Abstract
The clinical application of stem cells offers great promise as a potential avenue for therapeutic use in neurodegenerative diseases. However, cell loss after transplantation remains a major challenge which currently plagues the field. Based on our previous findings that fibroblast growth factor 21 (FGF-21) protected neurons from glutamate excitotoxicity and that upregulation of FGF-21 in a rat model of ischemic stroke was associated with neuroprotection, we proposed that overexpression of FGF-21 protects bone marrow-derived mesenchymal stem cells (MSCs) from apoptosis. To test this hypothesis, we examined whether the detrimental effects of apoptosis can be mitigated by the transgenic overexpression of FGF-21 in MSCs. FGF-21 was transduced into MSCs by lentivirus and its overexpression was confirmed by quantitative PCR. Moreover, FGF-21 overexpression did not stimulate the expression of other FGF family members, suggesting it does not activate a positive feedback system. The effects of hydrogen peroxide (H2O2), tumor necrosis factor-α (TNF-α), and staurosporine, known inducers of apoptosis, were evaluated in FGF-21 overexpressing MSCs and mCherry control MSCs. Caspase 3 and 7 activity was markedly and dose-dependently increased by all three stimuli in mCherry MSCs. FGF-21 overexpression robustly suppressed caspase activation induced by H2O2 and TNF-α, but not staurosporine. Moreover, the assessment of apoptotic morphological changes confirmed the protective effects of FGF-21 overexpression. Taken together, these results provide compelling evidence that FGF-21 plays a crucial role in protecting MSCs from apoptosis induced by oxidative stress and inflammation, and merits further investigation as a strategy for enhancing the therapeutic efficacy of stem cell-based therapies.
Keywords: Caspase activation, oxidative stress, hydrogen peroxide, TNF-α
1. Introduction
Stem cell-based therapy has recently emerged as a feasible therapeutic approach for the treatment of neurodegenerative diseases. However, the poor survival of transplanted cells including mesenchymal stem cells (MSCs) presents a significant obstacle for enhancing the efficacy of stem cell-based therapy. Excessive oxidative stress has been implicated in the pathogenesis of numerous diseases including neurodegenerative disorders. In diseased tissues, increased levels of reactive oxygen species (ROS) can overwhelm endogenous antioxidant defense systems and lead to increased damage to DNA, proteins, and lipids (Butterfield & Halliwell, 2019; Karihtala & Soini, 2007). Furthermore, a common feature of a damaged microenvironment are tissues that express hypoxic, apoptotic or inflamed regions (Olson et al., 2012). Because MSCs migrate towards areas of tissue damage, the identification of factors that counteract oxidative stress-induced apoptosis is essential for enhancing the therapeutic application of stem cells. Therefore, strategies to bolster the survival and efficacy of stem cells may lead to improved clinical outcomes. One possible strategy to accomplish this goal is by the genetic modification of MSCs with growth factors that protect against apoptotic stimuli such as oxidative stress and inflammation.
Fibroblast growth factor (FGF-21), is a novel metabolic regulator that acts as a circulating hormone in regulating gluconeogenesis and lipid metabolism (Staiger et al., 2017). Although it is highly expressed in the pancreas and liver, other tissues such as brown and white adipose tissue also express FGF-21. Alterations of FGF-21 levels in humans have been associated with type 2 diabetes, coronary heart disease, and obesity (Degirolamo et al., 2016). Our previous research demonstrated that FGF-21 was significantly upregulated in primary neurons and astrocytes by the mood stabilizers lithium and VPA through Akt-1 dependent mechanisms, and that it provided robust protection from excitotoxic cell death induced by glutamate treatment and ischemic stroke (Leng et al., 2016; Leng et al., 2015; Z. Wang et al., 2016).
Several studies have demonstrated the importance of FGF-21 in protecting against apoptosis. For example, the overexpression of FGF-21 in cardiomyocytes protected against ER stress-mediated apoptosis (Liang et al., 2017). Doxirubicin-induced cardiotoxicity was alleviated by FGF-21 via regulation of the SIRT1/LKB1/AMPK signaling pathway (Wang et al., 2017). Under diabetic conditions, deletion of FGF-21 in mice exacerbated apoptosis as indicated by enhanced TUNEL staining and caspase 3 activation (Yang et al., 2018). Conversely, the administration of FGF-21 reversed this effect. Taken together, these studies indicate that FGF-21 plays a crucial role in protecting against apoptosis. Herein, we investigated whether MSCs genetically engineered to overexpress FGF-21 would render protection against apoptotic stimuli encountered after transplantation in a pathological tissue including oxidative stress by hydrogen peroxide, inflammation by TNF-α, and protein kinase C inhibition by staurosporine.
