Abstract
Epitope mapping of outer surface protein C (OspC) by using sera from patients with neuroborreliosis led to the identification of one single major immunodominant epitope within the C-terminal 10 amino acid residues. Peptide binding studies and alanine replacement scanning of the C-terminal decapeptide, PVVAESPKKP, revealed a critical role for the PKKP sequence and its terminal carboxyl group for the binding of immunoglobulin M (IgM) antibodies from patients with Lyme borreliosis. Electron microscopy of antibody-labeled spirochetes indicated that the C-terminal region is exposed on the surface of the spirochete. Based on homology to proteins of known function, this region most probably adopts a polyproline II-like helix, which is found in surface-exposed structures involved in protein-protein interactions. This structural motif is highly conserved in Borrelia species causing Lyme borreliosis and subjected to purifying selection. We suggest that the abundance of the C-terminal region of OspC on the surface of B. burgdorferi allows a multimeric high-avidity interaction between the spirochete and surface Igs on B cells. The resulting cross-linking of surface Igs on B cells may induce a T-cell-independent B-cell activation without IgM-to-IgG switching, thus explaining the lack of IgG antibodies to OspC in neuroborreliosis.
Outer surface protein C (OspC) of the Lyme borreliosis agents Borrelia burgdorferi sensu stricto, Borrelia garinii, and Borrelia afzelii is the major outer surface lipoprotein expressed during the early stages of disease and one of the primary targets for the human immune response against these spirochetes (47). The corresponding ospC gene is present in a single copy on a 26-kb circular plasmid (23, 35). Although the ospC gene is present in all B. burgdorferi sensu lato isolates, the corresponding gene product is produced in only 40 to 45% of European strains (41, 48) and very rarely in U.S. strains upon in vitro isolation. It is known that ospC expression is temperature dependent and may be induced by raising the temperature of the growth medium (36, 38, 47). Accordingly, OspC is not produced by spirochetes in unfed ticks (7) but expression is turned on during the blood meal (36) and in the vertebrate host (28). It has been proposed that OspC is involved in the migration of B. burgdorferi from the midgut of the tick to the salivary glands and also in the survival of B. burgdorferi in the vertebrate host (36).
OspC homologs have been found in other Borrelia species (24). Most notable is the similarity to the variable major lipoprotein, Vmp33, of Borrelia hermsii (2). It has been suggested that these OspC homologs are functionally related (2, 24, 25, 40, 41); however, no shared function has yet been proposed for this family of proteins.
Since OspC elicits an early immune response in the human host, several attempts have been made to develop diagnostic assays based on OspC (10, 11, 31, 44, 45) and to examine its vaccine potential (1, 12, 17, 32, 33). The very high variability encountered in OspC (18, 19, 22, 37, 40, 41) may, however, complicate the further use of this antigen. More knowledge is therefore needed about the epitopes in OspC before OspC can be used rationally in vaccine formulations or in diagnostic assays. In this study, we present the identification and characterization of a single major epitope in the conserved C-terminal region of OspC, defined by immunoglobulin M (IgM) antibodies in sera from patients with neuroborreliosis (NB). We provide data which indicate that the C-terminal region is surface exposed.
MATERIALS AND METHODS
Expression of recombinant OspC fragments.
For the structures of the various plasmids used in this study, see Fig. 1. All plasmid constructs were verified by DNA sequencing.
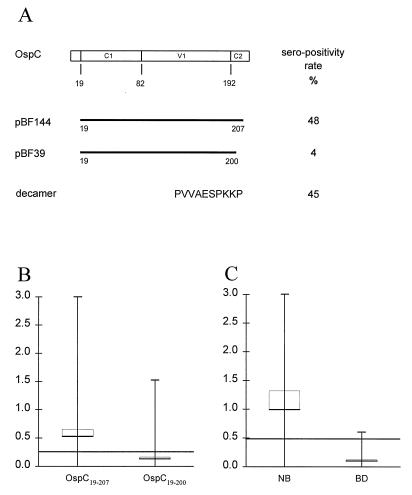
FIG. 1.
Identification of the major epitope of OspC. (A) A schematic representation of the two recombinant OspC proteins encoded by plasmids pBF144 and pBF39. Below is indicated the sequence (in single-letter code) of a synthetic peptide comprising the C-terminal 10 amino acids. The open box indicates the amino acid sequence of OspC from B. garinii DK6. C1 and C2 indicate relatively conserved regions, and V1 indicate the relatively variable region (22, 40). The NH2-terminal 19 amino acids constitute the signal peptide. Numbers below the box indicate amino acid residues. To the right is listed the percentage of sera from NB patients with a response greater than the 98% percentile for the 100 Danish blood donors. (B and C) Mean (horizontal bars) and range (vertical bars) of the IgM reactivity against OspC19–207 and OspC19–200 of the 100 serum samples from NB patients (B) and the anti-PVVAESPKKP IgM reactivity of sera from the 100 NB patients and from Danish blood donors (BD) (C); the thin brackets represent the 95% confidence interval. The 98% specific cutoff levels based on the examination of 100 Danish blood donors were optical densities of 0.230 for anti-OspC19–207 and anti-OspC19–200 IgM reactivity and 0.450 for anti-peptide IgM reactivity.
(i) pBF39.
The partial ospC gene lacking the codons encoding the C-terminal 7 amino acids was amplified from genomic DNA by using standard PCR conditions and the two primers BF22 (5′-ATA GAT ATC AAT AAT TCA GGT GGG GAT TC) (nucleotides 58 to 77, counting from the A of the ATG start codon) and BF23 (5′-TTT GAT ATC TCA CAC AAC AGG ATT TGT AAG CTC TTT AAC) (nucleotides 600 to 574), cut with EcoRV (underlined), and ligated into pMST24 digested with SmaI. This vector provides the OspC coding sequence with an in-frame start codon and a hexahistidyl tag (42). Recombinant protein OspC19–200 was purified by metal ion affinity chromatography on a Ni+ column (42).
