Abstract
The induction of local T helper type 1 (Th1)-mediated cellular immunity is crucial for resistance of mice to genital infection by the obligate intracellular bacterium Chlamydia trachomatis. We tested the hypothesis that the route of immunization that elicits relatively high numbers of chlamydia-specific, gamma interferon (IFN-γ)-secreting T lymphocytes (ISTLs) in the genital tract would induce optimal protective immunity against reinfection. Female BALB/c mice were infected intravaginally (i.v.), intranasally (i.n.), orally (p.o.), or subcutaneously (s.c.) with C. trachomatis. At days 7, 14, 21, and 28 postinfection, T cells isolated from the genital tract tissues were restimulated with chlamydial antigen in vitro, and the amounts of IFN-γ induced were measured by a sandwiched enzyme-linked immunosorbent assay method. At day 7 postinfection, i.n.- and i.v.-immunized mice had high levels of chlamydia-specific ISTLs in their genital tracts (203.58 ± 68.1 and 225.5 ± 12.1 pg/ml, respectively). However, there were no detectable ISTLs in the genital tracts of p.o.- or s.c.-infected mice. When preinfected mice were challenged i.v. 70 days later, animals preexposed by the i.n. route were highly resistant to reinfection, with greatly reduced chlamydial burden, and suffered an attenuated infection that resolved by day 6 postchallenge. Animals preexposed by the i.v. route were modestly protected, whereas p.o. and s.c. groups were indistinguishable in this regard from control mice. The resistance of i.n.-immunized mice (and to some extent the i.v.-exposed mice) to reinfection was associated with early appearance (within 24 h) of high levels of genital ISTLs compared with mice preinfected by other routes. Furthermore, although i.n. and i.v.-immunized mice had comparable levels of chlamydia-specific immunoglobulin A (IgA) antibodies in their vaginal washes, the levels of IgG2a were four- sixfold higher in i.n.-immunized mice than in any of the other groups. The results suggested that immunization routes that foster rapid induction of vigorous genital mucosal cell-mediated immune (CMI) effectors (e.g., IFN-γ), the CMI-associated humoral effector, IgG2a, and to some extent secretory IgA produce protective immunity against chlamydial genital infection. Therefore, i.n. immunization is a potential delivery route of choice in the development of a vaccine against Chlamydia.
Genital infection by the obligate intracellular bacterium Chlamydia trachomatis is common, and the sequelae of the infection, including pelvic inflammatory disease, ectopic pregnancy, and infertility, have considerable psychological, public health, and economic implications. Epidemiological studies in the United States have revealed a high incidence of infections, approximately 4 million cases per year, and an annual health care cost of $2.18 billion (7). The frequent asymptomatic incidence of chlamydial genital infection is a confounding factor in the management of chlamydial disease because such insidious infections make diagnosis and application of antibiotic chemotherapy relatively late and ineffective to control the sequelae associated with infections. Therefore, a reliable prophylactic measure, such as vaccine administration, has been recommended for controlling Chlamydia (40). However, the design of a vaccine would require a detailed understanding of the pathogenesis and immunobiology of chlamydial disease, including the relevant host immune parameters that control Chlamydia, the mechanisms of chlamydial inhibition, the antigens that elicit protective immunity, and the most effective route for vaccine delivery. The obvious limitations in human experimentation have led to the development of animal models for defining the requirements for developing a protective experimental vaccine from which findings potentially may be extrapolated to humans.
An important goal in controlling the spread of sexually transmitted diseases is the development and administration of protective vaccines that induce local genital tract immune effectors relevant to the control of the appropriate pathogen. This combined requirement is crucial because even the most promising vaccine formulations may fail to establish protective immunity if the route of administration is not optimal for induction of the appropriate local immune responses in the targeted mucosal tissue. In this respect, previous studies have indicated that secretory immunoglobulin A (IgA) and IgG have protective roles during genital chlamydial infection (1–4, 21, 38). However, recent studies using experimental animal models of genital chlamydial disease have clearly established that T-cell-mediated immunity (CMI), usually involving gamma interferon (IFN-γ), is crucial for chlamydial control (5, 8, 13, 14, 16, 18, 29, 30, 33, 34, 36, 37, 41, 44, 45, 49, 52). Additional studies revealed that the resistance of experimental animals to genital reinfection by Chlamydia was associated with the presence of relatively high intensity of antigen-specific T lymphocytes in the genital tract tissue (15). Taken together, the foregoing studies indicated that the most effective vaccine against Chlamydia is likely to be one that elicits a strong local CMI, involving especially chlamydia-specific, IFN-γ-secreting T lymphocytes (ISTLs), in the genital tract.