2. Materials and methods
2.1. Mesenchymal stem cell (MSC) culture
Gibco® C57BL/6 mouse MSCs derived from bone marrow were obtained from Invitrogen Life Technologies (Carlsbad, CA) as reported (Linares et al., 2016). MSCs were cultured in DMEM/F-12 GlutaMAX™ + 10% FBS + 5 μg/ml gentamicin (Invitrogen Life Technologies). Cells from passage 4–7 were used in the experiments. Hydrogen peroxide (H2O2) and staurosporine were purchased from Sigma-Aldrich (St. Louis, MO). Tumor necrosis factor alpha (TNF-α) was obtained from PeproTech Incorporated (Rocky Hill, NJ).
2.2. Generation of FGF-21 overexpressing (OE) MSCs
MSCs were transduced with lentivirus particles containing a bicistronic (IRES) vector cassette with an mCherry reporter gene (control) or the coding sequence of Mus musculus FGF-21 containing mCherry reporter gene (GenCopoeia, Rockville, MD) under the control of the human elongation factor 1-α (EF1-α) promoter. MSCs were seeded at 5,000 cells/well in a 12-well plate and were transduced at a multiplicity of infection (MOI) of 30 as described (Linares et al., 2016). Genetic modification using lentivirus did not alter the endogenous properties of the MSCs such as the ability to differentiate into osteoblasts and adipocytes as determined by alizarin red mineralization assay and Oil Red O staining, respectively. Moreover, differences in morphology and proliferation were unaltered by FGF-21 overexpression (Shahror et al., 2020).
2.3. Flow cytometry analysis
mCherry control and FGF-21 overexpressing MSCs underwent three rounds of fluorescence-activated cell sorting (FACS) to isolate a highly purified population of mCherry positive cells following lentiviral transduction as previously reported (Linares et al., 2016).
2.4. RNA extraction
Total RNA was isolated from mCherry control MSCs and FGF-21 overexpressing MSCs using a RNeasy mini kit according to the manufacturer’s instructions (Qiagen, Valencia, CA). Following extraction, the RNA samples were DNase-treated with a DNA-free kit (Ambion ® Invitrogen Life Technologies). The RNA concentration was determined using Nano Drop spectrophotometer (Wilmington, DE) and all samples had absorbance measurements (OD A260/A280 ratio) ranging from 1.8–2.0.
2.5. Gene expression analysis
Quantitative real-time RT-PCR was performed as previously described (Linares et al., 2011). Primers were used to amplify Mus musculus FGF-2 (forward: 5’-ATGAAGGAAGATGGACGG CT-3’, reverse: 5’-TTCTGTCCAGGTCCCGTTTT-3’), FGF-9 (forward: 5’- TCCCTCTCTGTC TGCAACTG-3’, reverse: CGTCCTGCACACCGAAATAG-3’), FGF-15 (forward: 5’-CATCTT CATCCAGGCCAAGC-3’, reverse: GAACGGATCCATGCTGTCAC-3’), and FGF-17 (forward: 5’-AACTACACGGCCTTCCAGAA-3’, reverse: 5’-TTCAGCGTGGTTGGGAAAAG-3’) and FGF-21 (forward: 5’-TGGAGATCAGGGAGGATGGA-3’, reverse: 5’-ATTGTAACCGTCCTC CAGCA-3’). Relative gene expression levels were examined and the housekeeping gene peptidylprolyl isomerase A (PPIA) was used as an internal control. The fold change was calculated according to the formula 2−ΔΔCt.
