Abstract
Objective:
To assess (i) the impact of changes in body weight on changes in joint-adjacent subcutaneous fat (SCF) and cartilage thickness over 4-years and (ii) the relation between changes in joint-adjacent SCF and knee cartilage thickness.
Design:
Individuals from the Osteoarthritis Initiative (total=399) with >10% weight gain (n=100) and >10% weight loss (n=100) over 4 years were compared to a matched control cohort with less than 3% change in weight (n=199). 3.0T MRI of the right knee was performed at baseline and after 4 years to quantify joint-adjacent SCF and cartilage thickness. Linear regression models were used to evaluate the associations between the (i) weight change group and 4-year changes in both knee SCF and cartilage thickness, and (ii) 4-year changes in knee SCF and in cartilage thickness. Analyses were adjusted for age, sex, baseline BMI, tibial diameter (and weight change group in analysis (ii)).
Results:
Individuals who lost weight over 4-years had significantly less joint-adjacent SCF (beta range, medial/lateral joint sides: 2.2mm to 4.2mm, p<0.001) than controls; individuals who gained weight had significantly greater joint-adjacent SCF than controls (beta range: −1.4mm- −3.9mm, p<0.001). No statistically significant associations were found between weight change and cartilage thickness change. However, increases in joint-adjacent SCF over 4-years were significantly associated with decreases in cartilage thickness (p=0.04).
Conclusions:
Weight change was associated with joint-adjacent SCF, but not with change in cartilage thickness. However, 4-year increases in joint-adjacent SCF were associated with decreases in cartilage thickness independent of baseline BMI and weight change group.
Keywords: Knee Subcutaneous Fat, Cartilage Thickness, Weight Change
INTRODUCTION
Osteoarthritis (OA) is the most common cause of disability in the United States, affecting over 32.5 million adults 1. One important modifiable risk factor for knee OA is obesity: weight loss is protective for the development of symptomatic knee OA 2, while weight gain may exacerbate knee OA symptoms 3 and increase the risk for knee replacement 4. Recently, joint-adjacent subcutaneous fat (SCF) has gained interest as an independent risk factor and a potential biomarker of OA progression.
While most research studies on obesity and OA have focused on BMI measurements as exposure variables 5, BMI has inherent limitations as it does not capture the distribution of fat around the body and cannot distinguish adipose tissue from non-adipose body mass 3. In contrast, joint-adjacent knee SCF is a localized measure of the amount of fat surrounding a joint that may provide additional insights (relative to BMI) on the effects of adipose tissue change on OA progression.
Recent studies have investigated the impact of localized fat depots including joint-adjacent SCF in the thigh and surrounding the knee joint on OA. These studies reported that SCF thickness was significantly higher in individuals presenting with chondromalacia 6, increases in thigh SCF over 2-years were associated with the progression of knee OA in men 7, and greater joint-adjacent SCF levels at baseline were associated with higher odds for cartilage and meniscal structural progression over 4 years, independent of baseline BMI 8. Thus, understanding the impact of localized adipose changes on adjacent knee joint tissue may provide novel insights OA pathogenesis, that are beyond the systemic effects of overall weight change.
The overarching goal of this study is to examine both weight change and change in joint-adjacent SCF in relation to knee OA progression (independent of BMI), using imaging data from the Osteoarthritis Initiative (OAI), a multi-center, longitudinal study OA study (sponsored by the US National Institutes of Health (NIH)). Since the relationship between weight loss and cartilage thickness change has shown varied results 9-12, the assessment of localized changes in SCF in relation to cartilage thickness change may provide novel insights on the effects of adipose tissue on knee joint degeneration. Thus, the clinical motivation for this study is to gain an understanding of how longitudinal changes in body weight and changes in localized adipose tissue are related to changes in cartilage thickness. Specifically, this study will assess (i) the impact of body weight on joint-adjacent subcutaneous fat (SCF) and cartilage thickness over 4-years and (ii) the relationship between joint-adjacent SCF and knee cartilage thickness.
METHOD
Subject Selection
This study utilized data from the Osteoarthritis Initiative (OAI; https://nda.nih.gov/oai) 13, a multi-center, longitudinal study of individuals aged 45-79 years at enrollment. The OAI dataset includes MRI and radiographic knee images of participants over eight years. The study protocol, amendments, and informed consent documentation were reviewed and approved by the local institutional review boards of all participating centers.
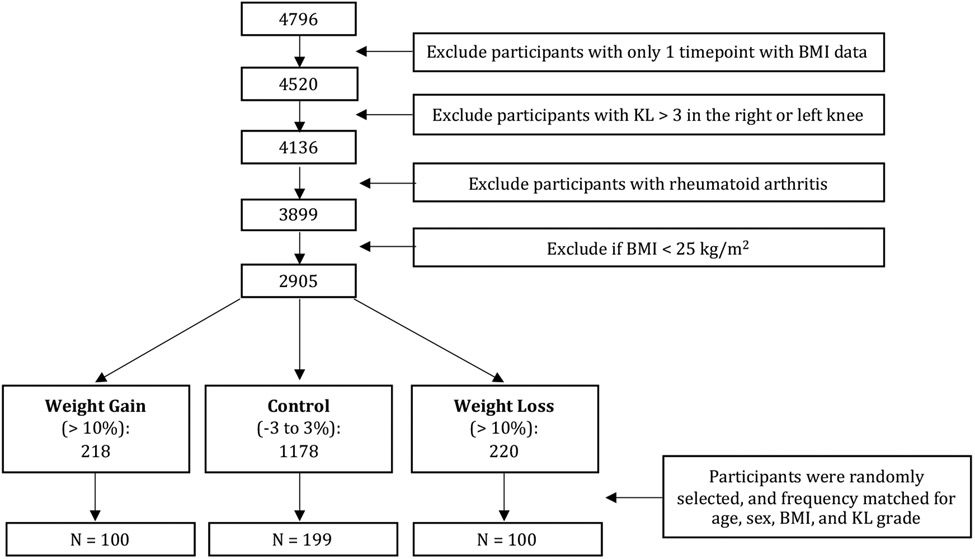
The present study analyzed participants enrolled in the OAI with the following inclusion criteria: (i) individuals with at least 2 BMI timepoints available from baseline to 4-year follow-up (ii) a baseline Kellgren Lawrence score (KL) ≤ 3 in the right or left knee, (iii) baseline BMI > 25. Participants with rheumatoid arthritis were excluded. Based on these criteria, individuals were classified in three groups: weight gain (>10%, n=221), weight loss (>−10%, n=227) and controls (−3 to 3% change, n=1237). The cut off values were chosen based on previously published studies including Messier et al. 14 who reported that “long-term weight loss between 10-19.9% of baseline body weight has substantial clinical and mechanistic benefits compared to less weight loss” when analyzing data from Intensive Diet and Exercise for Arthritis (IDEA) randomized controlled clinical trial 14. For this study, participants were randomly selected and frequency matched for age, sex, BMI, and KL grade at baseline, yielding a total of 399 individuals: weight gain (>10%, n=100), weight loss (>10%, n=100) and controls (−3 to 3% change, n=199), Figure 1.
