Abstract
Long-term use of sodium oxybate (SXB), (also called gamma-hydroxybutyrate [GHB]) attenuates the cataplexy and sleepiness of human narcolepsy. We had previously found that chronic opiate usage in humans and long-term opiate administration to mice significantly increased the number of detected hypocretin/orexin (Hcrt) neurons, decreased their size, and increased Hcrt level in the hypothalamus. We also found that opiates significantly decreased cataplexy in human narcoleptics as well as in narcoleptic mice and that cessation of locus coeruleus neuronal activity preceded and was tightly linked to cataplectic attacks in narcoleptic dogs. We tested the hypothesis that SXB produces changes similar to opiates and now report that chronic SXB administration significantly increased the size of Hcrt neurons, the reverse of what we had seen with opiates in humans and mice. Levels of Hcrt in the hypothalamus were nonsignificantly lower, in contrast to the significant increase in hypothalamic Hcrt level after opiates. SXB decreased tyrosine hydroxylase levels in the locus coeruleus, the major descending projection of the hypocretin system, also the reverse of what we saw with opioids. Therefore despite some similar effects on narcoleptic symptomatology, SXB does not produce anatomical changes similar to those elicited by opiates. Analysis of changes in other links in the cataplexy pathway might further illuminate SXB’s mechanism of action on narcolepsy.
Keywords: Xyrem, sodium oxybate, hypocretin, orexin, tyrosine hydroxylase, locus coeruleus, narcolepsy
Graphical Abstract
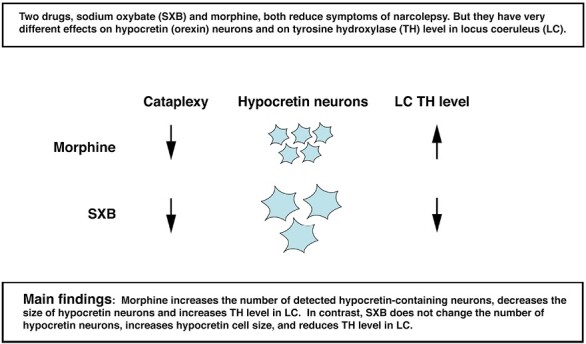
Statement of Significance.
We test the hypothesis that sodium oxybate (SXB), a well established, but poorly understood treatment for narcolepsy, acts by inducing changes in hypocretin (orexin) and locus coeruleus neurons similar to those we have shown are caused by morphine, which is effective in reversing symptoms in both murine and human narcolepsy. We conclude that this is not the case.
Introduction
It is now well established, by work in humans as well as in mice, that the loss of Hcrt (orexin) neurons is sufficient to cause narcolepsy [1, 2], with our immunohistochemical study showing an average 90% loss of these neurons in human narcolepsy with cataplexy [2, 3] and a smaller loss in human narcolepsy without cataplexy [4]. Murine narcolepsy can be induced by eliminating the Hcrt peptide [5] or, even more effectively, by eliminating neurons containing the peptide [6]. Inactivation of Hcrt receptor-2 (HcrtR2) induces narcolepsy in dogs [7].
Human narcoleptics not only show a 90%, on average, reduction in the number of Hcrt neurons, but also have an increased number of histamine-producing neurons throughout the posterior hypothalamus [8, 9]. Human narcoleptics show a decrease in the number of corticotropin-releasing hormone neurons in the paraventricular nucleus, but not of corticotropin-releasing hormone neurons in other brain regions [10]. It is unclear if these changes are a cause or a consequence of the loss of Hcrt neurons.
In 2018 we found that human heroin addicts had an average 54% increase in the number of “detected” Hcrt neurons and a 22% shrinkage in the size of these neurons, compared to human controls [11]. We also showed that morphine administration to mice for 2 or more weeks produced a similar 38% increase in the number of “detected” Hcrt neurons and a 23% shrinkage in the average size of these neurons [11].
We studied the effect of morphine on transgenic “DTA” mice in which Hcrt neurons could be selectively deleted by withdrawing doxycycline from the diet on which they had been raised [8]. At a 40% level of Hcrt neuron depletion, giving these mice 50 mg/kg of morphine daily for 2 weeks restored detected Hcrt neuron number to a normal level and prevented cataplexy [11]. We found that opiates also suppressed narcoleptic symptomatology in human narcoleptics [11, 12].
The increase in “detected” Hcrt neurons in mice produced by opioids was not due to neurogenesis, but rather to increased Hcrt production in a population of Hcrt neurons that produced amounts of Hcrt below the sensitivity of standard immunohistochemistry. These neurons could be revealed by administration of colchicine to mice. Colchicine increases the Hcrt level in Hcrt neurons by blocking axonal transport of Hcrt (and all other peptides) out of neurons. Colchicine treatment increases the number of detected Hcrt neurons in mice by 40% [11, 13].