2.6. Determination of apoptosis
Apoptosis was assessed by evaluating caspase 3,7 activity using Caspase-Glo 3,7 assay (Promega, Madison, WI) as reported (Linares et al., 2009). To induce apoptosis, mCherry control and FGF-21 overexpressing MSCs were treated with H2O2 (50 μM, 100 μM, 200 μM, and 400 μM), TNF-α (10 ng/ml, 20 ng/ml, and 50 ng/ml), staurosporine (2 nM, 10 nM, and 50 nM) or vehicle for 24 hours. Caspase 3,7 activity was determined after a 30-minute incubation by measuring luminescence in white-walled plates. Apoptotic morphology was evaluated by examining the presence of apoptotic bodies using a phase contrast inverted microscope and condensed nuclei by Hoechst 33258 (Invitrogen, Carlsbad, CA) staining in mCherry control and FGF-21 overexpressing MSCs that were exposed to 200 μM H2O2 for 24 hours. Media was removed and MSCs were rinsed with 1X PBS followed by a 15-minute incubation with 4% paraformaldehyde. MSCs were rinsed 3 times with 1X PBS, permeabilized with 0.1 % Triton X-100, and incubated with Hoechst 33528 (1:1000) for 10 minutes. Cells were imaged at 20X magnification by phase contrast (bright field) and fluorescent microscopy (nuclear images) using UV/488 dual excitation.
2.7. Statistical analysis
Results are expressed as means ± SEM and were analyzed using Student’s t-test or ANOVA (one-way) (GraphPad Prism, San Diego, CA) as appropriate. Post hoc analysis was performed using Tukey’s test. Values were considered significant when P < 0.05.
3. Results
3.1. FGF-21 overexpression in MSCs
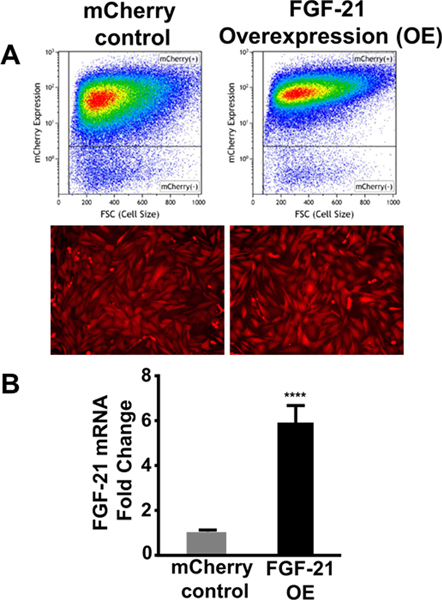
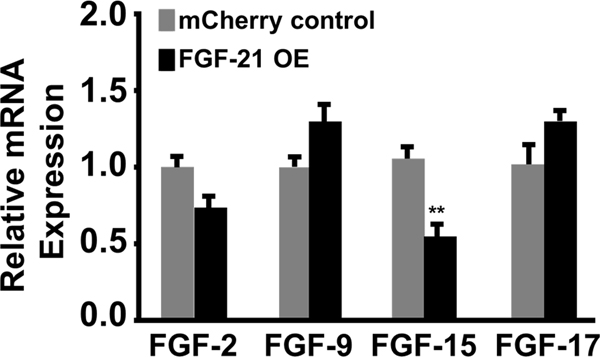
To evaluate the efficacy of FGF-21 in protecting bone marrow-derived MSCs from apoptotic stimuli, FGF-21 was transgenically overexpressed in MSCs using lentivirus. Following the primary transduction, mCherry control and FGF-21 overexpressing (OE) MSCs underwent three successive rounds of FACS to isolate a highly enriched population of mCherry expressing cells. Flow cytometric analysis and imaging by fluorescence microscopy demonstrated that over 97% of the MSCs exhibited mCherry fluorescence in the mCherry control group and the FGF-21 overexpression group (Figure 1A). Figure 1B shows increased FGF-21 mRNA expression (approximately 6-fold) as determined by quantitative RT-PCR in MSCs transduced with lentiviral vector expressing FGF-21 compared to mCherry control. FGF-21 protein expression was 7-fold higher in FGF-21 overexpressing MSCs relative to mCherry control MSCs as determined by ELISA (Shahror et al., 2020). Moreover, the mRNA expression levels of four other important FGF family members (FGF-2, FGF-9, FGF-15, and FGF-17) were not significantly upregulated (Figure 2), suggesting that a positive feedback loop was not induced by FGF-21 overexpression. Interestingly, the levels of FGF-15 was decreased by almost 50% in the FGF-21 overexpressing MSCs group compared with the mCherry control, suggesting a possible negative cross-talk between these two close members of the FGF family.
Figure 1.