Figure 1:
Participant Selection from the OAI. Abbreviations: KL: Kellgren-Lawrence, BMI Body Mass Index.
Group Definitions
BMI measurements were used to determine the rate of change in BMI over 4 years in each participant using all BMI data available from baseline to 4 years. The slope of the regression line (in units of change per year) was multiplied by four to determine magnitude of BMI change over 4 years, and the percentage change in BMI over 4 years was calculated. We employed a regression line to quantify change in BMI over 4-years (rather than only baseline and 4-year data) to comprehensively assess the overall change BMI using all available data. Individuals were classified into three groups based on their changes in BMI: weight loss (>10% change), weight gain (>10% change), and controls without weight change (−3 to 3% change).
Clinical Questionnaires
Knee pain was assessed using the WOMAC (Western Ontario McMaster Universities Osteoarthritis) Index, a standard questionnaire used to evaluate symptoms related to knee OA, including pain 15-17.
The participants’ physical activity levels were determined using a Physical Activity Scale for the Elderly (PASE) with a range of 0 to 400. This is a well-established, reliable, validated questionnaire that has been used to measure physical activity in individuals of similar age to those investigated in the current study 18-21.
Radiographs
Standardized bilateral standing posterior-anterior fixed flexion knee radiographs were acquired in all participants in the OAI. For eligibility and to assess baseline disease burden, knee Kellgren Lawrence (KL) gradings22 from the OAI baseline visit were scored as has been previously described23.
MR Imaging Acquisition and Analyzed Parameters
MR imaging was performed using 3T MRI scanners (Trio, Siemens, Erlangen, Germany) at four centers as part of the imaging OAI protocol at baseline and after 4 years. The following sequences of the right knee were analyzed in this study: 1) coronal 3D fast low angle shot with water excitation (FLASH WE) [7.57 ms/20 ms; 0.313 mm × 0.313 mm; 160 mm; 1.5 mm; 0 mm] and 2) sagittal 3D dual-echo steady state sequence with water excitation (DESS WE) [4.7 ms/16.3 ms; 0.365 mm × 0.456 mm; 140 mm; 1.5 mm; 0 mm] with axial and coronal reformations. Joint-adjacent SCF was measured on coronal 3D FLASH WE MRI sequence, while the DESS sequence was used for cartilage thickness measurements. Additional details on image acquisition parameters have been previously published 24.
Joint-adjacent SCF Quantification
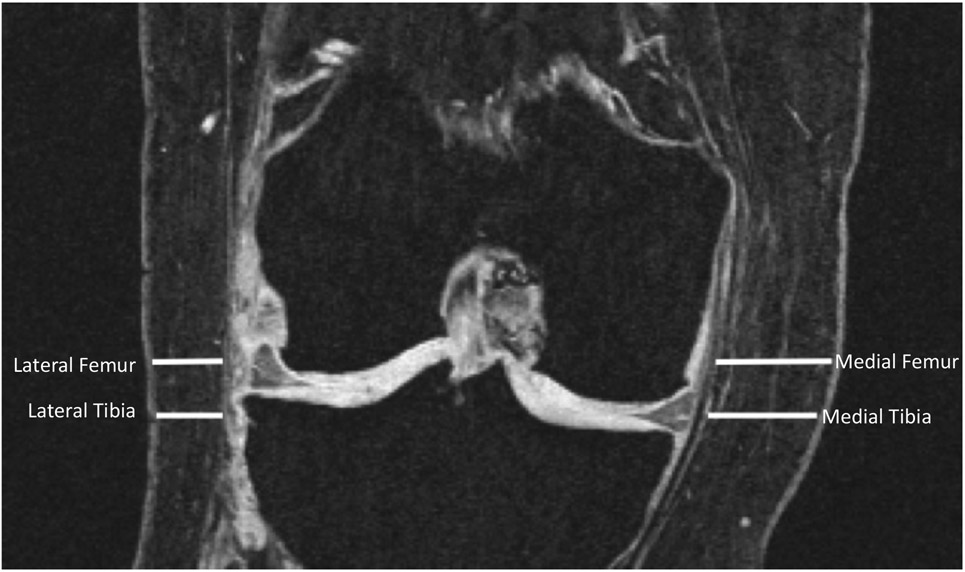
Joint-adjacent SCF was measured on coronal 3D FLASH WE MRI sequences at four locations on the medial and lateral sides of the knee joint (Figure 2) by two observers (M.T. and G.A., both 1 year of experience), who were trained by an experienced musculoskeletal radiologist (T.M.L., 25 years of experience). Measurements were performed at baseline and 4-year follow-up in the right knee. The 3D coronal flash sequence was chosen for its precise delineation of the joint-adjacent SCF boundaries and the larger field of view compared to the other available coronal sequences. A section centered on the medial tibial spine was selected, using sagittal and axial reformations of the DESS sequence. SCF thickness was measured on the coronal section at the level of the medial joint space and the superior boundary of the medial tibial spine, both medially and laterally 8. The inter- and intra-observer reproducibility of SCF measurements has been previously measured 8 and demonstrated good reproducibility (CVinter-observer = 2.72%; CVintra-observer = 2.01%). The difference between the baseline and 4-year follow-up SCF measurements were quantified.
