Abstract
Three antigenic variants of the K88 fimbrial adhesin exist in nature, K88ab, K88ac, and K88ad. Enterotoxigenic Escherichia coli (ETEC) strains that produce these fimbriae cause life-threatening diarrhea in some but not all young pigs. The susceptibility of pigs to these organisms has been correlated with the adherence of bacteria to isolated enterocyte brush borders. Whether that correlation holds for multiple K88 variants and over a broad genetic base of pigs is unknown and was the impetus for this study. We also desired to examine the correlation of the expression of a porcine intestinal brush border mucin-type glycoprotein (IMTGP) which binds K88ab and K88ac with the susceptibility of piglets to K88+ ETEC. Of 31 neonatal gnotobiotic pigs inoculated with K88ab+ or K88ac+ ETEC, 13 developed severe diarrhea, became dehydrated, and died or became moribund. Another pig became severely lethargic but not dehydrated. In vitro brush border adherence analysis was not possible for 10 of the severely ill pigs due to colonization by challenge strains. However, of the 17 pigs that did not become severely ill, 8 (47%) had brush borders that supported the adherence of K88ab+ and K88ac+ bacteria in vitro, suggesting a poor correlation between in vitro brush border adherence and piglet susceptibility to K88+ ETEC. By contrast, the expression of IMTGP was highly correlated with susceptibility to K88+ ETEC. Of the 12 pigs that produced IMTGP, 11 developed severe diarrhea. The other pig that produced IMTGP became lethargic but not severely diarrheic. Only 2 of 18 pigs that did not produce IMTGP became severely diarrheic. Colonizing bacteria were observed in histologic sections of intestines from all pigs that expressed IMTGP except for the one that did not develop severe diarrhea. However, colonizing bacteria were observed in histologic sections from only one pig that did not produce IMTGP. The bacterial concentration in the jejuna and ilea of pigs expressing IMTGP was significantly greater (P < 0.005) than that in pigs not expressing IMTGP. These observations suggest the IMTGP is a biologically relevant receptor for K88ab+ and K88ac+ E. coli or a correlate for expression for such a receptor.
Enterotoxigenic Escherichia coli (ETEC) strains that express K88 fimbriae are a major cause of diarrhea and death in neonatal and young pigs (18, 23). Three major antigenic variants of K88 fimbriae have been identified, K88ab, K88ac, and K88ad (17). However, K88ac is the most common type expressed on E. coli strains isolated from diarrheic pigs (10, 22). The K88 fimbriae are filamentous surface appendages that enable the bacterium to adhere to intestinal brush border cells (15). This adhesion is believed to prevent removal of the bacterium by intestinal peristalsis, thus facilitating colonization of the small intestine (reviewed in reference 14). Sellwood et al. (21) demonstrated that bacteria expressing K88 fimbriae bound to isolated enterocytes of some but not all pigs. This ability of piglet enterocytes to support the adherence of K88+ E. coli was found to be inherited in a simple Mendelian fashion as a dominant trait. Furthermore, fimbrial binding was found to correlate with the susceptibility of pigs to K88+ E. coli infection (20). Thus, susceptibility to enterotoxigenic colibacillosis caused by K88+ E. coli was shown to be an inherited characteristic. In other studies, Bijlsma et al. (2) found that the three antigenic variants of K88 differed in their porcine enterocyte brush border binding specificities in that brush borders that bound E. coli expressing one K88 variant did not necessarily bind E. coli expressing another K88 variant. These investigators identified five patterns of K88+ E. coli adhesion to brush borders among pigs. Those adhesin patterns (previously called phenotypes) were designated A (K88ab, K88ac, K88ad), B (K88ab, K88ac), C (K88ab, K88ad), D (K88ad), and E (no fimbriae). In a similar study, Rapacz and Hasler-Rapacz (19) identified four patterns of adhesion to brush borders among pigs. Those were the same patterns of brush border adhesion reported by Bijlsma et al., except that pattern C was not identified. More recently, we determined patterns of adhesion of K88+ E. coli to brush borders from a number of 3- to 5-week-old pigs of several breeds and confirmed the existence of the adhesion patterns reported by Bijlsma et al. and identified pigs with an additional adhesion pattern, F (bound K88ab only) (1). Correlation of the adhesion of K88ab+, K88ac+, and K88ad+ E. coli to brush borders as described by Bijlsma et al., Rapacz and Hasler-Rapacz, and us with susceptibility to disease has been assumed but has not been proven.
The presence of as many as six patterns of K88+ E. coli adhesion among brush borders from different pigs suggests the existence of several K88 receptors, expressed individually or in various combinations. Previously, we identified one such receptor from porcine enterocyte membranes, which we characterized as a pair of intestinal mucin-type sialoglycoproteins (IMTGP) which bind K88ab and K88ac (3–5, 11). This receptor typically appears in Western blots stained with biotinylated K88ab or K88ac fimbriae as two broad bands with approximate molecular masses of 210 and 240 kDa. The IMTGPs are found on brush borders exhibiting adhesion pattern B and most but not all brush borders exhibiting adhesion pattern A (3).
