Abstract
In this study, we demonstrate that Porphyromonas gingivalis fimbriae use molecules of β2 integrin (CD11/CD18) on mouse peritoneal macrophages as cellular receptors and also show that the β chain (CD18) may play a functional role in signalling for the fimbria-induced expression of interleukin-1β (IL-1β) and tumor necrosis factor alpha (TNF-α) genes in the cells. Using a binding assay with 125I-labeled fimbriae, we observed that fimbrial binding to the macrophages was inhibited by treatment with CD11a, CD11b, CD11c, or CD18 antibody but not by that with CD29 antibody. Western blot assays showed that the fimbriae bound to molecules of β2 integrin (CD11/CD18) on the macrophages. Furthermore, Northern blot analyses showed that the fimbria-induced expression of IL-1β and TNF-α genes in the cells was inhibited strongly by CD18 antibody treatment and slightly by CD11a, CD11b, or CD11c antibody treatment. Interestingly, intracellular adhesion molecule 1 (ICAM-1), a ligand of CD11/CD18, inhibited fimbrial binding to the cells in a dose-dependent manner. In addition, ICAM-1 clearly inhibited the fimbria-induced expression of IL-1β and TNF-α genes in the cells. However, such inhibitory action was not observed with laminin treatment. These results suggest the importance of β2 integrin (CD11/CD18) as a cellular receptor of P. gingivalis fimbriae in the initiation stage of the pathogenic mechanism of the organism in periodontal disease.
Porphyromonas gingivalis is a predominant periodontal pathogen. The microorganism has been shown to adhere to human gingival fibroblasts and monocytes/macrophages via its fimbriae (8, 16, 23, 29, 35). Interestingly, a recent study (6) demonstrated clearly that mutation of the fimA gene, encoding fimbrillin, the major subunit of the fimbriae, prevents bacterial adherence to host cells. Therefore, P. gingivalis fimbriae are an important cell structure involved in the adherence of bacteria to host cells. On the other hand, several investigators (15, 18–20, 22, 27, 28) have shown that P. gingivalis is able to bind to the extracellular matrix. In fact, we (18, 27) recently demonstrated a role for fibronectin, one of the matrix proteins, as a regulatory protein in the fimbria-mediated pathogenesis of the organism.
In addition, our previous studies (8, 10, 11, 26) showed that P. gingivalis fimbriae are able to induce the expression of inflammatory cytokines in human gingival fibroblasts and mouse peritoneal macrophages and suggested that N-acetyl-d-galactosamine may play a functional role in the interaction of macrophages with fimbriae. However, the cellular receptor for the fimbriae on the macrophages was not identified in these studies.
Recently, many investigators (1–3, 5, 13, 14, 21, 25, 30–34) reported that integrin functions as a cellular receptor for several bacterial cell surface components. Therefore, in the present study, we examined whether β2 integrin (CD11/CD18) on monocytes/macrophages functions as a cellular receptor for P. gingivalis fimbriae on macrophages and which subunit, α or β, of the molecule plays a central role in fimbrial signalling.
We found that P. gingivalis fimbriae are able to bind to mouse peritoneal macrophages via β2 integrin and that the β chain (CD18) may play a central role in the signalling required for the fimbria-induced expression of interleukin-1β (IL-1β) and tumor necrosis factor alpha (TNF-α) genes in the cells.
MATERIALS AND METHODS
Preparation of P. gingivalis fimbriae and antibody.
P. gingivalis ATCC 33277 fimbriae were prepared and purified from cell washings by the method of Yoshimura et al. (36) as described previously (8). We (17) previously demonstrated that purified fimbriae were able to induce several biological activities that could not be attributed to contaminants in the preparation. The protein content of the fimbriae was measured by the method of Bradford (4). A monoclonal antibody against P. gingivalis fimbriae was used as described previously (17).
Antibodies.
Rat anti-mouse CD11a monoclonal antibody (clone 8-6-2; Cedarlane, Hornby, Ontario, Canada), rat anti-mouse CD11b monoclonal antibody (clone MI/70.15.1; Serotec, Oxford, England), hamster anti-mouse CD11c monoclonal antibody (clone HL3; Pharmingen, San Diego, Calif.), rat anti-mouse CD18 monoclonal antibody (clone C71/16; Cedarlane), and rat anti-mouse CD29 monoclonal antibody (clone KM16; Pharmingen) were used in this study.
Preparation of mouse peritoneal macrophages.
Thioglycolate-stimulated peritoneal exudate cells from 6- to 8-week-old BALB/c mice were harvested. Peritoneal macrophages were prepared and purified as described earlier (9). The prepared macrophages were treated for selected times with test samples.