The available routes of administration of a protective vaccine include systemic and local mucosal delivery. In general, systemic immunization routes do not induce significant antigen-specific, secretory IgA or protective immunity in mucosal tissues (11, 22–24). However, it is becoming clearer that optimal induction of mucosal immunity in general requires targeting antigens to the specialized antigen-presenting cells of mucosa-associated lymphoid tissues (nasal lymphoid tissue [NALT], gut-associated lymphoid tissue, and bronchus-associated lymphoid tissue [25, 51]) or mucosal inductive sites. The essential tenets of the common mucosal immune system are that immune stimulation at one mucosal inductive site can generate immune responses or protective immunity at certain other mucosal effector sites that include the gut, genital tract, buccal cavity, upper respiratory tract (nasal mucosae), and lower respiratory tract (tracheobronchial mucosae) (22, 23). On the basis of this knowledge, experimentally designed, mucosally targeted vaccines have been administered orally (p.o.) or intragastrically, intranasally (i.n.), intrarectally, and intravaginally (i.v.), and the efficacy of each route has been determined by measurement of immune responses or protective immunity against specific pathogens or nominal antigens at mucosal sites of interest (19, 20, 25, 46, 51). In the case of genital chlamydial infection, experimental protective studies of mice revealed that i.n. immunization with either live or acellular vaccine preparation could induce protection against vaginal challenge as assessed by prevention of infertility in exposed animals (26, 27). Although secretory IgA and/or IgG were detected in the vaginal washes of protected subjects in the foregoing and other studies (9, 39, 51), the role of CMI was not investigated. Since CMI is crucial for chlamydial control, we investigated the hypothesis that an immunization route(s) leading to the induction of a relatively high intensity of chlamydia-specific ISTLs in the genital tract tissue would produce protection against challenge infection. The results indicated that protective immunity produced by i.n. exposure of mice to Chlamydia is associated with the rapid induction of ISTLs into genital tract tissues.
MATERIALS AND METHODS
Animals.
Female BALB/c mice (H-2d), 5 to 8 weeks old, were obtained from Harlan-Sprague Dawley (Indianapolis, Ind.). The animals were fed with food and water ad libitum and maintained in laminar flow racks under pathogen-free conditions of 12-h light and 12-h darkness.
Chlamydia stocks and antigens.
Stocks of C. trachomatis agent of mouse pneumonitis (MoPn) for infecting mice in vivo were prepared by propagating elementary bodies (EBs) in McCoy cells as previously described (34). Stocks were titered by infecting McCoy cells with various dilutions of EBs, and the infectious titer was expressed as inclusion-forming units (IFU) per milliliter (34). Chlamydial antigen was prepared by growing MoPn in HeLa cells and purification of the EBs over Renografin gradients, followed by inactivation under UV light for 3 h (6, 12).
Infection protocols.
Mice were infected i.v., i.n., p.o., and subcutaneously (s.c.) with 105 IFU of MoPn per mouse in a volume of 30 μl of phosphate-buffered saline (PBS) while under phenobarbital anesthesia. To ensure the effectiveness of each route of infection, mice in different groups were handled identically, at the same time, and administered equal volumes, equal doses of IFUs, and identical stocks of MoPn. The course of the infection was monitored by periodic (every 3 days) cervicovaginal swabbing of individual animals. Chlamydia was isolated from the swabs in tissue culture according to standard methods, and inclusions were visualized and enumerated by immunofluorescence (32, 34). The mice were monitored for 4 to 6 weeks, a time period that spans the course of MoPn infection in mice (29). Infected mice showed no clinical evidence of overt pathology other than the shedding of chlamydiae in their genital tracts, suggesting that the inoculum was not lethal for the animals. Experiments were repeated to give 10 or 12 animals per experimental group.
Cytokines, monoclonal antibodies, and other reagents.
Enzyme-linked immunosorbent assay (ELISA) kits for quantitating the amounts of murine cytokines in biological and culture fluids were purchased from BioSource International, Camarillo, Calif. Chlamydial isolation from cervicovaginal swabs in tissue culture was assayed by staining infected monolayers of McCoy cells with fluorescein isothiocyanate-labeled, genus-specific antichlamydial antibodies (Kallestad Diagnostics, Chaska, Minn.) to detect chlamydial inclusions by direct immunofluorescence (34).
Preparation of T cells from the genital tracts of infected mice and assessment of amount of IFN-γ secreted into culture supernatants.
Immune T cells were prepared from the genital tract tissues of infected mice by the collagenase digestion method as previously described (15, 17). Briefly, at the indicated time after infection, animals in each group were sacrificed and the genital tract between the vagina and ovaries (i.e., the cervix, uterus, and fallopian tubes) was excised and placed in sterile HEPES-buffered RPMI 1640 culture medium (Atlanta Biologicals, Norcross, Ga.). Explants were transferred to 7 ml of filter-sterilized type I collagenase (0.6 mg/ml; Atlanta Biologicals). The tissues were minced with surgical scalpel blades, incubated at 37°C for 45 to 60 min, then teased with forceps, and passed through a cell strainer. Following washing, the cells were enriched for T cells by the nylon wool adherence method as previously described (14, 15). Purified genital tract cells contained at least 97% CD3+ cells, as determined by fluorescence-activated cell sorting analysis.
The level of response of chlamydia-specific ISTLs induced in genital tissues was assessed by seeding purified T cells into 96-well tissue culture plates (Costar, Cambridge, Mass.) at 2 × 105 cells per well, in the presence or absence of UV-inactivated MoPn EBs as antigen. After 5 days of incubation in humidified incubators at 37°C and 5% CO2, the supernatants were collected and stored at −70°C until assayed for IFN-γ content by a quantitative ELISA procedure. It was previously shown that IFN-γ derived from culture by this procedure possesses biological activity as determined by the ability of IFN-γ-containing supernatants to protect L929 cells from infection by encephalomyocarditis virus (14).
The amounts of IFN-γ contained in supernatants derived from culture-stimulated cells and controls were measured by using a commercial ELISA kit (Cytoscreen immunoassay kit; BioSource) according to the supplier’s instructions. The concentration of the cytokine in each sample was obtained by extrapolation from a standard calibration curve generated simultaneously. Data were calculated as the mean values (± standard deviation) of triplicate cultures for each experiment. The results were derived from at least three independent experiments.