Figure 2:
A coronal reformation of the dual echo steady state (DESS) sequence. Subcutaneous fat (SCF) measurements are shown at the medial femur, medial tibia and lateral femur and lateral tibia. The tip of the medial tibial spine is used to define the axial slice level, on which medial and lateral measurements are taken.
Cartilage Thickness
A fully-automatic method was developed and validated by our group for reliable cartilage segmentation and thickness measurement of knee MRI volumes as previously described 25. Three identical 3D VNet architectures and three 2D UNet-like architectures were trained to segment DESS sequence volumes. Cartilage segmentation was sub-segmented into lateral tibia, medial tibia, patella, lateral femur, and medial femur regions in the right knee. Per compartment and per sagittal slice, a Euclidean distance transformation and skeletonization was performed. The value of the distance map was sampled at each skeleton point, and all points across all slices were averaged to calculate mean thickness. Lateral and medial femoral compartments underwent Euclidean distance transform and skeletonization before sub-segmentation. Only the weight-bearing region was included in the mean thickness calculation for the lateral and medial femur25. Cartilage thickness was quantified at baseline and at 4-year follow-up.
Statistical Analysis
Descriptive statistics were performed using a SAS Studio (version 3.8, SAS Institute Inc., Cary, NC, USA) macro program called “Tablen”26. Differences in continuous parameters between weight change groups were assessed using Kruskal Wallis tests, and differences in categorical parameters between groups were assessed using Chi-squared tests.
The primary statistical analyses were performed using STATA version 17 software (StataCorp LP, College Station, TX, USA) with significance set to p<0.05. Changes in SCF and cartilage thickness, respectively, were defined by subtracting the baseline measurement from the 4-year follow-up measurement. Linear regression models were used to evaluate the associations between (1) weight change group and 4-year changes in both knee SCF and cartilage thickness and (2) 4-year changes in knee SCF and 4-year changes in cartilage thickness. Analysis (2) was conducted on a standardized scale so that the beta coefficients represent the standard deviation change of the outcome, per standard deviation change of the predictor. All analyses were adjusted for age, sex, baseline BMI, and tibial diameter (and weight change group in analysis (2)).
The measurement variables were designated as primary or exploratory to address potential issues stemming from multiple testing. The primary joint adjacent SCF variables were 4-year changes in the medial femur and medial tibia SCF. The lateral SCF variables were designated as exploratory. The primary cartilage thickness variables were the average of all regions, the medial femur, and the medial tibia. The medial compartment of the knee was chosen as primary because medial OA occurs more frequently than lateral OA 27, 28, data from the OAI have shown that decreases in cartilage thickness over one year were greater in the medial compartment than in the lateral compartment 29, cartilage lesions are more prevalent on the medial side of the joint 28, and the medial femur is a concentrated region of weight-bearing 28.
Three sensitivity analyses were performed: First, a group-sex interaction was added to analysis 1, to assess whether the effects of group on fat outcomes and thickness outcomes differed by sex. Second, a SCF-sex interaction was added to analysis 2 to assess whether the effects of SCF change on thickness change outcomes differed by sex. Third, an additional adjustment for PASE was added to both analyses to assess whether physical activity had an impact on the relationship between group and SCF/thickness outcomes and between SCF and thickness outcomes.
RESULTS
Participant Characteristics
A total of 399 participants were included in this study; of those 100 had >10% weight gain over 4 years, 100 had >10% weight loss over 4 years, and 199 were matched controls with −3 to 3% weight change over 4 years. The participant characteristics are listed in Table 1. There were no significant differences in baseline BMI between weight change groups (weight gain: 31.2±4.13 kg/m2; weight loss: 31.5±3.93 kg/m2; controls: 31.2±4.11 kg/m2; p = 0.68). There were no significant differences in the age between groups (p=0.19), with the greatest age in participants who lost weight (62.3±9.93 years). There were no significant differences in the distribution of race (p=0.50) and knee KL grade (pright_knee = 0.88, pleft_knee = 0.87) between weight change groups.
Table 1:
Participant characteristics at the baseline timepoint. Abbreviations: KL: Kellgren Lawrence, PASE: physical activity scale for the elderly. WOMAC: Western Ontario McMaster Universities Osteoarthritis.
