ABSTRACT
Candida auris, an emerging multi-drug-resistant fungal pathogen, uniquely colonizes the human skin long term, leading to subsequent development of life-threatening invasive infections in humans. The factors regulating skin colonization of C. auris are not well understood. In this study, we established an intradermal mouse model of C. auris infection to define the innate and adaptive immune response to this emerging pathogen and compare it to Candida albicans. Our results indicate that compared to C. albicans-infected mice, C. auris-infected mouse skin tissue had significantly higher fungal load after 3 and 14 days post-infection. C. auris infection was associated with a significantly decreased accumulation of CD11b+ Ly6G+ neutrophils and increased numbers of CD11b+ Ly6 Chi inflammatory monocytes and CD11b+ CD207+ Langerhans cells at the site of infection. Furthermore, a significant decrease in the absolute numbers of type 3 innate lymphoid cells and Th17 cells was observed in C. auris-infected skin tissue. Taken together, our findings indicate that the skin immune responses are different between C. auris- and C. albicans-infected mice. The increased fungal load observed in C. auris-infected mouse skin tissue is associated with less potent innate and adaptive immune responses induced by this emerging pathogen relative to C. albicans.
IMPORTANCE
Candida auris is a globally emerging fungal pathogen that transmits among individuals in hospitals and nursing home residents. Unlike other Candida species, C. auris predominantly colonizes and persists in skin tissue, resulting in outbreaks of nosocomial infections. Understanding the factors that regulate C. auris skin colonization is critical to develop novel preventive and therapeutic approaches against this emerging pathogen. We established a model of intradermal C. auris inoculation in mice and found that mice infected with C. auris elicit less potent innate and adaptive immune responses in the infected skin compared to C. albicans. These findings help explain the clinical observation of persistent C. auris colonization in skin tissue.
KEYWORDS: Candida auris, Candida albicans, skin colonization, immune response
INTRODUCTION
Candida auris was recently categorized as an urgent threat by the U.S. Centers for Disease Control and Prevention (CDC) and classified in the critical priority fungal pathogen group by the World Health Organization (1 – 3). Unlike other Candida species which colonize in the intestine, C. auris colonizes the human skin, which leads to nosocomial transmission and outbreaks of invasive fungal infections (4 – 6). Furthermore, C. auris not only colonizes the outer layer of the skin but also enters the dermis and deeper tissues, including hair follicles, a phenomenon that was not observed previously with other Candida species (4, 7). Recent findings indicate that C. auris was detected in the skin tissues of mice even after 4 months of initial epicutaneous inoculation, indicative of the persistent colonization in the deep skin tissue, which may evade routine clinical surveillance (4). However, the immune factors that contribute to the unique propensity of C. auris for colonization and long-term persistence in the skin tissue remain unclear.
To address this question, we developed an intradermal mouse model of C. auris infection and compared the skin immune response of mice infected with C. auris or Candida albicans. Our results indicate that mice intradermally infected with C. auris had significantly higher fungal load in the skin. Furthermore, C. auris elicited significantly less potent immune responses compared to C. albicans. Our findings provide the first comparison of the immune cells that accumulate in the skin of C. auris-infected versus C. albicans-infected mice in vivo. Furthermore, they indicate that increased persistence of C. auris in the mouse skin tissue is associated with a less potent host immune response elicited by this emerging fungal pathogen.
MATERIALS AND METHODS
Strains and reagents
