Abstract
Pathogenic strains of Vibrio cholerae are lysogens of the filamentous phage CTXφ, which carries the genes for cholera toxin (ctxAB). We found that the titers of infective CTXφ in culture supernatants of El Tor CTXφ lysogens increased rapidly during exponential growth but dropped to undetectable levels late in stationary-phase growth. When CTXφ transducing particles were mixed with stationary-phase culture supernatants of El Tor strains, CTXφ infectivity was destroyed. Our data indicate that this growth phase-regulated factor, designated CDF (CTXφ-destroying factor), is the secreted hemagglutinin/protease (HA/P) of V. cholerae. A strain containing a disrupted hap gene, which encodes HA/P of V. cholerae, did not produce CDF activity in culture supernatants. Introduction of the HA/P-expressing plasmid pCH2 restored CDF activity. Also, CDF activity in culture supernatants of a variety of pathogenic V. cholerae isolates varied widely but correlated with the levels of secreted HA/P, as measured by immunoblotting with anti-HA/P antibody. CDF was purified from V. cholerae culture supernatants and shown to contain a 45-kDa polypeptide which bound anti-HA/P antibodies and which comigrated with HA/P in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The production of high levels of secreted HA/P by certain V. cholerae strains may be a factor in preventing CTXφ reinfection in natural environments and in the human host.
The diarrheal disease cholera is caused by infection with the gram-negative bacterium Vibrio cholerae. Nearly all of the features of the disease can be accounted for by the potent enterotoxin synthesized and secreted by V. cholerae upon successful colonization of the small intestine (19). Recently, Waldor and Mekalanos showed that the genes encoding cholera toxin reside on a novel filamentous phage called CTXφ (27). The CTXφ receptor is the toxin coregulated pilus (TCP), a bundle-forming pilus that is also an essential intestinal colonization factor (26, 27). Unlike the Escherichia coli F-specific filamentous phages such as M13, which replicate extrachromosomally as plasmids, CTXφ can lysogenize El Tor biotype V. cholerae by integration of its circular chromosome at a unique attachment site (attRS) on the V. cholerae chromosome (28). CTXφ will replicate extrachromosomally as a plasmid if it infects classical biotype strains of V. cholerae or El Tor strains with the attRS site and resident CTXφ copies deleted.
The 6.9-kbp CTXφ genome has been divided into core and RS2 regions. The core region encodes phage morphogenesis functions and cholera toxin. RS2 encodes a repressor protein (RstR) with sequence similarity to lambdoid phage repressors, plus two replication/integration functions encoded by rstA and rstB (28). In lysogens, RstR represses the transcription of rstA, a gene required for CTXφ replication (17), and may repress the expression of other CTXφ genes.
Initially, CTXφ lysogens appeared to be highly stable or quiescent since overnight culture supernatants of El Tor strain SM44 (8), harboring a single integrated copy of CTX-Knφ (CTXφ carrying a kanamycin resistance gene in place of ctxAB), contained no Knr transducing activity. This observation suggested to us that the regulation of lysogeny in CTXφ is distinct from that of other lysogenic phage, such as lambda (λ). In phage λ, lysogeny is regulated such that stable lysogens occasionally enter the lytic pathway of development, with the result that cultures of λ lysogens contain moderately high phage titers (20). Phage production by CTXφ lysogens would be expected to be a factor in the horizontal gene transfer of cholera toxin genes (ctxAB) and in the creation of new pathogenic strains of V. cholerae. Therefore, we have reexamined this issue and show here that integrated CTXφ lysogens do produce infectious particles during growth. However, when growth reaches stationary phase, CTXφ lysogens are rapidly inactivated by the major secreted hemagglutinin/protease (HA/P) of V. cholerae.
MATERIALS AND METHODS
Growth.