| Controls (N=199) |
Weight Gain (>10%) (N=100) |
Weight Loss (>−10%) (N=100) |
Total (N=399) |
P-value | |
|---|---|---|---|---|---|
| Age | 0.191 | ||||
| Mean (SD) | 60.8 (9.07) | 60.0 (8.68) | 62.3 (8.93) | 60.9 (8.95) | |
| BMI | 0.681 | ||||
| Mean (SD) | 31.2 (4.11) | 31.2 (4.13) | 31.5 (3.93) | 31.3 (4.06) | |
| Sex, n (%) | 0.982 | ||||
| Males | 88 (44.2%) | 45 (45.0%) | 44 (44.0%) | 177 (44.4%) | |
| Females | 111 (55.8%) | 55 (55.0%) | 56 (56.0%) | 222 (55.6%) | |
| PASE score | 0.121 | ||||
| Mean (SD) | 168.6 (86.36) | 168.0 (91.05) | 148.5 (83.11) | 163.4 (86.98) | |
| WOMAC Pain Score, right knee | 0.0191 | ||||
| Mean (SD) | 2.2 (2.91) | 3.0 (3.48) | 2.9 (3.13) | 2.6 (3.14) | |
| WOMAC Pain score, left knee | 0.0321 | ||||
| Mean (SD) | 2.1 (3.20) | 3.0 (3.79) | 2.4 (3.32) | 2.4 (3.39) | |
| Race, n (%) | 0.502 | ||||
| 0 – Other-non-white | 1 (0.5%) | 2 (2.0%) | 1 (1.0%) | 4 (1.0%) | |
| 1 – White or Caucasian | 150 (75.4%) | 76 (76.0%) | 70 (70.0%) | 296 (74.2%) | |
| 2 – Black or African American | 47 (23.6%) | 22 (22.0%) | 27 (27.0%) | 96 (24.1%) | |
| 3 - Asian | 1 (0.5%) | 0 (0.0%) | 2 (2.0%) | 3 (0.8%) | |
| KL grade right knee, n (%) | 0.882 | ||||
| 0 | 53 (26.6%) | 32 (32.0%) | 23 (23.0%) | 108 (27.1%) | |
| 1 | 48 (24.1%) | 21 (21.0%) | 26 (26.0%) | 95 (23.8%) | |
| 2 | 54 (27.1%) | 27 (27.0%) | 29 (29.0%) | 110 (27.6%) | |
| 3 | 44 (22.1%) | 20 (20.0%) | 22 (22.0%) | 86 (21.6%) | |
| KL grade left knee, n (%) | 0.872 | ||||
| 0 | 69 (34.7%) | 33 (33.0%) | 29 (29.0%) | 131 (32.8%) | |
| 1 | 41 (20.6%) | 20 (20.0%) | 20 (20.0%) | 81 (20.3%) | |
| 2 | 52 (26.1%) | 32 (32.0%) | 30 (30.0%) | 114 (28.6%) | |
| 3 | 32 (16.1%) | 13 (13.0%) | 16 (16.0%) | 61 (15.3%) |
Weight change and knee joint-adjacent SCF
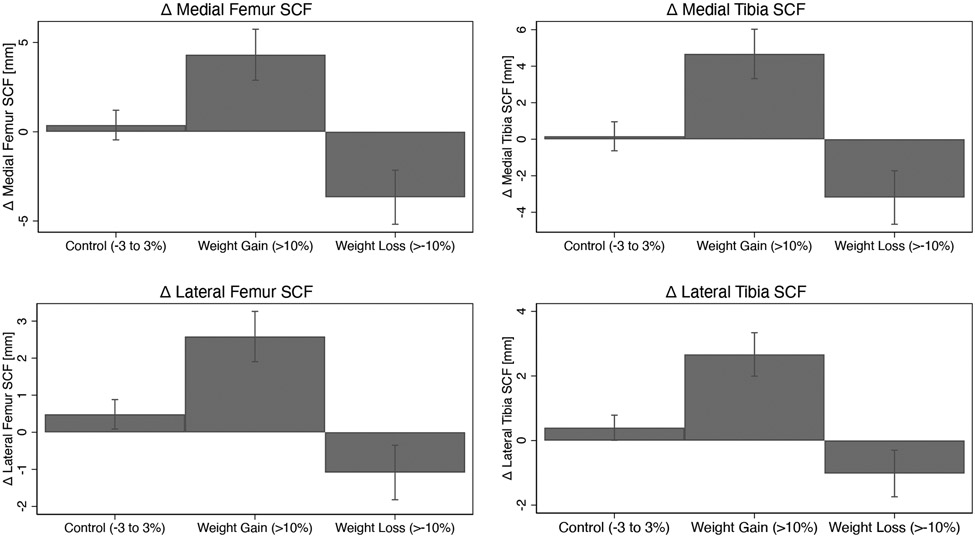
Individuals who gained weight over 4-years had significantly greater increases in joint-adjacent SCF than controls after 4 years (p<0.001 for all regions), while individuals who lost weight had significantly greater decreases in joint-adjacent SCF than controls (p<0.001 for all regions) as shown in Table 2 and Figure 3. For the weight gain group, the greatest increases in joint adjacent SCF were in the medial tibia SCF (adjusted mean: 4.86mm, 95%CI = 3.48-6.23) compared to controls (adjusted mean: 0.097mm, 95%CI = −0.70-0.89), Beta = 4.76, p<0.001), while the smallest increases were in the lateral femur SCF (adjusted mean: 2.52mm, 95%CI = 1.84-3.22) compared to controls (adjusted mean: 0.49mm, 95%CI = 0.09-0.89), Beta = 4.76, p< 0.001). For the weight loss group, the greatest decreases in joint-adjacent SCF were in the medial femur SCF (adjusted mean: −3.64mm, 95%CI = −5.17- −2.10) compared to controls (adjusted mean: 0.30mm, 95%CI = −0.53, −1.13), Beta = −3.94, p<0.001), while the smallest decreases were in the lateral tibia SCF (adjusted mean: −1.01mm, 95%CI = −1.73, −0.27) compared to controls (adjusted mean: 0.40mm, Beta = −1.41, 95%CI = 0.01-0.79), p<0.001). The between-group differences and 95% confidence intervals are listed in Table 2. The results were similar after adjusting for PASE. There were no statistically significant interactions (p-value range: 0.08 to 0.97) between weight change group and sex on 4-year changes in joint-adjacent SCF outcomes.