This work suggested to us that SXB might reduce narcoleptic symptomatology by a “mechanism of action” similar to that produced by opiates, i.e. increasing the number of Hcrt-producing neurons and decreasing their size [11] and increasing Tyrosine hydroxylase (TH) expression in the locus coeruleus [14]. Similar to the opiate effects on narcolepsy, which take 2 or more weeks to develop [11], optimal symptom reversal by sodium oxybate takes up to several months in humans to maximally reverse symptoms of narcolepsy [15].
In prior work, to identify cell groups that might be responsible for triggering cataplexy, the distinctive symptom of narcolepsy, we recorded neuronal activity in narcoleptic (HcrtR-2 mutant [7]) dogs. We examined three neuronal groups that are known to fire continuously in waking and cease firing in REM sleep in non-narcoleptic animals, i.e. “REM-off” neurons. One such group contains histamine. We found that these neurons continued firing at the normal waking rate or even elevated their discharge rate during cataplexy [8]. We examined serotonin-containing neurons in the dorsal raphe nucleus. These neurons decrease their discharge rate to the rate seen in non-REM sleep during cataplexy, but did not show the complete cessation they show in REM sleep [16]. In contrast to these two groups, noradrenergic neurons of the locus coeruleus cease activity immediately prior to and during cataplexy [17], similar to the greatly decreased norepinephrine release in the spinal ventral horn and hypoglossal nucleus during muscle tone suppression in both REM sleep and cataplexy [18, 19]. We found that a subpopulation of neurons in the central and basal nucleus of the amygdala discharges before and during cataplexy [20, 21]. A population of wake–active neurons localized to the cortical nucleus of the amygdala decreases discharge before and during cataplexy [20]. A population of GABAergic and glycinergic neurons linked to motor inhibition in the medial medulla region is known to be critically involved in the inhibition of muscle tone in REM sleep, fires prior to and during cataplexy [19, 22]. Heart rate increases precede and continue during cataplectic attacks in narcoleptic dogs [23].
To summarize, hypocretin neuronal loss is sufficient to cause narcolepsy. The number and size of Hcrt neurons are greatly affected by opioids. The largest descending projection of the Hcrt neuronal group is to the locus coeruleus [24], which ceases activity immediately prior to and during cataplexy.
Broughton and Mamelak showed that narcolepsy-cataplexy in human narcoleptics could be successfully treated with SXB (also called gamma-hydroxybutyrate) [25–27]. Chronic administration of this drug over a period of 4 weeks or longer produces a progressive decrease in the symptoms of narcolepsy including cataplexy and daytime sleepiness [28]. SXB appears to act through the endogenous opioid system, and its metabolic and pharmacological effects are blocked by naloxone [29], although its exact mechanism of action of SXB in the reduction of the symptoms of narcolepsy is unclear. As in human narcoleptics, SXB decreases cataplexy and consolidates waking in Hcrt-depleted (narcoleptic) mice [30–32]. These studies have guided the dosages used in the current study. Because of the central role of Hcrt neurons and their descending projections to the locus coeruleus in narcolepsy [17, 24], we examined the effects of chronic SXB administration on these neuronal groups.
Methods
Animals
Wild-type C57BL/6J male mice from Jackson Labs, between 10 and 12 weeks old were used. They were housed at a constant temperature of 23 ± 2°C with lights on at 07:00 am and lights off at 07:00 pm. All procedures were approved by the Institutional Animal Care and Use Committee of the University of California at Los Angeles and the Veterans Administration Greater Los Angeles Healthcare System.
Dose–response relation of sodium oxybate to the number and morphology of hypocretin cells in WT mice
Ten-week-old mice, 6 per group, were treated with either saline or sodium oxybate (supplied as sodium oxybate oral solution, 500 mg/mL) (Xyrem, Jazz Pharmaceuticals) at 150, 300, 600, or 1200 mg/kg, IP for 14 days. Two injections at each dose were given per day at ZT 2 (09:00 am) and ZT 6 (01:00 pm). Two hours after the last dose on the 14th day of treatment, the animals were euthanized with Fatal Plus (100 mg/kg), and then perfused transcardially with saline followed by 4% paraformaldehyde. Injections were staggered so that six animals (1–2 animals from each dose) were euthanized per day over 5 days during a 1.5-hour window. Brains were removed from the skull and immersed in 4% paraformaldehyde for 24 hours. The brains were then transferred to 20% sucrose until they sunk, then to 30% sucrose for 3 days. Brains were cut into 40 μm sections with a freezing microtome and saved in cryoprotectant until immunohistochemical processing.