Quantitation of chlamydia-specific secretory IgA and IgG2a in vaginal washes.
Vaginal washes were performed at different times after challenge of preinfected mice with 250 μl of PBS as previously described (32) and stored at −70°C until assayed. The levels of secretory IgA and IgG2a antibodies in vaginal washes were measured by a modified standard ELISA procedure as previously described (26, 27). Briefly, Maxisorb 96-well plates (Costar) were coated overnight with MoPn EBs (10 μg/ml) in 100 μl of PBS at 4°C. For generating a standard calibration curve, wells were similarly coated in triplicate with IgA or IgG2a standard (0.0, 12.5, 25, 50, 100, 250, 500, and 1,000 ng/ml). The plates were washed with PBS containing 0.05% Tween 20 and blocked with 1% bovine serum albumin–5% goat serum in PBS for 1 h at room temperature. After washing, 50 μl of twofold serially diluted vaginal washes was added to wells containing EBs and incubated for 2 h at room temperature. Control wells contained the buffer used for vaginal washing. Another washing step was followed by addition of 100 μl of horseradish peroxidase-conjugated goat anti-mouse IgA or IgG2a for 1 h at room temperature. A final washing step was followed by the addition to each well of 200 μl of substrate (o-phenylenediamine), incubation in the dark for 30 min, and addition of 50 μl of H2SO4 to stop the reaction. The absorbance associated with color development was measured at 492-nm wavelength in a Microplate Autoreader spectrophotometer (Bio-tex Instruments, Inc., Winooski, Vt.). Results represent the mean of triplicate wells for each set of samples obtained from different experiments.
Statistical analysis.
The levels of IFN-γ, IgA, and IgG2a in samples from different experiments were analyzed and compared by performing a one- or two-tailed t test, and the relationship between different experimental groupings was assessed by analysis of variance. Minimal statistical significance was judged at P < 0.05.
RESULTS
Induction of ISTLs after primary i.v., i.n., p.o., or s.c. infection.
Initially, to test our hypothesis regarding the relationship between local genital tract ISTLs and immunity, we investigated the kinetics of recruitment of chlamydia-specific ISTLs into the genital mucosa following i.v., i.n., p.o., and s.c. inoculation of MoPn into BALB/c female mice. As presented in Table 1, i.v. and i.n. infection resulted in high levels of ISTLs in the genital mucosae of mice by 7 days postinfection (225.50 [i.v.] and 203.58 [i.n.] pg/ml). IFN-γ secretion by ISTLs peaked by 14 days after i.v. infection and 7 days after i.n. infection. On the other hand, no significant IFN-γ was measured in cultures containing T cells derived from the genital tract tissues of mice infected p.o. or s.c. throughout the 4 weeks of the studies (Table 1). Similarly, genital T cells from uninfected mice or from infected mice that were not restimulated with chlamydial antigen, stimulated with an irrelevant antigen (bovine serum albumin) or HeLa cell cultures that were not infected with MoPn, did not secrete detectable IFN-γ in response to chlamydial antigen (data not shown). The results suggested that i.v. and i.n. immunization represent two mucosal routes of inoculation that result in induction of ISTLs into the genital mucosa site, and immunization via these routes is likely to support the induction of protective immunity against genital chlamydial infection.
TABLE 1.
IFN-γ secreted by MoPn-specific T cells from the genital tracts of BALB/c mice infected with MoPn by different routes
| Day postinfection | Mean IFN-γ concn (pg/ml) ± SEMa
|
|
|---|---|---|
| i.v. | i.n. | |
| 7 | 225.50 ± 12.1 | 203.58 ± 68.1 |
| 14 | 330.70 ± 92.1 | 121.90 ± 9.3 |
| 21 | 235.80 ± 6.4 | 9.10 ± 9.8 |
| 28 | 25.64 ± 5.4 | 4.56 ± 1.20 |
Nylon wool-purified genital T cells were isolated at the indicated time points from mice infected by different routes. The cells were stimulated with chlamydial antigen for 5 days, and the amounts of IFN-γ in the culture supernatants were measured by ELISA as described in Materials and Methods. No IFN-γ was detected in samples from p.o.- and s.c.-immunized mice.
Course of chlamydial genital disease in mice preinfected by the i.v., i.n., p.o., or s.c. route.
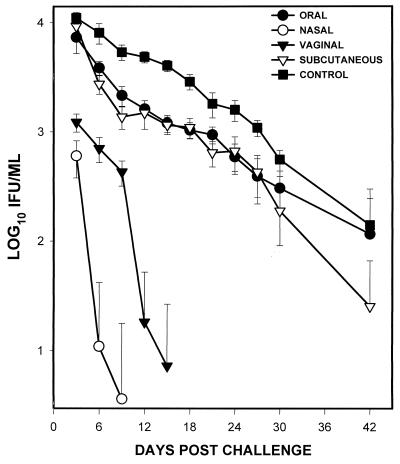
To directly test the hypothesis that the route of infection that fosters recruitment of ISTLs into the genital tract is likely to produce local immunity against chlamydial genital infection, mice were exposed to MoPn by the i.v., i.n., p.o., or s.c. route and then challenged i.v. 70 days postinfection. It was previously demonstrated that animals become susceptible to reinfection by this time after a primary genital chlamydial infection (29). The course of the infection was followed by isolation of live MoPn from cervicovaginal swabs as described in Materials and Methods. Figure 1 shows that the i.n.-preinfected group suffered attenuated genital disease upon challenge, with at least 1-log-lower chlamydial burden (IFU/milliliter) than the control group by day 3, and the infection was essentially resolved by day 6 postchallenge. Compared to the i.n.-preinfected group, the i.v.-preinfected mice suffered a higher chlamydial burden that resolved by day 12 postchallenge. However, p.o.- and s.c.-preinfected mice exhibited a course of challenged infection essentially indistinguishable from that in control (naive) mice. The results indicated that i.n. immunization is superior to i.v., p.o., or s.c. immunization for the induction of long-term genital immunity against Chlamydia in mice.