The purpose of the present study was to correlate the adhesion of K88ab+ and K88ac+ E. coli to isolated porcine brush borders and the expression of IMTGP with pig susceptibility to K88ab+ and K88ac+ ETEC. This study was done with gnotobiotic piglets challenged with wild-type ETEC strains expressing K88ab and K88ac. Enterocyte brush borders prepared from those pigs were tested for presence of IMTGP and adhesion of K88+ E. coli. Contrary to expectation, the results of this study do not show a strong correlation between piglet susceptibility to ETEC expressing a particular K88 variant and the ability of that bacterium to attach to brush borders vesicles prepared from the intestines of the challenged pig. However, a strong correlation was observed between the expression of IMTGP and susceptibility of piglets to K88ab+ and K88ac+ E. coli. The results of this study suggest that IMTGP is a biologically relevant receptor for K88ab and K88ac or a correlate for the expression of such a receptor.
MATERIALS AND METHODS
Bacterial strains.
Two ETEC strains and one nonpathogenic E. coli strain were used for animal inoculation in this study. Those strains were 263 (O8:K87:K88ab) (1), 3030-2 (O157:K88ac) (8) and G58-1 (O101:K28:NM) (7). The two K88+ strains were verified as expressing K88ab and K88ac, respectively, by an enzyme-linked immunosorbent assay using variant-specific monoclonal antibodies produced by us. In addition, they were shown by PCR to contain nucleic acid sequences consistent with the K88 variant indicated (9). The K88+ strains were both probe positive for heat-labile enterotoxin and heat-stable enterotoxin B, as determined by Linda Shultz, University of Missouri, Columbia, Mo. Strain G58-1, which was used as a nonpathogenic control, does not produce adhesive fimbriae or enterotoxins and has been shown to be nonpathogenic for pigs (7). Strains were maintained in liquid nitrogen until needed and then cultured first on blood agar and then in tubes containing 3 ml of tryptic soy broth and incubated for 18 h before inoculation of pigs. Each tube contained approximately 3 × 109 bacterial cells.
Piglets.
Thirty-four gnotobiotic pigs were used in this study. To maximize genetic diversity, piglets were selected at random from six litters derived from full-term pregnant commercial-grade sows obtained from several different sources. Some littermates from most of the litters were used for other purposes. The piglets were obtained from the sows by closed hysterotomy and maintained in rigid tub isolators as previously described (16). They were fed a sterile commercial piglet formula (SPF-Lac; PetAg, Inc., Hampshire, Ill.) and inoculated per os between 24 and 48 h of age with 3 ml of tryptic soy broth containing the appropriate E. coli strain. They were observed at least three times daily for signs of illness, including diarrhea, lethargy, and dehydration. When pigs developed severe dehydration or lethargy as determined clinically, they were euthanized and subjected to postmortem examination. Animals that did not become lethargic or dehydrated were euthanized 4 days (approximately 96 h) postinoculation (p.i.).
Postmortem examination, histopathology, and culture.
Specimens collected at necropsy from piglets included segments of proximal jejunum for brush border adhesion assays and receptor identification and segments of mid-jejunum and distal ileum for bacteriologic culture and immunofluorescence testing for K88+ E. coli. In addition, specimens for histologic examination were collected from duodenum, mid-jejunum, distal ileum, cecum, spiral colon, rectum, lungs, liver, and spleen; fixed in 10% neutral buffered formalin; processed by routine methods; sectioned; and stained with hematoxylin and eosin. Histologic sections were examined blind with regard to individual animal treatment or clinical presentation. Sections of intestine with bacteria in clusters of 10 or more organisms were considered positive for bacterial colonization. Widely scattered adherent bacteria in groups of fewer than 10 were not considered significant. Intestinal specimens collected for culture were split longitudinally and rinsed in phosphate-buffered saline for the removal of feces, ground, diluted 1:10 (wt/vol) in phosphate-buffered saline, serially diluted in the same buffer, and cultured on tryptic soy agar for the enumeration of CFU of E. coli. Ileum specimens were also cultured on blood agar under aerobic and anaerobic conditions to verify the identity of the challenge strain and to check for bacterial contamination.
Adhesion of K88+ E. coli to brush borders.
Brush borders prepared from the intestines of piglets and their dams were tested for the adhesion of K88ab+, K88ac+, and K88ad+ E. coli as described previously (1). Included among the strains used in this assay were the three challenge strains used in the present study: 263 (O8:K87:K88ab), 3030-2 (O157:K88ac), and G58-1 (O101:K28:NM). Individual brush border vesicles were considered adhesive when more than two bacteria adhered to the brush border membrane. Brush border specimens containing at least 10% adhesive brush borders were considered positive for adherence. The percentage of adhesive brush borders was determined by counting at least 20 brush borders. Brush border vesicles were prepared from specimens collected from proximal portions of the small intestines of inoculated pigs to minimize the chance that they would be colonized with bacteria as a result of piglet challenge. In addition, the mean and standard deviation of the number of bacteria of the challenge strains that adhered to brush border vesicles in brush border adherence assays was determined by enumerating the bacteria adherent to 10 brush borders per animal.
Analysis of brush borders for IMTGP.
