Abstract
Cryptomeriajaponicavar.sinensis Miquel in south China is currently overwhelmingly infested by a native caterpillar species, Dendrolimushoui (Lepidoptera), which is causing severe economic losses and ecological disasters in both planted and natural forests. Our results include report of five parasitoid species and eight parasitoid flies within D.houi and a dominant endoparasitoid species Kriechbaumerelladendrolimi, which attacks pupae of D.houi with a high parasitism rate. This result might be helpful to improve better identification and application in the future for potential biological control of D.houi in the forests of east Asia.
Keywords: Cryptomeriajaponica , Dendrolimushoui , parasitoid, natural enemy, defoliator
Introduction
Dendrolimushoui (Lajonquiere) (Lepidoptera, Lasiocampidae) is a species of caterpillar that feeds on the leaves and branch tips of coniferous species, such as Cryptomeriajaponicavar.sinensis Miquel, Pinusyunnanensis Faranch. and Pinuskesiya Royle ex Gordon (Kong et al. 2007), causing large losses of branches and leaves, preventing the normal growth and development of hosts and eventually resulting in dying forests across South Asian regions including India, Burma, Sri Lanka, Indonesia and the south of China. Caterpillars have reportedly destroyed 50,000 hectares of C.japonica in Zhejiang Province, China, in 2013 (Zhang 2013); thousands of hectares of Cupressusfunebris Endl. in Sichuan Province (He et al. 2018); and 8800 hectares of P.kesiya west of Yunnan Province (Yang 2015), which has significantly reduced the local forest quality and negatively affected the scenic landscape, especially in some national parks and natural reserve zones. Additionally, these caterpillars often cause painful allergies amongst tourists when contacting the venomous seta densely adhering on the larval skin (Luo et al. 1998).
The commercial insecticide Sendebao (0.18% abamectin plus Bacillusthuringiensis at 108 spores/g) has been broadly used to control D.houi in large areas, which has effectively reduced the population of caterpillars and protected local forests (Zhang 2013). Unfortunately, this chemical control has also been proven to have a negative impact on populations of natural enemy species of this caterpillar and overuse of this pesticide might lead to negative effects, including chemical resistance and resurgence of the pest species in the environment (Caniço et al. 2020). Biological control has gradually attracted the attention of entomologists due to its environmentally friendly nature. These successful examples suggest that biological control using parasitoid species can be a sustainable and effective tool for the suppression of pest populations without repeated biocontrol agent release (Vargas et al. 2007, Vargas et al. 2013). In the past three decades, 22 species of parasitic wasps and parasitic flies within D.houi have been discovered and identified (Lin et al. 2017, Liang et al. 2018). However, to date, little is known about the biology of any parasitoid species or about the morphology of adult and immature stages.
Here, we conducted a survey of the various parasitoid species that attack D.houi and further collected and identified all natural enemies of D.houi after laboratory rearing. The biological characteristics of parasitic species were also examined to preliminarily assess their potential for release as biological control agents (Holthouse et al. 2020, Kinyanjui et al. 2021).
Materials and Methods
Summary of sampling sites
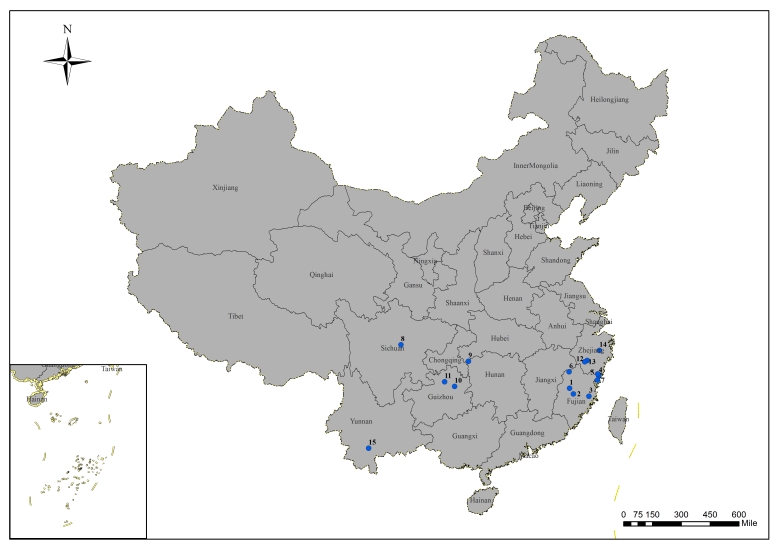
