Abstract
Background:
It is of utmost importance to monitor any change in the epidemiology of fungal diseases that may arise from a change in the number of the at-risk population or the availability of local data.
Objective:
We sought to update the 2015 publication on the incidence and prevalence of serious fungal diseases in Uganda.
Methods:
Using the Leading International Fungal Education methodology, we reviewed published data on fungal diseases and drivers of fungal diseases in Uganda. Regional or global data were used where there were no Ugandan data.
Results:
With a population of ~45 million, we estimate the annual burden of serious fungal diseases at 4,099,357 cases (about 9%). We estimated the burden of candidiasis as follows: recurrent Candida vaginitis (656,340 cases), oral candidiasis (29,057 cases), and esophageal candidiasis (74,686 cases) in HIV-infected people. Cryptococcal meningitis annual incidence is estimated at 5553 cases, Pneumocystis pneumonia at 4604 cases in adults and 2100 cases in children. For aspergillosis syndromes, invasive aspergillosis annual incidence (3607 cases), chronic pulmonary aspergillosis (26,765 annual cases and 63,574 5-year-period prevalent cases), and prevalence of allergic bronchopulmonary aspergillosis at 75,931 cases, and severe asthma with fungal sensitization at 100,228 cases. Tinea capitis is common with 3,047,989 prevalent cases. For other mycoses, we estimate the annual incidence of histoplasmosis to be 646 cases and mucormycosis at 9 cases.
Conclusion:
Serious fungal diseases affect nearly 9% of Ugandans every year. Tuberculosis and HIV remain the most important predisposition to acute fungal infection necessitating accelerated preventive, diagnostic, and therapeutic interventions for the management of these diseases.
Keywords: cryptococcal meningitis, chronic pulmonary aspergillosis, fungal diseases, Uganda
Plain language summary
How common are serious fungal infections in Uganda?
Why was the study done?
This study was conducted to provide an updated understanding of the occurrence and impact of serious fungal diseases in Uganda. The aim was to monitor changes in the epidemiology of fungal diseases related to shifts in the at-risk population or the availability of local data.
What did the researchers do?
Utilizing the Leading International Fungal Education methodology, the research team systematically reviewed published data on fungal diseases in Uganda. In instances where Ugandan data was unavailable, regional, or global data were incorporated. This method allowed for a thorough examination of the incidence and prevalence of various serious fungal diseases, considering the local context.
What did the researchers find?
With a population of approximately 45 million, the study estimated that nearly 9% of Ugandans, totalling around 4,099,357 individuals, are affected by serious fungal diseases annually. Notable findings include the prevalence of recurrent Candida vaginitis, oral candidiasis, and oesophageal candidiasis in HIV-infected individuals. Cryptococcal meningitis and Pneumocystis pneumonia were identified as significant contributors, along with various aspergillosis syndromes and widespread cases of tinea capitis.
What do the findings mean?
These findings underscore the substantial impact of serious fungal diseases on the health of almost 9% of the Ugandan population each year. Recognizing tuberculosis and HIV as major predisposing factors, the study calls for urgent interventions to prevent, diagnose, and treat these diseases effectively. The identified targets, including improved access to essential antifungal medications, training of health care workers on fungal diseases, and increasing access to essential diagnostics. These interventions can significantly contribute to improving public health outcomes in Uganda.
Introduction
Hippocrates first described what we now call oral thrush in 500BC in his classic writing on ‘apthae’ – meaning sores in the mouth. 1 Despite being among the oldest recognized causes of infection in humans, fungal diseases are the great neglected diseases of medical history.2–4 Therefore, their burden in terms of frequency (incidence, prevalence, morbidity, and mortality), severity (disability-adjusted life years and health-related quality of life), and economic impact (visible and invisible costs) remain unknown in most countries of the world. The World Health Organization (WHO) has recently recognized the importance of fungal disease to human health and has established a list of priority fungal pathogens to be targeted for research and development to ameliorate sufferings and deaths for serious fungal diseases. 5 Serious fungal infections denote fungal disease entities with major consequences, either death or severe morbidity. 3 This includes tinea capitis in children – which is associated with exclusion from school, stigma, severe pustular scalp infection (kerion), multiple treatment applications, household and school transmission, and permanent hair loss. 6 In addition to opportunistic and endemic fungal diseases, serious fungal infections also include recurrent vulvovaginal candidiasis (rVVc) (four or more episodes annually) and fungal keratitis, both with significant morbidity.7,8 Continuous epidemiological surveillances are important in our understanding of the global, regional, and local incidence, prevalence, and trend of this frequently under-recognized and difficult-to-detect class of diseases. 9
The first-ever estimate of the burden of serious fungal diseases in Uganda was published in 2015, highlighting important opportunistic fungal infections (OFIs) in human immunodeficiency virus (HIV) infection and knowledge gaps. 10 Since then, additional data on HIV-related OFIs,11–14 deep mycoses,15,16 and studies on chronic pulmonary aspergillosis (CPA) in post-tuberculosis (TB) 17 and in active TB18,19 populations have been published, providing us with more reflective local data to improve the robustness of the estimate of CPA. Also, several high-quality local data have been published on other important risk factors for fungal diseases including asthma20–24 and chronic obstructive pulmonary disease (COPD) 25 and the population size has changed significantly. Furthermore, intensive care unit (ICU) services and beds have expanded across the country, 26 HIV treatment outcomes have significantly improved, 27 significantly reducing the number of individuals at risk of major opportunistic infections, and finally the incidence of cancers and associated mortality remains high,28,29 reflecting an understudied high-risk population for severe fungal diseases.
In view of the above, we aimed to re-evaluate the incidence and prevalence of serious fungal diseases in Uganda based on locally available data to inform clinicians, researchers public health practitioners, and policymakers on areas of priorities for advocacy toward universal access to diagnostics and antifungals in Uganda.
Materials and methods
Study design
Using a mix-method approach, we re-evaluate the burden of serious fungal diseases in Uganda using the Leading International Fungal Education (LIFE) program (https://en.fungaleducation.org) methodologies. The LIFE methodology utilizes a deterministic approach based on locally, regionally, or globally published literature to model estimates of the burden of serious fungal diseases. For this study, we conducted a literature review at the end of 2021 and updated it in June 2023.
Data sources
To identify specific fungal disease incidences and epidemiological reports from Uganda, we performed a systematic search of the English literature for published data on fungal diseases in Uganda using PubMed, African Journal Online, and Google Scholar databases. Our search strings included the serious fungal diseases, including those associated with HIV, tuberculosis, and chronic lung diseases; ‘chronic pulmonary aspergillosis’ OR ‘allergic bronchopulmonary aspergillosis’ OR ‘severe asthma with fungal sensitization’ OR ‘cryptococcosis’ OR ‘Pneumocystis pneumonia’ OR ‘ esophageal candidiasis’ OR ‘ oropharyngeal candidiasis’ OR ‘ fungal keratitis’ OR ‘ invasive aspergillosis’ OR ‘candidemia’ OR ‘Candida peritonitis’ OR ‘vulvovaginal candidiasis’ AND Uganda. The data were used to estimate the incidence or prevalence of fungal infections based on the specific population at risk and the reported incidences for these risk groups. For all estimates, the most recent epidemiological data available were obtained for this study. Where national or local data were unavailable, data were extrapolated from other sources in the order of decreasing preference: from other countries in East Africa, elsewhere in Africa, non-African middle-income countries, and non-African non-middle-income countries. Published papers, including review articles with no clear epidemiological implications, were excluded.
We used population at-risk groups for infections and deterministic modeling to derive national incidence and prevalence estimates for the most serious fungal diseases. We used the United Nations Population and Woldometer databases to obtain population data. 30 Data on HIV were obtained from The Joint United Nations Programme on HIV/AIDS (UNAIDS) and TB from the WHO and Ministry of Health Uganda reports.31,32 Data on chronic obstructive pulmonary disease (COPD) 25 and asthma,20,21,33 ICU beds26,34 were obtained from locally published studies. Data on cancers were obtained from Globocan. 35
Results
Country profile
Uganda (officially the Republic of Uganda) is a landlocked country in East Africa with an estimated population of 45,741,000 (the basis of our estimate). In 2018, 23.8% of Ugandans were living in urban cities and towns up from 18.4% in 2008. Kampala, the capital city is the most populated with a population of over 1.5 million inhabitants. The median age of Ugandans is 15 years, making it one of the world’s youngest populations. 36 The average life expectancy of Ugandans at birth is about 53 years. Table 1 summarizes population characteristics and underlying comorbidities in Uganda.
Table 1.
Population characteristics and underlying comorbidities in Uganda.
| Population characteristic | Number | Data source (reference) |
|---|---|---|
| Total population, 2020 | 45,741,000 | Worldometers 36 |
| Women aged 15–49 years | 10,939,000 | Worldometers 36 |
| Children (5–14 years) | 13,252,128 | Worldometers 36 |
| Adults | 24,693,000 | Worldometers 36 |
| Persons living with HIV | 1,400,000 | UNAIDS, 2020 28 |
| Children living with HIV | 100,000 | UNAIDS, 2020 |
| HIV patients on ART | 1,300,000 | UNAIDS, 2020 |
| AIDS-related deaths | 22,000 | UNAIDS, 2020 |
| Pulmonary tuberculosis | 85,500 | WHO, 2020 29 |
| COPD prevalence | 16.2% age > 30 years; 4.1% of population | van Gemert et al. 25 |
| Asthma rates in adults and adolescents | 12.3% (9.1–15.5) | Kirenga et al.20,21 |
| Lung cancer/year | 486 | Globocan 35 |
| Acute myelogenous Leukemia/year | 1372 | Globocan 35 ; Natukunda et al. 29 |
| ICU beds nationally | 55 (pre-COVID-19) | Atumanya et al. 34 |
| Abdominal surgeries/year | 3000 | Estimate |
ART, anti-retroviral therapy; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; Globocan, Global Cancer Observatory; ICU, intensive care unit; UNAIDS, The Joint United Nations Programme on HIV/AIDS.