Brush border specimens were tested for the presence of IMTGP by the biotinylated-adhesin overlay assay as described elsewhere (5). Briefly, brush border proteins were solubilized by heating in Tris-HCl buffer (pH 6.8) containing β-mercaptoethanol and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Thereafter, they were transferred to nitrocellulose sheets, which were then incubated with biotinylated K88ac fimbriae. Bound biotinylated fimbriae were detected with horseradish peroxidase conjugated to streptavidin and an appropriate substrate. IMTGP usually appears as two broad bands with approximate molecular masses of 210 and 240 kDa (3), although occasionally the 210-kDa band is missing (3a). Proteins in bands less than 200 kDa are expressed without regard to the adhesiveness of the brush borders for K88+ E. coli and do not represent receptor glycoproteins (5).
IFAT for K88+ E. coli.
Indirect fluorescent-antibody tests (IFAT) for detection of K88+ E. coli in jejunal and ileal impression smears were performed as described elsewhere (6), except that monoclonal antibodies that recognized all three K88 variants were used in place of absorbed rabbit antiserum and fluorescein isothiocyanate-conjugated rabbit anti-mouse immunoglobulin G serum was used in place of fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin G serum. Smears were scored based on an estimate of the number of fluorescing bacteria per typical ×400 microscopic field: fewer than 1/field = 0; 1 to 10/field = 1+; 11 to 100/field = 2+; 101 to 400 = 3+, and >400/field = 4+.
RESULTS
Relationship between adhesion of K88+ E. coli to brush borders and susceptibility of piglets to diarrhea and dehydration.
Bacterial contaminants were cultured from pigs from two litters. The contaminants in one litter were alpha-hemolytic Streptococcus and gram-negative enteric bacilli. These contaminants probably originated from the vagina of the sow, since one of the piglets was found in the birth canal during hysterotomy. Nonhemolytic Staphylococcus was found in several piglets from the other contaminated litter. The source of this contamination was not determined. The contaminants appeared not to be pathogenic or to affect the course of the enteric disease caused by E. coli challenge strains. Piglets in contaminated litters exhibited no signs of disease before challenge with ETEC. Both contaminated litters contained pigs that succumbed to ETEC infection and pigs that were minimally affected. The clinical disease and lesions in these pigs were consistent with those observed in pigs from uncontaminated litters. Neither contaminated litter included pigs not inoculated with ETEC.
Brush borders from 11 pigs in the study were determined by the brush border adherence assay to be of adhesion pattern A (adhesive to E. coli expressing any K88 variant); brush borders from 1 pig were determined to be of adhesion pattern C (adhesive to K88ab+ and K88ad+ E. coli), and brush borders from 12 pigs were determined to be of adhesion pattern D (adhesive to K88ad+ E. coli (Table 1). Brush borders from 10 other pigs were not tested for K88+ E. coli adhesion, because they contained adherent bacteria as a consequence of colonization following animal challenge. Had they been testable, these brush borders probably would have exhibited adhesion pattern A, because the bacterial strains that bound to them were K88ab+ or K88ac+. In addition, brush borders from the piglets’ dams and all or most of their littermates were of adhesion pattern A. Also, brush borders from each pig contained IMTGP, which has been found only in brush borders of adhesion patterns A and B (3, 5). The brush borders exhibiting adhesion pattern A, isolated from the 11 pigs noted above, bound bacterial cells of challenge strains 263 (K88ab+) and 3030-2 (K88ac+) in large numbers (overall mean number of bacteria/brush border ± standard deviation = 14 ± 6 and 12 ± 5, respectively; Table 1, Fig. 1). By contrast, few individual brush border vesicles from the 13 pigs whose vesicles exhibited adhesion patterns C or D bound even one bacterial cell of strain 3030-2 (Table 1). Brush border vesicles from most of these pigs also failed to bind bacterial cells of strain 263. However, brush borders from two pigs bound small numbers of bacteria of that strain (the number [mean ± standard deviation] of strain 263 bacteria bound by brush borders from these two pigs was 1 ± 2 and 2 ± 3). The K88-negative challenge control strain, G58-1, did not adhere to brush borders vesicles from any pig in the study.
TABLE 1.