FIG. 1.
Course of chlamydial genital disease in mice preinfected by various routes. Female BALB/c mice were infected with MoPn by the indicated routes and challenged i.v. 70 days later. The course of the infection was monitored by periodic (every 3 days) cervicovaginal swabbing of individual animals. Chlamydia was isolated from the swabs in tissue culture according to standard methods, and inclusions were visualized and enumerated by immunofluorescence (32, 34). Experiments were repeated two times to obtain data for 10 or 12 animals per group.
Induction of ISTLs after vaginal challenge.
It might be inferred that the ability to induce genital mucosal ISTLs after i.n. infection was responsible for the attenuated genital infection in the protected mice. However, it was important to experimentally demonstrate that the efficacy of i.n. preinfection in protecting mice from prolonged disease after a challenged infection is directly related to the relative capacity to foster the rapid recruitment of ISTLs into the genital mucosae. This is because the presence of ISTLs in the genital tract appeared to have waned with time after exposure (Table 1), and it is not clear whether long-term memory was preserved in NALT or other mucosal inductive site after i.n. infection. To investigate whether i.v. challenge could stimulate the rapid recruitment of ISTLs into the genital mucosae after previous i.n. exposure, we studied the detailed kinetics of ISTL induction into the genital mucosae after challenge. Mice preinfected by the i.v., i.n., p.o., or s.c. route were challenged on day 70; 24, 48, 72, 144, 288, and 360 h postchallenge, the levels of IFN-γ secretion by ISTLs in the genital tract tissues were assessed as previously described. Table 2 reveals that within 24 h of challenge of the i.n.-preinfected group, significant ISTLs were present in the genital tract of each mouse. Within the first 6 days postchallenge, there was two- to fourfold-higher intensity of ISTLs (assessed by IFN-γ secretion) in the i.n.-preinfected group than in the i.v.-preinfected group. The p.o.- and s.c.-preinfected groups exhibited ISTL profiles similar to those mice that received primary genital infection of MoPn (compare Tables 1 and 2). The results indicated that the attenuated genital chlamydial disease in i.n.-preinfected mice may be due to the rapid recruitment of ISTLs into the genital tract after challenge.
TABLE 2.
IFN-γ secreted by MoPn-specific T cells from genital tracts of BALB/c mice preinfected with MoPn by different routes and then challenged i.v.
| Time (h) postchal- lenge | Mean IFN-γ concn (pg/ml) ± SEMa
|
|||
|---|---|---|---|---|
| p.o. | i.v. | i.n. | s.c. | |
| 24 | 0.0 ± 0.0 | 25.50 ± 6.2 | 88.50 ± 6.1 | 0.0 ± 0.0 |
| 48 | 6.0 ± 2.0 | 50.20 ± 18.1 | 160.21 ± 9.3 | 2.0 ± 0.40 |
| 72 | 14.0 ± 2.0 | 89.80 ± 16.4 | 364.12 ± 58.8 | 10.0 ± 2.0 |
| 144 | 196.0 ± 23.0 | 125.64 ± 25.4 | 256.6 ± 18.20 | 26.0 ± 3.0 |
| 288 | 140.0 ± 20.6 | 288.74 ± 22.0 | 240.46 ± 26.0 | 134.0 ± 21.6 |
| 360 | 196.0 ± 23.0 | 322.24 ± 32.0 | 202.24 ± 42.5 | 296.0 ± 15.0 |
Nylon wool-purified genital T cells were isolated at the indicated time points from mice infected by different routes. The cells were stimulated with chlamydial antigen for 5 days, and the amounts of IFN-γ in the culture supernatants were measured by ELISA as described in Materials and Methods.
Induction of chlamydia-specific secretory IgA and IgG2a after challenge.
Previous studies have indicated that secretory IgA and IgG have protective roles during genital chlamydial infection (2–4, 21, 35, 38), although a recent report showed that vaginal secretory IgA could not protect IFN-γ receptor knockout mice from overwhelming genital chlamydial disease (16). To investigate the levels of chlamydia-specific IgA and IgG2a induced into the genital tract after challenge of mice previously exposed to MoPn, the vaginal washes were assayed by a quantitative chlamydia-specific ELISA procedure as previously described (26, 27). IgG2a was measured because of its association with T-cell responses mediated by Th1 cells (42), which is relevant to chlamydial immunity. Table 3 shows that i.n.- and i.v.-preinfected groups had comparable levels of MoPn-specific IgA in their vaginal washes during the first 3 weeks following the challenge infection (i.e., 4.68, 16.20, and 19.84 [i.n.] and 5.4, 15.32, and 14.80 [i.v.] ng/ml by days 7, 14, and 21 postchallenge, respectively). However, the levels of IgA in the p.o.- and s.c.-preinfected groups were significantly lower during the same time points (i.e., 3.34, 2.58, and 2.29 [p.o.] and 0.6, 0.94, and 7.08 [s.c.] ng/ml by days 7, 14, and 21 postchallenge, respectively) (P > 0.001). On the other hand, whereas there was no significant difference between the levels of IgG2a in the genital washes from the i.v.- and p.o.-preinfected groups during the first 2 weeks following challenge (i.e., 5.96 and 6.80 [i.v.] and 6.85 and 6.24 [p.o.] ng/ml by days 7 and 14 postchallenge, respectively) (P > 0.340), the levels of IgG2a in the genital washes from the i.n.-preinfected group were four- to sixfold higher than for any of the other groups during the same period (Table 4). The results suggested that immunization routes leading to the induction of protective immunity against chlamydial genital infection foster enhanced induction of genital mucosal CMI effectors (e.g., IFN-γ), the CMI-associated humoral effector, IgG2a, and to a limited extent secretory IgA.
