Highlights
-
•
This is an “Inspirational Profile” – as such I do not think it is necessary to include highlights as part of the submission.
Keywords: Tandem mass spectrometry, Acylcarnitines, Newborn screening, Clinical diagnostics, Metabolomics
Abstract
This article offers a personal account of a remarkable journey spanning over 30 years of applied mass spectrometry in a clinical setting. It begins with the author's inspiration from a clinician's story of rescuing a child from near death with a revolutionary therapeutic intervention. Motivated by this experience, the author delved into the field of chemistry and mass spectrometry to solve an analytical challenge. The breakthrough came with the development of the first front-line diagnostic test performed by MS/MS, which focused on analyzing acylcarnitines to detect and diagnose inherited disorders related to fatty acid and branched-chain amino acid catabolism. Building upon this success, the author expanded the application of the method to dried blood spots, incorporating additional analytical components such as essential amino acids. The result was a groundbreaking multiplex assay capable of screening newborns for more than 30 inherited metabolic conditions with just one test. This novel approach laid the foundation for a targeted metabolomics platform that facilitated the identification of new animal models of metabolic disease through screening the offspring of genetically modified adults. The development and utilization of MS/MS with UPLC has led to the creation of new assays for biomarkers of metabolic disease, benefiting both the diagnosis and therapeutic monitoring of these conditions. The article provides compelling examples from the author's laboratory, highlighting the value and vast applications of these methods in the field of metabolic disease research.
Introduction
I was deeply honored to receive the Inaugural Distinguished Contribution Award from MSACL in 2015. It was a truly humbling experience to be recognized for my contributions to the field. In my award lecture at the MSACL Conference in San Diego, California, I had the opportunity to share my journey – one that took me from a reluctant organic chemist to a pioneer in the application of tandem mass spectrometry (MS/MS) to various disciplines such as biochemical genetics, clinical chemistry, neonatal screening, and metabolomics.
The widespread application of MS/MS in clinical chemistry and public health was inevitable, but it happened sooner than expected, driven by a few visionary individuals and fortunate circumstances. I was fortunate to be among those dedicated individuals who embraced the potential of MS/MS early on. Inspired by others with vision and a shared mindset, I was able to contribute to some of the most significant advancements in MS/MS within the health sciences.
The foundation for my journey was laid during my undergraduate studies in organic chemistry at the University of Liverpool, where I first encountered the field of organic mass spectrometry. Under the guidance of Dr. Robert Johnstone, I became fascinated by the potential of mass spectrometry (MS) and photochemistry to solve problems beyond chemistry. This led me to pursue a PhD (1966–1969) focused on the application of MS to peptide structure, followed by post-doctoral work in natural products research with Kenneth L. Rinehart (University of Illinois, 1969–71) and Tony Jackson (University of Cardiff, 1971–73).
My first “real” job was as Head of Mass Spectrometry at the Tenovus Institute, University Hospital of Wales (Cardiff, UK, 1973–76), where I developed methods to determine hormonal steroid concentrations using MS. The realization that a deeper understanding of MS was essential for my career in the life sciences led me to spend several years at VG Micromass (Manchester, UK), where I gained invaluable knowledge and collaborated with talented scientists worldwide. During this time, I gained firsthand experience with all aspects of double-focusing sector mass spectrometers including early MS/MS methods such as “MIKES” [1] and “Constant B/E Ratio Scanning” [2], as well as fast atom bombardment – a new ionization method developed in 1979 that greatly increased the scope of MS to include compounds of much higher polarity and molecular weight than were previously accessible [3]. In this article I will refer to this ionization method by its alternative name – liquid secondary ionization (LSI). At this time, the quadrupole MS was still in its infancy and the triple quadrupole, as we know it today, was an idea in the minds of Chris Enke and Rick Yost at Michigan State University [4].With this background, I begin my story as a recently appointed Research Faculty member in the School of Public Health at the University of North Carolina (Chapel Hill, USA, 1981–83).
An inspirational story
I owe my transition back to academia in the USA, as well as the introduction to my subsequent mentor, Charles Roe M.D., to Dr. J. Ronald Hass. At the time, Dr. Hass was the Head of Mass Spectrometry at the National Institute of Environmental Health Sciences in the Research Triangle Park, North Carolina. I had previously met Dr. Hass as a scientist and customer of VG Micromass. Notably, Dr. Hass was also part of the UNC School of Public Health faculty. He successfully convinced them to invest in a GC-high resolution MS instrument and hire a qualified scientist at the faculty level to fully realize its potential in the environmental health sciences.
I was asked to meet with Dr. Roe, or Charlie as he preferred to be called, sometime in the spring of 1982 to discuss a problem. Charlie had recently returned from a sabbatical year at the Medical Research Council in London, England, where he had studied GC–MS with Drs. Ronald Chalmers and Alex Lawson. During his time there, he even contributed by editing the index to their seminal publication “Organic Acids in Man” [5]. Charlie, who had initially trained as a cardiologist before becoming a pediatrician at Duke University Medical Center (DUMC) in Durham, NC, was particularly interested in applying methods for the diagnosis of inherited metabolic disorders (IMD). He had established a clinical laboratory equipped with GC (for analysis of amino acids), GC–MS (for analysis of urinary organic acids), electrophoresis (for analysis of glycosaminoglycans), as well as methods for analyzing certain therapeutic drugs and CK-MB isozymes.
Over lunch, Charlie shared an extraordinary and compelling story with me. He had recently diagnosed an infant with propionic acidemia (PA), a devastating inherited metabolic disorder caused by a deficiency of a mitochondrial enzyme. Charlie used his newly acquired GC–MS instrument for the diagnosis. Despite the limited treatment options available at the time to alleviate the symptoms, the infant’s condition continued to deteriorate until it reached a critical stage.
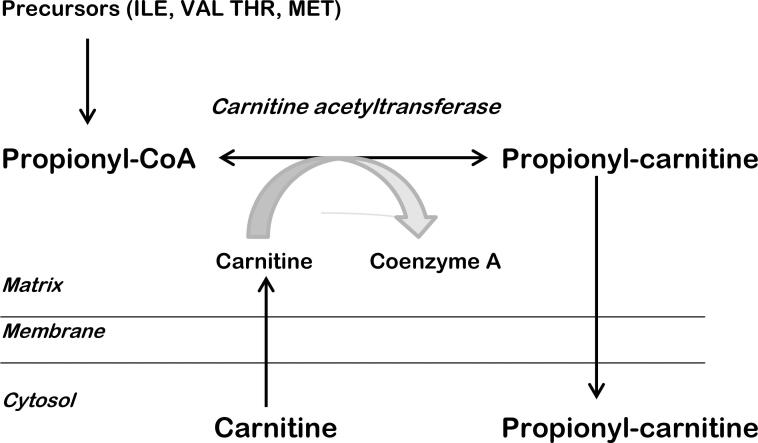
During his time at the MRC, Charlie, along with Ron Chalmers, had been contemplating a novel treatment approach for PA and a related disorder called methylmalonic aciduria (MMA). Their idea involved using L-carnitine to “detoxify” the mitochondria. L-carnitine would conjugate with propionyl-coenzyme A, restoring normal homeostasis, releasing coenzyme A for its vital intra-mitochondrial functions, and excreting propionylcarnitine as a harmless by-product (Fig. 1). The next step was to put this approach into practice, even though it had never been attempted before. Charlie obtained a supply of L-carnitine from Sigma Tau in Pomezia, Italy, and formulated it as an IV injectable.