Brush border adhesion patterns, clinical presentation, and the adherence of E. coli challenge strains to isolated piglet enterocyte brush borders
| Challenge strain and animal no. | Brush bordera adhesion pattern | Severe diarrhea and dehydration | No. of adherent bacteria/ brush border (mean ± SD)
|
||
|---|---|---|---|---|---|
| G58-1 (K88−) | 263 (K88ab+) | 3030-2 (K88ac+) | |||
| G58-1 | |||||
| 13035 | D | − | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 13036 | D | − | 0 ± 0 | 0 ± 1 | 0 ± 0 |
| 13037 | A | − | 0 ± 0 | 20 ± 7 | 18 ± 6 |
| 263 | |||||
| 4762 | A | + | NDb | ND | ND |
| 4763 | A | + | ND | ND | ND |
| 4765 | A | + | ND | ND | ND |
| 4767 | A | + | ND | ND | ND |
| 5197 | A | − | 0 ± 0 | 13 ± 3 | 14 ± 5 |
| 5198 | A | − | 0 ± 0 | 19 ± 5 | 12 ± 3 |
| 7198 | D | + | 0 ± 0 | 1 ± 2 | 0 ± 0 |
| 7201 | D | − | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 7202 | D | − | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 3030-2 | |||||
| 4764 | A | + | ND | ND | ND |
| 4766 | A | + | ND | ND | ND |
| 4770 | A | − | 0 ± 0 | 18 ± 6 | 14 ± 7 |
| 4771 | A | − | 0 ± 0 | 8 ± 6 | 6 ± 4 |
| 5199 | C | − | 0 ± 0 | 2 ± 3 | 0 ± 0 |
| 6585 | A | + | ND | ND | ND |
| 6586 | A | − | 0 ± 0 | 10 ± 3 | 11 ± 2 |
| 7197 | D | + | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 7199 | D | − | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 7200 | D | − | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 10997 | A | + | ND | ND | ND |
| 10998 | A | + | ND | ND | ND |
| 10999 | A | + | ND | ND | ND |
| 11000 | A | + | 0 ± 0 | 12 ± 2 | 9 ± 4 |
| 11001 | A | −c | 0 ± 0 | 11 ± 2 | 11 ± 2 |
| 11002 | D | − | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 11003 | A | − | 0 ± 0 | 14 ± 3 | 12 ± 5 |
| 11004 | A | − | 0 ± 0 | 14 ± 5 | 12 ± 4 |
| 13040 | A | − | 0 ± 0 | 20 ± 6 | 12 ± 3 |
| 13041 | D | − | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 13042 | D | − | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 13043 | D | − | 0 ± 0 | 0 ± 0 | 0 ± 0 |
Ten pigs whose brush borders were colonized with K88+ E. coli were assumed, but not proven, to be of adhesion pattern A (see the text for the reason for making that assumption).
ND, not done. The presence of adherent bacteria on brush borders in consequence of bacterial colonization after piglet challenge precluded conducting in vitro bacterial adherence assays.
The piglet became lethargic but not severely diarrheic or dehydrated.
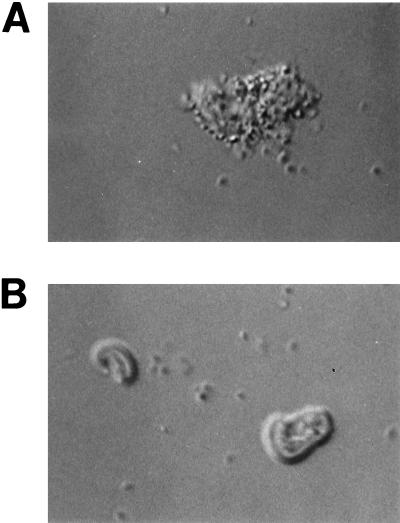
FIG. 1.
Hoffman modulation contrast microscopy of isolated brush borders adhesive and nonadhesive for ETEC 3030-2 (K88ac+). (A) Brush borders with numerous adherent bacteria (from piglet 11004, IMTGP negative, exhibiting adhesion pattern A). (B) Brush borders with no adherent bacteria (from piglet 11002, IMTGP negative, exhibiting adhesion pattern D). Brush borders that are IMTGP positive and exhibit adhesion pattern A are indistinguishable from that shown in panel A. Magnification, ×400.
Of the 31 piglets challenged with ETEC 263 (K88ab+) or 3030-2 (K88ac+), 13 developed severe diarrhea (typically by 12 h p.i.), became markedly dehydrated and lethargic, and died or became moribund (Table 2). One additional pig became severely lethargic but not dehydrated. Sixteen pigs developed mild diarrhea (20 to 72 h p.i.) but remained hydrated and active to the conclusion of the study at 4 days p.i. One piglet inoculated with strain 3030-2 and the three piglets inoculated with the K88− control strain, G58-1, developed no diarrhea following challenge. No differences in virulence were noted between strains 263 (K88ab+) and 3030-2 (K88ac+). Of the 13 pigs that became severely diarrheic and dehydrated, 1 had brush borders that exhibited K88 adhesion pattern A, 2 had brush borders that exhibited adhesion pattern D, and 10 had brush borders that were presumed to be of adhesion pattern A but were not tested because they contained adherent bacteria as a consequence of animal challenge. The piglet that became lethargic but not severely diarrheic or dehydrated had brush borders that exhibited K88 adhesion pattern A. Of the 17 ETEC-challenged pigs that did not develop severe diarrhea and dehydration or lethargy, 8 had brush borders exhibiting adhesion pattern A, 1 had brush borders exhibiting adhesion pattern C, and 8 had brush borders exhibiting adhesion pattern D.
TABLE 2.
Piglets with brush borders exhibiting adhesion patterns A, C, and D that became severely dehydrated when inoculated with K88ab+, K88ac+, or K88− E. coli
| Challenge strain | Dehydration (no. dehydrated/total no.) of pigs with Brush border adhesion patterna:
|
||
|---|---|---|---|
| A | C | D | |
| 263 (K88ab+) | 4/6 | 0/0 | 1/3 |
| 3030-2 (K88ac+) | 7/14b | 0/1 | 1/7 |
| G58-1 (K88−) | 0/1 | 0/0 | 0/2 |
Four and six pigs challenged with 263 and 3030-2, respectively, were assumed but not proven to have brush borders adhesive for E. coli expressing each of the K88 variants (brush border adhesion pattern A [see the text for reasons for the assumption of this adhesion pattern]).
