Abstract
Objective
We aimed to evaluate the sputum culture conversion time of DR-TB patients and its related factors.
Methods
PubMed, The Cochrane Library, Embase, CINAHL, Web of Science, CNKI, Wan Fang, CBM and VIP databases were electronically searched to collect studies on sputum culture conversion time in patients with DR-TB. Meta-analysis was performed by using the R 4.3.0 version and Stata 16 software.
Results
A total of 45 studies involving 17373 patients were included. Meta-analysis results showed that the pooled median time to sputum culture conversion was 68.57 days (IQR 61.01,76.12). The median time of sputum culture conversion in patients with drug-resistant tuberculosis was different in different WHO regions, countries with different levels of development and different treatment schemes. And female (aHR = 0.59,95%CI: s0.46,0.76), alcohol history (aHR = 0.70,95%CI:0.50,0.98), smoking history (aHR = 0.58,95%CI:0.38,0.88), history of SLD use (aHR = 0.64,95%CI:0.47,0.87), BMI < 18.5 kg/m2 (aHR = 0.69,95%CI:0.60,0.80), lung cavity (aHR = 0.70,95%CI:0.52,0.94), sputum smear grading at baseline (Positive) (aHR = 0.56,95%CI:0.36,0.87), (grade 1+) (aHR = 0.87,95%CI:0.77,0.99), (grade 2+) (aHR = 0.81,95%CI:0.69,0.95), (grade 3+) (aHR = 0.71,95%CI:0.61,0.84) were the related factor of sputum culture conversion time in patients with DR-TB.
Conclusion
Patients with DR-TB in Europe or countries with high level of economic development have earlier sputum culture conversion, and the application of bedaquiline can make patients have shorter sputum culture conversion time. Female, alcohol history, smoking history, history of SLD use, BMI < 18.5 kg/m2, lung cavity, sputum smear grading at baseline (Positive, grade 1+, grade 2+, grade 3+) may be risk factors for longer sputum culture conversion time.
This systematic review has been registered in PROSPERO, the registration number is CRD42023438746.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-09009-5.
Keywords: DR-TB, Sputum culture conversion time, Risk factors, Meta-analysis
Introduction
At present, the global situation of DR-TB (drug-resistant tuberculosis) is grim.
According to the 2022 Global Tuberculosis report, it is estimated that the number of new cases of MDR/RR-TB (multidrug-resistant/rifampin-resistant tuberculosis) reaches 450,000 [1]. Compared with drug-sensitive tuberculosis, drug-resistant tuberculosis, especially multidrug-resistant tuberculosis, has the characteristics of longer course of disease (18–24 months), heavy economic burden, more adverse reactions and poor therapeutic effect. A Meta analysis shows that the current success rate of MDR-TB treatment is only 58.4% [2], which is a major challenge in the field of tuberculosis treatment.
Because the treatment results of MDR-TB can not be obtained until 18–24 months after treatment, the effectiveness of the treatment regimen can not be evaluated in time, which to a certain extent affects the timely and effective adjustment of the treatment regimen. Therefore, the early prediction of treatment outcome of MDR-TB is very important [3]. The current evidence shows that sputum culture conversion time and status can be regarded as effective alternative indicators of treatment outcomes in patients with drug-resistant tuberculosis [4]. The study found that the earlier the sputum negative conversion, the better the efficacy [5], and faster conversion of sputum culture can increase patient comfort by reducing the duration of injectable drug use and simplifying patient treatment [6, 7]. In addition, the shorter sputum culture negative conversion time means that DR-TB patients have less chance of transmitting Mycobacterium tuberculosis, which has clinical and public health significance for controlling the spread of DR-TB [7]. Therefore, it is particularly important to evaluate the sputum culture conversion time and explore its influencing factors.
There are many studies on the sputum culture conversion time and its influencing factors in patients with DR-TB, but according to the current research results, it is found that there are differences in sputum culture conversion time among different studies, and the influencing factors are not consistent. At present, a systematic review describing the sputum culture conversion time and influencing factors in patients with MDR-TB is only for East African countries, and the inclusion of literature is limited, which limits the Meta analysis of influencing factors [8]. Therefore, we have the motivation to explore the sputum culture conversion time and related factors in the treatment of DR-TB patients. To further understand the level of treatment in each region and the differences between them. At the same time, to help medical staff identify the factors affecting the sputum culture conversion time, and intervene in time to improve the clinical outcome.
Methods
Search strategy
PubMed, The Cochrane Library, EMbase, CINAHL, Web of Science, CNKI, WanFang, CBM, VIP databases were electronically searched and retroactively included in the references of the study. The search time limit is from the establishment of the database to May 2023. Language restrictions are Chinese and English. During the search process, the authors used the following keywords and MeSH terms: “Drug-Resistant Tuberculosis/DR-TB/MDR-TB/Multidrug-Resistant Tuberculosis/MDR Tuberculosis/Extensively drug resistant pulmonary tuberculosis/XDR-TB/RR-TB” and “Sputum culture conversion time/Sputum conversion”.
Selection criteria
Inclusion criteria: On the one hand, the subjects were clearly diagnosed as DR-TB patients, and the content of the study reported the median time of sputum culture conversion (Median time, IQR) during the treatment of DR-TB patients, on the other hand, the type of study was a cohort study. Sputum culture conversion is defined as two consecutive negative sputum cultures at an interval of at least one month (or four weeks) after the initial positive sputum culture. The negative conversion time of sputum bacteria was the collection time of sputum culture negative samples for the first time [9].
Exclusion criteria: (1) reviews or case reports; (2) duplicate studies; (3) original texts were not in English or Chinese; (4) data were incomplete; (5) the full text can not be obtained.
Study selection
References were stored and managed using Endnote X9. The articles retrieved from the databases were imported to Endnote X9, and then duplicates were removed. Two researchers independently conducted the screening of the research literature. Articles were first screened based on the title and abstract, and then the literature was re-screened by reading the full text. In case of disagreement between the two researchers, a third researcher was consulted.
Data extraction and quality assessment
Data extraction using a standardized Microsoft Excel data extraction tool was carried out by two independent authors for each study, and inconsistencies were resolved by consultation with a third author. The contents of data extraction included the first author of the literature, year of publication, country, region according to WHO, data year, type of study, sample size, average/median age of patients, median time of sputum culture (IQR), 2-month negative conversion rate, overall negative conversion rate, treatment scheme, influencing factors of negative conversion time. Newcastle–Ottawa scale (Newcastle–Ottawa Scale, NOS) was used to evaluate the quality of the literature [10].