Fig. 1.
Basis for a therapeutic role for carnitine in propionic acidemia. Due to a metabolic block, propionyl-coenzyme A accumulates in the mitochondrial matrix. An alternative pathway exists to remove this toxic intermediate as propionyl-carnitine, a harmless by-product, but there is insufficient endogenous carnitine available. Supplemental L-carnitine enhances this pathway, thereby releasing coenzyme-A for its vital functions.
The infant's condition had deteriorated to the point where survival overnight seemed unlikely. Charlie, after waiting for a priest administering the last rites to leave, asked the parents for permission to administer the carnitine. Prior to that, he had sought permission from the internal review board (IRB) to administer the drug on compassionate grounds. At that time, the IRB was an individual who also happened to be the Chairman of Pediatrics. It took some time to convince him that the biochemistry was sound and that L-carnitine, being a natural substance synthesized in the body, but not in sufficient amounts to achieve the therapeutic goal, was safe to use. The miraculous result was that the child awoke from his coma approximately 3–4 h post-infusion, sat up, and smiled happily for the astonished onlookers and returned home. This is not to say that he was “cured”, but the new therapy significantly improved his quality of life until he eventually succumbed to a fungal infection approximately 2 years later. The introduction of this new therapeutic approach for PA was welcome news for those involved with caring for affected patients [6].
A solution looking for a problem – First results
After sharing this captivating story, which had certainly captured my full attention, Dr. Roe posed a question to me. He asked if it would be possible to identify propionylcarnitine by GC–MS in a freeze-dried sample of the patient's urine, which he produced from his shirt pocket. The sample in the test tube had a yellowish-white deposit and was collected post-carnitine administration. Intrigued, I requested that he draw the structure for me. Recognizing that carnitine is the betaine form of 3-hydroxy-4-aminobutyric acid, I informed him that the problem would require a tandem mass spectrometer equipped with a fast-atom bombardment ion (LSI) source, rather than GC–MS. Curious to learn more, Dr. Roe asked “what’s a tandem mass spectrometer?” and I responded “it’s a solution looking for a problem.” I think that was a fair assessment of MS/MS at the time. Commercially available MS/MS machines were double-focusing sector instruments with either “forward” (E-B) or “reverse” (B-E) geometry, depending on the order in which the ion beam traversed the electric (E) and magnetic (B) fields. These instruments were invariably complex and finicky, lacking computer control and user-friendly interfaces. While MS/MS was promoted as a versatile analytical tool capable of analyzing mixtures without prior separation, there were few practical examples in the literature, and those that did exist were contrived and of limited practical value. Despite these challenges, it seemed that this type of instrument was the best hope for solving the problem, cumbersome though it was.
Another obstacle we faced was the lack of commercial sources for acylcarnitines. To overcome this, we assigned the task of synthesizing acetyl and propionyl carnitines, as well as their isotopically labeled analogs, to David Maltby, a talented chemist and mass spectrometrist in my lab at UNC. Several months after the lunch meeting with Dr. Roe, we were prepared to proceed. We were granted access to a VG ZAB-2F “MIKES” MS/MS system equipped with LSI in Dr. Hass’s lab, where we obtained encouraging preliminary results. The standards we synthesized proved to be chemically and isotopically pure, confirming their expected molecular weights and structures through both NMR and mass spectral analysis. When spiked into urine samples, both acetyl and propionyl carnitines were detectable.
The crucial experiment was conducted on a VG 7070 mass spectrometer equipped with LSI, which Dr. Roe obtained second-hand from the University of Illinois. At this point, both David Maltby and I had been recruited by Dr. Roe into the Department of Pediatrics at DUMC. My appointment as the first non-MD faculty appointee in Pediatrics and one of the few non-MD faculty members at DUMC in 1983 was a significant leap of faith for me. However, it was a decision I have never regretted, and it represented a leap of faith for Dr. Roe as well.
The acquisition of the second-hand instrument marked the introduction of the first organic MS system at Duke University and the first of its kind in a clinical laboratory worldwide. We were able to finance this purchase through Dr. Roe's Division account, which had sufficient funds primarily from the CK-MB isozyme test. This test had proven to be the gold standard for diagnosing myocardial infarction and was highly sought after by cardiologists throughout the United States. Fortunately, this stroke of good fortune meant that we did not need to seek funding from government agencies. Given the nature of our proposal, it is doubtful that such funding would have been readily available to us.
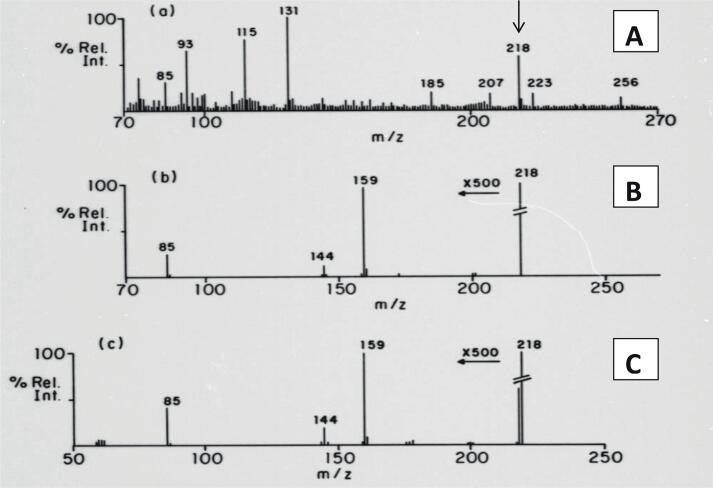
The freeze-dried urine sample from the PA patient was reconstituted in water, and a small volume was applied to the LSI probe coated with glycerol. The resulting mass normal spectrum displayed a prominent signal at m/z 218, which corresponded to the molecular mass of protonated propionylcarnitine (Fig. 2(a)). Most of the other signals at the low mass end of the spectrum originated from the glycerol matrix. The fact that acylcarnitines are quaternary ammonium salts made them ideal for LSI-MS analysis due to their pre-formed cations.
Fig. 2.
Detection of propionyl-carnitine in urine from a patient with propionic acidemia by tandem mass spectrometry. (A): normal mass spectrum from the patient’s urine using LSI with a glycerol matrix. Note the prominent signal at m/z 218, corresponding to the mass pf the protonated molecule.(B): B/E linked scan showing the fragmentation pattern of the putative biomarker. (C): B/E linked scan from the molecular signal of authentic propionyl-carnitine. This is the first example of the application of MS/MS to identify a novel disease biomarker in humans.
To obtain the fragmentation pattern, a constant B/E ratio linked-field scan [2] was applied to the signal at m/z 218 (Fig. 2(b)). The observed fragmentation pattern was found to be in good agreement with that of the authentic standard (Fig. 2(c)). It is important to note that these mass spectra were reproduced from original oscillographic recordings on UV-sensitive paper, as it was the only method available to us at that time.
In addition, the precise mass of the m/z 218 signal was determined through high-resolution mass measurement, which corresponded to the elemental formula for propionylcarnitine. This important achievement marked the first time that a novel biologically significant compound was unequivocally identified in a human specimen without prior isolation or separation, using only MS/MS. The groundbreaking results obtained from this study were published in 1984 [7], [8].
