Abstract
Objective
To investigate the effect of combined repetitive peripheral magnetic stimulation and transcranial magnetic stimulation on upper extremity function in subacute stroke patients.
Design
Pilot study.
Subjects
Subacute stroke patients.
Methods
Included patients were randomized into 3 groups: a central-associated peripheral stimulation (CPS) group, a central-stimulation-only (CS) group, and a control (C) group. The CPS group underwent a new paired associative stimulation (combined repetitive peripheral magnetic stimulation and transcranial magnetic stimulation), the CS group underwent repetitive transcranial magnetic stimulation, and the C group underwent sham stimulation. All 3 groups received physiotherapy after the stimulation or sham stimulation. The treatment comprised 20 once-daily sessions. Primary outcome was the Fugl-Meyer Assessment Upper Extremity (FMA-UE) score, and secondary outcomes were the Barthel Index and Comprehensive Functional Assessment scores, and neurophysiological assessments were mainly short-interval intracortical inhibition. A 3-group (CPS, CS, C) × 2-time (before, after intervention) repeated measures analysis of variance was conducted to determine whether changes in scores were significantly different between the 3 groups.
Results
A total of 45 patients were included in the analysis. Between-group comparisons on the FMA-UE demonstrated a significant improvement (group × time interaction, F2,42 = 4.86; p = 0.013; C vs CS, p = 0.020; C vs CPS, p = 0.016; CS vs CPS, p = 0.955). Correlation analysis did not find any substantial positive correlation between changes in FMA-UE and short-interval intracortical inhibition variables (C, r = –0.196, p = 0.483; CS, r = –0.169, p = 0.546; CPS, r = –0.424, p = 0.115).
Conclusion
This study suggests that the real-stimulus (CS and CPS) groups had better outcomes than the control (C) group. In addition, the CPS group showed a better trend in clinical and neurophysiological assessments compared with the CS group.
LAY ABSTRACT
Enhancing the recovery of arm function after a stroke may help patients regain their independence and improve their overall quality of life. This study examined a new method aiming to help patients recovering from stroke regain the use of their arm. Two groups underwent treatment with different types of magnetic stimulation (repetitive peripheral magnetic stimulation combined with transcranial magnetic stimulation, or repetitive transcranial magnetic stimulation alone) together with physiotherapy and the results were compared with those for a control group who only underwent physiotherapy. Arm function in the 2 treatment groups improved significantly compared with the control group. This suggests that a combined therapy might be a valuable addition to rehabilitation programmes for treatment of patients with arm hemiparesis following a stroke.
Key words: ipsilateral hemisphere, motor cortex excitability, motor function, paired associative stimulation, repetitive peripheral magnetic stimulation, repetitive transcranial magnetic stimulation, stroke, upper limb
Stroke is the leading cause of disability worldwide (1) and has become a global health burden because of the increasing population of survivors with persistent motor dysfunction (2). Upper-extremity motor impairment, a primary determinant of functional dependence, is one of the significant factors associated with poor recovery of activities of daily living (ADL) (3, 4). Recovery of the paretic extremity is affected by bilateral imbalance in the excitability of the cerebral hemispheres after stroke (5, 6). Numerous preliminary studies have identified the benefits of repetitive transcranial magnetic stimulation (rTMS) in motor recovery related to modulated cortical reorganization and induced neural plasticity (7–10). The lesioned hemisphere’s primary motor cortex (M1) is intensely involved in cortical reorganization of the motor functional network during recovery after stroke (11, 12). Consistent with the interhemispheric inhibition model, high-frequency rTMS can both partially restore the excitability of the ipsilesional M1 and suppress contralesional M1 excitability (13, 14).
Paired associative stimulation (PAS), which involves combining rTMS of the motor cortex with repetitive peripheral electrical stimulation (rPES) of the affected limb, has been used to accelerate motor recovery in patients with stroke (15, 16). Preliminary data show that PAS can partially restore corticospinal excitability in patients with stroke (17). These changes were dependent on the timing of the magnetic stimulation relative to the afferent input (18). However, PAS did not alter the excitability of spinal motor neurones nor the motor-evoked potential (MEP) observed following brainstem stimulation (19). Enhanced M1 excitability has been observed when peripheral stimulation preferentially activates proprioceptive afferents (7, 20). In previous studies, electrical stimulation was used to activate the excitability of peripheral nerves and muscles; however, high-intensity electrical stimulation can be perceived as uncomfortable (13, 21). Therefore, using repetitive peripheral magnetic stimulation (rPMS) instead of electrical stimulation to activate the afferent fibres would offer great advantages over conventional methods (15, 22). It is possible that high-frequency rTMS associated with rPMS may have stronger and more lasting effects on corticospinal and intracortical excitability (19, 23) than does the current best therapy.
Therefore, this pilot randomized controlled trial investigated the therapeutic effect of rPMS combined with rTMS in the affected hemisphere in patients with subacute stroke. It was hypothesized that this new paired associative therapy would improve the upper limb motor performance of patients with subacute stroke. To gain insight into the possible underlying neurophysiological mechanisms the study investigated motor cortical excitability and neurophysiological changes.
MATERIALS AND METHODS
Patients
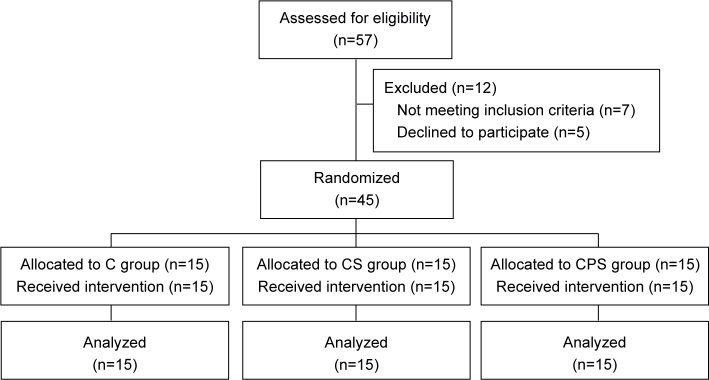
This randomized, single-blind, controlled pilot trial was registered (ChiCTR2000033495) in the Chinese Clinical Trial Registry (www.chictr.org.cn). The study was approved by the Ethics Committee of Fudan University (NO. KY2019-609). Informed consent was obtained from all patients before enrolment, and all patients with stroke were recruited from the Department of Rehabilitation at Huashan Hospital, Fudan University. Fig. 1 shows the patient flowchart.