One additional pig became lethargic but not dehydrated and was euthanized before the end of the study.
In summary, 86% of the pigs that became severely ill following challenge with K88ab+ or K88ac+ E. coli had brush borders that were known or believed to be of K88 adhesion pattern A. However, only 60% of the challenged pigs known or presumed to have brush borders of K88 adhesion pattern A became severely ill. Of the pigs having brush borders exhibiting adhesion pattern A, 40% failed to develop severe illness despite the ability of their brush borders to bind large numbers of bacteria of the challenge strain (Fig. 1). This result shows a poor correlation between brush border binding and disease susceptibility.
Relationship between the expression of IMTGP and piglet susceptibility to diarrhea and dehydration.
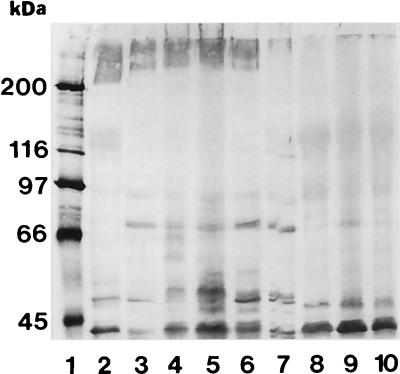
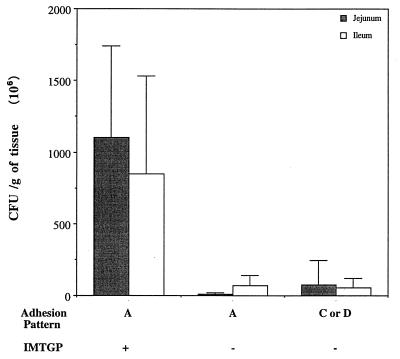
Western blots of brush borders from 11 pigs, stained with biotinylated K88ac fimbriae, exhibited the presence of bands with apparent molecular masses of approximately 210 and 240 kDa (Fig. 2). A 240-kDa band, but not a 210-kDa band, was observed in a brush border preparation from one other pig. Similar bands were not observed in Western blot preparations of brush borders from 21 pigs in the study. One pig was not tested due to limited sample availability. The 210- and 240-kDa bands were interpreted to represent IMTGP. All except one of the pigs that produced IMTGP developed severe diarrhea when challenged with K88ab+ or K88ac+ ETEC and became severely dehydrated (Table 3). The other IMTGP+ pig became lethargic but not severely diarrheic or dehydrated. Only two of the pigs challenged with K88ab+ or K88ac+ ETEC that did not produce IMTGP developed severe diarrhea and became dehydrated. Brush borders from the 12 pigs that produced IMTGP were shown or presumed to be of adhesion pattern A; however, brush borders from another 9 pigs, which did not produce IMTGP, were also shown to be of adhesion pattern A. Brush borders from the two pigs that became severely diarrheic and dehydrated, but that did not express IMTGP, were of adhesion pattern D (Table 3). The intestines of these two pigs were severely hyperemic, and congestion and hemorrhage were observed microscopically. This suggests that these pigs may have been septicemic in addition to being diarrheic. Adherent bacteria were observed in histologic sections of the duodenum, jejunum, and ileum from 10 of 12 pigs that expressed IMTGP (data not shown) and in the duodenum and ileum in 1 other IMTGP-positive pig. Adherent bacteria were observed the ileum only, in one IMTGP-negative pig challenged with strain 3030-2. However, that pig did not develop diarrhea. Adherent bacteria were not observed in histologic sections from any other pig. The concentrations of E. coli in the small intestines of the pigs that expressed IMTGP was significantly greater (P < 0.005) than those in the small intestines of IMTGP-negative pigs (Fig. 3). Interestingly, the bacterial concentration in intestines of pigs that did not produce IMTGP and whose brush borders exhibited adhesion pattern A was not significantly different (P > 0.2) from that in intestines of pigs whose brush borders exhibited adhesion patterns C and D. The IFAT results for K88+ bacteria on jejunal and ileal smears reflected the results of bacterial concentration assays. The mean IFAT scores for jejunal and ileal impression smears for IMTGP-expressing pigs were 3.5 and 3.9, respectively. The mean IFAT scores for jejunal and ileal smears for IMTGP-negative pigs whose brush borders exhibited adhesion pattern A were 0.9 and 2.0, and those for IMTGP-negative pigs whose brush borders exhibited adhesion pattern C or D were 1.0 and 1.7, respectively.
FIG. 2.
