language development at age 4 (Emerson et al. 2016) by demonstrating cognitive process-specific relationships between FC in infancy and school-age performance of oral language and literacy skills. Overall, such an interdisciplinary approach integrating developmental psychology, cognitive sciences, and pediatric (infant) imaging methodologies can provide a valuable opportunity to identify early brain functional characteristics linked to individual variation in core processes of high-order cognitive functions. This highlights the potential of developing imaging markers to identify specific weaknesses in at-risk populations for targeted early intervention (Gilmore et al. 2018).
Our study represents the first evidence of prospective associations between infant functional topologies and long-term development of language and foundational literacy skills measured at school-age. However, the results should be interpreted with caution. First, the infants investigated in the current study had a wide age range at our initial scan (4–18 months old), due to challenges associated with the recruitment and retention of a longitudinal infant MRI cohort (Turesky et al. 2021). This imposes challenges for both data processing and result interpretation (Turesky et al. 2021). Based on the mean age of the infant sample (10.2 ± 3.5 months), the 1-year-old AAL atlas was utilized in the current study (Shi et al. 2011). Due to the significant growth and myelination of the human brain within the first 2 years of life (Deoni et al. 2012; Sanchez et al. 2012), applying one atlas to an infant sample of 4–18 months old could potentially introduce registration errors. To minimize this risk, visual inspection was conducted to ensure the correct mapping of every cortical region to the structural image of each infant. Moreover, despite the risk of overlooking functional subdivisions within each cortical region, the current approach of adopting the mean time series of cortical regions defined by anatomical parcellation is more resistant to registration errors than a more fine-grained parcellation derived from a different age group. Furthermore, several approaches were adopted to minimize the potential confounding effects of age at scan, including modeling age at scan and the infant’s language environments as covariates in the main analyses, and performing conformational analyses in a subgroup of younger infants. Nevertheless, the current project design limits a clear distinction between neural mechanisms established at birth and contributions of postnatal growth/experiences.
Second, the participants of our study had a relatively high SES background, with more than 90% of their parents achieving a Bachelor’s degree or higher. The narrow range of the SES characteristics therefore limits the generalizability of the current findings. Future studies with a more diverse SES background are essential for unraveling the interaction between socioeconomic status and early brain development and its associations with long-term cognitive development, such as oral language and reading skills. Answers to this question carry critical implications for the protracted development of the language and reading neural network as a dynamic and plastic trajectory shaped by various linguistic inputs associated with the environment of a child. Third, the current analyses focused on seed regions in the temporal lobe since it is typically involved in speech and language perception processing in infants, suggesting an early functional engagement. Nevertheless, other key component regions of the language/reading network observed in adults, such as the inferior frontal gyrus (Cattinelli et al. 2013; Fedorenko and Thompson-Schill 2014), should also be considered in future studies in order to fully characterize the neural trajectories supporting the protracted development of language and reading skills that reaches proficiencies beyond childhood. Finally, it is important to emphasize that to objectively evaluate the generalizability of the observed FC correlates of long-term language and preliteracy skills, an independent out-of-sample dataset is essential. Nevertheless, due to challenges in performing longitudinal infant MRI studies over 5–6 years that also limited the sample size of the current study, the prospective associations identified in the current study were evaluated using the hold-out random resampling procedure and further confirmed by a crossvalidation approach (see details in the Supplementary Information). Since both approaches train and test the SVR models using different subsamples randomly drawn from the entire sample, we expect the obtained results to have a low risk of the overfitting issue compared to in-sample association analyses, as suggested by Poldrack et al. (2020). Future longitudinal investigation starting in neonates, along with behavioral and neural characterization at multiple developmental stages in a longitudinal design, are needed to independently evaluate the robustness of the observed prospective associations, and more importantly, unravel neural trajectories underlying language and literacy development and key components that shape this process.
