Abstract
Diffusely adhering Escherichia coli (DAEC) C1845 (clinical isolate) harboring the fimbrial adhesin F1845 can infect cultured human differentiated intestinal epithelial cells; this process is followed by the disassembly of the actin network in the apical domain. The aim of this study was to examine the mechanism by which DAEC C1845 promotes F-actin rearrangements. For this purpose, we used a human embryonic intestinal cell line (INT407) expressing the membrane-associated glycosylphosphatidylinositol (GPI) protein-anchored decay-accelerating factor (DAF), the receptor of the F1845 adhesin. We show here that infection of INT407 cells by DAEC C1845 can provoke dramatic F-actin rearrangements without cell entry. Clustering of phosphotyrosines was observed, revealing that the DAEC C1845-DAF interaction involves the recruitment of signal transduction molecules. A pharmacological approach with a subset of inhibitors of signal transduction molecules was used to identify the cascade of signal transduction molecules that are coupled to the DAF, that are activated upon infection, and that promote the F-actin rearrangements. DAEC C1845-induced F-actin rearrangements can be blocked dose dependently by protein tyrosine kinase, phospholipase Cγ, phosphatidylinositol 3-kinase, protein kinase C, and Ca2+ inhibitors. F-actin rearrangements and blocking by inhibitors were observed after infection of the cells with two E. coli recombinants carrying the plasmids containing the fimbrial adhesin F1845 or the fimbrial hemagglutinin Dr, belonging to the same family of adhesins. These findings show that the DAEC Dr family of pathogens promotes alterations in the intestinal cell cytoskeleton by piracy of the DAF-GPI signal cascade without bacterial cell entry.
Structural changes in the cytoskeleton of eukaryotic host cells have been extensively documented during the past 6 years by examination of the postattachment invasion stage of enterovirulent microorganisms (11). Diffusely adhering Escherichia coli (DAEC) is a pathogenic organism that adheres to host cells. As has been recently reported, DAEC C1845 expressing the fimbrial adhesin F1845 (5, 6) (i) infects cultured, fully differentiated human intestinal cells (16, 17); (ii) interacts with the brush border-associated decay-accelerating factor (DAF), inducing dramatic changes in the architecture of the microvilli (MV) (limited to the point of bacterial contact with the MV, showing disruption of the tip of the MV and then nucleation) (2); and (iii) induces apical F-actin disorganization (2). These morphological alterations in the host cells suggest that the pathogen signals the host cells. The observation that F-actin rearrangements occur after the attachment of DAEC C1845 to the brush border-associated DAF suggests that a transducing signal coupled to the DAF and linked to the host cell cytoskeleton could be activated. This hypothesis is consistent with the fact that the human DAF is a 70- to 75-kDa membrane-associated glycosylphosphatidylinositol (GPI)-anchored protein able to transduce signals (19, 20). We decided to examine how the interaction of DAEC C1845 expressing the F1845 adhesin with the DAF in human intestinal cells leads to the disorganization of the actin network.
Enteropathogenic Escherichia coli induces attaching-effacing lesions after the intimate attachment stage following the initial adherence stage in the brush border of enterocytes. Enteropathogenic E. coli-induced cytoskeletal protein rearrangements and signal transduction have been examined with nonpolarized HEp-2 cells (for a review, see references 10 and 11). We have chosen the same strategy to examine DAEC C1845-induced F-actin rearrangements and signal transduction by using human nonpolarized undifferentiated INT407 intestinal cells (13) expressing the DAF (3) and possessing a single F-actin network organized in stress fibers. We show that cytoskeletal F-actin rearrangements can be provoked upon DAEC C1845 infection, demonstrating that the membrane-associated GPI-anchored DAF can participate in the reorganization of the host cytoskeleton after the infecting pathogen stimulates its coupled signal transduction.
MATERIALS AND METHODS
Cell culture.
Human embryonic intestinal cell line INT407 was isolated from the jejunum and ileum of a human embryo at about 2 months of gestation (13). The cells were cultured as a monolayer to confluence for 5 days (in culture flasks or on coverslips) in Eagle’s basal medium supplemented with 10% newborn calf serum, 100 IU each of penicillin and streptomycin per ml, 2 μg of amphotericin B per ml, and 1% nonessential amino acids. The cells were kept at 37°C in a 5% CO2–95% air atmosphere.
Bacterial strains.
DAEC C1845 harboring the fimbrial adhesin F1845 (5, 6) was stored in CFA-glycerin at −80°C. The strain was grown on CFA agar containing 1% Casamino Acids (Difco Laboratories, Detroit, Mich.), 0.15% yeast extract, 0.005% magnesium sulfate, and 0.0005% manganese chloride in 2% agar for 18 h at 37°C. The laboratory strain E. coli HB101 transformed with plasmid pSSS1 producing the F1845 adhesin (5) was grown at 37°C for 18 h on Luria agar. The laboratory strain E. coli K-12 EC901 carrying recombinant plasmid pBNJ406 expressing the Dr hemagglutinin (22) was grown at 37°C for 18 h on Luria agar. E. coli HB101 was used as a control. Bacterial cells were collected from the plates, and a washed suspension of the cells was made with phosphate-buffered saline (PBS).
Cell infection.