According to the distribution of C.japonica forest and its infestation by D.houi, 15 representative sites were located and investigated in five provinces in China (Fig. 1): Fujian (26°35′33.23″ N, 117°31′17.18″ E, 882 m a.s.l., Guiyang Village, Jiangle County, Fujian Province, FJG; 26°8′28.94″ N, 117°45′31.43″ E, 901 m a.s.l., Chengqian Village, Sha County, Fujian Province, FSC; 25°46′29.49″ N, 118°59′8.37″ E, 915 m a.s.l., Duishan Village, Yongtai County, Fujian Province, FYD; 27°7′41.18″ N, 120°5′5.14″ E, 422 m a.s.l., Youkeng Village, Fuding County, Fujian Province, FFY; 27°16′46.26″ N, 120°2′13.21″ E, 603 m a.s.l., Chayang Village, Fuding County, Fujian Province, FFC; 27°48′52.32″ N, 117°42′36.16″ E, 1108 m a.s.l., Wuyi Mountain, Wuyishan County, Fujian Province, FWW; and 26°49′47.74″ N, 119°54′28.67″ E, 578 m a.s.l., Yangmeiling Forest Park, Xiapu County, Fujian Province, FXY), Sichuan (30°45′1.26″ N, 103°28′30.72″ E, 662 m a.s.l., Zuling Temple, Chongzhou County, Sicuan Province, SCZ), Hubei (29°24′45.20″ N, 109°18′5.50″ E, 895 m a.s.l., Guimao Mountain, Laifeng County, Hubei Province, HLG), Guizhou (27°34'56.05″ N, 108°1'49.70″ E, 787 m a.s.l., Sanxing Village, Sinan County, Guizhou Province, GSS; 27°56′55.15″ N, 107°11′56.94″ E, 1022 m a.s.l., Longli County, Qiangnan County, Guizhou Province, GQL), Yunnan (22°59′36.1 3″ N, 101°05′31.39″ E, 561 m a.s.l. Jingdong Yi Autonomous County, Pu'er City, Yunnan Province, YPJ) and Zhejiang (28°24′46.33″ N, 119°22′35.09″ E, 462 m a.s.l., Nandai Village, Songyang County, Zhejiang Province, ZSN; 28°21′21.1″ N, 119°8′40.91″ E, 1002 m a.s.l., Guiyang Village, Suichang County, Zhejiang Province, ZSG; 28°58′57.84″ N, 120°33′4.8″ E, 698 m a.s.l., Dapan Forest Farm, Pan'an County, Zhejiang Province, ZPD). Different samples of D.houi were individually collected, numbered and reared separately.
Figure 1.
Blue dots indicate deployment and collection sites of wild larvae and pupae masses in China, 2016-2019. Dendrolimushoui was discovered at Sites 1 and 15. Geographical coordinates are as follows: Site 1 (FJG): 26°35'33.23" N, 117°31'17.18" E; Site 2 (FSC): 26°8'28.94" N, 117°45'31.43" E; Site 3 (FYD): 25°46'29.49" N, 118°59'8.37" E; Site 4 (FFY): 27°7'41.18" N, 120°5'5.14" E; Site 5 (FFC): 27°16'46.26" N, 120°2'13.21" E; Site 6 (FWW): 27°48'52.32" N, 117°42'36.16" E; Site 7 (FXY): 26°49'47.74" N, 119°54'28.67" E; Site 8 (SCZ): 30°45'1.26" N, 103°28'30.72" E; Site 9 (HLG): 29°24'45.20" N, 109°18'5.50" E; Site 10 (GSS): 27°34'56.05" N, 108°1'49.70" E; Site 11 (GQL): 27°56'55.15" N, 107°11'56.94" E; Site 12 (ZSN): 28°24'46.33" N, 119°22'35.09" E; Site 13 (ZSG): 28°21'21.1" N, 119°8'40.91" E; Site 14 (ZPD): 28°58'57.84" N, 120°33'4.8" E; Site 15 (YPJ) 22°59'36.13" N, 101°05'31.39" E.
Collection, rearing and identification of parasitic wasps and parasitic flies
In the places described, branches containing pupae of D.houi were removed and observed in the lab. The hosts were maintained at 26 ± 1℃, 50 ± 10% relative humidity (R.H.) and 12/12 h light/dark (L.D.) photoperiod for 25 days during which they emerged (Fig. 2). All parasitoids were collected and fed with 100% honey in a screened plastic box (29 × 17 × 9 cm) at 25℃ and 12:12 h L.D. photoperiod after emerging from the hosts (Fig. 3). Then, all the specimens were kept in 75% ethanol. The morphological characteristics of parasitoids were observed under a microscope (SZ760B, Optec, Chongqing, China). Some specimens were sent to senior taxonomists for species identification after all specimens were preliminarily identified, based on morphological characteristics according to Huang (2003a), Huang (2003b), Yang et al. (2015) and Zhang (2016). Voucher specimens were preserved at Fujian Agriculture and Forestry University.
Figure 2.
Mass rearing of Dendrolimushoui.
Figure 3.
Rearing of parasitoid wasps.
Biological characteristics of parasitoids and determination of dominant parasitoids
During the rearing process (26 ± 1°C, 50 ± 10% R.H.), after adults emerged, the number of offspring was recorded every day, distinguishing between male and female by ovipositor or body size. The important parasitoid species were selected from a list of parasitic wasps and parasitic flies, based on mean criteria including: parasitism rate, sex ratio, longevity and fecundity. The parasitism rate was calculated by dividing all the emerged parasitoids by all the host pupae.
Parasitoid emergence rate was calculated as the number of parasitoids emerging from the host divided by the total number of pupae multiplied by 100. The sex ratio was calculated as the number of females divided by the number of males. Longevity was the time period from emergence of parasitoids to death. Fecundity was the number of offspring per pupa.