For Hcrt immunostaining, brain sections were washed in PBS, treated with 0.5% H2O2 for 30 minutes, then washed in PBS again. Sections were incubated in blocking agents (1% normal goat serum + 0.3% TritonX-100 in PBS) for 2 hours followed by Hcrt-1 antibody (Phoenix Pharmaceuticals, Burlingame, CA USA, Cat # H-003–30, Lot 01651-10, Rabbit; 1:10 000) for 72 hours at 5°C. Hcrt-1 and Hcrt-2 can be found co-expressed in the majority of hypocretin neurons.
After 72 hours, sections were washed in PBS, incubated for 2 hours in secondary antibody (0.3% TritonX-100 + 1% normal goat serum + 1:500 anti-rabbit secondary antibody), followed by avidin-biotin-peroxidase complex (ABC Elite Kit, Vector Laboratories, Burlingame, CA USA), for 2 hours each at room temperature. The tissue-bound peroxidase was visualized by diaminobenzidine (DAB) (Vector Laboratories).
Hypocretin cell number and distribution were determined using stereological techniques
Brains were coded and analyses were always carried out by investigators blind to treatment condition, using stereological techniques on a 1-in-3 series. We used a Nikon E600 microscope with three-axis motorized stage, video camera, Neurolucida interface, and Stereo Investigator software (MicroBrightField Corp., Williston, VT USA). Nine sections were counted per animal. The average surface area of 127.2 ± 24.8 Hcrt cells per animal from representative sections was quantified.
Western blot analysis of hypocretin protein in the hypothalamus
Twelve WT mice (n = 6/group) received a daily intraperitoneal administration of either saline (0.05 mL) or SXB (600 mg/kg, twice per day at ZT2 and ZT6) for 14 days. Animals (six each, three from each group, over two days) were euthanized 2 hours after the last dose at the end of the experiments with an overdose of Fatal Plus (100 mg/kg). The brain was removed from the skull and the hypothalamus was extracted. Total proteins (25 µg) for each sample were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 4%–20% 10-well TGX gels (Bio-Rad, Cat. # 456-1094) and blotted on to polyvinylidene fluoride (PVDF) membranes (Bio-Rad, Cat. # 162-0174). After blocking, membranes were incubated with primary antibodies (Prepro-Hcrt, Millipore, Cat. # AB3096; and β-actin, Millipore, Cat # MABT523, respectively) overnight at 4°C. Horseradish peroxidase-conjugated mouse anti-rabbit IgG (Invitrogen, Cat. # 31460) was used to enhance chemiluminescence (West Femto, Fisher Scientific, Cat. # 34094). Signal was detected by CL-XPosure™ X-ray films (Thermo Scientific, Cat. # 34090) and quantified through optical density measurements (Image J, NIH). The immunoreactivity of Prepro-Hcrt was normalized to that of β-actin. Each experiment was repeated four times and averaged for the final result.
TH fluorescence immunohistochemistry
We examined TH expression intensity as a function of SXB administration in locus coeruleus. Hcrt neurons project to virtually all brain regions, ending on Hcrt-receptors 1 and 2. The distribution of these receptors varies between brain regions [33–35]. TH is a rate-limiting factor in the synthesis of dopamine and norepinephrine. All immunohistochemical procedures were performed on 40 μm free-floating sections. These sections were taken from animals treated with 300 and 1200 mg/kg SXB and used in Hcrt cell number/size experiments. The sections were incubated in primary antibody (sheep anti-TH, ab-113, Abcam, USA, 1:1000, Lot # GR3277795-16) overnight at room temperature in PBS with 0.25% Triton X-100 (PBST), followed by incubation with lights off and samples wrapped in aluminum foil, in the corresponding secondary antibody tagged with fluorophores that match our microscope filters (Alexa Fluor 488 donkey anti-sheep, Cat # A-11015, ThermoFisher Scientific). Tissues were mounted and coverslipped using Vector Shield anti-fade mounting media (H 1000, Vector Laboratories, Lot # 2E00806). To quantify the number of norepinephrine-producing neurons, the same mounting media containing 4’, 6-diamidino-2-phenylindole (DAPI) was used (H 1200, Vector Laboratories, Lot # 2E0815). All tissue sections from experimental and control animals were stained at the same time and with the same antibody lot.