Fig. 1.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram of patients enrolled in the trial for randomization. CPS: central-associated peripheral stimulation group; CS: central-stimulation-only group; C: control group.
Inclusion and exclusion criteria
The inclusion criteria were: (i) first stroke (ischaemic or haemorrhagic) meeting the diagnostic criteria of the Chinese Classification of Cerebrovascular Disease 2019 (24) and confirmed by computed tomography or magnetic resonance imaging; (ii) male or female patient aged 18–80 years; (iii) residual upper-limb impairment; and (iv) enrolled < 3 months after the stroke event. The exclusion criteria were: (i) severe heart, liver, or kidney disease; (ii) history of seizures; (iii) presence of intracranial metallic components or implanted pacemakers; (iv) concomitant motor impairment disease (e.g. Parkinson’s disease or peripheral neuropathy); or (v) severe dementia, aphasia, apraxia, or other serious disorders influencing the therapeutic effect.
Sample size and randomization
The study protocol was based on a previously published study of cortical plastic changes and observed changes on the Fugl-Meyer Assessment Upper Extremity (FMA-UE) score induced by rTMS (25). These data were used in a power analysis to calculate the sample size required for the study. For the 3-group comparisons, an effect size of 0.6 and a power of 0.80 were used. Given a dropout rate of 20%, 45 patients were required. These patients were randomly assigned to either a central-associated peripheral stimulation (CPS) group, which received physiotherapy, rTMS and rPMS; a central-stimulation-only (CS) group, which received physiotherapy and rTMS; or a control (C) group, which received physiotherapy and sham rTMS. All patients were allocated to groups based on the same proportions using a computer-generated random list of numbers. Each evaluation was performed by a clinician proficient in operating transcranial magnetic stimulation (TMS) machines and performing clinical assessments, who was blinded to the baseline data and group allocation.
Experimental design and protocol
Preparation for evaluation of motor-cortex excitability. Each patient was asked to sit in a comfortable reclining chair and relax as much as possible. The patient’s head was strapped to a headrest.
Repetitive transcranial magnetic stimulation procedure. Magnetic stimulation was performed by a researcher trained in repeatability according to the protocol, using a YRD CCY-II transcranial magnetic device (Wuhan Yiruide Medical Equipment New Technology Co., Ltd, Wuhan, China) equipped with a double-ended circular coil. For the groups receiving CS (CPS and CS), the coil handle was placed 45° posterior to the mid-sagittal line and moved forward to the scalp over the hand area of the primary motor cortex. The hotspot for optimal stimulation was determined by visual observation of the evoked abduction of the contralateral abductor pollicis brevis. All patients exhibited high excitability in the unaffected hemisphere.
Repetitive transcranial magnetic stimulation intervention. The study combined rTMS and movement assessment to measure patterns of cortical excitability over M1 and the seventh cervical nerve root of the affected hemisphere. Once the stimulation site was identified, the resting motor threshold (rMT) and MEP threshold were determined. The rMT was evaluated by gradually reducing the output stimulation intensity and was defined as the minimum stimulus intensity that produced an MEP response of at least 50 µV amplitude in the target muscle at rest in at least 5 out of 10 consecutive stimulations. The MEP was then measured at 120% of the rMT intensity. If 10 successive stable waveforms were obtained, the amplitude (peak-to-peak) and latency (the period from the onset of the stimulation to the start of the MEP) were recorded. The mean amplitude of the MEPs for each individual were calculated before and after the intervention or sham treatment. If a MEP could not be obtained from the affected hemisphere, then a location symmetrical to the contralesional site was defined as the hotspot. Finally, the short-interval intracortical inhibition (SICI) was measured using a suprathreshold TMS pulse followed by a subthreshold TMS pulse 2 ms later. The subthreshold stimulation was 70% of the rMT. The suprathreshold stimulation intensity was the same as that used to record MEPs. The SICI was calculated as the percentage change in the mean amplitude of the MEP evoked by paired-pulse TMS from the MEP evoked by a single-pulse TMS at rest. The order of recordings (rMT, latency, MEP, and SICI) was consistent before and after the neuromodulation intervention for all participants.
Repetitive peripheral magnetic stimulation intervention. Patients in the CPS and CS groups received rTMS, delivered as 5-Hz paired repetitive magnetic stimulation, applied daily to the hotspot of the lesional hemisphere for 20 min (a total of 1200 pulses). In addition, the CPS group received rPMS; delivered as the seventh cervical nerve root was stimulated in 2-s training epochs with 8-s intervals between training sessions, for 20 min in total (1200 pulses) to improve excitability. Each peripheral stimulation was delivered 20 ms after rTMS.
Placebo stimulation was performed in the C group, with the coil held vertically to the scalp for 20 min to reproduce the noise of the 5-Hz stimulation. All patients were unaware of the stimulation received during treatment.
