Abstract
Reactive nitrogen intermediates were synthesized spontaneously in cultures of macrophages from Trypanosoma brucei brucei-infected mice by an inducible nitric oxide (NO) synthase. This was inhibited by the addition of nitro-l-arginine. In this paper, we report the kinetics of the fixation of macrophage-derived NO on bovine serum albumin by using an enzyme-linked immunosorbent assay. S nitrosylation was confirmed by the Saville reaction, using mercuric chloride. It is known that reactive oxygen intermediates (ROI) are also synthesized by stimulated macrophages. The fact that NO is able to bind cysteine only under aerobic conditions led us to investigate the role of macrophage-derived ROI in the formation of S-nitrosylated proteins by activated macrophages. The immunoenzymatic signal decreased by 66 and 30% when superoxide dismutase and catalase, respectively, were added to the culture medium of macrophages from infected mice. In addition, the decrease in S-nitrosylated albumin formation correlated with the protection of extracellular trypanosomes from the cytostatic and cytotoxic activity of NO. Melatonin, a hydroxyl radical scavenger resulting from the decomposition of peroxynitrous acid, had no effect. All these data support the concept that an interaction between NO and ROI promoted the production of S-nitroso-albumin by activated macrophages from infected mice.
Nitric oxide (NO) is an important bioregulatory mediator and possesses many physiological functions. NO and reactive nitrogen intermediates (RNI) are implicated in macrophage-derived cytostasis/cytotoxicity against tumor cells (15) and various intracellular and extracellular pathogens (1, 21, 22, 54) but also in the mechanisms of immunosuppression (45). NO circulates in plasma as S-nitrosothiols (16), mainly S-nitroso-albumin (49), and previous studies have elucidated the role of these intermediates in the long-distance effects of NO. Endothelium-derived relaxing-factor activities (19, 25, 34), inhibition of platelet functions (51), apoptosis (33), and anti-parasite activities of NO (36) are mediated through nitrosylated albumin. However, the mechanism of the in vivo formation of S-nitrosothiols remains unclear. It has been established that, under anaerobic conditions, NO does not react with cysteine, glutathione (26, 58), or serum albumin (6, 26). Therefore, RNI species seem to be needed for the nitrosylation process.
Macrophages from Trypanosoma brucei brucei-infected mice produce high levels of NO (30). In addition, reactive oxygen intermediates (ROI), such as superoxide anion (O2−) and O2−-derived hydrogen peroxide (H2O2), are synthesized as a result of the oxidative burst (47) by macrophages from T. b. brucei-infected mice (12) or macrophages exposed to opsonized T. b. brucei (55). Since NO reacts with ROI (50), we investigated the interaction of the l-arginine→NO metabolism with the NADPH oxidase pathway leading to S nitrosylation of bovine serum albumin (BSA), resulting in the death of extracellular parasites.
MATERIALS AND METHODS
Mice.
Female Swiss mice (8 to 12 weeks old) were purchased from Iffa Credo (Saint-Germain-sur-l’Arbresle, France).
Parasites.
The Antat 1.1.E. clone of T. b. brucei (Institute of Tropical Medicine, Antwerp, Belgium) was used in all the experiments. Parasites (5 × 103 per mice) were injected intraperitoneally, and trypanosomes were purified from the blood of infected mice by chromatography on a DEAE-cellulose column, as previously described (28).
Cells.
Peritoneal cells from control mice or from 10-day-infected mice were collected after intraperitoneal injection of Dulbecco’s modified Eagle’s medium (DMEM; BioWhittaker, Verviers, Belgium) supplemented with HEPES (20 mM), l-glutamine (2 mM), sodium pyruvate (2 mM), and gentamicin (10 μg/ml). Macrophages were purified by washing nonadherent cells after a 1-h incubation and cultured at 37°C in a 5% CO2-enriched atmosphere.
Cocultures of macrophages and trypanosomes.
Macrophages (106 per ml) from control or T. b. brucei-infected mice were cocultured with T. b. brucei (105 per ml) in supplemented Dulbecco modified Eagle medium containing BSA (4 mg/ml) in 24-well plates (Nunc Inc., Naperville, Ill.). Superoxide dismutase (SOD) (100 U/ml; Sigma Chemical Co., St. Louis, Mo.), catalase (100 U/ml; Sigma), melatonin (1 or 10 μg/ml), and nitro-l-arginine (l-NA) (1 mM; Sigma) were added when required. Parasites were counted daily.
Enzyme-linked immunosorbent assay (ELISA).