A quantitative assay of the binding of E. coli to cultured intestinal cells was conducted with metabolically labeled bacteria (2). E. coli was radiolabeled by the addition of 14C-acetic acid (Amersham; 94 mCi/mmol; 100 μCi per 10-ml tube) to CFA broth. Cell monolayers were infected with radiolabeled bacteria (108 CFU/ml; 50,000 to 70,000 cpm) and incubated at 37°C in 10% CO2–90% air for 3 h. The monolayers were then washed three times with sterile PBS. Adhering bacteria and intestinal cells were dissolved in a 0.2 N NaOH solution. The level of bacterial adhesion was evaluated by liquid scintillation counting. Each adhesion assay was conducted in duplicate with three successive cell passages. Inhibition of adhesion was conducted with chloramphenicol (20 μg/ml) and anti-DAF monoclonal antibodies (MAbs) IF7 and IA10 (diluted 1:20 in PBS). Before the bacterial adhesion assay, the cell monolayers were preincubated for 1 h at 37°C with chloramphenicol or each antibody; they were then incubated with radiolabeled DAEC C1845.
Gentamicin survival assay.
DAEC C1845 internalization was determined by quantitative determination of bacteria located within infected postconfluent-growth INT407 cell monolayers with the aminoglycoside assay. After infection, monolayers were washed twice with sterile PBS and then incubated for 60 min in a medium containing 50 μg of gentamicin per ml. Bacteria that adhered to the infected cells were rapidly killed, whereas those located within the cells were not. The monolayers were washed with PBS and lysed with sterilized H2O. Appropriate dilutions were plated on tryptic soy agar to determine the number of viable cell-associated bacteria by bacterial colony counts. Each assay was conducted in triplicate with three successive passages of INT407 cells.
Antibodies.
Mouse MAb CY-DAF raised against human DAF was obtained from Valbiotech (Paris, France). Ascites fluid containing antibody IF7 directed against the SCR3 domain of DAF was from J. M. Bergelson (Dana-Farber Cancer Institute, Harvard Medical School, Boston, Mass.). MAb IA10 directed against the SCR1 domain of DAF was from V. Nussenzweig (New York University Medical Center, New York). MAb PY20 directed against phosphotyrosine was from ICN Biochemicals, Inc., Costa Mesa, Calif. Fluorescein isothiocyanate (FITC)-phalloidin was from Molecular Probes, Inc., Eugene, Oreg. Rabbit polyclonal antibody directed against the purified adhesin F1845 was from S. S. Bilge (University of Washington, Seattle).
Immunofluorescence.
Monolayers of cells were prepared on glass coverslips, which were placed in six-well tissue culture plates (Corning Glass Works, Corning, N.Y.). Adhering E. coli and DAF were revealed by indirect immunofluorescence labeling of unpermeabilized cell layers as previously described (2). Preparations were fixed for 10 min at room temperature in 3.5% paraformaldehyde in PBS. Cell monolayers were incubated with a specific primary antibody for 30 min at room temperature, washed, and then incubated with the respective secondary fluorescein-conjugated antibody. Primary antibodies were diluted 1:20 to 1:100 (rabbit polyclonal anti-Dr and anti-F1845 sera, 1:20; CY-DAF, 1:50) in 2% bovine serum albumin–PBS. Secondary antibodies were either FITC- or tetramethyl rhodamine isothiocyanate-conjugated goat anti-mouse immunoglobulin G (IgG) from Immunotech (Luminy, France) or FITC-conjugated goat anti-rabbit IgG from Institut Pasteur Productions (Paris, France) and were diluted 1:20 in 2% bovine serum albumin–PBS.
When F actin was to be visualized in intestinal cells, coverslips were incubated with or without 0.2% Triton X-100 in PBS for 4 min before incubation with FITC-phalloidin for 45 min at 22°C and then were washed three times with PBS.
Phosphotyrosine was visualized with MAb PY20 followed by anti-mouse FITC-coupled goat immunoglobulins (Institut Pasteur Productions). Immunolabeling was performed at 25°C for 45 min for each antibody. In all immunolabeling experiments with primary and secondary antibodies, no fluorescence staining was observed when nonimmune serum was used and when the primary antibody was omitted.
Specimens were examined by epifluorescence microscopy and by interference contrast microscopy by use of a Leitz Aristoplan microscope with epifluorescence. All photographs were taken on Kodak T-MAX 400 black-and-white film (Eastman Kodak Co., Rochester, N.Y.).
Scoring of the F-actin rearrangements.
F-actin distribution in control and infected INT407 cells was examined by fluorescence microscopy after FITC-phalloidin staining. For the analysis of F-actin stress fiber disassembly and F-actin ruffling, the scoring method described by Kotani et al. (18) was used. In brief, cells which showed a well-organized F-actin network were scored as 1 point. Cells which showed atypical or equivocal F-actin disorganization were scored as 0.5 point. Cells which showed no well-organized F-actin network were scored as 0. More than 200 individual cells were examined for each assay. Apical F-actin alteration indices were calculated by dividing total points by the total number of cells examined and multiplying the result by 100.
Inhibitors.