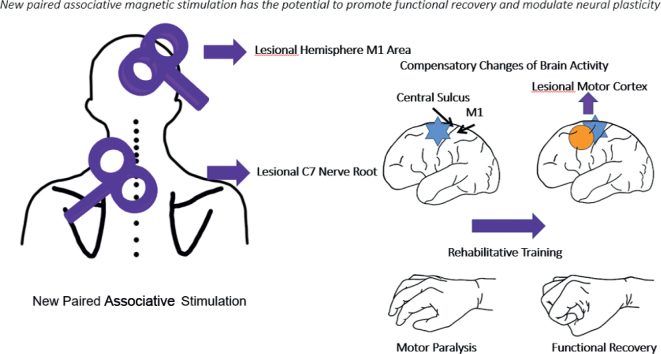
During stimulation, the patients were repeatedly observed and asked about their sensations and self-feelings, in order to maintain their alertness. Fig. 2 illustrates the mechanism of the new paired associative stimulation.
Fig. 2.
Mechanism of the new paired associative stimulation. Blue areas indicate affected regions, and yellow circle indicates brain recovery. As the brain recovers, upper extremity motor function also improves, and repetitive transcranial magnetic stimulation (rTMS) treatment accelerates this process.
Physiotherapy intervention. All 3 groups received standard physiotherapy after the stimulation or sham stimulation. Physiotherapy consisted of exercises designed to promote recovery of voluntary motor activity and activities of daily living (ADL), including muscle stretching, active-assisted mobilizations, progressive neuromuscular facilitation training exercises, and motor relearning training. The therapeutic programme comprised 20 once-daily sessions, 40 min each session, on 5 consecutive days per week, for 4 weeks.
Clinical evaluation
Clinical effectiveness was assessed using the FMA-UE score as the primary outcome (score ranged from a minimum of 0 to a maximum of 66) (26). The Comprehensive Functional Assessment (FCA) and the Barthel Index (BI) were secondary outcomes. Functional assessment of the affected upper extremities was clinically performed at baseline and after completion of therapy at 4 weeks, based on the same scales and using the FMA-UE, which is a highly reliable and sensitive test comprising 33 tasks for the upper extremities (26). All groups were evaluated at baseline and after treatment or sham treatment. The BI was used to compare the functional status and disability of patients.
Outcomes
Functional assessment of the affected upper extremities was clinically performed at baseline and after completion of therapy at 4 weeks based on the same scales (FMA-UE, FCA, BI). For efficacy analyses, the primary outcome was the change in FMA-UE score to assess pre-post treatment of upper-extremity motor function. Secondary endpoints were changes in the total scores of the FCA and BI, which were used to assess changes in locomotion, functional status, and disability. Neurophysiological parameter analysis was performed using the SICI, which was calculated as a critical mediating variable in the change in motor cortex excitability before and after treatment. Therefore, the MEP, latency, and rMT changes from baseline to post-treatment were also measured as secondary endpoints.
Statistical analysis
All analyses were performed using SPSS 20.0 for Windows (SPSS Inc., Chicago, IL, USA). The Kolmogorov–Smirnov statistic was used to test the normality of all variables. Demographic and clinical characteristic differences were calculated at baseline assessment using 1-way analysis of variance (ANOVA) or χ2 test. Two-way ANOVA was used to investigate the 3 groups’ clinical scales and neurophysiological performances, with between-group factors FMA-UE, FCA, BI, and TMS, as well as the within-group factor experimental time (baseline vs 4 weeks post-intervention). Modulation of corticospinal excitability was assessed with Pearson’s correlation analysis to determine the correlation between motor performance as FMA-UE scores and neurophysiological changes (MEP latency, MEP amplitude, rMT and SICI). Statistical significance was set at p < 0.05.
RESULTS
Fifty-seven patients were assessed for eligibility, of whom 50 were invited to join the study. Five patients declined to participate, hence a final total of 45 patients were included in the study and completed the treatment.
At baseline, no significant differences were observed among the 3 groups in terms of age, sex, time since the stroke event, lesion side, or stroke subtype (Table I). All participants tolerated the study well and no significant adverse effects were reported in any group. The practical training times were similar across all groups.
Table I.
Demographic and clinical characteristics among the 3 groups (N = 45)
| Characteristics | C group (n = 15) | CS group (n = 15) | CPS group (n = 15) | p-value |
|---|---|---|---|---|
| Age, years, mean (SD) | 54.3 (7.6) | 54.1 (9.6) | 56.6 (9.1) | 0.699 |
| Sex, n (%) | ||||
| Male | 10 (66.7) | 11 (73.3) | 10 (66.7) | 0.902 |
| Female | 5 (33.3) | 4 (26.7) | 5 (33.3) | |
| Time after stoke, months, mean (SD) | 2.5 (1.4) | 2.3 (1.5) | 2.3 (1.8) | 0.779 |
| Impairment side, n (%) | ||||
| Left hemisphere | 10 (66.7) | 8 (53.3) | 6 (40) | 0.343 |
| Right hemisphere | 5 (33.3) | 7 (46.7) | 9 (60) | |
| Subtype of stroke, n (%) | ||||
| Cerebral infarction | 8 (53.3) | 8 (53.3) | 5 (33.3) | 0.448 |
| Cerebral haemorrhage | 7 (46.7) | 7 (46.7) | 10 (66.7) | |
SD: standard deviation. All p-values are >0.05, indicating that there are no statistically significant differences in demographic and clinical characteristics between the groups at baseline.
Motor performance
Main outcome. A significant improvement was demonstrated in FMA-UE among the 3 groups (group × time interaction, F2,42 = 4.86; p = 0.013; η2 = 0.188). Effectiveness analysis compared with baseline assessment showed that FMA-UE scores improved from 10.93 ± 7.63 to 22.47 ± 12.67 (CPS group), 10.60 ± 8.36 to 22.20 ± 12.85 (CS group), and 10.53 ± 2.77 to 13.87 ± 2.61 (CS group). In addition, The FMA-UE score was significantly higher in the real-rTMS groups than in the sham group after treatment; however, no differences were observed between the experimental-rTMS groups (C vs CS, p = 0.020; C vs CPS, p = 0.016; CS vs CPS, p = 0.955).