Identification of IMTGP (which appears as broad bands with molecular masses of approximately 210 and 240 kDa [see reference 3 for a discussion]) in brush borders from piglets. Solubilized membrane proteins (40 μg per lane) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (7% polyacrylamide) and transferred to nitrocellulose. The IMTGP was detected with biotinylated K88ac adhesin. Bands less than 200 kDa are not phenotype specific and do not represent receptor glycoproteins (5). Lanes 1, molecular mass standards; 2, IMTGP-positive control pig; 3 to 7, pigs 10997, 10998, 10999, 11000, and 11001, all of which expressed IMTGP; 8 to 10, pigs 11002, 11003, and 11004, none of which expressed IMTGP. Brush borders from all these pigs except 11002 were adhesive for K88ab+ and K88ac+ E. coli. Pig 11001 became lethargic but did not develop severe diarrhea or become dehydrated.
TABLE 3.
IMTGP-positive and -negative pigs with brush borders exhibiting adhesion patterns A, C, and D that became severely dehydrated when inoculated with K88ab+, K88ac+, or K88− E. coli
| Challenge strain | Dehydration (no. dehydrated/total no.) of pigs witha:
|
||
|---|---|---|---|
| IMTGP+, pattern A | IMTGP−, pattern A | IMTGP−, patterns C and D | |
| 263 (K88ab+) | 4/4 | 0/2 | 1/3 |
| 3030-2 (K88ac+) | 7/8b | 0/6 | 1/8 |
| G58-1 (K88−) | 0/0 | 0/1 | 0/2 |
Four and six pigs challenged with 263 and 3030-2, respectively, were assumed but not proven to have brush borders adhesive for E. coli expressing each of the K88 variants (brush border adhesion pattern A [see the text for reasons for the assumption of this adhesion pattern]).
One pig became lethargic but not dehydrated and was euthanized before the end of the study.
FIG. 3.
Concentration of E. coli in the jejuna and ilea of IMTGP-positive pigs whose brush borders exhibited adhesion pattern A, IMTGP-negative pigs whose brush borders exhibited adhesion pattern A, and IMTGP-negative pigs whose brush borders exhibited adhesion pattern C or D. The bars represent the means and standard deviations of the CFU of bacteria per gram of washed intestine. The mean concentrations of bacteria in the jejuna and ilea of the IMTGP-positive pigs whose brush borders exhibited adhesion pattern A was significantly greater (P < 0.005) than those in jejuna or ilea of either of the other groups. Differences in mean intestinal bacterial concentrations between the two groups without IMTGP-positive pigs were not significant (P > 0.2; Student’s unpaired t test).
DISCUSSION
In this study, expression of IMTGP in the intestines of gnotobiotic pigs was correlated with susceptibility of the pigs to severe diarrhea and dehydration caused by K88ab+ or K88ac+ ETEC. Adherent bacteria were observed in histologic sections of small intestines from all except one of the pigs that expressed IMTGP but in only one section from only one pig that did not produce that glycoprotein. Furthermore, piglets that expressed IMTGP exhibited significantly higher concentrations of bacteria in their jejuna and ilea than did pigs that did not express IMTGP. These observations suggest that IMTGP is a biologically relevant receptor for K88ab and K88ac fimbriae or a correlate for the expression of such a receptor. If IMTGP is, in fact, a biological receptor for K88ab and K88ac, it may not be the only cell surface component required for colonization by bacteria. It is of interest that brush borders from a number of pigs bound large numbers of K88ab+ and K88ac+ bacteria in vitro despite the lack of IMTGP production (Fig. 1). This suggests the presence of another receptor besides IMTGP in these brush borders. IMTGP and the other K88 receptor may be used coordinately by K88+ ETEC in colonizing piglet intestinal epithelium, with each having a different function but both being necessary for bacterial colonization.
Most of the ETEC-challenged pigs in this study that did not become severely ill, did develop mild diarrhea. Since no adherent bacteria were observed in histologic sections of intestines from these pigs, it does not appear that adhesin receptors contributed to the production of this mild diarrhea. Lack of protective substances from sow’s colostrum or milk and lack of a competing intestinal flora may have allowed sufficient proliferation of ETEC, with consequent enterotoxin production, to result in the observed diarrhea. The two IMTGP-negative pigs that became dehydrated had higher concentrations of bacteria in their jejuna (3 × 108 and 5 × 108/g) than did other IMTGP-negative pigs. Perhaps this bacterial overgrowth contributed to the more severe disease observed in those pigs. The cause of the lethargy in the only markedly ill pig that did not become dehydrated was not determined. It is of interest that brush borders from that pig (pig 11001) contained IMTGP, although perhaps at a lower concentration than did brush borders from other IMTGP-expressing pigs from the same litter (Fig. 2). The concentration of bacteria in the intestines of that pig was lower than that in many pigs that were only mildly affected by challenge strains.