(ii) pBF147.
The coding region for the full-length OspC protein was amplified from genomic DNA by using standard PCR conditions and the two primers BF22 and BF65 (5′-TTT GAT ATC TCA AGG TTT TTT TGG ACT TTC TGC) (nucleotides 621 to 601). The amplified gene was cut with EcoRV (underlined) and subsequently cloned into pMST24 digested with SmaI. Recombinant protein OspC19–207 was purified as described above.
Peptide synthesis and conjugation.
Solid-phase peptide synthesis was performed by multiple-column peptide synthesis (9, 16). All peptides were synthesized with 9-fluorenylmethoxycarbonyl (Fmoc) amino acids (MilliGen and Calbiochem-Novabiochem), using O-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate and N-hydroxy-benzotriazole as coupling agents. An acid-labile H-Pro-2-ClTrt resin (Novabiochem; s = 0.8 mmol/g) was used to prepare C-terminal proline-containing peptide carboxylates. Peptide structure and homogeneity were confirmed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) with a Fisons TofSpec E instrument and by high-pressure liquid chromatography on a C18 reversed-phase column (Waters Rad-Pak Delta-Pak C18). The concentrations of the peptide samples were determined by amino acid analysis performed with the PICOTAG system (Waters). For preparation of conjugates, peptides were synthesized with an additional cysteine residue at the amino terminus and the resulting peptide was added to purified protein derivative (Statens Serum Institut) by means of the heterobifunctional reagent SPDP [N-succinimidyl-3-(2-pyridylthio)propionate; Sigma, St. Louis, Mo.] by the method of Granier et al. (29).
Sera.
Sera were obtained from 100 consecutive Danish patients with NB. All the patients had been hospitalized in 1994 (58 males and 42 females between 4 and 80 years of age; median age, 49 years). The selection was based primarily on a positive B. burgdorferi specific intrathecal antibody synthesis (13). The diagnosis was further based on clinical evidence and on the presence of lymphocytic pleocytosis in cerebrospinal fluid (CSF). The diagnosis was specified by the presence of lymphocytic meningoradiculitis with typical painful radiculitis, mononeuritis multiplex (Bannwarth’s syndrome), radiculomyelitis, monosymptomatic facial palsy (all children), subacute lymphocytic meningitis, and chronic progressive encephalomyelitis. Before treatment, all but two of the patients had lymphocytic pleocytosis in CSF, with counts of 5 × 106 to 1,200 × 106 cells per liter (median cell count, 124 × 106 cells per liter); in two patients, CSF cytology was not examined. Of the 100 patients, 48 recalled a previous encephalomyelitis-like skin lesion. The duration of disease, defined as the time from onset of neurological symptoms until diagnostic blood and CSF samples were taken, ranged from 1 to 26 weeks (median duration, 3 weeks).
The antipeptide antiserum was obtained by priming female BALB/c mice (6 to 8 weeks old) with 106 CFU of Mycobacterium bovis BCG (Copenhagen strain; Statens Serum Institut) per mouse delivered intraperitoneally in 500 μl of phosphate-buffered saline (PBS) followed by three intraperitoneal injections, at 2-week intevals, of PPD-PVVAESPKKP conjugate adsorbed to alum. The rabbit antiserum raised against purified OspC from DK6 has been described previously (41).
ELISA.
In the recombinant enzyme-linked immunosorbent assay (ELISA), microtiter plates (Maxisorb; Nunc, Roskilde, Denmark) were coated overnight at 4°C with OspC19–207, or OspC19–200 at 0.4 μg/ml and blocked with 3% (wt/vol) milk powder (Matas) in PBS–0.05% (vol/vol) Tween 20.
In the peptide ELISA, microtiter plates were coated overnight at 4°C with streptavidin (Zymed, South San Francisco, Calif.) at 2.5 μg/ml in citrate buffer (pH 5), washed, and then incubated overnight at 4°C with the biotinylated peptide at 0.1 μg/ml in PBS containing 0.37 M NaCl, 0.5% (vol/vol) Tween 20, and 1% (wt/vol) milk powder. Serum was incubated at a 1:200 dilution in PBS containing 0.7 M NaCl, 0.1% (vol/vol) Tween 20, and 1% (wt/vol) milk powder. Antibody binding was detected with peroxidase-conjugated rabbit anti-human IgM (no. P215; Dako, Copenhagen, Denmark). The 98% specific diagnostic cutoff levels were based on results from 100 healthy Danish blood donors and were optical densities of 0.230 for the recombinant ELISA and 0.450 for the peptide ELISA.
Competition ELISA.
Serial dilutions of OspC19–207, OspC19–200, overlapping synthetic peptides covering the C-terminal region, or analogs of this region with alanine substitutions were added to sera diluted 1,000-fold (serum a) or 200-fold (sera b to f) in 1% (wt/vol) milk powder in PBS–0.1% (vol/vol) Tween 20. The mixtures were incubated overnight at 4°C, and the effect on the binding to OspC19–207-coated ELISA plates was determined. The six serum samples used were randomly selected among the anti-OspC high-titer sera from NB patients.
Electron microscopy.