Secondary outcomes. Similarly, in all 3 groups an increase was observed in BI score (group × time interaction, F2,42 = 6.502; p = 0.003; η2 = 0.236) and FCA score (group × time interaction, F2,42 = 8.879; p = 0.001; η2 = 0.657). However, no difference was observed between the 2 real-rTMS groups; BI score (C vs CS, p = 0.049; C vs CPS, p = 0.019; CS vs CPS, p = 0.939), and FCA score (C vs CS, p = 0.044; C vs CPS, p = 0.025; CS vs CPS, p = 0.893).
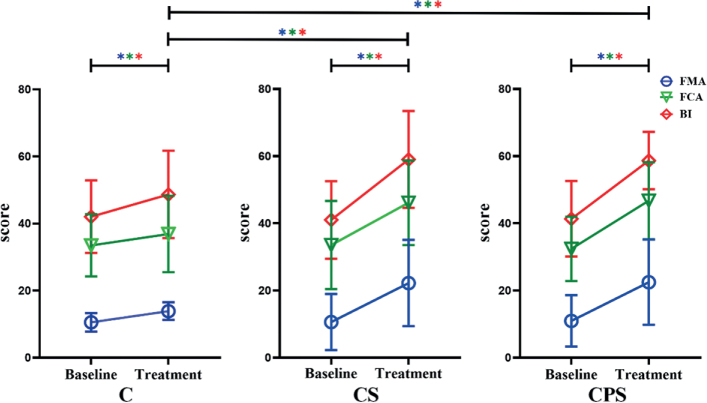
Baseline scores, follow-up scores and clinical scores are shown in Table II and Fig. 3.
Table II.
Results of clinical outcomes in the 3 group (N = 45)
| Variables Mean (SD) | Baseline | Treatment | p-value | ||||
|---|---|---|---|---|---|---|---|
| C group (n = 15) | CS group (n = 15) | CPS group (n = 15) | C group (n = 15) | CS group (n = 15) | CPS group (n = 15) | ||
| FMA-UE | 10.53 (2.77) | 10.60 (8.36) | 10.93 (7.63) | 13.87 (2.61) | 22.20 (12.85) | 22.47 (12.67) | 0.013* |
| FCA | 33.47 (9.30) | 33.53 (13.10) | 32.40 (9.57) | 36.87 (11.37) | 46.13 (12.61) | 46.73 (11.47) | 0.001* |
| BI | 42.00 (10.82) | 41.00 (11.53) | 41.33 (11.25) | 48.67 (13.02) | 59.00 (14.42) | 58.67 (8.55) | 0.003* |
CPS: central-associated peripheral stimulation group; CS: central-stimulation-only group; C: control group; FMA-UE: Fugl–Meyer Assessment of Upper Limb (percentages of maximum points in the upper limb (66 points)); FCA: Functional Comprehensive Assessment; BI: Barthel Index; SD: standard deviation. A significant improvement was demonstrated in FMA-UE, FCA, and BI among the 3 groups by 2-way analysis of variance (ANOVA),
p < 0.05.
Fig. 3.
Changes in mean rating of the Fugl–Meyer Assessment Upper Limb (FMA-UE) score, Functional Comprehensive Assessment Motor Score (FCA-MS) and Barthel Index (BI) among the 3 groups from baseline to post-treatment. *p < 0.05, different colours indicate different scores (blue = FMA-UE; green = FCA; red = BI). A significant improvement was demonstrated in FMA-UE among the 3 groups after treatment. Similarly, after treatment, the FMA-UE score was significantly higher in groups undergoing repetitive transcranial magnetic stimulation (rTMS) than in the sham group. However, no differences were observed between the experimental-rTMS groups (Including FMA-UE score, BI score and FCA score). CPS: central-associated peripheral stimulation group; CS: central-stimulation-only group; C: control group.
Neurophysiological outcomes. No notable changes were observed in rMT from pre- to post-treatment (group × time interaction, F2,42 = 3.104, p = 0.055, η2 = 0.129), MEP latency (group × time interaction, F2,42 = 1.379, p = 0.263, η2 = 0.062), or MEP amplitude (group × time interaction, F2,42 = 2.092, p = 0.136, η2 = 0.091) (Table III), which indicated no significant excitation of the affected hemisphere. Specifically, the assessment of change from pre- to post-treatment show-ed that rMT improved by 15.04% (CS) and 29.33% (CPS) in the real-rTMS groups and by 10.12% in the sham group (C).
Table III.
Results of neurophysiological parameters before and after resting motor threshold (rTMS) (N = 45)
| Variables Mean (SD) | Baseline | Treatment | p-values | ||||
|---|---|---|---|---|---|---|---|
| C group | CS group | CPS group | C group | CS group | CPS group | ||
| rMT (%) | 52.20 (10.84) | 52.00 (11.27) | 51.73 (13.47) | 47.40 (15.38) | 45.20 (9.78) | 40.00 (13.19) | 0.055 |
| Latency (ms) | 22.34 (1.60) | 22.10 (1.16) | 22.24 (1.37) | 22.28 (1.56) | 21.67 (1.17) | 21.84 (1.43) | 0.263 |
| MEP (mV) | 0.71(0.15) | 0.74 (0.17) | 0.72 (0.10) | 0.77 (0.15) | 0.84 (0.17) | 0.86 (0.19) | 0.091 |
| SICI (%) | 0.71 (0.25) | 0.70 (0.18) | 0.72 (0.23) | 0.80 (0.22) | 0.47 (0.36) | 0.46 (0.27) | 0.003* |
CPS: central-associated peripheral stimulation group; CS: central-stimulation-only group; C: control group; MEP: motor-evoked potential; SICI: short-interval intracortical inhibition; SD: standard deviation. A significant improvement was demonstrated in SICI among the 3 groups by 2-way analysis of variance (ANOVA),
p < 0.05.