Conclusion
Utilizing a 5-year longitudinal neuroimaging project starting in infancy, the current study provides the first empirical evidence of prospective associations between FC patterns in infancy and toddlerhood and the development of oral language and phonological skills in school-age children. Moreover, we further show that emergent language skills assessed at the time of MR scanning contribute to these identified longitudinal associations, suggesting a critical role of early linguistic experiences in shaping the functional network characteristics associated with development of high-order language and foundational literacy abilities. Overall, this body of work reveals early emerged functional network mechanisms in infancy and toddlerhood that underlie the long-term development of complex cognitive abilities with a protracted neuro-trajectory that starts early in life or even in utero.
Funding
Eunice Kennedy Shriver National Institute of Child Health and Human Development (#R01HD65762-01 awarded to N.G. and P.E.G.); the Fundamental Research Funds for the Central Universities (awarded to X.Y.); the Charles H. Hood Foundation (awarded to N.G.); and the Boston Children’s Hospital Pilot Grant (awarded to N.G.).
Notes
We sincerely thank all the families for their participation in this longitudinal study, and acknowledge the assistance of Carolyn King and Tengda Zhao for reading this manuscript. Conflict of Interests: The authors declare no competing financial interests.
Data availability
All documentation and code used in these analyses will be shared with research teams upon request. Due to Institutional Review Board regulations at Boston Children’s Hospital at the time of consent, our data cannot presently be uploaded to a permanent third-party archive. However, data sharing can be initiated through a Data Usage Agreement upon request.
Supplementary Material
Contributor Information
Xi Yu, State Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University, Beijing 100875, China; Laboratories of Cognitive Neuroscience, Division of Developmental Medicine, Department of Medicine, Boston Children’s Hospital, Boston, MA 02115, USA.
Silvina L Ferradal, Department of Intelligent Systems Engineering, Indiana University, Bloomington, IN 47408, USA.
Danielle D Sliva, Laboratories of Cognitive Neuroscience, Division of Developmental Medicine, Department of Medicine, Boston Children’s Hospital, Boston, MA 02115, USA; Department of Neuroscience, Brown University, Providence, RI 02912, USA.
Jade Dunstan, Laboratories of Cognitive Neuroscience, Division of Developmental Medicine, Department of Medicine, Boston Children’s Hospital, Boston, MA 02115, USA.
Clarisa Carruthers, Laboratories of Cognitive Neuroscience, Division of Developmental Medicine, Department of Medicine, Boston Children’s Hospital, Boston, MA 02115, USA.
Joseph Sanfilippo, Laboratories of Cognitive Neuroscience, Division of Developmental Medicine, Department of Medicine, Boston Children’s Hospital, Boston, MA 02115, USA.
Jennifer Zuk, Laboratories of Cognitive Neuroscience, Division of Developmental Medicine, Department of Medicine, Boston Children’s Hospital, Boston, MA 02115, USA; Harvard Medical School, Boston, MA 02115, USA; Department of Speech, Language & Hearing Sciences, Boston University, Boston, MA 02215, USA.
Lilla Zöllei, A.A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Boston, MA 02129, USA.
Emma Boyd, A.A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Boston, MA 02129, USA.
Borjan Gagoski, Harvard Medical School, Boston, MA 02115, USA; Fetal Neonatal Neuroimaging and Developmental Science Center, Boston Children’s Hospital, Boston, MA 02115, USA; Department of Radiology, Boston Children's Hospital, Boston, MA 02215, USA.
Yangming Ou, Harvard Medical School, Boston, MA 02115, USA; Fetal Neonatal Neuroimaging and Developmental Science Center, Boston Children’s Hospital, Boston, MA 02115, USA; Department of Radiology, Boston Children's Hospital, Boston, MA 02215, USA; Computational Health Informatics Program, Boston Children's Hospital, Boston, MA 02115, USA.
P Ellen Grant, Harvard Medical School, Boston, MA 02115, USA; Fetal Neonatal Neuroimaging and Developmental Science Center, Boston Children’s Hospital, Boston, MA 02115, USA; Department of Radiology, Boston Children's Hospital, Boston, MA 02215, USA; Department of Medicine, Boston Children's Hospital, Boston, MA 02115, USA.