Subsequently, Dr. Roe acquired urine samples from patients with other IMDs (after an oral carnitine bolus), which allowed us to identify more novel diagnostic acylcarnitines [9]. One of the crucial discoveries among these was medium-chain acyl-coenzyme A dehydrogenase (MCAD) deficiency, a disorder affecting fatty acid catabolism that was known to cause sudden unexplained death in some affected patients [10]. Diagnosis of MCAD deficiency using GC–MS analysis was challenging. Establishing a reliable diagnostic method for MCAD and other IMDs based on acylcarnitine analysis became a significant objective of our research throughout the 1980s [11].
By the end of the decade, our laboratory remained one of the few using MS/MS. Despite our publications on the subject, there was significant skepticism and criticism from many of our peers regarding the use of such an “expensive laboratory curiosity” in a clinical setting. However, we remained steadfast in our belief that MS/MS had immense potential for clinical diagnosis and were determined to prove its value in the field of IMDs.
Emergence of the triple quadrupole and development of the “acylcarnitine profile” test in human plasma
Undoubtedly, the transition from a manually operated double-focusing sector instrument to the computer-controlled triple quadrupole played a crucial role in the development of our novel diagnostic methods. The entertaining account of the triple quadrupole's development by Dr. Yost has been previously documented in this Journal in the form of an inspirational profile [4]. We were fortunate enough to acquire a commercial prototype from VG, known as the TRIO-3, for our initial research. It was later replaced by a bench-top version, the VG Quattro, both of which came equipped with LSI sources and computer control.
The triple quadrupole offered a range of scan functions, including product ion, precursor ion, and neutral loss, which were facilitated by accumulating multiple scans over a predefined mass range for a period of 60–90 s. After peak smoothing, centroiding, and baseline subtraction, the resulting spectra exhibited an excellent signal-to-noise ratio. This approach worked satisfactorily for urine samples. However, when it came to plasma analysis, the acylcarnitine concentrations were much lower than in urine, and chemical interference from proteins, lipids, and other components was significant.
We tackled this challenge in stages. First, we applied partial purification by precipitating proteins using methanol, followed by evaporation of the extract to dryness. Subsequently, we converted the acylcarnitines to their corresponding methyl esters using anhydrous methanolic-HCl. The addition of an ion-pairing reagent, specifically heptanesulfonic acid, proved to be the “magic bullet”. This reagent brought the cationic acylcarnitine derivatives to the surface layers of the glycerol substrate on the LSI probe, greatly enhancing sensitivity.
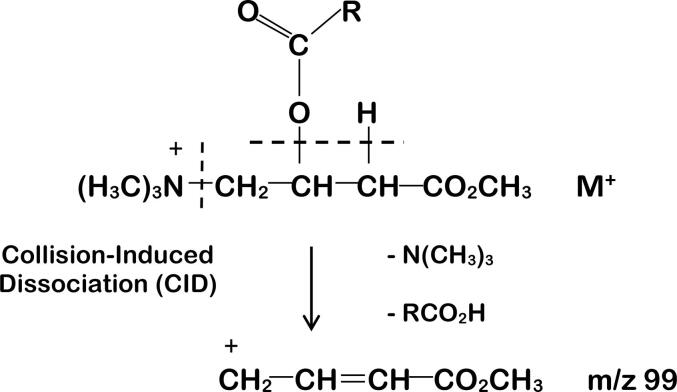
The selective analysis of methylated acylcarnitines using the triple quadrupole (Q1Q2Q3) instrument involved the precursors of m/z 99 scan function. The rationale behind this approach is shown in Fig. 3. In this process, the intact molecular species transmitted by Q1 undergoes collision-induced dissociation (CID) in Q2, resulting in the production of a common product ion with m/z 99. The driving force behind this reaction is likely the thermodynamic advantage gained from the loss of stable, intact species (trimethylamine and a carboxylic acid derived from the acyl group attached to carnitine) and the formation of a resonance-stabilized cation detected by Q3.
Fig. 3.
Fragmentation of acylcarnitine methyl esters by collision-induced dissociation (CID) in the triple quadrupole. The molecular species loses the neutral molecules N(CH3)3 and RCO2H to leave a resonance-stabilized carbonium ion having m/z 99. The same species is generated from isotope-enriched internal standards, regardless of the position of the label in either the acyl group or the trimethyl-amino group since both groups are lost in the transition to m/z 99.
By setting Q3 to transmit m/z 99 while scanning Q1 over the mass range of m/z 200–500, we were able to generate a spectrum of all acylcarnitine species in the plasma extract with remarkable clarity and minimal chemical interference. By this stage, we had also synthesized isotope-labeled acetyl, propionyl, butyryl, octanoyl, and palmitoyl carnitines to serve as internal standards. These labeled compounds were added to the plasma sample prior to the work-up. Their signals were clearly observable in the mass spectrum and used to quantify the levels of endogenous acylcarnitines.
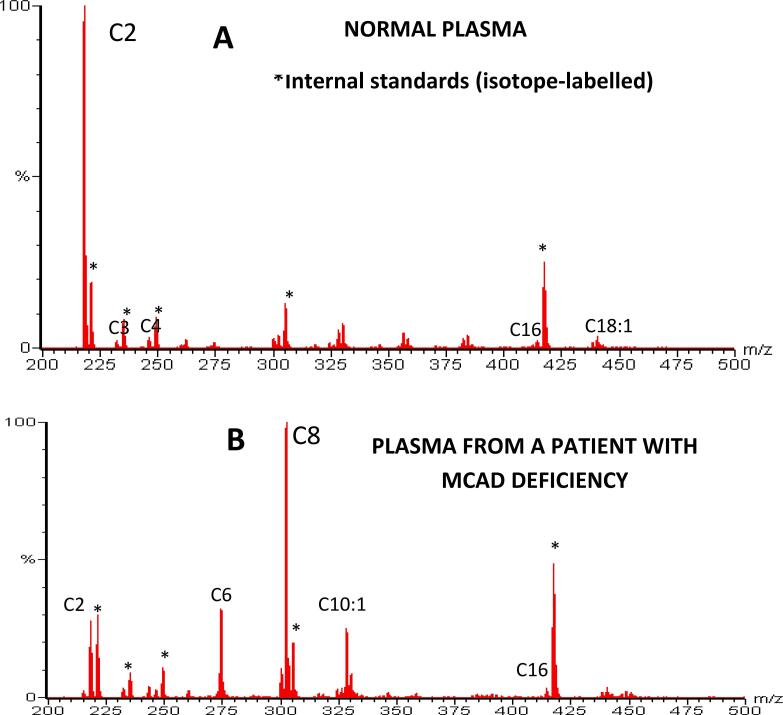
In Fig. 4, the acylcarnitine profile from normal plasma is compared with that from a patient with MCAD deficiency. The normal profile shows a prominent signal for acetylcarnitine (C2) along with a few minor signals resulting from the catabolism of branched-chain amino acids and fatty acids. In contrast, the MCAD patient's profile exhibits prominent signals from the octanoyl (C8), hexanoyl (C6) and decenoyl (C10:1) species of acylcarnitines. This distinction is due to the metabolic block at the medium-chain fatty acyl-coA level characteristic of MCAD deficiency.
Fig. 4.