Results
Some parasitoid species within D.houi pupae and other hosts
A total of 13 species from five families and two orders of parasitoids were identified: five parasitoid species from three families containing Xanthopimplakonowi (Krieger), Habronyxpyretorum (Cameron), Theroniadepressa (Gupta), Kriechbaumerelladendrolimi (Sheng et Zhong) and Dibrachysyunnanensis (Yang). There are eight parasitoid flies from five genera and two families containing Carcelia (Carcelia) illiberisi Chao et Liang, Carcelia (Carcelia) nigrantennata Chao et Liang, Carcelia (Carcelia) flavimaculata Sun et Chao, Drino (Palexorista) inconspicuoides (Baranov), Blepharipazebina (Walker), Mikiatepens (Walker), Sarcophaga (Sarcorohdendorfia) gracilior (Chen) and Sarrorohdeneantelope (Bottcher), of which, H.pyretorum, C.flavimaculata and M.tepens were new parasitoids recorded for D.houi (Table 1).
Table 1.
Parasitc species within D.houi pupae and other hosts.
| NO. | Order | Family | Species | Hosts | References |
| 1 | Hymenoptera | Ichneumonidae | Xanthopimplakonowi | D.houi, D.punctatus, Attacusarlas, Antheraeapernyi, Philosamiacynthia, Saturniapyretorm, Antheraeafrithi, Antheraeapolyphemus, Attacusdohertyi, Criculatrifenestrata, Malacosomaneustriatestacea | Huang (2003a) |
| 2 | Habronyxpyretorum | *D.houi, Dictyoplocajaponica, E.pyretorum | Huang (2003a) | ||
| 3 | Theroniadepressa | D.houi, Artonafuneralis | Huang (2003a) | ||
| 4 | Chalcididae | Kriechbaumerelladendrolimi | D.houi, D.kikuchii, D.punctatus | Tong and Ni (1986) | |
| 5 | Pteromalidae | Dibrachysyunnanensis | D.houi, Tomicuspiniper | Jiao et al. (2017) | |
| 6 | Diptera | Tachinidae | Carceliailliberisi | D.houi, Illiberispruni | Zhao and Liang (2002) |
| 7 | Carcelianigrantennata | D.houi, Lymantriadispar, Euproctissimiles | Huang (2003b) | ||
| 8 | Carceliaflavimaculata | *D.houi, Lymantriaxylina, Diprionjingyuanensis | Zhao and Liang (2002) | ||
| 9 | Drinoinconspicuoides | D.houi, L.xylina | Zhang (2016) | ||
| 10 | Blepharipazebina | D.houi, D.punctata, D.kikuchii, Dendrolimussuperans, Antheraeamylitta, Cephonodeshylas, Papiliodemoleus, Andracabipunctata, Hepialusyunnanensis, Ivelaochropoda, Dasychiraaxutha | Zhang (2016) | ||
| 11 | Mikiatepens | *D.houi | Zhang (2016) | ||
| 12 | Sarcophagidae | Sarrorohdenegracilior | D.houi | Fan (1992) | |
| 13 | Sarrorohdeneantelope | D.houi | Fan (1992) |
* New parasitoid wasps or parasitoid flies recorded within Dendrolimushoui.
Regional distribution, parasitism and host stage of parasitoids within D.houi
A total of 4537 pupae of D.houi were collected in the field and 417 pupae were parasitised. Five parasitoid species emerged in D.houi pupal stage, these being X.konowi, H.pyretorum, T.depressa, K.dendrolimi and D.yunnanensis (Table 2) and eight parasitic flies: C.illiberisi, C.nigrantennata, C.flavimaculata, D.inconspicuoides, M.tepens, B.zebina, S.gracilior and S.antelope (Table 3). We currently found that H.pyretorum and S.antelope were newly reported in Fujian Province and K.dendrolimi was newly reported in Guizhou Province, China.
Table 2.
Percent parasitism and distribution of parasitoid wasps within pupae of D.houi in China. (Guiyang Village, Jiangle County, Fujian Province, FJG; Chengqian Village, Sha County, Fujian Province, FSC; Duishan Village, Yongtai County, Fujian Province, FYD; Youkeng Village, Fuding County, Fujian Province, FFY; Chayang Village, Fuding County, Fujian Province, FFC; Wuyi Mountain, Wuyishan County, Fujian Province, FWW; Yangmeiling Forest Park, Xiapu County, Fujian Province, FXY; Zuling Temple, Chongzhou County, Sicuan Province, SCZ; Guimao Mountain, Laifeng County, Hubei Province, HLG; Sanxing Village, Sinan County, Guizhou Province, GSS; Nandai Village, Songyang County, Zhejiang Province, ZSN; Guiyang Village, Suichang County, Zhejiang Province, ZSG; Dapan Forest Farm, Pan'an County, Zhejiang Province, ZPD)
| Parasitism rate (%) in different localities during the pupae stage | ||||||||||||||
| Species | Offspring | FJG | FSC | FYD | FFY | FFC | FXY | FWW | SCZ | HLG | GSS | ZSN | ZSG | ZPD |
| X.konowi | 1 | 0.52 | 0.22 | 0.36 | 0.16 | |||||||||
| H.pyretorum | 1 | 0.48 | 0.36 | 0.79 | 4.35 | 2.44 | 0.62 | 0.98 | 4.17 | 0.47 | ||||
| T.depressa | 1-9 | 0.86 | 0.48 | 0.36 | 0.63 | 4.35 | ||||||||
| K.dendrolimi | 26-58 | 4.30 | 1.39 | 0.32 | 11.59 | 0.79 | 3.92 | 5.14 | ||||||
| D.yunnanensis | 33-49 | 0.48 | ||||||||||||
Table 3.