V. cholerae strains were grown in 10 ml of L broth in 100-ml flasks. The cultures were shaken at 250 rpm at 37°C. Antibiotics were used at the following concentrations: kanamycin, 50 μg/ml; tetracycline, 12.5 μg/ml. Strain stocks were stored at −70°C in 20% glycerol.
CDF bioassay.
Culture supernatants containing CTXφ-destroying factor (CDF) were prepared from 1.5-ml overnight cultures grown in Luria (L) broth at 37°C. Cells were pelleted briefly in a microcentrifuge, and the resulting supernatants were filtered through 0.2-μm-pore-size Millex-GV filters (Millipore, Bedford, Mass.) and stored at 4°C. In a typical assay, 25-μl supernatant samples were mixed with 25 μl of a CTX-Knφ lysate prepared from V. cholerae O395 containing pCTX-Kn, the replicative form of CTX-Knφ. The mixtures were incubated at 37°C for 3 h. The titer of CTX-Knφ transducing particles remaining in the mixtures was determined by transduction of agglutinated O395 cells to kanamycin resistance. A 50-μl aliquot of a fresh overnight agglutinated culture of V. cholerae O395 was added to each mixture. After phage adsorption for 25 min at room temperature with occasional vortexing, the samples were diluted and plated on L agar containing kanamycin. Knr transductants arose after overnight incubation at 37°C. CDF activity was determined as (Knr titerinitial/Knr titerfinal).
Purification of CDF.
Cultures (100 ml) of the El Tor CTXφ− V. cholerae strain 2740-80 were grown in L broth at 37°C for 16 to 18 h. The cultures were centrifuged for 30 min at 5,000 × g at 4°C. Supernatants were collected and filtered through 0.2-μm-pore-size filters (Corning Costar, Cambridge, Mass.) and chilled in an ice-water bath. With stirring, solid ammonium sulfate was added to 50% saturation (310 g/liter) and stirring was continued for several hours on ice. The precipitate was collected by centrifugation at 7,500 × g for 15 min and redissolved in 10 ml of buffer A (20 mM Tris HCl, 20 mM NaCl [pH 8.0]). Following extensive dialysis at 4°C in buffer A, insoluble material was removed by centrifugation. The soluble portion (fraction I) was stored at 4°C. Portions (1 ml) of fraction I (approximately 1 absorption at 260 nm unit) were loaded on a MonoQ (Pharmacia Biotech, Uppsala, Sweden) ion-exchange column, and chromatography was carried out with an FPLC G-250 pump/controller (Pharmacia Biotech) with a 30-ml linear salt gradient from 20 mM to 1 M NaCl. Fractions (1 ml) were collected and assayed for CDF activity. Samples of each fraction were precipitated with 10% trichloroacetic acid (TCA). The pellets were solubilized in sodium dodecyl sulfate (SDS) sample buffer and fractionated by SDS-polyacrylamide gel electrophoresis (PAGE) on 4 to 14% gels (Novex, San Diego, Calif.). Protein bands were stained with Coomassie blue as specified by the manufacturer. Western blot analysis was carried out with a 1:1,000 dilution of rabbit anti-HA/P antiserum (7). Alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G antiserum (Sigma), diluted 1:2,000, was used to detect the binding of the rabbit anti-HA/P antibodies. Binding of the antibody-alkaline phosphatase conjugate was detected with 5-bromo-4-chloro-3-indolyl phosphate and nitro blue tetrazolium, as previously described (11).
RESULTS
CTXφ production by lysogens.