The distribution of TH cells was assessed using a Zeiss LSM 900 confocal microscope (Imager.Z2 AX10, Jena, Germany) equipped with the appropriate lasers. Every section of the locus coeruleus was imaged at 1 μm optical planes. Superstack fluorescent images collected with standardized parameters were obtained using Zeiss ZEN Blue software. Quantification was performed bilaterally. Mean Fluorescence Intensity (MFI) and area measurements were done using the open-source imaging software Fiji [36]. The area was defined by the presence of the TH+ neuronal bodies in each structure. Representative sections of the locus coeruleus at the plane containing the maximum density of TH+ neurons were selected for comparison across treatment groups. An area of interest was defined around the cell cluster. The MFI of the area in the stack of the selected section was used for analysis and bilateral areas were averaged.
In data sets that were not normally distributed or with outlier data points (e.g. cell number and cell size), we employed nonparametric tests of significance. In the remaining studies, we employed parametric tests.
Results
Cell number analysis
There was, on average, a decrease of ~15% in the number of detectable Hcrt cell neurons after repeated SXB treatments for 14 days. This difference was not statistically significant, (Fligner–Wolfe nonparametric test comparing the difference between saline and all SXB conditions, FW = 342, p > 0.05) (Figure 1).
Figure 1.
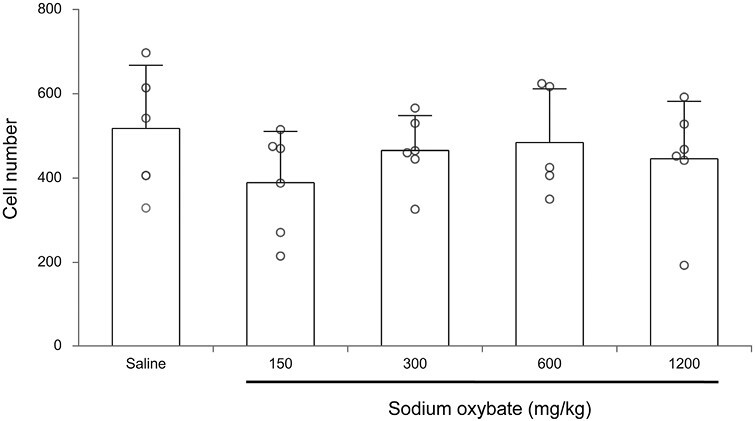
Average number of Hcrt-1 expressing cells after 2 weeks, twice daily, treatment with saline or different doses of SXB. The differences are not significant.
Cell size analysis
The size of Hcrt neurons in SXB-treated mice increased significantly by an average of 8% across doses relative to saline controls. This difference was significant (Fligner–Wolfe test, FW = 420, p = 0.019) (Figures 2 and 3).
Figure 2.
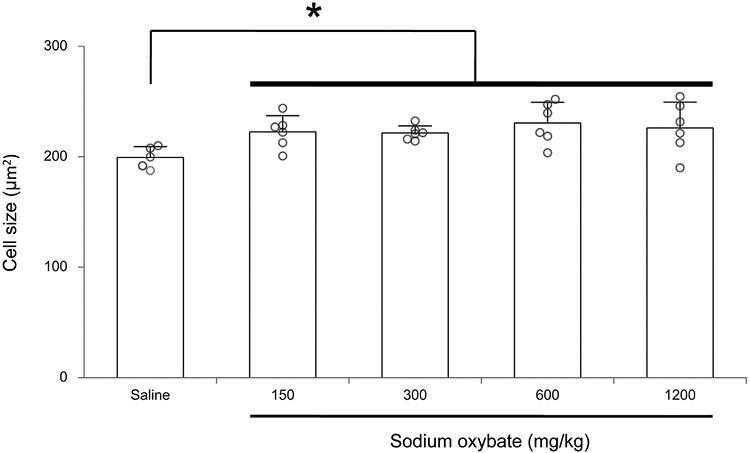
Average size of Hcrt-1 expressing cells after 2 weeks, twice daily, treatment with saline or different doses of SXB. Hcrt neuron size was increased by SXB (*p < 0.02, Fligner-Wolfe test, compared to saline control).
Figure 3.