B. garinii DK6 was grown to mid-log phase in BSKII medium, and 108 cells were harvested by centrifugation at 3,000 × g for 20 min at room temperature. The cells were washed once by centrifugation at 3,000 × g in Tyrode’s buffer (14) and gently resuspended in 1 ml of the same buffer. This treatment has been shown to keep the surface layer intact (14). Formvar-coated, carbon-reinforced 400-mesh copper grids were irradiated for 10 min with UV light and then incubated for 5 min on drops of B. garinii DK6 in Tyrode’s buffer. The grids were blocked for 30 min in PBS containing 3% (wt/vol) bovine serum albumin (Sigma) and then incubated with 50 μl of antiserum diluted 1:20 in PBS containing 1% (wt/vol) bovine serum albumin and 1% (vol/vol) Tween 20 (PBST). After five 5-min washes in PBST, the grids were incubated in 50 μl of goat anti-mouse Igs conjugated to 10-nm-diameter gold spheres (no. G441; Dako) and diluted 1:30 in PBST and then washed sequentially in PBST and distilled water. The cells were negatively stained with 1% (wt/vol) ammonium molybdate solution (pH 7.4) for 10 s, air dried, and examined by transmission electron microscopy with a Philips 201 C instrument operated at 60 kV.
RESULTS
The C-terminal region contains the major epitope recognized by patient serum antibodies.
OspC is composed of conserved N- and C-terminal regions and a variable central region (Fig. 1). To locate the immunodominant regions on OspC, the full-length OspC comprising amino acids 19 to 207 and a truncated OspC comprising amino acids 19 to 200 were used in an IgM ELISA testing 100 sera from patients with active NB. Of these sera, 48 showed a significant antibody reactivity with OspC19–207 and only 4 reacted with OspC19–200 (Fig. 1A and B). The C-terminal 7 amino acids are thus critical for IgM binding. This conclusion was confirmed by measuring the antibody reactivity in the 100 serum samples toward a synthetic peptide comprising the C-terminal 10 amino acid residues (Fig. 1A and C). Of these sera, 45 displayed antipeptide reactivity and 42 of the 45 also reacted with OspC19–207.
In contrast to the results obtained above, anti-OspC antibodies obtained by immunizing rabbits with purified OspC from DK6 (41) did not display antipeptide reactivity.
Competition experiments.
The IgM antibody response to OspC was analyzed in detail in six anti-OspC high-titer serum samples from NB patients by competition experiments.
In the first set of experiments, each of the two recombinant OspC polypeptides were added to the sera and the effect on the binding to OspC19–207-coated ELISA plates was determined. Figure 2 shows the results obtained for two representative serum samples. Only the addition of recombinant OspC19–207 inhibited the anti-OspC reactivity, while the addition of OspC19–200 had no effect on the binding of IgM antibodies to OspC19–207 (Fig. 2A).
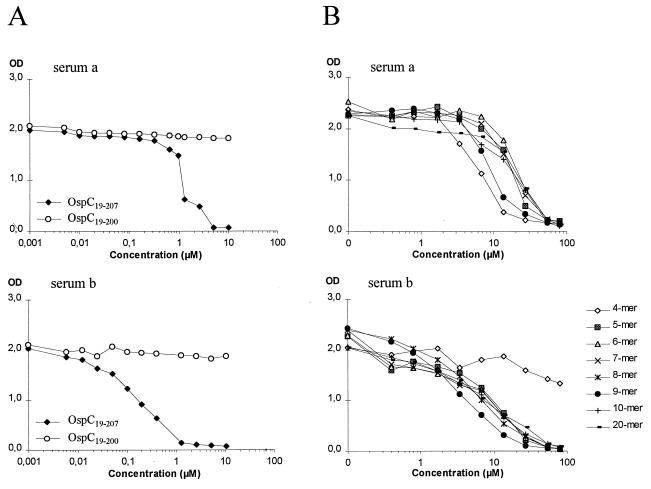
FIG. 2.
Differential serum IgM recognition of recombinant proteins and overlapping synthetic peptides derived from the C-terminal region. ELISA plates were coated with OspC19–207. Shown are the results obtained for two serum samples from patients with NB (serum a and serum b) diluted 200-fold and preincubated with the recombinant proteins OspC19–207 (○) and OspC19–200 (⧫) (A) or with eight overlapping synthetic peptides comprising the C-terminal region (B) at the indicated concentrations before addition to the wells. OD, optical density.
In the second set of experiments, synthetic peptides corresponding to the C-terminal 4, 5, 6, 7, 8, 9, 10, and 20 amino acids were tested for their ability to inhibit the binding of serum IgM antibodies to OspC19–207. The avidity of the sera against the above peptides, compared to the avidity against OspC19–207, was generally lower by a factor of 5 to 50 (compare Fig. 2A and B). In four of the six serum samples, the anti-OspC antibody reactivity was inhibited by all peptides; one example is shown in Fig. 2B (serum a). The anti-OspC antibody reactivity in serum b (Fig. 2B) was only slightly affected by the addition of the 4-mer but was inhibited by the 5-mer and longer peptides. The last serum sample, however, was unaffected by the addition of any of the peptides, even though it also displayed antipeptide reactivity in the peptide ELISA (data not shown). In conclusion, the major antigenic determinant recognized by anti-OspC antibodies in five of the six sera is associated with the C-terminal 5 amino acids.
Fine specificity of anti-OspC IgM antibodies in patient sera.