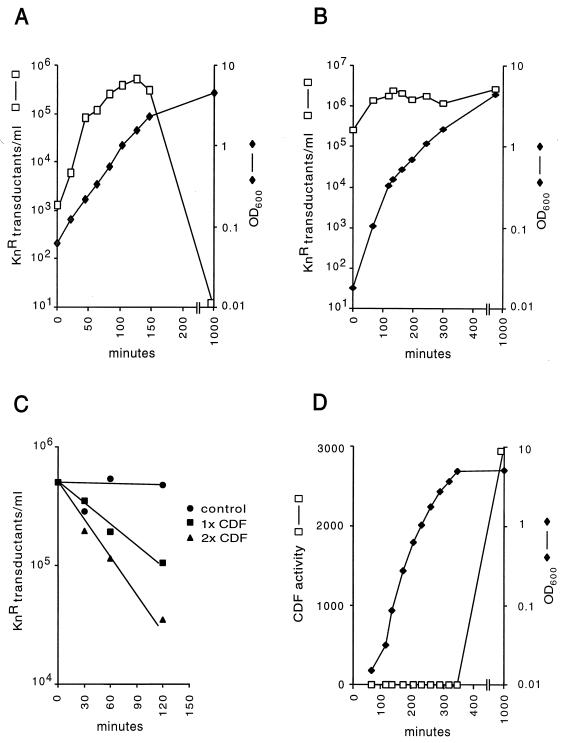
To study the production of CTXφ during growth of lysogens, we used strain HK138. This El Tor lysogen is a derivative of the CTXφ− attRS+ strain 2740-80 (23) and contains a single integrated copy of CTX-Knφ at the chromosomal attRS attachment site (27). CTX-Knφ is a CTXφ derivative carrying the Knr gene in place of ctxAB. Since CTXφ does not form visible plaques in bacterial lawns, the use of CTX-Knφ allowed us to quantitate phage production by a Knr transduction assay. We first investigated whether lysogens produced Knr transducing activity during growth. In filtered supernatants from cultures of HK138, the titer of Knr transducing activity increased rapidly during exponential growth, slowed with the onset of stationary phase, and finally decreased to nearly undetectable levels after overnight growth (Fig. 1A). Cell lysis was not observed, consistent with the fact that filamentous bacteriophages are extruded from host cells without killing. This rapid increase and subsequent decrease in Knr transducing titers was also observed with the El Tor CTX-Knφ lysogenic strain SM115, derived from a 1978 Bahrain clinical isolate (8), and with Peru15(pCTX-Kn), an El Tor vaccine strain lacking the attRS site required for CTXφ integration but carrying the pCTX-Kn plasmid (16) (data not shown). Unlike the El Tor strains examined here, CTX-Knφ transducing activity in supernatants from the classical strain O395(pCTX-Kn), which carries the replicative plasmid form of CTX-Knφ, showed no significant decrease after the onset of stationary-phase growth (Fig. 1B).
FIG. 1.
(A) Growth kinetics and CTX-Knφ production by El Tor strain HK138. Overnight cultures were diluted 1:1,000 into fresh medium. Growth was carried out with shaking aeration at 37°C. Samples (1 ml) were taken, and OD600 measurements were carried out on 100-μl subsamples. The remainder was chilled on ice and sterilized by filtration through 0.2-μm-pore-size filters. CTX-Knφ transducing titers were determined by using freshly agglutinated cultures of V. cholerae O395, as described in Materials and Methods. (B) Growth kinetics and CTX-Knφ production by the classical strain O395(pCTXKn). OD600 measurements and CTX-Knφ transducing titers were determined as described for panel A. (C) Kinetics of CDF activity. CDF assays were performed as described in Materials and Methods, using a partially purified preparation of CDF. The reactions were stopped by the addition of 5 mM EDTA. (D) Kinetics of CDF production by strain 2740-80. Cell-free culture supernatants were prepared by filtration through 0.2-μm filters. Samples were assayed for CDF activity as described in Materials and Methods.
CDF.