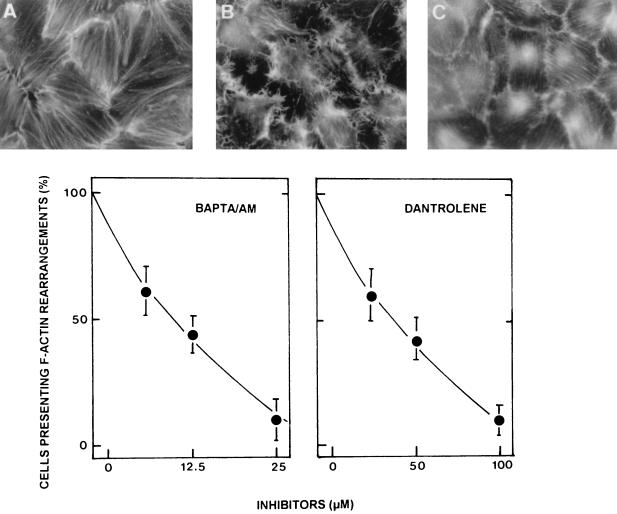
The protein tyrosine kinase (PTK) inhibitors used were genistein (4′,5,7-trihydroxyisoflavone) (1) (Sigma), erbstatin (methyl-2,5-dihydroxycinnamate) (14) (Biomol), and tyrphostin 25 (3,4,5-trihydroxy-cis-cinnamonitrile) (12). To study the role of phospholipase Cγ (PLCγ) in DAF-associated signal transduction, the aminosteroid U 73122 {1-[6-[[17-betha-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione} or its inactive analog U 73343 {1-[6-[[17-betha-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H- pyrrolidine-2,5-dione} (27) (both from Biomol) was used. The phosphatidylinositol 3-kinase (PI 3-kinase) inhibitor used was wortmannin (30) (Sigma). The protein kinase C (PKC) inhibitor used was H7 (1-[5-isoquinolinylsulfonyl]-2-methylpiperazine) (7) (Sigma). The G-protein blocker used was pertussis toxin (Sigma). To examine the role of intracellular Ca2+, a blocker of Ca2+ release from the sarcoplasmic reticulum, dantrolene {1-[(5-[p-nitrophenyl]furfurylidene)-amino]hydantoin} (8) (Sigma), was used. To chelate intracellular Ca2+, a cell-permeable Ca2+ chelator, BAPTA/AM {1,2-bis[2-amino-phenoxy]ethane-N,N,N′,N′-tetraacetic acid tetrakis [acetoxymethyl] ester} (Sigma), was used.
Genistein, tyrphostin 25, or erbstatin in dimethyl sulfoxide (DMSO) was added to the culture medium 3, 24, or 1 h before infection, respectively. U 73122 or the inactive analog U 73343 was added to the culture medium 30 min before infection. Wortmannin, stored in DMSO at −20°C in the dark and diluted with the incubating medium just before use, was added to the culture medium 40 min before infection. Dantrolene in DMSO or BAPTA/AM in methanol-dimethylformamide (50:50, vol/vol) was added to the culture medium 30 or 60 min before infection, respectively. Treatment of the cells with pertussis toxin was conducted 18 h before infection. The concentrations of all blockers were maintained during the infection.
RESULTS
Recognition of DAF by DAEC C1845 in human embryonic intestinal INT407 cells is followed by F-actin rearrangements.
We previously reported that cultured human embryonic intestinal INT407 cells expressing DAF could be infected by DAEC C1845 (clinical isolate) (3). The binding of metabolically radiolabeled DAEC C1845 to INT407 cells was inhibited by preincubating the cells with anti-DAF MAbs CY-DAF and IF7, recognizing the SCR3 domain of DAF (adhesion was inhibited by 71% ± 5% and 73% ± 3%, respectively), whereas MAb IA10, recognizing the SCR1 domain of DAF, failed to inhibit adhesion. DAEC C1845 entry into infected INT407 cells was examined with an aminoglycoside antibiotic which is known to kill extracellular bacteria but which does not reach bactericidal concentrations inside infected cells. We found no entry of DAEC C1845 after infection of INT407 cells.
In permeabilized preconfluent INT407 control cells (Fig. 1), F actin stained by FITC-phalloidin was organized in regular stress fibers. Moreover, cell-to-cell contacts were well organized in a continuous beaded pattern. Preconfluent DAEC C1845-infected cells showed dramatic alterations in F-actin distribution. F-actin stress fibers located centrally in the cells disappeared and were replaced by disorganized short actin filaments or central lucent zones. F actin at cell-to-cell contacts appeared highly dense. F-actin rearrangements were quantitatively assessed with the scoring system (ruffling index) defined by Kotani et al. (18). Disappearance of F-actin stress fibers and appearance of F-actin ruffles centrally in the cells and at cell-to-cell contacts were observed in 95% ± 2% and 98% ± 3% of preconfluent and postconfluent infected cells, respectively. Altogether, these results demonstrate that cytoskeletal rearrangements in intestinal cells could be induced after the attachment of a noninvasive pathogen to its membrane-associated receptor.
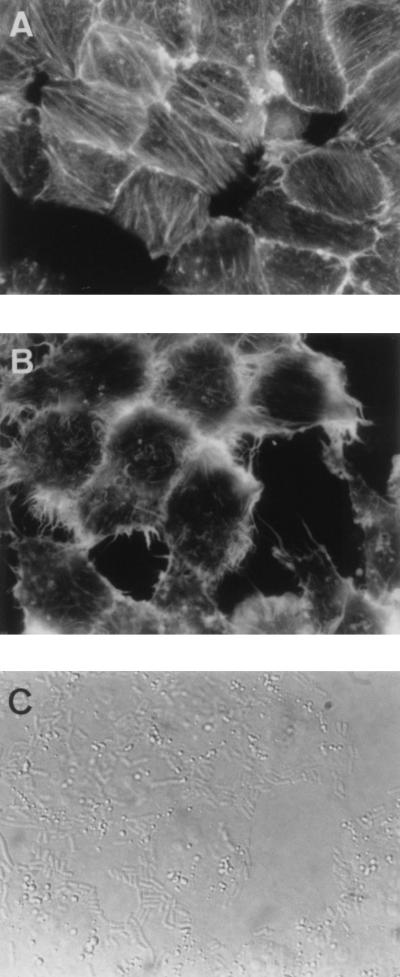
FIG. 1.