Nadine Gaab, Laboratories of Cognitive Neuroscience, Division of Developmental Medicine, Department of Medicine, Boston Children’s Hospital, Boston, MA 02115, USA; Harvard Medical School, Boston, MA 02115, USA; Harvard Graduate School of Education Boston, Boston, MA 02115, USA.
References
- Alcauter S, Lin W, Smith JK, Short SJ, Goldman BD, Reznick JS, Gilmore JH, Gao W. 2014. Development of thalamocortical connectivity during infancy and its cognitive correlations. J Neurosci. 34(27):9067–9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhamud A, Tisdall MD, Hess AT, Hasan KM, Meintjes EM, van der Kouwe AJ . 2012. Volumetric navigators for real-time motion correction in diffusion tensor imaging. Magn Reson Med. 68(4):1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrmann M, Plaut DC. 2015. A vision of graded hemispheric specialization. Ann N Y Acad Sci. 1359(1):30–46. [DOI] [PubMed] [Google Scholar]
- Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS. 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 34(4):537–541. [DOI] [PubMed] [Google Scholar]
- Brem S, Bach S, Kucian K, Kujala JV, Guttorm TK, Martin E, Lyytinen H, Brandeis D, Richardson U. 2010. Brain sensitivity to print emerges when children learn letter–speech sound correspondences. Proc Natl Acad Sci. 107(17):7939–7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattinelli I, Borghese NA, Gallucci M, Paulesu E. 2013. Reading the reading brain: a new meta-analysis of functional imaging data on reading. J Neurolinguistics. 26(1):214–238. [Google Scholar]
- Centanni TM, King LW, Eddy MD, Whitfield-Gabrieli S, Gabrieli JD. 2017. Development of sensitivity versus specificity for print in the visual word form area. Brain Lang. 170:62–70. [DOI] [PubMed] [Google Scholar]
- Centanni TM, Norton ES, Park A, Beach SD, Halverson K, Ozernov-Palchik O, Gaab N, Gabrieli JDE. 2018. Early development of letter specialization in left fusiform is associated with better word reading and smaller fusiform face area. Dev Sci. 21(5):e12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C-C, Lin C-J. 2011. LIBSVM: a library for support vector machines. ACM Trans Intell Syst Technol (TIST). 2(3):1–27. [Google Scholar]
- Chen Y, Liu S, Salzwedel A, Stephens R, Cornea E, Goldman BD, et al. 2020. The subgrouping structure of newborns with heterogenous brain–behavior relationships. Cereb Cortex. 31(1):301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chyl K, Kossowski B, Dębska A, Łuniewska M, Banaszkiewicz A, Żelechowska A, Frost SJ, Mencl WE, Wypych M, Marchewka A, et al. 2018. Prereader to beginning reader: changes induced by reading acquisition in print and speech brain networks. J Child Psychol Psychiatry. 59(1):76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AL, Fair DA, Dosenbach NU, Miezin FM, Dierker D, van Essen DC, Schlaggar BL, Petersen SE. 2008. Defining functional areas in individual human brains using resting functional connectivity MRI. Neuroimage. 41(1):45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. 2013. Statistical power analysis for the behavioral sciences. Academic press. [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehéricy S, Dehaene-Lambertz G, Hénaff M-A, Michel F. 2000. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 123(2):291–307. [DOI] [PubMed] [Google Scholar]
- Cole MW, Ito T, Bassett DS, Schultz DH. 2016. Activity flow over resting-state networks shapes cognitive task activations. Nat Neurosci. 19(12):1718–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristia A, Seidl A, Junge C, Soderstrom M, Hagoort P. 2014. Predicting individual variation in language from infant speech perception measures. Child Dev. 85(4):1330–1345. [DOI] [PubMed] [Google Scholar]
- Cunningham AE, Stanovich KE. 1997. Early reading acquisition and its relation to reading experience and ability 10 years later. Dev Psychol. 33(6):934. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L. 2011. The unique role of the visual word form area in reading. Trends Cogn Sci. 15(6):254–262. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. 2002. Functional neuroimaging of speech perception in infants. Science. 298(5600):2013–2015. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz, G., Fló, A., and Peña, M. (2020). Infants' early competence for language and symbols. The social brain: a developmental perspective. Cambridge, MA: MIT Press. Reprinted from: HAL Id: hal-02545079 https://hal.archives-ouvertes.fr/hal-02545079.