Generation of FGF-21 overexpressing (OE) MSCs. MSCs were stably transduced with lentiviral vector encoding mCherry reporter (control) or the coding sequence of Mus musculus FGF-21 and mCherry reporter contained in a bicistronic vector. Transduced MSCs underwent FACS to enrich the population of mCherry expressing MSCs. A) Flow cytometric analysis and in vitro fluorescent microscopy confirms robust mCherry expression in FGF-21 overexpressing MSCs and mCherry control MSCs. B) Expression of FGF-21 in transduced MSCs as determined by quantitative real time RT-PCR analysis. Values are presented as mean ± SEM (n=6 per group); **** P < 0.0001 vs mCherry control (Student’s t-test).
Figure 2.
FGF-21 overexpression does not induce the expression of other FGF family members. The mRNA expression levels of FGF-2, FGF-9, FGF-15, and FGF-17 as determined by quantitative real time RT-PCR analysis. Values are presented as mean ± SEM (n=6 per group); ** P < 0.001 vs mCherry control (Student’s t-test).
3.2. FGF-21 overexpressing MSCS attenuate caspase 3,7 activation in the presence of apoptotic stimuli
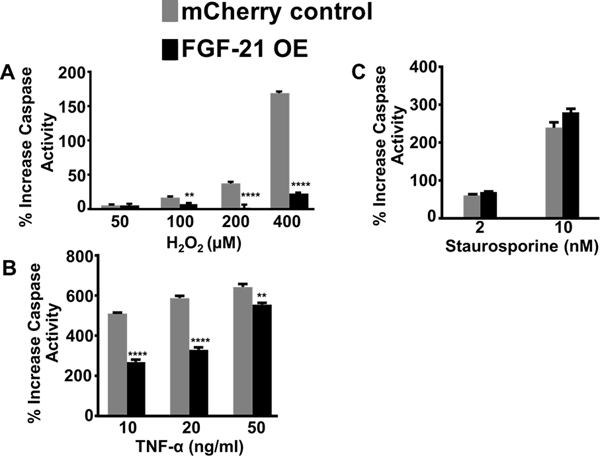
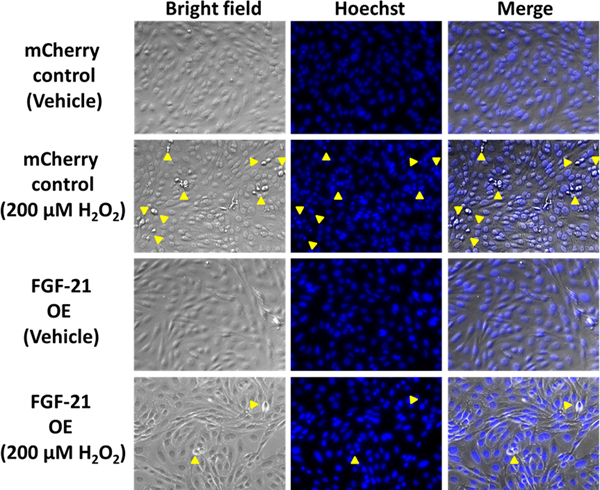
A common feature of a damaged microenvironment are tissues that express hypoxic, apoptotic or inflamed regions (Olson et al., 2012). To mimic the harsh microenvironment (reactive oxygen species and local inflammation) that MSCs encounter after transplantation in a pathological tissue, FGF-21 overexpressing MSCs and mCherry MSCs were exposed to different concentrations of H2O2 and TNF-α. These apoptotic stimuli were also selected because H2O2 and TNF-α are well-known inducers of cell death and caspase-dependent apoptosis (Byun et al., 2005). As expected, caspase 3,7 activity was robustly elevated in a dose-dependent manner in mCherry control MSCs following treatment with H2O2 for 24 hours (Figure 3A). FGF-21 overexpressing MSCs dramatically suppressed H2O2-induced caspase activation at 100 μM, 200 μM, and 400 μM doses, specifically with an 86% reduction at 400 μM compared to mCherry control MSCs (Figure 3A). Caspase 3, 7 activity was increased by 5–6-fold in mCherry control MSCs treated with 10 ng/ml, 20 ng/ml, and 50 ng/ml of TNF-α. FGF-21 overexpression decreased TNF-α induced apoptosis by 44% and 50% in the respective 10 ng/ml and 20 ng/ml treatment groups, but only by 27% at the highest dose used (Figure 3B). We next tested the effects of FGF-21 overexpression on staurosporine, a potent protein kinase inhibitor that induces apoptosis. Staurosporine treatment for 24 hours at 2 nM and 10 nM significantly induced caspase activation by 50% and 220%, respectively, in the mCherry control MSCs. However, FGF-21 overexpression failed to confer protection (Figure 3C). Given that hallmark features of apoptosis include the formation of round apoptotic bodies and condensed/fragmented nuclei, we evaluated the effects of 200 μM H2O2 on inducing cellular morphological changes in mCherry control and FGF-21 overexpressing MSCs. mCherry control MSCs exhibited a high number of round apoptotic bodies and fragmentation of apoptotic/condensed nuclei detected by using a phase contrast inverted microscope and fluorescent microscopy after Hoechst 33258 staining (Figure 4), whereas FGF-21 overexpression markedly abrogated these apoptotic-induced morphological alterations. Taken together, these data strongly support the notion that FGF-21 exerts a critical role in protecting MSCs from apoptosis and may create a more permissive microenvironment for MSC survival in damaged tissues.