Disappearance of F-actin stress fibers and F-actin accumulation in ruffles in DAEC C1845-infected preconfluent cultured human embryonic intestinal INT407 cells. After bacterial adhesion (3 h at 37°C) and three washes to remove the nonadhering bacteria, the cells were fixed, permeabilized with Triton X-100, and stained with FITC-phalloidin to show F-actin filaments. (A) F-actin distribution in control cells showing well-ordered F-actin stress fibers and regular margins of cells. (B) Disappearance of F-actin stress fibers and induction of F-actin ruffles at the margins of DAEC C1845-infected cells. (C) Adhesion of DAEC C1845 in the same cells (B) observed by phase-contrast microscopy.
Signaling molecules involved in DAEC C1845-induced F-actin rearrangements.
We used an antiphosphotyrosine antibody to examine changes in the patterns of phosphorylated proteins upon infection of INT407 cells with DAEC C1845 (Fig. 2). No phosphorylated proteins were observed in INT407 control cells. In contrast, induction of phosphorylated proteins was observed at the periphery of preconfluent DAEC C1845-infected cells.
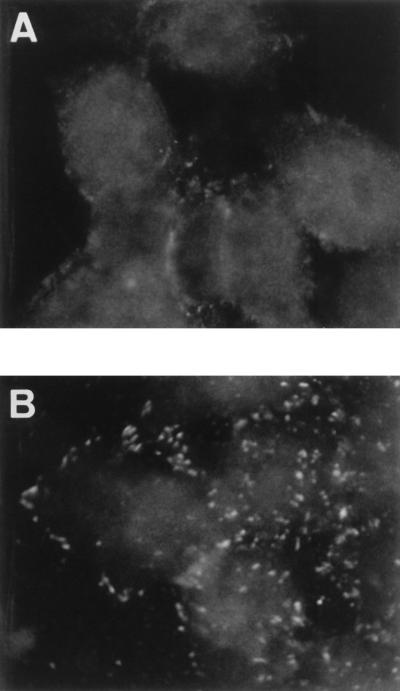
FIG. 2.
Phosphotyrosine clustering induced in DAEC C1845-infected preconfluent cultured human embryonic intestinal INT407 cells. After bacterial adhesion (3 h at 37°C), phosphotyrosines were localized with antiphosphotyrosine MAb PY20 followed by FITC-labeled goat anti-mouse IgG. (A) Uninfected control cells. (B) DAEC C1845-infected cells.
Several signaling molecules, such as the Src family member tyrosine kinase, PLCγ, PKC, and the secondary messenger Ca2+, have been identified as being associated with DAF signal transduction in hematopoietic cells (20, 28, 29). In order to determine if these signaling molecules are involved in F-actin disassembly in DAEC C1845-infected INT407 cells, we used the cellular and pharmacological approach developed by Chrzanowska-Wodnicka and Burridge (7) to demonstrate the requirement for PTK in the reorganization of the cytoskeleton during serum stimulation of quiescent Swiss 3T3 fibroblasts. Treatment of INT407 cells with inhibitors at the concentrations and preincubation times used here did not change the level of adhesion of DAEC C1845 (data not shown). Moreover, in uninfected control cells treated with inhibitors, no change in F-actin distribution was observed (data not shown).
Two different tyrosine kinase inhibitors, i.e., genistein and erbstatin, prevented DAEC C1845-induced F-actin stress fiber disassembly and completely inhibited F-actin ruffle induction (Fig. 3 and 4). Quantitative assessment of F-actin rearrangements in treated infected cells showed that the response to the tyrosine kinase inhibitors was dose dependent (Fig. 4).
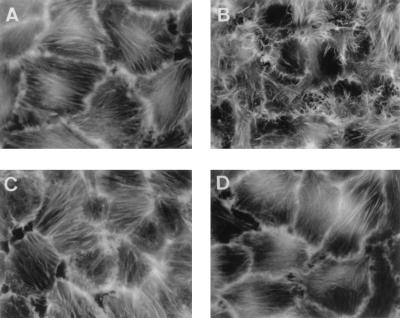
FIG. 3.
The tyrosine kinase inhibitor erbstatin blocks DAEC C1845-induced F-actin ruffles and F-actin stress fiber disappearance in infected postconfluent cultured human embryonic intestinal INT407 cells. After bacterial adhesion (3 h at 37°C), F-actin filaments were localized with FITC-phalloidin. Cells were treated prior to infection with erbstatin (1 h, 150 μM), and the inhibitor remained present in the incubation medium during the bacterial infection. (A) Uninfected control cells. (B) DAEC C1845-infected cells. (C) Uninfected control cells treated with erbstatin. (D) DAEC C1845-infected cells treated with erbstatin.
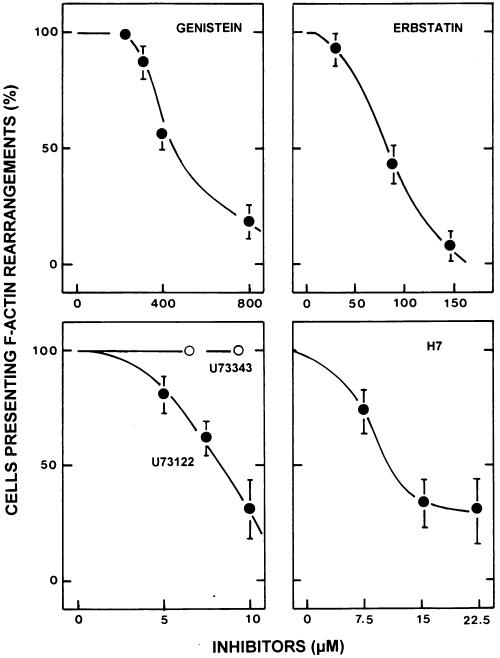
FIG. 4.