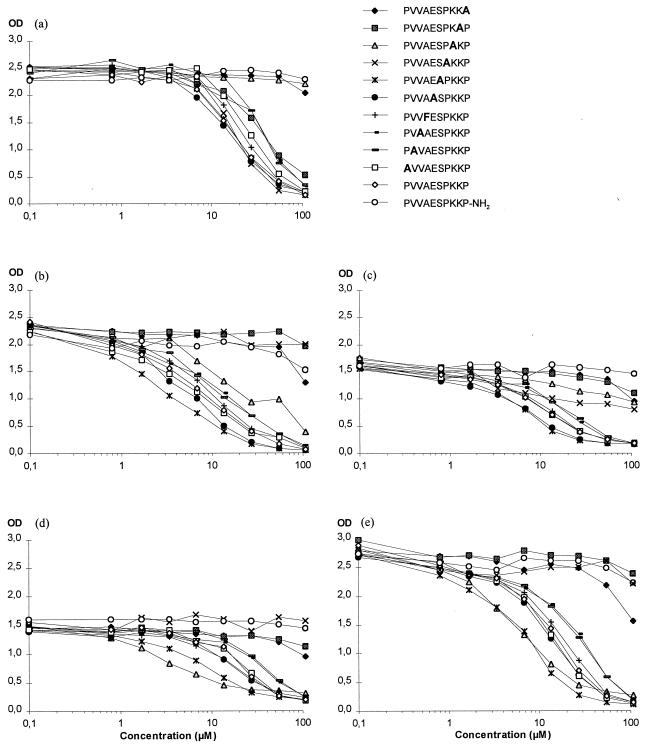
To identify amino acids that are important for binding to anti-OspC antibodies, a series of peptides were synthesized in which each of the 9 nonalanine residues in the C-terminal region was sequentially replaced with alanine; in all cases the remaining nine residues were maintained, and in the case of the single alanine residue (residue 201), it was replaced with phenylalanine. These peptides were tested for their ability to compete with OspC19–207 for the binding of IgM antibodies in the five serum samples (serum a to serum e) from NB patients which were all inhibited by the decamer (Fig. 3). An alanine substitution at position 207 (Pro-207) or replacement of the carboxyl group with an amine group greatly reduced the ability of the peptides to compete with OspC19–207 for the binding of IgM antibodies in all five serum samples (Fig. 3). Substitutions at positions 204, 205, and 206 (Pro-204, Lys-205, and Lys-206) resulted in peptides that were impaired in their ability to compete with OspC19–207 in four serum samples (b, c, d, and e), two serum samples (a and c), and four serum samples (b, c, d, and e), respectively. Alanine substitutions at residues 198, 199, 200, 202, and 203 and the phenylalanine substitution at residue 201 had no effect on the binding of serum antibodies in any of the five serum samples (Fig. 3). Thus, the C-terminal 4 amino acids, as well as the terminal carboxyl group, play a critical role in the binding of IgM antibodies to OspC.
FIG. 3.
Fine specificity of OspC-specific IgM antibodies in patient sera. Serum samples from five patients with NB were diluted 1,000-fold (serum a) or 200-fold (sera b to e) and preincubated with the peptides PVVAESPKKP-CONH2, PVVAESPKKP-CO2H, or alanine-substituted analogs for each residue at the indicated concentrations before addition to wells coated with OspC19–207. OD, optical density.
Sequence variation in the C-terminal region of OspC.
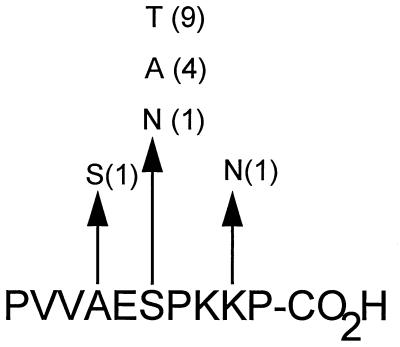
Sequence variation in the C-terminal conserved region was analyzed by retrieving the nucleotide sequences of ospC of 23 B. garinii isolates from the EMBL database. The deduced amino acid sequences of the C-terminal region were compared with that of OspC from DK6. In 11 of the strains, the C-terminal 10 amino acid residues were identical to those in the DK6 sequence. Ten strains contained a single amino acid substitution and two isolates contained two substitutions in this region. The locations of the substitutions are shown in Fig. 4. All amino acid substitutions except the lysine-to-asparagine substitution at position 206 correspond to evolutionarily conserved replacements. The proportion of nonsynonymous (amino acid-altering) nucleotide substitutions per nonsynonymous site (pN) and the proportion of synonymous nucleotide substitutions per synonymous site (pS) in the C-terminal region were calculated. If this region is subjected to purifying selection, meaning that there is a selection against nonsynonymous substitutions due to functional constraints, pS is expected to exceed pN. The mean values for pS and pN and the standard error of the mean calculated by the method of Nei and Gojobori (30) were 0.1510 ± 0.0155 and 0.0434 ± 0.0299, respectively. Thus, pS is significantly larger than pN (P < 0.01, Student’s t test), demonstrating that this region of OspC from B. garinii is under purifying selection. A similar analysis performed on ospC sequences obtained from B. burgdorferi sensu stricto and B. afzelii strains showed that this region of OspC is also conserved in these species.
FIG. 4.
Amino acid polymorphism in the C-terminal epitope. The deduced amino acid sequence of OspC from DK6 is shown. Polymorphic positions and the amino acid substitutions are indicated. The number in parentheses next to a substitution represents the number of substitutions among OspC alleles from 23 B. garinii isolates.