Percent parasitism and distribution of parasitic flies within pupae of D.houi in China. (Guiyang Village, Jiangle County, Fujian Province, FJG; Chengqian Village, Sha County, Fujian Province, FSC; Duishan Village, Yongtai County, Fujian Province, FYD; Yangmeiling Forest Park, Xiapu County, Fujian Province, FXY; Zuling Temple, Chongzhou County, Sicuan Province, SCZ; Guimao Mountain, Laifeng County, Hubei Province, HLG; Longli County, Qiangnan County, Guizhou Province, GQL; Jingdong Yi Autonomous County, Pu'er City, Yunnan Province, YPJ; Dapan Forest Farm, Pan'an County, Zhejiang Province, ZPD)
| Species | Offspring | FJG | FSC | FYD | FXY | SCZ | HLG | GQL | YPS | ZPD |
| C.illiberisi | 1-3 | 0.32 | ||||||||
| C.nigrantennata | 1-5 | 3.79 | 1.39 | 1.29 | 2.44 | 1.85 | 5.78 | |||
| C.flavimaculata | 1-5 | 0.32 | ||||||||
| D.inconspicuoides | 1 | 0.55 | ||||||||
| B.zebina | 1-11 | 3.79 | 5.54 | 2.89 | 3.33 | |||||
| M.tepens | 1 | 1.54 | ||||||||
| S.gracilior | 4-8 | 1.89 | 8.31 | 3.38 | 4.27 | 0.31 | 3.69 | |||
| S.antelope | 3-8 | 2.41 |
Biology of dominant species
K.dendrolimi had parasitism rates of 3.60% during the pupal stage with various elevation distributions (462-901 m). The sex ratio (female : male) was 1 : 0.25, it had the female and male longevity within 11 d and 10 d, respectively (Table 4). Further findings showed that multiparasitism frequently and naturally occurs between K.dendrolimi and T.depressa and between K.dendrolimi and D.yunnanensis. Consequently, K.dendrolimi was identified as the important pupal parasitoid species, based on its parasitism rates (0.79-11.59%) in Hymenoptera species and offspring (26–58/pupa), which might lead to a synergistic effect on the suppression of the large pupae of D.houi and be a promising species for mass release to suppress caterpillars in C.japonica forests.
Table 4.
Biology of high parasitism rates parasitoid species.
| Species | Total parasitism rates (%) | Order | A.S.L. (m) | Offspring | Ratio female : male | Longevity (d) |
| K.dendrolimi | 3.19 | Hymenoptera | 462-901 | 26-58 | 1 :0.25 | 8-16 |
| S.gracilior | 3.59 | Diptera | 578-915 | 1~14 | 1 :0.67 | 8-12 |
Morphology of immature stages
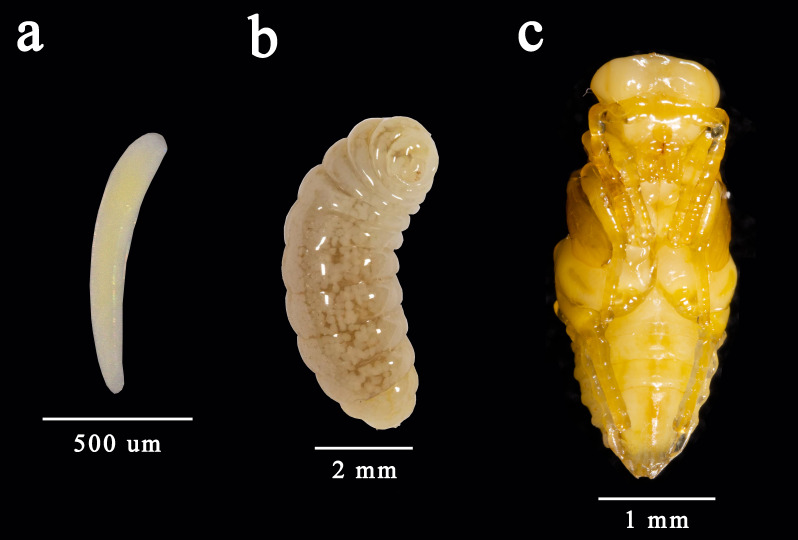
Egg clavate, light yellow, length 0.8-1.0 mm, one end is round and the other is thin and transparent. Early-instar larvae length is 1.8-5.0 mm, tawny, the whole body is covered with a transparent membrane, appears like a spindle with pointed ends. Late-instar larvae length is 6.1-8.5 mm, the body is yellowish-white, blunt round head and the tail is thin. Free pupa, with 5.5-8.0 mm length. Initial pupa stages with white, later brown (Fig. 4) and black before emergence.