Historical recordings showing “acylcarnitine profiles” from (A): a normal plasma sample generated by a “precursors of m/z 99” scan on the triple quadrupole using LSI-MS/MS. Note the lack of chemical interference and the relatively low abundance of long-chain species and the main signal due to the methyl ester of acetylcarnitine (C2); and (B): a plasma sample form a patient with medium-chain acyl-coenzyme A dehydrogenase (MCAD) deficiency, showing prominent signals for methyl esters of octanoylcarnitine (C8) and for hexanoyl (C6) and decenoyl (C10:1) that are characteristic of this metabolic disorder of fatty acid catabolism.
We introduced the term “acylcarnitine profile” for this newly developed test, which quickly became a front-line diagnostic test for diagnosing disorders of fatty acid oxidation (FAO), particularly for conditions like MCAD deficiency and several long-chain FAOs that were not reliably detectable (or detectable at all) using GC–MS [12]. The common thread in these IMDs is the mitochondrial accumulation of at least one acyl-coenzyme A species, which is reflected as an acylcarnitine in extracellular fluid. Apart from using electrospray ionization (ESI) with flow-injection analysis (FIA), the basic method for acylcarnitine profiling has seen few changes and has remained largely consistent since it was first published.
The true value of this test lies in its ability to identify approximately 20 IMDs involving mitochondrial coenzyme A deficiencies. This includes at least 12 disorders related to amino acid catabolism including PA, MMA and eight FAO disorders such as MCAD deficiency [13]. All that we required was a small volume of plasma, eliminating the need for a carnitine bolus administration to the patient and, of course, a triple quadrupole instrument.
Breaking new ground – MS/MS threatens to disrupt neonatal screening
In 1989, one of my colleagues in the Division of Medical Genetics at DUMC, Dr. Stephen Kahler, approached me with the question: “David, have you thought of applying your method to dried blood spots?”, to which my reply was: “What’s a dried blood spot?”. He was referring to the heel-prick specimens collected from newborns, where a few drops of blood were obtained and dried on a special cotton fiber card known as a Guthrie card. These cards were then sent to the State public health lab for testing for a small number of IMDs. The concept of newborn screening was championed by Robert Guthrie, who proposed testing all newborns in New England for phenylketonuria (PKU). PKU is an IMD that causes irreversible brain damage if not diagnosed and treated within the first few weeks of life [14]. The treatment consisted of a simple dietary therapy to maintain blood levels of phenylalanine below a critical value. The proposed test was a cost-effective microbiological assay that could be performed on a large scale [15]. Over the next 30 years, newborn screening for PKU and a handful of other IMDs that required early intervention to prevent irreversible damage became widespread in the United States and many other countries. It proved to be highly successful, identifying and treating at least 200 cases of PKU annually in the USA alone. Newborn screening became a mandated public health program unless parents objected for religious reasons.
During those days, the world of newborn screening was relatively small. In addition to a few dedicated biochemical geneticists and endocrinologists to whom affected newborns were referred for confirmatory diagnostic testing, clinical evaluation, and treatment, there were few health professionals aware of the program’s existence. Dr. Kahler, being on the Advisory Board for the North Carolina Public Health Lab, introduced me to the lab staff, and I observed firsthand how the dried blood spot (DBS) specimens were processed. For each test, a small sample from the DBS, around 2 mm in diameter and containing about 3 µL of whole blood, was punched out by a machine into a 96-well plate. The samples were then analyzed in dedicated laboratories for biomarkers associated with each condition being screened for. In 1990, the North Carolina Lab was testing for congenital hypothyroidism, congenital adrenal hyperplasia, galactosemia, sickle-cell disease, and PKU. Similar numbers of conditions were being screened for in most state programs at that time.
Fortuitously, the director of the Newborn Screening (NBS) Lab, Dr. Lindsay Hofman, was a biochemist who understood our proposal and graciously allowed us to access anonymized leftover DBS samples for method development. Dr. Kahler was aware of several cases that had been diagnosed later in infancy or childhood after the affected individuals became symptomatic. We were fortunate that the state lab had a policy of storing leftover DBS samples for up to 10 years – albeit at ambient temperature. With parental consent, we were able to retrieve the original DBS specimens from several patients with confirmed IMD diagnoses, as well as age-matched controls, to test our method. Our laboratory blindly analyzed these DBS specimens and successfully deduced the diagnoses of the affected patients based on their abnormal acylcarnitine profiles. Our preliminary findings were published in 1990 [16], setting the stage for further development.
Thanks again to Dr. Hofman’s kindness, I was invited to speak at the 8th International Newborn Screening Symposium in Leura, Australia in November 1991. The symposium gathered a small group, including Dr. Guthrie himself, but my talk received little enthusiasm from the NBS community, with only a few exceptions. The same lack of enthusiasm greeted my presentation at another NBS meeting in Raleigh, NC the following year. It became clear to us that acylcarnitine analysis of DBS samples using MS/MS (Fig. 5) was unlikely to persuade the NBS community about its potential. Fig. 6.
Fig. 5.
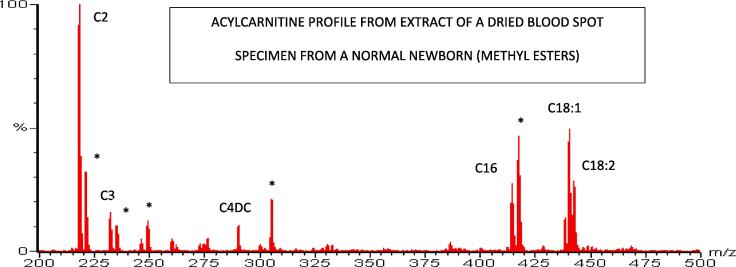
Historical recording showing the analysis of acylcarnitines in extracts of dried blood spots (DBS) from a normal newborn by LSI-MS/MS. Note the presence of long-chain acylcarnitines compared with the lack of them in plasma (Fig. 4). The signals from the isotope-labeled internal standards facilitates quantitative analysis of the target analytes. Acylcarnitines are now typically targeted simultaneously with amino acids as either butyl esters or non-derivatized using selected reaction monitoring (SRM/MRM), which enables selective targeting of metabolites for programs that prefer that option.
Fig. 6.
The application of tandem mass spectrometry in newborn screening, showing the process of punching DBS specimens into 96-well plates, injection into the electrospray ion source of the triple quadrupole system (after extraction and derivatization), recording and reporting of results and, ultimately, successful diagnosis and treatment of affected newborns. (Figure courtesy of Sigma Xi, from Millington, DS, Amer. Sci. 90:40–47;2002).
Meanwhile, we secured funding from the NC State NBS program to further develop the method for MS/MS analysis of DBS samples and added Dr. Donald Chace to the faculty. We realized that including amino acids, such as phenylalanine, methionine, citrulline, and others, would greatly enhance the value of MS/MS. Previously, we had demonstrated that butylation enhances the sensitivity of LSI-MS for analyzing citrulline and argininosuccinic acid in urine [17]. Additionally, we showed that a neutral loss scan function [18] could detect multiple butylated amino acids simultaneously using MS/MS.
Dr. Chace applied the same method to DBS samples and, to our delight, a clean spectrum of signals from the butylated alpha-amino acids was revealed. Moreover, butylated acylcarnitines generated a common product ion at m/z 85, allowing us to interlace two scan functions. After data reduction, we were able to simultaneously produce an acylcarnitine profile and an amino acid profile from the same DBS extract in less than two minutes [18]. The commercial availability of isotopically labeled amino acids facilitated their quantification in DBS extracts. This laid the foundation for the first multiplex NBS test for IMDs.