- Dehaene-Lambertz G, Hertz-Pannier L, Dubois J, Mériaux S, Roche A, Sigman M, Dehaene S. 2006. Functional organization of perisylvian activation during presentation of sentences in preverbal infants. Proc Natl Acad Sci. 103(38):14240–14245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Monzalvo K, Dehaene S. 2018. The emergence of the visual word form: longitudinal evolution of category-specific ventral visual areas during reading acquisition. PLoS Biol. 16(3):e2004103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Pena M. 2001. Electrophysiological evidence for automatic phonetic processing in neonates. Neuroreport. 12(14):3155–3158. [DOI] [PubMed] [Google Scholar]
- Denney MK, English JP, Gerber MM, Leafstedt J, Ruz ML. 2001. Family and home literacy practices: mediating factors for preliterate english learners at risk. Paper presented at the Annual Meeting of the American Educational Research Associations, April 10–14, 2001. Seattle, WA.
- Deoni SC, Dean DC III, O'Muircheartaigh J, Dirks H, Jerskey BA. 2012. Investigating white matter development in infancy and early childhood using myelin water faction and relaxation time mapping. Neuroimage. 63(3):1038–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni SC, O’Muircheartaigh J, Elison JT, Walker L, Doernberg E, Waskiewicz N, Dirks H, Piryatinsky I, Dean DC III, Jumbe NL. 2016. White matter maturation profiles through early childhood predict general cognitive ability. Brain Struct Funct. 221(2):1189–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz NA, Jones KM, Gareau L, Zeffiro TA, Eden GF. 2005. Phonological decoding involves left posterior fusiform gyrus. Hum Brain Mapp. 26(2):81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria V, Beckmann CF, Arichi T, Merchant N, Groppo M, Turkheimer FE, Counsell SJ, Murgasova M, Aljabar P, Nunes RG, et al. 2010. Emergence of resting state networks in the preterm human brain. Proc Natl Acad Sci. 107(46):20015–20020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff FJ, Reen G, Plunkett K, Nation K. 2015. Do infant vocabulary skills predict school-age language and literacy outcomes? J Child Psychol Psychiatry. 56(8):848–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ece Demir-Lira Ö, Applebaum LR, Goldin-Meadow S, Levine SC. 2019. Parents’ early book reading to children: relation to children's later language and literacy outcomes controlling for other parent language input. Dev Sci. 22(3):e12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson RW, Gao W, Lin W. 2016. Longitudinal study of the emerging functional connectivity asymmetry of primary language regions during infancy. J Neurosci. 36(42):10883–10892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, et al. 2009. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 5(5):e1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Thompson-Schill SL. 2014. Reworking the language network. Trends Cogn Sci. 18(3):120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald A, Perfors A, Marchman VA. 2006. Picking up speed in understanding: speech processing efficiency and vocabulary growth across the 2nd year. Dev Psychol. 42(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flax JF, Realpe-Bonilla T, Roesler C, Choudhury N, Benasich A. 2009. Using early standardized language measures to predict later language and early reading outcomes in children at high risk for language-learning impairments. J Learn Disabil. 42(1):61–75. [DOI] [PubMed] [Google Scholar]