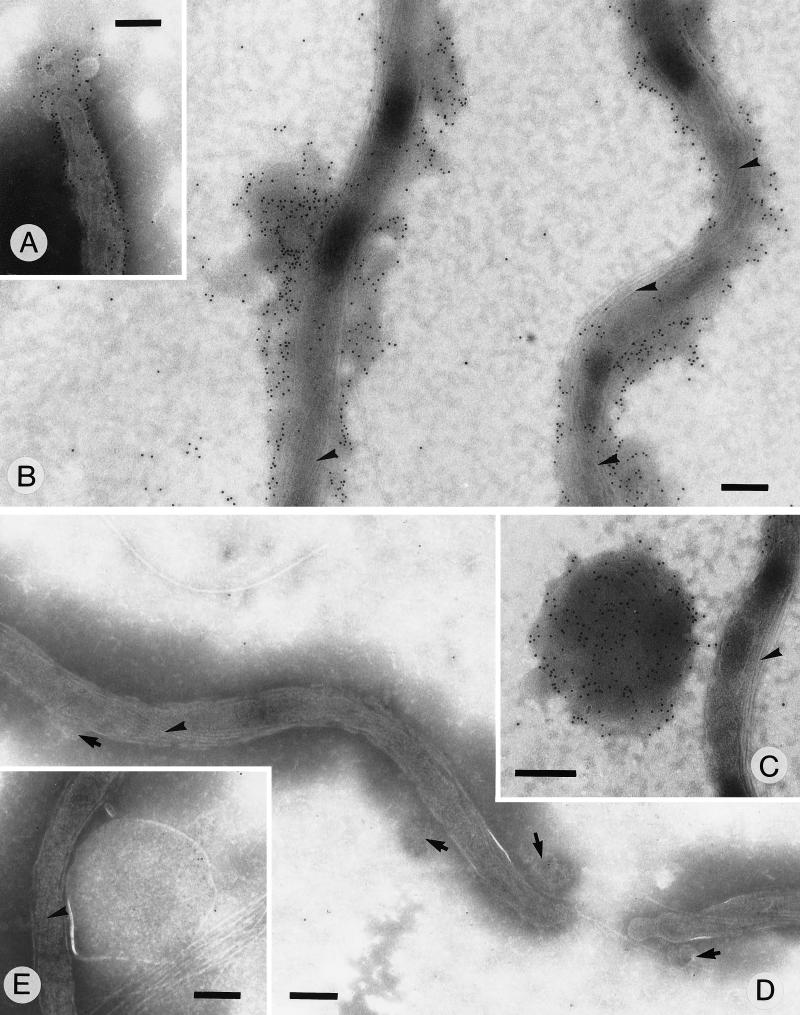
Localization of the C terminus of OspC by electron microscopy.
Immunofluorescence studies with antisera against recombinant OspC have led to the conclusion that the majority of OspC is not surface exposed on cultured spirochetes (1, 4). To determine whether the C-terminal region is accessible for interaction with antibodies, we have raised an antiserum against a synthetic peptide corresponding to the C-terminal 10 amino acid residues of OspC. In a Western blot analysis of a DK6 cell lysate, this antiserum recognized a single band corresponding to OspC (data not shown). When this antiserum was used as a probe, intense labeling was detected on the surface of unfixed B. garinii DK6 cells (Fig. 5A and B). The labeling was associated with the outermost layer surrounding intact cells. Labeling was also associated with flakes of detached S-layer material (Fig. 5C). This layer appears similar to the surface layer described previously (14). No labelling was detected when the preimmune serum was used as the probe (Fig. 5D and E). Thus, the C-terminal region of OspC on cultured spirochetes is accessible for interaction with Igs.
FIG. 5.
Immunoelectron micrograph of unfixed cells. B. garinii DK6 cells negatively stained with 1% ammonium molybdate are shown. The cells were labeled with a mouse anti-PVVAESPKKP antiserum (A to C) or with the preimmune serum (D and E). Localization of mouse antibodies was detected with goat anti-mouse IgG conjugated to 10-nm-diameter gold beads. (A and B) The surface layer on intact organisms is seen decorated with gold particles. (C) A labelled flake of the surface layer is seen detached from the organism. (D) Intact organisms are seen with unlabelled surface layers (arrows). (E) A large unlabelled flake is shown. Bands of endoflagella are marked by arrowheads. Bars, 0.2 pm.
DISCUSSION
Epitope mapping studies with sera from patients with NB identified a single major epitope of OspC located in the C-terminal region. The amino acid sequence comprising this epitope is highly conserved among Borrelia species causing Lyme borreliosis and has not been identified in other B. burgdorferi proteins. The C-terminal decapeptide contains the structural motif PXXP, found in polyproline II-like helices. We have shown that the C-terminal region of OspC, in particular the prolines in residues 198, 204, and 207 involved in the PXXP motif, is subjected to purifying selection, suggesting a functional constraint on this region of OspC.
Although OspC is located in the outer membrane of B. burgdorferi (46), several studies have indicated that this protein has a limited surface exposure on cultured spirochetes (1, 4). The results presented here indicate that the C-terminal region of OspC decorates the surface of unfixed B. garinii DK6 cells.
The C-terminal heptapeptide in OspC is immunodominant, since the majority of sera from patients with NB react with OspC19–207 but not with OspC19–200. Furthermore, the analysis of specificity revealed that in five of six serum samples, the prolines in residue 204 and 207 are critical to binding. The immunological reactivity thus seems to depend on residues critical for the maintenance of a polyproline II-like helical structure.
We conclude that the C terminus of OspC is surface exposed and immunodominant and that the purifying selection of the PXXP motif reflects that the C terminus adopts an elongated polyproline II helix of importance for the spirochete. We propose that in the vertebrate host, the surface of B. burgdorferi is a repetitive structure with the C-terminal region of OspC as the basic unit.
A polyproline II helix is the preferred conformation of proline-rich regions characterized by a rigid extended structure with ϕ = −78° and ψ = +146° (3). Due to the restriction on these angles, the polyproline II-like helix can be considered a “sticky arm” which binds rapidly and reversibly to other proteins (for a review, see reference 43). The polyproline II helix has been identified as a common structural element of various proteins engaged in host-parasite interactions (21, 34, 49) or in signal transduction (5, 39). For example, alanine replacement of proline residues in the hypothetical polyproline II-like helix of neuropeptide Y showed that this structure is involved in both potency and affinity to central nervous system receptors (8).
The C-terminal region of the OspC homolog, Vmp33, from the relapsing-fever agent B. hermsii contains the PXXP motif repeated four times (2). Relapsing fever, like Lyme borreliosis, may involve the nervous system; however, it is not yet known whether the C-terminal regions of OspC and Vmp33 play the same role.