Preparation of membrane fractions of mouse peritoneal macrophages.
The cells were treated with homogenization buffer (20 mM Tris-HCl [pH 8.0], 0.5 mM CaCl2, 25 mM NaCl) and then centrifuged at 200 × g for 10 min to remove nuclei. The supernatant was centrifuged at 100,000 × g for 60 min at 4°C. In addition, the pellets were suspended in binding buffer (50 mM HEPES, 128 mM NaCl, 5 mM KCl, 5 mM MgCl2, 1.2 mM CaCl2) containing 1% Nonidet P-40 and 0.25 mM phenylmethylsulfonyl fluoride (PMSF) and centrifuged at 100,000 × g for 60 min at 4°C. The resulting supernatant was used as the soluble membrane fraction.
Preparation of 125I-labeled fimbriae.
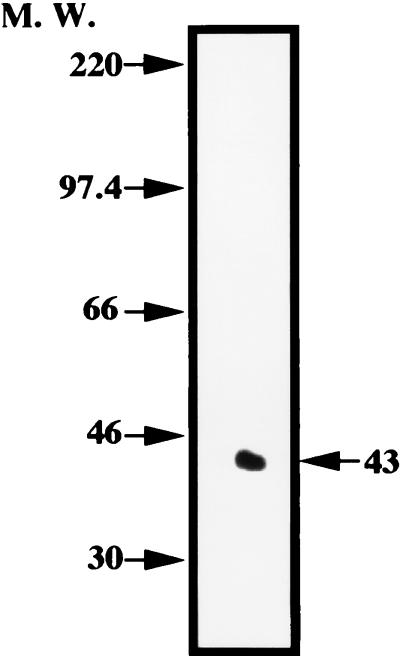
Iodination of purified fimbriae was performed with Iodo-Beads iodination reagent (N-chloro-benzenesulfonamide-derivatized nonporous polystyrene beads; Pierce, Rockford, Ill.). In brief, fimbriae (100 μg of protein) were added to phosphate-buffered saline (PBS) solution containing three Iodo-Beads and 125I-labeled Na (18.5 MBq). The reaction was stopped 15 min after it was begun, and the beads were applied to a Sephadex G-25 column to remove the free iodine. The labeled fimbriae were detected as a single band following sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) (Fig. 1).
FIG. 1.
Autoradiogram of 125I-labeled fimbriae of P. gingivalis on SDS-PAGE. Arrows show the positions of proteins used as apparent molecular weight (M. W.) markers.
Binding of 125I-labeled fimbriae to mouse peritoneal macrophages.
Macrophage monolayers formed by mouse peritoneal exudate cells (2 × 105) seeded into each well of a 96-well multiple microculture plate were fixed with 8% formalin. The fixed cells were washed five times with PBS and kept overnight at 4°C. Then, 125I-labeled fimbriae (1 μg of protein) were inoculated into each cell monolayer, and incubation was carried out for 4 h at 4°C in the absence or presence of each antibody. Thereafter, the monolayer was washed 10 times with 15 mM phosphate buffer (pH 7.2). The amount of radioactivity bound to the macrophages was measured with a gamma counter. The experiment was carried out in triplicate, and the results were expressed as the mean ± standard deviation (SD) percent inhibition.
Immunoprecipitation with a fimbrial monoclonal antibody.
Macrophage membrane fractions (500 μg of protein) were incubated for 12 h at 4°C with fimbriae (10 μg of protein) in binding buffer containing 1% Nonidet P-40 and 0.25 mM PMSF. Then, the mixtures were treated for 12 h at 4°C with a monoclonal antibody specific for the fimbriae in the presence of EDTA (5 mM). Thereafter, the test samples were incubated for 6 h at 4°C with protein A-labeled beads (20 μl) and then washed four times with immunoprecipitation buffer (20 mM Tris [pH 7.9], 1% Nonidet P-40, 150 mM NaCl, 20 mM EDTA, 0.25 mM PMSF, 10 mg of leupeptin per ml).
Western blot analysis with several kinds of antibody.