Data processing and analysis
Meta-analysis of sputum culture conversion time of DR-TB patients was performed with R software version 4.3.0 and the combination of the median time (median, IQR) to sputum culture conversion was realized by QE method (Quantile estimation, QE); Meta-analysis of the influencing factors of sputum culture conversion time was performed with software version 16 and the effect was combined with hazard ratio (HR) and its 95% confidence interval (CI). Heterogeneity was assessed by computing p-values of Higgins’s I2test statistics and Q-statistics among reported median time of culture conversion. If P > 0.1 and I2 < 50%, it shows that there is no statistical heterogeneity among the studies, so choose the fixed effect model, otherwise suggest that there is statistical heterogeneity, and choose the random effect model. The Higgins’s I2 statistic measures the difference between sample quartile estimation, which is due to heterogeneity due to random error rather than to sampling error. In this case, the pooled effect was estimated with a random-effects meta-analysis model. Subgroup analyses were performed to identify possible sources of heterogeneity by considering WHO region the study belonged to, treatment regimens, and national development level. The heterogeneity of the results was analyzed by χ2 test (the test level was α = 0.1) when the factors affecting the sputum culture conversion time were analyzed. If there was no statistical heterogeneity among the results (p > 0.1, I2 < 50%), the fixed-effects model was used for Meta-analysis. If there was statistical heterogeneity among the results (p ≤ 0.1, I2 ≥ 50%), random effects model was used for Meta-analysis. The test level of Meta analysis was 0. 05. The publication bias was analyzed by funnel chart.
Results
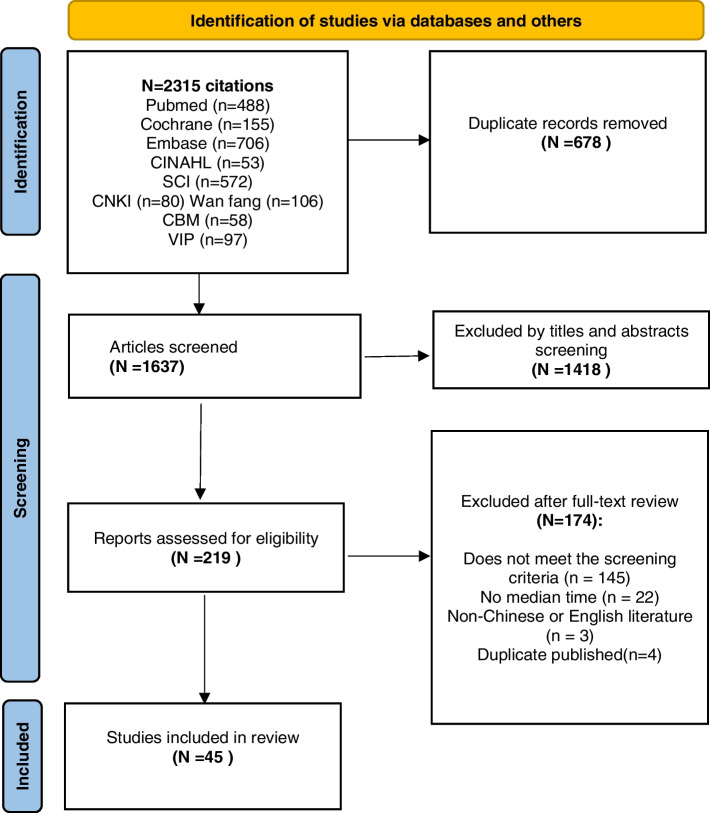
A total of 2315 articles were retrieved, and 45 studies including 17,373 DR-TB patients were finally included after layer-by-layer screening. The flow chart and results of literature screening are shown in Fig. 1.
Fig. 1.
Flow chart of selecting articles for systematic review and meta-analysis
Characteristics of the included studies
The 45 articles included were published from 2011 to 2022, from Ethiopia, Nigeria, Indonesia, China, Georgia, South Africa, Tanzania, Kenya, Egypt, Peru, South Korea, Pakistan, Botswana, Nepal, Liwan Tao, India, Guinea, Germany, Myanmar, Dominica 20 countries. Distributed in Africa, Southeast Asia, Western Pacific, Europe, America, Eastern Mediterranean six WHO regions. All were cohort studies, with data from 1990 to 2020 and study sample sizes ranging from 16 to 3712 with a cumulative total of 17,373 patients. The basic characteristics of the included literatures are shown in Table 1. (As the width of the table exceeds the letter landscape page, please see the Additional files).
Table 1.