- Fraga-González G, Pleisch G, Di Pietro SV, Neuenschwander J, Walitza S, Brandeis D, et al. 2021. The rise and fall of rapid occipito-temporal sensitivity to letters: transient specialization through elementary school. Dev Cogn Neurosci. 49:100958. 10.1016/j.dcn.2021.100958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Skiöld B, Horsch S, Nordell A, Blennow M, Lagercrantz H, Åden U. 2007. Resting-state networks in the infant brain. Proc Natl Acad Sci. 104(39):15531–15536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Alcauter S, Elton A, Hernandez-Castillo CR, Smith JK, Ramirez J, Lin W. 2015a. Functional network development during the first year: relative sequence and socioeconomic correlations. Cereb Cortex. 25(9):2919–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Alcauter S, Smith JK, Gilmore JH, Lin W. 2015b. Development of human brain cortical network architecture during infancy. Brain Struct Funct. 220(2):1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Knickmeyer RC, Gao W. 2018. Imaging structural and functional brain development in early childhood. Nat Rev Neurosci. 19(3):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, Petersen SE. 2016. Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb Cortex. 26(1):288–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AM, Rasmussen JM, Entringer S, Ben Ward E, Rudolph MD, Gilmore JH, Styner M, Wadhwa PD, Fair DA, Buss C. 2019. Maternal cortisol concentrations during pregnancy and sex-specific associations with neonatal amygdala connectivity and emerging internalizing behaviors. Biol Psychiatry. 85(2):172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Weiner KS. 2014. The functional architecture of the ventral temporal cortex and its role in categorization. Nat Rev Neurosci. 15(8):536–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S, Satterthwaite TD, Medaglia JD, Yang M, Gur RE, Gur RC, Bassett DS. 2015. Emergence of system roles in normative neurodevelopment. Proc Natl Acad Sci. 112(44):13681–13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttorm TK, Leppänen PH, Poikkeus A-M, Eklund KM, Lyytinen P, Lyytinen H. 2005. Brain event-related potentials (ERPs) measured at birth predict later language development in children with and without familial risk for dyslexia. Cortex. 41(3):291–303. [DOI] [PubMed] [Google Scholar]
- Hatcher PJ, Hulme C, Snowling MJ. 2004. Explicit phoneme training combined with phonic reading instruction helps young children at risk of reading failure. J Child Psychol Psychiatry. 45(2):338–358. [DOI] [PubMed] [Google Scholar]
- Hood M, Conlon E, Andrews G. 2008. Preschool home literacy practices and children's literacy development: a longitudinal analysis. J Educ Psychol. 100(2):252. [Google Scholar]
- Hulme C, Snowling MJ. 2013. Learning to read: what we know and what we need to understand better. Child development perspectives. 7(1):1–5. doi: 10.1111/cdep.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung J, Wang X, Wang X, Bi Y. 2020. Functional subdivisions in the anterior temporal lobes: a large scale meta-analytic investigation. Neurosci Biobehav Rev. 115:134–145. [DOI] [PubMed] [Google Scholar]
- Huttenlocher J, Haight W, Bryk A, Seltzer M, Lyons T. 1991. Early vocabulary growth: relation to language input and gender. Dev Psychol. 27(2):236. [Google Scholar]
- Hutton JS, Horowitz-Kraus T, Mendelsohn AL, DeWitt T, Holland SK, Consortium C-MA. 2015. Home reading environment and brain activation in preschool children listening to stories. Pediatrics. 136(3):466–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Behrens T, Robson M, Drobnjak I, Rushworth M, Brady J, Smith SM, Higham DJ, Matthews PM. 2004. Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proc Natl Acad Sci. 101(36):13335–13340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH. 2001. Functional brain development in humans. Nat Rev Neurosci. 2(7):475–483. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. 1997. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 17(11):4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman A, Kaufman N. 2004. Brief intelligence test. Minneapolis, MN: NCS Pearson, p. 2.
