Abstract
Background
Recent acute anaphylaxis guideline updates have identified remaining unmet needs based on currently available therapeutic options as a critical focus.
Objective
We compared the pharmacokinetic, pharmacodynamic, safety, and tolerability profiles of intranasal epinephrine with intramuscular epinephrine administered by autoinjector and manual syringe.
Methods
An open-label, 3-period crossover study was conducted in 116 healthy adult volunteers to assess the bioavailability of a single 13.2 mg intranasal dose of epinephrine compared to a 0.3 mg intramuscular autoinjector and a 0.5 mg manual syringe. Patients with epinephrine concentrations of 50, 100, and 200 pg/mL at 10, 20, 30, and 60 minutes after dosing were also evaluated.
Results
Pharmacokinetic parameters for the 13.2 mg intranasal dose exceeded those of the 0.3 mg autoinjector with a rapid and higher maximum observed concentration (intranasal, 429.4 pg/mL; autoinjector, 328.6 pg/mL) and greater systemic exposure (AUC0-360; intranasal, 39,060 pg∙min/mL; autoinjector, 17,440 pg∙min/mL). Similar results were observed compared to the 0.5 mg manual syringe. Pharmacokinetic parameters for opposite-nostril and same-nostril dosing were higher than both intramuscular doses, except time to reach maximum observed concentration, which was bracketed between the 2 intramuscular doses (intranasal opposite and same nostril, 20 minutes; autoinjector, 14.9 minutes; manual syringe, 45 minutes). Similar effects on blood pressure and heart rate were observed for intranasal and autoinjector administration. Intranasal epinephrine was safe and well tolerated. No serious or unexpected adverse events were reported, confirming results from earlier clinical studies.
Conclusions
Bidose epinephrine spray addresses the unmet medical and patient needs for a needle-free, convenient, and effective dose-delivery system for self-administration of epinephrine that is as good as or better than the 0.3 mg autoinjector.
Key words: Anaphylaxis, intranasal, epinephrine, delivery system, NDS1C, Bryn Pharma, self-administration, pharmacokinetics, pharmacodynamics, food allergy, venom allergy
Anaphylaxis, a rapid-onset type 1 allergic reaction that can lead to acute, life-threatening respiratory failure, represents the most severe form of an allergic reaction. It is characterized by sudden onset and rapid progression of symptoms as well as airway, breathing, or circulation problems and often skin or mucosal changes, the latter of which may be subtle or absent in 10% to 20% of reactions.1
The incidence of anaphylaxis is between 50 and 112 episodes per 100,000 person-years.2 In the United States, between 2004 and 2016, the incidence was 2.1 per 1000 person-years, with one quarter of anaphylactic reactions affecting children younger than 17 years old. Notably, the incidence of anaphylaxis peaks in children 2 to 12 years of age and adults between 50 and 69 years of age.3 Additionally, most anaphylactic reactions occur outside the hospital setting.3
Lifetime prevalence is estimated to be 0.3% to 5.1%, depending on the definitions used, study methodology, and geographical area evaluated.2 Importantly, the rate of occurrence of anaphylaxis in the general population is increasing approximately 3% to 5% per year, especially in people in their first 20 years of life. In addition, food allergy and anaphylaxis appear to be increasing in the United States, especially in young children. Among allergens leading to severe allergic reactions, food allergies are highly prevalent, occurring in approximately 6% to 8% of children and 2% to 5% of adults ≥18 years of age.4,5
A 2019 study reported that at least 10.8% (>26 million) of US adults are food allergic, and approximately 19% of adults believe that they have a food allergy.6 Notably, this is higher than other reports, which typically cite a 6% to 8% incidence in the adult population. This study demonstrated that among food-allergic adults, 51.1% experienced a severe food allergy reaction, 45.3% were allergic to multiple foods, and 48.0% developed food allergies as an adult. Among these patients, 24.0% reported a current epinephrine prescription and 38.3% reported at least 1 food allergy–related lifetime emergency department visit, demonstrating the impact on health care utilization.6
In the treatment of anaphylaxis, the current outpatient standard of care is an epinephrine autoinjector for self-administration or the use of intramuscular or intravenous epinephrine in a health care setting. The autoinjector is often referred to as an EpiPen (a trade name) as a result of the historical popularity of the brand; however, generic autoinjectors have become the mainstay of these prescribed devices, with different designs being used that can affect drug delivery efficiency and lead to the need for redosing to alleviate symptoms.7 As a result of the acute and life-threatening nature of anaphylaxis, delivery of a clinically meaningful dose of epinephrine in a timely fashion is critical to optimizing patient outcomes.
Recent updates to the guidelines for the acute management of anaphylaxis have identified both best practice and remaining unmet needs based on available therapeutic options.2,7 Because needle-based epinephrine devices are the only products available, intramuscular epinephrine remains the first-line treatment for anaphylaxis, either by autoinjector or prefilled syringe. Pharmacokinetic (PK) data are provided for all epinephrine autoinjector devices, supporting the benefits of acute and rapid bioavailability (BA) of epinephrine via autoinjectors as first-line management for patients experiencing an acute anaphylactic event.
An ad hoc committee speaking on behalf of the World Health Organization released a statement regarding the use of epinephrine in the treatment of anaphylaxis. They noted that epinephrine is currently underused, is often dosed suboptimally, and is underprescribed for self-administration. Many reasons proposed to withhold its use are flawed, and the therapeutic benefits of epinephrine exceed the risk when provided in appropriate intramuscular doses.8 The guidelines also mention the limited availability of epinephrine autoinjectors in many countries as well as their affordability for some patients. Overall, this signals an unmet need to improve the management of patients at risk for anaphylaxis and an acknowledgment that current autoinjector use remains suboptimal.
Current treatment guidelines list epinephrine as an essential medication for the treatment of anaphylaxis. As a result of the low oral BA of epinephrine, intramuscular administration is considered the first line of treatment.9, 10, 11 Three major issues hindering subjects’ compliance with carrying and using the intramuscular autoinjectors include reluctance to use self-injectors (eg, needle-use anxiety), reluctance to carry as a result of autoinjector size, and application error. Consequently, only 30% to 40% of individuals experiencing anaphylaxis receive epinephrine through autoinjectors.10 With the suboptimal use of emergency epinephrine in both outpatient and clinical settings, recommendations for the clinical management of anaphylaxis have been recently updated with emphasis on repeating intramuscular epinephrine doses after 5 to 20 minutes if symptoms do not resolve. However, there is a dosing restriction for use of no more than 2 autoinjectors. Patients are advised to seek medical attention if symptoms do not resolve, highlighting the importance of timely and appropriate administration in an emergent situation.
Although epinephrine is extremely effective in the treatment of anaphylaxis, there remain challenges that, if addressed, could improve patient outcomes, reduce health care utilization and cost of care by minimizing delay to treatment, and provide consistent, clinically effective doses of epinephrine. Identification of anaphylaxis is often delayed or missed in up to 50% of patients even in a health care setting.1 Recently there have been recommendations for improved recognition of anaphylaxis, epinephrine autoinjector design, and alternative non-needle products, as well as the need to address unmet needs in special populations.