Dose-response effects of tyrosine kinase, PLCγ, and PKC inhibitors on DAEC C1845-induced F-actin rearrangements in infected postconfluent cultured human embryonic intestinal INT407 cells. The tyrosine kinase inhibitors were genistein and erbstatin; the PLCγ inhibitors were U 73122 and its inactive analog U 73343; and the PKC inhibitor was H7. Cells were treated prior to infection with genistein for 3 h, erbstatin for 24 h, U 73122 and U 73343 for 0.5 h, or H7 for 1 h. Inhibitors remained present in the incubation medium during the bacterial infection. After bacterial adhesion (3 h at 37°C), F-actin filaments were localized with FITC-phalloidin. F-actin alterations were scored as described by Kotani et al. (18).
To elucidate if the DAF signaling pathway activated in DAEC C1845-infected INT407 cells involves PLCγ, we studied the effects of a PLCγ inhibitor, the aminosteroid U 73122, and its inactive analog, U 73343. We found that U 73122 prevented DAEC C1845-induced F-actin rearrangements dose dependently (Fig. 4). In contrast, the inactive analog of the inhibitor, U 73343, had no effect, since both DAEC C1845-induced F-actin stress fiber disassembly and F-actin ruffle induction remained unchanged after U 73343 treatment.
Using the PKC inhibitor H7, we examined the involvement of PKC in DAEC C1845-induced F-actin rearrangements at cell-to-cell contacts in infected INT407 cells. We found that DAEC C1845-induced F-actin rearrangements were sensitive to H7 (Fig. 4).
Considering the Ca2+ sensitivity of host cell cytoskeletal F actin, it is reasonable to speculate that F-actin rearrangements result from IP3 production through PLCγ activation, which probably releases Ca2+ from intracellular stores through IP3-sensitive channels (4). The requirement for extracellular and intracellular Ca2+ in DAEC C1845-induced F-actin rearrangements was examined. By using the PLC-induced Ca2+ flux inhibitors dantrolene and BAPTA/AM, we demonstrated that the F-actin rearrangements in DAEC C1845-infected INT407 cells resulted from the release of Ca2+ from intracellular stores, since treatment of the cells with both dantrolene and BAPTA/AM prior to infection blocked DAEC C1845-induced F-actin rearrangements dose dependently (Fig. 5).
FIG. 5.
Dose-response effects of a calcium inhibitor or chelator on DAEC C1845-induced F-actin rearrangements in infected postconfluent cultured human embryonic intestinal INT407 cells. Cells were treated prior to infection with the calcium inhibitor dantrolene (0.5 h) or the calcium chelator BAPTA/AM (1 h). Drugs remained present in the incubation medium during the bacterial infection. After bacterial adhesion (3 h at 37°C), F-actin filaments were localized with FITC-phalloidin. F-actin alterations were scored as described by Kotani et al. (18). (A) Uninfected control cells. (B) DAEC C1845-infected cells. (C) DAEC C1845-infected cells treated with BAPTA/AM (25 μg/ml).
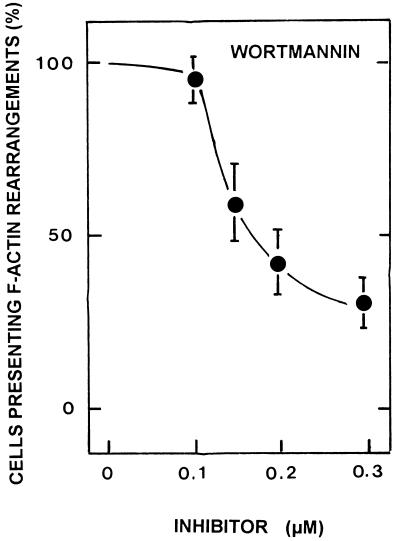
The association of PI 3-kinase with F-actin rearrangements has been reported (18). In order to obtain insight into the possible role of PI 3-kinase in DAEC C1845-induced F-actin rearrangements, we used wortmannin, a highly specific and potent inhibitor of the catalytic subunit of mammalian PI 3-kinase. As shown in Fig. 6, wortmannin inhibited the F-actin rearrangements induced by DAEC C1845 infection dose dependently.
FIG. 6.
The PI 3-kinase inhibitor wortmannin blocks DAEC C1845-induced F-actin rearrangements in infected postconfluent cultured human embryonic intestinal INT407 cells dose dependently. Cells were treated prior to infection with wortmannin for 0.75 h. The inhibitor remained present in the incubation medium during the bacterial infection. After bacterial adhesion (3 h at 37°C), F-actin filaments were localized with FITC-phalloidin. F-actin alterations were scored as described by Kotani et al. (18).
A family of related bacterial adhesins was able to activate the DAF-associated transducing signal promoting F-actin rearrangements.