Phage readsorption is an unlikely explanation for the decreases in CTX-Knφ titers observed in El Tor culture supernatants since the TCP pilus, the CTXφ receptor, is not expressed under the growth conditions used here (21). To investigate whether a substance secreted by V. cholerae destroys CTX-Knφ transducing activity, cell-free culture supernatants were prepared from overnight cultures of El Tor strains HK138 and 2740-80 and classical strain O395. Supernatants were mixed with a known quantity of CTX-Knφ transducing particles and incubated at 37°C. After incubation, CTX-Knφ titers were determined by Knr transduction of TCP-expressing cultures of V. cholerae O395. An approximately 100- to 1,000-fold loss in Knr transducing activity was observed when CTX-Knφ was mixed and incubated with supernatants of strains HK138 or 2740-80, whereas no decrease in CTX-Knφ titers was observed with O395 culture supernatants. In time course experiments, Knr transducing activity decreased with time in a log-linear fashion and the rate of this reaction increased with increasing concentrations of culture supernatant (Fig. 1C). In control experiments, treatment of TCP-expressing O395 cultures with culture supernatants before the addition of CTX-Knφ did not alter their transducibility, indicating that the supernatant activity did not alter the ability of recipient cells to be infected with CTXφ. The results of these experiments demonstrate that CDF is secreted into the culture media and degrades or alters one or more components of the CTX virion required for infectivity. CDF activity was abolished by heating to 65°C for 15 min and by treatment with 10 mM EDTA or 10 mM EGTA but was resistant to the nonionic detergent Triton X-100 (1%, wt/vol) and was stable at 4°C.
CDF in stationary phase.
Our observation that CTX-Knφ transducing activity decreased dramatically in El Tor lysogens suggests that CDF is synthesized or secreted only during stationary-phase growth. We examined this more closely by measuring CDF activity in culture supernatants at various times during growth of a 2740-80 culture. CDF activity was not detected during the exponential phase of growth but was detected after entry into stationary phase growth, at an optical density at 600 nm (OD600) of 4.9. Maximal CDF activity was found in supernatants of overnight (16-h) cultures (Fig. 1D). SDS-PAGE analysis of TCA-precipitated proteins from culture supernatants showed that the appearance of CDF activity coincided with the appearance of an abundant 45-kDa secreted polypeptide (data not shown).
Identification of CDF.
Taken together, our observations suggested that CDF could be the V. cholerae hemagglutinin/protease known as HA/P (4). HA/P is a secreted zinc-dependent metalloprotease (2) with a molecular mass of approximately 45 kDa (12). It was recently shown that the hap gene is transcribed only late in stationary phase growth (14), strikingly similar to the pattern of CDF appearance in culture supernatants.
We investigated this possible link by first measuring both CDF and HA/P levels in a variety of pathogenic V. cholerae strains, as well as in well-characterized hap+ and hap strains. HA/P levels in culture supernatants of these strains were determined semiquantitatively by immunoblot analysis of SDS-PAGE-fractionated proteins with anti-HA/P antisera. A wide range of HA/P levels was observed in the culture supernatants of pathogenic strains (Table 1), in agreement with previous studies of HA/P distribution in V. cholerae (3). Generally, El Tor biotype strains exhibited high levels of secreted HA/P whereas the two classical strains examined here did not express HA/P. Two O139 serogroup strains secreted low levels of HA/P. Similarly, CDF levels varied widely in this collection of strains (Table 1). Among these strains, there was a strong correlation between HA/P levels and CDF activity levels. Two O139 serogroup strains, MO10 and Bengal 15, did secrete low levels of HA/P but showed no detectable CDF activity.
TABLE 1.