Once identified, the treatment of anaphylaxis may be delayed further as a result of patients not carrying the device, stigma associated with needle use, and patient dislike of needles.12 Delayed treatment represents a major issue that allows symptoms to worsen to such a degree that they cannot be controlled with a single dose. The treatment of choice in an outpatient setting is intramuscular epinephrine delivered by autoinjector. However, some devices are challenged by less than user-friendly designs or may pose the risk of injury, especially in young patients, as the result of poor device design and can be difficult to use safely in these patients. Also, the 0.3 mg autoinjector may underdose compared to the 0.5 mg prefilled syringe, contributing to the need for a second dose as well as increasing the number of emergency room visits.
Even when anaphylaxis is identified quickly and intramuscular epinephrine is promptly self-administered, some barriers remain relating to correct dosing based on insufficient training and challenges with intramuscular autoinjectors.1 Consequently, some people experiencing anaphylaxis may require a second injection to address symptoms, yet many people at risk of anaphylaxis do not regularly carry a single, much less a second, autoinjector with them.
Finally, although relatively rare, <5% of anaphylactic reactions occur in a biphasic manner with recurrence of anaphylaxis even after treatment of the initial reaction, which may require access to a second dose of epinephrine. It has been reported that delayed administration of epinephrine is associated with a higher rate of biphasic anaphylaxis, supporting the emphasis on early and efficient epinephrine administration.1 Identification of anaphylaxis, as well as rapid and effective dosing of epinephrine, remain areas with significant unmet needs that have implications for patient outcomes.
In summary, current outpatient epinephrine administration options are associated with underutilization and delay to treatment, and potentially contribute to unfavorable outcomes for patients. There are high psychosocial burden and anxiety for patients and caregivers regarding use of autoinjectors, similar to that observed in patients with type 1 diabetes. As alternatives to epinephrine autoinjectors evolve, patients and clinicians will have access to viable, non-needle, user-friendly, and safe alternatives to autoinjectors.
In response to these remaining unmet needs in the treatment of acute anaphylactic reactions, intranasal delivery was explored as an alternative route of administration. Results from previous studies with different potencies of intranasal epinephrine, compared to a 0.3 mg intramuscular autoinjector, have suggested that intranasal spray is a viable alternative.13,14
Common adverse reactions to systemically administered epinephrine include anxiety, apprehensiveness, restlessness, tremor, weakness, dizziness, sweating, palpitations, pallor, nausea and vomiting, headache, and respiratory difficulties. These symptoms occur in some people receiving therapeutic doses of epinephrine but are more likely to occur in patients with hypertension or hyperthyroidism.15 In previous studies, systemic exposure over 6 hours (AUC0-360, where AUC is area under the concentration–time curve) and maximum observed concentration (Cmax) obtained after 13.2 mg intranasal administration of the bidose epinephrine spray NDS1C (ie, 2 × 6.6 mg intranasal sprays) to opposite nostrils suggested a slightly higher plasma level than that of the reference 0.3 mg intramuscular autoinjector dose. The current study was structured to confirm these results and support the validity of NDS1C as a potential treatment option for the acute management of anaphylaxis.
Methods
Study design
The study was conducted using an open-label, 3-period, 2-cohort crossover design to assess the PK profile of a single intranasal dose of epinephrine 13.2 mg consisting of 2 consecutive 6.6 mg sprays, compared to an intramuscular 0.3 mg autoinjector and 0.5 mg manual syringe. All subjects within a given cohort received the same intranasal treatment during period 1, followed by 1 of 2 intramuscular treatments during period 2 and the other intramuscular treatment during period 3, according to the randomization scheme. Each dose was separated by a 24-hour washout period.
Study population
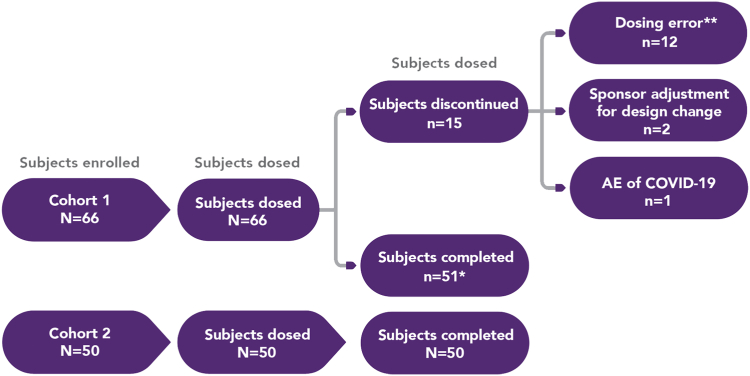
A total of 116 healthy men and women were enrolled onto the study, with 66 subjects enrolled onto cohort 1 and 50 subjects enrolled onto cohort 2. Fifteen subjects enrolled onto cohort 1 were subsequently discontinued (Fig 1), resulting in 51 subjects completing the protocol in cohort 1 and 50 in cohort 2.
Fig 1.
Subject disposition. ∗One subject who completed the study in cohort 1 was only included in a subset of study results. This patient received doses before protocol amendment 3 and had received 3 doses, but the last one was the same as the first. ∗∗Subjects received a dose inconsistent with that specified by the protocol.
Inclusion and exclusion criteria reflected standards for the conduct of a clinical trial. In addition, as a result of the coronavirus disease 2019 (COVID-19) pandemic, subjects were required to have a negative COVID-19 PCR test to be enrolled onto the study.
Cohorts and dosing
Each of the 2 cohorts in the study included 3 dosing groups; cohort 1 received treatments A, B, and C, and cohort 2 received treatments D, E, and F (Table I). Treatments A and D were supplied as a 6.6 mg epinephrine nasal spray (0.11 mL × 60 mg/mL NDS1C) administered as 2 consecutive intranasal sprays. Subjects were required to blow their nose to clear their nostrils immediately before dosing with the intranasal nasal spray, which was subsequently administered by a trained clinical professional at the clinic. The 2 consecutive intranasal sprays were administered within 10 seconds of each other.
Table I.
Key secondary end points
| • Proportion of subjects reaching unadjusted and baseline-adjusted epinephrine concentrations of 50, 100, and 200 pg/mL at 10, 20, 30, and 60 minutes after dosing. |
| • Unadjusted and baseline-adjusted TrefCmax. |
| • Unadjusted and baseline-adjusted AUEC0-t, Emax, and TEmax for SBP and DBP and HR. |
| • PD effects (expressed as changes in BP and HR) of intranasal administrations of epinephrine compared to epinephrine administered via intramuscular injection; unadjusted and change from baseline AUEC0-t, Emax, and TEmax for BP (SBP and DBP) and HR. |
| • Adverse events, 12-lead electrocardiograms, vital signs, telemetry, injection-site reaction assessment, clinical laboratory tests, and physical examinations. |
Treatments B and E were supplied as 0.3 mg epinephrine intramuscular injection (epinephrine injection, USP autoinjector 0.3 mg, an authorized generic for Mylan’s EpiPen). Treatments C and F were supplied as 0.5 mg intramuscular injection (0.5 mL × 1 mg/mL epinephrine injection, USP solution) using a manual syringe.
Screening of subjects occurred within 28 days before the first dose. On day 1 of period 1, subjects received a single intranasal dose of epinephrine as 2 consecutive sprays in opposite nostrils (cohort 1) or the same nostril (cohort 2). On day 1 of periods 2 and 3, subjects received an intramuscular dose of epinephrine either by autoinjector or manual syringe according to the randomization scheme. There was a washout of approximately 1 day between the epinephrine dosing in each period.
In each study period, PK sampling was conducted before dosing and for 360 minutes (6 hours) after dosing. PK time-matched blood pressure (BP) and heart rate (HR) measurements were collected in each period as pharmacodynamic (PD) markers. Safety was monitored throughout the study by repeated clinical and laboratory evaluations.