Macrophages (106 per ml) were plated in 24-well plates. Cells were incubated in Hanks balanced salt solution without calcium, magnesium, or phenol red (Life Technologies, Paisley, Scotland) but supplemented with BSA (4 mg/ml), l-arginine (1 mM), l-NA (1 mM), SOD (100 U/ml), and/or catalase (100 U/ml). Enzymes heated at 70°C for 1 h were used as controls. Supernatants were collected at the indicated times and coated in polystyrene well plates (Maxisorb; Nunc). Anti-NO acetylated cysteine antibody (Ab) (1/1,000) (36) or anti-nitrotyrosine Ab (1/100 to 1/1,000) (Transduction Laboratories, Lexington, Ky.) and horseradish peroxidase-labeled goat anti-rabbit immunoglobulin (Diagnostic Pasteur, Paris, France) were used. o-Phenylenediamine was used as the chromogen. The optical density was measured at 492 nm. Mercuric chloride (HgCl2; 1 mM) was used in supernatants before coating to confirm the S nitrosylation (43).
Assay for H2O2 release.
H2O2 release was induced by using phorbol 12-myristate 13-acetate (PMA) in cultures of macrophages (5 × 105 macrophages per well) from control or T. b. brucei-infected mice. The concentration of H2O2 in each cell supernatant was measured by spectrofluorimetry in the presence of scopoletin and peroxidase (41, 53).
Measurement of nitrite production.
In each cell culture supernatant, the concentration of nitrite (NO2−), the stable oxidized derivative of NO (18), was determined spectrophotometrically at 540 nm after reaction with the Griess reagent as previously described (14).
RESULTS
Kinetics of the S nitrosylation of BSA by NO from macrophages of infected mice.
Nitrite accumulation was detected by the Griess reaction, in culture supernatants of macrophages from T. b. brucei-infected mice (Table 1). After a 6-h incubation, the presence of S-nitroso-BSA in these supernatants was revealed by ELISA. A decrease in NO production and an inhibition of protein nitrosylation were induced by adding l-NA to cultures. The addition of HgCl2 abolished the immunoenzyme signal and confirmed the S nitrosylation of BSA. When supernatants from control macrophages were used with or without l-NA or HgCl2, nitrite and S-nitroso-BSA were not detected. In addition, nitrotyrosine was not detected by ELISA in activated or control macrophage supernatants at the indicated times (data not shown).
TABLE 1.
NO2− production and S-nitroso-BSA formation in supernatants of murine macrophages after a 6-h incubation
| Source of macrophages | Supplemen- tation with:
|
Concn of NO2− (μM)a | OD492 of S-nitrosoBSAb | |
|---|---|---|---|---|
| l-NA | HgCl2 | |||
| T. b. brucei-infected mice | − | − | 13 ± 3 | 0.32 ± 0.03 |
| + | − | 2 ± 1 | 0.07 ± 0.02 | |
| − | + | 17 ± 5 | 0.09 ± 0.03 | |
| Control mice | − | − | 2 ± 1 | 0.06 ± 0.01 |
| + | − | 2 ± 1 | 0.06 ± 0.02 | |
Mean ± standard deviation for at least three experiments performed in duplicate.
Mean ± standard error of the mean for at least three experiments performed in duplicate. OD492, optical density at 492 nm.
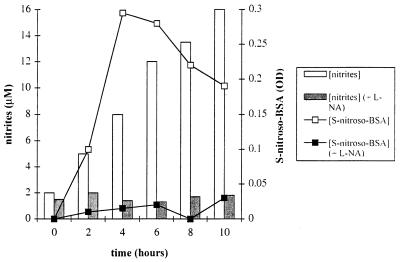
We performed a kinetic study of S-nitroso-BSA formation to investigate the mechanism of S nitrosylation. Figure 1 shows that the formation of S-nitroso-BSA increased during the first 4 h. Then, in spite of continuous production of NO by activated macrophages, the concentration of S-nitroso-BSA gradually decreased after 4 h but was still significant after 10 h.
FIG. 1.
Representative data of the kinetics of NO2− and S-nitroso-BSA production by macrophages from T. b. brucei-infected mice. Activated macrophages were cultured with or without l-NA. Supernatants were collected at the indicated times. NO2− and S-nitroso-BSA concentrations were determined for each sample.
Effect of oxygen-derived species on S-nitroso-BSA formation.
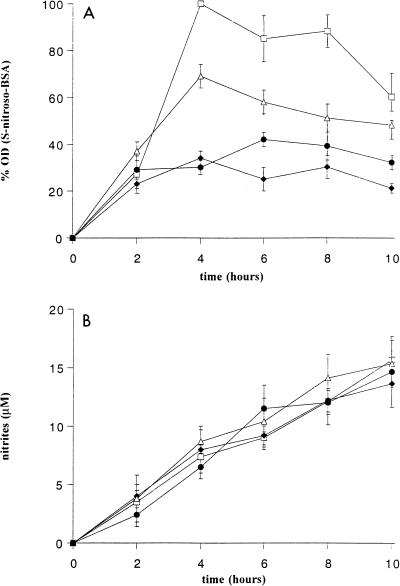
After a 4-h incubation, the concentration of S-nitroso-BSA decreased by 66 and 21% in SOD- and catalase-treated macrophage cultures from infected mice, respectively (Fig. 2A), whereas nitrite production was unaffected (Fig. 2B). The two enzymes used together had no synergistic effect with respect to SOD or catalase alone. Heated enzymes, used as controls, had no effect on BSA nitrosylation. Supernatants from control macrophage cultures treated with SOD and/or catalase, with or without l-NA, did not contain S-nitroso-BSA.