Descriptive summary of included studies
| Authors | Year (yr) |
Country | Region by World Health Organization | Year of the data (yr) | Study design | Sample size |
Mean/median, age (yrs) | Type of TB | Median time (IQR) | 2nd-month Conversion (%) |
Overall conversion (%) | Treatment scheme | Influencing factors of conversion time |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Akalu TY | 2018 | Ethiopia | Africa | 2010–2016 | Retrospective cohort | 392 | 29.5 |
RR-TB MDR-TB XDR-TB |
65 days (60–70) | - | 86.7% | - | 3, 8, 12, 13, 14 |
| Akinsola OJ | 2018 | Nigeria | Africa | 2012–2016 | Retrospective cohort | 413 | 36.8 ± 12.7 | MDR-TB | 5.5 months (1.5–11.5) | - | 58.4% | - | 1, 6, 15 |
| Putri FA | 2014 | Indonesia | Southeast Asia | 2009–2011 | Retrospective cohort | 212 | 37 ± 12 |
MDR-TB XDR-TB |
2 months (1–3) | - | 81% | A + B | 2, 5, 8, 14, 17, 19 |
| Lu P | 2017 | China | Western Pacific region | 2011–2014 | Prospective cohort | 139 | 51 | MDR-TB | 92 days (34–111) | 28% | 76.3% | A + B | - |
| Li Q | 2019 | China | Western Pacific region | 2011–2015 | Retrospective cohort | 365 | - | MDR-TB | 85 days (42.0–106.5) | - | 90.96% | A + B | - |
| Kurbatova EV | 2012 | Multiple countries | Multi-region | 2000–2003 | Retrospective cohort | 1416 | 38 |
MDR-TB XDR-TB |
3 months (2.0–5.0) | 33% | 85.4% | - | - |
| Li Q | 2020 | China | Western Pacific region | 2011–2015 | Retrospective cohort | 384 | 41.7 ± 15.4 | MDR-TB | 85 days (40–112) | - | 93.5% | - | 1 |
| Liu Q | 2018 | China | Western Pacific region | 2011–2014 | Prospective cohort | 139 | 51 | MDR-TB | 91.5 days (34.0–110.8) | - | 76.3% | A + B | 1, 2, 3, 4, 8,19 |
| Magee MJ | 2014 | Georgia | Europe | 2009–2011 | Prospective cohort | 1366 | 35.1 | MDR-TB | 68 days (50–120) | - | 70.7% | B | 1, 2, 3, 4, 5, 6, 8, 10, 12, 18 |
| Ncha R | 2019 | South Africa | Africa | 2012–2014 | Retrospective cohort | 371 | 38 |
MDR-TB XDR-TB |
58.2 days (29–113) | - | 70% | - | 2, 3, 4, 5, 8, 12, 14 |
| Mpagama SG | 2013 | Tanzania | Africa | 2009–2011 | Retrospective cohort | 61 | 36 ± 13 | MDR-TB | 2 months (1–3) | - | 85% | A | - |
| Huerga H | 2017 | Kenya | Africa | 2006–2012 | Retrospective cohort | 169 | 29 | MDR-TB | 2 months (2–3) | - | 73.9% | A + B | - |
| Bade AB | 2021 | Ethiopia | Africa | 2013–2019 | Retrospective cohort | 200 | 32.9 ± 9.5 | MDR-TB | 31 days (30–61) | 79.5% | 100% | - | - |
| Brust JC | 2011 | South Africa | Africa | 2008–2009 | Retrospective cohort | 45 | 33 | MDR-TB | 62 days (48–111) | - | 89% | A | - |
| Magee MJ | 2017 | South Africa | Africa | 2011–2015 | Prospective cohort | 91 | 35 | MDR-TB | 82 days (53–143) | 55.5% | 96% | A | - |
| Brust JC | 2013 | South Africa | Africa | 2008–2010 | Retrospective cohort | 56 | 36 | MDR-TB | 66 days (43–96) | 32% | 95% | A | - |
| Gadallah MA | 2016 | Egypt | Africa | 2006–2010 | Prospective cohort | 228 | 37 | MDR-TB | 60 days (58–121) | 21.3% | 87.5% | B | - |
| Zheng X | 2022 | China | Western Pacific region | 2016–2019 | Prospective cohort | 197 | 42.0 ± 9.9 | MDR-TB | 4 months (2–14) | 44.7% | 79.2% | A | - |
| Tierney DB | 2014 | Peru | America | 1990–2002 | Retrospective cohort | 592 | 28.7 | MDR-TB | 59 days (31–92) | - | 87.7% | B | - |
| Lee M | 2019 | South Korea | Southeast Asia | 2011–2015 | Retrospective cohort | 2472 | 47 ± 17.0 | MDR-TB | 61 days (28–109) | - | - | B | - |
| Javaid A | 2018 | Pakistan | Eastern Mediterranean | 2012–2014 | Retrospective cohort | 428 | 30.7 ± 14.35 | MDR-TB | 58 days (30–90) | 56.8% | - | B | 8, 11, 12, 15, 16, 19 |
| Hafkin J | 2013 | Botswana | Africa | 2005–2009 | Retrospective cohort | HIV-infected:40;non-HIV-infected:30 | 38 | MDR-TB |
HIV-infected:78 days (42–186) non-HIV-infected:95 days (70–133) |
- | 84% | B | 7 |
| Ghimire S | 2020 | Nepal | Southeast Asia | 2014–2016 | Retrospective cohort | 98 | 29 | MDR-TB | 60 days (60–90) | - | 82.6% | A | - |
| Diktanas S | 2021 | Li Wantao | Europe | 2016–2019 | Retrospective cohort | 115 | 48 ± 12 |
MDR-TB RR-TB XDR-TB |
1.1 months (0.9–1.8) | 65.2% | 89.6% | - | 1, 2, 3, 4, 8, 11, 12, 14 |
| Meshesha MD | 2022 | Ethiopia | Africa | 2014–2018 | Retrospective cohort | 145 | 29.6 ± 12.4 | MDR/RR-TB | 2 months (1–3) | 69.7% | - | A + B | 2, 9, 10, 12, 14 |
| Tekalegn Y | 2020 | Ethiopia | Africa | 2012–2017 | Retrospective cohort | 228 | 28 | All DR-TB | 61 days (34–92) | 46.9% | - | A | 5, 12, 14 |
| Velayutham B | 2016 | India | Southeast Asia | 2009–2011 | Retrospective cohort | 787 | - | MDR/RR-TB | 91.3 days (91.3–121.7) | - | 83% | A | - |
| Diallo A | 2020 | Guinea | Africa | 2016–2018 | Retrospective cohort | 118 | 34 | RR-TB | 59 days (31–61) | - | 89% | A | - |
| Reimann M | 2019 | Germany | Europe | 2012–2017 | Retrospective cohort | non-smoker:20; smoker:45 | 36.5/37.4 | MDR-TB XDR-TB |
non-smoker:53 days (19–89) smoker:60.7 days (33.3–76) |
- | - | - | - |
| Shibabaw A | 2018 | Ethiopia | Africa | 2011–2016 | Retrospective cohort | 235 | 30 | MDR/RR-TB | 72 days (44–123) | - | 85.5% | A | 5, 7 |
| Ding CH | 2021 | China | Western Pacific region | 2018–2020 | Prospective cohort | 79 | ≥ 18 |
MDR-TB XDR-TB pre-XDR-TB |
4 weeks (2–8) | 70.9% | 91.1% | C | - |
| Htun YM | 2018 | Myanmar | Southeast Asia | 2014 | Retrospective cohort | 330 | 39.45 ± 13.28 | MDR-TB | 147 days (94–241) | - | - | A | - |
| Shi ZY | 2021 | China | Western Pacific region | 2018–2019 | Retrospective cohort | 38 | 31 |
MDR-TB/ XDR-TB |