TABLE 3.
Secretory IgA antibodies in the genital tract after i.v. challenge of preexposed BALB/c mice with MoPn
| Day postchallenge | Mean IgA antibody concn (ng/ml) ± SEMa
|
|||
|---|---|---|---|---|
| p.o. | i.v. | i.n. | s.c. | |
| 7 | 3.34 ± 0.3 | 5.40 ± 0.4 | 4.68 ± 0.2 | 0.60 ± 1.2 |
| 14 | 2.58 ± 0.6 | 15.32 ± 1.1 | 16.20 ± 1.2 | 0.94 ± 1.8 |
| 21 | 2.29 ± 1.6 | 14.80 ± 1.0 | 19.84 ± 2.6 | 7.08 ± 2.2 |
Female BALB/c mice were infected with MoPn by the indicated routes and then challenged i.v. 70 days later. Vaginal washes were performed at different times after challenge of preinfected mice with 250 μl of PBS as previously described (32) and stored at −70°C until assayed. The levels of chlamydia-specific secretory IgA antibodies in vaginal washes were measured by a standard ELISA procedure as previously described elsewhere (26, 27). Results represent the means of triplicate wells for each set of samples obtained from different experiments. Experiments were repeated two times to obtain data for 10 or 12 animals per group.
TABLE 4.
Secretory IgG2a in the genital tract after i.v. challenge of preexposed BALB/c mice with MoPn
| Day postchallenge | Mean IgG2a antibody concn (ng/ml) ± SEMa
|
|||
|---|---|---|---|---|
| p.o. | i.v. | i.n. | s.c. | |
| 7 | 6.85 ± 1.8 | 5.96 ± 0.4 | 26.00 ± 1.9 | 3.46 ± 0.7 |
| 14 | 6.24 ± 0.6 | 6.80 ± 0.34 | 35.53 ± 5.6 | 3.96 ± 0.61 |
| 21 | 3.71 ± 0.1 | 7.53 ± 1.2 | 55.28 ± 11.4 | 17.53 ± 2.8 |
Female BALB/c mice were infected with MoPn by the indicated route and then challenged i.v. 70 days later. Vaginal washes were performed at different times after challenge of preinfected mice with 250 μl of PBS as previously described (32) and stored at −70°C until assayed. The levels of chlamydia-specific secretory IgG2a antibodies in vaginal washes were measured by a modified standard ELISA procedure as previously described (26, 27). Results represent the mean of triplicate wells for each set of samples obtained from different experiments. Experiments were repeated two times to obtain data for 10 or 12 animals per group.
DISCUSSION
The requirements for developing an effective vaccine against chlamydial genital disease include the identification of the appropriate antigen(s) that elicits long-term protective immunity and the selection of a suitable route for vaccine delivery. Although the mucosal route of immunization is likely to foster the induction of appropriate immune effectors against genitally acquired infectious diseases such as chlamydial infection, the concept of compartmentalization within the common mucosal immune system (25, 51) imposes limitation on the number of mucosal inductive sites available for immunizing against different infections. It is therefore necessary to examine the different delivery routes available to determine the route that would promote the induction of effective immune effectors to a desired mucosal site. In this respect, it has become established by studies using experimental animal models of genital chlamydial disease that CMI, usually involving IFN-γ secretion, is crucial for chlamydial control (5, 8, 13, 14, 16, 18, 29, 30, 33, 34, 36, 37, 41, 44, 45, 49, 52). Additional studies have revealed that the resistance of experimental animals to genital reinfection by Chlamydia was associated with the presence of relatively high intensity of antigen-specific T lymphocytes in the genital tract tissue (15). This finding indicated that a potentially effective vaccine against genital chlamydial disease should elicit critical numbers of ISTLs in the genital mucosae. The present study attempted to identify the route of chlamydial inoculation that would foster the induction of relatively high intensity of chlamydia-specific ISTLs in the genital tracts of infected animals. The i.n. route of immunization was more effective at protecting mice from vaginal challenge by Chlamydia than either the p.o., i.v., or s.c. route. The efficacy of i.n. immunization against Chlamydia is its ability to rapidly induce ISTLs into the genital mucosae. It was previously reported that i.n. immunization causes rapid generation of effector lymphocytes detectable within 2 days (50) and was superior to vaginal, gastric, peritoneal, or rectal immunization for the induction of mucosal anti-human immunodeficiency virus or anti-herpes simplex virus antibody responses (10, 43). Our findings appear to provide a cellular and molecular immunologic explanation for previous reports showing that i.n. immunization with live Chlamydia or an acellular outer membrane complex preparation protected mice against genital chlamydial disease (26–28). In terms of compartmentalization of the common mucosal immune system, it would appear that there is a strong link between NALT and genital mucosal effector site, as previously suggested (51).