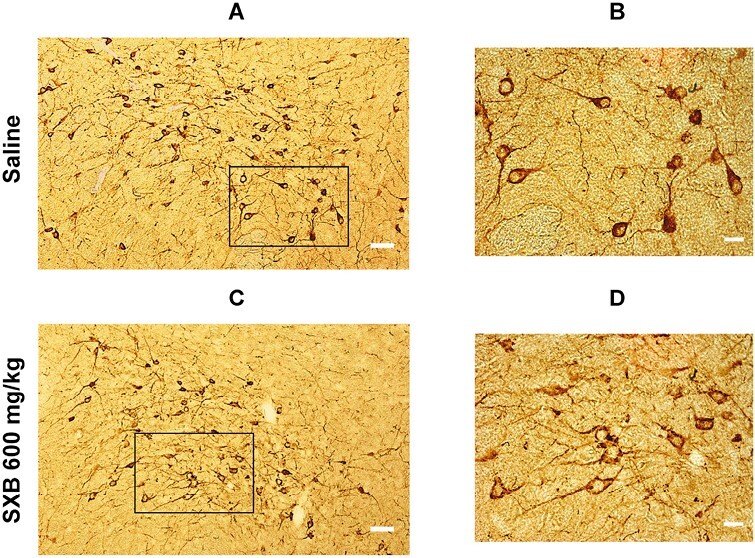
Light microscope pictures of Hcrt cell (brown) distribution in the hypothalamus of a saline control (A and B) and a SXB (600 mg/kg) treated (C and D) animal. (A) Picture from a saline control animal taken at 10x magnification. (B) 40x magnification of the box area in A. (C) Picture from a SXB-treated animal taken at 10x. (D) 40x magnification of the box area in C. White scale bars: 50 μm in A and C, 20 μm in B and D.
Western blot analysis
There was a nonsignificant reduction of Prepro-Hcrt level in the hypothalamus of SXB-treated animals (t-test, df = 9, t = 1.993, p = 0.077). Figure 4 shows western blot assessment of Prepro-Hcrt level in the hypothalamus compared to β-actin (control “housekeeping” protein) after twice daily doses of 600 mg/kg of SXB for 2 weeks (n = 6 in each group).
Figure 4.
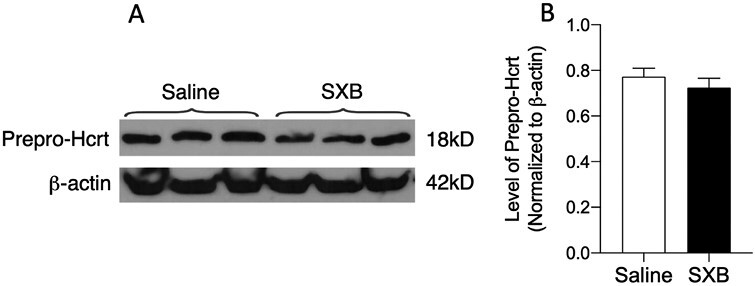
Western blots. (A) Prepro-Hcrt and β-actin after 2-week daily dose of 600 mg/kg of SXB. (B) Level of prepro-Hcrt in saline and SXB conditions normalized to β-actin. SXB did not produce a significant change in Hcrt level.
Locus coeruleus immunofluorescence
SXB at 300 and 1200 mg/kg produced a reduction in the TH fluorescent intensity in the locus coeruleus, with a decrease of 37.1% and 47.7%, respectively compared to the saline condition. (ANOVA, F(2,15) = 5.901, p = 0.013). Tukey post hoc comparisons showed significant differences between saline and SXB 300 mg/kg conditions (p = 0.041) and between saline and SXB 1200 mg/kg conditions (p = 0.016). Figure 5A shows the median of MFI across sections of the locus coeruleus following 14 days of saline or SXB. Figure 5B shows representative confocal TH immunofluorescent images of locus coeruleus.
Figure 5.
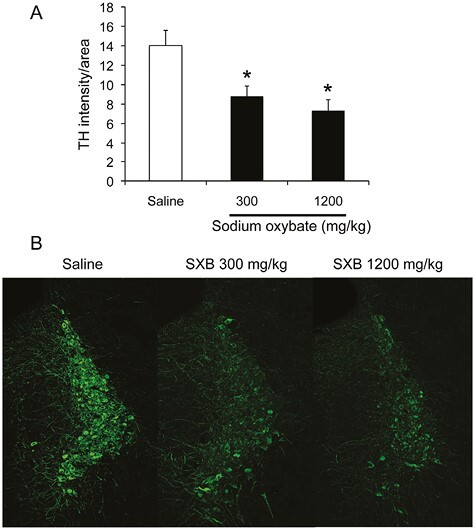
(A) Median TH Mean Fluorescence Intensity (MFI) in the locus coeruleus of saline and SXB-treated animals. MFI was significantly decreased by SXB (p < 0.05, Tukey post-hoc, ciompared to saline control). (B) Representative confocal images of fluorescent TH cells in the locus coeruleus of saline and SXB-treated animals.