Table 2:
Differences in joint-adjacent SCF change over 4 years in the weight gain (<10%) and weight loss (>−10%) groups compared to controls (−3 to 3% change in weight). The beta coefficient represents the differences in change in SCF (mm) over 4 years between the group with weight change (weight loss or weight gain) and the reference group (controls).
| Δ Medial Femur SCF | Δ Medial Tibia SCF | Δ Lateral Femur SCF* | Δ Lateral Tibia SCF* | |||||
|---|---|---|---|---|---|---|---|---|
| Group | Beta | p | Beta | p | Beta | p | Beta | p |
| Controls (−3 to 3% change) | Reference | Reference | Reference | Reference | ||||
| Weight Gain (>10%) |
4.21
(2.52 – 5.89) |
<0.001 |
4.76
(3.16 – 6.36) |
<0.001 |
2.03
(1.23 – 2.83) |
<0.001 |
2.23
(1.43 – 3.02) |
<0.001 |
| Weight Loss (>−10%) |
−3.94
(−5.69 – −2.19) |
<0.001 |
−3.29
(−4.97 – −1.60) |
<0.001 |
−1.57
(−2.42 – −0.73) |
<0.001 |
−1.41
(−2.24 – −0.58) |
0.001 |
Linear regression adjusted for age, sex, BMI, race, and tibial diameter at baseline
Note fat measurements are changes between baseline and 4-year follow-up
denotes exploratory variables
Figure 3:
Changes in subcutaneous fat (SCF) by weight change group over 4 years. Adjusted means are shown (adjustments: age, sex, BMI, race, tibia diameter) with error bars representing 95% confidence intervals.
Weight change and cartilage thickness
No statistically significant (p>0.05) associations were found between weight change group and cartilage thickness change in any cartilage region over 4 years. For the weight gain group, the coefficients of cartilage thickness change compared to the control group ranged from −0.009mm in the patella (p=0.68, 95%CI =−0.05 to 0.03)) to 0.02mm in the medial femur (p=0.19, 95%CI=−0.01 to 0.05). For the weight loss group, the coefficients of cartilage thickness change compared to the control group ranged from −0.008mm in the lateral femur (p=0.64, 95%CI=−0.04 to 0.03) to 0.01mm in the medial tibia (p=0.25, 95%CI=−0.01 to 0.04). The results were similar after adjusting for PASE. There were no statistically significant interactions (p-value range: 0.08 to 0.99) between weight change group and sex on 4-year changes in thickness outcomes.
Joint-adjacent SCF and knee cartilage thickness
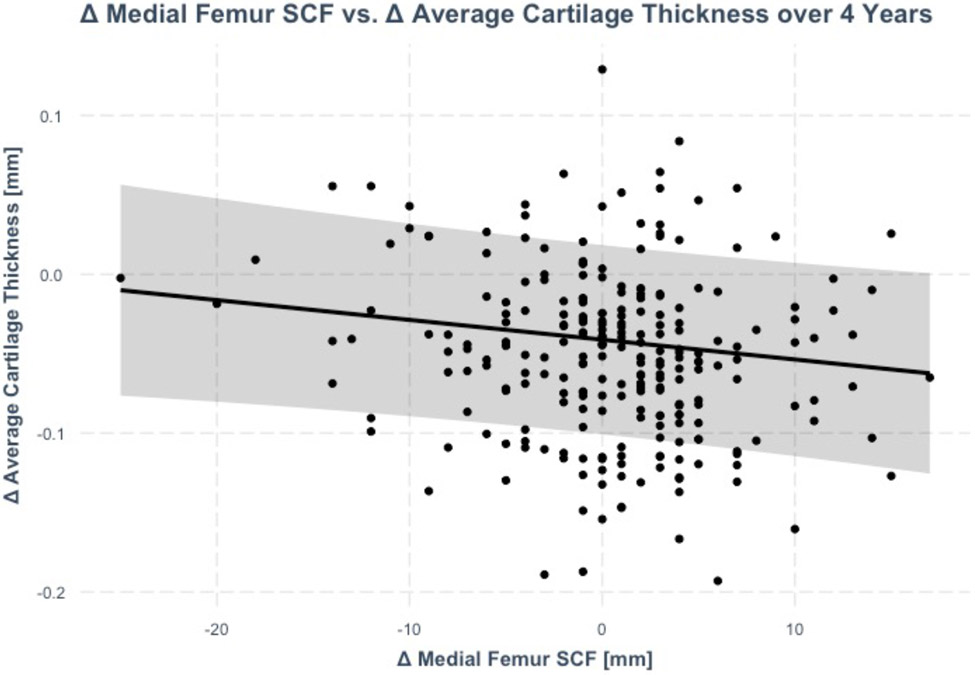
Increases in joint-adjacent SCF over 4-years were significantly associated with decreases in cartilage thickness (a 1 SD increase in medial femur SCF was associated with 0.14 SD decrease in average thickness, p=0.04) as shown in Figure 4 and Table 3. In addition to the average cartilage thickness, increases in medial femur SCF were significantly associated with decreases in medial femur cartilage thickness (coeff_standardized.=−1.5, p=0.03), lateral tibia thickness (coeff_standardized.=−0.17, p=0.01), and patella thickness (coeff_standardized.=−0.14, p=0.04). The remaining associations between joint-adjacent SCF changes and cartilage thickness changes over 4 years were not statistically significant (p>0.05). The results were similar after adjusting for PASE. There were no statistically significant interactions (p-value range: 0.07 to 0.87) between joint adjacent SCF change and sex on 4-year changes in thickness outcomes.
Figure 4:
Association between changes in medial femur SCF and average cartilage thickness over 4 years (beta: 1mm increase in medial femur SCF was associated with 0.001mm decrease average in thickness (standardized beta = −0.14, p=0.04)). The regression line is adjusted for age, sex, BMI, race, tibia diameter, and weight change group. The shaded area represents the 95% confidence interval.