Figure 3.
Overexpression of FGF-21 attenuates apoptosis in MSCs. Caspase 3,7 activities in mCherry control and FGF-21 overexpressing MSCs were determined at 24 hours after exposure to A) H2O2 (50 μM, 100 μM, 200 μM, and 400 μM), B) TNF-α (10 ng/ml, 20 ng/ml, and 50 ng/ml), or C) staurosporine (2 nM and 10 nM). Values are presented as % increase from corresponding vehicle-treated control ± SEM (n=6–8 per group); ** P< 0.01 and **** P < 0.0001 between FGF-21 overexpressing MSCs and mCherry control groups (Student’s t-test or one-way ANOVA, posthoc-Tukey’s test).
Figure 4.
FGF-21 overexpression reduces hydrogen peroxide-induced apoptotic morphological changes. mCherry control and FGF-21 overexpressing MSCs were treated with H2O2 (200 μM) for 24 hours. Round apoptotic bodies (bright field) and Hoechst 33528 staining of condensed/fragmented nuclei are indicated by yellow arrowheads. Representative images were taken at 20X magnification.
4. Discussion
In the present study, we generated a highly homogeneous population of bone marrow-derived MSCs overexpressing FGF-21 and demonstrated that FGF-21 overexpression effectively attenuated caspase activation in MSCs induced by treatment with H2O2 or TNF-α, but not staurosporine. Previously, we demonstrated that exogenous FGF-21 is protective against glutamate-induced neuronal death (Leng et al., 2015). This finding led us to hypothesize that FGF-21 would be protective against apoptotic stimuli such as hydrogen peroxide and TNF-α. However, the administration of recombinant FGF-21 may not be the best strategy for long-term protection because FGF-21 has a short half-life of only 1–2 hours (Kharitonenkov et al., 2005). Therefore, utilizing MSCs that overexpress FGF-21 as a delivery system would be advantageous for transplantation studies since they provide a continuous and steady supply of FGF-21 to the surrounding harsh microenvironment. This type of a system would be preferable in terms of translational application to the clinical setting.
These results suggest some similarities and differences in the mechanisms underlying caspase activation and apoptosis induced by these three apoptotic stimuli (H2O2, TNF-α, and staurosporine). In this context, H2O2 is known to cause cell death by triggering the generation of ROS and inducing oxidative stress as well as subsequent damage to DNA and other macromolecules (Uhl et al., 2015). Cell death caused by the pleiotropic inflammatory cytokine TNF-α appears to involve ROS accumulation and sustained JNK activation, and is prevented by antioxidant treatment (Kamata et al., 2005), suggesting that its underlying mechanisms overlap with those of H2O2. The mechanism by which staurosporine induces death is less clear, but it has been shown to involve both apoptosis and necrosis and to be associated with calpain-mediated Bax cleavage and plasma membrane permeabilization; both are prevented by calbindin-D28K (Choi & Oh, 2014). Given the protection by FGF-21 overexpression in MSCs against apoptosis induced by H2O2 and TNF-α, one may surmise that suppression of ROS and subsequent oxidative stress and/or inflammation are key targets for the protective effects.