- King LS, Camacho MC, Montez DF, Humphreys KL, Gotlib IH. 2021. Naturalistic language input is associated with resting-state functional connectivity in infancy. J Neurosci. 41(3):424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK. 2004. Early language acquisition: cracking the speech code. Nat Rev Neurosci. 5(11):831–843. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Cipolotti L, Manes F, Patterson K. 2010. Taking both sides: do unilateral anterior temporal lobe lesions disrupt semantic memory? Brain. 133(11):3243–3255. [DOI] [PubMed] [Google Scholar]
- Leppänen P, Hämäläinen J, Guttorm T, Eklund K, Salminen H, Tanskanen A, Torppa M, Puolakanaho A, Richardson U, Pennala R, et al. 2012. Infant brain responses associated with reading-related skills before school and at school age. Neurophysiologie Clinique/Clin Neurophysiol. 42(1–2):35–41. 10.1016/j.neucli.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Li J, Osher DE, Hansen HA, Saygin ZM. 2020. Innate connectivity patterns drive the development of the visual word form area. Sci Rep. 10(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudzadeh M, Dehaene-Lambertz G, Fournier M, Kongolo G, Goudjil S, Dubois J, Grebe R, Wallois F. 2013. Syllabic discrimination in premature human infants prior to complete formation of cortical layers. Proc Natl Acad Sci. 110(12):4846–4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchman VA, Fernald A. 2008. Speed of word recognition and vocabulary knowledge in infancy predict cognitive and language outcomes in later childhood. Dev Sci. 11(3):F9–F16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Passingham RE, Jbabdi S. 2018. Connectivity fingerprints: from areal descriptions to abstract spaces. Trends Cogn Sci. 22(11):1026–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melby-Lervåg M, Lyster S-AH, Hulme C. 2012. Phonological skills and their role in learning to read: a meta-analytic review. Psychol Bull. 138(2):322. [DOI] [PubMed] [Google Scholar]
- Mullen EM. 1995. Mullen scales of early learning. MN: AGS Circle Pines. [Google Scholar]
- Nation K, Hulme C. 2011. Learning to read changes children’s phonological skills: evidence from a latent variable longitudinal study of reading and nonword repetition. Dev Sci. 14(4):649–659. [DOI] [PubMed] [Google Scholar]
- Norton ES, Wolf M. 2012. Rapid automatized naming (RAN) and reading fluency: implications for understanding and treatment of reading disabilities. Annu Rev Psychol. 63:427–452. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. 2007. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 8(12):976–987. [DOI] [PubMed] [Google Scholar]
- Pena M, Maki A, Kovac̆ić D, Dehaene-Lambertz G, Koizumi H, Bouquet F, Mehler J. 2003. Sounds and silence: an optical topography study of language recognition at birth. Proc Natl Acad Sci. 100(20):11702–11705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perani D, Saccuman MC, Scifo P, Anwander A, Spada D, Baldoli C, Poloniato A, Lohmann G, Friederici AD. 2011. Neural language networks at birth. Proc Natl Acad Sci U S A. 108(45):18566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasta SB, Wagner RK. 2010. Developing early literacy skills: a meta-analysis of alphabet learning and instruction. Read Res Quart. 45(1):8–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce LJ, Genesee F, Delcenserie A, Morgan G. 2017. Variations in phonological working memory: linking early language experiences and language learning outcomes. Appl Psycholing. 38(6):1265–1300. [Google Scholar]