The immunoprecipitates obtained with the fimbrial monoclonal antibody as described above were suspended in sample buffer (0.06 M Tris [pH 7.9], 2% SDS, 6% 2-mercaptoethanol, 10% glycerol), boiled for 2 min, and then electrophoresed on 5% polyacrylamide gels (SDS-PAGE). The proteins were transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, Mass.) by means of a semidry Transblot system (Atto Co., Tokyo, Japan) with transfer buffer composed of 0.025 M Tris, 0.192 M glycine, and 20% methanol. The membrane was then washed with 0.1% Tween 20 in Tris-buffered saline (TBS; 100 mM Tris [pH 7.9], 150 mM NaCl) and blocked with 2% skim milk in TBS containing 0.05% Tween 20 for 12 h. The membrane was subsequently washed with TBS containing 0.1% Tween 20 and incubated with each antibody in TBS for 18 h. Rat immunoglobulin G (IgG) was used as a control antibody. After a wash with TBS containing 0.1% Tween 20, the membrane was incubated with 2 μCi of 125I-labeled sheep anti-rat IgG antibody (Amersham Japan, Tokyo, Japan) or 125I-labeled protein A (Amersham Japan) per ml in TBS for 3 h. Finally, the membrane was washed with 0.1% Tween 20 in TBS and exposed to X-ray film.
cDNA hybridization probes.
Plasmids containing mouse TNF-α and IL-1β cDNA sequences were provided by T. Hamilton. In addition, a plasmid bearing β-actin cDNA was obtained from the Japanese Cell Resource Bank (JCRB), Tokyo, Japan. The methods used for plasmid preparation were described earlier (24).
Preparation of total RNA and Northern blot analysis.
Macrophage monolayers prepared from mouse peritoneal exudate cells (107) were incubated in the presence or absence of fimbriae (5 μg of protein/ml) that had been pretreated or not pretreated for 2 h at room temperature with CD11a, CD11b, and CD11c antibodies or CD18 antibody. After 1 h of incubation, the monolayers were washed five times with PBS to remove the test samples. Total RNA was then extracted, and the expression of IL-1β and TNF-α genes in the macrophages was analyzed by the Northern blot assay as described previously (7). β-Actin was used as an internal standard for the quantification of total mRNA on each lane of the gel.
RESULTS
P. gingivalis fimbrial binding to mouse peritoneal macrophages is inhibited by CD11/CD18 antibodies.
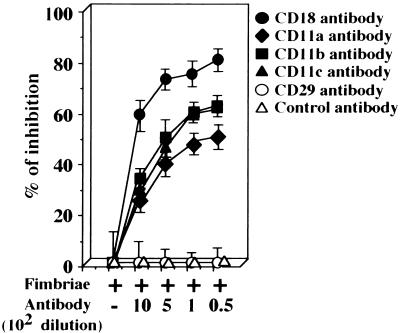
First, using purified 125I-P. gingivalis fimbriae (Fig. 1) and several antibodies against β2 integrin (CD11/CD18) or an antibody against CD29, we examined whether fimbriae could utilize molecules of β2 integrin on peritoneal macrophages as cellular receptors. As shown in Fig. 2, fimbrial binding to the cells was inhibited by each antibody to β2-integrin subunits in a dilution-dependent manner. The inhibitory activity of the CD18 antibody was stronger than that of the other antibodies. However, no inhibitory effect was observed with the CD29 antibody. These results suggest that P. gingivalis fimbriae are able to bind to molecules of β2 integrin on peritoneal macrophages.
FIG. 2.
Binding of 125I-labeled fimbriae to mouse peritoneal macrophage is inhibited by several antibodies against β2 integrin. 125I-labeled fimbriae (1 μg of protein) were added to peritoneal macrophage monolayers formed in each well of a 96-well multiple microculture plate as described in Materials and Methods. Then, each monolayer was incubated at 4°C in the absence or presence of each antibody. The binding of 125I-labeled fimbriae to cells was measured after 4 h. Rat IgG was used as a control antibody. Each experiment was performed in triplicate. The results are expressed as the mean ± SD percent inhibition of binding of 125I-labeled fimbriae (49,050 ± 2,571 cpm) in the absence of antibody. An identical experiment performed independently gave similar results.
P. gingivalis fimbriae bind to molecules of the β2-integrin (CD11/CD18) family on mouse peritoneal macrophages.
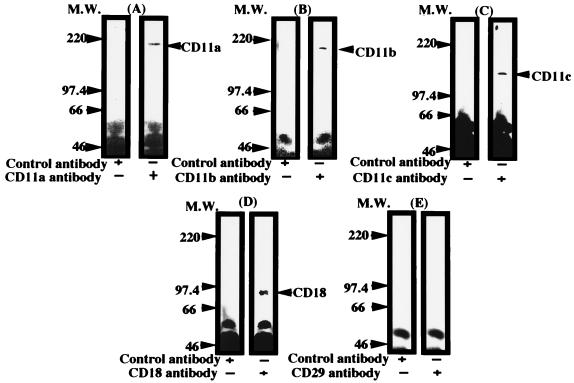
Next, we examined by the Western blot assay whether the fimbriae actually were able to bind to the macrophage-derived β2 integrin (CD11/CD18) and β1 integrin (CD29) on peritoneal macrophages. As shown in Fig. 3A to D, the fimbriae bound to each molecule of β2 integrin in the membrane fraction prepared from the cell homogenates by centrifugation at 100,000 × g. However, as shown in Fig. 3E, no fimbrial binding to the CD29 molecule in the membrane fraction was detected.