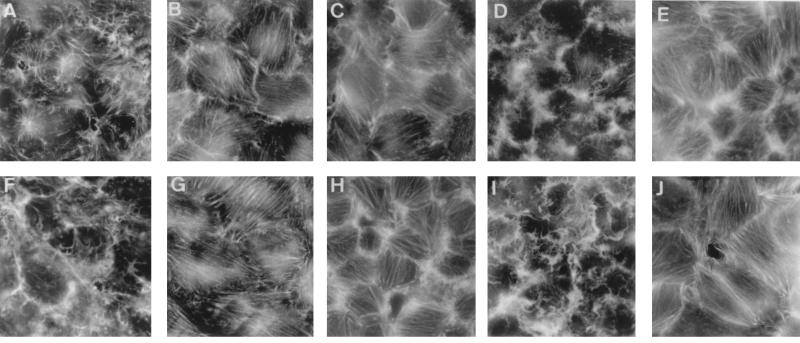
The Dr family of E. coli expresses several adhesins: afimbrial AFA-I and AFA-III adhesins, Dr hemagglutinin, and fimbrial adhesin F1845 (22). All of these E. coli adhesins recognize as a receptor DAF at its SCR3 domain (21). It can be hypothesized that this family of E. coli adhesins shares similar mechanisms of pathogenicity. To document this hypothesis, we examined the cellular events occurring in INT407 cells infected with recombinant E. coli HB101(pSSS1) (F1845+ E. coli) expressing fimbrial adhesin F1845 (5, 6) and recombinant E. coli HB101 (pBNJ406) expressing Dr hemagglutinin (Dr+ E. coli) (22). Binding of metabolically radiolabeled and Dr+ E. coli to INT407 cells was inhibited by preincubation of the cells with anti-DAF MAb IF7 (76% ± 6% and 74% ± 4% inhibition, respectively). Binding of F1845+ or Dr+ E. coli to confluent INT407 cells was followed by the induction of characteristic F-actin rearrangements similar to those observed with clinical isolate DAEC C1845 (Fig. 7). Since these E. coli strains expressed only the F1845 or the Dr adhesin, this result suggested that F-actin rearrangements resulted from F1845 and Dr adhesin-DAF interactions. Both F1845+ and Dr+ E. coli cell infections inducing dramatic F-actin rearrangements were inhibited by treatment of the cells with the PTK inhibitor erbstatin and the PLCγ inhibitor U 73122 and by blockage of intracellular Ca2+ (Fig. 7). In contrast, U 73343, the inactive analog of the PLCγ inhibitor U 73122, failed to block F1845+ and Dr+ E. coli-induced F-actin rearrangements.
FIG. 7.
Tyrosine kinase, PLCγ, and Ca2+ inhibitors block E. coli(pSSS1) (expressing F1845)- and E. coli(pBNJ406) (expressing Dr)-induced F-actin rearrangements in infected postconfluent cultured human embryonic intestinal INT407 cells. Cell infection, cell treatments, and F-actin staining and scoring of the cells were as described in the legends to Fig. 3, 4, and 5. (A to E) E. coli(pSSS1)-infected cells. (F to J) E. coli(pBNJ406)-infected cells. (A and F) Infected cells. (B and G) Infected cells treated with erbstatin (1 h, 150 μM). (C and H) Infected cells treated with U 73122 (20 μM). (D and I) Infected cells treated with U 73343 (20 μM). (E and J) Infected cells treated with BAPTA/AM (25 μg/ml).
DISCUSSION
Enterovirulent bacteria cause disease through the expression of adhesins required for host cell colonization, through perturbation of host cell activity by the production of toxins, or through invasion of cells to trigger major cytoskeletal rearrangements. Hijacking host cell signal transduction mechanisms is the mechanism through which several species of enterovirulent bacteria promote host cell structural injuries (for a review, see reference 11). The results reported here show that infection of the human embryonic intestinal INT407 cell line by a group of virulent E. coli strains is followed by cytoskeletal F-actin rearrangements without bacterial cell entry. When examining the mechanism through which Dr+ E. coli promoted the cytoskeletal F-actin injuries, we demonstrated that the interaction of the F1845 or Dr adhesin with the membrane-associated DAF receptor was the cellular event which initiated the cytoskeletal F-actin rearrangements.
Human DAF is a 70- to 75-kDa membrane glycoprotein involved in protecting cells against lysis by homologous complement (19, 20). The molecule has four contiguous short consensus repeat domains followed by a serine/threonine-rich, heavily O-glycosylated C-terminal domain. A GPI anchor attaches the molecule to the outer leaflet of the cell membrane. DAF functions to transmit signals from the GPI anchor. In human T cells, cross-linking of DAF with an anti-DAF MAb and a secondary antibody induces cell proliferation when the cells are costimulated with phorbol esters (9). Moreover, the activation of DAF by cross-linking results in the stimulation of human monocytes (26). Cytoskeletal protein reorganization, including clustering of actin, tubulin, and vimentin beneath capped DAF, has been reported after cross-linking of DAF on T lymphocytes (15). It was recently documented that the activation of DAF leads to the recruitment of a megacomplex of cytosolic PTKs. p56lck, a PTK related to the Src family of kinases, associates with DAF in human T cells (28, 29). In murine thymoma EL-4 cells transfected with DAF cDNA, the cross-linking of DAF triggers the production of IL-4 and the phosphorylation of 40-, 56- to 60-, and 85-kDa DAF-associated proteins, including the Scr family PTKs p56lck and p59fyn, while no DAF-associated kinase activity is found in cells transfected with a transmembrane form of DAF (25).