Correlation of CDF activity with HA/P production by V. cholerae
| Strain | Description (reference) | CDF activitya | HA/P levelsb |
|---|---|---|---|
| O395 | Classical, India (21) | 0.5 | − |
| RV508 | Classical, derivative of 569B (21) | 1 | − |
| 2740-80 | El Tor, U.S. Gulf Coast, CTXφ− (23) | 666 | ++++ |
| CO945 | El Tor, India (26) | 9 | ++ |
| C6709 | El Tor, Peru (28) | 55 | +++ |
| P27459 | El Tor, Bangladesh (21) | 38 | +++ |
| Bang 15 | El Tor, CTXφ− nonmotile derivative of P27459 (23) | 1,408 | ++++ |
| MO45 | O139, India (29) | 0.6 | − |
| MO10 | O139, India (29) | 0.8 | + |
| Bengal 15 | O139, CTXφ− nonmotile derivative of MO10 | 0.7 | + |
| 3083 | El Tor, Vietnam | 52 | +++ |
| HAP-1 | 3083 derivative, hap::kan (12) | 1 | − |
| HAP-1(pCH2) | 200 | NDc |
CDF activities (arbitrary units) were determined by a bioassay as described in Materials and Methods.
Total protein was precipitated from 1-ml portions of 16-h culture supernatants by addition of 10% TCA. SDS-solubilized protein was fractionated by SDS-PAGE, and HA/P levels were determined semiquantitatively by Western blot analysis with rabbit anti-HA/P antiserum, as described in Materials and Methods.
ND, not determined.
CDF activity was readily detected in culture supernatants of the hap+ El Tor strain 3083 but not in supernatants of HAP-1, a hap derivative of 3083 carrying an insertion of Knr in the hap coding sequence (12). CDF activity was restored when plasmid pCH2 (12), carrying a functional hap gene, was introduced into HAP-1 (Table 1). These data strongly suggest that HA/P secreted into culture supernatants is responsible for the CDF activity. Alternatively, HA/P could be required for the processing or activation of another secreted enzyme, which itself is responsible for CDF activity.
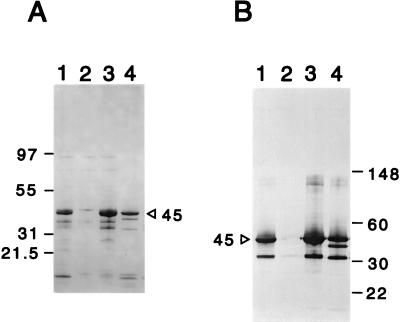
To distinguish between these possibilities, CDF activity was purified from culture supernatants of strain 2740-80 by a combination of ammonium sulfate precipitation and MonoQ ion-exchange chromatography. The majority of the CDF activity eluted in the flowthrough fraction of the MonoQ column (data not shown). SDS-PAGE analysis indicated that the flowthrough fractions contained a major 45-kDa polypeptide and three minor proteins of 37.5, 32, and 9 kDa. The 45-kDa polypeptide bound anti-HA/P antibodies and comigrated with the major anti-HA/P binding polypeptide in crude culture supernatants of strains 3083 and 2740-80 (Fig. 2). All three minor bands also bound anti-HA/P antibody, suggesting that they are proteolytic degradation or processing products of the 45-kDa polypeptide. In other flowthrough fractions, the 32-kDa polypeptide was the predominant species. This form is most probably identical to the major 32-kDa processed product of HA/P previously described for the purified enzyme (7). These data show that the major components of purified CDF are HA/P and possibly HA/P degradation products. Therefore, HA/P itself must directly proteolyze or alter a component of the CTXφ virion required for infectivity.
FIG. 2.
SDS-PAGE and immunoblot analysis of purified CDF. (A) SDS-PAGE with Coomassie blue protein stain. (B) Immunoblot analysis with anti-HA/P antiserum. Lanes (both panels): 1, 3083 hap+ supernatant; 2, 3083 hap::kan supernatant; 3, 2740-80 supernatant; 4, fast protein liquid chromatography MonoQ flowthrough fraction 2. For lanes 1 to 3, supernatants were prepared from 16-h cultures. The volumes loaded in each lane represent supernatant from approximately the same OD600 units of culture.