8 weeks (4–16) | 68.4% | 84.2% | C | - |
| Kim CT | 2018 | South Korea | Southeast Asia | 2015–2017 | Retrospective cohort | 55 | 52 |
MDR-TB XDR-TB |
119 days (52.5–198.5) | - | 70.9% | C | - |
| Abubakar M | 2022 | Pakistan | Eastern Mediterranean | 2010–2017 | Retrospective cohort | 355 | 32.99 ± 14.54 | XDR-TB | 91 days (59–156) | 27.3% | 63.6% | B | 1, 8, 10, 15, 16, 20 |
| Salindri AD | 2016 | Georgia | Europe | 2011–2014 | Prospective cohort | 52 | 47 |
MDR-TB XDR-TB |
62 days (32–94) | - | 84.6% | - | 1, 2, 4, 6, 7, 8, 12 |
| Wu GL | 2021 | China | Western Pacific region | 2018–2020 | Prospective cohort | 16 | 26 ~ 27 |
MDR-TB XDR-TB |
8 weeks (4–12) | - | 94% | C | - |
| Pei Y | 2021 | China | Western Pacific region | 2018–2020 | Prospective cohort | 44 | 38 |
MDR-TB XDR-TB |
22 days (18–59) | - | 95% | C | - |
| Kim J | 2016 | South Korea | Southeast Asia | 2009–2012 | Retrospective cohort | 35 | 41 |
MDR-TB XDR-TB |
56 days (0–92) | 60% | - | A | - |
| Shi L | 2021 | China | Western Pacific region | 2018–2019 | Retrospective cohort |
DM group: 76; non-DM group: 61 |
49.8 ± 10.5 49.3 ± 9.7 |
MDR-TB XDR-TB |
56 days (28–63) 56 days (28–84) |
- |
95.6% 98.2% |
C | - |
| Rodriguez M | 2013 | Dominica | America | 2006–2010 | Retrospective cohort | 289 | 35 | MDR-TB | 2 months (2–3) | - | 86.5% | A + B | - |
| Parmar MM | 2018 | India | Southeast Asia | 2007–2011 | Retrospective cohort | 3712 | 35 | MDR-TB | 100 days (92–125) | - | 73.6% | - | 1, 2, 5, 6 |
| Heyckendorf J | 2018 | Germany | Europe | 2013–2016 | Prospective cohort | 29 | 36 |
MDR-TB XDR-TB |
39 days (6–85) | 61% | 95% | B + C | - |
| Gao M | 2021 | China | Western Pacific region | 2018.02–12 | Prospective cohort | 177 | 40 |
MDR-TB XDR-TB |
4 weeks (2–8) | 67.2% | 85.3% | C | - |
| Borisov SE | 2017 | Multiple countries | Multi-region | 2008–2016 | Retrospective cohort | 428 | 35 |
MDR-TB XDR-TB |
60 days (33–90) | 56.7% | 91.2% | C | - |
A = a standardized regimen; B = an individualized regimen; C = bedaquiline-containing regimens
Note:- = Not described; 1 = Age; 2 = Sex; 3 = Alcohol; 4 = Smoking status; 5 = BMI, kg/m2; 6 = Diabetes; 7 = HIV status; 8 = Sputum smear grading at baseline; 9 = Baseline Hemoglobin (g/dl); 10 = TB treatment history; 11 = History of SLD use; 12 = lung cavity; 13 = Consolidation; 14 = Type of resistance; 15 = Number of resistant drugs; 16 = Resistance to all five first lines drugs; 17 = Resistant to any injectable(s); 18 = Any 2nd line resistance; 19 = Resistance to ofloxacin; 20 = Use of high dose isoniazid
Literature quality evaluation
All the included studies were evaluated strictly according to NOS standards, with 13 of medium quality and 32 of high quality (Table 2).
Table 2.
Results of bias risk assessment (Score)
| Name | Selection | Comparability | Outcome | Score | Quality Grade | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ① | ② | ③ | ④ | ⑤ | ⑥ | ⑦ | ⑧ | ||||
| 1 | Akalu TY | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 | High quality |
| 2 | Akinsola OJ | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 | High quality |
| 3 | Putri FA | 1 | 1 | 1 | 0 | 2 | 0 | 1 | 1 | 7 | Moderate quality |
| 4 | Lu P | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | High quality |
| 5 | Li Q | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 | High quality |
| 6 | Kurbatova EV | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 | High quality |
| 7 | Li Q | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 | High quality |
| 8 | Liu Q | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | High quality |
| 9 | Magee MJ | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | High quality |
| 10 | Ncha R | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 | High quality |
| 11 | Mpagama SG | 1 | 1 | 1 | 0 | 2 | 0 | 1 | 1 | 7 | Moderate quality |
| 12 | Huerga H | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 | High quality |
| 13 | Bade AB | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 8 | High quality |
| 14 | Brust JC | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 | Moderate quality |
| 15 | Magee MJ | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | High quality |
| 16 | Brust JC | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 | High quality |
| 17 | Gadallah MA | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | High quality |
| 18 | Zheng X | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | High quality |
| 19 | Tierney DB | 1 | 1 | 1 | 0 | 2 | 0 | 1 | 1 | 7 | Moderate quality |
| 20 | Lee M | 1 | 1 | 1 | 0 | 2 | 1 | 0 | 1 | 7 | Moderate quality |
| 21 | Javaid A | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 | High quality |
| 22 | Hafkin J | 1 | 1 | 1 | 0 | 2 | 0 | 0 | 1 | 6 | Moderate quality |
| 23 | Ghimire S | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 | High quality |
| 24 | Diktanas S | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 | High quality |
| 25 | Meshesha MD | 1 | 1 | 1 | 0 | 2 | 0 | 0 | 1 | 6 | Moderate quality |
| 26 | Tekalegn Y | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 | High quality |
| 27 | Velayutham B | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 | High quality |
| 28 | Diallo A | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 | High quality |
| 29 | Reimann M | 1 | 1 | 1 | 0 | 2 | 1 | 0 | 1 | 7 | Moderate quality |
| 30 | Shibabaw A | 1 | 1 | 1 | 0 | 2 | 1 | 0 | 1 | 7 | Moderate quality |