Our hypothesis, i.e., that the surface of B. burgdorferi is covered by a repetitive unit composed of the C-terminal region of OspC (assuming an extended polyproline II helical structure), may explain why the humoral immune response against OspC in NB is primarily of the IgM type and why very few patients have IgG antibodies against OspC (26, 45, 47). An ordered display of multiple identical epitopes is thought to induce T-cell-independent activation of B cells by cross-linking surface Igs (for a review, see reference 27). Thus, immunization with synthetic amino acid polymers (9, 15) or with live Escherichia coli expressing multiple copies of a recombinant epitope on the cell surface (20) stimulates T-cell-independent antibody responses. However, when this latter epitope was expressed as part of a periplasmic protein, the response became T-cell dependent (20). T-cell-independent humoral responses (non-major histocompatibility complex restricted) are considered less efficient than the major histocompatibility complex-restricted responses due to the absence of Ig switching, affinity maturation, and memory formation. Thus, the antibody response elicited by a repetitive structure would be expected to be of the IgM type, of low avidity toward the organism, and nonneutralizing. Indeed, as shown in this report, IgM antibodies against OspC in NB sera are of low affinity, and it is characteristic of the Lyme borreliosis infection that IgG antibodies against OspC are rare (26, 45, 47). Moreover, CD40L-deficient mice infected with B. burgdorferi are capable of eliciting an antibody response against OspC which is T-cell independent (6). The surface expression of OspC in the vertebrate host may thus suppress the development of protective immunity against B. burgdorferi by inducing a nonneutralizing IgM response without memory formation. Our suggested mechanism may be the driving principle behind the purifying selection of the C-terminal region of OspC and, in particular, the proline residues critical for the maintenance of a polyproline II helical structure.
Due to the surface exposure and immunological availability, the C-terminal region of OspC would seem to be a good vaccine candidate. However, two observations indicate that it may be difficult to induce IgG antibodies against this region. First, few Lyme borreliosis sera have IgG antibodies against OspC (26, 45, 47), and second, rabbits immunized with gel-purified native OspC in Freund’s complete adjuvant do not produce antibodies to the C-terminal region. This latter observation supports our hypothesis that the spatial organization of OspC in the outer membrane is decisive for the development of naturally occurring antibodies. Thus, to obtain a high-titer, long-lasting immune response, it may prove necessary to couple the C-terminal region of OspC to a strong T-cell epitope, e.g., purified protein derivative.
In conclusion, we have shown that the C-terminal region of OspC is surface exposed and immunodominant and that a PXXP motif contained in the sequence is conserved within Lyme borreliosis spirochetes and is subjected to purifying selection. Moreover, the residues important to the PXXP motif are critical for the maintenance of the immunological reactivity of the C terminus of OspC. Finally, we offer a novel hypothesis to explain how B. burgdorferi evades host immunity by presenting an epitope that induces T-cell independent B-cell activation, leading to a failure in affinity maturation and memory formation.
ACKNOWLEDGMENTS
We thank M. Paulli Andersen and H. Hasselager for technical assistance and N. Axelsen for his many useful suggestions concerning the manuscript.
This work was supported by the Research Center for Medical Biotechnology under the Danish Biotechnology Research Program.
REFERENCES
- 1.Bockenstedt L K, Hodzic E, Feng S, Bourrel K W, de-Silva A, Montgomery R R, Fikrig E, Radolf J D, Barthold S W. Borrelia burgdorferi strain-specific OspC-mediated immunity in mice. Infect Immun. 1997;65:4661–4667. doi: 10.1128/iai.65.11.4661-4667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carter C J, Bergstrom S, Norris S J, Barbour A G. A family of surface-exposed proteins of 20 kilodaltons in the genus Borrelia. Infect Immun. 1994;62:2792–2799. doi: 10.1128/iai.62.7.2792-2799.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cowan P M, McGavin S. Structure of poly-l-proline. Nature. 1955;176:501. doi: 10.1038/1761062a0. [DOI] [PubMed] [Google Scholar]
- 4.Cox D L, Akins D R, Bourell K W, Lahdenne P, Norgard M V, Radolf J D. Limited surface exposure of Borrelia burgdorferi outer surface proteins. Proc Natl Acad Sci USA. 1996;93:7973. doi: 10.1073/pnas.93.15.7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darbon H, Bernassau J M, Deleuze C, Chenu J, Roussel A, Cambillau C. Solution conformation of human neuropeptide Y by 1H nuclear magnetic resonance and restrained molecular dynamics. Eur J Biochem. 1992;209:765–771. doi: 10.1111/j.1432-1033.1992.tb17346.x. [DOI] [PubMed] [Google Scholar]
- 6.Fikrig E, Barthold S W, Chen M, Grewal I S, Craft J, Flavell R A. Protective antibodies in murine Lyme disease arise independently of CD40 ligand. J Immunol. 1996;157:1–3. [PubMed] [Google Scholar]