The beneficial effects of FGF-21-overexpressing MSCs against apoptotic cell death support our previous results that exogenous FGF-21 protects primary CNS neurons from apoptosis induced by the excitotoxin glutamate (Leng et al., 2015). In our previous paradigm, FGF-21-mediated neuroprotection against excitotoxicity requires activation of the cytoprotective factor Akt-1, and inhibition of the cytotoxic factor GSK-3β by enhancing their serine phosphorylations at specific loci (Leng et al., 2015). The roles of Akt activation and GSK-3 inhibition in suppressing oxidative stress and inflammation have been well documented and multiple mechanisms have been proposed (Dai et al., 2017; Jope et al., 2007; Mahmoud et al., 2017). The exact molecular events underlying the protection against H2O2 and TNF-α-induced apoptosis by FGF-21 overexpression in MSCs require future investigation. It should be noted that recent reports indicate additional important actions of FGF-21. These include the ability of FGF-21 to promote the elongation of neurite-like processes in primary astrocytes (Leng et al., 2016), to reduce brain edema and alleviate sensorimotor deficits after TBI (Chen et al., 2018), and to promote remyelination following toxin-induced demyelination in rodents (Kuroda et al., 2017). Furthermore, in an experimental model of stroke, we found that FGF-21 levels were drastically reduced in the brain after stroke and restoration of the brain FGF-21 levels by pharmacological treatment markedly reduced brain infarction and neurological deficits (Wang et al., 2016). Taken together, FGF-21 seems to be a promising molecule to counteract pathological conditions associated with diseases, notably brain disorders.
The overexpression of FGF-21 in MSCs induced a decrease in the expression of FGF-15. It is possible that FGF-21 induced the expression of a negative regulator that antagonized the expression of FGF-15. It is also feasible that FGF-21 could bind to the FGF-15 promoter thereby inhibiting its subsequent transactivation and transcription. The notion that FGF-21 and FGF-15 display opposite effects has been observed in the regulation of bile acid synthesis. Namely, high levels of serum FGF-21 correlate with low levels of FGF-15 (Zhang et al., 2017). Further experiments would be needed to determine the biological function of this mechanism in the context of MSCs.
MSCs have become a useful tool for the therapeutic intervention of diseases including CNS disorders. Among the benefits of MSCs are their low risk of inducing tumorgenesis and immunogenic rejection as well as their ability to serve as a vehicle carrying specific gene(s) to target diseased tissues. Preconditioned MSCs can also be delivered to the brain via intranasal application in a non-invasive manner to produce behavioral improvement in an animal model of Huntington’s disease (Linares et al., 2016). To assess the therapeutic effects of FGF-21 overexpression in vivo, we used the lines that we generated in a parallel study using a mouse model of traumatic brain injury (TBI). We demonstrated that hippocampal-dependent learning and spatial memory was markedly improved in TBI mice that received FGF-21 overexpressing MSCs, compared with control MSCs (Shahror et al., 2020). This effect was observed at 3–4 weeks post-transplantation, indicating that the therapeutic effects are maintained in vivo. In addition, we showed that the loss of neurogenesis and dendritic complexity following TBI was robustly restored by treatment with FGF-21 overexpressing MSCs, but not control MSCs.
5. Conclusions
The present study shows that overexpression of FGF-21 protected MSCs from apoptosis induced by oxidative stress-related insults such as treatment with H2O2 or TNF-α. In view of the evidence that oxidative stress has been linked to the pathophysiology of a variety of diseases including neurodegenerative disorders (Liguori et al., 2018), our results strongly suggest that overexpression of FGF-21 in MSCs can prolong the survival of transplanted MSCs in the harsh disease microenvironment and thus enhance the therapeutic efficacy of MSCs for pathological intervention. Further preclinical and clinical investigations are essential to substantiate this working hypothesis.
Acknowledgments
We are grateful for the support of the intramural research program of the National Institute of Mental Health, (NIMH), NIH (1ZIAMH002468-25). Fluorescence-activated cell sorting was performed at the Flow Cytometry core facility (National Institute of Neurological Disorders and Stroke). All other work was performed at the facilities provided by the National Institutes of Health. This work was written as part of the authors’ official duties as Government employees (affiliated with Annual Report MH002468). The views expressed in this article do not necessarily represent the views of the NIMH, NIH, HHS, or the United States Government.