During the early 1990s, our group published several additional studies that further demonstrated the value of MS/MS for recognizing IMDs, including those characterized by amino acid biomarkers in DBS samples [19], [20], [21]. With the LSI-MS probe, we could analyze samples in intervals of less than three minutes with no carry-over. However, it was a labor-intensive process and not suitable for high-throughput analysis, although that was about to change. We were fortunate to receive valuable commercial support during this period from VG and Sciex, who provided loaner instruments and collaborated in developing software to enhance data analysis.
Movers and shakers
Between 1990 and 1996, our numerous publications and presentations at various symposia gradually increased interest in MS/MS within the NBS community. Notably, Dr. Edwin Naylor, who attended the Leura meeting, acquired a triple quadrupole instrument with LSI and began using MS/MS for newborn screening in 1993 at the Magee Women’s Hospital in Pittsburgh, PA. In 1994, he established NeoGen Screening, Inc., a private company in Pittsburgh, and began collaborating with birthing centers across Pennsylvania to send DBS specimens to his laboratory for analysis. Dr. Bridget Wilken, also an attendee in Leura, began using MS/MS for newborn screening in Sydney, Australia. Dr. Nestor Chamoles established a private metabolic center in Buenos Aires, Argentina, and implemented MS/MS for screening newborns and at-risk pediatric patients.
In 1994, Drs. P. Ozand and Mohamed Rashed acquired MS/MS at the King Faisal Hospital in Riyadh, Saudi Arabia, and initiated newborn and at-risk infant screening for IMDs. Their use of electrospray ionization (ESI) with triple quadrupole MS/MS for NBS marked an important milestone, and their seminal publications in 1995 [22] and 1997 [23], describing the automated analysis of derivatized DBS extracts from 96-well plates using flow-injection analysis (FIA)-ESI-MS/MS, represented the final stage of method development for high-throughput multiplexed NBS. Dr. Harvey Levy also played a valuable role as a collaborator and promoter of the concept of what became known as “expanded newborn screening”. He advocated for population NBS in the New England states (USA). However, the North Carolina NBS program, led by Dr. Kahler, conducted the first full-scale pilot program for MS/MS NBS in 1997, with collaboration from Neogen Screening. It became the first state program in the US to officially adopt and mandate the new MS/MS method in 1998. Serendipity played a part in this development, as a tax surplus in North Carolina enabled the State NBS Lab to purchase three ESI-MS/MS instruments, along with ancillary equipment, and hire the necessary support staff for expansion. The New England program quickly followed suit, and the era of expanded NBS using MS/MS was officially under way.
Impact of expanded NBS
The introduction of MS/MS in NBS brought a mix of excitement and confusion. The sudden expansion of potential new cases with IMDs threatened to overwhelm an already overloaded system. No one was certain about the number or variety of conditions that could be reliably detected. The lack of effective treatment options for some of these conditions also posed challenges to the accepted norms of introducing a population screening test.
To manage this, some NBS programs initially limited the analyte panel to target IMDs that closely aligned with established screening protocols. Others, however, decided to cast a wide net in order to gain experience with rare IMDs that could be detected before symptoms appeared. Valuable experience was gained during the pilot phase and early years of expanded NBS. The first publications from state and national programs began to appear [24], [25], showing promising and consistent results across programs despite variations in methodology and equipment. The North Carolina NBS program, for instance, published its first report in 2006 based on almost one million newborns screened by MS/MS [26]. It revealed 219 confirmed diagnoses, including 99 FAO disorders, 58 amino acid catabolism disorders (“organic acidurias”) and 62 aminoacidopathies (mostly PKU). The overall incidence of an IMD was 1:4300, with a positive predictive value of around 4 % based on the initial screening test. These figures were considered acceptable and manageable, and they compared favorably with data reported by other programs, particularly those with a predominantly Caucasian population.
In 2005, a consortium of experts convened in the US to review the overall NBS program and make recommendations, which were subsequently published in 2006 and are now generally accepted and followed [27]. One significant outcome was the establishment of a list of 29”core”conditions (IMDs) that were designated as primary targets for all NBS programs. Of these, 20 were on the MS/MS panel (Table 1). The consortium also identified an additional 26 “secondary” conditions that might be incidentally detected, with 22 of them being on the MS/MS panel (Table 1), although they were not listed as primary targets [27], [28]. This list of primary targets is referred to as the recommended uniform screening panel (RUSP). Furthermore, the expert group recommended a consensus-based system for adding additional conditions to the RUSP.
Table 1.
Disorders detectable by tandem mass spectrometry (MS/MS) newborn screening based on the ACMG Recommended Uniform Screening Panel (RUSP) [27], [28]1.
 |
Notes: 1 The other core conditions that make up the 29 on the original RUSP are congenital hypothyroidism (CH), classic galactosemia (GALT), congenital adrenal hyperplasia (CAH), sickle cell anemia (SS), sickle cell diseases (SB and SC-Thal), biotinidase deficiency (BIOT), cystic fibrosis (CF), hearing loss (HEAR).
*This condition is now more correctly referred to as mitochondrial nicotinamide adenine dinucleotide phosphate NADP(H) deficiency (see Houten SM et al. Hum Mol Genet. 2014;23(18):5009–5016).
The use of MS/MS in NBS programs has experienced rapid global expansion. Currently, it is estimated that at least 25 million newborns are screened annually by MS/MS worldwide, with many private programs that do not participate in the CDC quality control and assurance program also utilizing this technology [29]. One of the most significant contributions of MS/MS to NBS is an increased awareness of its life-saving potential and the benefits it brings to families. This awareness has stimulated the development of new therapies, such as enzyme replacement, stem cell transplant, and gene replacement, to address rare diseases. In fact, the development of new therapies for rare diseases has become a major driving force behind the addition of more conditions to the RUSP. The original 29 conditions on the RUSP have now increased to 36, with some US programs successfully advocating for the addition of even more IMDs. This expansion has further emphasized the growing role of MS/MS in NBS.
Some of the recently added conditions, such as adrenoleukodystrophy, can be screened by adding biomarkers to the existing panel of acylcarnitines and amino acids. This approach preserves the simplicity and robustness of the original method based on flow-injection analysis (FIA).