- 7.Fingerle V, Hauser U, Liegl G, Petko B, Preac-Mursic V, Wilske B. Expression of outer surface proteins A and C of Borrelia burgdorferi in Ixodes ricinus. J Clin Microbiol. 1995;33:1867–1869. doi: 10.1128/jcm.33.7.1867-1869.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forest M, Martel J C, St-Pierre S, Quirion R, Fournier A. Structural study of the N-terminal segment of neuropeptide tyrosine. J Med Chem. 1990;33:1615–1619. doi: 10.1021/jm00168a014. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs S, Mozes E, Maoz A, Sela M. Thymus independence of a collagen-like synthetic polypeptide and of collagen, and the need for thymus and bone marrow-cell cooperation in the immune response to gelatin. J Exp Med. 1974;139:148–158. doi: 10.1084/jem.139.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fung B P, McHugh G L, Leong J M, Steere A C. Humoral immune response to outer surface protein C of Borrelia burgdorferi in Lyme disease: role of the immunoglobulin M response in the serodiagnosis of early infection. Infect Immun. 1994;62:3213–3221. doi: 10.1128/iai.62.8.3213-3221.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerber M A, Shapiro E D, Bell G L, Sampieri A, Padula S J. Recombinant outer surface protein C ELISA for the diagnosis of early Lyme disease. J Infect Dis. 1995;171:724–727. doi: 10.1093/infdis/171.3.724. [DOI] [PubMed] [Google Scholar]
- 12.Gilmore R D, Kappel K J, Dolan M C, Burkot T R, Johnson B J. Outer surface protein C (OspC), but not P39, is a protective immunogen against a tick-transmitted Borrelia burgdorferi challenge: evidence for a conformational protective epitope in OspC. Infect Immun. 1996;64:2234–2239. doi: 10.1128/iai.64.6.2234-2239.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen K, Lebech A M. Lyme neuroborreliosis: a new sensitive diagnostic assay for intrathecal synthesis of Borrelia burgdorferi-specific immunoglobulin G, A, and M. Ann Neurol. 1991;30:197–205. doi: 10.1002/ana.410300212. [DOI] [PubMed] [Google Scholar]
- 14.Hayes S F, Burgdorfer W. Ultrastructure of Borrelia burgdorferi. In: Weber K, Burgdorfer W, editors. Aspects of Lyme borreliosis. Berlin, Germany: Springer-Verlag KG; 1993. pp. 29–43. [Google Scholar]
- 15.Hillman K, Shapira-Nahor O, Blackburn R, Hernandez D, Golding H. A polymer containing a repeating peptide sequence can stimulate T-cell-independent IgG antibody production in vivo. Cell Immunol. 1991;134:1–13. doi: 10.1016/0008-8749(91)90326-7. [DOI] [PubMed] [Google Scholar]
- 16.Holm A, Meldal M. Multiple column peptide synthesis. In: Jung G, Bayer E, editors. Peptides 1988. New York, N.Y: Walter de Gruyter; 1989. pp. 208–210. [Google Scholar]
- 17.Hughes C A, Engstrom S M, Coleman L A, Kodner C B, Johnson R C. Protective immunity is induced by a Borrelia burgdorferi mutant that lacks OspA and OspB. Infect Immun. 1993;61:5115–5122. doi: 10.1128/iai.61.12.5115-5122.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jauris-Heipke S, Fuchs R, Motz M, Preac-Mursic V, Schwab E, Soutschek E, Will G, Wilske B. Genetic heterogenity of the genes coding for the outer surface protein C (OspC) and the flagellin of Borrelia burgdorferi. Med Microbiol Immunol. 1993;182:37–50. doi: 10.1007/BF00195949. [DOI] [PubMed] [Google Scholar]
- 19.Jauris-Heipke S, Liegl G, Preac-Mursic V, Rossler D, Schwab E, Soutschek E, Will G, Wilske B. Molecular analysis of genes encoding outer surface protein C (OspC) of Borrelia burgdorferi sensu lato: relationship to ospA genotype and evidence of lateral gene exchange of ospC. J Clin Microbiol. 1995;33:1860–1866. doi: 10.1128/jcm.33.7.1860-1866.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leclerc C, Charbit A, Martineau P, Deriaud E, Hofnung M. The cellular location of a foreign B cell epitope expressed by recombinant bacteria determines its T cell-independent or T cell-dependent characteristics. J Immunol. 1991;147:3545–3552. [PubMed] [Google Scholar]
- 21.Lee C H, Saksela K, Mirza U A, Chait B T, Kuriyan J. Crystal structure of the conserved core of HIV-1 Nef complexed with a Src family SH3 domain. Cell. 1996;85:931–942. doi: 10.1016/s0092-8674(00)81276-3. [DOI] [PubMed] [Google Scholar]
- 22.Livey I, Gibbs C P, Schuster R, Dorner F. Evidence for lateral transfer and recombination in OspC variation in Lyme disease Borrelia. Mol Microbiol. 1995;18:257–269. doi: 10.1111/j.1365-2958.1995.mmi_18020257.x. [DOI] [PubMed] [Google Scholar]
- 23.Marconi R T, Samuels D S, Garon C F. Transcriptional analyses and mapping of the ospC gene in Lyme disease spirochetes. J Bacteriol. 1993;175:926–932. doi: 10.1128/jb.175.4.926-932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marconi R T, Samuels D S, Schwan T G, Garon C F. Identification of a protein in several Borrelia species which is related to OspC of the Lyme disease spirochetes. J Clin Microbiol. 1993;31:2577–2583. doi: 10.1128/jcm.31.10.2577-2583.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Margolis N, Hogan D, Cieplak W J, Schwan T G, Rosa P A. Homology between Borrelia burgdorferi OspC and members of the family of Borrelia hermsii variable major proteins. Gene. 1994;143:105–110. doi: 10.1016/0378-1119(94)90613-0. [DOI] [PubMed] [Google Scholar]
- 26.Mathiesen M J, Hansen K, Axelsen N H, Halkier S L, Theisen M. Analysis of the human antibody response to OspC of Borrelia burgdorferi sensu stricto, Borrelia garinii, and Borrelia afzelii. Microbiol Immunol. 1996;185:121. doi: 10.1007/s004300050021. [DOI] [PubMed] [Google Scholar]
- 27.Mond J J, Vos Q, Lees A, Snapper C M. T cell independent antigens. Curr Opin Immunol. 1995;7:349–354. doi: 10.1016/0952-7915(95)80109-x. [DOI] [PubMed] [Google Scholar]