FIG. 2.
Effect of macrophage-derived ROI on S nitrosylation. S-Nitroso-BSA (A) and NO2− (B) concentrations were determined in activated macrophage supernatants incubated in Hanks balanced salt solution plus BSA (4 mg/ml) alone (□) or supplemented with SOD (100 U/ml) (⧫), catalase (100 U/ml) (▵), or SOD plus catalase (•). Each point represents the mean ± standard error of the mean of three experiments, each performed on two mice. OD, optical density.
H2O2 release by macrophages.
The production of H2O2 was observed in the first 4 h of culture when adherent macrophages from infected mice were stimulated with PMA (1.1 ± 0.22 nmol/106 cells/5 min). When these macrophages were cultured for 10 h, a dramatic decrease in H2O2 release was observed (0.14 ± 0.07 nmol/106 cells/5 min). Supernatants from control macrophages or macrophages of T. b. brucei-infected mice not treated with PMA did not contain H2O2 (0.04 ± 0.02 nmol/106 cells/5 min).
Effect of SOD and catalase on trypanosome survival.
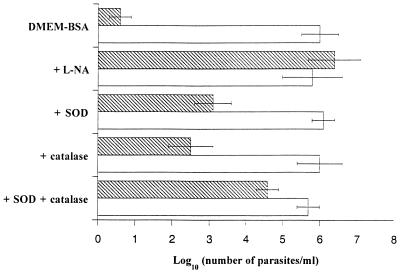
Previous studies had shown the involvement of nitrosylated albumin in trypanostatic and trypanocidal activity. Observation of the inhibition of S-nitroso-BSA formation in the presence of SOD and catalase led us to test the effect of these two enzymes on trypanosome survival. Parasites were cocultured with macrophages from infected mice. Two days later, a parasite count revealed that the trypanocidal activity of NO was partially inhibited by SOD and catalase (Fig. 3). These two enzymes used together acted more efficiently to protect trypanosomes. Nevertheless, SOD and catalase were not as efficient as l-NA in inhibiting the antiparasite effect. l-NA, SOD, and catalase had no effect on trypanosomes cocultured with control macrophages or with activated macrophages in a BSA-free medium (data not shown).
FIG. 3.
Protective effect of SOD and/or catalase on T. b. brucei survival. Trypanosomes were cocultured in DMEM plus BSA (4 mg/ml) with control (open bars) or activated (hatched bars) macrophages. l-NA (1 mM), SOD (100 U/ml), and/or catalase (100 U/ml) was added to the culture. A parasite count was performed after 48 h of incubation, and the results represent the mean ± standard error of the mean of four experiments.
Role of melatonin in the protection of trypanosomes.
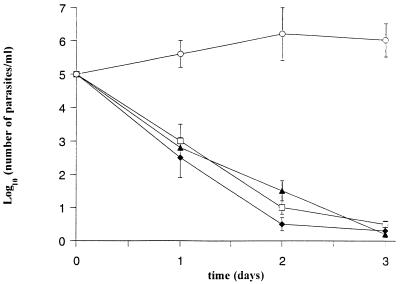
Melatonin, a specific hydroxyl radical (OH) scavenger (32), was used in cocultures of macrophages and trypanosomes. As shown in Fig. 4, trypanosome lysis was not compromised by the addition of melatonin (1 or 10 μg/ml) to supernatants of stimulated macrophages. Melatonin added to control macrophages had no effect on trypanosome growth. These data indicate either that stimulated macrophages did not produce OH or that cell-derived OH in culture were not involved in the cytotostatic and cytotoxic effect of macrophages on extracellular trypanosomes.
FIG. 4.
Trypanosome killing by activated macrophages and the effect of an OH scavenger. Trypanosomes were cocultured with activated macrophages in DMEM plus BSA alone (□) or supplemented with melatonin at 1 μg/ml (⧫) or 10 μg/ml (▴) or with l-NA (○). A parasite count was performed daily, and the results represent the mean ± standard deviation of two experiments.
DISCUSSION
Our results demonstrate that (i) biosynthesis of S-nitroso-BSA in macrophage supernatants is a rapid and transitory mechanism and (ii) interaction between NO and ROI from activated macrophages is involved in the synthesis of S-nitroso-BSA, which has antiparasite effects.