The strains used in the study were Candida albicans SC5314 (obtained from Dr. Andrew Koh, University of Texas Southwestern Medical Center, USA) and Candida auris isolates (South Asian AR0387, East Asian AR0381, African AR0383, and South American AR0385, obtained from CDC AR Isolate Bank, USA). The reagents and chemicals used in this study, purchased from the following vendors, were yeast peptone dextrose (YPD, 242810; BD Difco, Franklin Lakes, NJ, USA); deoxyribonuclease I (bovine pancreas, DN25-100mg; Sigma-Aldrich, St. Louis, MO, USA); CHROMagar (CA222; CHROMagar, Paris, France); agar (BP1423-500; Fisher Bioreagents, Pittsburgh, PA, USA); phosphate buffer solution (PBS) (10×) (BP3994, Fisher Bioreagents); ampicillin (14417; Cayman Chemicals, Ann Arbor, MI, USA); kanamycin-sulfate (BP9069-5, Fisher Bioreagents); liberase TL (05401020001; Roche, Basel, Switzerland); streptomycin (100556; MP Biomedicals, Santa Ana, CA, USA); U-bottom 96-well plate (229190; Cell Treat, Pepperell, MA, USA); 10-mL syringes (302995, BD Difco); 40-µm cell strainer (22–363-547, Fisher Scientific); 0.15-M NaCl (BP-358–212; Fisher Bioreagents); trypsin-1-mM EDTA (25200–056; Gibco, Thermo Fisher Scientific, Waltham, MA, USA); RPMI-1640 (SH30027.02; Cytiva, Marlborough, MA, USA); and fetal bovine serum (FBS-500-HI; CPS Serum, Parkville, MO, USA). For intradermal infection, the following items were used: 27-G hypodermic needle (NEZ27114; Air-Tite, VA, USA); 1-mL syringes (14–826-87, BD Syringes; Fisher Scientific); isoflurane (Dechra, Fort Worth, TX, USA); veterinary trimmer (Wahl; Kent Scientific, Torrington, CT, USA); chemical depilatory cream (Nair, Ewing, NJ, USA); and cotton-tipped applicators (19–062-715; Puritan Medical Products, Guilford, ME). For immune cell staining, anti-mouse antibodies were purchased from BioLegend (San Diego, CA, USA): CD207 (PerCP/Cyanine5.5, 4C7); CD11c (APC, N418); Ly6C (Pacific Blue, HK1.4); CD11b (PE, M1/70); Ly-6G (PE/Cyanine7, 1A8); CD45 (FITC, 30-F11); CD64 (PE/Dazzle 594, F54-5/7.1); TCR γ/δ (Brilliant Violet 421, GL3); CD4 (PerCP/Cyanine5.5, GK1.5); TCR β (PE/Cyanine7, H57-597); IL-17F (Alexa Fluor 488, 9D3.1C8); IL-17A (PE/Dazzle 594, TC11-18H10.1); IFN-γ (Alexa Fluor 700, XMG1.2); CD45 (Brilliant Violet 421, 30-F11); CD90.2 (Thy-1.2) (PE/Dazzle 594, 53–2.1); and IL-17A (PE/Cyanine7, TC11-18H10.1). The following lineage marker anti-mouse antibodies were purchased from BioLegend: TCR γ/δ (APC, GL3); CD3ε (APC, 145–2C11); CD19 (APC, 6D5); CD11c (APC, N418); CD11b (APC, M1/70); NK1.1 (APC, PK136); IL-22 (APC, IL-22JOP); and IL-22 (PerCP-eFluor 710, IL-22JOP). LIVE/DEAD Fixable Yellow Dead (L34959) were purchased from Invitrogen, Waltham, MA, USA. Anti-mouse CD8b (PE, H35-17.2) was purchased from BD Biosciences, La Jolla, CA, USA. Anti-mouse MHC II (Alexa Fluor 700, M5/114.15.2), IL-22 (APC, IL-22JOP), and IL-22 (PerCP-eFluor 710, IL-22JOP) were purchased from Thermo Fisher. The cell staining buffer (420201), cell activation cocktail without brefeldin A (500×) (423302), monensin (1,000×) (420701), intracellular staining permeable wash buffer (10×) (421002), and fixation buffer (420801) were purchased from BioLegend.
Intradermal infection in mice
All animal studies were approved by the Institutional Animal Care and Use Committee at Purdue University. Six- to eight-week-old C57BL/6 J mice of both male and female sexes were used in the study. On the day of infection, mice were anesthetized using isoflurane; hair was removed using a trimmer; and chemical depilatory cream was applied for destroying hair follicles on the trimmed area. The shaved area was wiped clean with sterile water. Mice were then injected intradermally with 27-G hypodermic needle of inoculum size, 0.1 mL of 1×–2× 107 colony-forming units (CFU) per mice with live C. albicans SC5314, C. auris AR0381, C. auris AR0383, C. auris AR0385, C. auris AR0387 or sterile 1× PBS.
Determining fungal burden in skin tissue
After 3 and 14 days post-infection, mice from all groups were euthanized and skin tissues were collected. Skin tissues were weighed and homogenized using 1× PBS. Fifty-microliter tissue homogenate was either serially diluted or directly plated on YPD-agar plates containing antibiotics, 50-µg/mL kanamycin, 100-µg/mL streptomycin, and 100-µg/mL ampicillin. Plates were incubated at 37°C for 24 hours to determine the fungal burden in skin (CFU per gram of tissue). The skin homogenates were also plated on CHROMagar plates to confirm C. auris and C. albicans colonies.