A statistically significant change in SICI was observed (group × time interaction, F2,42 = 6.501, p = 0.003, η2 = 0.236) when comparing different experimental conditions (Table III), revealing a decrease in cortical inhibition with transcranial magnetic stimulation relative to stimulation by sham stimulation.
Relationship between motor performance and cortical excitability. Correlation analysis revealed no substantial positive correlation between pre-post-treatment changes in FMA-UE and SICI variables, indicating that a more significant improvement in the affected upper limb, as measured by FMA-UE, was not associated with a more substantial increase in corticospinal excitability in the unaffected hemisphere as indexed by SICI (C group, r = –0.196, p = 0.483; CS group, r = –0.169, p = 0.546; CPS group, r = –0.424, p = 0.115).
DISCUSSION
This pilot study found that the application of rTMS to the affected motor cortex, either alone, or in combination with rPMS, facilitates recovery of upper-limb hemiparesis in subacute stroke patients. In contrast to previous research (15, 27), the current study demonstrated the effectiveness of rPMS associated with rTMS (CPS group), as evidenced by an increase in FMA-UE score and a decrease in the inhibition of cortical excitability in the contralesional hemisphere shown by SICI analysis. These results support the study hypothesis that the application of rPMS combined with rTMS to inhibit the unaffected motor cortex significantly improves the recovery of paretic hand function.
Clinical performance: functional parameter increases
From a functional viewpoint, rPMS combined with rTMS (CPS group) increased the FMA-UE score from 10 to 22 points, varying from a level at which patients could hardly move their paretic extremity to a group capable of executing gross motor actions as the patients selected for the study were all in the subacute phase. Notably, this increase in FMA-UE score illustrates a slight improvement in motor performance, which was greater than that in the other groups (CS group and C group) and led to enhancement of individual quality of life, since the BI score (CPS group) also improved (from 41 to 59) (28).
This result could be attributed to several factors. First, upper limb improvement is to be expected in the subacute stage of stroke, regardless of the interventions applied. This improvement is important, given that upper-limb motor function improves after a transient initial decline from stroke onset (26) and such early impairment is associated with long-term disability and impaired quality of life (4). However, in the current study this area exhibited rapid improvement, as shown by the significant differences observed among the 3 groups. Secondly, compared with sham stimulation, real stimulation accelerated recovery with increasing treatment duration (12, 29). Crucially, such improvement was achieved in a relatively short period of training (4 weeks). This suggests that rTMS associated with rPMS may be beneficial, leading to low-cost, effective, and relatively rapid protocols for upper-extremity recovery (30, 31).
Modulation of corticospinal excitability: short-interval intracortical inhibition ratio increases
From a neurophysiological perspective, both of the real-stimulation groups exhibited an increasing tendency in rMT intensity and latency and a decreasing trend in MEP amplitudes in the contralesional hemisphere. However, the neurophysiological parameters showed no significant intergroup differences. Numerous studies have shown that the contralesional hemisphere exerts an inhibitory effect on the affected hemisphere after stroke and disturbs the interhemispheric balance of activity, thereby influencing recovery (32, 33). Increasing the activity of the affected hemisphere is one solution to restore the imbalance by directly applying high-frequency stimulation over the lesional hemisphere, which affects the excitability of the contralesional hemisphere (34). A meta-analysis of inhibitory rTMS for stroke-induced upper limb motor deficits showed an enhancing effect on the rMT in the unaffected hemisphere (33). Nonetheless, in the current study, the contralesional rMT did not increase significantly in the trial group, and the MEP latency and amplitude did not change significantly in either group. The intensity of stimulation is a factor that might have affected the current results (35). The current study used 80% of rMT (contralesional hemisphere) as all included patients were expected to tolerate this intensity; however, other studies have applied higher stimulation intensities to the affected hemisphere (13). It is possible that insufficient stimulation intensity might not be beneficial to post-stroke recovery (25). Another factor is that changes in the excitability of the lesioned hemisphere have little influence over the excitability of the contralesional hemisphere (36). Moreover, since the motor threshold of the affected hemisphere could not be measured in 4 patients (2 in the CPS group, 1 in the CS group, and 1 in the C group), it was assumed that the location of the stimulation would be opposite to the “hotspot” in the unaffected hemisphere, which might have influenced the results of the current study (8).
Notably, in contrast to a previous study of the effect of New Paired Associative Stimulation (NPAS) on these intracortical measures in neurologically intact participants, the current study observed a significant change in the SICI ratio after NPAS between the different experimental conditions, which revealed a decrease in cortical inhibition. NPAS-induced increases in corticomotor excitability after stroke may have occurred subcortically, possibly through changes in thalamocortical connectivity (29). Moreover, unlike MEP amplitude, SICI is a ratio value, as such, it improves the significance of the statistical analysis results to some extent.
Intracortical inhibition
Previous research in patients with stroke has suggested that, during rTMS therapy, improvements occur in motor function and brain plasticity (10, 13, 36). However, the underlying mechanisms of high-frequency rTMS treatment remain elusive (37). High-frequency rTMS has been shown to strengthen neurogenesis in rat models of ischaemic stroke (38). This process might be related to stimulated release of brain-derived neurotrophic factor, which accelerates functional recovery (39). Moreover, high-frequency 5-Hz rTMS applied to the M1 area may enhance the cortical excitability of the affected hemisphere while exerting a modulatory influence on subsequent motor performance (30, 40). Similarly, rPMS could increase the neural excitability of the cortex, thereby improving the motor function of patients with stroke (41). In addition, the conventional PAS technique may modulate abnormal activity in intracortical circuits, which could be beneficial for increasing cortical excitability and motor behaviour (17). In the current study, rPMS was coordinated with rTMS, which could generate more proprioceptive information during muscle contraction than could peripheral neuromuscular electrical stimulation; the additional proprioceptive feedback could improve the sensorimotor system to influence brain plasticity (42).