Assumptions and modeling approach
The estimating approach varied by fungal disease and at-risk denominator. Annual incidence rates of candidemia, intra-abdominal candidiasis (Candida peritonitis), mucormycosis, and fungal keratitis were based on the overall population in 2020 (Table 2). rVVc prevalence was based on females aged 15–50 years, with a rate per 100,000 of all females in Uganda based on a recent estimate (Table 2). 8 Tinea capitis prevalence was based on children under the age of 15 years (Table 2). Mycetoma was based on a recent review of documented cases in Uganda. 16 Both allergic bronchopulmonary aspergillosis (ABPA) and severe asthma with fungal sensitization (SAFS) prevalence were based on adults with asthma, and this was derived on recent work from Uganda indicating an asthma prevalence rate of 12% (Table 2).20,21 Invasive aspergillosis (IA) annual incidence was derived from deaths from AIDS (4%) (UNAIDS), lung cancer (2.6%) (Globocan), 10% of acute myeloid leukemia cases which represent 50% of all leukemia-linked IA (Globocan) (so an equal number of IA cases linked to all other hematological cancers, multiple myeloma, and lymphoma), and 1.3% of COPD patients admitted to hospital (10.5% of all COPD cases GOLD stage 2–4) (Table 2). 37 No solid organ or allogeneic stem cell transplants are done in Uganda.
Table 2.
Epidemiological and clinical studies in Uganda data and assumptions based on literature made in assessing fungal disease burden.
| Disease | Underlying disease(s) | Incidence/prevalence used to estimate burden | Comments | Reference |
|---|---|---|---|---|
| Cryptococcosis | Advanced HIV disease | Cryptococcal antigenemia, 19% | CD4 < 100 | Oyella et al. 38 |
| Cryptococcal antigenemia, 8.2% 8.6% in CD4 < 100, 2.3% in CD4 > 100 |
ART-naïve | Meya et al. 39 | ||
| Cryptococcal antigenemia, 3.0% | HIV treatment experience with virologic failure | Mpoza et al. 12 | ||
| Meningitis, 6.5% | CD4<100 | Oyella et al. 38 | ||
| Meningitis, 67.8% | Among meningitis suspects | Ellis et al. 40 | ||
| Meningitis, 63% | Among meningitis suspects | Flynn et al. 41 | ||
| Cryptococcosis, 1.6% | Among HIV patients | Rubaihayo et al. 14 | ||
| Meningitis mortality, 20−50% | Among meningitis patients | Kambugu et al. 42 | ||
| Meningitis mortality, 45% versus 30% | COAT trial | Boulware et al. 43 | ||
| Meningitis mortality, 15.7% | All-cause mortality among HIV-infected patients | Kiragga et al. 13 | ||
| Meningitis mortality, 19% | All-cause mortality among hospitalized HIV-infected patients | Namutebi et al. 44 | ||
| Pneumocystis jirovecii pneumonia | Advanced HIV disease | PCP, 0.3% | Among HIV-infected adults | Rubaihayo et al. 14 |
| PCP, 3.9%; 11.4% among newly diagnosed | Among patients admitted with pneumonia | Taylor et al. 45 | ||
| PCP, 15.4% | Meta-analysis, Sub-Saharan Africa; mainly in patients | Wasserman et al. 46 | ||
| PCP mortality, 7.1% | All-cause mortality among HIV-infected patients | Kiragga et al. 13 | ||
| Oropharyngeal candidiasis | HIV | 19.4% | Among patients with advanced HIV disease | Rubaihayo et al. 14 |
| Esophageal candidiasis | Advanced HIV disease | 8.0% | Among patients with advanced HIV disease | Rubaihayo et al. 14 |
| Invasive aspergillosis | Advanced HIV disease, COPD admissions to hospital, hematological malignancy | 4% of deaths from HIV/AIDS 13% rate in AML, number in non-AML same as AML patients. 2.6% of lung cancer patients 1.3% of the 10.5% of COPD patients admitted to hospital |
Global data | Lortholary et al.
47
Denning and Morgan 48 |
| Disseminated histoplasmosis | Advanced HIV disease | 1.2–1.3% | Among patients with suspected meningitis and outpatient clinic | Bahr et al. 49 ; Sekar et al. 50 |
| Chronic pulmonary aspergillosis | Tuberculosis | 6.5% annually in those with TB cavities and 0.2% with no cavities, assumed to be 67% of all TB-related. CPA 20% of TB cases with persistent symptoms (CPA-TB co-infection): Definitive CPA cases were 6.3% among HIV+ and 6.1% among HIV− 8% at end of TB treatment |
The largest study in Africa on post-TB CPA was done in Uganda. All patients were symptomatic. Indonesian study |
Page et al.
17
Namusobya et al. 19 Setianingrum et al. 51 |
| Allergic bronchopulmonary aspergillosis | Adult patients living with asthma | 2.5% of adult asthmatics 3.2% of adult asthmatics with severe asthma |
Among African population Uganda | Benatar et al.
52
Kwizera et al. 23 |
| Severe asthma with fungal sensitization | Adult patients living with asthma | 33% of the worst 10% of adult asthmatics 34.6% of asthma patients have severe persistent asthma |
Among African population East African cohort |
Kwizera et al.
23
Kirenga et al. 33 |
| Candidemia | Intensive care, invasive procedures | 5/100,000 | Country dependent varies from 2 to 11/100,000 | Arendrup
53
Bongomin et al. 54 |
| Candida peritonitis | Post-abdominal surgery, pancreatitis | 50% annual incidence of candidemia in ICU, itself assumed to be 33% of all candidemia | No reliable local and regional data | Montravers 55 |
| Mucormycosis | Uncontrolled diabetes, hematological malignancies | 0.6% | Among patients with deep mycoses diagnosed histologically | Kwizera et al. 15 |
| Mycetoma | Trauma | 0.32/100,000 annual prevalence | Kwizera et al.15,16 | |
| Fungal keratitis | Trauma | 13.3/100,000 | Brown et al. 7 | |
| Tinea capitis | School-age children | 7.1% of children <10 years of age | No locally published data. May be an underestimate | Komba and Mgonda 56 |
| Recurrent vulvovaginal candidiasis (4 or more/year) | Women of reproductive age | 6% of adult women | The prevalence varies between 5% and 8% | Denning et al. 8 |
AML, acute myelogenous leukemia; ART, anti-retroviral therapy; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; PCP, Pneumocystis pneumonia.
In contrast to the prior estimate which only estimated CPA following pulmonary tuberculosis (PTB), we have also modeled mistaken TB diagnoses (19% in HIV-uninfected and 10% in HIV-infected patients, Nigeria), 57 dual CPA and TB infections (6.3% in HIV-infected and 6.2% in HIV-uninfected, Uganda), 19 and CPA arising during or immediately after completion of anti-TB therapy (8%, Indonesia). 51 In TB survivors, we have estimated CPA occurs annually in 6.5% of those left with a cavity (30%) and 0.2% of those without a cavity (Uganda) (Table 2). 17 To calculate prevalence, a 20% year 1 mortality (or surgical resection) was assumed, and 7.5% annual mortality in years 2–5 (overall 50%). 58
The at-risk group of HIV-infected adults was assumed to be <200 × 106/L CD4 count, and this was derived by assuming a 7-year fall in CD4 counts in those not on anti-retroviral therapy (ART) (39,500) and 36% of those without viral load suppression (25,000). 59 The annual incidence of oral candidiasis was assumed to occur in 90% and esophageal candidiasis in 20% of this group and 5% of those on ART.14,60 In adults with advanced HIV disease, the annual incidence of cryptococcal antigenemia was assumed to be 8.6% (Table 2) and Pneumocystis jirovecii pneumonia (PCP) 11% (Table 2) (but a 2-year risk period for PCP).12,14,38,39,46 For PCP in children, it was assumed that 21% of deaths (4800) were attributable to PCP and this was 40% of all PCP cases among the 100,000 HIV-infected children in the country. 61 Disseminated histoplasmosis was estimated at 1% based on recently published data on histoplasmosis in advanced HIV disease in Uganda.15,50,62,63 Table 2 summarizes the epidemiology data and assumptions made in assessing the burden of serious fungal diseases in Uganda.
Estimate of serious fungal diseases in Uganda
We estimate a total of 4,099,357 (about 9% of the Ugandan population) cases of serious fungal diseases a year in Uganda, Table 3.
Table 3.