Previous studies have shown that NO is transported to extracellular targets by proteins such as albumin (46) or hemoglobin (11, 23). These intermediates may release NO to other thiol-containing species by transnitrosylation (25, 39, 44). The first part of our results confirmed that NO products of macrophages from T. b. brucei-infected mice yield S-nitroso-BSA under physiological conditions, in agreement with the spectrophotometric findings of Simon et al. (48). However, the mechanism of NO binding to thiols remains unclear. Biochemical investigations have demonstrated that NO cannot react directly with thiols. S nitrosylation is more probably due to RNI, the reactive products of NO and O2 or ROI (50, 57). However, all these data were obtained in cell-free experiments.
Our kinetic study showed that the S-nitroso-BSA level in culture supernatants reached its maximum after 6 h of incubation and then decreased. Then, although NO2− accumulated continuously, the S-nitroso-BSA concentration gradually decreased. Consequently, we have concluded that NO is not a limiting factor in the S-nitrosylation process. We have also shown that the in vitro production of H2O2 by macrophages of parasitized mice was a transitory phenomenon. This decrease in ROI generation was probably due to the inhibition of NADPH oxidase by an NO- and/or R-S-NO-dependent mechanism. Several studies have shown that NO inactivates NADPH oxidase and consequently impairs ROI production in phagocytes (5, 9, 38). In murine microglial cell cultures, the gamma interferon-mediated suppression of the oxidative burst was reversed by an NO synthase inhibitor. The addition of S-nitroso-N-acetylpenicillamine, an NO-releasing compound, caused a gradual reduction in the oxidative burst during the first few hours in culture (27). The NADPH oxidase is composed of membrane-bound proteins, including cytochrome b558, that may be gradually altered through the effects of RNI (4, 40). This time dependence of ROI led us to speculate that the NADPH oxidase metabolism pathway was involved in S-nitroso-BSA biosynthesis. Indeed, when SOD or SOD plus catalase was used in cultures of macrophages from infected mice, the formation of S-nitrosylated albumin was significantly inhibited, but catalase alone had less effect than did SOD or SOD plus catalase. This suggests that macrophage-derived ROI, and more particularly O2−, are involved in the formation of S-nitroso-BSA. The question raised in these experiments is how O2− and/or H2O2 interact with NO to favor S nitrosylation.
Peroxynitrite (ONOO−) is produced by the reaction of NO with O2−. Its formation in an extracellular environment leads to protein oxidation and to a decrease in the yield of nitrosothiols (56), but it is not involved in S nitrosylation (58). However, our results indicate that NO and O2− are both involved in the formation of S-nitroso-BSA. In addition, an immunoenzymatic assay was unable to detect nitrotyrosine, an ONOO− marker (20), in stimulated macrophage supernatants. The protonated form of ONOO−, peroxynitrous acid (ONOOH), may be indirectly implicated in the S-nitrosylation mechanism since NO2 is produced by the decomposition of ONOOH (ONOOH → OH + NO2). This radical may also be formed by oxidation of NO by H2O2 (H2O2 + NO → NO2 + H2O). NO2 reacts with NO to form nitrous anhydride (N2O3), which decomposes rapidly in aqueous solutions (N2O3 + H2O → 2 NO2− + 2 H+). The molecule N2O3 has been shown to be a good nitrosylating agent of intracellular thiol molecules (26). All these data led us to hypothesize that S-nitrosylated compounds, such as S-nitroso-glutathione, formed in the intracellular medium by an ROI-dependent mechanism could transfer NO to extracellular BSA. However, RNI, resulting from the interaction between ROI and NO may also be released by activated macrophages in the extracellular medium and could be involved in the S nitrosylation.
The effect of ROI on the killing of extracellular trypanosomes was also investigated. ROI may act directly or indirectly, through RNI or nitrosylated albumin, on trypanosome development. Although macrophage-derived ROI mediate the killing of intracellular parasites (17). SOD or catalase had no significant effect on the growth of trypanosomes cocultured with activated macrophages in BSA-free medium. In a medium with BSA, the addition of SOD and/or catalase to macrophage cultures led to a decrease in S-nitroso-BSA formation and an inhibition of the trypanostatic effect of NO. Catalase alone has a similar protective effect on the growth of trypanosomes to that of SOD, although the inhibition of S-nitroso-BSA synthesis by SOD was more marked than that of catalase. Recently, it was shown that NO and R-S-NO cooperate with H2O2 to favor cell lysis (8, 31). Thus, one hypothesis would be that the cytotoxic activity of H2O2 on trypanosomes is potentiated by the presence of S-nitroso-BSA. We also hypothesized that the strong oxidizing species, OH, resulting from the decomposition of HONOO, may also act as an extracellular trypanostatic/trypanocidal agent. However, melatonin, an OH scavenger, had no protective activity. It is thus likely that the main trypanostatic effect is due to S-nitroso-BSA rather than ROI. The fact that interaction between NO and ROI was needed to kill different pathogens was also established (29, 35). We propose that this interaction acts through the formation of S-nitroso-BSA.