The current study observed a decreasing tendency of SICI in both of the real-stimulation groups, indicating that the cortical excitability of the unaffected hemisphere had decreased (43). However, no positive correlation was observed between FMA-UE and SICI, which indicated that FMA-UE scores were not reflected in change in neurophysiological measures (44). Moreover, 4 patients had no detectable MEP in the lesioned side. The absence of MEPs in the lesional side implies poor motor functional state, which will influence subsequent motor recovery (45).
Study limitations
This study has several limitations. As it is a pilot study, only a small number of patients was included in each group. However, the numbers determined in the sample size analysis were included. As mentioned above, it is not clear if the stimulation intensity was sufficiently high; this was determined subjectively based on previous studies and the absence of abnormal reactions in patients. The observations in this study cannot effectively determine the effectiveness of rTMS and rPMS, and the comparison of which effect is better can be used only to guide potential research direction. Moreover, the angle of the coil and the ability to find the identical hotspot at pre- and post-test may slightly affect the results.
Randomization was performed to ensure that the patients were randomly distributed in 3 groups with regard to known confounders. It is clear from Table I that the randomization was successful regarding age, sex, etc. However, the patients in the 3 groups may differ in other respects than those presented, and a larger samples size is preferable. Although no significant difference was observed among the 3 groups in the current study, with regard to the side of the paretic extremity, the injury was on the dominant side in most of the patients (C group, 50%; CS group, 66.7%; CPS group, 57.1%). Handedness is shown to affect how the hemisphere executes movements and is a possible confounder for the observed effect of TMS treatment on upper limb function. However, no significant difference was observed among the 3 groups regarding the side of the paretic extremity in our previous study (33).
In addition, the current study assessed outcomes twice in 4 weeks rather than performing a long-term follow-up. Thus, it is not possible to compare long-term changes in the observed effects between groups. Moreover, given the lack of neuroimaging examinations and guidance for coil placement, it was not possible precisely to locate the hotspot or assess the structural changes in the hemisphere (46). As far as possible, the researcher ensured that the position remained the same.
Conclusion
This pilot study suggests that simultaneous stimulation of the lesional motor cortex and the paretic upper extremity by high-frequency rPMS combined with rTMS may be relevant for treating upper limb motor function in patients with subacute stroke in early neurorehabilitation. Further research is required including a larger number of subjects to allow stratification of the analysis by different variables, such as the degree of hand motor function or cortical activation.
ACKNOWLEDGEMENTS
This work was supported by the National Project of Clinical Key Specialty Development (Pro.20211231084249000238).
The authors thank all the patients for their voluntary contribution and for completing this study.
This pilot randomized, single-blind, controlled trial was approved by the Ethics Committee of Fudan University (No. KY2019-609). The study was registered in the Chinese Clinical Trial Registry (www.chictr.org.cn; ChiCTR2000033495).
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019; 394: 1145–1158. DOI: 10.1016/s0140-6736(19)30427-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaji R. Global burden of neurological diseases highlights stroke. Nat Rev Neurol 2019; 15: 371–372. DOI: 10.1038/s41582-019-0208-y [DOI] [PubMed] [Google Scholar]
- 3.Coscia M, Wessel M, Chaudary U, Millán J, Micera S, Guggisberg A, et al. Neurotechnology-aided interventions for upper limb motor rehabilitation in severe chronic stroke. Brain 2019; 142: 2182–2197. DOI: 10.1093/brain/awz181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolf S, Holm S, Ingwersen T, Bartling C, Bender G, Birke G, et al. Pre-stroke socioeconomic status predicts upper limb motor recovery after inpatient neurorehabilitation. Ann Med 2022; 54: 1265–1276. DOI: 10.1080/07853890.2022.2059557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leocani L, Cohen L, Wassermann E, Ikoma K, Hallett M. Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain 2000; 123: 1161–1173. DOI: 10.1093/brain/123.6.1161 [DOI] [PubMed] [Google Scholar]
- 6.Koski L, Mernar T, Dobkin B. Immediate and long-term changes in corticomotor output in response to rehabilitation: correlation with functional improvements in chronic stroke. Neurorehabil Neural Repair 2004; 18: 230–249. DOI: 10.1177/1545968304269210 [DOI] [PubMed] [Google Scholar]
- 7.Kamo T, Wada Y, Okamura M, Sakai K, Momosaki R, Taito S. Repetitive peripheral magnetic stimulation for impairment and disability in people after stroke. Cochrane Database Syst Rev 2022; 9: CD011968. DOI: 10.1002/14651858.CD011968.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiang H, Sun J, Tang X, Zeng K, Wu X. The effect and optimal parameters of repetitive transcranial magnetic stimulation on motor recovery in stroke patients: a systematic review and meta-analysis of randomized controlled trials. Clin Rehabil 2019; 33: 847–864. DOI: 10.1177/0269215519829897 [DOI] [PubMed] [Google Scholar]