In our initial reports of the identification and characterization of IMTGP, we indicated a perfect correlation between presence of IMTGP in brush borders and the adhesion of K88ab+ and K88ac+ E. coli to those brush borders (4, 5). The pigs used in those studies were mostly adults. Several younger (juvenile) pigs, but no nursing pigs, were also included in that study. Subsequently, when studying 3- to 5-week-old pigs, we reported the identification of one pig whose brush borders were adhesive to K88ab+ and K88ac+ E. coli but did not contain IMTGP (3). Brush borders from a number of animals in the present study did not contain detectable IMTGP despite their support of adhesion by K88ab+ and K88ac+ E. coli. Animals used in this study were all younger than 1 week of age. Because animals in each of the studies came from multiple sources, the disparity in results is not likely to be attributable to animal population heterogeneity. A great difference in the expression of IMTGP relative to the age of the animal population sampled suggests that animal age may influence the expression of one or more receptors manifested by K88 adhesion to brush borders. It appears either that IMTGP is expressed in the brush borders of more pigs as they become older or that the receptor responsible for the adhesion of K88ab+ and K88ac+ E. coli in IMTGP-negative brush borders is not expressed in older pigs. There is some evidence that expression of receptors responsible for brush border adhesiveness does change with the age of the pig. Hu et al. (12) reported that a low-affinity receptor for K88ad, found in the brush borders of some young pigs, was not present in the brush borders of pigs older than 16 weeks. We have also observed age-associated differences in K88ad receptor expression. Brush borders were obtained by surgical laparotomy from a pig that was about 6 weeks old and were adhesive for K88ad (K88 adhesion pattern D). Brush borders taken from intestinal tissue collected from the same animal as an adult were not adhesive for K88ad (6a).
Brush borders from all of the pigs used in the present study (and their littermates, which were used for other purposes) supported the adhesion of at least one K88 variant. In contrast, Srinivasappa (21a) identified 2 (6%) of 36 7- to 11-day-old piglets whose brush borders failed to bind any K88 variant (adhesion pattern E). Previously, we identified 27 (28%) of 96 3- to 5-week-old pigs whose brush borders failed to bind any K88 variant (1). The increasing percentage, with age, of pigs whose brush borders fail to bind K88+ E. coli suggests loss of the expression of a K88 receptor as pigs mature. The receptor used in the adhesion of F18 E. coli fimbriae to brush borders of weaned pigs is apparently not expressed on brush borders of newborn pigs (13). Thus, some adhesion receptors on pig brush borders disappear as piglets mature, while others appear. As indicated above, the differential between neonatal and adult pigs in the correlation between K88+ E. coli adherence to brush borders and IMTGP expression could be explained by either the loss of an uncharacterized K88ab and K88ac receptor with piglet age or the appearance of IMTGP in more pigs with age.
This study is the second report of pigs whose brush borders supported the adherence of K88+ E. coli but which were resistant to disease caused by those organisms. Rutter et al. (20) reported the identification of such pigs in a challenge study, although the percentage of brush border adherence-positive, disease-resistant animals in their study (9%) was lower than that in our study (40%). Perhaps the difference in the frequency of observation was due to chance differences in the percentages of pigs with genes for the expression of IMTGP. The lack of a strong correlation between bacterial adherence to isolated intestinal brush border vesicles and piglet susceptibility to K88+ E. coli suggests that the brush border adhesion assay is not an accurate predictor of the susceptibility of pigs to K88+ E. coli. The high correlation between expression of IMTGP and piglet susceptibility to K88+ E. coli suggests that tests for the presence of that glycoprotein may be a better predictor of piglet susceptibility. Studies are under way in our laboratory to fully elucidate the structure of IMTGP and, thereafter, to identify the genes responsible for its production. Characterization of K88 fimbria-epithelial cell surface interactions is a long-term goal.
ACKNOWLEDGMENTS
We gratefully acknowledge financial assistance for this work through USDA grant 94-02419, NSF grant OSR-9452894, the South Dakota Future Fund, and the South Dakota Agricultural Experiment Station.
REFERENCES
- 1.Baker D R, Billey L O, Francis D H. Distribution of K88 Escherichia coli-adhesive and nonadhesive phenotypes among pigs of four breeds. Vet Microbiol. 1997;54:123–132. doi: 10.1016/s0378-1135(96)01277-1. [DOI] [PubMed] [Google Scholar]
- 2.Bijlsma I G W, de Nijs A, van der Meer C, Frik J F. Different pig phenotypes affect adherence of Escherichia coli to jejunal brush borders by K88ab, K88ac, and K88ad antigen. Infect Immun. 1982;37:891–894. doi: 10.1128/iai.37.3.891-894.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Billey L O, Erickson A K, Francis D H. Multiple receptors on porcine intestinal epithelial cells for the three variants of Escherichia coli K88 fimbrial adhesin. Vet Microbiol. 1998;59:203–212. doi: 10.1016/s0378-1135(97)00193-4. [DOI] [PubMed] [Google Scholar]
- 3a.Erickson, A. K. Personal communication.
- 4.Erickson A K, Baker D R, Bosworth B T, Casey T A, Benfield D A, Francis D H. Characterization of porcine intestinal epithelial receptors for the K88ac fimbrial adhesin of Escherichia coli as mucin-type sialoglycoproteins. Infect Immun. 1994;62:5404–5410. doi: 10.1128/iai.62.12.5404-5410.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erickson A K, Willgohs J A, McFarland S Y, Benfield D A, Francis D H. Identification of two porcine brush border glycoproteins that bind the K88ac adhesin of Escherichia coli and correlation of these binding glycoproteins with the adhesive porcine phenotype. Infect Immun. 1992;60:983–988. doi: 10.1128/iai.60.3.983-988.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francis D H. Use of immunofluorescence, gram staining, histologic examination, and seroagglutination in the diagnosis of porcine colibacillosis. Am J Vet Res. 1983;44:1884–1888. [PubMed] [Google Scholar]
- 6a.Francis, D. H. Unpublished data.