FIG. 3.
Western blot analysis of binding of P. gingivalis fimbriae to β2-integrin molecules on mouse peritoneal macrophages. Test samples of the cell membrane fraction prepared by immunoprecipitation of a fimbria-treated fraction with fimbrial antibody as described in Materials and Methods were separated by SDS-PAGE and blotted onto polyvinylidene difluoride membranes. The membranes were incubated for 18 h with a rat monoclonal antibody against each mouse β2-integrin molecule or β1 integrin (A to E), and then the bound antibody was detected with 125I-labeled sheep anti-rat IgG antibody. Rat IgG was used as a control antibody. An identical experiment performed independently gave similar results. M.W., molecular weight.
The CD18 molecule plays a central role in the gene expression of inflammatory cytokines in P. gingivalis fimbria-treated mouse peritoneal macrophages.
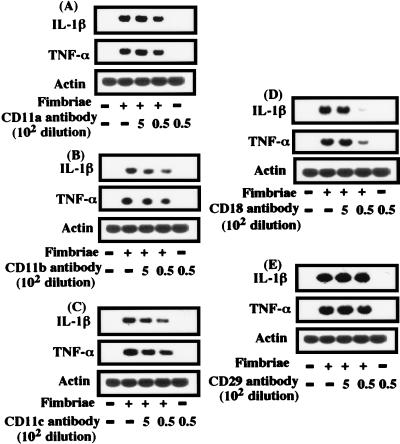
Since our previous studies (11, 26) had demonstrated fimbria-induced expression of IL-1β and TNF-α genes in peritoneal macrophages, it was of interest to examine which molecules of β2 integrin play a central role in the fimbria-induced expression of these genes in cells. Therefore, we examined this point by using the Northern blot assay. Macrophages were pretreated or not pretreated for 2 h with each antibody, incubated in the presence or absence of fimbriae, and then analyzed for the expression of IL-1β and TNF-α genes. As shown in Fig. 4A, B, and C, the fimbria-induced expression of IL-1β and TNF-α genes in cells was slightly inhibited by CD11a, CD11b, or CD11c antibody treatment. Interestingly, CD18 antibody treatment markedly inhibited the expression of both genes (Fig. 4D). However, the CD29 antibody had no effect on the expression of these genes in fimbria-treated cells (Fig. 4E). Likewise, a control antibody (rat IgG) also had no effect (data not shown). These results strongly suggest a central role for CD18 (β chain) in the signalling of the fimbria-induced expression of the IL-1β and TNF-α genes in cells and indicate that CD11 (α chain) is not deeply involved in signalling, although the chain is able to bind to fimbriae.
FIG. 4.
Effect of β2-integrin antibodies on the fimbria-induced expression of IL-1β and TNF-α genes in peritoneal macrophages. Peritoneal macrophage monolayers prepared as described in Materials and Methods were incubated with or without fimbriae (4 μg of protein/ml) that had been pretreated or not pretreated for 2 h with each antibody (A to E). After 1 h of incubation, total RNAs were prepared, and Northern blot analysis was performed with IL-1β, TNF-α, and β-actin cDNAs as probes. An identical experiment performed independently gave similar results.
ICAM-1, a ligand of CD11/CD18, also inhibits fimbrial binding to mouse peritoneal macrophages.
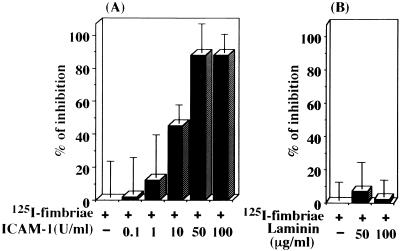
Since intracellular adhesion molecule 1 (ICAM-1) is a ligand of CD11/CD18, we considered the possibility that fimbrial binding to macrophages may be inhibited by ICAM-1 treatment. As we expected, ICAM-1 (Ancell Corp., Bayport, Minn.) indeed inhibited fimbrial binding to cells in a dose-dependent manner (Fig. 5A). However, laminin (Koken Co., Tokyo, Japan), a ligand of β1 integrin, did not inhibit fimbrial binding to cells (Fig. 5B). These results suggest that the fimbriae as well as ICAM-1 uses β2 integrin on macrophages as a cellular receptor.
FIG. 5.