Isolation and staining of immune cells from mouse skin tissue
Immune cells from whole skin tissue were isolated and stained as described elsewhere (8, 9). Briefly, the whole skin excised from mice dorsal section was collected in cold sterile 1 mL of digestion media (RPMI-1640 with 0.25-mg/mL Liberase TL and 1-µg/mL DNase). Each skin sample was placed in a 12-well tissue culture plate with 1 mL of digestion media. Tissue was minced using an ophthalmic scissors, and then 1.5 mL of digestion media was added in each well and the samples were incubated in a CO2 incubator for 2 hours at 37°C. Ten minutes before the reaction ends, 0.5 mL of 0.25% trypsin-1-mM EDTA was added in each well. The reaction was deactivated by adding 2 mL of 5% chilled Fluorescence-activated cell sorting (FACS) buffer (5% FBS in 1× PBS) in each well. The resulting digest was mechanically dissociated with a 10-mL syringe by pumping moderately 10 times for each sample. Single-cell suspension was collected in 50-mL centrifuge tubes after straining through a 40-µm cell strainer. Tubes were centrifuged at 400 × g for 10 min. The cell pellet was resuspended in 8 mL of 5% chilled FACS buffer and centrifuged again at 400 × g for 10 min. The cell suspensions were transferred to a 15-mL centrifuge tube for all the centrifugation steps. The final cell pellet was resuspended in 500 µL of 5% chilled FACS buffer. Cells were transferred in a U-bottom 96-well plate for the staining procedure. For surface antibody staining, cells were washed with cell staining buffer and live cells were determined by staining the cells with LIVE/DEAD Fixable Yellow Dead stain. Next, the Fc receptors were blocked with anti-mouse CD16/32 antibody. The surface markers were stained with indicated surface antibodies in a ratio of 1:200, except MHC II (1:2,000). Cells were fixed after the staining procedure with fixation buffer. After fixation, cells were stained with CD207 antibody (1:200) in 1× intracellular staining permeable wash buffer for 1 hour in ice. For adaptive T cells and innate lymphoid cell staining, mononuclear cells were stimulated with cell activation cocktail without brefeldin (1:500) and monensin (1:1,000) for 4.5 hours at 37°C at 5% CO2. Cells were stained with respective surface antibodies and were fixed in fixation buffer overnight. For innate lymphoid cells (ILCs), the following lineage antibodies were used: TCR γ/δ (APC, GL3), CD3ε (APC, 145–2C11), CD19 (APC, 6D5), CD11c (APC, N418), CD11b (APC, M1/70), and NK1.1 (APC, PK136). On the next day, cells were washed and incubated with 1× intracellular staining permeabilization wash buffer with indicated intracellular antibodies (1:50). Cells were washed and suspended in cell staining buffer, and the flow cytometer data were acquired through a Attune NxT Flow Cytometer (Invitrogen, Carlsbad, CA, USA) and analyzed using FlowJo software (Eugene, OR, USA).
Statistics analysis
All the data were presented as mean ± standard error of the mean. The data were statistically analyzed using Mann-Whitney U test (or) Kruskal-Wallis test followed by Dunn’s multiple comparisons test using GraphPad Software. P values of ≤ 0.05 were considered significant.
RESULTS
Mice intradermally infected with C. auris had significantly higher fungal load compared to C. albicans-infected groups
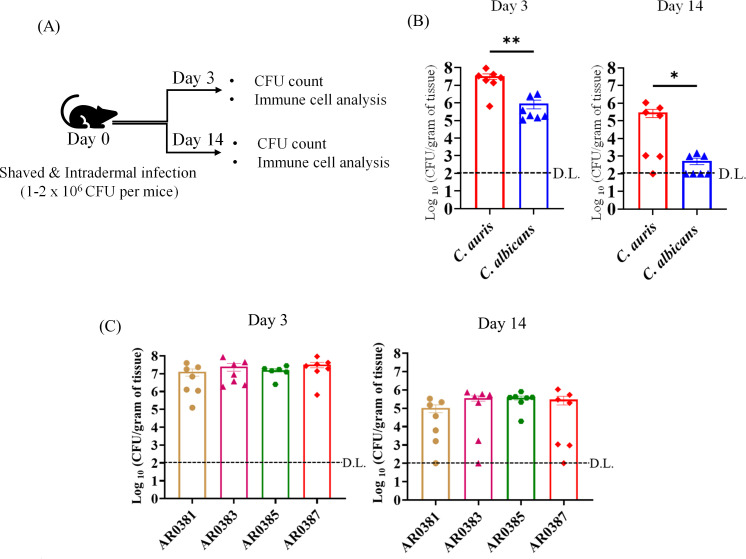
Groups of mice were infected with C. auris South Asian AR0387 strain or C. albicans at a dose of 1–2 × 106 CFU per mouse. Skin tissues were collected on day 3 and 14 post-infection to determine the tissue fungal load (Fig. 1A). C. auris-infected mice had significantly higher fungal load compared to C. albicans groups (Fig. 1B). At day 3 post-infection, the average fungal load in skin tissue of C. auris-infected mice was 7.51 ± 0.70 log10 CFU/g of tissue, whereas that of C. albicans-infected mice was ~5.97 ± .57 log10 CFU/g of tissue (Fig. 1B). At day 14 post-infection, only three out of seven mice infected with C. albicans had detectable fungal colonies with an average fungal load about 2.74 ± 0.23 log10 CFU/g of tissue calculated from all mice. On the other hand, six out of seven mice infected with C. auris had detectable fungal colonies with an average fungal load about 5.47 ± 0.51 log10 CFU/g of tissue calculated from all mice (Fig. 1B). Next, we compared the fungal load among four clades of C. auris (East Asian AR0381, African AR0383, South American AR0385, and South Asian AR0387). No significant difference in the fungal load was observed between the four clades of C. auris after 3 and 14 days post-infection using the intradermal route of infection (Fig. 1C). Collectively, our findings indicate that mice intradermally infected with C. auris had significantly greater fungal load compared to C. albicans-infected groups. Furthermore, persistence of C. auris in mouse skin tissue was observed for 2 weeks in more than 80% of infected mice.