- Pobric G, Jefferies E, Ralph MAL. 2010. Amodal semantic representations depend on both anterior temporal lobes: evidence from repetitive transcranial magnetic stimulation. Neuropsychologia. 48(5):1336–1342. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Huckins G, Varoquaux G. 2020. Establishment of best practices for evidence for prediction: a review. JAMA Psychiat. 77(5):534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 59(3):2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SJ, Wang Y, Beach SD, Sideridis GD, Gaab N. 2016. Examining the relationship between home literacy environment and neural correlates of phonological processing in beginning readers with and without a familial risk for dyslexia: an fMRI study. Ann Dyslexia. 66(3):337–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. 2003. The myth of the visual word form area. Neuroimage. 19(3):473–481. [DOI] [PubMed] [Google Scholar]
- Puolakanaho A, Poikkeus A-M, Ahonen T, Tolvanen A, Lyytinen H. 2004. Emerging phonological awareness differentiates children with and without familial risk for dyslexia after controlling for general language skills. Ann Dyslexia. 54(2):221–243. [DOI] [PubMed] [Google Scholar]
- Ralph MAL, Jefferies E, Patterson K, Rogers TT. 2017. The neural and computational bases of semantic cognition. Nat Rev Neurosci. 18(1):42–55. [DOI] [PubMed] [Google Scholar]
- Raschle N, Zuk J, Ortiz-Mantilla S, Sliva DD, Franceschi A, Grant PE, Benasich AA, Gaab N. 2012. Pediatric neuroimaging in early childhood and infancy: challenges and practical guidelines. Ann N Y Acad Sci. 1252(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner K, Reichle ED. 2010. Models of the reading process. Wiley Interdis Rev: Cogn Sci. 1(6):787–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CE, Sylvester CM, Mintz C, Kenley JK, Shimony JS, Barch DM, Smyser CD. 2017. Neonatal amygdala functional connectivity at rest in healthy and preterm infants and early internalizing symptoms. J Am Acad Child Adolesc Psychiatry. 56(2):157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RR, Segaran J, Leonard JA, Robinson ST, West MR, Mackey AP, Yendiki A, Rowe ML, Gabrieli JDE. 2018. Language exposure relates to structural neural connectivity in childhood. J Neurosci. 38(36):7870–7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe ML. 2012. A longitudinal investigation of the role of quantity and quality of child-directed speech in vocabulary development. Child Dev. 83(5):1762–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph MD, Graham AM, Feczko E, Miranda-Dominguez O, Rasmussen JM, Nardos R, Entringer S, Wadhwa PD, Buss C, Fair DA. 2018. Maternal IL-6 during pregnancy can be estimated from newborn brain connectivity and predicts future working memory in offspring. Nat Neurosci. 21(5):765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez CE, Richards JE, Almli CR. 2012. Neurodevelopmental MRI brain templates for children from 2 weeks to 4 years of age. Dev Psychobiol. 54(1):77–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansavini A, Bertoncini J, Giovanelli G. 1997. Newborns discriminate the rhythm of multisyllabic stressed words. Dev Psychol. 33(1):3. [DOI] [PubMed] [Google Scholar]
- Sato H, Hirabayashi Y, Tsubokura H, Kanai M, Ashida T, Konishi I, Uchida‐Ota M, Konishi Y, Maki A. 2012. Cerebral hemodynamics in newborn infants exposed to speech sounds: a whole-head optical topography study. Hum Brain Mapp. 33(9):2092–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin ZM, Osher DE, Norton ES, Youssoufian DA, Beach SD, Feather J, Gaab N, Gabrieli JDE, Kanwisher N. 2016. Connectivity precedes function in the development of the visual word form area. Nat Neurosci. 19(9):1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarborough HS, Neuman S, Dickinson D. 2009. Connecting early language and literacy to later reading (dis) abilities: evidence, theory, and practice. Approach Difficul Lit Dev: Assess Pedagogy Program. 10:23–38. [Google Scholar]
- Schrank FA, Wendling BJ. 2015. Woodcock-Johnson® IV tests of early cognitive and academic development. Rolling Meadows, IL: Riverside.