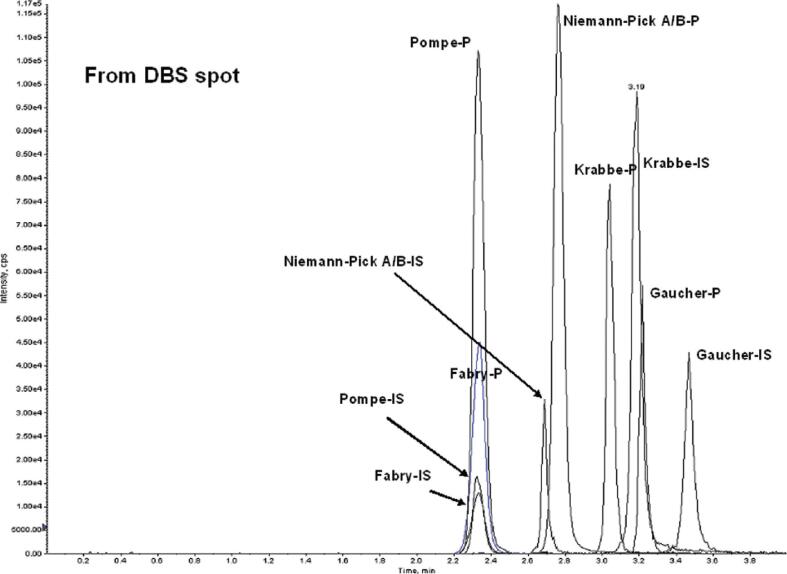
For several lysosomal storage disorders (LSD), including Pompe disease, Hurler syndrome, and Hunter disease, which are also recent additions to the RUSP, the primary screening test involves measuring residual enzyme activity in DBS extracts. These measurements can be performed simultaneously using MS/MS or fluorometry with digital microfluidics, another novel technology recently introduced to NBS [30]. MS/MS methods for LSD NBS were pioneered by Dr. Michael Gelb at the University of Washington in Seattle [31], [32]. These methods are gaining popularity in part because MS/MS is already widely used in NBS for analyte screening. However, additional equipment is required for LSD screening methods, as they are not compatible with the existing method for amino acids and acylcarnitines.Furthermore, FIA does not adequately prevent contaminants from the work-up of enzymatic products from interfering in the LSD assays, which limits the efficiency of the ion source. Currently, the most efficient methods for LSD screening are based on UPLC-MS/MS [33], as practiced in the Illinois NBS program, which screens for six LSDs using a modified version of this method [34]. UPLC in this application ensures high throughput, with a total analysis time of about 3 min per sample (Fig. 7). Once again, the power of MS/MS to multiplex similar assays on the same platform is strikingly evident.
Fig. 7.
Application of UPLC-MS/MS in NBS for lysosomal storage disorders (LSD). Enzyme-specific reagents are incubated with DBS extracts and their reaction products are separated by UPLC and detected using SRM, together with their corresponding internal standards (IS). The figure shows extracted ion current chromatograms for 5 enzyme products within the same analytical run from a normal newborn – the LSDs to which they correspond are shown. (Figure reproduced by kind permission of ACS Publications from la Marca, G, et al. Anal. Chem. 81:6113–6121;2009).
Another significant contribution of MS/MS to the NBS community is the development of second-tier tests. These tests, which are more specific and applicable to the original DBS, greatly improve the positive predictive value (PPV) of the primary NBS screen. By implementing second-tier tests, false positive result reporting is minimized. A notable example of this is the second-tier test developed for congenital adrenal hyperplasia (CAH), caused by 21-hydroxylase deficiency. The primary screening test for CAH is a kit-based immunoassay known for its low PPV. By re-analyzing DBS extracts of all presumptive positives, specifically targeting the steroids in the metabolic pathway using UPLC-MS/MS with stable-isotope-labelled internal standards[35], the false positive rate was cut by a factor of 30, improving the accuracy, and saving unnecessary costs and parental anxiety [36]. Several other valuable second-tier methods for conditions screened by MS/MS have also been developed and published [37].
More recently, LSD screening has experienced high false positive rates. Second-tier testing for biomarkers in the original DBS extracts, utilizing UPLC-MS/MS and other post-analytical tools, has proven advantageous in limiting the reporting of false positives [38], [39]. Because second-tier testing involves re-analysis of relatively few specimens on a daily basis, multiple dedicated systems are not necessary. This approach also enables NBS programs to tolerate more aggressive critical values for the primary screening test, reducing the risk of false-negative results, which is crucial for effective screening.
Tandem mass spectrometry in the biochemical genetics laboratory
After babies are identified as being at risk for IMDs, they are referred by follow-up coordinators in the NBS program to regional genetics services for clinical evaluation and diagnostic testing to confirm or rule out the suspected diagnosis. Diagnostic tests are typically performed in certified biochemical genetics laboratories, including both commercial labs (such as LabCorp and ARUP) and labs affiliated with teaching hospitals, such as the DUMC Biochemical Genetics Lab which I directed from 1983 to 2015.
Follow-up tests for IMDs detected through the analysis of acylcarnitines and amino acids often include the analysis of urinary organic acids using GC–MS, amino acids in urine, plasma, and occasionally cerebrospinal fluid (CSF) using amino acid analyzers or LC-MS/MS, and acylcarnitines using MS/MS. The DUMC Lab, for example, has developed and published clinical diagnostic methods for carnitine [40] and acylcarnitines [41], as well as novel biomarker assays for Pompe disease [42] and the mucopolysaccharidoses (including Hurler syndrome and Hunter disease) [43], [44]. These assays have proven valuable for the follow-up of LSDs that have recently been added to the RUSP and most of them are based on UPLC-MS/MS using a triple quadrupole instrument.
Contributing to metabolomics
Metabolomics, which is the application of analytical chemical methods to analyze the metabolic end-products of genomic expression in a biological system, has emerged as an important tool for biomarker discovery [45]. Numerous commercial laboratories and academic institutions have established metabolomics facilities, employing various platforms based on LC-MS/MS and GC–MS. These platforms are capable of analyzing hundreds, if not thousands, of small molecules that comprise the metabolome. Most of these facilities use a combination of targeted and non-targeted approaches. Targeted methods often focus on analyzing metabolites such as amino acids, acylcarnitines, fatty acids, and organic acids using semi-quantitative methods that were previously developed for screening IMDs.
Metabolomics has produced early successes in various areas. One example is the identification of animal models of metabolic disease created by chemical mutagenesis through the application of MS/MS methods originally developed for NBS [46], [47]. Additionally, metabolomics has been instrumental in discovering metabolic signatures that differentiate lean and obese individuals, providing insights into insulin resistance [48].
The final word
In summary, this account demonstrates the power of collaboration and dedication in improving outcomes for individuals with genetic predispositions to progressive and devastating disorders. Through persistence, dedication, hard work, and a stroke of good fortune, institutional skepticism and resistance can be overcome, leading to a positive impact on the lives of many individuals who may have otherwise been forgotten or neglected. In this case, a disruptive technology incorporating the triple quadrupole MS/MS – in my opinion one of the world’s greatest technological inventions – was the real star of the show.
We are now on the brink of another transformative step in newborn screening, with the emergence of genome-wide mutational analysis, which is currently being piloted. However, it is unlikely that this development will eclipse or replace MS/MS. More than ever, biomarker analysis will be essential to understand gene expression at the whole-organism level, and it is difficult to envision a more effective method than LC-MS/MS to fulfill this role.
Declaration of competing interest
The author declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
I am indebted to many individuals responsible for making this research possible – especially the late Dr. Charles R. Roe who was the instigator-in-chief, aided and abetted by colleagues in the Division of Medical Genetics at Duke University Medical Center especially Drs Stephen G. Kahler, Y.-T. Chen and Donald Chace; The North Carolina Public Health Laboratory (especially Dr. Lindsay Hofman, Tom Vittaglione) whose support and funding was vital; to many post-doctoral fellows and laboratory staff who have worked on the project over a 15-year period; to Drs. Michael Morris, Normal Lynaugh of VG Analytical (now a division of Waters, Inc.) for generously providing equipment and software; to the many researchers who believed in the method and adopted it, especially Drs. Bridget Wilkin, Harvey Levy, Edwin Naylor, Nestor Chamoles, Mohamed Rashed, P. Ozand and many others who provided valuable samples from patients with rare metabolic disorders. Finally, thanks to MSACL and Dr. Yost for encouraging me to write this article, which I dedicate to the patients and their families who have benefitted from the expanded screening for IMDs made possible by tandem mass spectrometry.