Fig 1.
(A) Schematic of experimental design shown here. Mice were shaved and injected with PBS (or) infected intradermally with fungal cells on day 0. Fungal load in the skin of mice and immune cell isolation and analysis were determined on day 3 and day 14 post-infection. (B) Fungal load in the skin tissue of mice intradermally infected with C. auris AR0387 (or) C. albicans SC5314 after 3 and 14 days post-infection is shown here. (C) Fungal load in the skin tissue of mice intradermally infected with C. auris AR0381, C. auris AR0383, C. auris AR0385, or C. auris AR0387 after 3 and 14 days post-infection is shown here. Fungal load was determined by plating the homogenized skin tissue onto YPD agar containing the following antibiotics: ampicillin, streptomycin, and kanamycin. C. auris or C. albicans colonies were further confirmed by plating the homogenates onto CHROMagar plates. For days 3 and 14, each symbol represents the fungal load from each mouse (n = 6–7 per group). Data are represented as mean ± standard error of the mean for each group. Statistical significance was calculated using Mann-Whitney U test or the Kruskal-Wallis test followed by Dunn’s multiple comparisons test. *P ≤ 0.05 and **P ≤ 0.01 were considered as significant. D.L., detectable limit of fungal colonies.
C. auris infection is associated with significantly decreased accumulation of CD11b+ Ly6G+ neutrophils and increased numbers of CD11b+ Ly6 C hi inflammatory monocytes at the site of infection relative to C. albicans infection
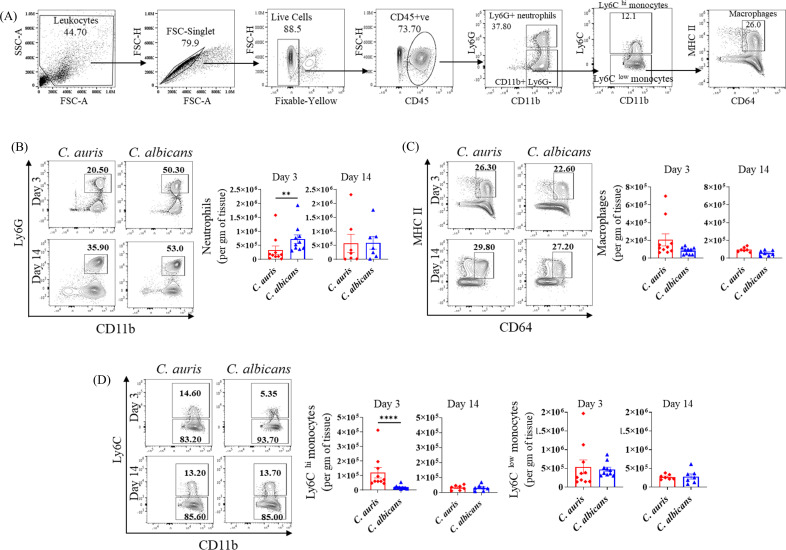
Phagocytic cells such as macrophages and neutrophils play a critical role in host defense against Candida (10 – 12). Furthermore, a recent study indicated that C. auris may elicit a less potent pro-inflammatory response in mononuclear phagocytes compared to C. albicans (13). Therefore, we examined the accumulation of various phagocytic cells in C. auris-infected mice and compared them with C. albicans-infected groups. Mice injected with PBS served as control. We examined CD11b+ Ly6G+ neutrophils, CD11b+ MHCII+ CD64+ macrophages, CD11b+ Ly6 Chi, and CD11b+ Ly6 Clow inflammatory monocytes as indicated in Fig. 2A. Compared to PBS controls, after 3 and 14 days post-infection, a significant increase in the absolute numbers of CD11b+ Ly6G+ neutrophils, CD11b+ MHCII+ CD64+ macrophages, CD11b+ Ly6 Chi and CD11b+ Ly6 Clow inflammatory monocytes was observed in C. auris-infected mice (Fig. S1). Next, we compared C. auris- and C. albicans-infected mice. At day 3 post-infection, C. auris infection was associated with a significantly decreased accumulation of CD11b+ Ly6G+ neutrophils (Fig. 2B). On the other hand, absolute numbers of CD11b+ Ly6 Chi inflammatory monocytes were significantly increased in C. auris groups compared to C. albicans infection (Fig. 2D). No significant changes were observed in the absolute numbers of CD11b+ MHCII+ CD64+ macrophages and CD11b+ Ly6 Clow inflammatory monocytes between C. auris- and C. albicans-infected groups (Fig. 2C and D). Taken together, compared with PBS groups, C. auris infection induces a significantly increased accumulation of neutrophils, monocytes, and macrophages in the skin tissue. Compared to C. albicans infection, C. auris-infected mice had decreased numbers of CD11b+ Ly6G+ neutrophils and increased numbers of CD11b+ Ly6 Chi inflammatory monocytes at the site of infection.