| 31 | Ding CH | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | High quality |
| 32 | Htun YM | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 | High quality |
| 33 | Shi ZY | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | High quality |
| 34 | Kim CT | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 6 | Moderate quality |
| 35 | Abubakar M | 1 | 1 | 1 | 0 | 2 | 1 | 0 | 1 | 7 | Moderate quality |
| 36 | Salindri AD | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | High quality |
| 37 | Wu GL | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 | High quality |
| 38 | Pei Y | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | High quality |
| 39 | Kim J | 1 | 1 | 1 | 0 | 2 | 1 | 0 | 1 | 7 | Moderate quality |
| 40 | Shi L | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 | High quality |
| 41 | Rodriguez M | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 | High quality |
| 42 | Parmar MM | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 7 | Moderate quality |
| 43 | Heyckendorf J | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | High quality |
| 44 | Gao M | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | High quality |
| 45 | Borisov SE | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 | High quality |
Note: ①Representativeness of the exposed cohort; ②selection of the non exposed cohort; ③Ascertainment of exposure; ④Demonstration that outcome of interest was not present at start of study; ⑤Comparability of cohorts on the basis of the design or analysis; ⑥Assessment of outcome; ⑦Was follow-up long enough for outcomes to occur; ⑧Adequacy of follow up of cohorts
Time to sputum culture conversion among DR-TB patients
The median time of sputum culture conversion was described in all 45 studies. Meta analysis showed that the pooled median time of sputum culture conversion was 68.57d (IQR 61.01,76.12). According to the Higgins I2 test (I2 = 99.32%, p < 0.0001), the pooled median time of sputum culture conversion in Meta analysis showed high heterogeneity. Therefore, we conducted a subgroup analysis to determine the source of heterogeneity. We considered the subgroup analysis of the characteristics of the inclusion study, such as WHO region, national development level, treatment scheme and so on. By region, Meta analysis showed that the shortest negative conversion time of sputum culture was 53.15 days (IQR 40.39,65.91) in Europe, and the longest negative conversion time of sputum culture was 85.94 days (IQR 63.00,108.88) in Southeast Asia. According to the national development level, Meta analysis shows that the negative conversion time of developed countries is 57.63 days (IQR 40.48,74.78), while that of developing countries is 69.97 days (IQR 61.35,78.59). According to the analysis of whether the treatment regimen contained bedaquiline or not, the results of Meta analysis showed that sputum culture conversion time was 49.39 days (IQR 34.95,63.83) in patients with bedaquiline and 73.36 days (IQR 65.68,81.04) in patients without bedaquiline in treatment regimen (Table 3).
Table 3.
Subgroup analysis of sputum culture conversion time among DR-TB patients
| Method | Pooled median time (IQR), d | Heterogeneity evaluation | |||
|---|---|---|---|---|---|
| T2 | I2 | Q | |||
| QE | 68.57 (61.01, 76.12) d | 663.34 | 99.32% | 5149.79 (P < 0.0001) | |
| Category | Subgroup | No. of studie | Sample size | Median time (IQR),d | P-val |
| World Health Organization regions | Africa | 15 | 2780 | 69.42d (56.35, 82.49) | < 0.0001 (I2 = 98.71%) |
| Europe | 5 | 1627 | 53.15d (40.39, 65.91) | < 0.0001 (I2 = 92.37%) | |
| Southeast Asia | 8 | 7701 | 85.94d (63.00, 108.88) | < 0.0001 (I2 = 99.73%) | |
| America | 2 | 881 | 59.64d (56.92, 62.37) | 0.73 (I2 = 0%) | |
| Eastern Mediterranean | 2 | 787 | 59.22d (54.56, 63.88) | 0.86 (I2 = 0%) | |
| Western Pacific Ocean | 11 | 1715 | 63.27d (46.78, 79.76) | < 0.0001 (I2 = 97.39%) | |
| National development level | developed country | 6 | 2771 | 57.63d (40.48, 74.78) | < 0.0001 (I2 = 96.12%) |
| developing country | 37 | 12,716 | 69.97d (61.35, 78.59) | < 0.0001 (I2 = 99.41%) | |
| Treatment regimen | without bedaquiline | 26 | 8895 | 73.36d (65.68, 81.04) | < 0.0001 (I2 = 97.76%) |
| Contain bedaquiline | 9 | 1003 | 49.39d (34.95, 63.83) | < 0.0001 (I2 = 96.67%) | |
Influencing factors of sputum culture conversion time
Among the 45 studies included in this study, 16 reported the adjusted HR values of the factors affecting the sputum culture conversion time, which were included in Meta analysis. Finally, 12 influencing factors were included in the analysis, including gender, alcohol, smoking status, TB treatment history, history of second-line drug (SLD) use, BMI, diabetes, lung cavity, HIV, sputum smear grading at baseline (Positive, grade 1+, grade 2+, grade 3+), resistance to ofloxacin, and resistance to all five first lines drugs.
The results of Meta analysis showed that female, alcohol history, smoking history, history of SLD use, BMI < 18.5 kg/m2, lung cavity and sputum smear grading at baseline (Positive, grade 1+, grade 2+, grade 3+) were the influencing factors of longer sputum culture conversion time, and fund to be statistically significant (P < 0.05). However, male, current smoking, TB treatment history, diabetes, HIV, resistance to ofloxacin, and resistance to all five first lines drugs were not the influencing factors of longer sputum culture conversion time (Table 4).
Table 4.