Figure 4.
K.dendrolimi. a egg; b late-instar larva ; c pupa.
Morphology of adults
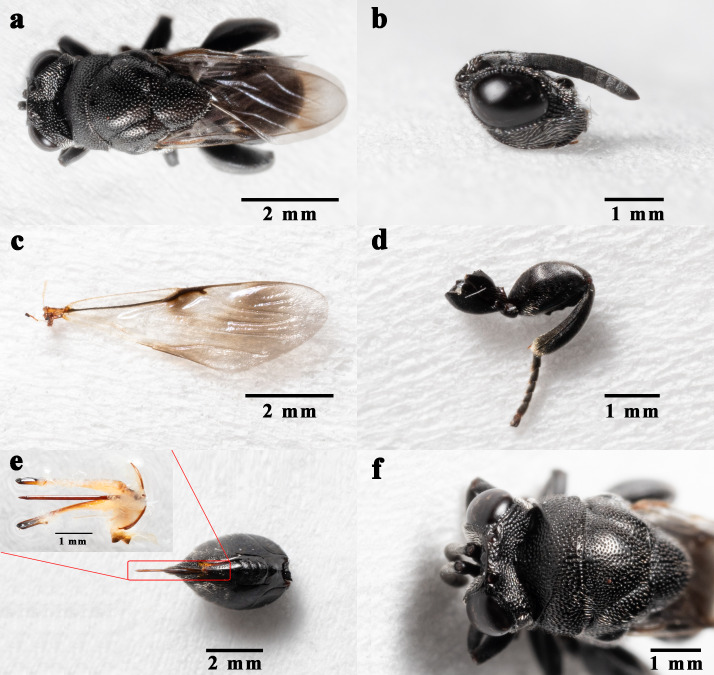
The length of females is 6.8-7.2 mm, the length of males is 4.2-5.7 mm. Antennae almost blackish, scapus and anelli kermesinus, tegula dark brown, forewings faintly smoky with brown patch, hind tarsus dark brown, scapus almost reaching middle ocelli, the length is equal to the 1-4 funiculur segments. OOL : POL = 9 : 28. Blunt triangle occiput, pits on pronotum were narrower than on mesoscutum and scutellum, frenal length greater than width, back end sleek without concave edge, forewings submarginal vein 1.5 times as long as marginal, stigmal vein stubby, coxa-3 dorsal lateral base with tuberculate protuberances, hind femur length about 1.5 times the width, first gastral tergite about 2/5 the length of the cercus. The ovipositor 2 mm in length (Fig. 5).
Figure 5.
K.dendrolimi, female. a whole body; b head, c forewing; d hind leg, e cercus and ovipositor; f head and mesosoma in dorsal view.
Discussion
In previous research, 22 different native parasitoid species from 16 genera and 11 families within D.houi were identified, containing seven parasitoid species that emerged from eggs: Mesopolobustabatae Ishii, M.albitarsis, A.gastropachae, Ooencyrtuskuwanae Howard, Telenomusdendrolimi Matsumura, T.dendrolimusi Chu and M.subfumatus (Ratzeburg); nine species of parasitoid species emerged from the pupae of D.houi, including K.dendrolimi, K.longiscutellaris Qian and He, BrachymeriaSecundaria Rushika, B.lasus, Monodontomerusminor Ratz, D.yunnanensis, T.depressa, Coccygomimuslaothoe Cameron and X.konowi. Here, we identified five species of parasitoid species, of which a new parasitoid species was discovered and identified compared with previous studies: Habronyxpyretorum (Lin et al. 2017). Seven different native parasitoid flies from four genera and two families within D.houi were identified, these being C.rasella, C.illiberisi, C.nigrantennata, D.inconspicuoides, B.zebina, S.gracilior and S.antilope. They parasitised D.houi larvae and emerged in its pupal stage. We identified eight species of parasitoid flies from five genera and two families, of which Carceliaflavimaculata and Mikiatepens were new parasitoid flies recorded for D.houi (Liang et al. 2018).
There are four species of parasitoid natural enemies distributed only in China, Habronyxpyretorum, Kriechbaumerelladendrolimi, Dibrachysyunnanensis and Carcelianigrantennata mainly distributed in south China. Carceliailliberisi and Carceliaflavimaculata have a naturally broad distribution in a large latitude crossing (Tong and Ni 1986, Zhao and Liang 2002, Huang 2003a, Huang 2003b). The following seven species of parasitic wasps and parasitoid flies are found in other countries besides China. Xanthopimplakonowi is distributed in Japan, India, Myanmar, Vietnam, Thailand, Malaysia and Indonesia (Huang 2003a). Theroniadepressa has been recorded in south China as well as in the Philippines (Lin et al. 2017). Drinoinconspicuoides is distributed in Japan and Melanesia; Blepharipazebina in addition to 29 provinces in China, with distribution in Russia, South Korea, India, Nepal, Myanmar, Thailand and Sri Lanka. Mikiatepens was a commonly distributed species in Russia, Japan, Kazakhstan, India, Bhutan, Nepal, Bangladesh, Vietnam and Malaysia (Zhang 2016). Sarcophagagracilior was distributed in south China and Nepal, while Sarrorohdeneantelope is distributed abroad in Russia, Korea, Japan and northern Oceania (Fan 1992).