ICAM-1 inhibits the binding of 125I-labeled fimbriae to mouse peritoneal macrophages. 125I-labeled fimbriae (1 μg of protein) were treated or not treated with ICAM-1 (A) or laminin (B) at the indicated doses in each well of a microculture plate containing formalin-fixed peritoneal macrophages. The binding of 125I-labeled fimbriae to cells was measured after 4 h. Each assay was carried out in triplicate. The results are expressed as the mean ± SD percent inhibition of binding of 125I-labeled fimbriae (40,050 ± 2,171 cpm) in the absence of test samples. An identical experiment performed independently gave similar results.
ICAM-1 inhibits the fimbria-induced expression of IL-1β and TNF-α genes in mouse peritoneal macrophages.
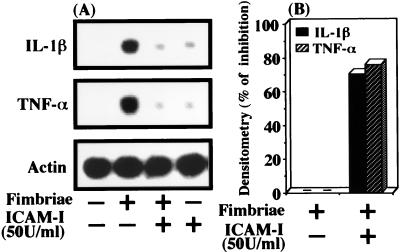
ICAM-1 inhibition of fimbrial binding to macrophages also suggested to us that the adhesion molecule may be able to inhibit the fimbria-induced expression of IL-1β and TNF-α genes in macrophages. Therefore, we explored this possibility. In preliminary experiments, we observed that peak expression of the IL-1β and TNF-α genes in cells occurred at 2 h after the initiation of ICAM-1 treatment and thereafter that the expression decreased to near baseline levels. We also found that the peak of fimbria-induced expression of both cytokine genes occurred at 1 h after the initiation of treatment (data not shown). Therefore, the cells were pretreated or not pretreated with ICAM-1 at 50 U/ml for 3 h before the addition of fimbriae and then were incubated for 1 h in the absence or presence of fimbriae. As shown in Fig. 6, ICAM-1 at 50 U/ml clearly inhibited the fimbria-induced expression of the IL-1β and TNF-α genes in cells. On the other hand, such inhibitory action was not observed with laminin pretreatment (data not shown).
FIG. 6.
ICAM-1 inhibits the fimbria-induced expression of IL-1β and TNF-α genes in peritoneal macrophages. (A) Peritoneal macrophages in each well of a microculture plate were pretreated or not pretreated for 3 h with ICAM-1 at 50 U/ml and then incubated for 1 h with or without fimbriae (4 μg of protein/ml). After incubation, total RNAs were prepared, and Northern blot analysis was performed with IL-1β, TNF-α, and β-actin cDNAs as probes. (B) Quantification of IL-1β and TNF-α gene expression in panel A was done by densitometry, and the results are expressed as percent inhibition. An identical experiment performed independently gave similar results.
DISCUSSION
Many studies (1–3, 5, 13, 14, 21, 25, 30–34) have shown that some integrins interact with bacterial cell surface components. Therefore, we investigated here whether P. gingivalis fimbriae use molecules of β2 integrin (CD11/CD18) as cellular receptors for binding to mouse peritoneal macrophages, because this integrin is predominantly expressed on these macrophages. Our findings clearly indicate such a role for β2-integrin molecules and a functional role for their β chain in fimbrial signaling.
In an assay for inhibition of 125I-labeled P. gingivalis fimbria binding with several antibodies against β2 integrin, we observed that the fimbriae were able to bind to molecules of β2 integrin (CD11/CD18) on the cells, suggesting to us that β2 integrin (CD11/CD18) may be the macrophage cellular receptor of fimbriae of this bacterium. This suggestion was validated by the results of the Western blot assay, because the fimbriae bound to β2-integrin (CD11/CD18) molecules localized in the macrophage membrane fraction.
We (8, 10, 11, 26) previously showed that P. gingivalis fimbriae are able to induce the expression of several inflammatory cytokines, such as IL-1α, IL-1β, TNF-α, JE/monocyte chemoattractant protein 1 (MCP-1), and KC, by mouse macrophages and human gingival fibroblasts. Svanborg et al. (33) also showed the inducing action of E. coli fimbriae on IL-6 production by urinary epithelial cells. Interestingly, they recently suggested the importance of ceramide, a secondary messenger of the sphingosine pathway, in cytokine expression by the cells (12). These investigations suggested that inflammatory cytokines induced via binding of fimbriae to their receptors on host cells play an important role in the pathogenic mechanism of bacterial fimbriae. Therefore, it was of interest to us to explore what kinds of cellular receptors on peritoneal macrophages are involved in P. gingivalis fimbria-induced expression of various inflammatory cytokines. Since it is known that β2-integrin molecules are expressed predominantly on macrophages and function as cellular receptors for several bacterial cell components and since our Western blot assay showed binding of the fimbriae to molecules of β2 integrin on mouse peritoneal macrophages, we used a Northern blot assay to determine if the fimbria-induced expression of IL-1β and TNF-α genes in the cells occurred via this integrin. CD18 antibody markedly inhibited their expression; however, such strong action was not detected following treatment with antibody to CD11a, CD11b, or CD11c. These observations suggest a significant involvement of the β chain (CD18) of β2 integrin in the initial stage of signalling in the fimbria-induced expression of IL-1β and TNF-α genes in these macrophages, although the α chain (CD11a, CD11b, or CD11c) is able to bind to the fimbriae.