Estimate of serious fungal diseases in Uganda.
| Serious fungal diseases | Measure | No underlying disease | HIV | Respiratory disease | Hematology/oncology | Critical care + surgery | Rate/100,000 | Total |
|---|---|---|---|---|---|---|---|---|
| Candidiasis | ||||||||
| Recurrent Candida vaginitis (⩾4×/year) | I | 656,340 | 2870 | 656,340 | ||||
| Oral candidiasis | I | 29,057 | 63.5 | 29,057 | ||||
| Esophageal candidiasis | I | 74,686 | 163.3 | 74,686 | ||||
| Candidemia | I | 1601 | 686 | 5.0 | 2287 | |||
| Candida peritonitis | I | 343 | 0.8 | 343 | ||||
| Cryptococcosis | ||||||||
| Cryptococcal meningitis | I | 5553 | 12.1 | 5553 | ||||
| Pneumocystosis | ||||||||
| Pneumocystis pneumonia in adults | I | 4604 | 10.1 | 4604 | ||||
| PCP in children | I | 2100 | 4.6 | 2100 | ||||
| Aspergillosis | ||||||||
| Invasive aspergillosis | I | 680 | 2570 | 357 | 7.9 | 3607 | ||
| CPA | I | 26,241 | 57.4 | 26,241 | ||||
| CPA | P | 63,574 | 137.1 | 63,574 | ||||
| ABPA in asthma | P | 75,931 | 166.0 | 75,931 | ||||
| SAFS | P | 100,228 | 219.1 | 100,228 | ||||
| Superficial and implantation mycoses | ||||||||
| Tinea capitis | P | 3,047,989 | 6664 | 3,047,989 | ||||
| Fungal keratitis | I | 6084 | 13.3 | 6084 | ||||
| Mycetoma | I | 79 | 0.3 | 79 | ||||
| Other mycoses | ||||||||
| Histoplasmosis | I | 646 | 1.4 | 646 | ||||
| Mucormycosis | I | 9 | 0.02 | 9 | ||||
| Total | 4,099,357 |
ABPA, allergic bronchopulmonary aspergillosis; CPA, chronic pulmonary aspergillosis; I, annual incidence; PCP, Pneumocystis jirovecii pneumonia; P, prevalence; SAFS, severe asthma with fungal sensitization.
Discussion
This study aimed to re-evaluate the burden of serious fungal diseases in Uganda. In comparison to the 2015 estimate of about 2.5 million cases of serious fungal diseases in Uganda, our updated analysis revealed an annual burden of 4.1 million, primarily driven by the significant prevalence of tinea capitis (74%). Uganda, being a young country with half of its population being children, predominantly of rural habitation and with poverty, experiences a substantial health impact of superficial mycoses. However, with the publication of more epidemiological data from Uganda, compared to the 2015 estimates, we observe a high burden of CPA (63,574 up from 3347), ABPA (75,931 from 18,700), SAFS (100,228 from 24,684), and cryptococcal meningitis (5553 from 4050), PCP (6704 down from 42,761), IA (3607 up from 389) contributing significantly to morbidity and mortality among Ugandans at risk.
Candidiasis
Candida species are opportunistic fungal pathogens mainly caused by Candida albicans, and other non-albicans Candida species such as C. glabrata (Nakaseomyces glabrata), C. parapsilosis, C. tropicalis, C. krusei (Pichia kudriavzevii), and C. auris. 64 Clinical presentation of candidiasis is broad ranging from mucocutaneous disease such as oropharyngeal, esophageal, and vaginal candidiasis to candidemia and invasive candidiasis.65–67 There are few publications on Candida and candidiasis from Uganda, based on culture.14,68 With an estimated 10,939,000 women of reproductive age in Uganda, we estimated 656,340 cases of recurrent vulvo-vaginal candidiasis (rVVC) based on global estimates of rVVC of 6% among adult women. 8 Our recent meta-analysis, pooling data of 15,723 women from sub-Saharan Africa, found the prevalence of vulvo-vaginal candidiasis (VVC) at 33%, 69 so a rVVC rate of 6% may be an underestimate. Our previous estimate of fungal disease burden in Uganda 3 included 651,600 cases of VVC in pregnancy, as 60% are colonized or infected. 70
With an estimated 1.4 million HIV cases in 2021, including 1.3 million adults, 200,000 not on ART and 100,000 not virally suppressed, 71 about 64,571 PLWH are probably at risk of developing serious OFIs including oropharyngeal candidiasis, esophageal candidiasis, PCP, cryptococcal meningitis, and histoplasmosis. From sub-Saharan Africa, our meta-analysis of HIV-associated esophageal candidiasis found an overall pooled prevalence of 12% in nine studies detailing 113,272 affected. 72 The prevalence was relatively higher in the pre-ART era compared to the ART era (34.1% versus 8.7%). However, the pooled data are small compared to the millions of PLWH in Africa, so this estimate is uncertain.
For non-HIV-associated cases of life-threatening candidiasis, that is candidemia and Candida peritonitis, estimates were based on at-risk populations, particularly patients with hematology-oncology conditions and those receiving critical care and post-abdominal surgery. We used an estimate of candidemia rate of 5/100,000 population, probably a conservative estimate based on other resource-constrained countries.53,54 Many will be undiagnosed as blood cultures are only done in major hospitals,73,74 and in any case are only ~40% sensitive for invasive candidiasis. Without local data, we cannot summarize the species distribution of Candida and other yeasts in bloodstream infections. Of the 686 estimated cases of candidemia following surgery or ICU admission, based on data from Montravers et al., 55 0.5% are estimated to develop Candida peritonitis; therefore, an estimated 343 (686 * 0.5) cases of Candida peritonitis probably occur annually in Uganda. The incidence of cancers has significantly increased in Uganda, with very high mortality rates. 29 Similarly, postoperative mortality following abdominal surgery in Uganda is more than 10%. 75 With limited access to anti-fungal agents such as echinocandins which are recommended for the treatment of candidemia, some of these mortalities might be attributed to undiagnosed, untreated OFIs including candidemia.
Cryptococcosis
Cryptococcosis, predominantly caused by Cryptococcus neoformans, a WHO critical priority pathogen, 5 accounts for 15–20% of AIDS-related deaths annually, with a disproportionate effect on PLWH in East, Central, Southern, and West Africa. 76 In Africa, our recent systematic review has shown that about 28% of people who died with HIV had Cryptococcus isolated from autopsy samples. 77 Cryptococcal meningitis is a common cause of meningitis among adults living with HIV in Uganda. 40 Among 200 Ugandans with culture-documented cryptococcal meningitis, none of the isolates was C. gattii complex. 78 Screening for cryptococcal antigenemia is routine in Uganda among people with advanced HIV disease, with the prevalence of cryptococcal antigenemia of about 8–20% among those with CD4 count of <100,38,40 3% for ART-experienced PLWH. 12 HIV-associated cryptococcal meningitis remains an important cause of mortality among PLWH in Uganda.13,42,43 In this study, we estimate 5553 annual cases of cryptococcal meningitis, assuming an 8.6% annual incidence rate among 64,571 PLWH at risk.
Uganda has made significant strides in the clinical study of cryptococcosis and data emanating from these studies have informed clinical practice including validation of the cryptococcal lateral flow antigen test, 79 timing of anti-retroviral therapy, 43 therapeutic lumbar puncture, 80 the use of single, high-dose amphotericin B, 81 the use of orally bioavailable amphotericin B, 82 and others.
Pneumocystis pneumonia
PCP is an important cause of acute respiratory distress syndrome among PLWH and commonly presents as an index opportunistic infection, particularly among those presenting to care with advanced HIV disease. 83 In a meta-analysis of hospital-based studies across 18 countries in sub-Saharan Africa, the overall prevalence of PCP was 15.4%, with a mortality rate of about 18%. 46 In a more recent meta-analysis that included studies from 15 African countries, the overall prevalence of laboratory-confirmed PCP was found to be 19% among adult PLWH experiencing respiratory symptoms. 84 This prevalence ranged from 15% in studies using microscopy to 22% in studies employing polymerase chain reaction for detection. Interestingly, the prevalence of laboratory-confirmed PCP has remained relatively consistent both in the pre-ART era (1995–2005) at 21% and in the ART era (2006–2020) at 18%. At autopsy, about 7% of people who died with HIV in Africa were found to have Pneumocystis. 77
In the current estimates, we estimate 4604 annual cases of PCP in adults and 2100 in children linked to HIV; we did not estimate non-HIV-related cases. This assumes an 8.6% annual incidence rate among 64,571 adults PLWH. We halved this number given the reduction in the incidence of PCP since the advent of ART and widespread cotrimoxazole prophylaxis in Uganda, thus 7361.1/2 = 3681 cases in adults. With 17,000 HIV-related deaths in 2021, including 4000 children (therefore, 13,000 adults), and an estimated prevalence of PCP of 7.1% from a study conducted in Uganda by Kiragga et al., 13 we estimated a total of 3681 + (13,000 × 0.071) = 4604. In children, we assumed that PCP causes deaths in about 21% 46 based on a recent meta-analysis from Africa, and this could be underestimated by about 2.5-fold. Therefore, PCP in children with HIV probably occurs in about 4000 * 0.21 * 2.5 = 2100 annual cases. Most cases of PCP in Uganda are clinically diagnosed, given the limited access to bronchoscopy and laboratory diagnostics for the definitive diagnosis of the disease. 85 However, the widespread use of co-trimoxazole both in ART care for newly diagnosed PLWH and over-the-counter for the treatment of community-acquired pneumonia has probably contributed to a significant decline in PCP incidence. There are no substantive data to confirm this supposition.
Our prior estimate of serious fungal disease in Uganda 5 also estimated PCP deaths in children with pneumonia, without HIV infection – 2.6% autopsy incidence in children dying of pneumonia (1950 children). Given the lack of up-to-date data on specific microbial causes of death in pneumonia in Uganda, we have not included this in this updated estimate.