Fig 2.
Absolute number of phagocytic cells in the skin tissue of C. auris- and C. albicans-infected mice groups. Mice were infected intradermally with C. auris 0387 or C. albicans SC5314 strains. Mice were euthanized after 3 and 14 days post-infection, and the phagocytic cells was examined by flow cytometry. (A) Flow gating strategy of phagocytic cells; CD11b+ Ly6G+ neutrophils, CD11b+ Ly6 Chi monocytes, CD11b+ Ly6 Clow monocytes, and CD11b+ CD64+ MHC-II+ macrophages are shown here. Representative flow plots and absolute number of (B) CD11b+ Ly6G+ neutrophils, (C) CD11b+ MHC-II+ CD64+ macrophages, and (D) CD11b+ Ly6C hi monocytes and CD11b+ Ly6C low monocytes in C. auris- or C. albicans-infected mice after 3 and 14 days post-infection are shown. Seven to ten mice were used for each group and data represented were mean ± standard error of the mean for each group. Statistical significance was calculated using Mann-Whitney U test. **P ≤ 0.01, ****P ≤ 0.0001 were considered as significant.
C. auris infection is associated with increased accumulation of CD11b+ CD207+ Langerhans cells
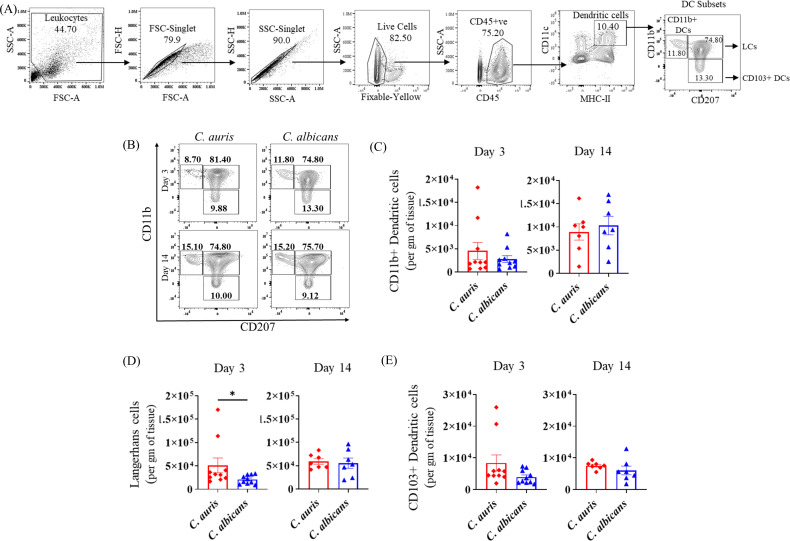
Antigen-presenting cells (APCs) such dendritic cells (DCs) play a major role in modulating the immune response to fungal pathogens in the skin (9, 14 – 16). We examined three major APC subsets gated from CD45+ CD11c+ MHCII+ cells: CD11b+ CD207− cells (CD11b+ dendritic cells), CD11b− CD207+ cells (CD103+ dendritic cells), and CD11b+ CD207+ Langerhans cells (LCs) as indicated in Fig. 3A and as described previously (9). At day 3 post-infection, compared to PBS-injected groups, C. auris infection resulted in a significant increase in the absolute numbers of CD11b+ DCs, CD103+DCs, and LCs. At day 14 post-infection, C. auris infection was associated with increased numbers of LCs and CD103+ DCs (Fig. S2). Next, we compared C. auris- and C. albicans-infected mice. At day 3 post infection, C. auris infection was associated with significantly increased accumulation of LCs compared to C. albicans infection (Fig. 3D). No significant changes were observed in the absolute numbers of CD11b+ DCs and CD103+ DCs between C. auris- and C. albicans-infected groups (Fig. 3B through E). Collectively, comparing with PBS groups, we observed a significant increase in the cutaneous accumulation of all three subsets of APCs in C. auris-infected mice. Comparing C. auris and C. albicans infection, we found that C. auris-infected mice elicited an increased number of LCs in the skin tissue after 3 days post-infection.
Fig 3.