- 9.Hummel F, Cohen L. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol 2006; 5: 708–712. DOI: 10.1016/s1474-4422(06)70525-7 [DOI] [PubMed] [Google Scholar]
- 10.Takeuchi N, Tada T, Toshima M, Matsuo Y, Ikoma K. Repetitive transcranial magnetic stimulation over bilateral hemispheres enhances motor function and training effect of paretic hand in patients after stroke. J Rehabil Med 2009; 41: 1049–1054. DOI: 10.2340/16501977-0454 [DOI] [PubMed] [Google Scholar]
- 11.Sakamoto D, Hamaguchi T, Murata K, Ito H, Nakayama Y, Abo M. Upper limb function recovery by combined repetitive transcranial magnetic stimulation and occupational therapy in patients with chronic stroke according to paralysis severity. Brain Sci 2023; 13: 284. DOI: 10.3390/brainsci13020284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juan Du, Yao W, Li J, Yang F, Hu J, Xu Q, et al. Motor network reorganization after repetitive transcranial magnetic stimulation in early stroke patients: a resting State fMRI study. Neurorehabil Neural Repair 2022; 36: 61–68. DOI: 10.1177/15459683211054184 [DOI] [PubMed] [Google Scholar]
- 13.Wang Q, Zhang D, Zhao Y, Hai H, Ma Y. Effects of high-frequency repetitive transcranial magnetic stimulation over the contralesional motor cortex on motor recovery in severe hemiplegic stroke: A randomized clinical trial. Brain Stimul 2020; 13: 979–986. DOI: 10.1016/j.brs.2020.03.020 [DOI] [PubMed] [Google Scholar]
- 14.Wang R, Wang F, Huang S, Yang Y. High-frequency repetitive transcranial magnetic stimulation enhanced treadmill training effects on gait performance in individuals with chronic stroke: a double-blinded randomized controlled pilot trial. Gait Posture 2019; 68: 382–387. DOI: 10.1016/j.gaitpost.2018.12.023 [DOI] [PubMed] [Google Scholar]
- 15.Rosso C, Moulton E, Kemlin C, Leder S, Corvol J, Mehdi S, et al. Cerebello-motor paired associative stimulation and motor recovery in stroke: a randomized, sham-controlled, double-blind pilot trial. Neurotherapeutics 2022; 19: 491–500. DOI: 10.1007/s13311-022-01205-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmer J, Wolf S, Borich M. Paired associative stimulation modulates corticomotor excitability in chronic stroke: a preliminary investigation. Restor Neurol Neurosci 2018; 36: 183–194. DOI: 10.3233/rnn-170785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silverstein J, Cortes M, Tsagaris K, Climent A, Gerber L, Oromendia C, et al. Paired associative stimulation as a tool to assess plasticity enhancers in chronic stroke. Front Neurosci 2019; 13: 792. DOI: 10.3389/fnins.2019.00792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tolmacheva A, Mäkelä J, Shulga A. Increasing the frequency of peripheral component in paired associative stimulation strengthens its efficacy. Sci Rep 2019; 9: 3849. DOI: 10.1038/s41598-019-40474-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang T, Li X, Xia P, Wang X, Lu J, Wang L. Effects of rTMS combined with rPMS on stroke patients with arm paralysis after contralateral seventh cervical nerve transfer: a case-series. Int J Neurosci 2023; 133: 999–1007. DOI: 10.1080/00207454.2022.2032044 [DOI] [PubMed] [Google Scholar]
- 20.Sun T, Zhu G, Zheng Y, Mao Y, Hu Q, Song G, et al. Effects of paired associative magnetic stimulation between nerve root and cortex on motor function of lower limbs after spinal cord injury: study protocol for a randomized controlled trial. Neural Regen Res 2022; 17: 2459–2464. DOI: 10.4103/1673-5374.339012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carson RG, Buick AR. Neuromuscular electrical stimulation-promoted plasticity of the human brain. J Physiol 2021; 599: 2375–2399. DOI: 10.1113/JP278298 [DOI] [PubMed] [Google Scholar]
- 22.Tarri M, Brihmat N, Gasq D, Lepage B, Loubinoux I, De BX, et al. Five-day course of paired associative stimulation fails to improve motor function in stroke patients. Ann Phys Rehabil Med 2018; 61: 78–84. DOI: 10.1016/j.rehab.2017.11.002 [DOI] [PubMed] [Google Scholar]
- 23.Kumru H, Albu S, Rothwell J, Leon D, Flores C, Opisso E, et al. Modulation of motor cortex excitability by paired peripheral and transcranial magnetic stimulation. Clin Neurophysiol 2017; 128: 2043–2047. DOI: 10.1016/j.clinph.2017.06.041 [DOI] [PubMed] [Google Scholar]
- 24.Liu Liping, Chen Weiqi, Zhou Hongyu, Duan Wanying, Li Shujuan, Huo Xiaochuan, et al. Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: executive summary and 2019 update of clinical management of ischaemic cerebrovascular diseases. Stroke Vasc Neurol 2020; 5(2): 159–176. DOI:10.1136/svn-2020-000378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noh J, Lim J, Choi T, Jang S, Pyun S. Effects and safety of combined rTMS and action observation for recovery of function in the upper extremities in stroke patients: a randomized controlled trial. Restor Neurol Neurosci 2019; 37: 219–230. DOI: 10.3233/rnn-180883 [DOI] [PubMed] [Google Scholar]
- 26.Liz L, Silva TG, Michaelsen SM. Validity, reliability, and measurement error of the remote Fugl-Meyer assessment by videoconferencing: Tele-FMA. Phys Ther 2023; 103: pzad054. DOI: 10.1093/ptj/pzad054 [DOI] [PubMed] [Google Scholar]
- 27.Beaulieu L, Milot M. Changes in transcranial magnetic stimulation outcome measures in response to upper-limb physical training in stroke: a systematic review of randomized controlled trials. Ann Phys Rehabil Med 2018; 61: 224–234. DOI: 10.1016/j.rehab.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 28.Scarpino M, Lanzo G, Salimova M, Lolli F, Del VA, Cossu C, et al. Efficacy of high-frequency (15Hz) repetitive transcranial magnetic stimulation (rTMS) of the left premotor cortex/dorsolateral prefrontal cortex in decreasing cocaine intake (the MagneTox study): a study protocol for a randomized placebo-controlled pilot trial. Neurophysiol Clin 2019; 49: 1–9. DOI: 10.1016/j.neucli.2018.10.002 [DOI] [PubMed] [Google Scholar]