Primary and secondary outcome measures
The primary objective of the study was to compare the PK of a single intranasal dose of epinephrine (consisting of 2 consecutive sprays administered in either the same or opposite nostrils) to that of a single intramuscular dose administered by 0.3 mg autoinjector. Primary end points included unadjusted and baseline-adjusted early BA parameters Cmax20, AUC0-10, AUC0-20, AUC0-30, AUC0-Tmax, and AUC0-60, standard BA parameters Cmax, Tmax, AUC0-t, AUC0-360, AUC0-inf, and the additional PK parameters elimination rate constant (Kel), half-life (t½), and percentage extrapolated AUC (AUC%extrap) for epinephrine in plasma. Table II summarizes these parameters.
Table II.
Noncompartmental PK parameters
| Parameter | Definition | Method of determination |
|---|---|---|
| AUC0-t, AUC0-t,adj | AUC from time 0 to time of last observed/measured nonzero concentration. | Calculated by linear trapezoidal with linear interpolation method. |
| AUC0-Tmax AUC0-Tmax,adj | AUC from time 0 to time of maximum observed plasma concentration. | Calculated by linear trapezoidal with linear interpolation method. |
| AUC0-10 AUC0-10,adj | AUC from time 0 to 10-minute postdose time point. | Calculated by linear trapezoidal with linear interpolation method. |
| AUC0-20 AUC0-20,adj | AUC from time 0 to 20-minute postdose time point. | Calculated by linear trapezoidal with linear interpolation method |
| AUC0-30 AUC0-30,adj | AUC from time 0 to 30-minute postdose time point. | Calculated by linear trapezoidal with linear interpolation method. |
| AUC0-60 AUC0-60,adj | AUC from time 0 to 60-minute postdose time point. | Calculated by linear trapezoidal with linear interpolation method. |
| AUC0-360 AUC0-360,adj | AUC from time 0 to 360-minute postdose time point. | Calculated by linear trapezoidal with linear interpolation method. |
| AUC0-inf AUC0-inf,adj | AUC from time 0 extrapolated to infinity. | AUC0-inf = AUC0-t + (Clast/Kel), where Clast was last observed/measured concentration. |
| AUC%extrap, AUC%extrap,adj | Percentage of AUC0-inf extrapolated. | AUC%extrap = (AUC0-inf − AUC0-t)/AUC0-inf × 100 |
| Cmax, Cmax,adj | Maximum observed concentration. | Taken directly from bioanalytic data. |
| Cmax20, Cmax20,adj | Maximum observed concentration within 20 minutes after dose. | Taken directly from bioanalytic data. |
| Tmax, Tmax,adj | Time to reach Cmax. | Taken directly from clinical data. Tmax is first occasion if Cmax is observed at multiple points. |
| TrefCmax, TrefCmax,adj | time after test drug administration to reach plasma concentrations equal to Cmax after reference drug administration. | Time after intranasal administration to reach epinephrine intramuscular injection Cmax (treatments B and E) using interpolation. |
| Kel, Keladj | Apparent terminal elimination rate constant from semilog plot of concentration–time curve. | Calculated by linear least-squares regression analysis using maximum number of points in terminal log-linear phase (eg, 3 or more nonzero concentrations), Cmax excluded. |
| t½, t½,adj | Apparent terminal elimination half-life. | Calculated as 0.693/Kel. |
The secondary objectives included evaluation of the BA, PD effect, and safety of intranasal epinephrine spray compared to that of intramuscular injection (0.3 mg). The secondary end points are listed in Table I.
Baseline assessments were conducted to correct for endogenous epinephrine levels for each subject. Subject served as their own controls.
Statistical analysis
PK analysis
Summary statistics, including sample size (n), arithmetic mean, standard deviation (SD), coefficient of variation (CV%), standard error of the mean (SEM), minimum, median, and maximum were calculated for all nominal concentration time points. Summary statistics (n, mean, SD, CV%, SEM, minimum, median, maximum, geometric mean, and geometric CV%) were calculated for plasma epinephrine PK parameters (Table II).
ANOVA was performed on the unadjusted and baseline-adjusted, ln-transformed AUC0-10, AUC0-20, AUC0-30, AUC0-60, AUC0-360, AUC0-Tmax, AUC0-t, AUC0-inf, Cmax, and Cmax20 for each cohort. The ANOVA model included sequence and treatment as fixed effects, and subject nested within sequence as a random effect. Each ANOVA included calculation of least-squares means (LSM) as well as the difference between treatment LSM. Ratios of LSM were calculated using the exponentiation of the difference between treatment LSM from analyses of the unadjusted and baseline-adjusted, ln-transformed AUC0-10, AUC0-20, AUC0-30, AUC0-60, AUC0-360, AUC0-Tmax, AUC0-t, AUC0-inf, Cmax, and Cmax20.
Consistent with the two 1-sided tests, 90% confidence intervals for the ratios were derived by exponentiation of the confidence intervals obtained for the difference between treatment LSM resulting from the analyses on the unadjusted and baseline-adjusted, ln-transformed AUC0-10, AUC0-20, AUC0-30, AUC0-60, AUC0-360, AUC0-Tmax, AUC0-t, AUC0-inf, Cmax, and Cmax20.
Comparisons of interest included, for cohort 1, treatment A compared to B and treatment A compared to C, and for cohort 2, treatment D compared to E and treatment D compared to F. ANOVA was also performed comparing treatment A to D for the PK analyses. The model for the cross-cohort comparison of treatment A versus D did not include sequence as a fixed effect.
PD analysis
Summary statistics, including sample size, mean, SD, CV%, SEM, minimum, median, and maximum, were calculated for all nominal collection time points (Table III).
Table III.
Noncompartmental PD parameters
| Parameter | Definition | Method of determination |
|---|---|---|
| AUEC0-t, AUEC0-t,adj | Area under effect time curve from time 0 to last measured time point. | Calculated by linear trapezoidal with linear interpolation method using Net AUEC from WinNonlin Drug Effect module, with baseline set to 0 for baseline-adjusted estimations. Time 0 was average of 3 predose values. |
| Emax, Emax,adj | Maximum positive effect level. | Taken directly from unadjusted and baseline-adjusted clinical data only with baseline set to 0 in Drug Effect module. |
| TEmax, TEmax,adj | Time to reach Emax. | Taken directly from unadjusted and baseline-adjusted clinical data only with baseline set to 0 in Drug Effect module. |
Results
Subject disposition summary
A total of 66 subjects were enrolled onto cohort 1 and were randomized to study treatment; 51 subjects completed the study. Of the 15 subjects discontinuing, 12 were due to not receiving the protocol-specified dose, 2 were due to sponsor adjustment for design change (protocol amendment 3), and 1 was due to an adverse event of COVID-19. Although discontinued, some data from these subjects were included in analyses, as appropriate, per protocol. A total of 50 subjects were enrolled onto cohort 2, and all these subjects completed the study (Fig 1).
In cohort 1, three subjects were dosed before protocol amendment 3 and followed a different dosing regimen. For these subjects, period 1 and period 2 data were included in summaries. However, for 1 subject, the period 3 data (second dose of treatment A) were not included in any summaries and were only listed in the concentration tables. It should be noted that the other 2 subjects were not dosed in period 3.