Meta analysis of the factors affecting the sputum culture conversion time among DR-TB patients
| The influence factors were included | Exposure factors | Number of articles included | Heterogeneity | aHR | 95%CI | P | ||
|---|---|---|---|---|---|---|---|---|
| I2 (%) | P | effects model | ||||||
| Sex | Male | 4 | 0 | 0.57 | Fixed | 0.99 | 0.91 ~ 1.07 | 0.80 |
| Female | 3 | 0 | 0.84 | Fixed | 0.59 | 0.46 ~ 0.76 | < 0.0001 | |
| Alcohol | Alcohol history | 3 | 49 | 0.14 | Fixed | 0.70 | 0.50 ~ 0.98 | 0.039 |
| Smoking status | Smoking history | 3 | 0 | 0.506 | Fixed | 0.58 | 0.38 ~ 0.88 | 0.01 |
| Current smoker | 2 | 79.2 | 0.028 | Random | 0.61 | 0.30 ~ 1.24 | 0.17 | |
| TB treatment history | 3 | 0 | 0.925 | Fixed | 0.94 | 0.83 ~ 1.09 | 0.46 | |
| History of SLD use | 2 | 0 | 0.96 | Fixed | 0.64 | 0.47 ~ 0.87 | 0.004 | |
| BMI | BMI < 18.5 kg/m2 | 4 | 0 | 0.89 | Fixed | 0.69 | 0.60 ~ 0.80 | < 0.0001 |
| Diabetes | 4 | 79.1 | 0.002 | Random | 0.77 | 0.50 ~ 1.17 | 0.22 | |
| lung cavity | 5 | 70.3 | 0.009 | Random | 0.70 | 0.52 ~ 0.94 | 0.016 | |
| HIV | 2 | 0 | 0.673 | Fixed | 0.76 | 0.42 ~ 1.21 | 0.36 | |
| Sputum smear grading | Positive | 3 | 50.3 | 0.13 | Random | 0.56 | 0.36 ~ 0.87 | 0.009 |
| grade 1 + | 3 | 0 | 0.94 | Fixed | 0.87 | 0.77 ~ 0.99 | 0.043 | |
| grade 2 + | 3 | 0 | 0.66 | Fixed | 0.81 | 0.69 ~ 0.95 | 0.009 | |
| grade 3 + | 3 | 0 | 0.94 | Fixed | 0.71 | 0.61 ~ 0.84 | < 0.0001 | |
| Resistance to ofloxacin | 3 | 58.6 | 0.09 | Random | 0.67 | 0.43 ~ 1.04 | 0.07 | |
| Resistance to all five first lines drugs | 2 | 0 | 0.973 | Fixed | 0.86 | 0.62 ~ 1.21 | 0.395 | |
In addition, age, type of resistance, number of resistant drugs, consolidation, resistant to any injectable(s), resistance to any second-line drug, baseline hemoglobin (g/dl) and use of high-dose isoniazid could not be Meta-analyzed, because the classification criteria are different or only mentioned in a single article.
Sensitivity analysis
In order to test the stability and reliability of the analysis results, the fixed effect model and random effect model were used to calculate HR and 95%CI respectively, and the stability of the results was discussed. The results showed that except for “Alcohol history”, “Current smoker” and “Resistance to ofloxacin”, the Meta analysis results of other risk factors did not change after the transformation effect model, which suggested that the results were reliable (Table 5).
Table 5.
The combined results of fixed effect model and random effect model
| The influence factors were included | Exposure factors | Fixed effects model | Random effect model |
|---|---|---|---|
| Gender | Male | 0.99 (0.91 ~ 1.07) | 0.99 (0.91 ~ 1.07) |
| Female | 0.59 (0.46 ~ 0.76) | 0.59 (0.46 ~ 0.76) | |
| Alcohol | Alcohol history | 0.70 (0.50 ~ 0.98) | 0.69 (0.43 ~ 1.13) |
| Smoking status | Smoking history | 0.58 (0.38 ~ 0.88) | 0.58 (0.38 ~ 0.88) |
| Current smoker | 0.79 (0.68 ~ 0.91) | 0.61 (0.30 ~ 1.24) | |
| TB treatment history | 0.94 (0.83 ~ 1.09) | 0.94 (0.83 ~ 1.09) | |
| History of SLD use | 0.64 (0.47 ~ 0.87) | 0.64 (0.47 ~ 0.87) | |
| BMI | BMI < 18.5 kg/m2 | 0.69 (0.60 ~ 0.80) | 0.69 (0.60 ~ 0.80) |
| Diabetes | 0.77 (0.50 ~ 1.17) | 0.77 (0.50 ~ 1.17) | |
| lung cavity | 0.70 (0.62 ~ 0.80) | 0.70 (0.52 ~ 0.94) | |
| HIV | 0.76 (0.42 ~ 1.21) | 0.76 (0.42 ~ 1.21) | |
| Sputum smear grading | Positive | 0.58 (0.45 ~ 0.76) | 0.56 (0.36 ~ 0.87) |
| grade 1 + | 0.87 (0.77 ~ 0.99) | 0.87 (0.77 ~ 0.99) | |
| grade 2 + | 0.81 (0.69 ~ 0.95) | 0.81 (0.69 ~ 0.95) | |
| grade 3 + | 0.71 (0.61 ~ 0.84) | 0.71 (0.61 ~ 0.84) | |
| Resistance to ofloxacin | 0.66 (0.53 ~ 0.80) | 0.67 (0.43 ~ 1.04) | |
| Resistance to all five first lines drugs | 0.86 (0.62 ~ 1.21) | 0.86 (0.62 ~ 1.21) |
Publication bias
We did not assess publication bias due to the limited number of studies (< 10) [11].
Discussion
This study comprehensively searched the study on the sputum culture conversion time in DR-TB, and finally included 45 articles that met the inclusion criteria. The included literatures come from 20 countries and are widely distributed in 6 WHO regions, with a total of 17,373 samples. All the literatures are cohort studies with strong causal argumentation intensity. The overall NOS quality scores included in the literature are all ≥ 6, indicating that the quality of literature methodology is medium or above, so the overall conclusion of the study is more reliable.
This study was divided into subgroups according to the characteristics of the literature, and discussed the sputum culture conversion time under different WHO regions, national development levels and treatment schemes, and used the adjusted HR value to analyze the influencing factors of sputum culture conversion time, so as to ensure the scientificity and reliability of the results. At the same time, this study is of great significance to explore the sputum culture conversion time and its influencing factors which are of great value in monitoring treatment results, preventing and controlling infection and adjusting patients’ treatment plan. Therefore, this study mainly focuses on the median time of sputum culture conversion in the treatment of DR-TB patients, and objectively analyzes the influencing factors of conversion time, in order to provide clinical reference.
The results of this study show that women have a longer conversion time of sputum culture than men. This difference may reflect the biological differences in patients with DR-TB.
Studies have shown that alcohol use is a key driver of poor response to tuberculosis treatment [12]. The results of this study showed that alcohol history was a risk factor for longer sputum culture negative conversion time in patients with DR-TB, which was consistent with the conclusions of previous studies. This may be due to the fact that alcohol can reduce the number and function of dendritic cells and neutrophils by inhibiting the phagocytic and bactericidal activity of macrophages, thus reducing the immune function of patients with DR-TB. In addition, some studies have pointed out that long-term heavy drinking is related to the inhibition of phagocytosis and the production of growth factors in innate immune cells in a dose-and time-dependent manner [13], indicating that long-term alcohol consumption has a greater adverse effect on the immune response of tuberculosis. Whether the length of drinking history and the severity of alcohol consumption further promote the delay of negative conversion time of sputum culture is still worthy of further exploration.