DISCUSSION
We observed that El Tor CTXφ lysogens readily produce new infectious particles during growth. The rate of production of new CTXφ by El Tor lysogens was greatest during exponential growth. Therefore, CTXφ shares the classical property of other lysogenic bacteriophage that lysogenic strains produce new infectious phage during growth (20). Although the regulation of this process is not understood for CTXφ, it seems likely that RstR repressor function is lost or inactivated at some frequency during active growth, thereby allowing the expression of RstA-mediated replication functions (28). E. coli lysogens of phage λ enter lytic growth at low frequency because cI repressor function and expression is antagonized by another phage repressor, Cro, which promotes lytic growth (24). Curiously, examination of the CTXφ DNA sequence revealed no Cro-like repressor gene. A Cro-like function may be encoded elsewhere in the V. cholerae genome. Alternatively, CTXφ has evolved a mechanism for regulating lysogeny distinct from that used by phage λ.
Our finding that El Tor CTXφ lysogens produce infectious phage during growth raises the possibility that lysogenization of nontoxinogenic V. cholerae strains by CTXφ is an ongoing process allowing the emergence of new toxinogenic V. cholerae clones. For example, V. cholerae O139, which emerged in 1992 as the first non-O1 strain of V. cholerae to give rise to epidemic cholera (29), may have arisen by infection of an O139 TCP+ CTXφ− strain by CTXφ. Infection of TCP+ CTXφ− V. cholerae strains could potentially occur either in the environment or within the human host. In fact, our recent work demonstrates that during infection of the suckling-mouse intestine, El Tor lysogens produce CTXφ virions capable of infecting recipient strains (18). If free CTXφ virions exist in water sources contaminated by V. cholerae, these virions would be expected to be relatively resistant to degradation and could transfer cholera toxin genes to other V. cholerae hosts. However, expression of TCP, the CTXφ receptor, may not occur frequently or efficiently in the environment, potentially limiting this phage-mediated horizontal exchange of the cholera toxin genes.
Although CTXφ phages are produced by growing El Tor lysogens, they are inactivated or destroyed by CDF. Several clues initially suggested that CDF might be the well-characterized secreted HA/P of V. cholerae. First, CDF activity, like HA/P activity (10), was detected in culture supernatants of El Tor strains only late in stationary-phase growth. CDF activity, like HA/P activity (2), was inhibited by EDTA and EGTA. Also, supernatants of the classical strain O395, which does not secrete HA/P, showed no detectable CDF activity. We confirmed that CDF was absent from cultures of the hap strain HAP-1 and that CDF activity in culture supernatants was restored after introduction of the HA/P-expressing plasmid pCH2 into HAP-1. Finally, CDF activity purified from culture supernatants consisted of a 45-kDa polypeptide which bound anti-HA/P antibody and comigrated with full-length HA/P in SDS-PAGE analysis.
Filamentous phages like fd are generally resistant to proteolysis (23). However, the gene III protein of fd, a minor polar protein required for phage adsorption to the host pilus, can be proteolyzed by subtilisin (25), leading to noninfectious particles. The gene III protein is probably susceptible to proteolysis because of its unique structure: a globular domain attached to a short stalk (9). We investigated the action of partially purified HA/P on fd-dog (22), an fd derivative carrying the gene for tetracycline resistance (Tetr). HA/P also inactivated the Tetr transducing activity of an fd-dog lysate (data not shown). Although we do not yet know the mechanism of HA/P inactivation of CTXφ or fd-dog, it is possible that HA/P proteolyzes the fd gene III protein in a fashion similar to subtilisin and may inactivate CTXφ by proteolyzing the ortholog of gene III protein, OrfU. HA/P may also influence the infectivity of other recently described filamentous bacteriophages of V. cholerae (13, 15).