In period 1, a total of 12 subjects received approximately half of the protocol-specified dose. As a result, these subjects were discontinued from the study and replaced with alternative subjects. Data from the discontinued subjects were not analyzed for epinephrine in plasma by the bioanalytic laboratory and hence are not included in the PK tables.
Primary outcome
Because epinephrine is an endogenous hormone, baseline epinephrine levels were assessed by 3 separate blood draws before study drug administration to obtain a reflective baseline. These were comparable between both cohorts (17-26 pg/mL). Consequently, the primary outcomes are reported as baseline-adjusted values.
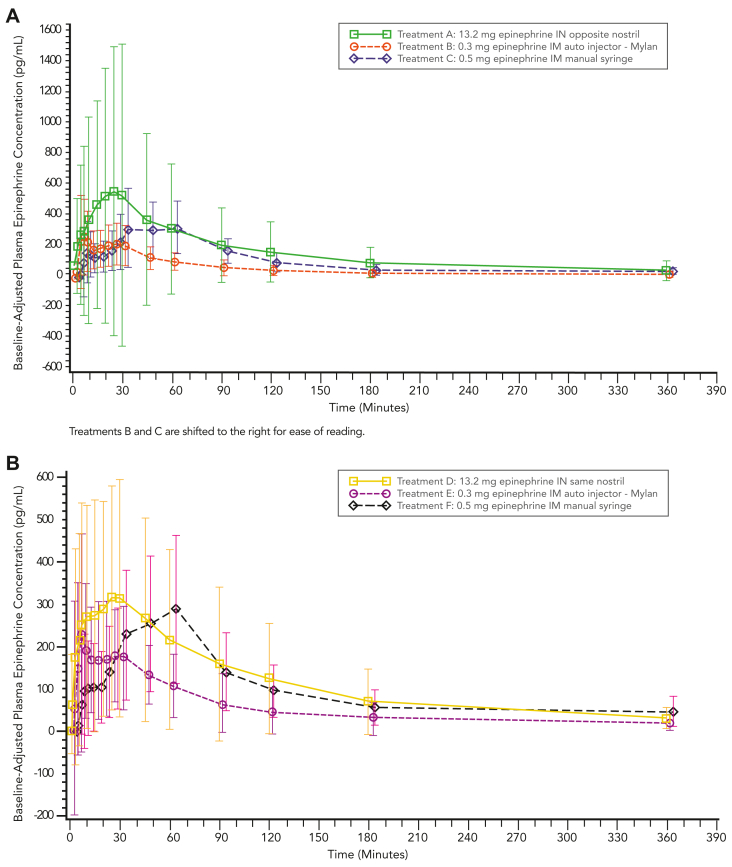
The highest exposure was obtained after administration of 13.2 mg epinephrine delivered to opposite nostrils and the second highest by administration in the same nostril, followed by the 0.5 mg epinephrine intramuscular manual syringe, and last the 0.3 mg epinephrine intramuscular autoinjector (Table II). Notably, 13.2 mg epinephrine intranasal administration demonstrated a single peak that was greater than the peaks observed after intramuscular administration (Figs 2 and 3). The 0.3 mg epinephrine intramuscular autoinjector demonstrated a profile with a double peak absorption, including a higher first peak mean observed at approximately 5 minutes after dosing and a secondary, lower peak at approximately 25 minutes after dosing. A similar trend was observed after the 0.5 mg epinephrine intramuscular manual syringe, with the first peak occurring at 5 minutes, followed by a second peak at 60 minutes after dosing in cohort 1.
Fig 2.
Mean baseline-adjusted plasma epinephrine concentration–time profiles from 0 to 360 minutes. (A) Cohort 1. AUC0-360,adjP values: treatment A versus B, P < .0001; treatment A versus C, P = .3403. Cmax,adjP values: treatment A versus B, P = .0581; treatment A versus C, P = .7384. (B) Cohort 2. AUC0-360,adjP values: treatment D versus E, P < .0001; treatment D versus F, P = .7462. Cmax,adjP values: treatment D versus E, P = .0064; treatment D versus F, P = .2759.
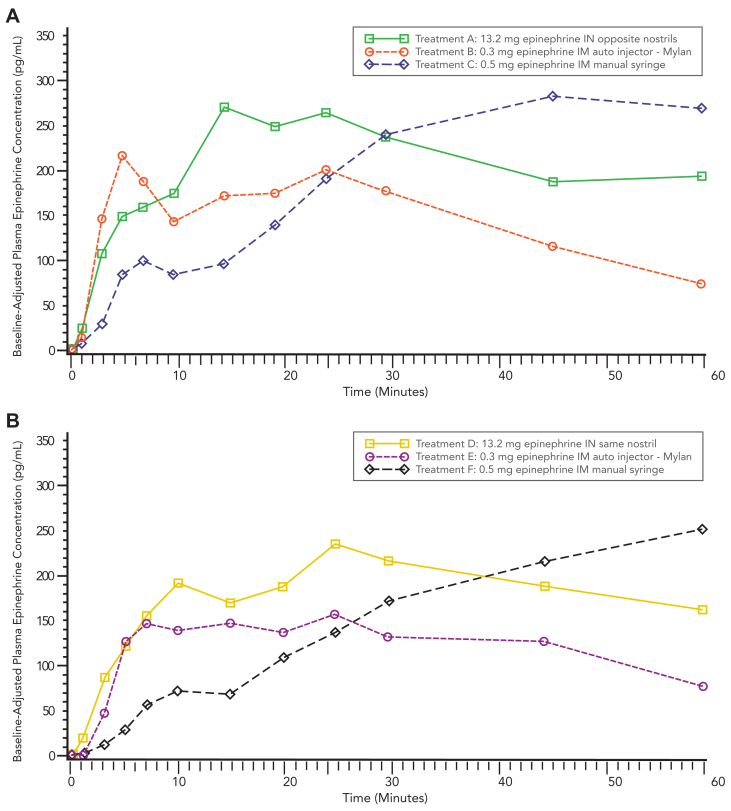
Fig 3.
Median baseline-adjusted plasma epinephrine concentration–time profiles from 0 to 60 minutes. (A) Cohort 1. AUC0-60,adjP values: treatment A versus B, P < .0001; treatment A versus C, P = .1643. Cmax0-20,adjP values: treatment A versus B, P = .1088; treatment A versus C, P < .0001. (B) Cohort 2. AUC0-60,adj P values: treatment D versus E, P = .0002; treatment D versus F, P = .0833. Cmax0-20,adjP values: treatment D versus E, P = .2102; treatment D versus F, P < .0001. Treatments E and F are shifted right to ease reading.
In cohort 2, the first and only peak was observed for the intranasal dose in the same nostril, occurring at 25 minutes. Peaks observed for the 0.3 mg intramuscular autoinjector and 0.5 mg manual syringe occurred at 5 minutes and 60 minutes after dosing, respectively. After peaks, epinephrine mean concentrations declined, heading toward baseline levels by 360 minutes after dosing (Fig 2).
Baseline-adjusted epinephrine parameters demonstrated that administration of 13.2 mg intranasal treatment to opposite nostrils resulted in a higher AUC and Cmax compared to either intramuscular treatment. When 13.2 mg epinephrine intranasal was administered in opposite nostrils compared to the 0.3 mg epinephrine intramuscular autoinjector, AUC and Cmax were higher for the intranasal treatment while the AUC0-10 was similar (Table II). When the comparison was made with the 0.5 mg epinephrine intramuscular manual syringe, the intranasal AUC and Cmax parameters were higher, while the AUC0-Tmax was lower (Table II). After the 13.2 mg intranasal dose was administered in opposite nostrils of the 54 subjects, 27 subjects reached the epinephrine levels observed after 0.3 mg epinephrine intramuscular autoinjector treatment, with a median time (TrefCmax) of approximately 8.2 minutes.