This study found that patients with a history of smoking had a longer negative conversion time of sputum culture than patients without a history of smoking. Published studies have shown that smoking can delay sputum culture transformation in tuberculosis patients, including XDR-TB [14], which is consistent with the findings of previous studies. It may be because smoking has a negative effect on the phagocytosis of alveolar macrophages, which leads to the spread of tuberculosis bacteria in the lungs and delays the clearance of bacteria [15].
The history of the use of second-line anti-tuberculosis drugs is the influencing factor of the sputum culture conversion time, which may be due to the more complex drug resistance caused by the exposure of patients to second-line anti-tuberculosis drugs, resulting in poor therapeutic effect and longer sputum culture conversion time [16]. BATOOL et al. also support this view [17]. In addition, BATOOL et al. also pointed out that the sputum culture conversion time of patients increased with the increase of previous exposure to SLD [17]. However, this study has not been explored because of the lack of relevant data in the literature, so it needs to be further studied.
The results of this study showed that malnutrition was a risk factor for longer sputum culture conversion time. A Meta analysis of the effect of malnutrition on sputum culture negative conversion time showed that malnutrition was significantly associated with longer sputum culture negative conversion time [18]. It is consistent with the conclusion of this study. In addition, some studies have found that obese patients with tuberculosis have a lower conversion rate than patients with ideal body mass index [19], so overweight and obesity may also delay the sputum culture conversion time, but this study only analyzed the effect of BMI < 18.5 kg/m2 on the sputum culture conversion time. The relationship between overweight, obesity and sputum culture negative conversion time needs to be further explored.
In this study, it was determined that lung cavity was a factor affecting the negative conversion time of sputum culture. On the one hand, it may be due to the high load of mycobacteria in patients with lung cavity [20]. On the one hand, it may be difficult for drugs to penetrate into these lung cavities. Reduce the drug permeability and antibacterial activity, and finally prolong the sputum conversion time [21]. In addition, the study [22] found that the median time of sputum culture transformation in patients with single lung cavity was shorter than that in patients with double lung cavity, but Tekaleg et al. found that the negative conversion time of sputum culture in patients with single lung cavity and double lung cavity was not statistically significant [22], so the relationship between the two needs to be further determined.
Patients with negative sputum smear at baseline took longer to turn negative than patients with sputum smear positive at baseline and sputum smear grades 1+, 2+ and 3+. It may be because of the high bacterial load, it takes a long time to remove the bacteria.
In this study, we have not found the correlation between the negative conversion time of sputum culture and the history of TB treatment, diabetes, HIV, resistance to ofloxacin and resistance to five first-line antituberculosis drugs. With regard to the history of TB treatment, most studies have found that retreated pulmonary tuberculosis patients have more bacterial load and later sputum bacteria conversion than newly treated pulmonary tuberculosis patients [23]. BADE et al. pointed out that patients with a previous history of TB treatment had a 4-fold higher risk of delayed culture conversion than patients with new MDR-TB [24]. It is not consistent with the conclusion of this study, which may be related to the lack of literature included in this study. Therefore, the relationship between the history of TB treatment and the negative conversion time of sputum culture needs further study. Previous studies showed that diabetes delayed the sputum culture conversion time of drug-sensitive tuberculosis [21], but this study showed that diabetes had no effect on the sputum culture negative conversion time of DR-TB. JAFRI et al. also indicated that the blood glucose level did not affect the sputum culture negative conversion rate of DR-TB patients when adopting the best regimen [23]. It is consistent with the conclusion of this study.
In addition, this study found that age, type of drug resistance, number of resistant drugs, consolidation, resistant to any injectable(s), resistance to any second-line drug, baseline hemoglobin (g/dl) and use of high dose isoniazid may be related to the negative conversion time of sputum culture in patients with DR-TB, but we were not able to pool to generate the effect size of these factors on sputum culture conversion time due to the different classification criteria or only mentioned in single study, so more studies are needed to confirm this furtherly.
Our limitations include: (1) Only Chinese and English literatures are included in this study, and there may be some selection bias; (2) The description of the median time of sputum culture conversion is not all in days. In this study, the conversion time in monthly /weekly units is converted into days, which may have some errors; (3) The description of the treatment schemes is not specific enough to further analyze its effect on the median time of negative conversion; (4) Some of the influencing factors can not be analyzed by Meta because of different classification criteria or only mentioned in a single article; (5) Since the number of studies included in the Meta analysis is less than 10, the funnel chart is not depicted, and there may be a potential publication bias. We found that in some studies, the monitoring frequency of sputum culture is not strictly once a month, which may affect the accuracy of sputum culture negative conversion time. Therefore, it is suggested that more prospective studies with high quality and large sample size be carried out in the future, strict monthly sputum examination, and further clarify the factors affecting the negative conversion time of sputum culture.
Conclusion
Therefore, the negative effects of female, alcohol history, smoking history, history of SLD use, BMI < 18.5 kg/m2, lung cavity and sputum smear grading at baseline (Positive, grade 1+, grade 2+, grade 3+) on sputum negative conversion time should be recognized. Patients with this characteristic should be prevented and reliable intervention programs should be adjusted to improve the prognosis of patients.
Supplementary Information
Acknowledgements
Not applicable.
Authors’ contributions
Y WL is responsible for the conception and design of the article, the collection and arrangement of research materials, and paper writing; WB and ZX are responsible for the revision, quality control and revision of the article, and responsible for the overall supervision and management of the article; W CT, SD and YX are responsible for data analysis and checking; Y QL, X XJ and YR are responsible for editing and collating forms.
Funding
This work is supported by Sichuan Nursing Association [Nursing Scientific Research Project Program of Sichuan Province, No.H22008] and Sichuan Provincial Health Committee [Medical Science and Technology Project of Sichuan Provincial Health Commission,No.21PJ156].
Availability of data and materials
The dataset(s) supporting the conclusions of this article is (are) included within the article (and its additional file(s)).
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yang Wenlu and Zhao Xia are co-first authors.