Discussion
Medically useful drugs are often found through trial and error, rather than through an understanding of the disease mechanism. This was the case with SXB, as outlined in the Introduction. SXB is a naturally occurring substance that is a precursor to GABA, glutamate, and glycine. It acts on the SXB (GHB) receptor and is a weak agonist at the GABAB receptor [32, 37–40].
We find that doses of SXB that significantly reduce TH expression in LC do not affect Hcrt cell number. These findings differ from the anatomical changes produced in these cell types by doses of morphine that reduce cataplexy in animal models of narcolepsy and in human narcoleptics [11, 12]. These findings lead us to reject the hypothesis that SXB reduces cataplexy by the same means as opiates. The changes we observe in LC may be direct results of SXB action on LC neurons or may be mediated by Hcrt or other systems projecting to or otherwise affecting LC. Whether changes in LC are critical to the changes in cataplexy that both drugs produce remains to be determined, although extensive evidence suggests that LC activity is tightly linked to cataplexy [17–19, 41].
Narcolepsy is now thought to be a disease of the REM sleep-generating system, caused by the loss of Hcrt neurons. Cataplexy is due to a pontomedullary system that removes noradrenergic facilitation and increases GABAergic and glycinergic inhibition of motoneurons. The major Hcrt descending projection is to the noradrenergic locus coeruleus. However, muscle tone suppression in REM sleep does not require Hcrt neurons as is demonstrated by the spontaneous, regular induction of muscle tone suppression in REM sleep in decerebrate animals, i.e. in animals in which Hcrt neurons have been disconnected or removed [42]. Since we had seen that opioids produce major changes in both Hcrt and locus coeruleus neurons, and a reduction in the symptoms of narcolepsy [11, 12] we wondered if SXB would cause similar changes. But clearly, the changes in Hcrt and locus coeruleus caused by doses of SXB that reverse cataplexy and sleepiness [30, 31] are not the same as those caused by opioids. Indeed in terms of neuronal morphology, they seem to be opposite.
Despite the differences in the effects of SXB and opioids on Hcrt and locus coeruleus neurons, ultimately both SXB and opioids have similar effects on the systems controlling both muscle tone and sleepiness. The point of “downstream” neuronal convergence of these drugs remains to be determined.
Contributor Information
Ming-Fung Wu, Department of Psychiatry and Biobehavioral Sciences, David Geffen School of Medicine, University of California, Los Angeles, CA, USA; Neurobiology Research, VA Greater Los Angeles Healthcare System, North Hills, CA, USA.
Thomas C Thannickal, Department of Psychiatry and Biobehavioral Sciences, David Geffen School of Medicine, University of California, Los Angeles, CA, USA.
Songlin Li, Department of Psychiatry and Biobehavioral Sciences, David Geffen School of Medicine, University of California, Los Angeles, CA, USA.
Ronald McGregor, Department of Psychiatry and Biobehavioral Sciences, David Geffen School of Medicine, University of California, Los Angeles, CA, USA.
Yuan-Yang Lai, Department of Psychiatry and Biobehavioral Sciences, David Geffen School of Medicine, University of California, Los Angeles, CA, USA.
Jerome M Siegel, Department of Psychiatry and Biobehavioral Sciences, David Geffen School of Medicine, University of California, Los Angeles, CA, USA; Neurobiology Research, VA Greater Los Angeles Healthcare System, North Hills, CA, USA; Brain Research Institute, University of California, Los Angeles, CA, USA.
Funding
Supported by Jazz Pharmaceuticals, NIH DA034748, and the Medical Research Service of the Department of Veterans Affairs.
Disclosure Statement
Financial disclosure: Supported by Jazz Pharmaceuticals, NIH DA034748, and the Medical Research Service of the Department of Veterans Affairs. Nonfinancial disclosure: No conflicts of interest.