The relatively reduced capacity of i.v. inoculation to induce mucosal immune responses may be explained by its lack of an organized mucosal inductive site (51). However, the failure of p.o. inoculation may be due to its tendency to promote humoral immune responses (22) that are ineffective against Chlamydia (29). Although i.v. immunization was less effective than i.n. immunization at inducing protective immunity, the former was more effective than p.o. or s.c. immunization at either inducing ISTLs or protecting mice from prolonged challenge infection. The ineffectiveness of p.o. immunization in these studies was not surprising because it has now been established that chlamydial control in mice is T-cell mediated, requiring CD4+ Th1 cells. These results are therefore in agreement with findings by others (19, 47) that i.v. administration of antigen was more effective than p.o. administration for generating local production of specific IgA and IgG in the cervices and vaginas of women.
Although the secretory IgA levels were comparable between i.n. and i.v. groups, there was an earlier and greater accumulation of IgG2a in the i.n. group than either the i.v. group or other groups. The association of IgG2a with T-cell immunity involving IFN-γ (42) and its early appearance at relatively high levels may suggest that it was involved in the attenuated infection suffered by i.n.-immunized mice. In addition, since vaginal secretory IgA could not protect IFN-γ receptor knockout mice from overwhelming genital chlamydial disease (16), these studies may provide a cellular and molecular basis for the effectiveness of i.n. immunization against genital chlamydial infection.
The induction of long-term protective immunity is a major challenge in chlamydial vaccine development. While these studies and others (26–28) reveal that i.n. immunization can enhance genital immunity against chlamydial infection, it is unclear whether the immunity is long lasting. The mucosal immune response to a vaccine can be affected by many factors, including the antigen, vector, adjuvant, route of delivery, and hormones associated with the estrous cycle (for genital mucosal response) (31, 48). Of particular importance to chlamydial genital infection are the factors that regulate the persistence of immune effectors at the genital mucosal site and so foster long-term genital mucosal immunity. The natures of such factors are presently unknown, but they will be important for the persistence of ISTLs in the genital mucosae and so will potentially regulate the expression and functions of certain adhesion molecules, including the intercellular adhesion molecules. A detailed knowledge of these factors is central to achieving long-term immunity against reinfection by Chlamydia.
Ongoing studies are attempting to clone ISTLs from the genital tracts of i.n.-infected mice. Such clones may recognize crucial protective epitopes on chlamydial proteins. The identification, mapping, and characterization of such protective epitopes may furnish valuable reagents for designing an experimental vaccine against Chlamydia.
ACKNOWLEDGMENTS
This study was supported by PHS grants AI41231 and RR03034 from the National Institutes of Health.
We thank Harlan Caldwell, Linda Perry, and Gordon B. Bailey for critiques and excellent suggestions.
REFERENCES
- 1.Barron A L, Rank R G, Moses E B. Immune response in mice infected in the genital tract with mouse pneumonitis agent (Chlamydia trachomatis biovar) Infect Immun. 1984;44:82–85. doi: 10.1128/iai.44.1.82-85.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batteiger B E, Rank R G. Analysis of the humoral immune response in chlamydial genital infection in guinea pigs. Infect Immun. 1987;55:1767–1773. doi: 10.1128/iai.55.8.1767-1773.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunham R C, Kuo C-C, Cles L, Holmes K K. Correlation of host immune response with quantitative recovery of Chlamydia trachomatis from the human endocervix. Infect Immun. 1983;39:1491–1494. doi: 10.1128/iai.39.3.1491-1494.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunham R C, Peeling R, Maclean I, McDowell J, Persson K, Osser S. Postabortal Chlamydia trachomatis salpingitis: correlating risk with antigen-specific serological responses and with neutralization. J Infect Dis. 1987;155:749–755. doi: 10.1093/infdis/155.4.749. [DOI] [PubMed] [Google Scholar]
- 5.Byrne G I, Krueger D A. Lymphokine-mediated inhibition of Chlamydia replication in mouse fibroblasts is neutralized by anti-gamma interferon immunoglobulin. Infect Immun. 1983;42:1152–1158. doi: 10.1128/iai.42.3.1152-1158.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caldwell H D, Schachter J. Antigenic analysis of the major outer membrane protein of Chlamydia spp. Infect Immun. 1982;35:1024–1031. doi: 10.1128/iai.35.3.1024-1031.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Chlamydia trachomatis genital infections—United States, 1995. Morbid Mortal Weekly Rep. 1997;46:193–198. [PubMed] [Google Scholar]
- 8.Cotter T W, Ramsey K H, Miranpuri G S, Poulsen C E, Byrne G I. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect Immun. 1997;65:2145–2152. doi: 10.1128/iai.65.6.2145-2152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallichan W S. Specific secretory immune responses in the female genital tract following intranasal immunization with a recombinant adenovirus expressing glycoprotein B of herpes simplex virus. Vaccine. 1995;13:1589–1595. doi: 10.1016/0264-410x(95)00100-f. [DOI] [PubMed] [Google Scholar]