Administration of the 13.2 mg intranasal treatment to the same nostril had higher AUC and Cmax values compared to both intramuscular treatments when adjusted for baseline. When compared to the 0.3 mg epinephrine intramuscular autoinjector, the intranasal PK parameters were consistently higher for same-nostril intranasal administration.
Additionally, when the intranasal treatment was compared to the 0.5 mg epinephrine intramuscular manual syringe, all parameters assessed were higher for intranasal administration, with the exception of AUC0-360, AUC0-t, and AUC0-Tmax (Tables IV and V).
Table IV.
Summary of baseline-adjusted plasma epinephrine PK parameters after 13.2 mg intranasal spray in cohort 1 compared to intramuscular injection (PK population)
| PK parameter | Treatment |
P value |
|||
|---|---|---|---|---|---|
| A [no.] | B [no.] | C [no.] | A vs B | A vs C | |
| AUC0-10,adj (pg∙min/mL) | 1130 (213.2) [54] | 1157 (163.2) [51] | 624.5 (158.5) [53] | .8213 | .0068 |
| AUC0-20,adj (pg∙min/mL) | 3730 (146.1) [54] | 2789 (105.9) [51] | 1757 (107.1) [53] | .1073 | <.0001 |
| AUC0-30,adj (pg∙min/mL) | 6789 (134.8) [54] | 4652 (85.8) [51] | 3699 (86.3) [53] | .0120 | <.0001 |
| AUC0-60,adj (pg∙min/mL) | 14240 (122.8) [54] | 8615 (66.6) [51] | 11970 (64.1) [53] | <.0001 | .1643 |
| AUC0-360,adj (pg∙min/mL) | 39060 (100.0) [53] | 17440 (52.0) [51] | 34620 (41.5) [53] | <.0001 | .3403 |
| AUC0-t,adj (pg∙min/mL) | 37860 (103.5) [54] | 17360 (52.5) [51] | 34760 (41.4) [53] | <.0001 | .5383 |
| AUC0-Tmax,adj (pg∙min/mL) | 3994 (212.1) [54] | 1813 (138.5) [51] | 6262 (129.2) [53] | .0001 | .0274 |
| AUC0-inf,adj (pg∙min/mL) | 45820 (99.5) [44] | 20850 (49.8) [31] | 43090 (35.4) [21] | <.0001 | .7997 |
| AUC%extrap,adj (%) | 13.60 ± 14.119 [44] | 11.77 ± 15.490 [31] | 11.54 ± 9.3210 [21] | — | — |
| Cmax0-20,adj (pg/mL) | 359.786 (132.3) [54] | 266.554 (111.2) [51] | 172.579 (113.6) [53] | .1088 | <.0001 |
| Cmax,adj (pg/mL) | 429.398 (121.7) [54] | 328.550 (74.2) [51] | 406.519 (63.7) [53] | .0581 | .7384 |
| TrefCmax,adj (min) | 8.171 (0.88, 30.14) [27] | — | — | — | — |
| Tmax,adj (min) | 20.475 (1.33, 180.03) [54] | 14.933 (2.98, 121.27) [51] | 45.100 (2.98, 125.60) [53] | — | — |
| Keladj (1/min) | 0.007314 ± 0.0041556 [44] | 0.01147 ± 0.0087269 [31] | 0.007982 ± 0.0078574 [21] | — | — |
| t½,adj (min) | 147.335 ± 159.6736 [44] | 143.845 ± 249.9399 [31] | 127.710 ± 64.8007 [21] | — | — |
AUCadj and Cmax,adj are presented as geometric mean (geometric CV%). Tmax,adj and TrefCmax,adj are presented as median (minimum, maximum). Other parameters are presented as arithmetic means ± SDs. Dash indicates value missing or not reportable.
Treatments were as follows: A, 13.2 mg epinephrine administered as 2 intranasal sprays of 6.6 mg (0.11 mL × 60 mg/mL NDS1C) to opposite nostrils; B, 0.3 mg epinephrine (0.3 mL × 1 mg/mL epinephrine injection) administered via intramuscular injection (autoinjector); and C, 0.5 mg epinephrine (0.5 mL × 1 mg/mL epinephrine injection) administered via intramuscular injection (manual syringe).
Table V.
Summary of baseline-adjusted plasma epinephrine PK parameters after 13.2 mg intranasal spray in cohort 2 compared to intramuscular injection (PK population)
| PK parameter | Treatment |
P value |
|||
|---|---|---|---|---|---|
| D [no.] | E [no.] | F [no.] | D vs E | D vs F | |
| AUC0-10,adj (pg∙min/mL) | 1012 (188.3) [49] | 937.4 (172.6) [50] | 389.7 (191.3) [50] | .7248 | <.0001 |
| AUC0-20,adj (pg∙min/mL) | 2975 (128.4) [49] | 2313 (109.9) [50] | 1323 (101.9) [50] | .1213 | <.0001 |
| AUC0-30,adj (pg∙min/mL) | 5402 (107.1) [49] | 3969 (80.4) [50] | 2854 (81.7) [50] | .0278 | <.0001 |
| AUC0-60,adj (pg∙min/mL) | 11930 (86.4) [49] | 7945 (58.8) [50] | 9923 (58.2) [50] | .0002 | .0833 |
| AUC0-360,adj (pg∙min/mL) | 31420 (79.0) [49] | 16920 (60.8) [50] | 32280 (42.3) [50] | <.0001 | .7462 |
| AUC0-t,adj (pg∙min/mL) | 31310 (79.2) [49] | 16860 (61.2) [50] | 32210 (42.8) [50] | <.0001 | .7362 |
| AUC0-Tmax,adj (pg∙min/mL) | 3219 (161.4) [49] | 1707 (143.4) [50] | 5697 (115.5) [50] | .0011 | .0031 |
| AUC0-inf,adj (pg∙min/mL) | 41130 (72.5) [40] | 21640 (67.9) [25] | 38370 (43.6) [25] | <.0001 | .5520 |
| AUC%extrap,adj (%) | 13.84 ± 10.578 [40] | 9.953 ± 12.468 [25] | 12.28 ± 10.924 [25] | — | — |
| Cmax0-20,adj (pg/mL) | 276.206 (113.2) [49] | 224.805 (106.9) [49] | 132.756 (95.8) [49] | .2102 | <.0001 |
| Cmax,adj (pg/mL) | 383.017 (82.5) [49] | 279.564 (78.4) [50] | 337.541 (57.3) [50] | .0064 | .2759 |
| TrefCmax,adj (min) | 6.234 (0.45, 58.61) [33] | — | — | — | — |
| Tmax,adj (min) | 20.200 (2.98, 120.22) [49] | 17.500 (1.03, 120.43) [50] | 45.275 (3.02, 359.97) [50] | — | — |
| Keladj (1/min) | 0.008862 ± 0.011261 [40] | 0.01309 ± 0.014495 [25] | 0.007504 ± 0.0051269 [25] | — | — |
| t½,adj (min) | 133.254 ± 78.1943 [40] | 111.268 ± 114.8136 [25] | 125.765 ± 69.4076 [25] | — | — |
AUCadj and Cmax,adj are presented as geometric mean (geometric CV%). Tmax,adj and TrefCmax,adj are presented as median (minimum, maximum). Other parameters are presented as arithmetic means ± SDs. Dash indicates value missing or not reportable.