Although ONOO− has been proposed as a cytotoxic factor against various pathogens (42, 52), the killing of Gardia trophozoites was not shown to be affected by this anion (7). In addition, immunocytochemical testing was unable to detect nitrotyrosine on the surface of trypanosomes after a 24-h incubation with activated macrophages (data not shown). The trypanostatic and trypanocidal effects may thus be explained by a transnitrosylation mechanism rather than ONOO− activity, since R-S-NO was found, in vitro, by using anti-NO-acetylated cysteine Ab on the surface of trypanosomes cocultured with activated macrophages (unpublished data). However, it was not possible to distinguish between S-nitroso-BSA bound on the parasites and S-nitroso proteins of trypanosomes. The cytostatic and cytotoxic activity of NO was probably linked to the release of NO from S-nitroso-BSA on other thiol-containing proteins or Fe-S clusters. The antiproliferative activity of NO is usually attributed to its effect on numerous enzymes, including protein kinase C (10), ribonucleotide reductase (13), or glyceraldehyde-3-phosphate dehydrogenase (37, 59). The modification of a single highly reactive cysteine led to a complete inhibition of phosphoenolpyruvate carboxykinase, a key enzyme in the energy metabolism of trypanosomes which lack functional mitochondria (24). Trypanothione reductase, which represents up to 13% of the total soluble proteins and is considered equivalent to glutathione reductase in mammals, has two cysteines which are essential for overall catalysis (2). Moreover, cysteine residues are conserved in variant surface glycoproteins and non-variant surface glycoprotein surface proteins of trypanosomes (3). All these data show the importance of cysteine integrity for trypanosomes. Furthermore, trypanosomes are lower eucaryotes, which constitute simpler but adequate models for studying the effects and targets of S nitrosylation in mammalian cells.
ACKNOWLEDGMENTS
This work was supported by grants from Le Conseil Regional d’Aquitaine and La Ligue Nationale Contre le Cancer (Comité Pyrénées Atlantique).
REFERENCES
- 1.Adams L B, Hibbs J B, Jr, Taintor R R, Krahenbuhl J L. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. J Immunol. 1990;144:2725–2729. [PubMed] [Google Scholar]
- 2.Borges A, Cunningham M L, Tovar J, Fairlamb H. Site-directed mutagenesis of the redox-active cysteines of Trypanosoma cruzi trypanothione reductase. Eur J Biochem. 1995;228:745–752. doi: 10.1111/j.1432-1033.1995.tb20319.x. [DOI] [PubMed] [Google Scholar]
- 3.Carrington M, Boothroyd J. Implications of conserved structural motifs in disparate trypanosome surface proteins. Mol Biochem Parasitol. 1996;81:119–126. doi: 10.1016/0166-6851(96)02706-5. [DOI] [PubMed] [Google Scholar]
- 4.Clancy R M, Leszczynska-Piziak J, Abramson S B. Nitric oxide, an endothelial cell relaxation factor, inhibits neutrophil superoxide anion production via a direct action on the NADPH oxidase. J Clin Invest. 1992;90:1116–1121. doi: 10.1172/JCI115929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clancy R M, Levartovsky D, Leszczynska-Piziak J, Yegudin J, Abramson S B. Nitric oxide reacts with intracellular glutathione and activates the hexone monophosphate shunt in human neutrophils: evidence for S-nitrosoglutathione as a bioctive intermediary. Proc Natl Acad Sci USA. 1994;91:3680–3684. doi: 10.1073/pnas.91.9.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeMaster E G, Quast B J, Redfern B, Nagasawa H T. Reaction of nitric oxide with the free sulfhydryl group of human serum albumin yields a sulfenic acid and nitrous oxide. Biochemistry. 1995;34:11494–11499. doi: 10.1021/bi00036a023. [DOI] [PubMed] [Google Scholar]
- 7.Fernandes P D, Assreuy J. Role of nitric oxide and superoxide in Giardia lamblia killing. Braz J Med Biol Res. 1997;30:93–99. doi: 10.1590/s0100-879x1997000100015. [DOI] [PubMed] [Google Scholar]