Adjusted means; Error Bars represent 95% CIs
Table 3:
Associations between increases in joint-adjacent SCF and decreases in cartilage thickness over 4 years. The beta coefficients represent the change in cartilage thickness outcome (in SD units) per one SD change in the predictor (SCF).
| Δ Average Thickness |
Δ Medial Femur Thickness |
Δ Medial Tibia Thickness |
Δ Lateral Femur Thickness |
Δ Lateral Tibia Thickness |
Δ Patella Thickness |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictors | Estimates | p | Estimates | p | Estimates | p | Estimates | p | Estimates | p | Estimates | p |
| Δ Medial Femur SCF |
−0.14
(−0.27 – −0.01) |
0.04 |
−0.15
(−0.28 – −0.01) |
0.03 | 0.05 (−0.08 – 0.19) |
0.45 | −0.09 (−0.22 – 0.04) |
0.19 |
−0.17
(−0.31 – −0.04) |
0.01 |
−0.14
(−0.28 – −0.00) |
0.04 |
| Δ Medial Tibia SCF | −0.09 (−0.22 – 0.04) |
0.18 | −0.11 (−0.24 – 0.03) |
0.12 | 0.07 (−0.06 – 0.21) |
0.3 | −0.09 (−0.23 – 0.04) |
0.17 |
−0.19
(−0.32 – −0.06) |
0.005 | −0.1 (−0.24 – 0.04) |
0.17 |
| Δ Lateral Femur SCF | −0.07 (−0.20 – 0.06) |
0.27 | 0.03 (−0.10 – 0.17) |
0.61 | 0.1 (−0.03 – 0.23) |
0.15 | −0.06 (−0.19 – 0.07) |
0.38 | −0.04 (−0.17 – 0.09) |
0.54 | −0.11 (−0.25 – 0.04) |
0.15 |
| Δ Lateral Tibia SCF | −0.04 (−0.18 – 0.09) |
0.5 | 0.06 (−0.07 – 0.19) |
0.39 | 0.09 (−0.05 – 0.22) |
0.21 | −0.01 (−0.14 – 0.12) |
0.83 | −0.01 (−0.14 – 0.12) |
0.88 | −0.11 (−0.25 – 0.03) |
0.12 |
Linear Regression adjusted for age, sex, BMI, race, and tibia diameter and weight change group
DISCUSSION
In this study, weight gain was associated with increases in joint-adjacent SCF, while weight loss was associated with decreases in joint-adjacent SCF, independent of baseline BMI. While there were no significant associations between weight change group and cartilage thickness change (all confidence intervals cross 0mm), 4-year increases in joint-adjacent SCF were associated with decreases in cartilage thickness (and vice versa) independent of baseline BMI and weight change group. Thus, cartilage thickness changes may be more sensitive to changes in joint-adjacent SCF compared to changes in BMI, potentially due to the localized nature of joint-adjacent SCF measurements.
While several studies have assessed the relationship between weight loss and cartilage thickness change 9-12, their conclusions were inconsistent. For reference, a previous study has shown in 3910 individuals that the average cartilage thickness in the femur is was 2.34 mm (standard deviation, 0.71; 95% confidence interval, 0.95-3.73) 30” Anandacoomarasamy et al. reported that after a 12-month follow-up and a mean weight loss of 9.3%, percentage weight loss was negatively associated with cartilage thickness loss in the medial knee compartment, but not the lateral knee compartment 10. However, Jafarzadeh et al. reported that 1-year after bariatric surgery, a majority of the cartilage regions (14/16) did not show significant changes in cartilage thickness, and there were “little if any” correlations between cartilage thickness change and weight change percentage 11. Moreover, Hunter et al. reported no significant associations between weight loss over 18 months (after various interventions including diet, diet and exercise, and exercise only) and cartilage thickness loss 12. The results from the current study, which show no significant associations between weight change and cartilage thickness change, are in agreement with Hunter et al. 12, and complementary to the results from Jafarzadeh et al. 11. This study further demonstrates that these associations hold true over 4 years, and are applicable to not only weight loss, but also to weight gain.
Many regions of lower limb SCF including thigh SCF and infrapatellar/suprapatellar fat pads have been investigated in relation to knee joint degeneration 31; in contrast, fewer studies have assessed joint adjacent SCF, which is a unique and motivating feature of this study. One previous study assessed joint adjacent SCF at baseline only, reporting a cross-sectional relationship between SCF and knee joint morphology (as measured by Whole-Organ Magnetic Resonance Imaging Score (WORMS)), and a positive relationship between baseline SCF and increases in cartilage and meniscus degeneration scores over 4 years 8. The current study focuses on the longitudinal changes in joint-adjacent SCF over 4 years, demonstrating that increases in joint-adjacent SCF and are associated with decreases in cartilage thickness. A majority of the associations were present in the medial femur SCF region in relation to cartilage thickness in the average all regions, medial femur, lateral tibia and the patella. Two notable findings are (1) the associations between joint-adjacent SCF and cartilage thickness held true despite statistically adjusting for baseline BMI and weight change group (thus suggesting that the relationship between SCF and cartilage thickness is independent of BMI and weight change) and (2) the association between weight change and cartilage thickness change was not statistically significant. Collectively, these two key findings emphasize that the localized nature of joint-adjacent SCF measurements may play a distinct role in the complex pathogenesis of cartilage degeneration in OA.
The mechanisms responsible for the associations between increases in joint adjacent SCF and decreases cartilage thickness are complex but may be attributed to localized inflammatory factors such as adipokines that are secreted from adipose tissue. Various adipokines are associated with cartilage degeneration including adiponectin, visfatin, and leptin 31. In particular, serum leptin levels are correlated with reduced cartilage thickness (both cross-sectionally and over 2.7 years), and “the associations between measures of adiposity and cartilage thickness are mediated by leptin, suggesting leptin may play a key role in cartilage thinning 32.” Since leptin is a hormone released from fat cells in adipose tissue, and in this study increased localized levels of adipose tissue were related to loss of cartilage thickness, leptin secretion may be a potential mechanism responsible for this relationship. In addition, visfatin inhibition has been shown protective for collagen-induced OA in mice 33, and adiponectin (produced by adipocytes) may be protective against inflammation 34. Overall, we hypothesize that joint-adjacent SCF may impact cartilage thickness by increasing localized inflammation.