- Schwartz MF, Kimberg DY, Walker GM, Faseyitan O, Brecher A, Dell GS, Coslett HB. 2009. Anterior temporal involvement in semantic word retrieval: voxel-based lesion-symptom mapping evidence from aphasia. Brain. 132(12):3411–3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Yap P-T, Wu G, Jia H, Gilmore JH, Lin W, Shen D. 2011. Infant brain atlases from neonates to 1-and 2-year-olds. PLoS One. 6(4):e18746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz S, Vouloumanos A, Bennett RH, Pelphrey K. 2014. Neural specialization for speech in the first months of life. Dev Sci. 17(5):766–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeide MA, Friederici AD. 2016. The ontogeny of the cortical language network. Nat Rev Neurosci. 17(5):323–332. [DOI] [PubMed] [Google Scholar]
- Swingley D. 2009. Contributions of infant word learning to language development. Philos Trans R Soc B: Biol Sci. 364(1536):3617–3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J, Rastle K, Davis MH. 2013. Can cognitive models explain brain activation during word and pseudoword reading? A meta-analysis of 36 neuroimaging studies. Psychol Bull. 139(4):766. [DOI] [PubMed] [Google Scholar]
- Telkemeyer S, Rossi S, Koch SP, Nierhaus T, Steinbrink J, Poeppel D, et al. 2009. Sensitivity of newborn auditory cortex to the temporal structure of sounds. J Neurosci. 29(47):14726–14733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torppa M, Lyytinen P, Erskine J, Eklund K, Lyytinen H. 2010. Language development, literacy skills, and predictive connections to reading in Finnish children with and without familial risk for dyslexia. J Learn Disabil. 43(4):308–321. 10.1177/0022219410369096. [DOI] [PubMed] [Google Scholar]
- Turesky TK, Vanderauwera J, Gaab N. 2020. Imaging the rapidly developing brain: current challenges for MRI studies in the first five years of life. Dev Cogn Neurosci. 47:100893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Marc J. 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 15(1):273–289. [DOI] [PubMed] [Google Scholar]
- van Zuijen TL, Plakas A, Maassen BA, Been P, Maurits NM, Krikhaar E, van Driel J, van der Leij A. 2012. Temporal auditory processing at 17 months of age is associated with preliterate language comprehension and later word reading fluency: an ERP study. Neurosci Lett. 528(1):31–35. [DOI] [PubMed] [Google Scholar]
- Visser M, Jefferies E, Lambon Ralph M. 2010. Semantic processing in the anterior temporal lobes: a meta-analysis of the functional neuroimaging literature. J Cogn Neurosci. 22(6):1083–1094. [DOI] [PubMed] [Google Scholar]
- Vouloumanos A, Werker JF. 2007. Listening to language at birth: evidence for a bias for speech in neonates. Dev Sci. 10(2):159–164. [DOI] [PubMed] [Google Scholar]
- Wagner R, Torgesen J, Rashotte C, Pearson NA. 1999. CTOPP-2: comprehensive test of phonological processing. 2nd ed. Austin, TX: Pro-ed. [Google Scholar]
- Wang X, Men W, Gao J, Caramazza A, Bi Y. 2020. Two forms of knowledge representations in the human brain. Neuron. 107(2):383–393. [DOI] [PubMed] [Google Scholar]
- Yeatman JD, Dougherty RF, Ben-Shachar M, Wandell BA. 2012. Development of white matter and reading skills. Proc Natl Acad Sci. 109(44):E3045–E3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Raney T, Perdue MV, Zuk J, Ozernov-Palchik O, Becker BL, Raschle NM, Gaab N. 2018. Emergence of the neural network underlying phonological processing from the prereading to the emergent reading stage: a longitudinal study. Hum Brain Mapp. 39(5):2047–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Chen C, Shao L, Wang Y, Xiao X, Chen C, Yang J, Zevin J, Xue G. 2017. Orthographic and phonological representations in the fusiform cortex. Cereb Cortex. 27(11):5197–5210. [DOI] [PubMed] [Google Scholar]
- Zuk J, Yu X, Sanfilippo J, Figuccio MJ, Dunstan J, Carruthers C, Sideridis G, Turesky TK, Gagoski B, Grant PE, et al. 2021. White matter in infancy is prospectively associated with language outcomes in kindergarten. Dev Cogn Neurosci. 50:100973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All documentation and code used in these analyses will be shared with research teams upon request. Due to Institutional Review Board regulations at Boston Children’s Hospital at the time of consent, our data cannot presently be uploaded to a permanent third-party archive. However, data sharing can be initiated through a Data Usage Agreement upon request.