- 8.Filep J G, Lapierre C, Lachance S, Chan J S. Nitric oxide cooperates with hydrogen peroxide in inducing DNA fragmentation and cell lysis in murine lymphoma cells. Biochem J. 1997;321:897–901. doi: 10.1042/bj3210897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujii H, Ichimori K, Hoshiai K, Nakazawa H. Nitric oxide inactivates NADPH oxidase in pig neutrophils by inhibiting its assembling process. J Biol Chem. 1997;272:32773–32778. doi: 10.1074/jbc.272.52.32773. [DOI] [PubMed] [Google Scholar]
- 10.Gopalakrishna R, Chen Z H, Gundimeda U. Nitric oxide and nitric oxide-generating agents induce a reversible inactivation of protein kinase C activity and phorbol ester binding. J Biol Chem. 1993;36:27180–27185. [PubMed] [Google Scholar]
- 11.Gow A J, Stamler J S. Reactions between nitric oxide and haemoglobin under physiological conditions. Nature. 1998;391:169–173. doi: 10.1038/34402. [DOI] [PubMed] [Google Scholar]
- 12.Grosskinsky C M, Ezekowitz R A B, Berton G, Gordon S, Askonas B A. Macrophage activation in murine african trypanosomiasis. Infect Immun. 1983;39:1080–1086. doi: 10.1128/iai.39.3.1080-1086.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haskin C J, Ravi N, Lynch J B, Münck E, Que L., Jr Reaction of NO with the reduced R2 protein of ribonucleotide reductase from Escherichia coli. Biochemistry. 1995;34:11090–11098. doi: 10.1021/bi00035a014. [DOI] [PubMed] [Google Scholar]
- 14.Hageman R H, Reed A J. Nitrate reductase from higher plants. Methods Enzymol. 1980;40:427–429. [Google Scholar]
- 15.Hibbs J B, Vavrin Z, Taintor R R. l-Arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition of target cells. J Immunol. 1987;138:550–565. [PubMed] [Google Scholar]
- 16.Hilliquin P, Borderie D, Hernvann A, Menkes C J, Ekindjian O G. Nitric oxide as S-nitrosoproteins in rheumatoid arthritis. Arthritis Rheum. 1997;40:1512–1517. doi: 10.1002/art.1780400820. [DOI] [PubMed] [Google Scholar]
- 17.Hughes H P A. Oxidative killing of intracellular parasites mediated by macrophages. Immunol Today. 1988;4:340–347. doi: 10.1016/0169-4758(88)90003-8. [DOI] [PubMed] [Google Scholar]
- 18.Ignarro L J, Fukuto J M, Griscavage J M, Rogers N E, Byrns R E. Oxidation of nitric oxide in aqueous solution to nitrite but not nitrate: comparison with enzymatically formed nitric oxide from L-arginine. Proc Natl Acad Sci USA. 1993;90:8103–8107. doi: 10.1073/pnas.90.17.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ignarro L J, Lippton H, Edwards J C, Baricos W H, Hyman H L, Kadowitz P J, Gruetter C A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981;218:739–749. [PubMed] [Google Scholar]
- 20.Ischiropoulos H, al-Mehdi A B. Peroxynitrite-mediated oxidative protein modifications. FEBS Lett. 1995;364:279–282. doi: 10.1016/0014-5793(95)00307-u. [DOI] [PubMed] [Google Scholar]
- 21.James S L, Glaven J. Macrophage cytotoxicity against schistosomula of Schistosoma mansoni involves arginine-dependent production of reactive nitrogen intermediates. J Immunol. 1989;143:4208–4212. [PubMed] [Google Scholar]
- 22.James S L, Nacy C. Effector functions of activated macrophages against parasites. Curr Opin Immunol. 1995;5:518–523. doi: 10.1016/0952-7915(93)90032-n. [DOI] [PubMed] [Google Scholar]
- 23.Jia L, Bonaventura C, Bonaventura J, Stamler J S. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature. 1996;380:221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 24.Jurado L A, Machin I, Urbina J A. Trypanosoma cruzi phospho enol pyruvate carboxykinase (ATP-dependent):transition metal iron requirement for activity and sulfhydryl group reactivity. Biochim Biophys Acta. 1996;1292:188–196. doi: 10.1016/0167-4838(95)00201-4. [DOI] [PubMed] [Google Scholar]
- 25.Keaney J F, Simon D I, Stamler J S, Jaraki O, Scharfstein J, Vita J A, Loscalzo J. NO forms an adduct with serum albumin that has endothelium-derived relaxing factor-like properties. J Clin Invest. 1993;91:1582–1589. doi: 10.1172/JCI116364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kharitonov V G, Sundquist A R, Sharma V S. Kinetics of nitrosation of thiols by nitric oxide in presence of oxygen. J Biol Chem. 1995;270:28158–28164. doi: 10.1074/jbc.270.47.28158. [DOI] [PubMed] [Google Scholar]