- 28.Montgomery R R, Malawista S E, Feen K J, Bockenstedt L K. Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. J Exp Med. 1996;183:261–269. doi: 10.1084/jem.183.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller S. Peptide-carrier conjugation. In: Burdon R H, van Knippenberg P H, editors. Laboratory techniques in biochemistry and molecular biology. Vol. 19. Amsterdam, The Netherlands: Elsevier; 1988. pp. 110–113. [Google Scholar]
- 30.Nei M, Gojobori T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol. 1986;3:418–426. doi: 10.1093/oxfordjournals.molbev.a040410. [DOI] [PubMed] [Google Scholar]
- 31.Padula S J, Dias F, Sampieri A, Craven R B, Ryan R W. Use of recombinant OspC from Borrelia burgdorferi for serodiagnosis of early Lyme disease. J Clin Microbiol. 1994;32:1733–1738. doi: 10.1128/jcm.32.7.1733-1738.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Preac-Mursic V, Wilske B, Patsouris E, Jauris S, Will G, Soutschek E, Rainhardt S, Lehnert G, Klockmann U, Mehraein P. Active immunization with pC protein of Borrelia burgdorferi protects gerbils against B. burgdorferi infection. Infection. 1992;20:342–349. doi: 10.1007/BF01710681. [DOI] [PubMed] [Google Scholar]
- 33.Probert W S, LeFebvre R B. Protection of C3H/HeN mice from challenge with Borrelia burgdorferi through active immunization with OspA, OspB, or OspC, but not with OspD or the 83-kilodalton antigen. Infect Immun. 1994;62:1920–1926. doi: 10.1128/iai.62.5.1920-1926.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raj P A, Edgerton M. Functional domain and poly-l-proline II conformation for candidacidal activity of bactenecin 5. FEBS Lett. 1995;368:526–530. doi: 10.1016/0014-5793(95)00712-i. [DOI] [PubMed] [Google Scholar]
- 35.Sadziene A, Wilske B, Ferdows M S, Barbour A G. The cryptic ospC gene of Borrelia burgdorferi B31 is located on a circular plasmid. Infect Immun. 1993;61:2192–2195. doi: 10.1128/iai.61.5.2192-2195.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevenson B, Barthold S W. Expression and sequence of outer surface protein C among North American isolates of Borrelia burgdorferi. FEMS Microbiol Lett. 1994;124:367–372. doi: 10.1111/j.1574-6968.1994.tb07310.x. [DOI] [PubMed] [Google Scholar]
- 38.Stevenson B, Schwan T G, Rosa P A. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:4535–4539. doi: 10.1128/iai.63.11.4535-4539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Terasawa H, Kohda D, Hatanaka H, Tsuchiya S, Ogura K, Nagata K, Ishii S, Mandiyan V, Ullrich A, Schlessinger J, et al. Structure of the N-terminal SH3 domain of GRB2 complexed with a peptide from the guanine nucleotide releasing factor Sos. Nat Struct Biol. 1994;1:891–897. doi: 10.1038/nsb1294-891. [DOI] [PubMed] [Google Scholar]
- 40.Theisen M, Borre M, Mathiesen M J, Mikkelsen B, Lebech A M, Hansen K. Evolution of the Borrelia burgdorferi outer surface protein OspC. J Bacteriol. 1995;177:3036–3044. doi: 10.1128/jb.177.11.3036-3044.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Theisen M, Frederiksen B, Lebech A M, Vuust J, Hansen K. Polymorphism in ospC gene of Borrelia burgdorferi and immunoreactivity of OspC protein: implications for taxonomy and for use of OspC protein as a diagnostic antigen. J Clin Microbiol. 1993;31:2570–2576. doi: 10.1128/jcm.31.10.2570-2576.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Theisen M, Vuust J, Gottschau A, Jepsen S, Hogh B. Antigenicity and immunogenicity of recombinant glutamate-rich protein of Plasmodium falciparum expressed in Escherichia coli. Clin Diagn Lab Immunol. 1995;2:30–34. doi: 10.1128/cdli.2.1.30-34.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williamson M P. The structure and function of proline-rich regions in proteins. Biochem J. 1994;297:249–260. doi: 10.1042/bj2970249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilske B, Fingerle V, Herzer P, Hofmann A, Lehnert G, Peters H, Pfister H W, Preac-Mursic V, Soutschek E, Weber K. Recombinant immunoblot in the serodiagnosis of Lyme borreliosis. Comparison with indirect immunofluorescence and enzyme-linked immunosorbent assay. Med Microbiol Immunol. 1993;182:255–270. doi: 10.1007/BF00579624. [DOI] [PubMed] [Google Scholar]
- 45.Wilske B, Fingerle V, Preac-Mursic V, Jauris-Heipke S, Hofmann A, Loy H, Pfister H W, Rossler D, Soutschek E. Immunoblot using recombinant antigens derived from different genospecies of Borrelia burgdorferi sensu lato. Med Microbiol Immunol. 1994;183:43–59. doi: 10.1007/BF00193630. [DOI] [PubMed] [Google Scholar]
- 46.Wilske B, Preac-Mursic V, Jauris S, Hofmann A, Pradel I, Soutschek E, Schwab E, Will G, Wanner G. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect Immun. 1993;61:2182–2191. doi: 10.1128/iai.61.5.2182-2191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilske B, Preac-Mursic V, Schierz G, Busch K V. Immunochemical and immunological analysis of European Borrelia burgdorferi strains. Zentbl Bakteriol Mikrobiol Hyg Ser A. 1986;263:92–102. doi: 10.1016/s0176-6724(86)80108-0. [DOI] [PubMed] [Google Scholar]
- 48.Wilske B, Preac-Mursic V, Schierz G, Kuhbeck R, Barbour A G, Kramer M. Antigenic variability of Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:126–143. doi: 10.1111/j.1749-6632.1988.tb31846.x. [DOI] [PubMed] [Google Scholar]
- 49.Wu X, Knudsen B, Feller S M, Zheng J, Sali A, Cowburn D, Hanafusa H, Kuriyan J. Structural basis for the specific interaction of lysine-containing proline-rich peptides with the N-terminal SH3 domain of c-Crk. Structure. 1995;3:215–226. doi: 10.1016/s0969-2126(01)00151-4. [DOI] [PubMed] [Google Scholar]