References
- 1. Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690 [DOI] [PubMed] [Google Scholar]
- 2. Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27(3):469–474. doi: 10.1016/s0896-6273(00)00058-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thannickal TC, Moore RY, Aldrich M, Albin R, Cornford M, Siegel JM. Human narcolepsy is linked to reduced number, size and synaptic bouton density in hypocretin-2 labeled neurons. Abstr Soc Neurosci. 2000;26:2061. [Google Scholar]
- 4. Thannickal TC, Nienhuis R, Siegel JM. Localized loss of hypocretin (orexin) cells in narcolepsy without cataplexy. Sleep. 2009 Aug 1;32(8):993–998. doi: 10.1093/sleep/32.8.993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999 Aug 20;98(4):437–451. doi: 10.1016/s0092-8674(00)81973-x [DOI] [PubMed] [Google Scholar]
- 6. Tabuchi S, Tsunematsu T, Black SW, et al. Conditional ablation of orexin/hypocretin neurons: a new mouse model for the study of narcolepsy and orexin system function. J Neurosci. 2014;34(19):6495–6509. doi: 10.1523/JNEUROSCI.0073-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lin L, Faraco J, Kadotani H, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor gene. Cell. 1999;98:365–376. [DOI] [PubMed] [Google Scholar]
- 8. John J, Thannickal TC, McGregor R, et al. Greatly increased numbers of histamine cells in human narcolepsy with cataplexy. Ann Neurol. 2013 Dec;74(6):786–793. doi: 10.1002/ana.23968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Valko PO, Gavrilov YV, Yamamoto M, et al. Increase of histaminergic tuberomammillary neurons in narcolepsy. Ann Neurol. 2013 Dec;74(6):794–804. doi: 10.1002/ana.24019 [DOI] [PubMed] [Google Scholar]
- 10. Shan L, Balesar R, Swaab DF, Lammers GJ, Fronczek R. Reduced numbers of corticotropin-releasing hormone neurons in narcolepsy type 1. Ann Neurol. 2022 Feb 1;91(2):282–288. doi: 10.1002/ana.26300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thannickal TC, John J, Shan L, et al. Opiates increase the number of hypocretin-producing cells in mouse and human brain, and reverse cataplexy in a mouse model of narcolepsy. Sci Transl Med. 2018;10(447):piieaao4953. doi: 10.1126/scitranslmed.aao4953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Donjacour CEHM, Lammers GJ, Siegel JM. Striking cessation of cataplexy by opioids. J Sleep Res. 2018;28(4):e12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McGregor R, Shan L, Wu MF, Siegel JM. Diurnal fluctuation in the number of hypocretin/orexin and histamine producing: implication for understanding and treating neuronal loss. PLoS One. 2017;12(6):e0178573. doi: 10.1371/journal.pone.0178573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McGregor R, Wu M-F, Holmes B, et al. Hypocretin/orexin interactions with norepinephrine contribute to the opiate withdrawal syndrome. J Neurosci. 2022;42:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bogan RK, Roth T, Schwartz J, Miloslavsky M. Time to response with sodium oxybate for the treatment of excessive daytime sleepiness and cataplexy in patients with narcolepsy. J Clin Sleep Med. 2015;11:427–432. doi: 10.5664/jcsm.4598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu MF, John J, Boehmer LN, Yau D, Nguyen GB, Siegel JM. Activity of dorsal raphe cells across the sleep-waking cycle and during cataplexy in narcoleptic dogs. J Physiol. 2004;554(1):202–215. doi: 10.1113/jphysiol.2003.052134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu MF, Gulyani S, Yao E, Mignot E, Phan B, Siegel JM. Locus coeruleus neurons: cessation of activity during cataplexy. Neuroscience. 1999;91:1389–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lai YY, Kodama T, Schenkel E, Siegel JM. Behavioral response and transmitter release during atonia elicited by medial medullary stimulation. J Neurophysiol. 2010;104(4):2024–2033. doi: 10.1152/jn.00528.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lai YY, Kodama T, Siegel JM. Changes in monoamine release in the ventral horn and hypoglossal nucleus linked to pontine inhibition of muscle tone: an in vivo microdialysis study. J Neurosci. 2001;21:7384–7391. doi: 10.1523/JNEUROSCI.21-18-07384.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gulyani S, Wu M-F, Nienhuis R, John J, Siegel JM. Cataplexy-related neurons in the amygdala of the narcoleptic dog. Neuroscience. 2002;112(2):355–365. doi: 10.1016/s0306-4522(02)00089-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hasegawa E, Miyasaka A, Sakurai K, Cherasse Y, Li Y, Sakurai T. Rapid eye movement sleep is initiated by basolateral amygdala dopamine signaling in mice. Science. 2022 Mar 4;375(6584):994–1000. doi: 10.1126/science.abl6618 [DOI] [PubMed] [Google Scholar]