Absolute number of APCs in the skin of C. auris- and C. albicans-infected mice groups. Mice were euthanized after 3 and 14 days post-infection to determine the APCs in the skin tissue. (A) Flow gating strategy of antigen-presenting cells gated from CD11c+ MHC-II+ cells is shown here. Representative flow plots (B) and absolute number of (C) CD11b+ CD207− cells (CD11b+ dendritic cells), (D) CD11b+ CD207+ cells (Langerhans cells), and (E) CD11b− CD207+ cells (CD103+ dendritic cells) in C. auris- or C. albicans-infected mice skin tissue after 3 and 14 days post-infection are shown. Seven to ten mice were used for each group and data represented were mean ± standard error of the mean for each group. statistical significance was calculated using Mann-Whitney U test. *P ≤ 0.05 was considered as significant. APC, antigen-presenting cell.
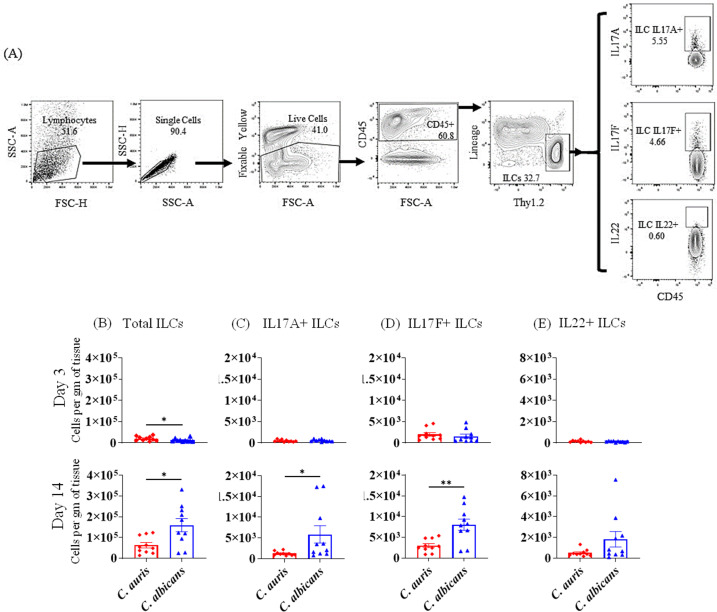
C. auris infection is associated with decreased accumulation of IL-17-producing ILCs
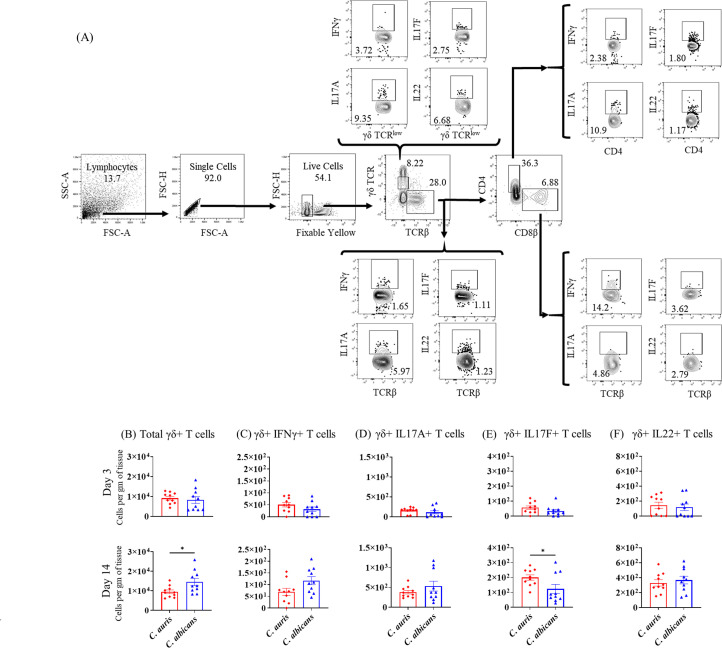
ILCs play a critical role in host defense against fungal pathogens (17). We measured the total numbers of ILCs, as well as IL-17A+, IL-17F+, and IL-22+ ILCs in skin tissue of PBS-injected, C. auris-infected, and C. albicans-infected mouse groups. ILCs were gated from CD45+ lineage-negative CD90.2+ cells as indicated in Fig. 4A. Compared to PBS injected mice, C. auris-infected animals had significantly increased numbers of IL-17F+ ILCs (day 3) and total ILCs (day 14) (Fig. S3). When comparing ILCs between C. auris-infected and C. albicans-infected mice, we noted an increase in the numbers of total ILCs in C. auris-infected skin compared to C. albicans-infected mice at day 3 post-infection (Fig. 4B). However, at day 14 post-infection, C. auris infection was associated with a significantly decreased accumulation of total ILCs, IL-17A+ ILCs, and IL-17F+ ILCs compared to C. albicans (Fig. 4B through E). Taken together, these data show that compared to C. albicans-infected mice, C. auris skin infection is associated with decreased accumulation of type 3 ILCs that are critical for anti-fungal defense in skin tissue (7).
Fig 4.