Cotrimoxazole (sulfamethoxazole in combination with trimethoprim) is the recommended first-line agent for both prevention and treatment of PCP.83,86 However, resistance to cotrimoxazole, mediated by mutations in the dihydropteroate synthase (DHPS) enzymes, the main target of sulfamethoxazole, which inhibits the synthetic pathway of folate, has emerged due to widespread use of the drug in both HIV and non-HIV populations.87,88 The anti-malarial agent, primaquine is an alternative medication for the treatment of PCP, when combined with clindamycin; however, its usage is challenged by the high burden of glucose-6-phosphate dehydrogenase (G6PD) deficiency in many African countries.89,90 The WHO has guided the use of rapid diagnostic tests for G6PD deficiency in the context of Plasmodium vivax malaria treatment with primaquine. 91 However, data on the utilization of rapid diagnostic tests for G6PD deficiency in the larger context of patients with PCP who would need primaquine for second-line treatment are scarce and are a subject for critical review and further research.
Aspergillosis
Aspergillosis is mainly caused by Aspergillus fumigatus, and occasionally by A. flavus, A. terreus, A. niger, and other species. 92 The disease spectrum is broad and varies significantly in severity from allergic disorders such as ABPA in individuals with asthma and cystic fibrosis, superficial diseases such as keratitis and onychomycosis in immunocompetent individuals, to acute, sub-acute, and chronic pulmonary diseases in persons with underlying pulmonary or systemic immunosuppression. 93
IA is a life-threatening infection that occurs in patients with underlying malignancies, advanced HIV disease, acutely ill patients in the ICU, and those with other conditions such as COPD.37,94,95 We used a prevalence rate of 4% 48 for IA in advanced HIV, to estimate the IPA prevalence rate among Ugandans who died of HIV in 2021 (0.04 × 17,000 = 680). With an estimated population of Ugandans older than 30 years of age at 11,563,325, and COPD prevalence of about 16.2% from van Gemert et al., 25 thus 1,873,259 COPD patients, with about 10.5% hospitalization rate per year, 196,692 admissions per year are anticipated. IA (all pulmonary) probably occurs in about 1.3% (196,692 × 0.013 = 2557) of hospitalized Ugandans with COPD every year. We estimate about 1372 cases of AML in Uganda and 501 cases of lung cancer, resulting in 13 cases of IA among lung cancer patients at a rate of 2.6% and 357 cases of IPA among leukaemia and lymphoma patients. In total, we estimate 3607 annual cases of IPA in Uganda. Orem et al. 96 reported a fatal case of disseminated aspergillosis in an immunocompetent Ugandan woman and Meya et al. 97 reported a case of renal aspergilloma in a Ugandan man who survived following nephrectomy and intravenous amphotericin B therapy.
CPA is a debilitating lung disease that occurs in patients with underlying structural lung diseases. 98 A prospective cohort study conducted by Page et al. 17 evaluated 398 Ugandans after completion of PTB treatment using clinical assessment, chest radiography, and Aspergillus-specific IgG measurement. Of these, 285 were resurveyed 2 years later, including computed tomography of the thorax in 73 with suspected CPA. CPA was confirmed in 14 resurvey patients. The annual rate of new CPA development between surveys was 6.5% in those with chest radiography cavitation and 0.2% in those without. Our recent study from Mulago Hospital (Kampala, Uganda) found that 32 (20%) of 162 patients with PTB who had persistent respiratory symptoms despite completing 2 months of TB treatment had CPA, 19 with patients with CPA-PTB co-infection having a much worse health-related quality of life at the time of TB treatment completion compared to those with PTB alone. 18 We reported death in a patient with CPA complicating PTB, with heart failure, 99 use of point of care lateral flow assay for Aspergillus specific immunoglobulin G and M (IgG/IgM) detection for early screening for CPA 100 and demonstrated that CPA is frequently misdiagnosed as PTB relapse among Ugandans with chronic respiratory symptoms following PTB treatment. 101
To estimate the CPA burden, we used the 2021 WHO TB profile data for Uganda showing an estimated 87,360 Ugandans developing TB every year, including 27,840 HIV-positive cases, and TB mortality of 12,000 including 5952 cases among PLWH, and 43% clinically diagnosed TB. 32 We conducted a multi-level estimate based on our current understanding of the spectrum of TB-associated CPA. We estimate an annual incidence of 26,765 CPA cases. Using an all-cause mortality rate of 20% from the study by Ohba et al., 102 a total of 12,503 patients survive (1759 die) at the end of 12 months. Therefore, CPA probably accounts for 14.7% (1759 of 12,000) of TB-related deaths in newly presenting cases. Our recent work has shown that Ugandans with CPA-PTB co-infection have a poorer health-related quality of life compared to those with only PTB. 18
In Uganda, Page et al. 17 showed that incident CPA cases occur at a rate of 6.5% among TB survivors with cavitation, and 0.2% among those without cavitation. With annual incident cases of 12,297, mortality at one year of 20%, 102 and in years 2–5 of 7.5%, 103 we estimate a 5-year CPA prevalence of 63,574. However, this estimate assumes that CPA only occurs in people who have active, presumed or previously treated TB. Only 60% of CPA cases in Africa are due to TB-related complications. 104 Moreover, positive isolation of Aspergillus from patients with TB is common in Uganda, and usually. A. niger, but additional diagnostic work-up with imaging and antibody detection is required to diagnose aspergillosis.105,106 Other factors such as COPD, recurrent pneumonias, and cavities caused by other infectious diseases such as echinococcosis and nocardiosis also contribute to CPA burden, 104 but are probably accounted for among the smear or GeneXpert-negative cases in Uganda, given the general lack of other diagnostics.
Fungal asthma encompasses ABPA, SAFS, and allergic bronchopulmonary mycosis (ABPM). Kirenga et al.20,21 report a 12.3% asthma rate among Ugandans with a slighter higher burden among PLWH. There is no documented case of cystic fibrosis in Uganda. In Uganda, ABPA occurs in about 3.2% of adult patients living with asthma,23,107 and SAFS in about 33% of most poorly-controlled (10%) of adult asthmatics among African population adult patients living with asthma. 23 ABPM was also documented as a discrete group in 2.9% 17 but we have not added this group to our overall estimate of fungal asthma. About 35% of asthma cohorts in East Africa have severe persistent asthma, with less than 15% having access to inhaled corticosteroids. 33 Previous studies showed that access and affordability to essential diagnostics and medicines for asthma care is sub-optimal in Uganda. 108 Therefore, asthma is underdiagnosed and undertreated with standard asthma therapies in Uganda. On top of this, fungal asthma is clearly a major problem in Uganda and one that would be responsive to antifungal therapy in many patients.
Superficial and implantation mycoses
Superficial fungal diseases contribute to over 1 billion cases annually. 54 However, most superficial fungal infections such as pityriasis versicolor and tinea corporis are mainly asymptomatic and not life-threatening. However, tinea capitis is associated with complications such as kerion and scaring alopecia which affects the quality of life of the affected individuals. A recent systematic review of the burden of tinea capitis in Africa found that about 136 million children suffer from the disease, with a pooled prevalence of 23%. 6 With an estimated 13,252,000 children in Uganda, the burden of tinea capitis is estimated at 3,048,000 cases every year. Recent diagnostic surveys across Africa have shown a marked improvement in the availability and access to essential diagnostics for superficial fungal infections. 109 However, epidemiologic data on tinea capitis in Uganda are generally lacking and many cases of tinea capitis are diagnosed clinically across the country.
The WHO has recognized the ‘implantation mycoses’, mycetoma, sporotrichosis, and chromoblastomycosis as neglected tropical diseases.110,111 A retrospective review of all the biopsy reports at the Pathology Reference Laboratory, Department of Pathology, Makerere University, Kampala, Uganda from January 1950 to September 2019 revealed 249 cases of mycetoma, 16 with 30 cases previously published, so a total of 279 cases of mycetoma published from Uganda. Further histological analysis of 697 cases of deep fungal infections identified subcutaneous ‘phycomycosis’ (the term used in the 1960s and 1970s) (26%), histoplasmosis (9.2%), chromoblastomycosis (4.6%), aspergillosis (3.3%), cryptococcosis (3.3%), blastomycosis (1.6%), coccidioidomycosis (0.6%), mucormycosis (0.6%), and sporotrichosis (0.1%). 15 However, these data do not reflect the national burden of deep fungal diseases in Uganda as the majority of these cases were drawn from a single region. Pathology services remain unavailable in most general hospitals and specialist pathologists tend to concentrate within the city and major tertiary centers. Enhanced histopathological practice and the use of a registry would improve the reporting of deep fungal diseases in Uganda.