ICAM-1 is a ligand for CD11/CD18 and has five Ig-like domains; domains 3 through 5 are essential for the binding of ICAM-1 to integrins. With this fact in mind, we examined whether the fimbriae have amino acid sequence homology with domains 3 through 5 of ICAM-1 and found that fimbrillin contains some regions in its amino acid sequence homologous to some sites in domains 3 through 5 of ICAM-1. A highly homologous sequence was located in the region from positions 331 to 346 (homology rate, 44 to 55%) of the fimbriae. This homology suggested to us the possibility that fimbrial binding to the macrophages might be inhibited by ICAM-1 treatment. In fact, this suggestion was proved by our observations that ICAM-1 significantly inhibited fimbrial binding to the peritoneal macrophages and also reduced the fimbria-induced expression of IL-1β and TNF-α genes in the cells, whereas laminin was unable to inhibit fimbrial binding or the fimbria-induced expression of IL-1β and TNF-α genes in the cells.
Several investigators (14, 32) have shown that molecules of β2 integrin serve as cellular receptors for bacterial cell components, such as Bordetella pertussis filamentous hemagglutinin and Escherichia coli pili and lipopolysaccharide. Also, an interesting study (13) indicated that Rhodococcus equi, an intracellular bacterium that causes disease in individuals with AIDS, requires CD11b/CD18 to bind to mammalian cells. However, to our knowledge, no earlier studies have demonstrated that the β2-integrin cellular receptor plays a functional role in cytokine expression by promoting adherence of pathogenic bacteria to mammalian cells. Our present study is the first to demonstrate that P. gingivalis fimbria-induced expression of IL-1β and TNF-α genes in mouse peritoneal macrophages occurs via β2-integrin molecules.
On the other hand, we observed that P. gingivalis fimbriae are also able to bind to human gingival fibroblasts and subsequently induce the production of several inflammatory cytokines, such as IL-6 and monocyte chemoattractant protein 1, by these cells (unpublished data). Therefore, in a future study, we propose to identify the cellular receptor and the signalling molecule of the fimbriae on the gingival fibroblasts.
In conclusion, we have demonstrated here that P. gingivalis fimbriae bind to mouse peritoneal macrophages via β2-integrin molecules and that the β chain (CD18) of these molecules plays an important role in signalling the fimbria-induced expression of IL-1β and TNF-α genes in these cells.
ACKNOWLEDGMENTS
We thank T. Hamilton for kindly providing mouse IL-1β and TNF-α cDNAs.
REFERENCES
- 1.Agace W W. The role of the epithelial cell in Escherichia coli induced neutrophil migration into the urinary tract. Eur Respir J. 1996;9:1713–1728. doi: 10.1183/09031936.96.09081713. [DOI] [PubMed] [Google Scholar]
- 2.Autenrieth I B, Kempf V, Sprinz T, Preger S, Schnell A. Defense mechanisms in Peyer’s patches and mesenteric lymph nodes against Yersinia enterocolitica involve integrins and cytokines. Infect Immun. 1996;64:1357–1368. doi: 10.1128/iai.64.4.1357-1368.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bermudez L E, Goodman J. Mycobacterium tuberculosis invades and replicates within type II alveolar cells. Infect Immun. 1996;64:1400–1406. doi: 10.1128/iai.64.4.1400-1406.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 5.Coburn J, Leong J M, Erban J K. Integrin alpha IIb beta 3 mediates binding of the Lyme disease agent Borrelia burgdorferi to human platelets. Proc Natl Acad Sci USA. 1993;90:7059–7063. doi: 10.1073/pnas.90.15.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamada N, Watanabe K, Sasakawa C, Yoshikawa M, Yoshimura F, Umemoto T. Construction and characterization of a fimA mutant of Porphyromonas gingivalis. Infect Immun. 1994;62:1696–1704. doi: 10.1128/iai.62.5.1696-1704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanazawa S, Takeshita A, Amano S, Semba T, Nirazuka T, Katoh H, Kitano S. Tumor necrosis factor-alpha induces expression of monocyte chemoattractant JE via fos and jun genes in clonal osteoblastic MC3T3-E1 cells. J Biol Chem. 1993;268:9526–9532. [PubMed] [Google Scholar]