Treatments were as follows: D, 13.2 mg epinephrine administered as 2 intranasal sprays of 6.6 mg (0.11 mL × 60 mg/mL NDS1C) to same nostril; E, 0.3 mg epinephrine (0.3 mL × 1 mg/mL epinephrine injection) administered via intramuscular injection (autoinjector); and F, 0.5 mg epinephrine (0.5 mL × 1 mg/mL epinephrine injection) administered via intramuscular injection (manual syringe).
After 13.2 mg intranasal dose administered in the same nostril, of 49 subjects in cohort 2, a total of 33 subjects (67%) had epinephrine levels observed after 0.3 mg epinephrine intramuscular autoinjector treatment, with a TrefCmax of approximately 6.2 minutes. One subject was excluded from the summary statistics because the subject did not have 3 consecutive measurable concentrations.
Baseline-adjusted data for the intranasal treatment administered in opposite nostrils resulted in exposures (AUCs and Cmax) approximately 9% to 30% higher than when the product was administered in the same nostril. Overall, the PK measurements observed with 13.2 mg intranasal were similar to or greater than those obtained using the 0.3 mg autoinjector (Tables IV and V).
Secondary outcomes
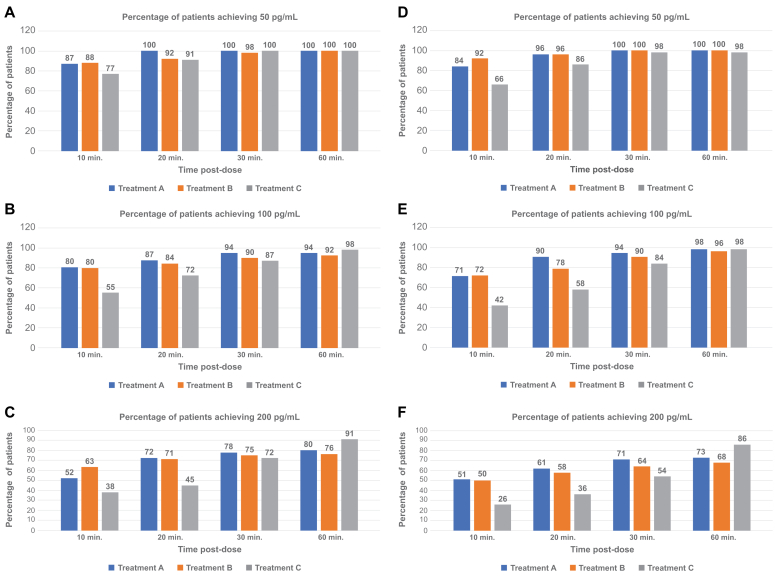
The ability to obtain clinically relevant plasma concentrations of epinephrine rapidly that are maintained over time was of particular interest as a secondary outcome in this study. In this context, the data showed that intranasal epinephrine administered in opposite nostrils consistently obtained a similar or greater percentage of subjects with 50, 100, and 200 pg/mL by 60 minutes compared to the intramuscular autoinjector, with a substantial number with these concentrations within the first 10 minutes after administration (Fig 4). Subjects evaluated in cohort 2 who received intranasal epinephrine in the same nostril exhibited similar or greater obtainment of therapeutic plasma concentrations of epinephrine of 50, 100, and 200 pg/mL compared to the 0.3 mg autoinjector from 10 to 60 minutes after dosing, except for the 50 pg/mL concentration at 10 minutes.
Fig 4.
Summary of baseline-adjusted plasma epinephrine PK parameters after 13.2 mg intranasal spray in cohorts 1 and 2 compared to intramuscular injection (PK population). (A) Cohort 1. (B) Cohort 2. (C) Proportion of subjects obtaining baseline-adjusted epinephrine plasma concentrations at or above 50, 100, or 200 pg/mL (PK population).
These data support the ability of 13.2 mg epinephrine intranasal to obtain clinically meaningful concentrations in subjects rapidly and to maintain those levels for a prolonged period of time at a similar or greater rate than those obtained using the intramuscular autoinjector.
Pharmacodynamics
The PD of intranasal epinephrine were evaluated in comparison to the 2 intramuscular dosing systems to evaluate if there were any differences in HR or BP. Because the PD of epinephrine are well known, these results were sought to determine if the intranasal delivery system resulted in a consistent PD profile to that observed with currently available intramuscular products. Overall, the observed PD profile for 13.2 mg epinephrine intranasal, administered in the opposite or same nostril, was similar to that observed with the 0.3 mg autoinjector and 0.5 mg manual syringe, with no significant deviations in PD parameters noted among the various modes of administration.
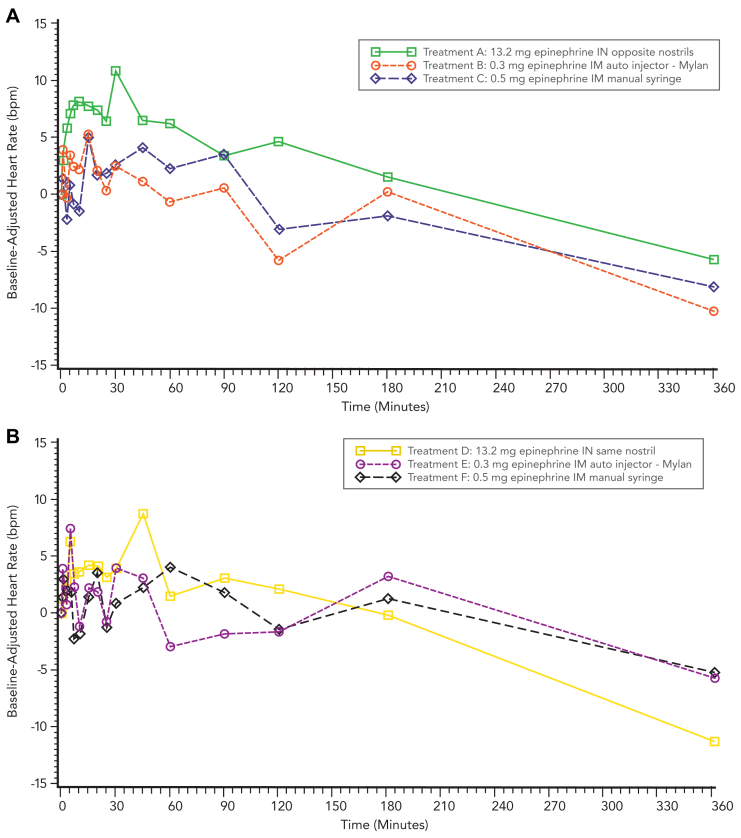
In cohort 1, mean changes from baseline HR values were slightly higher after treatment with 13.2 mg epinephrine intranasal (opposite nostrils) compared to treatment after 0.3 mg epinephrine intramuscular autoinjector and 0.5 mg epinephrine intramuscular manual syringe through 60 minutes after dosing. Otherwise, there were no remarkable differences in mean unadjusted HR values between treatments. In cohort 2, there were no remarkable differences in mean change from baseline HR values between treatments. Mean change from baseline HR values ranged from −11.2 bpm (360 minutes) to +8.7 bpm (45 minutes) after 13.2 mg epinephrine intranasal (same nostril), from −5.7 bpm (360 minutes) to +7.4 bpm (5 minutes) after 0.3 mg epinephrine intramuscular autoinjector, and from −5.2 bpm (360 minutes) to +4.1 bpm (60 minutes) after 0.5 mg epinephrine intramuscular manual syringe (Fig 5).