Mycotic (fungal) keratitis is a serious infection of the cornea that frequently leads to blindness and the loss of the affected eye. 7 This condition is predominating in regions with tropical and subtropical climates, affecting predominantly young agricultural workers with low socioeconomic status. About 1 million incident cases of fungal keratitis occur annually, particularly in Africa and Asia. 7 At a modest rate of 13.3/100,000 population, we estimate about 6084 annual cases of fungal keratitis in Uganda. Fungal keratitis contributes to over 60% of all cases of microbial keratitis in Uganda.112,113 Treatment remains a challenge, 114 with over 25% of the patients losing their sight or eye, with their overall and ocular quality of life grossly affected. 115 Across Africa, diagnostic procedures for sample collection for pathogen identification remain a major limitation in the diagnosis of fungal keratitis. 116
Other mycoses
Histoplasmosis is an invasive fungal disease that is prevalent in the Ohio and Mississippi river valleys in the United States, as well as in various regions, including Central and South America, Western Africa, Southern Africa, Eastern Africa, Central Africa, and Southeast Asia. The classical form of the disease is caused by Histoplasma capsulatum var capsulatum (under current names of Histoplasma capsulatum sensu stricto, Histoplasma mississippiense, Histoplasma ohiense, Histoplasma suramericanum), while the African type is attributed to Histoplasma capsulatum var duboisii. 117 However, there is uncertainty about species-level identification in this genus in Africa due to limited access to molecular diagnostics. The common mode of Histoplasma infection is through the inhalation of microconidia. In adults, particularly those with HIV/AIDS, this infection poses a significant risk and was officially classified as an AIDS-defining illness in 1987. 118 Histoplasmosis has been shown to occur in Uganda. From our review of histologically diagnosed fungal diseases, about 10% of the cases were due to histoplasmosis. 15 Also about 1.2% of HIV patients in the outpatient settings and those being investigated for acute meningitis have been estimated to have evidence of acute disseminated histoplasmosis.49,50 In our estimate, we assumed a 1% occurrence of disseminated histoplasmosis among the estimated 64,571 PLWH with low CD4 cell counts. This is a reasonable estimate given the available data. However, other manifestations of histoplasmosis such as chronic pulmonary histoplasmosis (CPH) and subclinical histoplasmosis remain to be investigated. A study in the 1950s in Uganda found histoplasmin sensitivity in 10 of 175 (5.7%) screened individuals, with 6 out of the 10 having features of CPH and features of co-infection with TB. 119 A follow-up survey in 1970 found a 3.8% histoplasmin (and 0% coccidioidin) sensitivity in Uganda. 120 However, the sensitivity was much higher among sawmill workers in the industrial city of Jinja, consistent with potentially higher exposure to Histoplasma spores. 121 A recent outbreak of acute pulmonary histoplasmosis following a visit to this area of high histoplasmin sensitivity has been described among short-term stay students who undertook their field trips to this region. 122
Mucorales fungi are responsible for causing mucormycosis, an invasive and swiftly progressing disease of people with diabetes mellitus and immunocompromised individuals but also affecting those with intact immune systems due to trauma.123–125 There are varied sites of involvement of mucormycosis infection with rhino-sino-orbital-cerebral, pulmonary, cutaneous, and gastrointestinal involvement being the most common sites of involvement.123–125
Osaigvovo et al. 126 identified 408 cases of mucormycosis in Africa between 1960 and 2022; most cases (80.9%) were reported from North Africa. The predominant clinical forms were rhino-orbital-cerebral (75.2%) and gastrointestinal (12.5%). Underlying risks included diabetes mellitus (49.8%), COVID-19 (24.8%), malignancies (15.9%), and neutropenia (13.0%). Histopathology was the primary diagnostic method for most cases (72.5%). From our review of histologically diagnosed fungal diseases in Uganda, we only identified four cases of mucormycosis. 15 Mucormycosis is largely unreported in Uganda and presents an opportunity for future research, particularly with the high burden of diabetes, which is poorly controlled in a significant proportion of the patients127,128; recent data on diabetes mellitus in Uganda are lacking.
For diagnostics, all the WHO-listed essential diagnostics for advanced HIV disease including cryptococcal antigen test, histoplasma antigen test, direct microscopy, and fungal culture are available in Uganda, in at least some centers. However, there is only occasional use of Aspergillus serology (for research purposes), and no/very limited access to galactomannan and beta-d-glucan used in the diagnosis of invasive fungal infections129–131 (Table 4). Access to antifungals remains limited. Echinocandins and newer triazoles including posaconazole and isavuconazole are not available in Uganda 129 (Table 5). Available triazoles such as itraconazole and voriconazole are not on the country’s essential medicine list. In addition, there are limited studies on antifungal resistance both in clinical and environmental settings, and is therefore an area for further studies.
Table 4.
Availability of laboratory diagnostic methods for the diagnosis of fungal infections in Uganda.
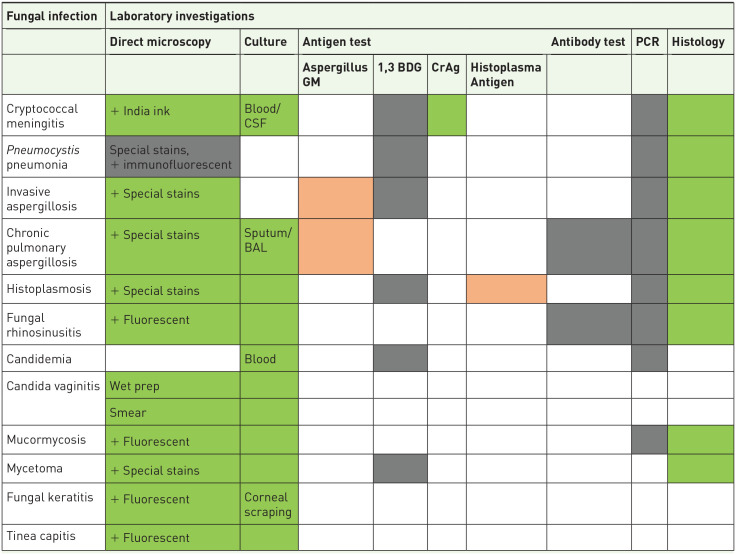
|
Key.
 Not available;
Not available;  Limited availability;
Limited availability;  Widely available; ☐ Not applicable
Widely available; ☐ Not applicable
BAL, bronchoalveolar lavage; 1,3 BDG, 13, beta-D-glucan; CrAg, cryptococcal antigen; CSF, cerebrospinal fluid; GM, galactomannan; PCR, polymerase chain reaction.
Table 5.
Availability of antifungal medications for the treatment of serious fungal infections in Uganda.
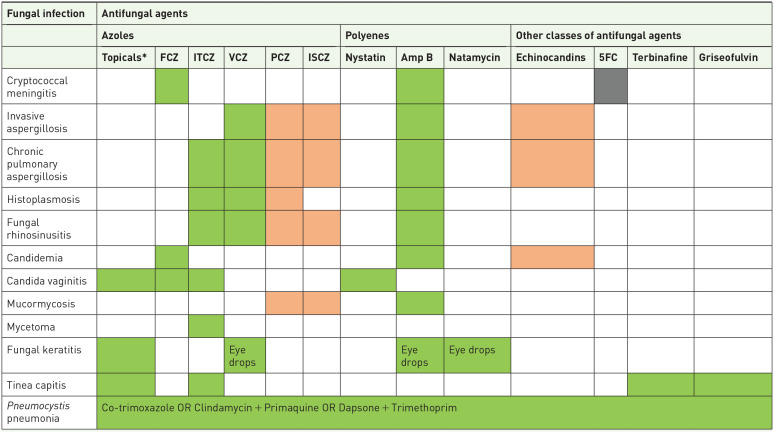
|
Key.
 Not available;
Not available;  Limited availability;
Limited availability;  Widely available; ☐ Not applicable
Widely available; ☐ Not applicable
Topical antifungals include econazole, miconazole, and clotrimazole.
Amp B, amphotericin B; 5FC, 5-fluoro-cytosine (Flucytosine); FCZ, fluconazole; ISCZ, isavuconazole; ITCZ, itraconazole; PCZ, posaconazole; VCZ, voriconazole.
Study limitations
This study is not without limitations. First, there are limited published data on most fungal diseases in Uganda. Cryptococcosis data, the most studied fungal disease in Uganda, are mainly derived from prospective clinical studies which may not translate into a good epidemiological estimate. Also, there is no registry for fungal diseases in Uganda, and fungal diseases are not notifiable to the Ministry of Health.
Conclusion
In conclusion, our study presents a re-evaluation of the burden of serious fungal diseases in Uganda, revealing a substantial annual burden of 4.1 million cases, notably driven by prevalent conditions such as tinea capitis. TB and HIV continue to be pivotal predisposing factors, maintaining a stable burden over the past 5 years. This underscores the urgency for accelerated preventive, diagnostic, and therapeutic interventions to address the considerable impact of fungal diseases on the health landscape of Uganda. Moving forward, future directions should prioritize targeted public health strategies, increased awareness, and enhanced healthcare infrastructure to effectively diagnose, manage, and reduce the burden of fungal diseases among at-risk Ugandans.
Acknowledgments
None.
Footnotes
ORCID iDs: Felix Bongomin  https://orcid.org/0000-0003-4515-8517
https://orcid.org/0000-0003-4515-8517
Richard Kwizera  https://orcid.org/0000-0002-5270-3539
https://orcid.org/0000-0002-5270-3539
Contributor Information
Felix Bongomin, Department of Medical Microbiology and Immunology, Faculty of Medicine, Gulu University, Gulu, Uganda; Manchester Fungal Infection Group, Division of Evolution, Infection and Genomics, Faculty of Biology, Medicine and Health, University of Manchester, Manchester, UK.
Richard Kwizera, Infectious Diseases Institute, School of Medicine, College of Health Sciences, Makerere University, Kampala, Uganda.
Martha Namusobya, Department of Clinical Epidemiology and Biostatistics, School of Medicine, College of Health Sciences, Makerere University, Kampala, Uganda.
Norman van Rhijn, Manchester Fungal Infection Group, Division of Evolution, Infection and Genomics, Faculty of Biology, Medicine and Health, University of Manchester, Manchester, UK.
Bruce J. Kirenga, Department of Medicine, School of Medicine, College of Health Sciences, Makerere University, Kampala, Uganda
David B. Meya, Infectious Diseases Institute, School of Medicine, College of Health Sciences, Makerere University, Kampala, Uganda Department of Medicine, School of Medicine, College of Health Sciences, Makerere University, Kampala, Uganda.
David W. Denning, Manchester Fungal Infection Group, CTF Building, The University of Manchester, Grafton Street, Manchester M13 9NT, UK.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Felix Bongomin: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Richard Kwizera: Writing – original draft; Writing – review & editing.
Martha Namusobya: Writing – original draft; Writing – review & editing.
Norman van Rhijn: Supervision; Writing – original draft; Writing – review & editing.