In vitro studies have demonstrated that HA/P cleaves a number of host substrate molecules, including ovomucin and fibronectin, found on the surfaces of intestinal epithelial cells, and lactoferrin, found in the intestinal lumen (6). In addition, it has been shown that HA/P can activate cholera toxin by nicking the cholera toxin A subunit (1). Experiments in an infant-rabbit model of cholera indicated that HA/P is not a virulence factor; however, it has been suggested that HA/P plays a role in promoting the detachment of V. cholerae from intestinal colonization sites, thereby facilitating its dissemination (5). Our data suggest that HA/P may also function to protect V. cholerae from infection by CTXφ, thereby limiting the emergence of new pathogenic strains of V. cholerae.
ACKNOWLEDGMENTS
We are grateful to Claudia Häse for kindly providing us with anti-HA/P antisera, the hap strain HAP-1, and plasmid pCH2 and for useful discussions. We also thank our collegues S. Lazar, A. Kane, and A. Camilli for valuable suggestions.
This work was supported by NIH grant AI42347-01, a NEMC GRASP Center Pilot Grant, and a Tupper Scholar Award.
REFERENCES
- 1.Booth B A, Boesman-Finkelstein M, Finkelstein R A. Vibrio cholerae hemagglutinin/protease nicks cholera enterotoxin. Infect Immun. 1984;45:558–560. doi: 10.1128/iai.45.3.558-560.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Booth B A, Boesman-Finkelstein M, Finkelstein R A. Vibrio cholerae soluble hemagglutinin/protease is a metalloenzyme. Infect Immun. 1983;42:639–644. doi: 10.1128/iai.42.2.639-644.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Booth B A, Finkelstein R A. Presence of hemagglutinin/protease and other potential virulence factors in O1 and non-O1 Vibrio cholerae. J Infect Dis. 1986;154:183–186. doi: 10.1093/infdis/154.1.183. [DOI] [PubMed] [Google Scholar]
- 4.Finkelstein R A, Arita M, Clements J D, Nelson E T. Proceedings of the 13th Joint Conference on Cholera. U.S.-Japan Cooperative Medical Science Program. NIH publication no. 781590. Bethesda, Md: National Institutes of Health; 1978. Isolation and purification of an adhesive factor (“cholera lectin”) from Vibrio cholerae; pp. 137–151. [Google Scholar]
- 5.Finkelstein R A, Boesman-Finkelstein M, Chang Y, Hase C C. Vibrio cholerae hemagglutinin/protease, colony variation, virulence, and detachment. Infect Immun. 1992;60:472–478. doi: 10.1128/iai.60.2.472-478.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finkelstein R A, Boesman-Finkelstein M, Holt P. Vibrio cholerae hemagglutinin/lectin/protease hydrolyzes fibronectin and ovomucin: F. M. Burnet revisited. Proc Natl Acad Sci USA. 1983;80:1092–1095. doi: 10.1073/pnas.80.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finkelstein R A, Hanne L F. Purification and characterization of the soluble hemagglutinin (cholera lectin) produced by Vibrio cholerae. Infect Immun. 1982;36:1199–1208. doi: 10.1128/iai.36.3.1199-1208.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldberg I, Mekalanos J J. Effect of recA mutation on cholera toxin gene amplification and deletion events. J Bacteriol. 1986;165:723–731. doi: 10.1128/jb.165.3.723-731.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray C W, Brown R S, Marvin D A. Direct visualization of adsorption protein of fd phage. J Supramol Struct. 1979;S3:91. [Google Scholar]