- 27.Kiprianova I, Schwab S, Fandrey J, Spranger M. Suppression of the oxidative burst in murine microglia by nitric oxide. Neurosci Lett. 1997;226:75–78. doi: 10.1016/s0304-3940(97)00235-8. [DOI] [PubMed] [Google Scholar]
- 28.Lanham S M. Separation of trypanosomes from the blood of infected rats and mice by anion exchangers. Nature. 1968;218:1273–1274. doi: 10.1038/2181273a0. [DOI] [PubMed] [Google Scholar]
- 29.Lin J Y, Chadee K. Macrophage cytotoxicity against Entamoeba histolytica trophozoïtes is mediated by nitric oxide from l-arginine. J Immunol. 1992;148:3999–4005. [PubMed] [Google Scholar]
- 30.Mabbott N A, Sutherland I A, Sternberg J M. Suppressor macrophages in Trypanosoma brucei infection: nitric oxide is related to both suppressive activity and lifespan in vivo. Parasite Immunol. 1995;17:143–150. doi: 10.1111/j.1365-3024.1995.tb01016.x. [DOI] [PubMed] [Google Scholar]
- 31.Marcinkiewicz J. Nitric oxide and antimicrobial activity of reactive oxygen intermediates. Immunopharmacology. 1997;37:35–41. doi: 10.1016/s0162-3109(96)00168-3. [DOI] [PubMed] [Google Scholar]
- 32.Matuszac Z, Reszac K, Chignell C F. Reaction of melatonin and related indoles with hydroxyl radicals: EPR and spin trapping investigations. Free Radical Biol Med. 1997;23:367–372. doi: 10.1016/s0891-5849(96)00614-4. [DOI] [PubMed] [Google Scholar]
- 33.Melino G, Bernassola F, Knight R A, Corasaniti M T, Nistico G, Finazzi-Agro A. S-nitrosylation regulates apoptosis. Nature. 1997;388:432–433. doi: 10.1038/41237. [DOI] [PubMed] [Google Scholar]
- 34.Minamiyama Y, Takemura S, Inoue M. Albumin is an important vascular tonus regulator as a reservoir of nitric oxide. Biochem Biophys Res Commun. 1996;225:112–115. doi: 10.1006/bbrc.1996.1138. [DOI] [PubMed] [Google Scholar]
- 35.Miyagi K, Kawakami K, Saito A. Role of reactive nitrogen and oxygen intermediates in gamma interferon-stimulated murine macrophage bactericidal activity against Burkholderia pseudomallei. Infect Immun. 1997;65:4108–4113. doi: 10.1128/iai.65.10.4108-4113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mnaimneh S, Gefffard M, Veyret B, Vincendeau P. Albumin nitrosylated by activated macrophages possesses antiparasitic effects neutralized by anti-NO-acetylated-cysteine antibodies. J Immunol. 1997;158:308–314. [PubMed] [Google Scholar]
- 37.Mohr S, Stamler J S, Brune B. Posttranslational modification of glyceraldehyde-3-phosphate dehydrogenase by S-nitrosylation and subsequent NADH attachment. J Biol Chem. 1996;271:4209–4214. doi: 10.1074/jbc.271.8.4209. [DOI] [PubMed] [Google Scholar]
- 38.Nakata M, Nasuda-Kouyama A, Isogai Y, Kanegasaki S, Tizuka T. Effect of aromatic nitroso-compounds on superoxide-generating activity in neutrophils. J Biochem. 1997;122:188–192. doi: 10.1093/oxfordjournals.jbchem.a021727. [DOI] [PubMed] [Google Scholar]
- 39.Pietraforte D, Mallozi C, Scorza G, Minetti M. Role of thiols in the targeting of S-nitroso thiols to red blood cells. Biochemistry. 1995;34:7177–7185. doi: 10.1021/bi00021a032. [DOI] [PubMed] [Google Scholar]
- 40.Rodenas J, Mitjavila M T, Carbonell T. Nitric oxide inhibits superoxide production by inflammatory polymorphonuclear leukocytes. Am J Physiol. 1998;274:C827–C830. doi: 10.1152/ajpcell.1998.274.3.C827. [DOI] [PubMed] [Google Scholar]
- 41.Root R K, Metcalf J, Oshino N, Chance B. H2O2 release from human granulocytes during phagocytosis. Documentation, quantitation, and some regulating factors. J Clin Invest 1975. 1975;55:945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubbo H, Denicola A, Radi R. Peroxynitrite inactivates thiol-containing enzymes of Trypanosoma cruzi energetic metabolism and inhibits cell respiration. Arch Biochem Biophys. 1994;308:96–102. doi: 10.1006/abbi.1994.1014. [DOI] [PubMed] [Google Scholar]
- 43.Saville B. A scheme for the colorimetric determination of microgram amounts of thiols. Analyst. 1958;83:670–672. [Google Scholar]