Funding
This work was funded by the intramural research program of the NIMH (1ZIAMH002468–25).
Abbreviations:
- FACS
Fluorescence-activated cell sorting
- FGF
Fibroblast growth factor
- H2O2
Hydrogen peroxide
- MSCs
Mesenchymal stem cells
- OE
Overexpressing
- ROS
Reactive oxygen species
- TNF-α
Tumor necrosis factor alpha
Footnotes
Conflict of interest
The authors have no conflicting financial interests.
References
- Butterfield DA, & Halliwell B. (2019). Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci, 20(3), 148–160. doi: 10.1038/s41583-019-0132-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun C, Koh J, Kim D, Park S, Lee K, & Kim G. (2005). Alpha-lipoic acid inhibits TNF-alpha-induced apoptosis in human bone marrow stromal cells. J Bone Miner Res, 20(7), 1125–1135. [DOI] [PubMed] [Google Scholar]
- Chen J, Hu J, Liu H, Xiong Y, Zou Y, Huang W, . . . Lin L. (2018). FGF21 Protects the Blood-Brain Barrier by Upregulating PPARγ via FGFR1/β-klotho after Traumatic Brain Injury. J Neurotrauma, 35(17), 2091–2103. doi: 10.1089/neu.2017.5271 [DOI] [PubMed] [Google Scholar]
- Choi WS, & Oh YJ (2014). Calbindin-D28K Prevents Staurosporin-induced Bax Cleavage and Membrane Permeabilization. Exp Neurobiol, 23(2), 173–177. doi: 10.5607/en.2014.23.2.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai P, Mao Y, Sun X, Li X, Muhammad I, Gu W, . . . Huang S. (2017). Attenuation of Oxidative Stress-Induced Osteoblast Apoptosis by Curcumin is Associated with Preservation of Mitochondrial Functions and Increased Akt-GSK3β Signaling. Cell Physiol Biochem, 41(2), 661–677. doi: 10.1159/000457945 [DOI] [PubMed] [Google Scholar]
- Degirolamo C, Sabbà C, & Moschetta A. (2016). Therapeutic potential of the endocrine fibroblast growth factors FGF19, FGF21 and FGF23. Nat Rev Drug Discov, 15(1), 51–69. doi: 10.1038/nrd.2015.9 [DOI] [PubMed] [Google Scholar]
- Jope RS, Yuskaitis CJ, & Beurel E. (2007). Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem Res, 32(4–5), 577–595. doi: 10.1007/s11064-006-9128-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata H, Honda S, Maeda S, Chang L, Hirata H, & Karin M. (2005). Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell, 120(5), 649–661. doi: 10.1016/j.cell.2004.12.041 [DOI] [PubMed] [Google Scholar]
- Karihtala P, & Soini Y. (2007). Reactive oxygen species and antioxidant mechanisms in human tissues and their relation to malignancies. APMIS, 115(2), 81–103. [DOI] [PubMed] [Google Scholar]
- Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, . . . Shanafelt AB (2005). FGF-21 as a novel metabolic regulator. J Clin Invest, 115(6), 1627–1635. doi: 10.1172/JCI23606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda M, Muramatsu R, Maedera N, Koyama Y, Hamaguchi M, Fujimura H, . . . Yamashita T. (2017). Peripherally derived FGF21 promotes remyelination in the central nervous system. J Clin Invest, 127(9), 3496–3509. doi: 10.1172/JCI94337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Y, Wang J, Wang Z, Liao HM, Wei M, Leeds P, & Chuang DM (2016). Valproic Acid and Other HDAC Inhibitors Upregulate FGF21 Gene Expression and Promote Process Elongation in Glia by Inhibiting HDAC2 and 3. Int J Neuropsychopharmacol, 19(8). doi: 10.1093/ijnp/pyw035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Y, Wang Z, Tsai LK, Leeds P, Fessler EB, Wang J, & Chuang DM (2015). FGF-21, a novel metabolic regulator, has a robust neuroprotective role and is markedly elevated in neurons by mood stabilizers. Mol Psychiatry, 20(2), 215–223. doi: 10.1038/mp.2013.