- 10.Hanne L F, Finkelstein R A. Characterization and distribution of the hemagglutinins produced by Vibrio cholerae. Infect Immun. 1982;36:209–214. doi: 10.1128/iai.36.1.209-214.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 12.Hase C C, Finkelstein R A. Cloning and nucleotide sequence of the Vibrio cholerae hemagglutinin/protease gene and construction of an HA/protease-negative strain. J Bacteriol. 1991;173:3311–3317. doi: 10.1128/jb.173.11.3311-3317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honma Y, Ikema M, Toma C, Ehara M, Iwanaga M. Molecular analysis of a filamentous phage (fs1) of Vibrio cholerae O139. Biochim Biophys Acta. 1997;1362:109–115. doi: 10.1016/s0925-4439(97)00055-0. [DOI] [PubMed] [Google Scholar]
- 14.Jobling M G, Holmes R K. Characterization of hapR, a positive regulator of the Vibrio cholerae HA/protease gene hap, and its identification as a functional homologue of the Vibrio harveyi luxR gene. Mol Microbiol. 1997;26:1023–1034. doi: 10.1046/j.1365-2958.1997.6402011.x. [DOI] [PubMed] [Google Scholar]
- 15.Jouravleva E A, McDonald G A, Garon C F, Boesman-Finkelstein M, Finkelstein R A. Characterization and possible functions of a new filamentous bacteriophage from Vibrio cholerae O139. Microbiology. 1998;144:315–324. doi: 10.1099/00221287-144-2-315. [DOI] [PubMed] [Google Scholar]
- 16.Kenner J R, Coster T S, Taylor D N, Trofa A F, Barrera-Ora M, Hyman T, Adams J M, Beattie D T, Killeen K P, Spriggs D R, Mekalanos J J, Sadoff J C. Peru-15, an improved live attenuated oral vaccine candidate for Vibrio cholerae O1. J Infect Dis. 1995;172:1126–1129. doi: 10.1093/infdis/172.4.1126. [DOI] [PubMed] [Google Scholar]
- 17.Kimsey H H, Waldor M K. CTXφ immunity: application in the development of cholera vaccines. Proc Natl Acad Sci USA. 1998;95:7035–7039. doi: 10.1073/pnas.95.12.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lazar S, Waldor M K. ToxR-independent expression of cholera toxin from the replicative form of CTXφ. Infect Immun. 1998;66:394–397. doi: 10.1128/iai.66.1.394-397.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levine M M, Kaper J B, Black R E, Clements M L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983;47:510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lwoff A. Lysogeny. Bacteriol Rev. 1953;17:269–299. doi: 10.1128/br.17.4.269-337.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manning P A. The tcp gene cluster of Vibrio cholerae. Gene. 1997;192:63–70. doi: 10.1016/s0378-1119(97)00036-x. [DOI] [PubMed] [Google Scholar]
- 22.McCafferty J, Griffiths A D, Winter G, Chiswell D J. Phage antibodies: filamentous phage displaying antibody variable domains. Nature. 1990;348:552–554. doi: 10.1038/348552a0. [DOI] [PubMed] [Google Scholar]
- 23.Model P, Russel M. Filamentous bacteriophages. In: Calendar R, editor. The bacteriophages. Vol. 2. New York, N.Y: Plenum Press; 1988. pp. 375–456. [Google Scholar]
- 24.Ptashne M. A genetic switch. 2nd ed. Cambridge, Mass: Cell Press and Blackwell Scientific Publications; 1992. [Google Scholar]
- 25.Rossomando E F, Zinder N D. Studies on the bacteriophage f1. I. Alkali-induced disassembly of the phage into DNA and protein. J Mol Biol. 1968;36:387–399. doi: 10.1016/0022-2836(68)90163-0. [DOI] [PubMed] [Google Scholar]
- 26.Taylor R K, Miller V L, Furlong D B, Mekalanos J J. The use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci USA. 1987;84:2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waldor M K, Mekalanos J J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 28.Waldor M K, Rubin E J, Pearson G D N, Kimsey H, Mekalanos J J. Regulation, replication, and integration functions of the Vibrio cholerae CTXφ are encoded by region RS2. Mol Microbiol. 1997;24:917–926. doi: 10.1046/j.1365-2958.1997.3911758.x. [DOI] [PubMed] [Google Scholar]
- 29.Working C. Large epidemic of cholera-like disease in Bangladesh caused by Vibrio cholerae O139 synonym Bengal. Lancet. 1993;342:387–390. [PubMed] [Google Scholar]