Absolute number of ILCs in the skin of C. auris- and C. albicans-infected mice groups. Mice were infected intradermally with C. auris 0387 or C. albicans SC5314. Mice were euthanized after 3 and 14 days post-infection to determine ILCs. (A) Flow gating strategy for ILCs is shown here. Absolute numbers of (B) total ILCs, (C) IL-17A+ ILCs, (D) IL-17F+ ILCs, and (E) IL-22+ ILCs in C. auris- or C. albicans-infected mice skin tissue after 3 and 14 days post-infection are shown. Ten mice were used for each group and data represented were mean ± standard error of the mean for each group. Statistical significance was calculated using Mann-Whitney U test. *P ≤ 0.05, **P ≤ 0.01 were considered as significant. ILC, innate lymphoid cell.
C. auris infection induces significantly decreased numbers of IL-17- and IL-22-producing TCRβ+ T cells
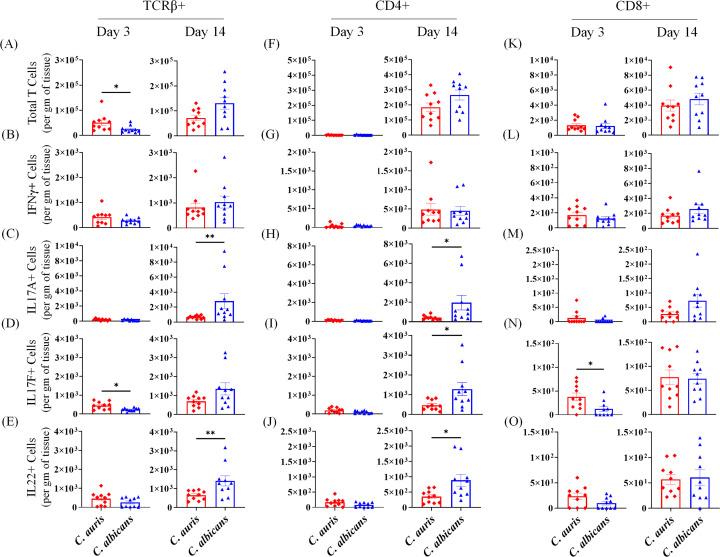
T cells such as TCRβ+ IL-17+ T cells are critical for host defense against Candida pathogens, including those in the skin (10, 18). We examined absolute numbers of total TCRγδ+ and TCRβ+ T cells, as well as IL-17-, IFN-γ-, and IL-22-producing CD4 and CD8 T-cell subsets in PBS-injected and C. auris- and C. albicans-infected mice as indicated in Fig. 5A. We analyzed T cells after 3 and 14 days post-infection. Analysis of T cells at the early timepoint of 3 days post-infection showed a mixed trend. Therefore, we focused on T-cell populations at 14 days post-infection. At that timepoint, compared to PBS-injected mice, C. auris infection elicited a significantly increased number of total γδ+ T cells and a decreased number of γδ+ IFN-γ+ T cells, γδ+ IL17A+ T cells, and γδ+ IL-22+ T cells (Fig. S4). Compared to PBS-injected mice, C. auris-infected mice had significantly increased numbers of total TCRβ+ T cells, TCRβ+ IFN-γ+ T cells, TCRβ+ IL-17A+ T cells, TCRβ+ IL-17F+ T cells, and TCRβ+ IL-22+ T cells (Fig. S5A through E). Similarly, a significant increase in the total numbers of CD4+ T cells, CD8+ T cells, CD4+ IFN-γ+ T cells, CD4+ IL-17A+ T cells, CD4+ IL-17F+ T cells, and CD4+ IL-22+ T cells was observed in C. auris-infected skin compared to PBS-injected mice (Fig. S5F through J and 5K).
Fig 5.
Absolute number of γδ+ cells in the skin of C. auris- and C. albicans-infected mice groups. Mice were infected intradermally with C. auris 0387 or C. albicans SC5314. Mice were euthanized after 3 and 14 days post-infection. (A) Flow gating strategy of T cells is shown here. (B–F) Absolute numbers of total γδ+ cells, γδ+ IFN-γ+, γδ+ IL-17A+, γδ+ IL-17F+, and γδ+ IL-22+ T cells in C. auris- or C. albicans-infected mice skin tissue after 3 and 14 days post-infection are shown, respectively. Ten mice were used for each group and data represented were mean ± standard error of the mean for each group. Statistical significance was calculated using Mann-Whitney U test. *P ≤ 0.05 was considered as significant.
Next, we compared TCRγδ+ and TCRβ+ T-mediated immune response in the skin of C. auris- and C. albicans-infected mice. C. auris infection elicited a significantly decreased number of total TCRγδ+ T cells and an increased number of TCRγδ+ IL-17F+ T cells (Fig. 5B through E). Mice infected with C. auris had significantly decreased numbers of TCRβ+ IL-17A+ T cells and TCRβ+ IL-22+ T cells compared to C. albicans-infected mice (Fig. 6C and E). Significantly decreased numbers of CD4+ IL-17A+ T cells, CD4+ IL-17F+ T cells, and CD+ IL-22+ T cells were observed in C. auris-infected skin tissue (Fig. 6H through J). No significant differences were noted in other TCRγδ+ and TCRβ+ cell types (Fig. 5 and 6). Taken together, C. auris skin infection induces significantly increased numbers of IL-17- and IL-22-producing TCRβ+ cells relative to PBS control. However, compared to C. albicans-infected mice, C. auris skin infection induces a significantly decreased number of IL-17- and IL-22-producing TCRβ+ cells that are important for host defense against fungal pathogens.