Further findings showed that multiparasitism frequently and naturally occurs between K.dendrolimi and T.depressa, between K.dendrolimi and D.yunnanensis and between parasitoid flies Carcelianigrantennata, Blepharipazebina and Sarcophagagracilior. The results showed that multiple natural enemies could attack the same large host at the same time, which might lead to a synergistic effect on the suppression of the large pupae of D.houi and be a promising species for mass release to suppress caterpillars in C.japonica forests. Obviously, multiple natural enemies co-exist in the pupae of D.houi, killing host pests through feeding, which has a combined control effect on the natural population. However, this phenomenon is contrary to the law of species competition, so it is considered as a strange and interesting phenomenon (Hackett-Jones et al. 2008). Due to the large size gap between natural enemies and hosts, a single parasitoid species cannot overcome the defence of hosts' immune systems and successfully inhibit host development and multiple natural enemies are required to participate at the same time. Therefore, polyparasitism or multiparasitism may have more potential for pest control (Yang et al. 2018). It is worthy of further study to provide reference for the protection and rational utilisation of natural enemies in the future.
Previous studies revealed that K.dendrolimi was originally recorded attacking large moths, such as D.punctatus, D.kikuchii and D.houi, while A.pernyi, Philosamiacynthia Walker and Felder, Lebedanobilis Walker, E.pyretorum and Trabalavishnou Lefebure are factitious hosts (Tong and Ni 1986). Unfortunately, little was known about the interaction between these host pests and parasitoid K.dendrolimi, including the mass rearing procedure (Suppl. materials 1, 2). Amongst the host pupae in this paper, A.pernyi might be associated with being larger and heavier and having more abundant nutrients within one pupa (King 1987), which is sufficient to simultaneously support hundreds of parasitoid larvae inside them to develop into adults. Additionally, A.pernyi pupae are easily and extensively accessible from the north of China at low cost for mass rearing of K.dendrolimi. A high ratio of female parasitic wasps may increase the outcome of offspring in the next generation, which is beneficial to the expansion of parasitoid population (Uckan and Gulel 2002).
The average annual temperature of K.dendrolimi collected in Guizhou Province was 15.3°C; therefore, K.dendrolimi was able to adapt to lower temperatures. The laboratory rearing data could predict its potential distribution and evaluate its pest control efficiency in the forest. In addition, K.dendrolimi were better reared under 24°C-30°C, which could be used to optimise and regulate rearing conditions and change their development process according to practical need. All results will be conducive to mass rearing and release in the future (Pereira et al. 2011, Bao et al. 2021).
As a native parasitoid species, K.dendrolimi has stronger adaptability to local stress factors than imported species and it has a high emergence rate from wild populations of D.houi with strong fertility and it specially attacks most Lasiocampidae pests. Therefore, this parasitoid species will be a potentially promising biological control agent for control of pine caterpillars in China. Using natural enemies to control D.houi has some advantages, such as stronger specificity, better environmental protection (Gurr et al. 2011) and K.dendrolimi can be industrialised to become an economic control agent by providing sufficient supply of alternative hosts. For releasing purpose, the population needs to be increased by mass rearing prior to the outbreak of D.houi and transported artificially before jump-like releasing or inundant releasing in the field (Rossi Stacconi et al. 2018).
Supplementary Material
The courtship behaviour of Kriechbaumerelladendrolimi
ZhengHao Chen
Data type
multimedia
File: oo_822946.mp4
Mating behaviour of Kriechbaumerelladendrolimi
Zheng-Hao Chen
Data type
multimedia
File: oo_823085.mp4
Acknowledgements
All parasitoid flies confirmed by Prof. Chuntian Zhang, Shenyang Normal University, Shenyang, CN); Kriechbaumerelladendrolimi (Sheng et Zhong) confirmed by Prof. Changming Liu (Fujian Agriculture and Forestry University, Fuzhou, China), three species of Ichneumonidae confirmed by Dr. Tao Li (National Forestry and Grassland Administration, Shenyang, China) and Dibrachysyunnanensis (Yang) confirmed by Dr. Qin Li, (Xinjiang University, Urumqi, CN).
All authors thank Eric B. Jang, from USDA-ARS, Daniel K. Inouye US Pacific Basin Agricultural Research Center, (Hilo, HI, USA), who provided linguistic editing for this manuscript, we thank Yuxuan Kuang and Tian Gan for picture editing of the manuscript and Jianhui Lin for representative sites map drawings. This research was funded by the National Natural Science Fund of China (No. 31870641), Science and Technology Department of Fujian Province-China (No. 2021N0002) and the Forestry Science and Technology Programs of Fuzhou City (No. 2021FZLY012 and 2022FZLY02).