References
- 1.Beynon J.H., Cooks R.G., Amy J.W., Baitinger W.E., Ridley T.Y. Design and Performance of a Mass-analyzed Ion Kinetic Energy (MIKE) Spectrometer. Anal. Chem. 1973;45:1023A. doi: 10.1021/ac60334a763. 1031A. [DOI] [Google Scholar]
- 2.Millington D.S., Smith J.A. Fragmentation Patterns by Fast Linked Electric and Magnetic Field Scanning. Org. Mass Spectrom. 1977;12:264–265. [Google Scholar]
- 3.Barber M.M., Bordoli R.S., Sedgewick R.D., Tyler A.N. Fast atom bombardment of solids (F.A.B.): a new ion source for mass spectrometry. J. Chem. Soc. Chem. Commun. 1981;7:325–327. doi: 10.1039/C39810000325. [DOI] [Google Scholar]
- 4.Yost R.A. The triple quadrupole: Innovation, serendipity and persistence. J. Mass Spectrom. Adv. Clin. Lab. 2022;24:90–99. doi: 10.1016/j.jmsacl.2022.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chalmers R.A., Lawson A. Chapman & Hall; London: 1982. Organic acids in man; p. 523. [Google Scholar]
- 6.C.R. Roe CR, T.P. Bohan, L-carnitine therapy in propionicacidaemia, Lancet 319 (1982)1411–1412. DOI: https://doi.org/10.1016/S0140-6736 (82)92524-7. [DOI] [PubMed]
- 7.Millington D.S., Roe C.R., Maltby D.A. Application of high resolution fast atom bombardment and constant B/E ratio linked scanning to the identification and analysis of acylcarnitines in metabolic disease. Biomed. Mass Spectrom. 1984;11:236–241. doi: 10.1002/bms.1200110508. [DOI] [PubMed] [Google Scholar]
- 8.Roe C.R., Millington D.S., Hoppel C.L., Maltby D.A., Bohan T.P. L-Carnitine enhances excretion of propionyl-CoA as propionylcarnitine in propionic academia. J. Clin. Invest. 1984;73:1785–1788. doi: 10.1172/JCI111387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roe C.R., Millington D.S., Maltby D.A. In: Clinical Aspects of Human Carnitine Deficiency. Borum P.R., editor. Pergamon Press; NY: 1986. Diagnostic and therapeutic implications of acylcarnitine profiling in organic acidurias associated with carnitine deficiency; pp. 97–107. [Google Scholar]
- 10.Roe C.R., Millington D.S., Maltby D.A., Kinnebrew P. Recognition of medium-chain acyl-CoA dehydrogenase deficiency in asymptomatic siblings of children dying from sudden infant death or Reye-like syndromes. J. Pediatr. 1986;108:1–13. doi: 10.1016/s0022-3476(86)80762-4. [DOI] [PubMed] [Google Scholar]
- 11.Millington D.S., Norwood D.L., Kodo N., Roe C.R., Inoue F. Application of fast atom bombardment with tandem mass spectrometry and liquid chromatography/mass spectrometry to the analysis of acylcarnitines in human urine, blood and tissue. Anal. Biochem. 1989;180:331–339. doi: 10.1016/0003-2697(89)90441-7. [DOI] [PubMed] [Google Scholar]
- 12.Millington D.S., Terada N., Chace D.H., Chen Y.-T., Ding J.-H., Kodo N., Roe C.R. The role of tandem mass spectrometry in the diagnosis of fatty acid oxidation disorders. Prog. Clin. Biol. Res. 1992;375:339–354. [PubMed] [Google Scholar]
- 13.Millington D.S., Terada N., Kodo N., Chace D.H. In: Matsumoto I., editor. Vol. 1. John Wiley & Sons; Chichester UK: 1992. A Review: carnitine and acylcarnitine analysis in the diagnosis of metabolic diseases. Advantages of tandem mass spectrometry; pp. 59–71. (Advances in Chemical Diagnosis and Treatment of Metabolic Disorders). [Google Scholar]
- 14.Wright S.W. Phenylketonuria. JAMA. 1957;165:2079–2083. doi: 10.1001/jama.1957.72980340006012. [DOI] [PubMed] [Google Scholar]
- 15.Guthrie R., Susi A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics. 1963;32(3):338–343. doi: 10.1542/peds.32.3.338. [DOI] [PubMed] [Google Scholar]
- 16.Millington D.S., Kodo N., Norwood D.L., Roe C.R. Tandem mass spectrometry: a new method for acylcarnitine profiling with potential for neonatal screening for inborn errors of metabolism. J. Inherit. Metab. Dis. 1990;13:321–324. doi: 10.1007/BF01799385. [DOI] [PubMed] [Google Scholar]
- 17.Millington D.S., Maltby D.A., Roe C.R. Rapid detection of argininosuccinic aciduria and citrullinuria by fast atom bombardment and tandem mass spectrometry. Clin. Chim. Acta. 1986;155:173–178. doi: 10.1016/0009-8981(86)90280-9. [DOI] [PubMed] [Google Scholar]
- 18.Millington D.S., Kodo N., Terada N., Chace D.H., Gale D. The analysis of diagnostic markers of genetic disorders in human blood and urine using tandem mass spectrometry with liquid SIMS. Int. J. Mass Spectrom. Ion Phys. 1991;111:211–228. [Google Scholar]
- 19.Chace D.H., Millington D.S., Terada N., Kahler S.G., Roe C.R., Hofman L.F. Rapid diagnosis of phenylketonuria by quantitative analysis of phenylalanine and tyrosine in neonatal blood spots using tandem mass spectrometry. Clin. Chem. 1993;39:66–71. [PubMed] [Google Scholar]
- 20.Chace D.H., Hillman S.L., Millington D.S., Kahler S.G., Roe C.R., Naylor E.W. Rapid diagnosis of maple syrup urine disease in blood spots from newborns by tandem mass spectrometry. Clin. Chem. 1995;41:62–68. [PubMed] [Google Scholar]
- 21.Chace D.H., Hillman S.L., Millington D.S., Kahler S.G., Adam B.W., Levy H.L. Rapid diagnosis of homocystinuria and other hypermethioninemias from newborns’ blood spots by tandem mass spectrometry. Clin. Chem. 1996;42:349–355. [PubMed] [Google Scholar]
- 22.Rashed M.S., Ozand P.T., Bucknall M.P., D., Little, D, Diagnosis of inborn errors of metabolism from blood spots by acylcarnitines and amino acids profiling using automated electrospray tandem mass spectrometry. Pediatr. Res. 1995;38:324–331. doi: 10.1203/00006450-199509000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Rashed M.S., Bucknall M.P., Little D., Awad A., Jacob M., Alamoudi M., et al. Screening blood spots for inborn errors of metabolism by electrospray tandem mass spectrometry with a microplate batch process and a computer algorithm for automated flagging of abnormal profiles. Clin. Chem. 1997;43:1129–1141. [PubMed] [Google Scholar]
- 24.Wiley V., Carpenter K., Wilcken B. Newborn screening with tandem mass spectrometry 12 months’ experience in NSW Australia. Acta Paediatr. Suppl. 1999;88:48–51. doi: 10.1111/j.1651-2227.1999.tb01157.x. [DOI] [PubMed] [Google Scholar]
- 25.Zytkovicz T.H., Fitzgerald E.F., Marsden D., Larson C.A., Shih V.E., Johnson D.M., et al. Tandem mass spectrometric analysis for amino, organic, and fatty acid disorders in newborn dried blood spots: a two-year summary from the New England Newborn Screening Program. Clin. Chem. 2001;47:1945–1955. [PubMed] [Google Scholar]
- 26.Frazier D.M., Millington D.S., McCandless S.E., Koeberl D.D., Weavil S.D., Chaing S.H., Muenzer J. The tandem mass spectrometry newborn screening experience in North Carolina: 1997–2005. J. Inherit. Metab. Dis. 2006;29:76–85. doi: 10.1007/s10545-006-0228-9. [DOI] [PubMed] [Google Scholar]
- 27.American College of Medical Genetics Newborn Screening Expert Group, Newborn screening: toward a uniform screening panel and system--executive summary, Pediatrics, 117(5 Pt 2) (2006) S296–S307. https://doi.org/10.1542/peds.2005-2633I. [DOI] [PubMed]
- 28.L. Sweetman, D.S. Millington, B.L. Therrell, W.H. Hannon, B. Popovich, M.S. Watson, et al., (2006). Naming and counting disorders (conditions) included in newborn screening panels, Pediatrics, 117(5 Pt 2) (2006) S308–S314. https://doi.org/10.1542/peds.2005-2633J. [DOI] [PubMed]
- 29.Personal Communication, Dr. J. Mei, CDC Quality Assurance Program, Atlanta, GA.