- 10.Gallichan W S, Rosenthal K L. Specific secretory immune responses in the female genital tract following intranasal immunization with a recombinant adenovirus expressing glycoprotein B of herpes simplex virus. Vaccine. 1995;13:1589–1595. doi: 10.1016/0264-410x(95)00100-f. [DOI] [PubMed] [Google Scholar]
- 11.Holmgren J, Czerkinsky C, Lycke N, Svennerholm A-M. Mucosal immunity: implications for vaccine development. Immunobiology. 1992;184:157–179. doi: 10.1016/S0171-2985(11)80473-0. [DOI] [PubMed] [Google Scholar]
- 12.Huss H, Jungkind D A, Amadio A P, Rubenfeld I. Frequency of Chlamydia trachomatis as the cause of pharyngitis. J Clin Microbiol. 1985;22:858–860. doi: 10.1128/jcm.22.5.858-860.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Igietseme J U, Magee D M, Williams D M, Rank R G. Role for CD8+ T cells in antichlamydial immunity defined by chlamydia-specific T-lymphocyte clones. Infect Immun. 1994;62:5195–5197. doi: 10.1128/iai.62.11.5195-5197.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Igietseme J U, Ramsey K H, Magee D M, Williams D M, Kincy T J, Rank R G. Resolution of murine chlamydial genital infection by the adoptive transfer of a biovar-specific, Th1 lymphocyte clone. Reg Immunol. 1993;5:317–324. [PubMed] [Google Scholar]
- 15.Igietseme J U, Rank R G. Susceptibility to reinfection after a primary chlamydial genital infection is associated with a decrease of antigen-specific T cells in the genital tract. Infect Immun. 1991;59:1346–1351. doi: 10.1128/iai.59.4.1346-1351.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansson M, Schon K, Ward M, Lycke N. Genital tract infection with Chlamydia trachomatis fails to induce protective immunity in gamma interferon receptor-deficient mice despite a strong local immunoglobulin A response. Infect Immun. 1997;65:1032–1044. doi: 10.1128/iai.65.3.1032-1044.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kincy-Cain T J, Rank R G. Local Th1-like responses are induced by intravaginal infection of mice with the mouse pneumonitis (MoPn) biovar of Chlamydia trachomatis. Infect Immun. 1995;63:1784–1789. doi: 10.1128/iai.63.5.1784-1789.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knight S C, Iqball S, Woods C, Stagg A, Ward M E, Tuffrey M. A peptide of Chlamydia trachomatis shown to be a primary T-cell epitope in vitro induces cell-mediated immunity in vivo. Immunology. 1995;85:8–15. [PMC free article] [PubMed] [Google Scholar]
- 19.Kozlowski P A, Cu-Uvin S, Neutra M R, Flanigan T P. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infect Immun. 1997;65:1387–1394. doi: 10.1128/iai.65.4.1387-1394.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marx P A, Compans R W, Gettie A, Staas J K, Gilley R M, Mulligan M J, Yamshchikov G V, Chen D, Eldridge J H. Protection against vaginal SIV transmission with microencapsulated vaccine. Science. 1993;260:1323–1327. doi: 10.1126/science.8493576. [DOI] [PubMed] [Google Scholar]
- 21.McComb D E, Nichols R L, Semine D Z, Evrard J R, Alpert S, Crockett V A, Rosner B, Zinner S H, McCormack W M. Chlamydia trachomatis in women: antibody in cervical secretions as a possible indicator of genital infection. J Infect Dis. 1979;139:628–633. doi: 10.1093/infdis/139.6.628. [DOI] [PubMed] [Google Scholar]
- 22.McGhee J R, Mestecky J, Dertzbaugh M T, Eldridge J H, Hirasawa M, Kiyono H. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine. 1992;10:75–88. doi: 10.1016/0264-410x(92)90021-b. [DOI] [PubMed] [Google Scholar]
- 23.Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987;7:265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- 24.Mestecky J, Jackson S. Reassessment of the impact of mucosal immunity in infection with the human immunodeficiency virus (HIV) and design of relevant vaccines. J Clin Immunol. 1994;14:259–272. doi: 10.1007/BF01540979. [DOI] [PubMed] [Google Scholar]
- 25.Moldoveanu Z, Russell M W, Wu H-Y, Huang W-Q, Compans R W, Mestecky J. Compartmentalization within the common mucosal immune system. Adv Exp Med Biol. 1995;371:97–101. doi: 10.1007/978-1-4615-1941-6_17. [DOI] [PubMed] [Google Scholar]
- 26.Pal S, Fielder T J, Peterson E M, de la Maza L M. Protection against infertility in a BALB/c mouse salpingitis model by intranasal immunization with a mouse pneumonitis biovar of Chlamydia trachomatis. Infect Immun. 1994;62:3354–3362. doi: 10.1128/iai.62.8.3354-3362.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pal S, Peterson E M, de la Maza L M. Intranasal immunization induces long-term protection in mice against a Chlamydia trachomatis genital challenge. Infect Immun. 1996;64:5341–5348. doi: 10.1128/iai.64.12.5341-5348.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pal S, Theodor I, Peterson E M, de la Maza L M. Immunization with an acellular vaccine consisting of the outer membrane complex of Chlamydia trachomatis induces protection against a genital challenge. Infect Immun. 1997;65:3361–3369. doi: 10.1128/iai.65.8.3361-3369.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patton D L, Rank R G. Animal models for the study of pelvic inflammatory disease. In: Quinn T C, editor. Sexually transmitted diseases. New York, N.Y: Raven Press, Ltd.; 1992. pp. 85–111. [Google Scholar]