- 22. Siegel JM, Nienhuis R, Fahringer H, et al. Neuronal activity in narcolepsy: identification of cataplexy related cells in the medial medulla. Science. 1991;252:1315–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Siegel JM, Tomaszewski KS, Fahringer H, Cave G, Kilduff T, Dement C. Heart rate and blood pressure changes during sleep-waking cycles and cataplexy in narcoleptic dogs. Am J Physiol. 1989;256:H111–H119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peyron C, Tighe DK, van den Pol AN, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18(23):9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Broughton R, Mamelak M. The treatment of narcolepsy-cataplexy with nocturnal gamma-hydroxybutyrate. Can J Neurol Sci. 1979;6:1–6. doi: 10.1017/s0317167100119304. [DOI] [PubMed] [Google Scholar]
- 26. Broughton R, Mamelak M. Gamma-hydroxybutyrate in the treatment of narcolepsy: a preliminary report. In: Guilleminault C, Dement W, eds. Narcolepsy. New York: Spectrum Publications, 1976:659–668. [Google Scholar]
- 27. Broughton R, Mamelak M. Effects of gamma hydroxy-butyrate on sleep/waking patterns in narcolepsy-cataplexy. Can J Neurol Sci. 1980;9:23–31. [PubMed] [Google Scholar]
- 28. Dauvilliers Y, Arnulf I, Foldvary-Schaefer N, et al. Safety and efficacy of lower-sodium oxybate in adults with idiopathic hypersomnia: a phase 3, placebo-controlled, double-blind, randomised withdrawal study. Lancet Neurol. 2022;21(1):53–65. doi: 10.1016/S1474-4422(21)00368-9 [DOI] [PubMed] [Google Scholar]
- 29. Mamelak MG. an endogenous regulator of energy metabolism. Neurosci Biobehav Rev. 1989;13:187–198. [DOI] [PubMed] [Google Scholar]
- 30. Hamieh M, Fraigne J, Peever J. The effect of sodium oxybate on cataplexy in orexin knockout mice. Sleep. 2021;44(suppl_2):A2–A3. doi: 10.1093/sleep/zsab072.005 [DOI] [Google Scholar]
- 31. Mochizuki T, Clark E, Scammell T. Sodium oxybate consolidates wakefulness in orexin knockout mice. 20th Annual Meeting of the Associated-Professional-Sleep-Societies 2006;29:A1. [Google Scholar]
- 32. Black SW, Morairty SR, Chen TM, et al. GABAB agonism promotes sleep and reduces cataplexy in murine narcolepsy. J Neurosci. 2014;34(19):6485–6494. doi: 10.1523/JNEUROSCI.0080-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marcus JN, Aschkenasi CJ, Lee CE, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190 [DOI] [PubMed] [Google Scholar]
- 34. Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438(1–2):71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- 35. Coleman P, De Lecea L, Gotter A, et al. Orexin receptors in GtoPdb v.2021.3. IUPHAR/BPS Guide to Pharmacology CITE. 2021;2021(3):10.2218/gtopdb/f51/2021.3. doi: 10.2218/gtopdb/f51/2021.3. Epub 2021 Sep 2. PMID: 34927075; PMCID: PMC8682808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schindelin J, Aganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maitre M, Humbert JP, Kemmel V, Aunis D, Andriamampandry C. Mecanismes d’action d’un medicament detourne: le gamma-hydroxybutyrate. Med Sci (Paris). 2005;21(3):284–289. doi: 10.1051/medsci/2005213284 [DOI] [PubMed] [Google Scholar]
- 38. Cash CD, Gobaille S, Kemmel V, Andriamampandry C, Maitre M. Gamma-hydroxybutyrate receptor function studied by the modulation of nitric oxide synthase activity in rat frontal cortex punches. Biochem Pharmacol. 1999;58(11):1815–1819. doi: 10.1016/s0006-2952(99)00265-8 [DOI] [PubMed] [Google Scholar]
- 39. Huang YS, Guilleminault CN. Action of two gamma-aminobutyric acid type B agonists, baclofen and sodium oxybate. Pediatr Neurol. 2009;41(1):9–16. [DOI] [PubMed] [Google Scholar]
- 40. Andriamampandry C, Taleb O, Kemmel V, Humbert JP, Aunis D, Maitre M. Cloning and functional characterization of a gamma-hydroxybutyrate receptor identified in the human brain. FASEB J. 2007;21(3):885–895. doi: 10.1096/fj.06-6509com [DOI] [PubMed] [Google Scholar]
- 41. Kodama T, Kimura M. Arousal effects of orexin-A correlate with GLU release from the locus coeruleus in rats. Peptides. 2002;23(9):1673–1681. doi: 10.1016/s0196-9781(02)00109-2 [DOI] [PubMed] [Google Scholar]
- 42. Siegel JM. Rapid eye movement sleep control and function. In: Kryger MK, Roth T, Goldstein CA, Dement WC, eds. Principles and Practice of Sleep Medicine. Seventh ed. Amsterdam, Netherlands: Elsevier, 2022:68–86. [Google Scholar]