Fig 5.
Mean baseline-adjusted HR time profiles after 13.2 mg epinephrine intranasal spray compared to intramuscular injection. (A) Cohort 1. (B) Cohort 2.
Blood pressure
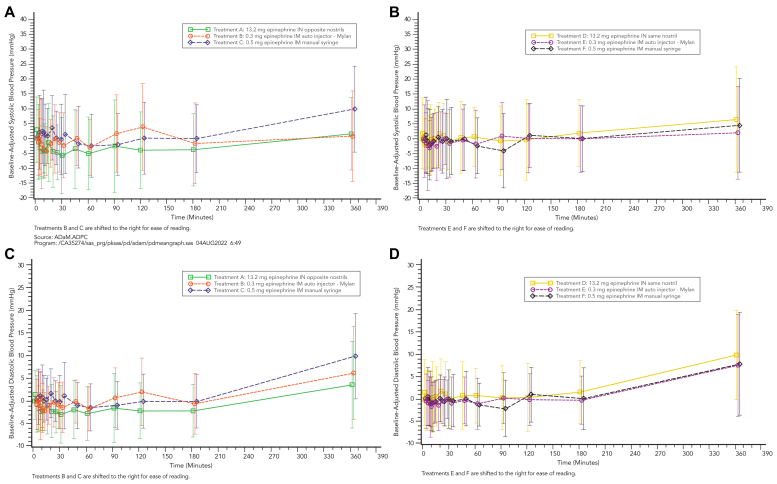
Both systolic BP (SBP) and diastolic BP (DBP) were evaluated for all treatment groups. In cohort 1, SBP change from baseline values were minimal and comparable across treatments, with slightly greater changes from baseline (decreases) observed after 13.2 mg epinephrine intranasal (opposite nostrils) from 20 minutes to 180 minutes after dosing compared to 0.3 mg epinephrine intramuscular autoinjector and 0.5 mg epinephrine intramuscular manual syringe. No changes from baseline for SBP were observed that were >20 mm Hg. Mean changes for SBP values ranged from −5.8 mm Hg (30 minutes) to +2.7 mm Hg (1 minute) after 13.2 mg epinephrine intranasal opposite nostrils, −4.4 mm Hg (10 minutes) to +3.8 mm Hg (120 minutes) after 0.3 mg epinephrine intramuscular autoinjector, and from −2.5 mm Hg (60 minutes) to +9.7 mm Hg (360 minutes) after 0.5 mg epinephrine intramuscular manual syringe (Fig 6, A).
Fig 6.
Mean baseline-adjusted SBP-time and DBP-time profiles after 13.2 mg epinephrine intranasal spray (opposite and same nostrils) compared to intramuscular injection (autoinjector and prefilled syringe) in cohorts 1 and 2 (linear scale). (A) SBP in cohort 1. (B) SBP in cohort 2. (C) DBP in cohort 1. (D) DBP in cohort 2.
For cohort 2, changes from baseline SBP values were minimal and comparable across treatments. There were no changes from baseline for SBP observed >20 mm Hg. Mean change from baseline SBP values ranged from −2.1 mm Hg (5 minutes) to +6.5 mm Hg (360 minutes) after 13.2 mg epinephrine intranasal (same nostril), from −3.0 mm Hg (7 minutes) to +2.1 mm Hg (360 minutes) after 0.3 mg epinephrine intramuscular autoinjector, and from −4.0 mm Hg (90 minutes) to +4.5 mm Hg (360 minutes) after 0.5 mg epinephrine intramuscular manual syringe (Fig 6, B).
For DBP, change from baseline DBP values in cohort 1 were minimal and comparable across treatments, with slightly greater changes from baseline (decreases) observed after 13.2 mg epinephrine intranasal (opposite nostrils) from 20 to 180 minutes after dosing compared to 0.3 mg epinephrine intramuscular autoinjector and 0.5 mg epinephrine intramuscular manual syringe. No changes from baseline for DBP were observed greater than ±10 mm Hg. Mean change from baseline DBP values ranged from −3.0 mm Hg (30 minutes) to +3.6 mm Hg (360 minutes) after 13.2 mg epinephrine intranasal (opposite nostrils), −2.3 mm Hg (10 minutes) to +6.2 mm Hg (360 minutes) after 0.3 mg epinephrine intramuscular autoinjector, and −1.4 mm Hg (60 minutes) to +9.9 mm Hg (360 minutes) after 0.5 mg epinephrine intramuscular manual syringe.
Change from baseline DBP values for cohort 2 were minimal and comparable across treatments, with slightly greater changes from baseline (decreases) observed after 13.2 mg epinephrine intranasal (opposite nostrils) from 20 to 180 minutes after dosing compared to 0.3 mg epinephrine intramuscular autoinjector and 0.5 mg epinephrine intramuscular manual syringe. There were no changes from baseline for DBP observed greater than ±10 mm Hg. Mean change from baseline DBP values ranged from −3.0 mm Hg (30 minutes) to +3.6 mm Hg (360 minutes) after 13.2 mg epinephrine intranasal opposite nostrils, from −2.3 mm Hg (10 minutes) to +6.2 mm Hg (360 minutes) after 0.3 mg epinephrine intramuscular autoinjector, and from −1.4 mm Hg (60 minutes) to +9.9 mm Hg (360 minutes) after 0.5 mg epinephrine intramuscular manual syringe (Fig 6, C).
Safety
The overall safety profile for intranasal epinephrine was as expected for an epinephrine delivery system. There were no deaths or serious adverse events reported by subjects participating in either cohort 1 or cohort 2. Overall, 49 subjects (74%) reported a total of 219 adverse events in cohort 1, and 33 subjects (66%) reported 105 adverse events in cohort 2. The majority of events were commonly associated with epinephrine administration. Overall, they were mild and transient, and were reported after 13.2 mg epinephrine intranasal (opposite nostrils), with 44 subjects (67%) reporting 136 events in cohort 1 and 25 subjects (50%) reporting a total of 68 treatment-emergent adverse events in cohort 2. The subject incidence for adverse event reporting was similar for 0.3 mg epinephrine intramuscular autoinjector and 0.5 mg manual syringe, although slightly lower than observed with intranasal administration. Injection-site events after 0.3 mg epinephrine intramuscular autoinjector and events related to intranasal administration were minimal.
Almost all reported events were considered by the investigators to be mild in severity for both cohorts. One hundred eighty-one events were deemed to be likely related to the various study treatments from both cohorts. Overall, the safety results for intranasal and intramuscular epinephrine administration routes were comparable, demonstrating no new safety signals for the intranasal route of administration.