- 30.Perry L L, Feilzer K, Caldwell H D. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J Immunol. 1997;158:3344–3352. [PubMed] [Google Scholar]
- 31.Prabhala R H, Wira C R. Sex hormone and IL-6 regulation of antigen presentation in the female reproductive mucosal tissues. J Immunol. 1995;155:5566–5573. [PubMed] [Google Scholar]
- 32.Ramsey K H, Newhall W J, Rank R G. Humoral immune response to chlamydial genital infection of mice with the agent of mouse peritonitis. Infect Immun. 1989;57:2441–2446. doi: 10.1128/iai.57.8.2441-2446.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramsey K H, Rank R G. Resolution of chlamydial genital infection with antigen-specific T-lymphocyte lines. Infect Immun. 1991;59:925–931. doi: 10.1128/iai.59.3.925-931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramsey K H, Soderberg L S F, Rank R G. Resolution of chlamydial genital infection in B-cell-deficient mice and immunity to reinfection. Infect Immun. 1988;56:1320–1325. doi: 10.1128/iai.56.5.1320-1325.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rank R G, Batteiger B E. Protective role of serum antibody in immunity to chlamydial genital infection. Infect Immun. 1989;57:299–301. doi: 10.1128/iai.57.1.299-301.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rank R G, Ramsey K H, Pack E A, Williams D M. Effect of gamma interferon on resolution of murine chlamydial genital infection. Infect Immun. 1992;60:4427–4429. doi: 10.1128/iai.60.10.4427-4429.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rank R G, White H J, Barron A L. Humoral immunity in the resolution of genital infection in female guinea pigs infected with the agent of guinea pig inclusion conjunctivitis. Infect Immun. 1979;26:573–579. doi: 10.1128/iai.26.2.573-579.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richmond S J, Milne J D, Hilton A L, Caul E O. Antibodies to Chlamydia trachomatis in cervicovaginal secretions: relation to serum antibodies and current chlamydial infection. Sex Transm Dis. 1980;7:11–15. doi: 10.1097/00007435-198001000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Russel M W, Moldoveanu Z, White P L, Sibert G J, Mestecky J, Michalek S M. Salivary, nasal, genital, and systemic antibody responses in monkeys immunized intranasally with a bacterial protein antigen and the cholera toxin B subunit. Infect Immun. 1996;64:1272–1283. doi: 10.1128/iai.64.4.1272-1283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schachter J. Overview of Chlamydia trachomatis infection and the requirements for a vaccine. Rev Infect Dis. 1985;7:713–716. doi: 10.1093/clinids/7.6.713. [DOI] [PubMed] [Google Scholar]
- 41.Shemer Y, Sarov I. Inhibition of growth of Chlamydia trachomatis by human gamma interferon. Infect Immun. 1985;48:592–596. doi: 10.1128/iai.48.2.592-596.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snapper C M, Paul W E. Interferon-γ and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 43.Staats H F, Montgomery S P, Palker T J. Intranasal immunization is superior to vaginal, gastric, or rectal immunization for induction of systemic and mucosal anti-HIV antibody responses. AIDS Res Hum Retroviruses. 1997;13:945–952. doi: 10.1089/aid.1997.13.945. [DOI] [PubMed] [Google Scholar]
- 44.Starnbach M N, Bevan M J, Lampe M F. Protective cytotoxic T lymphocytes are induced during murine infection with Chlamydia trachomatis. J Immunol. 1994;153:5183–5189. [PubMed] [Google Scholar]
- 45.Tuffrey M, Falder P, Taylor-Robinson D. Reinfection of the mouse genital tract with Chlamydia trachomatis: the relationship of antibody to immunity. Br J Exp Pathol. 1984;65:51–58. [PMC free article] [PubMed] [Google Scholar]
- 46.Walker R I. New strategies for using mucosal vaccination to achieve more effective immunization. Vaccine. 1994;12:387–400. doi: 10.1016/0264-410x(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 47.Wassen L, Schon K, Holmgren J, Jertborn M, Lycke N. Local intravaginal vaccination of the female genital tract. Scand J Immunol. 1996;44:408–414. doi: 10.1046/j.1365-3083.1996.d01-320.x. [DOI] [PubMed] [Google Scholar]
- 48.Wira C R, O’Mara B, Richardson J, Prabhala R. The mucosal immune system in the female reproductive tract: influence of sex hormones and cytokines on immune recognition and responses to antigen. Vaccine Res. 1992;1:151–167. [Google Scholar]
- 49.Woods M L, Mayer J, Evans T G, Hibbs J B., Jr Antiparasitic effects of nitric oxide in an in vitro murine model of Chlamydia trachomatis infection and an in vivo model of Leishmania major infection. Immunol Ser. 1994;60:179–195. [PubMed] [Google Scholar]
- 50.Wu H-Y, Nikolova E B, Beagley K W, Russell M W. Induction of antibody-secreting cells and T helper and memory cells in murine nasal lymphoid tissue. Immunology. 1996;88:493–500. doi: 10.1046/j.1365-2567.1996.d01-690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu H-Y, Russell M W. Nasal lymphoid tissue, intranasal immunization, and compartmentalization of the common mucosal immune system. Immunol Res. 1997;16:187–201. doi: 10.1007/BF02786362. [DOI] [PubMed] [Google Scholar]
- 52.Zhong G, Peterson E M, Czarniecki C W, Schreiber R D, de la Maza L M. Role of endogenous gamma interferon in host defense against Chlamydia trachomatis infections. Infect Immun. 1989;57:152–157. doi: 10.1128/iai.57.1.152-157.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]