Contributor Information
Hui Chen, Email: chenhuiyl@163.com.
Guang hong Liang, Email: fjlhg@126.com.
References
- Bao Ke Xin, Luo Li Ping, Wang Xiao Yi, Zhang Yan Long, Wei Ke, Zhang Yong An, Yang Zong Qi. Method for evaluating the comprehensive benefits of biological control of insect pests. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2021&filename=ZSWF202101005&uniplatform=NZKPT&v=_0GOkqKlxKxYWun4-BRwE-ehnj11BjtXnkk-A23lV6K9TL7YRgMEFYxaHA1PXZUI. Chinese Journal of Biological Control. 2021;37(1):38–51. doi: 10.16409/j.cnki.2095-039x.2021.01.024. Chinese. [DOI] [Google Scholar]
- Caniço Albasini, Mexia António, Santos Luisa. First report of native parasitoids of fall armyworm Spodopterafrugiperda Smith (Lepidoptera: Noctuidae) in Mozambique. Insects. 2020;11(9):615. doi: 10.3390/insects11090615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Zi De. Key to the common flies of China (second edition) Science Press; Beijing: 1992. 656-690. Chinese. [Google Scholar]
- Gurr Geoff M., Read Donna M. Y., Catindig Josie Lynn A., Cheng Jiuan, Liu Jian, Lan La Pham, Heong Kong Luen. Parasitoids of the rice leaffolder Cnaphalocrocismedinalis and prospects for enhancing biological control with nectar plants. Agricultural and Forest Entomology. 2011;14(1):1–12. doi: 10.1111/j.1461-9563.2011.00550.x. [DOI] [Google Scholar]
- Hackett-Jones E., Cobbold C., White A. Coexistence of multiple parasitoids on a single host due to differences in parasitoid phenology. Theoretical Ecology. 2008;2(1):19–31. doi: 10.1007/s12080-008-0025-1. [DOI] [Google Scholar]
- He Xiang, Zou Xue Mei, Shang Hui Yan, Yuan Shi Bin, He Heng Guo. The occurrence regularity and biocontrol research of Dendrolimushoui Lajonquiere in cypress forest of Guang’ an. Journal of China West Normal University (Natural Sciences) 2018;39(2):132–136. doi: 10.16246/j.issn.1673-5072.2018.02.004. Chinese. [DOI] [Google Scholar]
- Holthouse Mark, Schumm Zachary, Talamas Elijah, Spears Lori, Alston Diane. Surveys in northern Utah for egg parasitoids of Halyomorphahalys (Stål) (Hemiptera: Pentatomidae) detect Trissolcusjaponicus (Ashmead) (Hymenoptera: Scelionidae) Biodiversity Data Journal. 2020;8:e53363. doi: 10.3897/bdj.8.e53363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Bang Kan. Fauna of insects in Fujian province of China. Vol. 7. Fujian Science and Technology Publishing House; Fuzhou: 2003. 258-259. Chinese. [Google Scholar]
- Huang Bang Kan. Fauna of insects in Fujian province of China. Vol. 8. Fujian Science and Technology Publishing House; Fuzhou: 2003. 464-477. Chinese. [Google Scholar]
- Jiao Tian-yang, Yao Qin-ying, Hui Xiao. Review of Dibrachys Förster from China (Hymenoptera, Chalcidoidea, Pteromalidae) ZooKeys. 2017;656:123–149. doi: 10.3897/zookeys.709.20780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King Bethia Hurlbutt. Offspring sex ratios in parasitoid wasps. The Quarterly Review of Biology. 1987;62(4):367–396. doi: 10.1086/415618. [DOI] [Google Scholar]
- Kinyanjui G., Khamis F. M., Ombura F. L. O., Kenya E. U., Ekesi S., Mohamed S. A. Distribution, abundance and natural enemies of the invasive tomato leafminer, Tutaabsoluta (Meyrick) in Kenya. Bulletin of Entomological Research. 2021;111(6):658–673. doi: 10.1017/s0007485321000304. [DOI] [PubMed] [Google Scholar]
- Kong Xiang Bo, Zhang Zhen, Zhao Cheng Hua, Wang Hong Bin. Female sex pheromone of the Yunnan pine caterpillar moth DendrolimusHoui: First (E,Z)-isomers in pheromone components of Dendrolimus spp. Journal of Chemical Ecology. 2007;33(7):1316–1327. doi: 10.1007/s10886-007-9313-2. [DOI] [PubMed] [Google Scholar]
- Liang Guang Hong, Lin Hao Yu, Lu Ci Ding, Han Xiao Hong, Hua Yin, Huang Xin Jin, Xie Zuo Gui, Zhang Fei Ping, Zhang Chun Tian. Morphology and biology of seven parastic flies of Dendrolimushoui in China. Plant Protection. 2018;44(6):177–184. doi: 10.16688/j.zwbh.2018194. Chines. [DOI] [Google Scholar]