- 30.R.S. Sista RS, A.E. Eckhardt, T. Wang T, C. Graham, J.L. Rouse, S.M. Norton, et al., Digital microfluidic platform for multiplexing enzyme assays: implications for lysosomal storage disease screening in newborns, Clin. Chem. 57 (2011) 1444-1448. [DOI] [PMC free article] [PubMed]
- 31.Li Y., Scott R.C., Chamoles N.A., Ghavami A., Pinto P.M., F., Turecek, F,, M.H., Gelb, Direct multiplex assay of lysosomal enzymes in dried blood spots for newborn screening. Clin. Chem. 2004;50:1785–1796. doi: 10.1373/clinchem.2004.035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X.K., Elbin C.S., Chuang W.L., Cooper S.K., Marashio C.A., Beauregard C., et al. Multiplex enzyme assay screening of dried blood spots for lysosomal storage disorders by using tandem mass spectrometry. Clin. Chem. 2008;54:1725–1728. doi: 10.1373/clinchem.2008.104711. [DOI] [PubMed] [Google Scholar]
- 33.la Marca G., Casetta B., Malvagia S., Guerrini R., Zammarchi E. New strategy for the screening of lysosomal storage disorders: the use of the online trapping-and-cleanup liquid chromatography/mass spectrometry. Anal. Chem. 2009;81(15):6113–6121. doi: 10.1021/ac900504s. [DOI] [PubMed] [Google Scholar]
- 34.B.K. Burton BK, J. Charrow , G.E. Hoganson, B. Waggonner, D. Tinkle, S.R. Draddock, et al., Newborn screening for lysosomal storage disorders in Illinois: the initial 15-Month experience. J Pediatr. 190 (2017) 130-135. doi:10.1016/j.jpeds.2017.06.048. [DOI] [PubMed]
- 35.Lacey J.M., Minutti C.Z., Magera M.J., Tauscher A.L., Casetta B., McCann M., et al. Improved specificity of newborn screening for congenital adrenal hyperplasia by second-tier steroid profiling using tandem mass spectrometry. Clin Chem. 2004;50:621–625. doi: 10.1373/clinchem.2003.027193. [DOI] [PubMed] [Google Scholar]
- 36.Schwarz E., Liu A., Randall H., Haslip C., Keune F., Murray M., et al. Use of steroid profiling by UPLC-MS/MS as a second tier test in newborn screening for congenital adrenal hyperplasia: the Utah experience. Pediatr. Res. 2009;66:230–235. doi: 10.1203/PDR.0b013e3181aa3777. [DOI] [PubMed] [Google Scholar]
- 37.Ombrone D., Giocaliere E., Forni G., Malvagia S., la Marca G.G. Expanded newborn screening by mass spectrometry: New tests, future perspectives. Mass Spectrom. Rev. 2016;35:71–84. doi: 10.1002/mas.21463. [DOI] [PubMed] [Google Scholar]
- 38.Millington D.S., Bali D.S. Current state of the art of newborn screening for lysosomal storage disorders. Int. J. Neonatal Screen. 2018;4:24–35. doi: 10.3390/ijns4030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.M.M. Minter Baerg, S.D. Stoway, J. Hart, L. Mott, D.S. Peck, S.L. NettL, et al., Precision newborn screening for lysosomal disorders, Genet. Med. 20 (2018) 847-854. doi: 10.1038/gim.2017.194. Epub 2017 Nov 9. PMID: 29120458. [DOI] [PubMed]
- 40.Stevens R.D., Hillman S., Worthy L.S., Sanders D., Millington D.S. Assay for free and total carnitine in human plasma using tandem mass spectrometry. Clin. Chem. 2000;46:727–729. [PubMed] [Google Scholar]
- 41.Millington D.S. In: Methods in Molecular Biology: Metabolic Profiling Chapter 3. Metz T.O., editor. Humana Press (Springer); New York: 2011. Acylcarnitines: analysis in plasma and whole blood using tandem mass spectrometry. [DOI] [PubMed] [Google Scholar]
- 42.Young S.P., Stevens R.D., An Y., Chen Y.-T., Millington D.S. Analysis of a glucose tetrasaccharide elevated in Pompe disease by stable isotope dilution-electrospray ionization tandem mass spectrometry. Anal. Biochem. 2003;316:175–180. doi: 10.1016/s0003-2697(03)00056-3. [DOI] [PubMed] [Google Scholar]
- 43.Zhang H., Wood T., Young S.P., Millington D.S. A straightforward, quantitative ultra-performance liquid chromatography-tandem mass spectrometric method for heparan sulfate, dermatan sulfate and chondroitin sulfate in urine: an improved clinical screening test for the mucopolysaccharidoses. Mol. Genet. Metab. 2015;114(2):123–128. doi: 10.1016/j.ymgme.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 44.Zhang H.H., Young S.P., Millington D.S. Quantification of glycosaminoglycans in urine by isotope-dilution liquid chromatography-electrospray Ionization tandem mass spectrometry. Curr. Protoc. 2023;3(3):e701. doi: 10.1002/cpz1.701. [DOI] [PubMed] [Google Scholar]
- 45.Nicholson J.K., Lindon J.C. Systems biology: metabonomics. Nature. 2008;455:1054–1106. doi: 10.1038/4551054a. [DOI] [PubMed] [Google Scholar]
- 46.Wu J.-Y., Kao H.-J., Li S.-C., Stevens R., Hillman S., Millington D., Chen Y.-T. ENU mutagenesis identifies mice with mitochondrial branched-chain aminotransferase deficiency resembling human maple syrup urine disease. J. Clin. Invest. 2004;113:434–440. doi: 10.1172/JCI19574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kao H.-J., Cheng C.-F., Chen Y.-H., Hung S.-I., Huang C.-C., Millington D., et al. ENU mutagenesis identifies mice with cardiac fibrosis and hepatic steatosis caused by a mutation in the mitochondrial trifunctional protein beta-subunit. Hum. Mol. Genet. 2006;15(24):3569–3577. doi: 10.1093/hmg/ddl433. [DOI] [PubMed] [Google Scholar]
- 48.Newgard C.B., An J., Bain J.R., Muehlbauer M.J., Stevens R.D., Lien L.F., et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]