- 8.Hanazawa S, Hirose K, Ohmori Y, Amano S, Kitano S. Bacteroides gingivalis fimbriae stimulate production of thymocyte-activating factor by human gingival fibroblasts. Infect Immun. 1988;56:272–274. doi: 10.1128/iai.56.1.272-274.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanazawa S, Nakada K, Ohmori Y, Miyoshi T, Amano S, Kitano S. Functional role of interleukin-1 in periodontal disease: induction of interleukin-1 production by Bacteroides gingivalis lipopolysaccharide in peritoneal macrophages from C3H/HeN and C3H/HeJ mice. Infect Immun. 1985;50:262–270. doi: 10.1128/iai.50.1.262-270.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanazawa S, Murakami Y, Takeshita A, Nishida H, Ohta K, Amano S, Kitano S. Porphyromonas gingivalis fimbriae induce gene expression of a neutrophil chemotactic factor, KC, of mouse peritoneal macrophages through protein kinase C. Infect Immun. 1992;60:1544–1549. doi: 10.1128/iai.60.4.1544-1549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanazawa S, Murakami Y, Hirose K, Amano S, Kitano S. Porphyromonas (Bacteroides) gingivalis fimbriae activate mouse peritoneal macrophages and induce gene expression and production of interleukin-1. Infect Immun. 1991;59:1972–1977. doi: 10.1128/iai.59.6.1972-1977.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hedlund M, Svensson M, Nilsson A, Duan R, Svanborg C. Role of ceramide-signaling pathway in cytokine responses to P-fimbriated Escherichia coli. J Exp Med. 1996;183:1037–1044. doi: 10.1084/jem.183.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hondalus M K, Diamond M S, Rosenthal L A, Springer T A, Mosser D M. The intracellular bacterium Rhodococcus equi requires Mac-1 to bind to mammalian cells. Infect Immun. 1993;61:2919–2929. doi: 10.1128/iai.61.7.2919-2929.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishibashi Y, Claus S, Relman D A. Bordetella pertussis filamentous hemagglutinin interacts with a leukocyte signal transduction complex and stimulates bacterial adherence to monocyte CR3 (CD11b/CD18) J Exp Med. 1994;180:1225–1233. doi: 10.1084/jem.180.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isogai E, Hirose K, Fujii N, Isogai H. Three types of binding by Porphyromonas gingivalis and oral bacteria to fibronectin, buccal epithelial cells and erythrocytes. Arch Oral Biol. 1992;37:667–670. doi: 10.1016/0003-9969(92)90130-z. [DOI] [PubMed] [Google Scholar]
- 16.Isogai H, Isogai E, Yoshimura F, Suzuki T, Kagota W, Takano K. Specific inhibition of adherence of an oral strain of Bacteroides gingivalis 381 to epithelial cells by monoclonal antibodies against the bacterial fimbriae. Arch Oral Biol. 1988;33:479–485. doi: 10.1016/0003-9969(88)90028-3. [DOI] [PubMed] [Google Scholar]
- 17.Kawata Y, Hanazawa S, Amano S, Murakami Y, Matsumoto T, Nishida K, Kitano S. Porphyromonas gingivalis fimbriae stimulate bone resorption in vitro. Infect Immun. 1994;62:3012–3016. doi: 10.1128/iai.62.7.3012-3016.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawata Y, Iwasaka H, Kitano S, Hanazawa S. Porphyromonas gingivalis fimbria-stimulated bone resorption is inhibited through binding of the fimbriae to fibronectin. Infect Immun. 1997;65:815–817. doi: 10.1128/iai.65.2.815-817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kontani M, Ono H, Shibata H, Okamura Y, Tanaka T, Fujiwara T, Kimura S, Hamada S. Cysteine protease of Porphyromonas gingivalis 381 enhances binding of fimbriae to cultured human fibroblasts and matrix proteins. Infect Immun. 1996;64:756–762. doi: 10.1128/iai.64.3.756-762.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kubo K, Kamada T, Okamoto H, Izumi Y, Otsuji S, Sueda T. Lipopolysaccharide increases cell surface-associated fibronectin in fibroblasts in vitro. Oral Microbiol Immunol. 1996;11:29–34. doi: 10.1111/j.1399-302x.1996.tb00333.x. [DOI] [PubMed] [Google Scholar]