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P, Zhong L, Gong L, Wang J, Zhu Y, Liu W, & Yang J. (2017). Fibroblast growth factor 21 protects rat cardiomyocytes from endoplasmic reticulum stress by promoting the fibroblast growth factor receptor 1-extracellular signal-regulated kinase 1/2 signaling pathway. Int J Mol Med, 40(5), 1477–1485. doi: 10.3892/ijmm.2017.3140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, . . . Abete P. (2018). Oxidative stress, aging, and diseases. Clin Interv Aging, 13, 757–772. doi: 10.2147/CIA.S158513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares G, Xing W, Govoni K, Chen S, & Mohan S. (2009). Glutaredoxin 5 regulates osteoblast apoptosis by protecting against oxidative stress. Bone, 44(5), 795–804. doi:S8756-3282(09)00012-X [pii] 10.1016/j.bone.2009.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares GR, Chiu CT, Scheuing L, Leng Y, Liao HM, Maric D, & Chuang DM (2016). Preconditioning mesenchymal stem cells with the mood stabilizers lithium and valproic acid enhances therapeutic efficacy in a mouse model of Huntington’s disease. Exp Neurol, 281, 81–92. doi: 10.1016/j.expneurol.2016.04.003 [DOI] [PubMed] [Google Scholar]
- Linares GR, Xing W, Burghardt H, Baumgartner B, Chen ST, Ricart W, . . . Mohan S. (2011). Role of diabetes- and obesity-related protein in the regulation of osteoblast differentiation. Am J Physiol Endocrinol Metab, 301(1), E40–48. doi: 10.1152/ajpendo.00065.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson SD, Pollock K, Kambal A, Cary W, Mitchell GM, Tempkin J, . . . Nolta JA (2012). Genetically engineered mesenchymal stem cells as a proposed therapeutic for Huntington’s disease. Mol Neurobiol, 45(1), 87–98. doi: 10.1007/s12035-011-8219-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud AM, Wilkinson FL, McCarthy EM, Moreno-Martinez D, Langford-Smith A, Romero M, . . . Alexander MY (2017). Endothelial microparticles prevent lipid-induced endothelial damage. FASEB J, 31(10), 4636–4648. doi: 10.1096/fj.201601244RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahror RA, Linares GR, Wang Y, Hsueh SC, Wu CC, Chuang DM, . . . Chen KY (2020). Transplantation of Mesenchymal Stem Cells Overexpressing Fibroblast Growth Factor 21 Facilitates Cognitive Recovery and Enhances Neurogenesis in a Mouse Model of Traumatic Brain Injury. J Neurotrauma, 37(1), 14–26. doi: 10.1089/neu.2019.6422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiger H, Keuper M, Berti L, Hrabe de Angelis M, & Häring HU (2017). Fibroblast Growth Factor 21-Metabolic Role in Mice and Men. Endocr Rev, 38(5), 468–488. doi: 10.1210/er.2017-00016 [DOI] [PubMed] [Google Scholar]
- Uhl L, Gerstel A, Chabalier M, & Dukan S. (2015). Hydrogen peroxide induced cell death: One or two modes of action? Heliyon, 1(4), e00049. doi: 10.1016/j.heliyon.2015.e00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Wang Y, Zhang Z, Liu Q, & Gu J. (2017). Cardioprotective effects of fibroblast growth factor 21 against doxorubicin-induced toxicity via the SIRT1/LKB1/AMPK pathway. Cell Death Dis, 8(8), e3018. doi: 10.1038/cddis.2017.410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Leng Y, Wang J, Liao HM, Bergman J, Leeds P, . . . Chuang DM (2016). Tubastatin A, an HDAC6 inhibitor, alleviates stroke-induced brain infarction and functional deficits: potential roles of α-tubulin acetylation and FGF-21 up-regulation. Sci Rep, 6, 19626. doi: 10.1038/srep19626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Feng A, Lin S, Yu L, Lin X, Yan X, . . . Zhang C. (2018). Fibroblast growth factor-21 prevents diabetic cardiomyopathy via AMPK-mediated antioxidation and lipid-lowering effects in the heart. Cell Death Dis, 9(2), 227. doi: 10.1038/s41419-018-0307-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Gupte J, Gong Y, Weiszmann J, Zhang Y, Lee KJ, . . . Li Y. (2017). Chronic Over-expression of Fibroblast Growth Factor 21 Increases Bile Acid Biosynthesis by Opposing FGF15/19 Action. EBioMedicine, 15, 173–183. doi: 10.1016/j.ebiom.2016.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]