Irene Andia-Biraro: Writing – original draft; Writing – review & editing.
Bruce J. Kirenga: Data curation; Validation; Writing – original draft; Writing – review & editing.
David B. Meya: Writing – original draft; Writing – review & editing.
David W. Denning: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Carigest SA Conny Naeva Charitable Foundation as part of the ‘Chronic Pulmonary Aspergillosis: Optimization of Therapy, Immunogenetic Screening, and Diagnosis in Uganda [CPA_OPTIONS_Uganda]’ PhD studentship award to Dr Felix Bongomin at the University of Manchester, United Kingdom. CARIGEST SA did not play any role in the design, implementation, and analysis of the study.
The authors declare that there is no conflict of interest.
Availability of data and material: All underlying data have been included in the manuscript.
References
- 1. Ainsworth GC. Introduction to the history of mycology. Cambridge: Cambridge University Press, 1976. [Google Scholar]
- 2. Denning DW. Calling upon all public health mycologists: to accompany the country burden papers from 14 countries. Eur J Clin Microbiol Infect Dis 2017; 36: 923–924. [DOI] [PubMed] [Google Scholar]
- 3. Brown GD, Denning DW, Levitz SM. Tackling human fungal infections. Science 2012; 336: 647. [DOI] [PubMed] [Google Scholar]
- 4. Kumar R, Srivastava V. Application of anti-fungal vaccines as a tool against emerging anti-fungal resistance. Front Fungal Biol 2023; 4: 1241539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization. WHO fungal priority pathogens list to guide research, development and public health action. Geneva, Switzerland: World Health Organization, 2022. [Google Scholar]
- 6. Bongomin F, Olum R, Nsenga L, et al. Estimation of the burden of tinea capitis among children in Africa. Mycoses 2021; 64: 349–363. [DOI] [PubMed] [Google Scholar]
- 7. Brown L, Leck AK, Gichangi M, et al. The global incidence and diagnosis of fungal keratitis. Lancet Infect Dis 2021; 21: e49–e57. [DOI] [PubMed] [Google Scholar]
- 8. Denning DW, Kneale M, Sobel JD, et al. Global burden of recurrent vulvovaginal candidiasis: a systematic review. Lancet Infect Dis 2018; 18: e339–e347. [DOI] [PubMed] [Google Scholar]
- 9. Brandt ME, Park BJ. Think fungus–prevention and control of fungal infections. Emerg Infect Dis 2013; 19: 1688–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parkes-Ratanshi R, Achan B, Kwizera R, et al. Cryptococcal disease and the burden of other fungal diseases in Uganda; where are the knowledge gaps and how can we fill them? Mycoses 2015; 58: 85–93. [DOI] [PubMed] [Google Scholar]
- 11. Kitonsa J, Mayanja Y, Aling E, et al. Factors affecting mortality among HIV positive patients two years after completing recommended therapy for cryptococcal meningitis in Uganda. PLoS One 2019; 14: e0210287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mpoza E, Rajasingham R, Tugume L, et al. Cryptococcal antigenemia in human immunodeficiency virus antiretroviral therapy–experienced Ugandans with virologic failure. Clin Infect Dis 2020; 71: 1726–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kiragga AN, Mubiru F, Kambugu AD, et al. A decade of antiretroviral therapy in Uganda: what are the emerging causes of death? BMC Infect Dis 2019; 19: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rubaihayo J, Tumwesigye NM, Konde-Lule J, et al. Frequency and distribution patterns of opportunistic infections associated with HIV/AIDS in Uganda. BMC Res Notes 2016; 9: 501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kwizera R, Bongomin F, Lukande R. (eds). Deep fungal infections diagnosed by histology in Uganda: a 70-year retrospective study. Med Mycol 2020; 58: 1044–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kwizera R, Bongomin F, Meya DB, et al. Mycetoma in Uganda: a neglected tropical disease. PLoS Negl Trop Dis 2020; 14: e0008240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Page ID, Byanyima R, Hosmane S, et al. Chronic pulmonary aspergillosis commonly complicates treated pulmonary tuberculosis with residual cavitation. Eur Respir J 2019; 53: 1801184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Namusobya M, Bongomin F, Mukisa J, et al. The impact of chronic pulmonary aspergillosis co-infection on the health-related quality of life of patients with pulmonary tuberculosis in Uganda. Mycopathologia 2023; 188: 713–720. [DOI] [PubMed] [Google Scholar]
- 19. Namusobya M, Bongomin F, Mukisa J, et al. Chronic pulmonary aspergillosis in patients with active pulmonary tuberculosis with persisting symptoms in Uganda. Mycoses 2022; 65: 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kirenga BJ, Mugenyi L, de Jong C, et al. The impact of HIV on the prevalence of asthma in Uganda: a general population survey. Respir Res 2018; 19: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kirenga BJ, de Jong C, Katagira W, et al. Prevalence and factors associated with asthma among adolescents and adults in Uganda: a general population based survey. BMC Public Health 2019; 19: 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kwizera R, Bongomin F, Olum R, et al. Fungal asthma among Ugandan adult asthmatics. Med Mycol 2021; 59: 923–933. [DOI] [PubMed] [Google Scholar]
- 23. Kwizera R, Musaazi J, Meya DB, et al. Burden of fungal asthma in Africa: a systematic review and meta-analysis. PLoS One 2019; 14: e0216568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kwizera R, Bongomin F, Olum R, et al. Evaluation of an Aspergillus IgG/IgM lateral flow assay for serodiagnosis of fungal asthma in Uganda. PLoS One 2021; 16: e0252553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Gemert F, Kirenga B, Chavannes N, et al. Prevalence of chronic obstructive pulmonary disease and associated risk factors in Uganda (FRESH AIR Uganda): a prospective cross-sectional observational study. Lancet Glob Heal 2015; 3: e44–e51. [DOI] [PubMed] [Google Scholar]
- 26. Kwizera A, Sendagire C, Kamuntu Y, et al. Building critical care capacity in a Low-Income country. Crit Care Clin 2022; 38: 747–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nkolo EKK, Ejike JC, Sensalire S, et al. Clients in Uganda accessing preferred differentiated antiretroviral therapy models achieve higher viral suppression and are less likely to miss appointments: a cross-sectional analysis. J Int AIDS Soc 2023; 26(Suppl. 1): e26122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wabinga HR, Nambooze S, Amulen PM, et al. Trends in the incidence of cancer in Kampala, Uganda 1991–2010. Int J Cancer 2014; 135: 432–439. [DOI] [PubMed] [Google Scholar]
- 29. Natukunda B, Omoding A, Bongomin F, et al. One-Year survival and prognosticators of adults with acute leukemia at the Uganda cancer institute. J Glob Oncol 2023; 9: e2200244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. United Nations Population Dynamics. Department of Economic and Social Affairs, https://www.un.org/development/desa/en/key-issues/population.html (2020, accessed 3 May 2020).
- 31. The HIV and AIDS Uganda Country Progress Report. 2014. Minist Heal Uganda 2015, http://www.unaids.org/sites/default/files/country/documents/UGA_narrative_report_2015.pdf (2015, accessed 20 November 2017).
- 32. World Health Organization. Uganda tuberculosis profile. World Health Organization, Geneva, Switzerland, 2021. [Google Scholar]
- 33. Kirenga B, Chakaya J, Yimer G, et al. Phenotypic characteristics and asthma severity in an East African cohort of adults and adolescents with asthma: findings from the African severe asthma project. BMJ Open Respir Res 2020; 7: e000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Atumanya P, Sendagire C, Wabule A, et al. Assessment of the current capacity of intensive care units in Uganda; a descriptive study. J Crit Care 2020; 55: 95–99. [DOI] [PubMed] [Google Scholar]
- 35. International Agency for Research on Cancer (IARC). GLOBOCAN 2020: Estimated cancer incidence, mortality and prevalence worldwide in 2018. International Agency for Research on Cancer, http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx (2020, accessed 12 July 2020).