- 7.Francis D H, Collins J E, Duimstra J R. Infection of gnotobiotic pigs with an Escherichia coli O157:H7 strain associated with an outbreak of hemorrhagic colitis. Infect Immun. 1986;51:953–956. doi: 10.1128/iai.51.3.953-956.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francis D H, Willgohs J A. Evaluation of a live avirulent Escherichia coli vaccine for K88+, LT+ enterotoxigenic colibacillosis in weaned pigs. Am J Vet Res. 1991;52:1051–1055. [PubMed] [Google Scholar]
- 9.Franklin M A, Francis D H, Baker D, Mathew A G. A PCR-based method of detection and differentiation of K88+ adhesive Escherichia coli. J Vet Diagn Invest. 1996;8:460–463. doi: 10.1177/104063879600800410. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez E A, Vazques F, Ignacio J, Blanco J. Isolation of K88 antigen variants (ab, ac, ad) from porcine enterotoxigenic Escherichia coli belonging to different serotypes. Microbiol Immunol. 1995;39:937–942. doi: 10.1111/j.1348-0421.1995.tb03296.x. [DOI] [PubMed] [Google Scholar]
- 11.Grange P A, Erickson A K, Anderson T J, Francis D H. Characterization of the carbohydrate moiety of intestinal mucin-type sialoglycoprotein receptors for the K88ac fimbrial adhesin of Escherichia coli. Infect Immun. 1998;66:1613–1621. doi: 10.1128/iai.66.4.1613-1621.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu Z L, Hasler-Rapacz J, Huang S C, Rapacz J. Studies in swine on inheritance and variation in expression of small intestinal receptors mediating adhesion of the K88 enteropathogenic Escherichia coli variants. J Hered. 1993;84:157–165. doi: 10.1093/oxfordjournals.jhered.a111309. [DOI] [PubMed] [Google Scholar]
- 13.Imberechts H, Bertschinger H U, Nagy B, Deprez P, Pohl P. Fimbrial colonization factors F18ab and F18ac of Escherichia coli isolated from pigs with postweaning diarrhea and edema disease. In: Paul P S, Francis D H, Benfield D A, editors. Mechanisms in the pathogenesis of enteric diseases. New York, N.Y: Plenum Press; 1997. pp. 175–183. [DOI] [PubMed] [Google Scholar]
- 14.Isaacson R E. Molecular and genetic basis of adherence of enteric Escherichia coli in animals. In: Roth J A, editor. Virulence mechanisms of bacterial pathogens. Washington, D.C: American Society for Microbiology; 1988. pp. 28–44. [Google Scholar]
- 15.Jacobs A A C, Roosendaal B, van Breemen J F L, de Graaf F K. Role of phenylalanine 150 in the receptor-binding domain of the K88 fimbrillar subunit. J Bacteriol. 1987;169:4907–4911. doi: 10.1128/jb.169.11.4907-4911.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miniats O P, Jol D. Gnotobiotic pigs-derivation and rearing. Can J Comp Med. 1978;42:428–437. [PMC free article] [PubMed] [Google Scholar]
- 17.Mooi F R, de Graaf F K. Isolation and characterization of K88 antigen. FEMS Microbiol Lett. 1978;5:17–20. [Google Scholar]
- 18.Moon H W, Bunn T O. Vaccines for preventing enterotoxigenic Escherichia coli infections in farm animals. Vaccine. 1993;11:213–220. doi: 10.1016/0264-410X(93)90020-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rapacz J, Hasler-Rapacz J. Polymorphism and inheritance of swine small intestinal receptors mediating adhesion of three serological variants of Escherichia coli-producing K88 pilus antigen. Anim Genet. 1986;17:305–321. doi: 10.1111/j.1365-2052.1986.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 20.Rutter J M, Burrows M R, Sellwood R, Gibbons R A. A genetic basis for resistance to enteric disease caused by E. coli. Nature (London) 1975;257:135–136. doi: 10.1038/257135a0. [DOI] [PubMed] [Google Scholar]
- 21.Sellwood R, Gibbons R A, Jones G W, Rutter J M. Adhesion of enteropathogenic Escherichia coli to pig intestinal brush borders: the existence of two pig phenotypes. J Med Microbiol. 1975;8:405–411. doi: 10.1099/00222615-8-3-405. [DOI] [PubMed] [Google Scholar]
- 21a.Srinivasappa G B. Ph.D. dissertation. Brookings: South Dakota State University; 1995. [Google Scholar]
- 22.Westerman R B, Mills K W, Phillips R M, Fortner G W, Greenwood J M. Predominance of the ac variant in K88-positive Escherichia coli isolates from swine. J Clin Microbiol. 1988;26:149–150. doi: 10.1128/jcm.26.1.149-150.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson R A, Francis D H. Fimbriae and enterotoxins associated with E. coli serotypes isolated from clinical cases of porcine colibacillosis. Am J Vet Res. 1986;47:213–217. [PubMed] [Google Scholar]