- 29.Asao A, Wada K, Nomura T, Shibuya K. Time course changes in corticospinal excitability during repetitive peripheral magnetic stimulation combined with motor imagery. Neurosci Lett 2022; 771: 136427. DOI: 10.1016/j.neulet.2021.136427 [DOI] [PubMed] [Google Scholar]
- 30.Demirtas A, Alonso M, Shetty R, Ronen I, Pascual-Leone A, Fregni F. Long-term effects of contralesional rTMS in severe stroke: safety, cortical excitability, and relationship with transcallosal motor fibers. NeuroRehabilitation 2015; 36: 51–59. DOI: 10.3233/nre-141191 [DOI] [PubMed] [Google Scholar]
- 31.Emara T, Moustafa R, ElNahas N, ElGanzoury A, Abdo T, Mohamed S, et al. Repetitive transcranial magnetic stimulation at 1Hz and 5Hz produces sustained improvement in motor function and disability after ischaemic stroke. Eur J Neurol 2010; 17: 1203–1209. DOI: 10.1111/j.1468-1331.2010.03000.x [DOI] [PubMed] [Google Scholar]
- 32.Di G, Pellegrino G, Assenza G, Capone F, Ferreri F, Formica D, et al. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol 2014; 10: 597–608. DOI: 10.1038/nrneurol.2014.162 [DOI] [PubMed] [Google Scholar]
- 33.Le Q, Qu Y, Tao Y, Zhu S. Effects of repetitive transcranial magnetic stimulation on hand function recovery and excitability of the motor cortex after stroke: a meta-analysis. Am J Phys Med Rehabil 2014; 93: 422–430. DOI: 10.1097/phm.0000000000000027 [DOI] [PubMed] [Google Scholar]
- 34.Du J, Tian L, Liu W, Hu J, Xu G, Ma M, et al. Effects of repetitive transcranial magnetic stimulation on motor recovery and motor cortex excitability in patients with stroke: a randomized controlled trial. Eur J Neurol 2016; 23: 1666–1672. DOI: 10.1111/ene.13105 [DOI] [PubMed] [Google Scholar]
- 35.Khedr E, Etraby A, Hemeda M, Nasef A, Razek A. Long-term effect of repetitive transcranial magnetic stimulation on motor function recovery after acute ischemic stroke. Acta Neurol Scand 2010; 121: 30–37. DOI: 10.1111/j.1600-0404.2009.01195.x [DOI] [PubMed] [Google Scholar]
- 36.Du J, Yang F, Hu J, Hu J, Xu Q, Cong N, et al. Effects of high- and low-frequency repetitive transcranial magnetic stimulation on motor recovery in early stroke patients: Evidence from a randomized controlled trial with clinical, neurophysiological and functional imaging assessments. Neuroimage Clin 2019; 21: 101620. DOI: 10.1016/j.nicl.2018.101620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim WJ, Rosselin C, Amatya B, Hafezi P, Khan F. Repetitive transcranial magnetic stimulation for management of post-stroke impairments: An overview of systematic reviews. J Rehabil Med 2020; 52. DOI: 10.2340/16501977-2637. [DOI] [PubMed] [Google Scholar]
- 38.Gao B, Sun C, Xia G, Zhou S, Zhang Y, Mao Y, et al. Paired associated magnetic stimulation promotes neural repair in the rat middle cerebral artery occlusion model of stroke. Neural Regen Res 2020; 15: 2047–2056. DOI: 10.4103/1673-5374.282266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xing Y, Zhang Y, Li C, Luo L, Hua Y, Hu J, et al. Repetitive transcranial magnetic stimulation of the brain after ischemic stroke: mechanisms from animal models. Cell Mol Neurobiol 2023; 43: 1487–1497. DOI: 10.1007/s10571-022-01264-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bates K, Rodger J. Repetitive transcranial magnetic stimulation for stroke rehabilitation-potential therapy or misplaced hope? Restor Neurol Neurosci 2015; 33: 557–569. DOI: 10.3233/rnn-130359 [DOI] [PubMed] [Google Scholar]
- 41.Momosaki R, Yamada N, Ota E, Abo M. Repetitive peripheral magnetic stimulation for activities of daily living and functional ability in people after stroke. Cochrane Database Syst Rev 2017; 6: CD011968. DOI: 10.1002/14651858.CD011968.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sui Y, Tong L, Zhang X, Song Z, Guo T. Effects of paired associated stimulation with different stimulation position on motor cortex excitability and upper limb motor function in patients with cerebral infarction. J Clin Neurosci 2021; 90: 363–369. DOI: 10.1016/j.jocn.2021.06.028 [DOI] [PubMed] [Google Scholar]
- 43.Premoli I, Király J, Müller F, Zipser C, Rossini P, Zrenner C, et al. Short-interval and long-interval intracortical inhibition of TMS-evoked EEG potentials. Brain Stimul 2018; 11: 818–827. DOI: 10.1016/j.brs.2018.03.008 [DOI] [PubMed] [Google Scholar]
- 44.Mello E, Cohen L, Monteiro DAS, Conti J, Andrade K, Tovar MF, et al. Increase in short-interval intracortical facilitation of the motor cortex after low-frequency repetitive magnetic stimulation of the unaffected hemisphere in the subacute phase after stroke. Neural Plast 2015; 2015: 407320. DOI: 10.1155/2015/407320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferreiro AK, Conforto A. Decreased short-interval intracortical inhibition correlates with better pinch strength in patients with stroke and good motor recovery. Brain Stimul 2018; 11: 772–774. DOI: 10.1016/j.brs.2018.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harvey R, Edwards D, Dunning K, Fregni F, Stein J, Laine J, et al. Randomized sham-controlled trial of navigated repetitive transcranial magnetic stimulation for motor recovery in stroke. Stroke 2018; 49: 2138–2146. DOI: 10.1161/strokeaha.117.020607 [DOI] [PubMed] [Google Scholar]