- 36. Worldometers. Uganda Population, Dover, Delaware, 2020. [Google Scholar]
- 37. Hammond EE, McDonald CS, Vestbo J, et al. The global impact of Aspergillus infection on COPD. BMC Pulm Med 2020; 20: 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oyella J, Meya D, Bajunirwe F, et al. Prevalence and factors associated with cryptococcal antigenemia among severely immunosuppressed HIV-infected adults in Uganda: a cross-sectional study. J Int AIDS Soc 2012; 15: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Meya DB, Manabe YC, Castelnuovo B, et al. Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count < or = 100 cells/µL who start HIV therapy in resource-limited settings. Clin Infect Dis 2010; 51: 448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ellis J, Bangdiwala AS, Cresswell FV, et al. The changing epidemiology of HIV-associated adult meningitis, Uganda 2015–2017. Open Forum Infect Dis 2019; 6: ofz419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Flynn AG, Meya DB, Hullsiek KH, et al. Evolving failures in the delivery of human immunodeficiency virus care: lessons from a Ugandan meningitis cohort 2006–2016. Open Forum Infect Dis 2017; 4: ofx077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kambugu A, Meya D, Rhein J, et al. Outcomes of cryptococcal meningitis in Uganda before and after the availability of highly active antiretroviral therapy. Clin Infect Dis 2008; 46: 1694–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Boulware DR, Meya DB, Muzoora C, et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. New Engl J Med 2014; 370: 2487–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Namutebi AM, Kamya MR, Byakika-Kibwika P. Causes and outcome of hospitalization among HIV-infected adults receiving antiretroviral therapy in Mulago Hospital, Uganda. Afr Health Sci 2013; 13: 977–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Taylor SM, Meshnick SR, Worodria W, et al.; International HIV-associated Opportunistic Pneumonias (IHOP) Study. Low prevalence of Pneumocystis pneumonia (PCP) but high prevalence of Pneumocystis dihydropteroate synthase (dhps) gene mutations in HIV-Infected persons in Uganda. PLoS One 2012; 7(11): e49991. DOI: 10.1371/journal.pone.0049991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wasserman S, Engel ME, Griesel R, et al. Burden of Pneumocystis pneumonia in HIV-infected adults in sub-Saharan Africa: a systematic review and meta-analysis. BMC Infect Dis 2016; 16: 482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lortholary O, Meyohas M-C, Dupont B, et al. Invasive aspergillosis in patients with acquired immunodeficiency syndrome: report of 33 cases. French Cooperative Study Group on aspergillosis in aids. Am J Med 1993; 95: 177–187. [DOI] [PubMed] [Google Scholar]
- 48. Denning DW, Morgan EF. Quantifying deaths from aspergillosis in HIV positive people. J Fungi 2022; 8: 1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bahr NC, Sarosi GA, Meya DB, et al. Seroprevalence of histoplasmosis in Kampala, Uganda. Med Mycol 2016; 54: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sekar P, Nalintya E, Kwizera R, et al. Prevalence of histoplasma antigenuria among outpatient cohort with advanced HIV in Kampala, Uganda. J Fungi 2023; 9: 757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Setianingrum F, Rozaliyani A, Adawiyah R, et al. A prospective longitudinal study of chronic pulmonary aspergillosis in pulmonary tuberculosis in Indonesia (APICAL). Thorax 2022; 77: 821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Benatar SR, Keen GA, Du Toit Naude W. Aspergillus hypersensitivity in asthmatics in Cape Town. Clin Allergy 1980; 10: 285–291. [DOI] [PubMed] [Google Scholar]
- 53. Arendrup MC. Epidemiology of invasive candidiasis. Curr Opin Crit Care 2010; 16: 445–452. [DOI] [PubMed] [Google Scholar]
- 54. Bongomin F, Gago S, Oladele RO, et al. Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi 2017; 3: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Montravers P, Mira JP, Gangneux JP, et al.; AmarCand study group. A multicentre study of antifungal strategies and outcome of Candida spp. peritonitis in intensive-care units. Clin Microbiol Infect 2011; 17: 1061–1067. [DOI] [PubMed] [Google Scholar]
- 56. Komba EV, Mgonda YM. The spectrum of dermatological disorders among primary school children in Dar es Salaam. BMC Public Health 2010; 10: 765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Oladele RO, Irurhe NK, Foden P, et al. Chronic pulmonary aspergillosis as a cause of smear-negative TB and/or TB treatment failure in Nigerians. Int J Tuberc Lung Dis 2017; 21: 1056–1061. [DOI] [PubMed] [Google Scholar]
- 58. Denning DW, Cole DC, Ray A. New estimation of the prevalence of chronic pulmonary aspergillosis (CPA) related to pulmonary TB – a revised burden for India. IJID Reg 2023; 6: 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ewings FM, Bhaskaran K, McLean K, et al. Survival following HIV infection of a cohort followed up from seroconversion in the UK. AIDS 2008; 22: 89–95. [DOI] [PubMed] [Google Scholar]
- 60. Fungal Infection Trust. How common are fungal diseases ? 2017. https://fungalinfectiontrust.org/ (accessed 30 January 2024). [Google Scholar]
- 61. Schwartz IS, Boyles TH, Kenyon CR, et al. (eds). The estimated burden of fungal disease in South Africa. South African Med J 2019; 109: 885. [Google Scholar]
- 62. Nalwanga D, Henning L. If it looks like a duck, swims like a duck, and quacks like a duck—does it have to be a duck? PLoS Negl Trop Dis 2016; 10(4): e0004430. DOI: 10.1371/journal.pntd.0004430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kiggundu R, Nabeta H, Okia R, et al. Unmasking histoplasmosis immune reconstitution inflammatory syndrome in a patient recently started on antiretroviral therapy. Autops Case Reports 2016; 6: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kullberg BJ, Arendrup MC, Arendrup MC. Invasive candidiasis. New Engl J Med 2015; 373: 1445–1456. [DOI] [PubMed] [Google Scholar]
- 65. McCarty TP, Pappas PG. Invasive candidiasis. Infect Dis Clin North Am 2016; 30: 103–124. [DOI] [PubMed] [Google Scholar]
- 66. Vazquez JA, Sobel JD. Mucosal candidiasis. Infect Dis Clin North Am 2002; 16: 793–820. [DOI] [PubMed] [Google Scholar]
- 67. Pallotta F, Viale P, Barchiesi F. Candida auris: the new fungal threat. J Infez Med 2023; 31: 323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mukasa KJ, Herbert I, Daniel A, et al. Antifungal susceptibility patterns of vulvovaginal Candida species among women attending antenatal clinic at Mbarara Regional Referral Hospital, South Western Uganda. Br Microbiol Res J 2015; 5: 322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mushi MF, Olum R, Bongomin F. Prevalence, antifungal susceptibility and etiology of vulvovaginal candidiasis in sub-Saharan Africa: a systematic review with meta-analysis and meta-regression. Med Mycol 2022; 60: myac037 [DOI] [PubMed] [Google Scholar]
- 70. Tann CJ, Mpairwe H, Morison L, et al. Lack of effectiveness of syndromic management in targeting vaginal infections in pregnancy in Entebbe, Uganda. Sex Transm Infect 2006; 82: 285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. UNAIDS. UNAIDS Country factsheet-Uganda. UNAIDS Secretariat. Geneva, Switzerland, 2021. [Google Scholar]
- 72. Olum R, Baluku JB, Okidi R, et al. Prevalence of HIV-associated esophageal candidiasis in sub-Saharan Africa: a systematic review and meta-analysis. Trop Med Health 2020; 48: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tufa TB, Bongomin F, Fathallah A, et al. Access to the World Health Organization-recommended essential diagnostics for invasive fungal infections in critical care and cancer patients in Africa: a diagnostic survey. J Infect Public Health 2023; 16: 1666–1674. [DOI] [PubMed] [Google Scholar]
- 74. Bongomin F, Ekeng BE, Kibone W, et al. Invasive fungal diseases in Africa: a critical literature review. J Fungi 2022; 8: 1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Onen BC, Semulimi AW, Bongomin F, et al. Surgical Apgar score as a predictor of outcomes in patients following laparotomy at Mulago National Referral Hospital, Uganda: a prospective cohort study. BMC Surg 2022; 22: 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rajasingham R, Govender NP, Jordan A, et al. The global burden of HIV-associated cryptococcal infection in adults in 2020: a modelling analysis. Lancet Infect Dis 2022; 22: 1748–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bongomin F, Kibone W, Atulinda L, et al. Frequency of fungal pathogens in autopsy studies of people who died with HIV in Africa: a scoping review. Clin Microbiol Infect 2023: S1198-743X(23)00624-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wembabazi A, Nassozi DR, Akot E, et al. Prevalence of Cryptococcus gattii in Ugandan HIV-infected patients presenting with cryptococcal meningitis. PLoS One 2022; 17: e0270597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Boulware DR, Rolfes MA, Rajasingham R, et al. Multisite validation of cryptococcal antigen lateral flow assay and quantification by laser thermal contrast. Emerg Infect Dis 2014; 20: 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rolfes MA, Hullsiek KH, Rhein J, et al. The effect of therapeutic lumbar punctures on acute mortality from cryptococcal meningitis. Clin Infect Dis 2014; 59: 1607–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jarvis JN, Lawrence DS, Meya DB, et al. (eds). Single-dose liposomal amphotericin B treatment for cryptococcal meningitis. N Engl J Med 2022; 386: 1109–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Boulware D, Atukunda M, Kagimu E, et al. Oral lipid nanocrystal amphotericin B for cryptococcal meningitis: a randomized clinical trial. Clin Infect Dis 2023; 77: 1659–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Masur H, Michelis MA, Greene JB, et al. An outbreak of community-Acquired Pneumocystis carinii pneumonia. New Engl J Med 1981; 305: 1431–1438. [DOI] [PubMed] [Google Scholar]
- 84. Wills NK, Lawrence DS, Botsile E, et al. The prevalence of laboratory-confirmed Pneumocystis jirovecii in HIV-infected adults in Africa: a systematic review and meta-analysis. Med Mycol 2021; 59: 802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Global Action for Fungal Infections (GAFFI). Fungal Diagnostic Survey Africa 2022: Appendix 4 - Country Reports. Glob Action Fungal Infect 2022. https://gaffi.org/wp-content/uploads/GAFFI-Survey-Appendix-4-2022-Final_2.pdf (2022, accessed October 4, 2023).