In searching for the signal transduction proteins recruited through DAF activation in DAEC C1845-infected INT407 cells, we found that phosphorylated proteins appeared in the infected cells. We demonstrated that PTKs are functional in DAF signal transduction, since F-actin rearrangements upon infection were blocked by a subset of PTK blockers. A previous report suggested that PLCγ is associated with DAF transduction, since elevation of levels of the secondary messenger molecule IP3 was observed in monocytes upon cross-linking of DAF (26). PLC is a phospholipase catalyzing the cleavage of phosphatidylinositol bisphosphate into the secondary messengers inositol 1,4,5-triphosphate and diacylglycerol (23). We observed that the PLCγ inhibitor U 73122 completely blocked DAEC C1845-induced F-actin disassembly, while its inactive analog, U 73343, had no effect. The major PLC-associated secondary messenger is Ca2+, released from its intracellular stores through PLCγ activation (4). By using inhibitors of PLC-induced Ca2+ flux (dantrolene or a chelator of intracellular Ca2+, BAPTA/AM), we found that this secondary messenger is involved in DAEC C1845-induced F-actin rearrangements. Interestingly, in T cells an unidentified 85-kDa molecule containing PTK activity coimmunoprecipitated with GPI-anchored proteins such as DAF (28, 29). In this study, we present data suggesting that PI 3-kinase was involved in DAF signaling initiated by the binding of F1845-expressing E. coli to DAF. Using wortmannin, which binds to the 85-kDa catalytic subunit but not to the 110-kDa adaptor subunit, inhibiting PI 3-kinase activity both in vivo and in vitro, we found that DAEC-induced F-actin rearrangements were inhibited. This result indicated that PI 3-kinase, like PLCγ, functions as a relay enzyme in the DAF signaling pathway in human intestinal cells.
We observed increased density of F actin at cell-to-cell contacts in Dr+ E. coli-infected INT407 cells, developing in parallel with F-actin ruffling centrally in the cells. The PLC-associated secondary messenger diacylglycerol is a known activator of the phospholipid-dependent Ca2+-activated PKC linked to tight junctions in several polarized mammalian cells (31). PLC and PKC participate in assembly and sealing of tight junctions. Although INT407 cells are nonpolarized cells which do not form tight junctions, we examined the role of PKC in DAF signal transduction upon infection with DAEC C1845. By using the PKC inhibitor H7, we found inhibition of Dr+ E. coli-induced F-actin rearrangements, indicating that PKC is involved in DAF signal transduction in intestinal cells.
In conclusion, our results provide consistent data on the mechanism of pathogenicity of a family of pathogens which, through their adhesins, recognize as a receptor the membrane-associated GPI-anchored DAF and which in turn are able to induce the activation of the GPI-associated signal transduction molecule cascade. However, several questions remain to be elucidated. It is well established that DAF lacks intrinsic PTK activity (19, 20). As for all of the GPI-anchored protein transducing signals, the nature of the adaptor through which DAF is coupled to signaling molecules remains to be determined. Finally, DAEC-induced F-actin ruffles resemble the F-actin ruffles induced in response to growth factors involving the Ras-related small GTPase Rac (24). Further studies are required to elucidate the detailed molecular mechanism by which cytoskeletal F-actin disassembly occurs upon infection of human intestinal cells with Dr+ E. coli after the signal transduction molecules coupled to GPI-anchored DAF are activated.
REFERENCES
- 1.Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuyan M, Fukami Y. Genistein is a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- 2.Bernet-Camard M F, Coconnier M H, Hudault S, Servin A L. Pathogenicity of the diffusely adhering strain Escherichia coli C1845: F1845 adhesin-decay accelerating factor interaction, brush border microvillus injury, and actin disassembly in cultured human intestinal epithelial cells. Infect Immun. 1996;64:1818–1828. doi: 10.1128/iai.64.6.1918-1928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernet-Camard M F, Coconnier M H, Hudault S, Servin A L. Differential expression of complement proteins and regulatory decay accelerating factor in relation to differentiation of cultured human colon adenocarcinoma cell lines. Gut. 1996;38:248–253. doi: 10.1136/gut.38.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berridge M J. Elementary and global aspects of calcium signaling. J Physiol. 1997;499:291–306. doi: 10.1113/jphysiol.1997.sp021927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilge S S, Apostol J M, Jr, Fullner K J, Moseley S L. Transcriptional organization of the F1845 fimbrial adhesin determinant of Escherichia coli. Mol Microbiol. 1993;7:993–1006. doi: 10.1111/j.1365-2958.1993.tb01191.x. [DOI] [PubMed] [Google Scholar]
- 6.Bilge S S, Clausen C R, Lau W, Moseley S L. Molecular characterization of a fimbrial adhesin, F1845, mediating diffuse adherence of diarrhea-associated Escherichia coli to HEp-2 cells. J Bacteriol. 1989;171:4281–4289. doi: 10.1128/jb.171.8.4281-4289.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chrzanowska-Wodnicka M, Burridge K. Tyrosine phosphorylation is involved in reorganization of the actin cytoskeleton in response to serum or LPA stimulation. J Cell Sci. 1994;107:3643–3654. doi: 10.1242/jcs.107.12.3643. [DOI] [PubMed] [Google Scholar]