- 44.Scharfstein J S, Keaney J F, Slivka A, Welch G N, Vita J A, Stamler J S, Loscalzo J. In vivo transfer of nitric oxide between a plasma protein-bound reservoir and low molecular weight thiols. J Clin Invest. 1994;94:1432–1439. doi: 10.1172/JCI117480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schleifer K W, Mansfield J M. Suppressor macrophages in African trypanosomiasis inhibit T cell proliferative responses by nitric oxide and prostaglandins. J Immunol. 1993;151:5492–5503. [PubMed] [Google Scholar]
- 46.Scorza G, Pietraforte D, Minetti M. Role of ascorbate and protein thiols in the release of nitric oxide from S-nitroso-albumin and S-nitroso-glutathione in human plasma. Free Radical Biol Med. 1997;22:633–642. doi: 10.1016/s0891-5849(96)00378-4. [DOI] [PubMed] [Google Scholar]
- 47.Segal A W, Abo A. The biochemical basis of the NADPH oxidase of phagocytes. Trends Biochem Sci. 1993;18:43–47. doi: 10.1016/0968-0004(93)90051-n. [DOI] [PubMed] [Google Scholar]
- 48.Simon D I, Mullins M E, Jia L, Gaston B, Singel D J, Stamler J S. Polynitrosylated proteins: characterization, bioactivity, and functional consequences. Proc Natl Acad Sci USA. 1996;93:4736–4741. doi: 10.1073/pnas.93.10.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stamler J S, Jaraki O, Osborne J A, Simon D I, Keaney J F, Vita J, Singel D, Valeri C R, Loscalzo J. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc Natl Acad Sci USA. 1992;89:7674–7677. doi: 10.1073/pnas.89.16.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stamler J S, Singel D J, Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258:1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- 51.Stamler J S, Simon D I, Osborne J A, Mullins M E, Jaraki O, Michel T, Singel D J, Loscalzo J. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc Natl Acad Sci USA. 1992;89:444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vazquez-Torres A, Jones-Carson J, Balish E. Peroxynitrite contributes to the candidacidal activity of nitric oxide-producing macrophages. Infect Immun. 1996;64:3127–3133. doi: 10.1128/iai.64.8.3127-3133.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vincendeau P, Caristan A, Pautrizel R. Macrophage function during Trypanosoma musculi infection in mice. Infect Immun. 1981;34:348–381. doi: 10.1128/iai.34.2.378-381.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vincendeau P, Daulouede S. Macrophages cytostatic effect on Trypanosoma musculi involves an l-arginine-dependent mechanism. J Immunol. 1991;146:4338–4343. [PubMed] [Google Scholar]
- 55.Vray B, de Baetselier P, Ouaissi A, Carlier Y. Trypanosoma cruzi but not Trypanosoma brucei fails to induce a chemiluminescent signal in a macrophage hybridoma cell line. Infect Immun. 1991;59:3303–3308. doi: 10.1128/iai.59.9.3303-3308.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wink D A, Cook J A, Kim S Y, Vodovotz Y, Pacelli R, Krishna M C, Russo A, Mitchell J B, Jourd’heuil D, Miles A M, Grishnam M B. Superoxide modulates the oxidation and nitrosation of thiols by nitric oxide-derived reactive intermediates. J Biol Chem. 1997;272:11147–11151. doi: 10.1074/jbc.272.17.11147. [DOI] [PubMed] [Google Scholar]
- 57.Wink D A, Hanbauer I, Grisham M B, Laval F, Nims R W, Laval J, Cook J, Pacelli R, Liebmann J, Krishna M, Ford P C, Mitchell J B. Chemical biology of nitric oxide: regulation and protective and toxic mechanisms. Curr Top Cell Regul. 1996;34:159–187. doi: 10.1016/s0070-2137(96)80006-9. [DOI] [PubMed] [Google Scholar]
- 58.Wink D A, Nims R W, Darbyshire J F, Christodoulou D, Hanbauer I, Cox G W, Laval F, Laval J, Cook J A, Krishna M C, DeGraff W G, Mitchell J B. Reaction kinetics for nitrosation of cysteine and glutathione in aerobic nitric oxide solutions at neutral pH. Insights into the fate and physiological effects of intermediates generated in the NO/O2 reaction. Chem Res Toxicol. 1994;7:519–525. doi: 10.1021/tx00040a007. [DOI] [PubMed] [Google Scholar]
- 59.Zhang J, Snyder S H. Nitric oxide stimulates auto-ADP-ribosylation of glyceraldehyde-3-phosphate dehydrogenase. Proc Natl Acad Sci USA. 1992;89:9382–9385. doi: 10.1073/pnas.89.20.9382. [DOI] [PMC free article] [PubMed] [Google Scholar]