One potential clinical implication of this study is that spot reduction of subcutaneous fat around the knee could slow cartilage thickness loss. While the research on spot reduction of subcutaneous fat is limited and somewhat inconclusive, there have been two studies suggesting spot reduction is feasible through localized exercises 35, 36. If spot reduction can be achieved, it may help preserve cartilage thickness through decreases in localized levels of inflammation (in addition to general exercise, which is associated with decreases in metabolic and localized inflammation)37.
The primary limitations of this study are analysis of the OAI data in a retrospective manner (which does not allow for conclusions on causal associations), and that the reasons for a participant’s weight loss or weight gain were unknown (no data available in the OAI); a future study with a prospective design may address this limitation. In addition, the OAI did not provide data on adipokine levels, thereby precluding the analysis of these hormone levels in relation to weight change, joint-adjacent SCF, and cartilage thickness. Despite these limitations, this study also has pertinent strengths, particularly its longitudinal follow-up and quantitative cartilage thickness outcomes.
Overall, this study suggests that increases in joint-adjacent SCF are associated with progression of cartilage degeneration, while decreases in joint-adjacent SCF are associated with less cartilage loss. Weight loss was associated with decreases in joint-adjacent SCF, but not with changes in cartilage thickness. Changes in cartilage thickness were significantly associated with changes in joint-adjacent SCF (independent of BMI) while changes in BMI were not, suggesting that the localized nature of adipose tissue may play a vital role in the pathogenesis of cartilage loss in knee OA.
Funding Source:
This study was funded by NIH R01-AR064771, NIH R01-AR078917 and R01-AG070647. The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health.
Footnotes
Disclosure: The authors declared no conflict of interest.
REFERENCES
- 1.Callahan LF, Ambrose KR, Albright AL, Altpeter M, Golightly YM, Huffman KF, et al. Public health interventions for osteoarthritis-updates on the osteoarthritis action Alliance’s efforts to address the 2010 OA public health agenda recommendations. Clin Exp Rheumatol 2019; 37: 31–39. [PubMed] [Google Scholar]
- 2.Bliddal H, Leeds A, Christensen R. Osteoarthritis, obesity and weight loss: evidence, hypotheses and horizons–a scoping review. Obesity reviews 2014; 15: 578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanamas SK, Wluka AE, Davies-Tuck M, Wang Y, Strauss BJ, Proietto J, et al. Association of weight gain with incident knee pain, stiffness, and functional difficulties: a longitudinal study. Arthritis care & research 2013; 65: 34–43. [DOI] [PubMed] [Google Scholar]
- 4.Apold H, Meyer H, Nordsletten L, Furnes O, Baste V, Flugsrud G. Weight gain and the risk of knee replacement due to primary osteoarthritis: a population based, prospective cohort study of 225,908 individuals. Osteoarthritis and Cartilage 2014; 22: 652–658. [DOI] [PubMed] [Google Scholar]
- 5.King LK, March L, Anandacoomarasamy A. Obesity & osteoarthritis. Indian J Med Res 2013; 138: 185–193. [PMC free article] [PubMed] [Google Scholar]
- 6.Kok HK, Donnellan J, Ryan D, Torreggiani WC. Correlation between subcutaneous knee fat thickness and chondromalacia patellae on magnetic resonance imaging of the knee. Canadian Association of Radiologists Journal 2013; 64: 182–186. [DOI] [PubMed] [Google Scholar]
- 7.Dannhauer T, Ruhdorfer A, Wirth W, Eckstein F. Quantitative relationship of thigh adipose tissue with pain, radiographic status, and progression of knee osteoarthritis: longitudinal findings from the osteoarthritis initiative. Investigative Radiology 2015; 50: 268–274. [DOI] [PubMed] [Google Scholar]
- 8.Bodden J, Ok AH, Joseph GB, Nevitt MC, McCulloch CE, Lane NE, et al. Joint-adjacent Adipose Tissue by MRI is Associated With Prevalence and Progression of Knee Degenerative Changes: Data from the Osteoarthritis Initiative. J Magn Reson Imaging 2021; 54: 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daugaard C, Hangaard S, Bartels E, Gudbergsen H, Christensen R, Bliddal H, et al. The effects of weight loss on imaging outcomes in osteoarthritis of the hip or knee in people who are overweight or obese: a systematic review. Osteoarthritis and Cartilage 2020; 28: 10–21. [DOI] [PubMed] [Google Scholar]
- 10.Anandacoomarasamy A, Leibman S, Smith G, Caterson I, Giuffre B, Fransen M, et al. Weight loss in obese people has structure-modifying effects on medial but not on lateral knee articular cartilage. Annals of the rheumatic diseases 2012; 71: 26–32. [DOI] [PubMed] [Google Scholar]