- Lin Hao Yu, Fu Lie Qing, Lin Jian Hui, Hua Yin, Han Xiao Hong, Zheng Jun Xian, He Huan, Zhang Fei Ping, Liang Guang Hong. Main species of parasitic natural enemy insects within Dendrolimushoui (Lajonquiere) in the forest of Cryptomeriafortunei (Hooibrenk) Chinese Journal of Biological Control. 2017;33(6):842–848. doi: 10.16409/j.cnki.2095-039x.2017.06.018. Chinese. [DOI] [Google Scholar]
- Luo Song Gen, Xu Zhen Wang, Zhao Ren You, Hua Bao Song, Lin Shao Bo, et al. Biological Properties and Control of Dendrolimushoui. Journal OF Zhejiang Forest Science and technology. 1998;18(1):11–15. Chinese. [Google Scholar]
- Pereira FF, Zanuncio JC, Oliveira HN, Grance ELV, Pastori PL, Gava-Oliveira MD. Thermal requirements and estimate number of generations of Palmistichuselaeisis (Hymenoptera: Eulophidae) in different Eucalyptus plantations regions. Brazilian Journal of Biology. 2011;71(2):431–436. doi: 10.1590/s1519-69842011000300012. [DOI] [PubMed] [Google Scholar]
- Rossi Stacconi Marco Valerio, Grassi Alberto, Ioriatti Claudio, Anfora Gianfranco. Augmentative releases of Trichopriadrosophilae for the suppression of early season Drosophila suzukii populations. BioControl. 2018;64(1):9–19. doi: 10.1007/s10526-018-09914-0. [DOI] [Google Scholar]
- Tong Xin Wang, Ni Le Xiang. Biological studies of Eucepsis sp. in Hunan province. Chinese Journal of Biological Control. 1986;2(4):165–166. doi: 10.16409/j.cnki.2095-039x.1986.04.008. Chine'se. [DOI] [Google Scholar]
- Uckan F., Gulel A. Age-related fecundity and sex ratio variation in Apantelesgalleriae (Hym., Braconidae) and host effect on fecundity and sex ratio of its hyperparasitoid Dibrachysboarmiae (Hym., Pteromalidae) Journal of Applied Entomology. 2002;126(10):534–537. doi: 10.1046/j.1439-0418.2002.00706.x. [DOI] [Google Scholar]
- Vargas Roger I., Stark John D., Banks John, Leblanc Luc, Manoukis Nicholas C., Peck Steven. Spatial dynamics of two oriental fruit fly (Diptera: Tephritidae) parasitoids, Fopiusarisanus and Diachasmimorphalongicaudata (Hymenoptera: Braconidae), in a Guava Orchard in Hawaii. Environmental Entomology. 2013;42(5):888–901. doi: 10.1603/en12274. [DOI] [PubMed] [Google Scholar]
- Vargas Roger I, Leblanc Luc, Putoa Rudolph, Eitam Avi. Impact of introduction of Bactroceradorsalis (Diptera: Tephritidae) and classical biological control releases of Fopiusarisanus (Hymenoptera: Braconidae) on economically important fruit flies in French Polynesia. Journal of Economic Entomology. 2007;100(3):670–679. doi: 10.1603/0022-0493(2007)100[670:ioiobd]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Yang Chen. Occurrence rule of yunnan pine moth in Changning cigui forest region and its control methods. Forest Inventory and Planning. 2015;40(3):48–50. doi: 10.3969/j.issn.1671-3168.2015.03.010. Chinese. [DOI] [Google Scholar]
- Yang Jianquan, Cai Pumo, Chen Jia, Zhang HeHe, Wang Cong, Xiang Houjun, Wu Jian, Yang Yanchuan, Chen Jiahua, Ji Qinge, Song Dongbao. Interspecific competition between Fopiusarisanus and Psyttaliaincisi (Hymenoptera: Braconidae), parasitoids of Bactroceradorsalis (Diptera: Tephritidae) Biological Control. 2018;121:183–189. doi: 10.1016/j.biocontrol.2018.02.003. [DOI] [Google Scholar]
- Yang Zong Qi, Yao Yan Xia, Cao Liang Ming. Chalcidoidea parasitizing forest defoliators (Hymenoptera) Science Press; Beijing: 2015. [Google Scholar]
- Zhang Chun Tian. Tachinidae of Northeast China. Science Press; Beijing: 2016. 377-382. Chinese. [Google Scholar]
- Zhang Li Bin. Control experiments of two biological pesticides on Dendrolimushoui in the forests. Forest Science and Technology. 2013;5(4):31–33. doi: 10.13456/j.cnki.lykt.2013.04.005. Chinese. [DOI] [Google Scholar]
- Zhao Jian Ming, Liang En Yi. Review of the Chinese Carcelia Robineau-Desvoidy (Diptera: Tachinidae) Acta Zootaxonomica Sinica. 2002;27(4):807–848. doi: 10.3969/j.issn.1000-0739.2002.04.027. Chinese. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The courtship behaviour of Kriechbaumerelladendrolimi
ZhengHao Chen
Data type
multimedia
File: oo_822946.mp4
Mating behaviour of Kriechbaumerelladendrolimi
Zheng-Hao Chen
Data type
multimedia
File: oo_823085.mp4