References
- 1.Bagcchi S. WHO's global tuberculosis report 2022. Lancet Microbe. 2023;4(1):e20. doi: 10.1016/S2666-5247(22)00359-7. [DOI] [PubMed] [Google Scholar]
- 2.Chaves Torres NM, Quijano Rodriguez JJ, Porras Andrade PS, et al. Factors predictive of the success of tuberculosis treatment: A systematic review with meta-analysis. PLoS One. 2019;14(12):e0226507. doi: 10.1371/journal.pone.0226507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lv L, Li T, Xu K, et al. Sputum bacteriology conversion and treatment outcome of patients with multidrug-resistant tuberculosis. Infect Drug Resist. 2018;11:147–154. doi: 10.2147/IDR.S153499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Velayutham B, Nair D, Kannan T, et al. Factors associated with sputum culture conversion in multidrugresistant pulmonary tuberculosis. Int J Tuberc Lung Dis. 2016;20(12):1671–1676. doi: 10.5588/ijtld.16.0096. [DOI] [PubMed] [Google Scholar]
- 5.Lu P, Liu Q, Zhu LM, et al. Comparison of the effectiveness of sputum smear and sputum culture negative conversion as predictors of treatment outcome in MDR-TB patients. Prev Med Jiangsu Prov. 2019;30(04):360–363. doi: 10.13668/j.issn.1006-9070.2019.04.002. [DOI] [Google Scholar]
- 6.Iqbal Z, Khan MA, Aziz A, et al. Time for culture conversion and its associated factors in multidrug-resistant tuberculosis patients at a tertiary level hospital in Peshawar, Pakistan. Pakistan J Med Sci. 2022;38(4Part-II):1009. doi: 10.12669/pjms.38.4.5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.YihunieAkalu T, FentahunMuchie K, Alemu Gelaye K. Time to sputum culture conversion and its determinants among Multi-drug resistant Tuberculosis patients at public hospitals of the Amhara Regional State: A multicenter retrospective follow up study. PLoS One. 2018;13(6):e0199320. doi: 10.1371/journal.pone.0199320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Assemie MA, Alene M, Petrucka P, et al. Time to sputum culture conversion and its associated factors among multidrug-resistant tuberculosis patients in Eastern Africa: A systematic review and meta-analysis. Int J Infect Dis. 2020;98:230–236. doi: 10.1016/j.ijid.2020.06.029. [DOI] [PubMed] [Google Scholar]
- 9.Chinese Antituberculosis Association. Guidelines for chemotherapy of drug-resistant tuberculosis (2019 edition). Chin J of Antituber, 2019;41(10):1025–73.
- 10.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The New- castle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Oxford: The Ottawa Hospital Research Institute; 2000. [Google Scholar]
- 11.Sutton AJ, Duval SJ, Tweedie RL, et al. Empirical assessment of effect of publication bias on meta analyses. BMJ. 2000;320(7249):1574–1577. doi: 10.1136/bmj.320.7249.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Myers B, Bouton TC, Ragan EJ, et al. Impact of alcohol consumption on tuberculosis treatment outcomes: a prospective longitudinal cohort study protocol. BMC Infect Dis. 2018;18:1–9. doi: 10.1186/s12879-018-3396-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russkikh A, Korotych O, Sereda Y, et al. Factors associated with culture conversion among adults treated for pulmonary extensively drug-resistant tuberculosis during 2018-2019 in the Russian Federation: an observational cohort study. Monaldi Arch Chest Dis. 2021;91(1):1678. [DOI] [PubMed]
- 14.Javaid A, Ahmad N, Afridi AK, et al. Validity of time to sputum culture conversion to predict cure in patients with multidrug-resistant tuberculosis: a retrospective single-center study. Am J Trop Med Hyg. 2018;98(6):1629. doi: 10.4269/ajtmh.17-0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diktanas S, Korotych O, Sereda Y, et al. Factors associated with time to sputum culture conversion of rifampicin-resistant tuberculosis patients in Klaipeda, Lithuania in 2016-2019: a cohort study. Monaldi Arch Chest Dis. 2021;91(1):1675. [DOI] [PubMed]
- 16.Batool R, Khan SW, Imran M, et al. Culture conversion and six months interim outcomes in retreatment cases of pulmonary MDRTB—a six month interim analysis. J Pak Med Assoc. 2021;71(12):2710–2716. doi: 10.47391/JPMA.1325. [DOI] [PubMed] [Google Scholar]
- 17.Wagnew F, Alene KA, Kelly M, et al. The effect of undernutrition on sputum culture conversion and treatment outcomes among people with multidrug-resistant tuberculosis: a systematic review and meta-analysis. Int J Infect Dis. 2023;127:93–105. [DOI] [PubMed]
- 18.Gamachu M, Deressa A, Birhanu A, et al. Sputum smear conversion and treatment outcome among drug-resistant pulmonary tuberculosis patients in Eastern Ethiopia, 2022: a nine-year data. Front Med. 2022;12:3493. doi: 10.3389/fmed.2022.1007757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basit A, Ahmad N, Khan AH, et al. Predictors of two months culture conversion in multidrug-resistant tuberculosis: findings from a retrospective cohort study. PLoS One. 2014;9(4):e93206. doi: 10.1371/journal.pone.0093206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butov D, Myasoedov VV, Gumeniuk M, et al. Treatment effectiveness and outcome in patients with a relapse and newly diagnosed multidrug-resistant pulmonary tuberculosis. 2020. [DOI] [PubMed] [Google Scholar]
- 21.Restrepo BI, Fisher-Hoch SP, Smith B, et al. Mycobacterial clearance from sputum is delayed during the first phase of treatment in patients with diabetes. Am J Trop Med Hyg. 2008;79(4):541. doi: 10.4269/ajtmh.2008.79.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tekalegn Y, Woldeyohannes D, Assefa T, et al. Predictors of time to sputum culture conversion among drug-resistant tuberculosis patients in Oromia Region Hospitals, Ethiopia. Infect Drug Resist. 2020;13:2547–2556. doi: 10.2147/IDR.S250878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jafri S, Ahmed N, Saifullah N, et al. Liaison of sugar control with time to sputum culture conversion in multi-drug resistant tuberculosis. Cureus. 2020;12(7):e9395. doi: 10.7759/cureus.9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bade AB, Mega TA, Negera GZ. Malnutrition is associated with delayed sputum culture conversion among patients treated for MDR-TB. Infect Drug Resist. 2021;14:1659–1667. doi: 10.2147/IDR.S293461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset(s) supporting the conclusions of this article is (are) included within the article (and its additional file(s)).