- 21.Kumasaka T, Doyle N A, Quinlan W M, Graham L, Doerschuk C M. Role of CD 11/CD 18 in neutrophil emigration during acute and recurrent Pseudomonas aeruginosa-induced pneumonia in rabbits. Am J Pathol. 1996;148:1297–1305. [PMC free article] [PubMed] [Google Scholar]
- 22.Lantz M S, Allen R D, Duck L W, Blume J L, Switalski L M, Hook M. Identification of Porphyromonas gingivalis components that mediate its interactions with fibronectin. J Bacteriol. 1991;173:4263–4270. doi: 10.1128/jb.173.14.4263-4270.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J Y, Sojar H T, Bedi G S, Genco R J. Synthetic peptides analogous to the fimbrillin sequence inhibit adherence of Porphyromonas gingivalis. Infect Immun. 1992;60:1662–1670. doi: 10.1128/iai.60.4.1662-1670.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. pp. 365–389. [Google Scholar]
- 25.Mukaida N, Ishikawa Y, Ikeda N, Fujioka N, Watanabe S, Kuno K, Matsushima K. Novel insight into molecular mechanism of endotoxin shock: biochemical analysis of LPS receptor signaling in a cell-free system targeting NF-kappaB and regulation of cytokine production/action through beta2 integrin in vivo. J Leukocyte Biol. 1996;59:145–151. doi: 10.1002/jlb.59.2.145. [DOI] [PubMed] [Google Scholar]
- 26.Murakami Y, Hanazawa S, Nishida K, Iwasaka H, Kitano S. N-acetyl-d-galactosamine inhibits TNF-α gene expression induced in mouse peritoneal macrophages by fimbriae of Porphyromonas (Bacteroides) gingivalis, an oral anaerobe. Biochem Biophys Res Commun. 1993;192:826–832. doi: 10.1006/bbrc.1993.1489. [DOI] [PubMed] [Google Scholar]
- 27.Murakami Y, Iwahashi H, Yasuda H, Umemoto T, Namikawa I, Kitano S, Hanazawa S. Porphyromonas gingivalis fimbrillin is one of the fibronectin binding proteins. Infect Immun. 1996;64:2571–2576. doi: 10.1128/iai.64.7.2571-2576.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naito Y, Gibbons R J. Attachment of Bacteroides gingivalis to collagenous substrate. J Dent Res. 1988;67:1075–1080. doi: 10.1177/00220345880670080301. [DOI] [PubMed] [Google Scholar]
- 29.Pike R N, Potempa J, McGraw W, Coetzer T H, Travis J. Characterization of the binding activities of proteinase-adhesin complexes from Porphyromonas gingivalis. J Bacteriol. 1996;178:2876–2882. doi: 10.1128/jb.178.10.2876-2882.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramamoorthy C, Sasaki S S, Su D L, Sharar S R, Harlan J M, Winn R K. CD18 adhesion blockade decreases bacterial clearance and neutrophil recruitment after intrapulmonary E. coli, but not after S. aureus. J Leukocyte Biol. 1997;61:167–172. doi: 10.1002/jlb.61.2.167. [DOI] [PubMed] [Google Scholar]
- 31.Rao S P, Ogata K, Catanzaro A. Mycobacterium avium- M. intracellulare binds to the integrin receptor alpha v beta 3 on human monocytes and monocyte-derived macrophages. Infect Immun. 1993;61:663–670. doi: 10.1128/iai.61.2.663-670.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Register K B, Ackermann M R, Kehrli M., Jr Non-opsonic attachment of Bordetella bronchiseptica mediated by CD11/CD18 and cell surface carbohydrates. Microb Pathog. 1994;17:375–385. doi: 10.1006/mpat.1994.1083. [DOI] [PubMed] [Google Scholar]
- 33.Svanborg C, Agace W, Hedges S, Lindstedt R, Svensson M L. Bacterial adherence and mucosal cytokine production. Ann N Y Acad Sci. 1994;730:162–181. doi: 10.1111/j.1749-6632.1994.tb44247.x. [DOI] [PubMed] [Google Scholar]
- 34.Tran V N G, Isberg R R. Bacterial internalization mediated by beta 1 chain integrins is determined by ligand affinity and receptor density. EMBO J. 1993;12:1887–1895. doi: 10.1002/j.1460-2075.1993.tb05837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinberg A, Belton C M, Park Y, Lamont R J. Role of fimbriae in Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 1997;65:313–316. doi: 10.1128/iai.65.1.313-316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshimura F, Takahashi K, Nodasaka Y, Suzuki T. Purification and characterization of a novel type of fimbriae from the oral anaerobe Bacteroides gingivalis. J Bacteriol. 1984;160:949–954. doi: 10.1128/jb.160.3.949-957.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]