- 11.Jafarzadeh SR, Clancy M, Li JS, Apovian CM, Guermazi A, Eckstein F, et al. Changes in the structural features of osteoarthritis in a year of weight loss. Osteoarthritis Cartilage 2018; 26: 775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunter DJ, Beavers DP, Eckstein F, Guermazi A, Loeser RF, Nicklas BJ, et al. The Intensive Diet and Exercise for Arthritis (IDEA) trial: 18-month radiographic and MRI outcomes. Osteoarthritis Cartilage 2015; 23: 1090–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peterfy C, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis and Cartilage 2008; 16: 1433–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Messier SP, Resnik AE, Beavers DP, Mihalko SL, Miller GD, Nicklas BJ, et al. Intentional Weight Loss in Overweight and Obese Patients With Knee Osteoarthritis: Is More Better? Arthritis Care Res (Hoboken) 2018; 70: 1569–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellamy N, Buchanan W, Goldsmith C, et al. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to anti-rheumatic drug therapy in patients with osteoarthritis of the hip and knee. J Rheumatology 1988; 15: 1833–1840. [PubMed] [Google Scholar]
- 16.Link TM, Steinbach LS, Ghosh S, Ries M, Lu Y, Lane N, et al. Osteoarthritis: MR imaging findings in different stages of disease and correlation with clinical findings. Radiology 2003; 226: 373–381. [DOI] [PubMed] [Google Scholar]
- 17.Phan CM, Link TM, Blumenkrantz G, Dunn TC, Ries MD, Steinbach LS, et al. MR imaging findings in the follow-up of patients with different stages of knee osteoarthritis and the correlation with clinical symptoms. Eur Radiol 2006; 16: 608–618. [DOI] [PubMed] [Google Scholar]
- 18.Stehling C, Lane NE, Nevitt MC, Lynch J, McCulloch CE, Link TM. Subjects with higher physical activity levels have more severe focal knee lesions diagnosed with 3T MRI: analysis of a non-symptomatic cohort of the osteoarthritis initiative. Osteoarthritis Cartilage 2010; 18: 776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Washburn RA, Ficker JL. Physical Activity Scale for the Elderly (PASE): the relationship with activity measured by a portable accelerometer. J Sports Med Phys Fitness 1999; 39: 336–340. [PubMed] [Google Scholar]
- 20.Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The physical activity scale for the elderly (PASE): evidence for validity. J Clin Epidemiol 1999; 52: 643–651. [DOI] [PubMed] [Google Scholar]
- 21.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 1993; 46: 153–162. [DOI] [PubMed] [Google Scholar]
- 22.Kellgren J, Jeffrey M, Ball J. The epidemiology of chronic rheumatism. Atlas of standard radiographs of arthritis. Volume vii–11. Oxford, UK, Blackwell Scientific Publications; 1963. [Google Scholar]
- 23.Felson DT, Niu J, Yang T, Torner J, Lewis CE, Aliabadi P, et al. Physical activity, alignment and knee osteoarthritis: data from MOST and the OAI. Osteoarthritis Cartilage 2013; 21: 789–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage 2008; 16: 1433–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iriondo C, Liu F, Caliva F, Kamat S, Majumdar S, Pedoia V. Towards Understanding Mechanistic Subgroups of Osteoarthritis: 8 Year Cartilage Thickness Trajectory Analysis. J. Orthop. Res 2020; in press. [DOI] [PubMed] [Google Scholar]
- 26.Meyers J. Paper AD-088: Demographic Table and Subgroup Summary Macro %TABLEN. Pharmaceuticals SAS Users Group conference. San Francisco, CA2020. [Google Scholar]
- 27.Strover S. Understanding Arthritis of the Knee. Understanding arthritis - compartments: Associates Ltd 2008. [Google Scholar]
- 28.Takatalo J, Karppinen J, Niinimaki J, Taimela S, Nayha S, Jarvelin MR, et al. Prevalence of degenerative imaging findings in lumbar magnetic resonance imaging among young adults. Spine (Phila Pa 1976) 2009; 34: 1716–1721. [DOI] [PubMed] [Google Scholar]
- 29.Eckstein F, Maschek S, Wirth W, Hudelmaier M, Hitzl W, Wyman B, et al. One year change of knee cartilage morphology in the first release of participants from the Osteoarthritis Initiative progression subcohort: association with sex, body mass index, symptoms and radiographic osteoarthritis status. Ann Rheum Dis 2009; 68: 674–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah RF, Martinez AM, Pedoia V, Majumdar S, Vail TP, Bini SA. Variation in the Thickness of Knee Cartilage. The Use of a Novel Machine Learning Algorithm for Cartilage Segmentation of Magnetic Resonance Images. J Arthroplasty 2019; 34: 2210–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang J, Liao Z, Lu M, Meng T, Han W, Ding C. Systemic and local adipose tissue in knee osteoarthritis. Osteoarthritis Cartilage 2018; 26: 864–871. [DOI] [PubMed] [Google Scholar]
- 32.Stannus OP, Cao Y, Antony B, Blizzard L, Cicuttini F, Jones G, et al. Cross-sectional and longitudinal associations between circulating leptin and knee cartilage thickness in older adults. Ann Rheum Dis 2015; 74: 82–88. [DOI] [PubMed] [Google Scholar]
- 33.Gomez R, Lago F, Gomez-Reino J, Dieguez C, Gualillo O. Adipokines in the skeleton: influence on cartilage function and joint degenerative diseases. Journal of Molecular Endocrinology 2009; 43: 11–18. [DOI] [PubMed] [Google Scholar]
- 34.Bay-Jensen AC, Slagboom E, Chen-An P, Alexandersen P, Qvist P, Christiansen C, et al. Role of hormones in cartilage and joint metabolism: understanding an unhealthy metabolic phenotype in osteoarthritis. Menopause 2013; 20: 578–586. [DOI] [PubMed] [Google Scholar]
- 35.Kostek MA, Pescatello LS, Seip RL, Angelopoulos TJ, Clarkson PM, Gordon PM, et al. Subcutaneous Fat Alterations Resulting from an Upper-Body Resistance Training Program. Medicine & Science in Sports & Exercise 2007; 39: 1177–1185. [DOI] [PubMed] [Google Scholar]
- 36.Olson AL, Edelstein E. Spot reduction of subcutaneous adipose tissue. Res Q 1968; 39: 647–652. [PubMed] [Google Scholar]
- 37.Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol 2011; 11: 607–615. [DOI] [PubMed] [Google Scholar]