- 8.Danko S, Kim D H, Streter F A, Ikemoto N. Biochim. Biophys Acta. 1985;816:18–24. doi: 10.1016/0005-2736(85)90388-8. [DOI] [PubMed] [Google Scholar]
- 9.Davis L S, Patel S S, Atkinson J P, Lipsky P E. Decay-accelerating factor functions as a signal transducing molecule for human T cells. J Immunol. 1988;141:2246–2252. [PubMed] [Google Scholar]
- 10.Donnenberg M S, Kaper J B. Enteropathogenic Escherichia coli. Infect Immun. 1992;60:3953–3961. doi: 10.1128/iai.60.10.3953-3961.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gazit A, Yaish P, Gilon C, Levitski A. Inhibition of tyrosine protein kinase by synthetic erbstatin analogues. J Med Chem. 1989;32:2344–2352. doi: 10.1021/jm00130a020. [DOI] [PubMed] [Google Scholar]
- 13.Henle G, Deinhardt F. The establishment of strains of human cells in tissue culture. J Immunol. 1957;79:54–59. [PubMed] [Google Scholar]
- 14.Isshiki K, Imoto M, Sawa T, Umezawa K, Takeuchi T, Umezawa H, Tsuchida Y, Yoshioka T, Tatsuta K. Inhibition of tyrosine protein kinase by synthetic erbstatin analogues. J Antibiot. 1987;40:1209–1210. doi: 10.7164/antibiotics.40.1209. [DOI] [PubMed] [Google Scholar]
- 15.Kammer G M, Walter E I, Medof M E. Association of cytoskeletal re-organization with capping of the complement decay-accelerating factor on T lymphocytes. J Immunol. 1988;141:2924–2928. [PubMed] [Google Scholar]
- 16.Kernéis S, Bernet M F, Gabastou J M, Coconnier M H, Nowicki B J, Servin A L. Human cultured intestinal cells express attachment sites for uropathogenic Escherichia coli bearing adhesins of the Dr adhesin family. FEMS Microbiol Lett. 1994;119:27–32. doi: 10.1111/j.1574-6968.1994.tb06862.x. [DOI] [PubMed] [Google Scholar]
- 17.Kernéis S, Bilge S, Fourel V, Chauvière G, Coconnier M H, Servin A L. Use of purified F1845 fimbrial adhesin to study localization and expression of receptors for diffusely adhering Escherichia coli (DAEC) during enterocytic differentiation of human colon carcinoma cell lines HT-29 and Caco-2 in culture. Infect Immun. 1991;59:4013–4018. doi: 10.1128/iai.59.11.4013-4018.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotani K, Yonezawa K, Hara K, Ueda H, Kitamura Y, Sakaue H, Ando A, Chavanieu A, Calas B, Grigorescu F, Nishiyama M, Waterfiels M D, Kasuga K. Involvement of phosphoinositide 3-kinase in insulin- or IGF-1-induced membrane ruffling. EMBO J. 1994;13:2313–2321. doi: 10.1002/j.1460-2075.1994.tb06515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lublin D M. Glycosyl-phosphatidylinositol anchoring of membrane proteins. Curr Top Microbiol Immunol. 1992;178:141–162. doi: 10.1007/978-3-642-77014-2_9. [DOI] [PubMed] [Google Scholar]
- 20.Nicholson-Weller A, Wang C. Structure and function of decay-accelerating factor CD55. J Lab Clin Med. 1994;123:485–491. [PubMed] [Google Scholar]
- 21.Nowicki B, Hart A, Coyne K E, Lublin D M, Nowicki S. Short consensus repeat-3 domain of recombinant decay-accelerating factor is recognized by Escherichia coli recombinant Dr adhesin in a model of cell-cell interaction. J Exp Med. 1993;178:2115–2121. doi: 10.1084/jem.178.6.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nowicki B, Labigne A, Moseley S, Hull R, Moulds J. The Dr hemagglutinin, afimbrial adhesins AFA-I, AFA-II, and AFA-III, and F1845 fimbriae of uropathogenic and diarrhea-associated Escherichia coli belong to a family of hemagglutinins with Dr receptor recognition. Infect Immun. 1990;58:279–281. doi: 10.1128/iai.58.1.279-281.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhee S G, Choi K D. Regulation of inositol phospholipid-specific phospholipase C isoenzymes. J Biol Chem. 1992;267:12393–12396. [PubMed] [Google Scholar]
- 24.Ridley A J, Paterson H F, Johnston C L, Diekmann L, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 25.Shenoy-Scaria A M, Kwong J, Fijita T, Olszowy M W, Shaw A S, Lublin D M. Signal transduction through decay-accelerating factor. Interaction of glycosyl-phosphatidylinositol anchor and protein tyrosine kinases p56lck and p59fyn1. J Immunol. 1992;149:3535–3541. [PubMed] [Google Scholar]
- 26.Shibuya K, Abe T, Fujita T. Decay-accelerating factor functions as a signal transducing molecule for human monocytes. J Immunol. 1992;149:1758–1762. [PubMed] [Google Scholar]
- 27.Smith R J, Sam L M, Justen J M, Bundy G L, Bala G A, Bleasdale J E. Receptor-coupled signal transduction in human polymorphonuclear neutrophils: effects of a novel inhibitor of phospholipase C-dependent processes on cell responsiveness. J Pharmacol Exp Ther. 1990;253:688–692. [PubMed] [Google Scholar]
- 28.Stefanova I, Horejsi V, Ansotegui I J, Knapp W, Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science. 1991;254:1016–1019. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- 29.Stefanova I, Horejsi V. Association of the CD59 and CD55 cell surface glycoproteins with other membrane molecules. J Immunol. 1991;147:1587–1592. [PubMed] [Google Scholar]
- 30.Yano H, Nakanishi S, Kimura K, Hanai N, Saitoh Y, Fukui Y, Nonomura Y, Matsuda Y. Inhibition of histamine secretion by wortmannin through the blockade of phosphatidylinositol 3-kinase in RBL-2H3 cells. J Biol Chem. 1993;268:25846–25856. [PubMed] [Google Scholar]
- 31.Zigmond S H. Signal transduction and actin filament organization. Curr Opin Cell Biol. 1996;8:66–73. doi: 10.1016/s0955-0674(96)80050-0. [DOI] [PubMed] [Google Scholar]