Discussion
These results demonstrate that intranasal epinephrine provides an enhanced PK profile compared to the standard reference product, the epinephrine autoinjector. This is an important finding in consideration of intranasal epinephrine spray as a viable alternative to the autoinjector in a real-world outpatient setting. Recent treatment guideline updates highlight the need for alternative, safe, and effective epinephrine delivery devices to optimize the ability of patients to obtain the required concentration of epinephrine and alleviate symptoms quickly and efficiently. The inability to consistently obtain effective plasma concentrations of epinephrine, either as a result of challenges with autoinjector design or delay to treatment and exacerbation of symptoms requiring a second dose, remains a challenge. The ability to get higher plasma concentrations via intranasal delivery has the potential to circumvent some of the challenges observed with the use of autoinjectors, as well as the need for a second dose, especially in an outpatient setting. As demonstrated in this study, intranasal epinephrine has the potential for enhanced clinical utility based on obtaining therapeutic plasma levels (ie, >100 pg/mL) for twice as long as the autoinjector (Fig 2), with a PD profile that supports overall safety.
By providing an option for needle-free self-administration, it is possible that intranasal epinephrine may decrease the delay to administration that has been observed with autoinjectors, as well as an improved patient-carry rate. This can be obtained by eliminating the stigma associated with use of needles and by bypassing needle anxiety, providing a delivery device that is small, easy to carry, convenient, and easy to use, and with this intranasal device, obtaining a higher and more sustained PK profile compared to an autoinjector. Therefore, by ensuring sufficient epinephrine is delivered as quickly as with an autoinjector, with higher and more prolonged therapeutic levels of epinephrine plasma levels (ie, ≥100 pg/mL), intranasal epinephrine may provide greater clinical utility and optimize clinical outcomes.
Although this study was conducted in healthy adult subjects, the potential to provide benefit to the pediatric population cannot be ignored. According to a 2020 systematic review, in children, anaphylaxis incidence may be up to 761 per 100,000 person-years.2 Further, recurrence of reactions occurs in 26.5% to 54.0% of anaphylaxis patients during 1.5 to 5 years’ follow-up. Children are particularly at risk; the occurrence of anaphylaxis peaks around age 12, then for adults again at age 59 to 60. Additionally, existing autoinjectors vary in design; if used incorrectly, suboptimal delivery of epinephrine may result. This is of particular concern in the school setting, when children must rely on the availability and competent administration of epinephrine when an anaphylactic event occurs. Dose proportionality in the pediatric population must be considered; dose-proportionality analysis suggests that a single 6.6 mg intranasal spray demonstrates a PK profile that is slightly better than a 0.15 mg intramuscular autoinjector dose (EpiPen Jr).
Conclusions
The addition of a new and convenient option that does not require use of a needle for self-administration of epinephrine during acute anaphylaxis is an attractive possibility. As a delivery mechanism, intranasal administration has consistently demonstrated to be a user-accepted and effective method of drug delivery. On the basis of the greater performance of intranasal epinephrine compared to autoinjector in terms of PK/PD, as well as its acceptable safety and the tolerability parameters we evaluated, NDS1C offers a potential solution to some of the issues regarding delay in treatment and the need for a secondary dose in managing anaphylaxis. NDS1C may thus provide a viable alternative to intramuscular autoinjectors.
Disclosure statement
Supported by Bryn Pharma LLC.
Disclosure of potential conflict of interest: D. A. Dworaczyk reports a relationship with Bryn Pharma LLC that includes employment, equity or stocks, and travel reimbursement. A. L. Hunt, M. Di Spirito, M. Lor, M. J. Lamson, J. Pollock, and T. Ward report a relationship with Celerion Inc that includes employment. K. L. Dretchen reports a relationship with Mesa Science Associates that includes employment and equity or stocks.
References
- 1.Whyte A.F., Soar J., Dodd A., Hughes A., Sargant N., Turner P.J. Emergency treatment of anaphylaxis: concise clinical guidance. Clin Med. 2022;22:332–339. doi: 10.7861/clinmed.2022-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardona V., Ansotegui I.J., Ebisawa M., El-Gamal Y., Fernandez Rivas M., Finement S., et al. World Allergy Organization anaphylaxis guidance, 2020. World Allergy Organ J. 2020;13 doi: 10.1016/j.waojou.2020.100472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaaban M., Warren Z., Baillargeon J., Baillargeon G., Resto V., Kuo Y. Epidemiology and trends of anaphylaxis in the United States, 2004-2016. Int Forum Allergy Rhinol. 2019;9:607–614. doi: 10.1002/alr.22293. [DOI] [PubMed] [Google Scholar]
- 4.Barni S., Liccioli G., Sarti L., Giovannini M., Novembre E., Mori F. Immunoglobulin E (IgE)-mediated food allergy in children: epidemiology, pathogenesis, diagnosis, prevention, and management. Med Kaunas Lith. 2020;56:111. doi: 10.3390/medicina56030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iweala O.I., Choudhary S.K., Commins S.P. Food allergy. Curr Gastroenterol Rep. 2018;20:17. doi: 10.1007/s11894-018-0624-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta R.S., Warren C.M., Smith B.M., Jiang J., Blumestock J.A., Davis M., et al. Prevalence and severity of food allergies among US adults. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2018.5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muraro A., Worm M., Alviani C., Cardona V., DunnGalvin A., Garvey L.H., et al. EAACI guidelines: anaphylaxis (2021 update) Allergy. 2022;77:357–377. doi: 10.1111/all.15032. [DOI] [PubMed] [Google Scholar]
- 8.Kemp S.F., Lockey R.F., Simons F.E. World Allergy Organization Ad Hoc Committee on Epinephrine in Anaphylaxis. Epinephrine: the drug of choice for anaphylaxis. A statement of the World Allergy Organization. Allergy. 2008;63:1061–1070. doi: 10.1111/j.1398-9995.2008.01733.x. [DOI] [PubMed] [Google Scholar]
- 9.Simons F.E.R. Anaphylaxis: recent advances in assessment and treatment. J Allergy Clin Immunol. 2009;124:625–636. doi: 10.1016/j.jaci.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 10.Breuer C., Wachall B., Gerbeth K., Abdel-Tawab M., Fuhr U. Pharmacokinetics and pharmacodynamics of moist inhalation epinephrine using a mobile inhaler. Eur J Clin Pharmacol. 2013;69:1303–1310. doi: 10.1007/s00228-012-1465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dreskin S.C., Halsey N.A., Kelso J.M., Wood R.A., Hummell D.S., Edwards K.M., et al. International consensus (ICON): allergic reactions to vaccines. World Allergy Organ J. 2016;9:32. doi: 10.1186/s40413-016-0120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenberger P.A., Wallace D.V., Lieberman P.L., Gregory S.M. Contemporary issues in anaphylaxis and the evolution of epinephrine autoinjectors. Ann Allergy Asthma Immunol. 2017;119:333–338. doi: 10.1016/j.anai.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 13.Srisawat C., Nakponetong K., Benjasupattananun P., Suratannon C., Wachirutmanggur L., Boonchoo S., et al. A preliminary study of intranasal epinephrine administration as a potential route for anaphylaxis treatment. Asian Pac J Allergy Immunol. 2016;34:38–43. [PubMed] [Google Scholar]
- 14.Chen J, Yu J, Chilampalli C, Narayanan E, Wakaskar RR, Atiee GJ. An open label, 5-treatment, crossover, single-dose pharmacokinetic study of epinephrine nasal spray in comparison to EpiPen® intramuscular injection in healthy adults with seasonal allergies. Poster presented at: American Academy of Allergy, Asthma & Immunology 2019 annual meeting (AAAAI19); February 22-25, 2019; San Francisco, Calif.
- 15.US Food and Drug Administration (FDA) EpiPen and EpiPen Jr (epinephrine) injection full prescribing information. Reference ID 4722151. Revised December 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/019430s091lbl.pdf Available at: