Abstract
The in vitro glycolipid binding specificity of the gastric pathogen Helicobacter pylori is altered to include sulfated glycolipids (sulfatides) following brief exposure of the organism to acid pH typical of the stomach. This change is prevented by anti-hsp70 antibodies, suggesting that hsp70 may be a stress-induced surface adhesin, mediating sulfatide recognition. To facilitate investigation of the role of hsp70 in attachment, we have cloned and sequenced the H. pylori hsp70 gene (dnaK). The hsp70 gene was identified by probing a cosmid DNA library made from H. pylori 439 with a PCR amplicon generated with oligonucleotides synthesized to highly conserved regions of dnaK. The 1.9-kb H. pylori hsp70 gene encodes a product of 616 amino acids. Primer extension analysis revealed a single transcription start site, while Northern blot analysis established that hsp70 was preferentially induced by low pH rather than by heat shock. The ability of H. pylori to alter its glycolipid binding specificity following exposure to low pH by upregulating hsp70 and by expressing hsp70 on the bacterial surface may provide a survival advantage during periods of high acid stress.
Helicobacter pylori organisms have evolved several adaptive features which allow them to survive and establish chronic gastroduodenal infections. Their characteristic helical shape and motility in gastric mucus provide a means for escaping the extremely low pH of the gastric lumen (29), while the expression of a potent urease potentially neutralizes the bacterial microenvironment by producing ammonia from the urea present in mucosal secretions (10, 12, 19). Other mechanisms of adaptation to conditions in the stomach include expression of several adhesins which are involved in bacterial attachment (1, 6, 37, 49, 52, 57, 74) and the induction by acidic pH of the synthesis of products that inhibit acid secretion (5, 55). Finally, H. pylori produces a cytotoxin (VacA) whose expression is also induced by acid pH (7, 38).
The response of H. pylori to acid stress resembles the universal stress response manifest in the selective synthesis of a subset of proteins, heat shock proteins (hsps) or stress proteins, which function to facilitate cell adaptation to adverse conditions (8, 34, 48, 73). H. pylori synthesizes homologs of GroEL (hsp60), GroES (hsp10) (9), and a DnaK-related hsp70 (32). These proteins are selectively synthesized following heat shock (76) or following pH shock (32). It has been proposed that the H. pylori hsp60 may participate in protection and regulation of expression of urease (17, 70).
Viable H. pylori cells bind to the glycolipids gangliotetra- and gangliotriaosyl ceramide (Gg4 and Gg3) and to the phospholipid phosphatidylethanolamine (PE) separated by thin-layer chromatography and overlaid with H. pylori at neutral pH under microaerobic conditions (50–52). We found that H. pylori binding to eukaryotic cells was significantly reduced for cells deficient in PE (11, 24). PE binding may allow bacteria to preferentially adhere to apoptotic cells (4, 13). Apoptosis plays an integral role the homeostasis of the gastrointestinal mucosa (27) and has been found to increase following H. pylori infection (36).
This in vitro binding specificity is shared by a variety of pathogenic microorganisms (4, 40–42, 45, 46, 59). Among other putative H. pylori receptors that have been reported are sulfatides and ganglioside GM3 (64, 65), fucose-containing blood group antigens (Leb glycoprotein) (1, 18, 33), neuraminyl lactose-containing glycoconjugates (14–16), and sialylpolyglycosyl ceramides (57). Thus, the molecular basis of H. pylori attachment to host tissues may be complex and multifactorial. However, many of the binding studies identifying these receptors were not carried out under conditions which reflect in vivo colonization or optimal growth conditions and may therefore be of questionable relevance to H. pylori adherence in vivo.
Our initial receptor binding studies on H. pylori were performed under microaerobic conditions (50, 51) required to maintain the viability of this organism. We found a marked change in the receptor binding specificity when the binding assay was performed at low pH (equivalent to that of the stomach) or if the organisms were briefly exposed to low pH followed by binding analysis at neutral pH (32). Under such stress conditions, binding was primarily to the sulfated glycolipids sulfogalactosylceramide and 3′-sulfogalactosylglycerol, in addition to the binding of PE and of Gg3 or Gg4 characteristic of unstressed organisms. Under our culture and assay conditions, untreated organisms did not bind to sulfogalactolipids. A similar but less marked change in binding specificity was observed following brief heat shock of H. pylori, suggesting that this change was a result of a stress response. The change in receptor binding was prevented in the presence of inhibitors of protein synthesis or if the stressed organisms were pretreated with anti-hsp70 or anti-hsp60 antibodies (32). These studies led us to propose a binary receptor model for the attachment of H. pylori to the stomach mucosa whereby the low pH of the stomach induces surface hsp-mediated attachment of the organism to sulfated glycolipids contained within the mucous layer. Penetration of the mucous layer would allow subsequent attachment of the organism at neutral pH to gastric epithelial PE.
Increased surface expression of hsp70 and hsp60 was confirmed by immunofluorescence following low-pH shock, and increased content of hsp60 in a surface extract of H. pylori following heat shock was observed (32). These results are consistent with expression of the hsps on the bacterial surface following stress, to function as an adhesin mediating the induced recognition of sulfated glycolipids. hsp60 has also been implicated as an adhesin for H. pylori binding to gastric carcinoma cells (74, 75). Although the mechanism by which hsp70 is surface expressed is unknown, it is not via the absorption of proteins released from lysed organisms (72). We have observed a similar hsp-mediated effect on glycolipid binding specificity following heat shock of Haemophilus influenzae (28).
To begin to define the role of hsp70 in H. pylori adhesion and sulfatide recognition, we have characterized the H. pylori hsp70 gene and its expression under conditions of normal growth, heat shock, and acid shock.
MATERIALS AND METHODS
Antibodies against hsps.
Polyclonal rabbit anti-H. pylori hsp60 (9) was kindly provided by G. Perez-Perez (Vanderbilt University). The rabbit anti-Chlamydia trachomatis hsp70 (61), a generous gift from P. Wyrick (University of North Carolina), was selected for these studies based on its sequence homology and cross-reactivity with the H. pylori hsp70, demonstrated by Western blotting using protein extracts from H. pylori.
Bacterial strains and growth conditions.
H. pylori LC11 was obtained from P. Sherman (Hospital for Sick Children, Toronto, Ontario, Canada), and H. pylori 439 was obtained from the Victoria General Hospital, Halifax, Nova Scotia, Canada. H. pylori LC11 was grown on blood agar plates under a reduced-oxygen environment (10% CO2, 5% O2, 85% N2) at 37°C. Strain 439 was grown on Brucella agar plates supplemented with 10% fetal calf serum (FCS; Gibco-BRL) in a microaerobic incubator maintained at 7% O2 and 5% CO2. Liquid cultures were grown in Brucella broth supplemented with FCS in 125-ml screw-capped flasks. The medium was equilibrated with 7% O2 and 5% CO2 in a microaerobic incubator for 1 to 3 h prior to inoculation, and then the flask was sealed and placed on a rotary shaker at 150 rpm for 2 to 3 days at 37°C.
PCR-based cloning of hsp70.
A PCR-based protocol described by Galley et al. (22) was used to clone a fragment of hsp70. Briefly, 1 μg of genomic DNA isolated from H. pylori LC11 was used as the template, and degenerate oligonucleotide primers were designed from conserved regions of the hsp70 family of proteins; primer A (forward; 5′ CARGCNACNAARGAYGCNGG) was designed from the sequence QATKDAG (E. coli DnaK, amino acids 152 to 158), and primer B (reverse; 5′ GCNACNGCYTCRTCNGGRTT) was designed from amino acid sequence NPDEAVA (E. coli DnaK, amino acids 366 to 372). The PCR program (30 cycles) consisted of denaturation for 60 s at 93°C, annealing for 30 s at 58°C, and extension for 60 s at 72°C. The amplicon was cloned into pCRII vector (Invitrogen Corp., San Diego, Calif.) and introduced into Escherichia coli INVaF′ competent cells (Invitrogen). Recombinant clones were screened on LB plates supplemented with ampicillin and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal). The amplicon was sequenced by using primers for the Sp6 and T7 promoters present in the pCRII vector (56). The 660-bp DNA fragment showed homology with a number of bacterial hsp70 sequences, and the DNA fragment was used to screen an H. pylori genomic library. A cosmid library of genomic DNA from H. pylori 439 was constructed in Lorist6 as described elsewhere (25) and screened by colony Southern blotting. The 660-bp PCR fragment was labeled with [α-32P]dATP by the random priming method (66) and used to screen a library of ca. 900 clones.
DNA sequencing and analysis.
Sequencing of the hsp70 gene was performed in the Biotechnology Service Center, The Hospital for Sick Children, by the dideoxy-chain termination method of Sanger et al. (67); four differentially labeled fluorescent primers (Epicentre Technologies) complementary to sequences from the 660-bp amplicon were utilized for automated sequencing using a thermostable DNA polymerase (SequiTherm/Epicentre Technologies). Both strands were sequenced, and the contigs were assembled by using the Genetics Computer Group (University of Wisconsin) sequence analysis programs and DNA Strider (54).
RNA isolation and primer extension analysis.
Total RNA was isolated from H. pylori by the hot phenol method (31). Briefly, bacteria were grown in 100 ml of Brucella broth supplemented with FCS to an optical density of ca. 0.4 (108 bacteria/ml) and harvested by centrifugation at 4°C for 10 min, and the pellet was suspended in Tris-EDTA-sodium dodecyl sulfate (SDS) buffer at 95°C. Following phenol extractions (2) and precipitation, the RNA was dissolved in diethylpyrocarbonate-treated water and the total RNA concentration was determined at 260 nm. Purity and quality of the RNA were judged by agarose gel electrophoresis. For the primer extension studies, an oligonucleotide (5′-TCCGTAAAGGCTACAATAGA-3′) complementary to H. pylori hsp70 was labeled with 2 μl of T4 polynucleotide kinase (20 U) and 12 × 106 cpm of [γ-32P]ATP. H. pylori RNA (100 μg) was incubated with the 32P-labeled oligonucleotide and annealed under the following temperature conditions: 80°C for 2 min, 65°C for 5 min, 42°C for 10 min, and 37°C for 20 min. Following annealing, the DNA was precipitated with ethanol, dried, and resuspended in 7 μl of diethylpyrocarbonate-treated water. Thirteen microliters of reverse transcriptase buffer (50 mM Tris-HCl [pH 8.3], 60 mM KCl, 10 mM MgCl2, 10 mM each deoxynucleoside triphosphate 100 mg of actinomycin D per ml, 1 mM dithiothreitol) and 1 μl of Moloney murine leukemia virus reverse transcriptase enzyme (0.4 U) were added to the sample, and the reaction mixture was incubated at 37°C for 60 min; 1 μl of 0.5 M EDTA and 1 ml of RNase (10 mg/ml) were added, and the reaction mixture was incubated at 37°C for additional 30 min. The reaction was stopped by phenol-chloroform extraction followed by ethanol precipitation. The sample was then dried and resuspended in 10 μl of sequencing dye, and aliquots were loaded onto a 6% polyacrylamide sequencing gel.
Northern blot analysis.
Total RNA from H. pylori (107 bacteria in 200 μl per sample) incubated for 30 min in RPMI containing 10 mM urea, pH 7.0 at 37 or 42°C or pH 2.5 at 37°C, was isolated as described above. Five micrograms of total RNA was analyzed by Northern blotting in a formaldehyde-containing agarose gel (53). RNA was transferred to a positively charged nylon membrane (Boehringer Mannheim Canada, Laval, Quebec, Canada) and fixed to the membrane by baking for 30 min at 120°C. A 50-mer reverse primer complementary to nucleotides 350 to 400 of the H. pylori hsp70 sequence was labeled with digoxigenin (DIG) by the enzyme terminal transferase (Boehringer Mannheim Canada) and used as a probe in the Northern blot analysis.
One microgram of the DIG-oligonucleotide probe in 2 ml of hybridization buffer (5× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate] with 1% of the blocking reagent supplied, 0.1% N-lauroylsarcosine, 0.02% SDS) was hybridized for 16 h at 50°C in a hybridization oven. Detection of the DIG-DNA/RNA hybrid was performed by using anti-DIG antibodies conjugated with alkaline phosphatase. The approximate size of the transcript was estimated by comparing the relative mobility of the RNA band with RNA standards (GIBCO-BRL), stained with ethidium bromide in a separate track.
Pulse-labeling of H. pylori proteins under acidic conditions and Western blot analysis.
The method for radiolabeling the H. pylori proteins was a modification of the method reported by Yokota et al. (76). Briefly, two aliquots of 106 bacteria of an H. pylori LC11 suspension were resuspended in 500 μl of methionine-free RPMI 1640 (RPMI; Sigma, St. Louis, Mo.) (pH 7.0) or RPMI–0.1 N HCl (pH 2.5) and were incubated for 30 min at 37°C with 150 μCi of [35S]methionine (Amersham Canada, Oakville, Ontario, Canada), both suspensions containing urea at physiological concentrations (10 mM). Bacteria were centrifuged, rinsed with fresh RPMI (pH 7.0), and incubated in the same medium for an additional 30 min under reduced-oxygen concentration. Both the addition of urea at acidic pH and reduced oxygen concentration at neutral pH were used to favor H. pylori survival (12). Proteins were extracted by boiling the bacterial pellets 2 min in 10 mM Tris buffer (pH 7.0) containing 1% SDS. The protein content was determined by the bicinchoninic acid protein assay (3).
Five-microgram aliquots of proteins of each cell extract were separated by SDS-polyacrylamide gel electrophoresis (PAGE) as described previously (44), using a Mini-Protean II system (Bio-Rad, Hercules, Calif.). The SDS-polyacrylamide gel was stained, or proteins were transferred to nitrocellulose membranes (Schleicher & Schuell, Keene, N.H.) for Western blot analysis. Duplicate membranes were incubated with an antibody against either hsp70 or hsp60 as described elsewhere (2). Direct autoradiograms of the Western blots were then obtained. To characterize the proteins induced by heat and acid shock, antibody-reactive radiolabeled bands were identified by overlaying enlarged scanned images of the Western blots and their corresponding autoradiograms.
Nucleotide sequence accession number.
The sequence shown in Fig. 1B has been deposited in GenBank under accession no. AF053125.
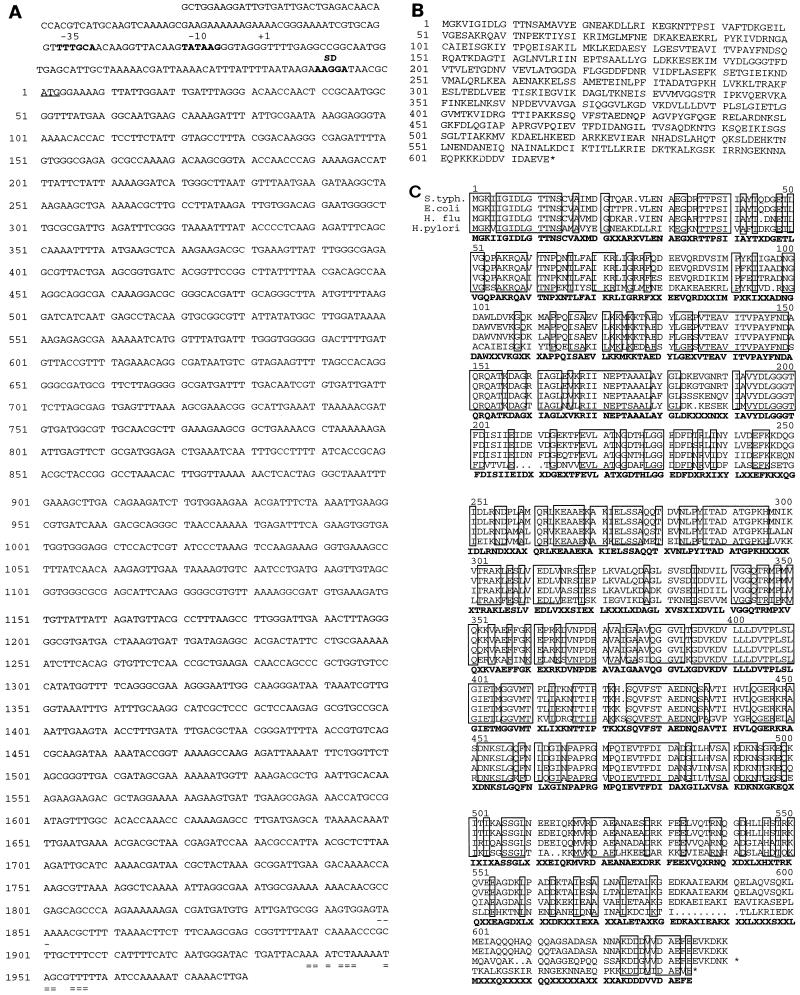
FIG. 1.
(A) DNA sequence of H. pylori hsp70. Indicated are the transcriptional start site (+1), a putative Shine-Dalgarno (SD) sequence, and −35 and −10 putative promoters. The 1,851-bp ORF starts at an ATG codon (underlined); a potential rho-independent transcription terminator sequence (doubly underlined) and a stop codon (underlined TA) are also shown. (B) Deduced amino acid sequence of H. pylori hsp70. Asterisks represent the positions of stop codons. (C) Amino acid sequence comparison between H. pylori hsp70 and homologs from Salmonella typhimurium (S.typh.), E. coli, and H. influenzae (H. flu). Amino acids 153 to 158 and 366 to 373 were used in the design of PCR primers. Identical amino acids are boxed, and the consensus sequence is shown in bold.
RESULTS
Cloning of the H. pylori hsp70 gene.
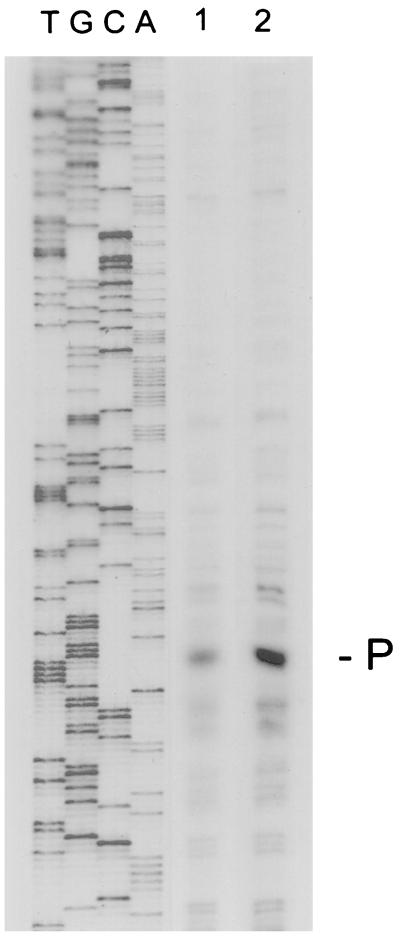
Degenerate oligonucleotides synthesized to two highly conserved regions of bacterial hsp70 genes were used to amplify a 660-bp DNA fragment from genomic DNA of H. pylori LC11. The nucleotide sequence of the DNA fragment was determined, and its deduced product showed homology with bacterial hsp70 proteins. A screening of a genomic Lorist6 cosmid library of H. pylori 439 with the 660-bp DNA fragment as a probe identified one clone (HPL20). The clone contained the entire hsp70 open reading frame (ORF) and flanking sequences. Subcloning and DNA sequencing identified a 1,848-bp ORF encoding a polypeptide of 616 amino acids (Fig. 1). The ORF was terminated by a TAA codon followed by a putative rho-independent transcription terminator sequence (63). The G+C content of the coding region is 42%, which is consistent with the G+C content of other H. pylori genes (43, 47, 68, 71). The ORF corresponds to HP0109 in the Institute for Genomic Research genome sequence (71). The hsp70 of H. pylori was highly homologous to the hsp70s from other bacteria (Fig. 1B) including H. influenzae (72% similarity and 58% identity) and E. coli (72% similarity and 57% identity). Primer extension analysis identified a single transcription start site 62 bp upstream of the translation initiation codon (Fig. 2) with putative RNA polymerase/ς70 recognition nucleotide sequences at −10 (TATAAG) and −35 (TTTGCA). Other, faster-migrating cDNA bands noted by primer extension may derive from weaker nonspecific start points or from degradation of RNA transcripts. Analysis of upstream sequences did not reveal any obvious regulatory sequences of known DNA binding proteins.
FIG. 2.
Primer extension analysis of the transcriptional start site of the H. pylori hsp70 gene. A [γ-32P]ATP-labeled oligonucleotide complementary to the 5′ end of H. pylori hsp70 was hybridized to 25 and 50 μg of total RNA (lanes 1 and 2, respectively). Lanes T, G, C, and A are products of sequencing reactions using the same oligonucleotide as the primer. The start site at position −62 (P) is indicated on the right. The upstream sequence of the antisense strand is shown in Fig. 1A.
hsp70 mRNA levels.
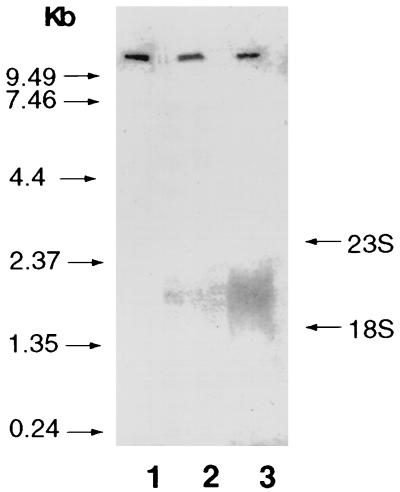
Since the TIGR genomic sequence identified grpE, encoding the hsp70 binding nucleotide exchange protein, upstream of hsp70 (71), we investigated the possibility that hsp70 is part of a heat shock operon. Northern blot analysis was performed with a 50-mer oligonucleotide probe designed from an internal sequence of the hsp70 gene. As seen in Fig. 3, no transcript was detected with mRNA prepared from bacteria cultured at 37°C (although hsp70 synthesis is seen under nonstressed conditions [Fig. 4]). However, mRNA obtained from heat-shocked (at 42°C) bacteria or acid-shocked (at pH 2.5) bacteria contained a single transcript of 1.9 kbp, indicating that hsp70 was not part of an operon. However, we could not exclude the possibilities that under nonstress conditions, a polycistronic message was produced in low amounts and that following a stress response, hsp70 mRNA was selectively synthesized. Acid shock led to a significantly greater increase (5.9-fold) in hsp70 mRNA compared with heat shock (Fig. 3; compare lane 2 with lane 3).
FIG. 3.
Northern blot analysis of hsp70 expression after stress. H. pylori mRNA was isolated before and after heat shock or acid pH shock. Northern hybridization was performed with 5 μg of total RNA per lane. A 50-mer synthetic oligonucleotide probe designed from the hsp70 gene was used as a probe. Lanes: 1, mRNA from H. pylori incubated at 37°C (control); 2, heat-shocked (at 42°C) organisms; 3, acid pH-shocked (at pH 2.0) organisms. The hybridization bands were quantitated by densitometry. The hsp mRNA was increased 5.9-fold following pH shock relative to heat-shocked organisms. Under the assay conditions, transcription in nonstressed organisms could not be detected.
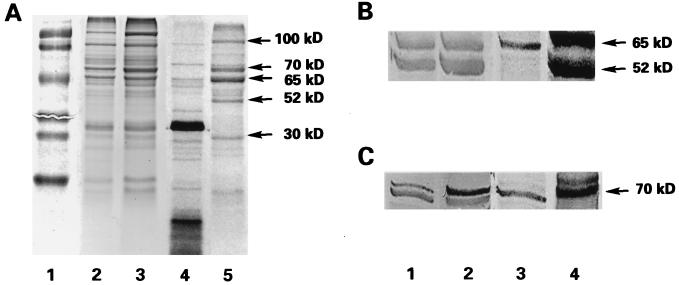
FIG. 4.
Comparison of 35S metabolic labeling and Western blotting using anti-hsp antibodies to identify pH 2.5-induced stress proteins. Each lane contained 5 μg of total protein extracted with 1% SDS. (A) SDS-PAGE. Lane 1, prestained MW standards (from top to bottom, phosphorylase [111 kDa], bovine serum albumin [77 kDa], ovalbumin [48.2 kDa], carbonic anhydrase [33.8 kDa], soybean trypsin inhibitor [28.6 kDa], and lysozyme [20.5 kDa]); lanes 2 and 3, total protein stained with Coomassie blue; lanes 4 and 5, autoradiograms corresponding to lanes 2 and 3. Lanes 2 and 4, control H. pylori; lanes 3 and 5, acid pH-shocked H. pylori. (B) Western blotting using anti-hsp60 antibody. Lanes 1 and 2, immunostain; lanes 3 and 4, autoradiograms of lanes 1 and 2, respectively. Lanes 1 and 3, control H. pylori; lanes 2 and 4, pH-shocked H. pylori. (C) Western blotting using anti-hsp70 antibody. Lanes 1 and 2, immunostain; lanes 3 and 4, autoradiograms of lanes 1 and 2, respectively. Lanes 1 and 3, control H. pylori; lanes 2 and 4, pH-shocked H. pylori.
Synthesis of proteins induced by acidic pH.
SDS-PAGE analysis of metabolically labeled proteins induced by incubation of H. pylori under acidic conditions (pH 2.5) showed a typical poststress pattern of protein synthesis (48), comprising reduced basal protein synthesis and increased synthesis of a small subset of proteins represented, in this case, by five species with apparent molecular masses of 100, 70, 65, 52, and 30 kDa (Fig. 4A, lane 5). These proteins were undetected or found at lower concentration in the extract from H. pylori incubated at neutral pH at 37°C (Fig. 4A, lane 4). A less marked difference was observed when the same protein extracts were stained with Coomassie blue (Fig. 4A, lanes 2 and 3). To investigate which of these bands correspond to hsps, particularly hsp60 and hsp70, we transferred proteins to nylon membranes and performed Western blot analysis. Results obtained by staining with polyclonal anti-H. pylori GroEL-related hsp (anti-hsp60) and anti-C. trachomatis DnaK-related hsp (anti-hsp70) are shown in Fig. 4B and C. Two bands, of 65 and 52 kDa, were reactive with antibodies against hsp60 in extracts from H. pylori incubated at both neutral and acidic pH (Fig. 4B, lanes 1 and 2, respectively). The intensities of both bands detected by Western blotting and of the corresponding bands detected in the autoradiogram (Fig. 4B, lanes 3 and 4), particularly the species of lower molecular weight (MW), is increased in H. pylori exposed to acidic conditions. Two species of similar MW were detected by anti-hsp70. The upper band increased after pH shock (Fig. 4C, lanes 1 and 2) and corresponds to the 70-kDa band observed after pH shock in the metabolic labeling experiment (Fig. 4C, lane 4). Only the lower-MW band was detected by this method in the extract from H. pylori incubated at neutral pH (Fig. 4C, lane 3). A band above 70 kDa, not recognized by anti-hsp70 antibodies but detected in the autoradiogram after pH shock, was observed (Fig. 4C, lane 4). Thus, only one pH-sensitive anti-hsp70-reactive species was detected.
DISCUSSION
We have cloned and sequenced the hsp70 from the reference strain HP439 of H. pylori. The HP439 hsp70 gene shows 91.3% homology with the single hsp70 gene from H. pylori 26695, for which the whole genome sequence has recently been released (71). Our upstream sequencing of HP439 hsp70 has identified an hsp60 gene 500 bp from the 5′ of the hsp70 sequence (unpublished data). A considerably greater distance (108.934 kb) separates these genes in strain 26695 (71). This difference is a further example of the high degree of genetic variability observed among different H. pylori strains (35).
The translation from nucleotide to amino acid sequence defined a 616-amino-acid protein highly homologous to several bacterial hsp70s, including that of C. trachomatis, the bacterium utilized to raise the anti-hsp70 antibodies used in this study. It is of interest that the closest homolog to the H. pylori hsp70 sequence that we have determined is the hsp70 from H. influenzae. These are the two organisms for which we have shown a stress-induced surface hsp-mediated induction of sulfatide binding (28).
The stress response in H. pylori produced by acidic pH induced a pattern of protein synthesis different from the pattern induced by heat shock, which consisted of the increased synthesis of only GroEL-related hsps (31a, 76). A distinctive post-acid shock change in gene expression is also observed in microorganisms such as Salmonella typhimurium and protozoans of the genus Leishmania, which must tolerate the acidic conditions of the phagolysosome (21, 77). pH-induced changes have also been reported for E. coli (30) and other microorganisms (26). In most cases, the induction of both hsp60 and hsp70 is observed, and they are considered to play an important role in the adaptation of these microorganisms to acidic conditions. It has been proposed (70) that an H. pylori GroEL-like protein, in addition to a GroES homolog, both encoded in the same bicistronic operon, plays an important role in the adaptation of H. pylori to acidic conditions by regulating the expression of the enzyme urease. Our results, at both the protein and mRNA levels, support a selective role of a DnaK-related protein in the adaptation of H. pylori to the pH of the stomach. In addition to a chaperone role of this hsp70, which would provide protection of proteins exposed to acidic conditions, the surface hsp70 would facilitate the association of H. pylori with the stomach mucus, in this way protecting the bacteria from the stomach peristalsis and long exposure to acid conditions. Our Northern hybridization shows that H. pylori hsp70 is preferentially (∼6-fold) upregulated by low pH rather than heat shock. Regulation of the hsp70 stress response is achieved via interplay between DnaK, DnaJ, GrpE, and ς32 (23). However, no ortholog of ς32 has been identified in H. pylori, (71), suggesting that the stress response is regulated differently in this organism.
Several studies, apart from our own, have proposed that surface hsps mediate cell attachment in both prokaryotes (60, 61) and eukaryotes (20, 58), and it is possible that sulfogalactolipid binding is involved in these systems also.
In addition to their potential role in H. pylori adhesion, bacterial hsps have been implicated in the mechanism of host tissue inflammation (62, 69). H. pylori induces chronic inflammation of the gastric epithelium (39), which may be exacerbated by the ability of this organism to express hsp70 on the cell surface. Our cloning of the H. pylori hsp70 gene will allow the definition of the role played by this stress protein in colonization and pathology.
ACKNOWLEDGMENT
This work was supported by MRC grant MT 12559.
REFERENCES
- 1.Borén T, Falk P, Roth K A, Larson G, Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993;262:1892–1895. doi: 10.1126/science.8018146. [DOI] [PubMed] [Google Scholar]
- 2.Boulanger J, Faulds D, Eddy E M, Lingwood C A. Members of the 70kDa heat shock protein family specifically recognize sulfoglycolipids: role in gamete recognition and mycoplasma related infertility. J Cell Physiol. 1995;165:7–17. doi: 10.1002/jcp.1041650103. [DOI] [PubMed] [Google Scholar]
- 3.Boulanger J, Huesca M, Arab S, Lingwood C A. Universal method for the facile production of glycolipid/lipid matrices for the affinity purification of binding ligands. Anal Biochem. 1994;217:1–6. doi: 10.1006/abio.1994.1075. [DOI] [PubMed] [Google Scholar]
- 4.Busse J, Hartmann E, Lingwood C A. Receptor affinity purification of a lipid-binding adhesin from Haemophilus influenzae. J Infect Dis. 1996;175:77–83. doi: 10.1093/infdis/175.1.77. [DOI] [PubMed] [Google Scholar]
- 5.Cave D R, Vargal M. Effect of a Campylobacter pylori protein on acid secretion by parietal cells. Lancet. 1989;ii:187–189. doi: 10.1016/s0140-6736(89)90372-3. [DOI] [PubMed] [Google Scholar]
- 6.Clyne M, Drumm B. Cell envelope characteristics of Helicobacter pylori: their role in adherence to mucosal surfaces and virulence. FEMS Immunol Microbiol. 1996;16:141–155. doi: 10.1111/j.1574-695X.1996.tb00130.x. [DOI] [PubMed] [Google Scholar]
- 7.Cover T L, Puryear W, Perez-Perez G, Blaser M. Effect of urease on HeLa cell vaculation induced by Helicobacter pylori cytotoxin. Infect Immun. 1991;59:1264–1270. doi: 10.1128/iai.59.4.1264-1270.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Craig E. Chaperones: helpers along the pathways to folding. Science. 1993;260:1902–1903. doi: 10.1126/science.8100364. [DOI] [PubMed] [Google Scholar]
- 9.Dunn B, Roop R, Sung C, Sharma S, Perez-Perez G, Blaser M. Identification of a cpn60 heat shock protein homolog from Helicobacter pylori. Infect Immun. 1992;60:1946–1951. doi: 10.1128/iai.60.5.1946-1951.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn B E, Cambell G P, Perez-Perez G I, Blaser M J. Purification and characterization of urease from Helicobacter pylori. J Biol Chem. 1990;265:9464–9469. [PubMed] [Google Scholar]
- 11.Dytoc M, Fedorko L, Huesca M, Gold B, Louie M, Crowe S, Lingwood C, Brunton J, Sherman P. Comparison of Helicobacter pylori and attaching-effacing Escherichia coli adhesion to eukaryotic cells. Infect Immun. 1993;61:448–456. doi: 10.1128/iai.61.2.448-456.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eaton K A, Brooks C L, Morgan D R, Krakowka S. Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect Immun. 1991;59:2470–2475. doi: 10.1128/iai.59.7.2470-2475.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emoto K, Toyama-Sorimachi N, Karasuyama H, Inoue K, Umeda M. Exposure of phosphatidylethanolamine on the surface of apoptotic cells. Exp Cell Res. 1997;232:430–434. doi: 10.1006/excr.1997.3521. [DOI] [PubMed] [Google Scholar]
- 14.Evans D, Karjalainen T, Evans D, Graham D, Lee C-H. Cloning, nucleotide sequence, and expression of an adhesin subunit protein of Helicobacter pylori. J Bacteriol. 1993;175:674–683. doi: 10.1128/jb.175.3.674-683.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans D G, Evans D J, Moulds J J, Graham D Y. N-Acetylneuraminyl lactose-binding fibrillar hemagglutinin of Campylobacter pylori: a putative colonization factor antigen. Infect Immun. 1988;56:2896–2906. doi: 10.1128/iai.56.11.2896-2906.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans D G, Lampert H C, Nakano H, Eaton K A, Burnens A P, Bronsdon M A, Evans D J., Jr Genetic evidence for host specificity in the adhesin-encoding genes haxA of Helicobacter acinonyx, hnaA of H. nemestrinae and hpaA of H. pylori. Gene. 1995;163:97–102. doi: 10.1016/0378-1119(95)00404-t. [DOI] [PubMed] [Google Scholar]
- 17.Evans D J, Evans D G, Engstrand L, Graham D. Urease-associated heat shock protein of Helicobacter pylori. Infect Immun. 1992;60:2125–2127. doi: 10.1128/iai.60.5.2125-2127.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falk P, Roth K A, Borén T, Westblom T U, Grodon J I, Normark S. An in vitro adherence assay reveals that Helicobacter pylori exhibits cell lineage-specific tropism in the human gastric epithelium. Proc Natl Acad Sci USA. 1993;90:2035–2039. doi: 10.1073/pnas.90.5.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrero R L, Lee A. The importance of urease in acid protection for the gastric-colonising bacteria Helicobacter pylori and Helicobacter felis. Microb Ecol Health Dis. 1991;4:121. [Google Scholar]
- 20.Foltz K R, Partin J S, Lennarz W J. Sea urchin receptor for sperm: sequence similarity of binding domain and Hsp 70. Science. 1993;259:1421–1425. doi: 10.1126/science.8383878. [DOI] [PubMed] [Google Scholar]
- 21.Foster J W. Salmonella acid shock proteins are required for the adaptive acid tolerance response. J Bacteriol. 1991;173:6896–6902. doi: 10.1128/jb.173.21.6896-6902.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galley K A, Bhag S, Gupta R S. Cloning of HSP70 (dnaK) gene from Clostridium perfringens using a general polymerase chain reaction based approach. Biochim Biophys Acta. 1992;1130:203–208. doi: 10.1016/0167-4781(92)90529-9. [DOI] [PubMed] [Google Scholar]
- 23.Gamer J, Multhaup G, Tomoyasu T, McCarty J, Rudiger S, Schonfeld H, Schirra C, Bujard H, Bukau B. A cycle of binding and release of the DnaK, DnaJ and GrpE chaperones regulates activity of the E. coli heat shock transcription factor ς32. EMBO J. 1996;5:607–617. [PMC free article] [PubMed] [Google Scholar]
- 24.Gold B D, Dytoc M, Huesca M, Philpott D, Kuksis A, Czinn S, Lingwood C A, Sherman P M. Comparison of Helicobacter mustelae and Helicobacter pylori adhesion to eukaryotic cells in vitro. Gastroenterology. 1995;109:692–700. doi: 10.1016/0016-5085(95)90375-5. [DOI] [PubMed] [Google Scholar]
- 25.Goodwin, A., D. Kersulyte, G. Sisson, S. van Zanten, D. Berg, and P. Hoffman. Metronidazole-resistance in Helicobacter pylori is due to null mutations in a gene (rdxA) that encodes an oxygen insensitive NADPH nitroreductase. Mol. Microbiol., in press. [DOI] [PubMed]
- 26.Hall H K, Karem K L, Foster J W. Molecular responses of microbes to environmental pH stress. Adv Microb Physiol. 1995;37:229–272. doi: 10.1016/s0065-2911(08)60147-2. [DOI] [PubMed] [Google Scholar]
- 27.Hall P, Coates P, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107:3569–3577. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- 28.Hartmann E, Lingwood C A. Brief heat shock induces a long-lasting alteration in the glycolipid receptor binding specificity of Haemophilus influenzae. Infect Immun. 1997;65:1729–1733. doi: 10.1128/iai.65.5.1729-1733.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hazell S L, Lee A, Brady L, Hennessy W. Campylobacter pyloridis and gastritis: association with intercellular spaces and adaptation to an environment of mucus as important factors in colonization of the gastric epithelium. J Infect Dis. 1986;153:658–663. doi: 10.1093/infdis/153.4.658. [DOI] [PubMed] [Google Scholar]
- 30.Heyde M, Portalier R. Acid shock proteins of Escherichia coli. FEMS Microbiol Lett. 1990;57:19–26. doi: 10.1016/0378-1097(90)90406-g. [DOI] [PubMed] [Google Scholar]
- 31.Hoffman P S, Ripley M, Weeratna R. Cloning and nucleotide sequence of a gene (ompS) coding the major outer membrane porin protein of Legionella pneumophila. J Bacteriol. 1992;174:914–920. doi: 10.1128/jb.174.3.914-920.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31a.Huesca, M. Unpublished data.
- 32.Huesca M, Borgia S, Hoffman P, Lingwood C A. Acidic pH changes receptor binding of Helicobacter pylori: a binary adhesion model in which surface heat-shock (stress) proteins mediate sulfatide recognition in gastric colonization. Infect Immun. 1996;64:2643–2648. doi: 10.1128/iai.64.7.2643-2648.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ilver D, Arnqvist A, Ogren J, Frick I-M, Kersulyte D, Incecik E T, Covacci A, Engstrand L, Borén T. Helicobacter pylori adhesin binding fucosylated hosto-blood group antigens revealed by retagging. Science. 1998;279:373–377. doi: 10.1126/science.279.5349.373. [DOI] [PubMed] [Google Scholar]
- 34.Itoh H, Tashima Y. The stress (heat shock) proteins. Int J Biochem. 1991;23:1185–1191. doi: 10.1016/0020-711x(91)90214-8. [DOI] [PubMed] [Google Scholar]
- 35.Jiang Q, Hiratsuka K, Taylor D E. Variability of gene order in different Helicobacter pylori strains contributes to genome diversity. Mol Microbiol. 1996;20:833–842. doi: 10.1111/j.1365-2958.1996.tb02521.x. [DOI] [PubMed] [Google Scholar]
- 36.Jones N L, Shannon P T, Cutz E, Yeger H, Sherman P M. Increase in proliferation and apoptosis of gastric epithelial cells early in the natural history of Helicobacter pylori infection. Am J Pathol. 1997;151:1695–1703. [PMC free article] [PubMed] [Google Scholar]
- 37.Kamisago S, Iwamori M, Tai T, Mitamura K, Yazaki Y, Sugano K. Role of sulfatides in adhesion of Helicobacter pylori to gastric cancer cells. Infect Immun. 1996;64:624–628. doi: 10.1128/iai.64.2.624-628.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karita M, Tummuru M K R, Wirth H-P, Blaser M J. Effect of growth phase and acid shock on Helicobacter pylori cagA expression. Infect Immun. 1996;64:4501–4507. doi: 10.1128/iai.64.11.4501-4507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karttunen R, Kartuuunen T, Ekre H P T, MacDonald T T. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut. 1995;64:2643–2648. doi: 10.1136/gut.36.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krivan H, Nilsson B, Lingwood C A, Ryu H. Chlamydia trachomatis and Chlamydia pneumoniae bind specifically to phosphatidylethanolamine in HeLa cells and to GalNacβ1-4Galβ1-4Glc sequences found in asialo-GM1 and asialo-GM2. Biochem Biophys Res Commun. 1991;175:1082–1089. doi: 10.1016/0006-291x(91)91676-4. [DOI] [PubMed] [Google Scholar]
- 41.Krivan H C, Ginsburg V, Roberts D D. Pseudomonas aeruginosa and Pseudomonas cepacia isolated from cystic fibrosis patients bind specifically to gangliotetraosylceramide (asialo GM1) and gangliotriaosylceramide (asialo GM2) Arch Biochem Biophys. 1988;260:493–496. doi: 10.1016/0003-9861(88)90473-0. [DOI] [PubMed] [Google Scholar]
- 42.Krivan H C, Roberts D D, Ginsburg V. Many pulmonary pathogenic bacteria bind specifically to the carbohydrate sequence GalNAcβ1-4 Gal found in some glycolipids. Proc Natl Acad Sci USA. 1988;85:6157–6161. doi: 10.1073/pnas.85.16.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Labigne A, Cussac V, Courcoux P. Shuttle cloning and nucleotide sequences of Helicobacter pylori genes responsible for urease activity. J Bacteriol. 1991;173:1920–1931. doi: 10.1128/jb.173.6.1920-1931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laemmeli U K. Cleavage of structural proteins during the assembly of head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 45.Lee K, Sheth H, Wong W, Serburne R, Paranchych W, Hodges R, C. L, Krivan H, Irvin R. The binding of Pseudomonas aeruginosa pili to glycosphingolipids is a tip associated event involving the C-terminal region of the structural pilin subunit. Mol Microbiol. 1994;11:705–713. doi: 10.1111/j.1365-2958.1994.tb00348.x. [DOI] [PubMed] [Google Scholar]
- 46.Lee K K, Yu L, Macdonald D L, Paranchych W, Hodges R S, Irvin R T. Anti-adhesin antibodies that recognize a receptor-binding motif (adhesintope) inhibit pilus/fimbrial-mediated adherence of Pseudomonas aeruginosa and Candida albicans to asialo-GM1 receptors and human buccal epithelial cell surface receptors. Can J Microbiol. 1996;42:479–486. doi: 10.1139/m96-065. [DOI] [PubMed] [Google Scholar]
- 47.Leying H, Suebaum S, Geis G, Haas R. Characterization of flaA, a Helicobacter pylori flagellin gene. Mol Microbiol. 1992;6:2863–2874. doi: 10.1111/j.1365-2958.1992.tb01466.x. [DOI] [PubMed] [Google Scholar]
- 48.Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- 49.Lingwood C A. H. pylori adhesins and receptors. In: Goodwin S, W. B, editors. Helicobacter pylori: biology and clinical practice. Boca Raton, Fla: CRC Press; 1993. pp. 209–222. [Google Scholar]
- 50.Lingwood C A, Huesca M, Kuksis A. The glycerolipid receptor for Helicobacter pylori (and exoenzyme S) is phosphatidylethanolamine. Infect Immun. 1992;60:2470–2474. doi: 10.1128/iai.60.6.2470-2474.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lingwood C A, Law H, Pellizzari A, Sherman P, Drumm B. A gastric glycerolipid as a receptor for Campylobacter pylori. Lancet. 1989;ii:238–241. doi: 10.1016/s0140-6736(89)90428-5. [DOI] [PubMed] [Google Scholar]
- 52.Lingwood C A, Wasfy G, Han H, Huesca M. Receptor affinity purification of a lipid-binding adhesin from Helicobacter pylori. Infect Immun. 1993;61:2474–2478. doi: 10.1128/iai.61.6.2474-2478.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maniatis T, Fritsch E, Sambrook J. Molecular: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 54.Marck C. “DNA Strider”: a ‘C’ program for fast analysis of DNA and protein sequences on the Apple Macintosh family of computers. Nucleic Acids Res. 1988;173:1829–1836. doi: 10.1093/nar/16.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McGowan C C, Cover T L, Blaser M. Helicobacter pylori and gastric acid: biological and therapeutic implications. Gastroenterology. 1996;110:926–938. doi: 10.1053/gast.1996.v110.pm8608904. [DOI] [PubMed] [Google Scholar]
- 56.Mead D A, Pey N K, Herrnstadt C, Marcil R A, Smith L M. A universal method for the direct cloning of PCR amplified nucleic acid. Bio/Technology. 1991;9:657–663. doi: 10.1038/nbt0791-657. [DOI] [PubMed] [Google Scholar]
- 57.Miller-Podraza H, Milh M A, Bergström J, Karlsson K-A. Recognition of glycoconjugates by Helicobacter pylori: an apparently high-affinity binding of human polyglycosylceramides, a second sialic acid-based specificity. Glycoconj J. 1996;13:453–460. doi: 10.1007/BF00731478. [DOI] [PubMed] [Google Scholar]
- 58.Multhoff G, Botzler C, Jenner L, Schmidt J, Ellwart J, Issels R. Heat shock protein 72 on tumor cells. A recognition structure for natural killer cells. J Immunol. 1997;158:4341–4350. [PubMed] [Google Scholar]
- 59.Paruchuri D K, Seifert H S, Ajioka R S, Karlsson K-A. Identification and characterization of a Neisseria gonorrhoeae gene encoding a glycolipid-binding adhesin. Proc Natl Acad Sci USA. 1990;87:333–337. doi: 10.1073/pnas.87.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ratnakar P, Rao S, Catanzaro A. Isolation and characterization of a 70 kDa protein from Mycobacterium avium. Microb Pathog. 1996;21:471–486. doi: 10.1006/mpat.1996.0077. [DOI] [PubMed] [Google Scholar]
- 61.Raulston J E, Davis C H, Schmiel D H, Morgan M W, Wyrick P B. Molecular characterization and outer membrane association of a Chlamydia trachomatis protein related to the hsp70 family of proteins. J Biol Chem. 1993;268:23139–23147. [PubMed] [Google Scholar]
- 62.Retzlaff C, Yamamoto Y, Okubo S, Hoffman P S, Friedman H, Klein T W. Legionella pneumophila heat shock protein induced increase of interleukin 1β mRNA involves protein kinase C signalling in macrophages. Immunology. 1996;156:1196–1206. doi: 10.1046/j.1365-2567.1996.d01-735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosenberg M, ••• C D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- 64.Saitoh T, Natomi H, Zhao W, Okuzumi K, Sugano K, Iwamori M, Nagai Y. Identification of glycolipid receptors for Helicobacter pylori by TLC-immunostaining. FEBS Lett. 1991;282:385–387. doi: 10.1016/0014-5793(91)80519-9. [DOI] [PubMed] [Google Scholar]
- 65.Saitoh T, Sugano K, Natomi H, Zhao W, Okuzumi K, Iwamori M, Yazaki Y. Glycosphingolipid receptors in human gastric mucosa for Helicobacter pylori. Eur J Gastroenterol Hepatol. 1992;4:S49–S53. [Google Scholar]
- 66.Sambrook J, Fritsch E, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 67.Sanger F, Nicklen S, Coulson A. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5468. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmitt W, Haas R. Genetic analysis of the Helicobacter pylori vacuolating cytotoxin: structural similarities with IgA protease type of exported protein. Mol Microbiol. 1994;12:307–319. doi: 10.1111/j.1365-2958.1994.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 69.Skeen M J, Miller M A, Shinnick T M, Ziegler H K. Regulation of murine macrophage IL-12 production: activation of macrophages in vivo, restimulation in vitro, and modulation by other cytokines. J Immunol. 1996;156:1196–1206. [PubMed] [Google Scholar]
- 70.Suerbaum S, Thiberge J-M, Kansau I, Ferrero R L, Labigne A. Helicobacter pylori hspA-hspB heat-shock gene cluster: nucleotide sequence, expression, putative function and immunogenicity. Mol Microbiol. 1994;14:959–974. doi: 10.1111/j.1365-2958.1994.tb01331.x. [DOI] [PubMed] [Google Scholar]
- 71.Tomb J-F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, McKenney K, Fitzegerald L M, Lee N, Adams M D, Hickey E K, Berg D E, Gocayne J D, Utterback T R, Peterson J D, Kelley J M, Cotton M D, Weidman J M, Fujii C, Bowman C, Watthey L, Wallin E, Hayes W S, Borodovsky M, Karp P D, Smith H O, Fraser C M, Venter J C. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 72.Vanet A, Labigne A. Evidence for specific secretion rather than autolysis in the release of some Helicobacter pylori proteins. Infect Immun. 1998;66:1023–1027. doi: 10.1128/iai.66.3.1023-1027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Watson K. Microbial stress proteins. Adv Microb Physiol. 1990;31:255–258. doi: 10.1016/s0065-2911(08)60122-8. [DOI] [PubMed] [Google Scholar]
- 74.Yamaguchi H, Osaki T, Kurihar N, Taguchi H, Hanawa T, Yamamoto Y, Kamiya S. Heat-shock protein 60 homologue of Helicobacter pylori is associated with adhesin of H. pylori to human gastric epithelial cells. J Med Microbiol. 1997;46:825–831. doi: 10.1099/00222615-46-10-825. [DOI] [PubMed] [Google Scholar]
- 75.Yamaguchi H, Osaki T, Taguchi H, Hanawa T, Yamoto T, Kamiya S. Flow cytometric analysis of the heat shock protein 60 expressed on the cell surface of Helicobacter pylori. J Med Microbiol. 1996;45:270–277. doi: 10.1099/00222615-45-4-270. [DOI] [PubMed] [Google Scholar]
- 76.Yokota K, Hira Y, Haque M, Hayashi S, Isogai H, Sugiyama T, Nagamachi E, Tsukada Y, Fujii N, Oguma K. Heat shock protein produced by Helicobacter pylori. Microbiol Immunol. 1994;38:403–405. doi: 10.1111/j.1348-0421.1994.tb01799.x. [DOI] [PubMed] [Google Scholar]
- 77.Zilberstein D. The role of pH and temperature in the development of Leishmania parasites. Annu Rev Microbiol. 1994;48:449–470. doi: 10.1146/annurev.mi.48.100194.002313. [DOI] [PubMed] [Google Scholar]