- 86. Wood AJJ, Masur H. Prevention and treatment of Pneumocystis pneumonia. New Engl J Med 1992; 327: 1853–1860. [DOI] [PubMed] [Google Scholar]
- 87. Ponce CA, Chabé M, George C, et al. High prevalence of Pneumocystis Jirovecii dihydropteroate synthase gene mutations in patients with a first episode of Pneumocystis pneumonia in Santiago, Chile, and clinical response to trimethoprim-sulfamethoxazole therapy. Antimicrob Agents Chemother 2017; 61: e01290-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Masur H, Brooks JT, Benson CA, et al. Prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: updated Guidelines from the Centers for Disease Control and Prevention, National Institutes of Health, and HIV Medicine Association of the Infectious Diseases Soc. Clin Infect Dis 2014; 58: 1308–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Youngster I, Arcavi L, Schechmaster R, et al. Medications and glucose-6-phosphate dehydrogenase deficiency. Drug Saf 2010; 33: 713–726. [DOI] [PubMed] [Google Scholar]
- 90. Zhang Z, Li Q, Shen X, et al. The medication for Pneumocystis pneumonia with glucose-6-phosphate dehydrogenase deficiency patients. Front Pharmacol 2022; 13: 957376. DOI: 10.3389/fphar.2022.957376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. World Health Organization (WHO). Guide to G6PD deficiency rapid diagnostic testing to support P. Vivax radical cure. World Heal Organ 2018; 34. https://www.who.int/publications/i/item/9789241514286 [Google Scholar]
- 92. Sugui JA, Kwon-Chung KJ, Juvvadi PR, et al. Aspergillus fumigatus and related species. Cold Spring Harb Perspect Med 2015; 5: a019786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax 2015; 70: 270–277. [DOI] [PubMed] [Google Scholar]
- 94. Segal BH. Aspergillosis. New Engl J Med 2009; 360: 1870–1884. [DOI] [PubMed] [Google Scholar]
- 95. Hope WW, Walsh TJ, Denning DW. Laboratory diagnosis of invasive aspergillosis. Lancet Infect Dis 2005; 5: 609–622. [DOI] [PubMed] [Google Scholar]
- 96. Orem J, Mpanga L, Habyara E, et al. Disseminated Aspergillus fumigatus infection: case report. East Afr Med J 1998; 75: 436–438. [PubMed] [Google Scholar]
- 97. Meya D, Lwanga I, Ronald A, et al. A renal aspergilloma–an unusual presentation of aspergillosis in an HIV patient. Afr Health Sci 2005; 5: 341–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Denning DW, Riniotis K, Dobrashian R, et al. Chronic cavitary and fibrosing pulmonary and pleural aspergillosis: case series, proposed nomenclature change, and review. Clin Infect Dis 2003; 37(Suppl. 3): S265–S280. [DOI] [PubMed] [Google Scholar]
- 99. Bongomin F, Kwizera R, Atukunda A, et al. Cor pulmonale complicating chronic pulmonary aspergillosis with fatal consequences: experience from Uganda. Med Mycol Case Rep 2019; 25: 22–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kwizera R, Katende A, Teu A, et al. Algorithm-aided diagnosis of chronic pulmonary aspergillosis in low- and middle-income countries by use of a lateral flow device. Eur J Clin Microbiol Infect Dis 2020; 39: 1–3. [DOI] [PubMed] [Google Scholar]
- 101. Kwizera R, Katende A, Bongomin F, et al. Misdiagnosis of chronic pulmonary aspergillosis as pulmonary tuberculosis at a tertiary care center in Uganda: a case series. J Med Case Rep 2021; 15: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ohba H, Miwa S, Shirai M, et al. Clinical characteristics and prognosis of chronic pulmonary aspergillosis. Respir Med 2012; 106: 724–729. [DOI] [PubMed] [Google Scholar]
- 103. Lowes D, Al-Shair K, Newton PJ, et al. Predictors of mortality in chronic pulmonary aspergillosis. Eur Respir J 2017; 49: 1601062. [DOI] [PubMed] [Google Scholar]
- 104. Olum R, Osaigbovo II, Baluku JB, et al. Mapping of chronic pulmonary aspergillosis in Africa. J Fungi 2021; 7: 790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Buyi J, Mwambi B, Taremwa IM. Prevalence of pulmonary aspergillosis and drug susceptibility testing amongst adult HIV patients at Kisugu Health Centre III, Makindye Division, in Uganda. Student’s J Heal Res Africa 2023; 4: 9. [Google Scholar]
- 106. Njovu IK, Musinguzi B, Mwesigye J, et al. Status of pulmonary fungal pathogens among individuals with clinical features of pulmonary tuberculosis at Mbarara University Teaching Hospital in Southwestern Uganda. Ther Adv Infect Dis 2021; 8: 20499361211042476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kwizera R, Bongomin F, Olum R, et al. Prevalence of Aspergillus fumigatus skin positivity in adults without an apparent/known atopic disease in Uganda. Ther Adv Infect Dis 2021; 8: 20499361211039040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kibirige D, Kampiire L, Atuhe D, et al. Access to affordable medicines and diagnostic tests for asthma and COPD in sub Saharan Africa: the Ugandan perspective. BMC Pulm Med 2017; 17: 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Badiane AS, Ramarozatovo LS, Doumbo SN, et al. Diagnostic capacity for cutaneous fungal diseases in the African continent. Int J Dermatol 2023; 62: 1131–1141. [DOI] [PubMed] [Google Scholar]
- 110. Hay R, Denning D, Bonifaz A, et al. The diagnosis of fungal neglected tropical diseases (Fungal NTDs) and the role of investigation and laboratory tests: an expert consensus report. Trop Med Infect Dis 2019; 4: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hotez PJ, Aksoy S, Brindley PJ, et al. What constitutes a neglected tropical disease? PLoS Negl Trop Dis 2020; 14: e0008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Arunga S, Kintoki GM, Mwesigye J, et al. Epidemiology of microbial keratitis in Uganda: a Cohort study. Ophthalmic Epidemiol 2020; 27: 121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Arunga S, Kwaga T, Leck A, et al. Bilateral Candida keratitis in an HIV patient with asymptomatic genitourinary candidiasis in Uganda. Med Mycol Case Rep 2018; 22: 14–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Arunga S, Mbarak T, Ebong A, et al. Chlorhexidine gluconate 0.2% as a treatment for recalcitrant fungal keratitis in Uganda: a pilot study. BMJ Open Ophthalmol 2021; 6(1): e000698. DOI: 10.1136/bmjophth-2020-000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Arunga S, Wiafe G, Habtamu E, et al. The impact of microbial keratitis on quality of life in Uganda. BMJ Open Ophthalmol 2019; 4(1): e000351. DOI: 10.1136/bmjophth-2019-000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Makangara Cigolo JC, Oladele RO, Kennedy SB, et al. Diagnostic capacity for fungal keratitis in Africa – survey in 50 African countries. Ocul Surf 2023; 30: 139–141. [DOI] [PubMed] [Google Scholar]
- 117. Kidd SE, Abdolrasouli A, Hagen F. Fungal nomenclature: managing change is the name of the game. Open Forum Infect Dis 2023; 10: ofac559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wheat LJ, Azar MM, Bahr NC, et al. Histoplasmosis. Infect Dis Clin North Am 2016; 30: 207–227. [DOI] [PubMed] [Google Scholar]
- 119. Ball JD, Evans PR. Histoplasmin sensitivity in Uganda. BMJ 1954; 2: 848–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Farsey S, Bezjak V. Prevalence of skin sensitivity to histoplasmin and coccidioidin in various Ugandan populations. Am J Trop Med Hyg 1970; 19: 664–669. [DOI] [PubMed] [Google Scholar]
- 121. Bezjak V. Histoplasmin tests in Ugandan sawmill workers. Trop Geogr Med 1971; 23: 71–78. [PubMed] [Google Scholar]
- 122. Cottle LE, Gkrania-Klotsas E, Williams HJ, et al. A multinational outbreak of histoplasmosis following a biology field trip in the Ugandan rainforest. J Travel Med 2013; 20: 83–87. [DOI] [PubMed] [Google Scholar]
- 123. Gamaletsou MN, Sipsas NV, Roilides E, et al. Rhino-orbital-cerebral mucormycosis. Curr Infect Dis Rep 2012; 14: 423–434. [DOI] [PubMed] [Google Scholar]
- 124. Chmel H, Grieco MH. Cerebral mucormycosis and renal aspergillosis in heroin addicts without endocarditis. Am J Med Sci 1973; 266: 225–231. [DOI] [PubMed] [Google Scholar]
- 125. Kennedy KJ, Daveson K, Slavin MA, et al.; Australia and New Zealand Mycoses Interest Group of the Australasian Society for Infectious Diseases. Mucormycosis in Australia: contemporary epidemiology and outcomes. Clin Microbiol Infect 2016; 22: 775–781. [DOI] [PubMed] [Google Scholar]
- 126. Osaigbovo II, Ekeng BE, Davies AA, et al. Mucormycosis in Africa: epidemiology, diagnosis and treatment outcomes. Mycoses 2023; 66: 555–562. [DOI] [PubMed] [Google Scholar]
- 127. Nambuya A, Otim M, Whitehead H, et al. The presentation of newly-diagnosed diabetic patients in Uganda. QJM 1996; 89: 705–711. [DOI] [PubMed] [Google Scholar]
- 128. Bateganya MH, Nambuya L, Jr, Otim AP. Morbidity and mortality among diabetic patients admitted to Mulago Hospital, Uganda. Malawi Med J 2003; 15: 91–94. [PMC free article] [PubMed] [Google Scholar]
- 129. Bongomin F, Ekeng BE, Kwizera R, et al. Fungal diseases in Africa: closing the gaps in diagnosis and treatment through implementation research and advocacy. J Med Mycol 2023; 33: 101438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Driemeyer C, Falci DR, Oladele RO, et al. The current state of clinical mycology in Africa: a European Confederation of Medical Mycology and International Society for Human and Animal Mycology survey. Lancet Microbe 2022; 3: e464–e470. [DOI] [PubMed] [Google Scholar]
- 131. Bongomin F, Govender NP, Chakrabarti A, et al. Essential in vitro diagnostics for advanced HIV and serious fungal diseases: international experts’ consensus recommendations. Eur J Clin Microbiol Infect Dis 2019; 38: 1581–1584. [DOI] [PubMed] [Google Scholar]