Fig 6.
Absolute number of TCRβ+ cells in the skin of C. auris- and C. albicans-infected mice groups. Mice were infected intradermally with C. auris 0387 or C. albicans SC5314. Mice were euthanized after 3 and 14 days post-infection. (A–E) Absolute numbers of total TCRβ+ cells, TCRβ IFN-γ+, TCRβ+ IL-17A+, TCRβ+ IL-17F+, and TCRβ+ IL-22+ T cells in C. auris- or C. albicans-infected mice skin tissue after 3 and 14 days post-infection are shown, respectively. (F–J) Absolute numbers of total of total CD4+ cells, CD4+ IFN-γ+, CD4+ IL-17A+, CD4+ IL-17F+, and CD4+ IL-22+ T cells in C. auris- or C. albicans-infected mice skin tissue after 3 and 14 days post-infection are shown, respectively. (K–O) Absolute numbers of total of total CD8+ cells, CD8+ IFN-γ+, CD8+ IL-17A+, CD8+ IL-17F+, and CD8+ IL-22+ T cells in C. auris- or C. albicans-infected mice skin tissue after 3 and 14 days post-infection are shown, respectively. Ten mice were used for each group and data represented were mean ± standard error of the mean for each group. Statistical significance was calculated using Mann-Whitney U test. *P ≤ 0.05, **P ≤ 0.01 were considered as significant.
Differences in the skin immune response among different clades of C. auris
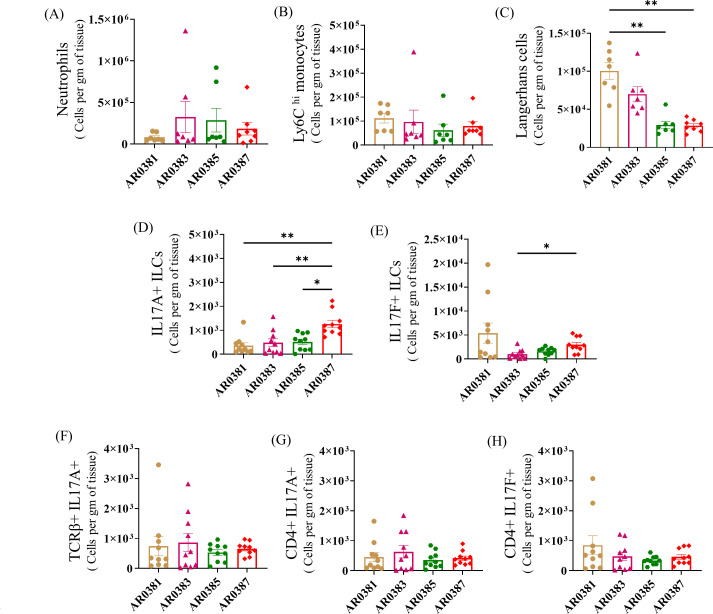
To examine if any differences exist in the skin immune response among isolates from different clades of C. auris, we compared innate and adaptive immune cell types such as neutrophils, monocytes, LCs, and IL-17+ T cells that are significantly different between C. auris and C. albicans mice using strains from four clades of C. auris (East Asian AR0381, African AR0383, South American AR0385, and South Asian AR0387). No significant differences in the absolute numbers of CD11b+ Ly6G+ neutrophils, CD11b+ Ly6 Chi inflammatory monocytes, TCRβ+ IL-17A+ T cells, CD4+ IL-17A+ T cells, and CD4+ IL-17F+ T cells were observed among the examined strains of the four clades of C. auris (Fig. 7A, B, and F through H). However, the absolute numbers of CD11b+ CD207+ LCs, IL-17A+, and IL-17F+ ILCs were significantly different among different clades of C. auris (Fig. 7C through E). Taken together, mice infected with different clades of C. auris elicit different levels of accumulation of LCs and ILCs. However, no significant difference in the absolute number of IL-17+ T cells was observed in the skin tissue among the four clades of C. auris.
Fig 7.
Innate and adaptive immune cells in skin tissue of mice infected with different clades of C. auris. Mice were infected intradermally with C. auris AR0381, C. auris AR0383, C. auris AR0385, or C. auris AR0387. Mice were euthanized after 3 and 14 days post-infection. Absolute numbers of (A) CD11b+ Ly6G+ neutrophils, (B) CD11b+ Ly6C hi monocytes, and (C) CD11b+ CD207+ LCs in mice infected with different clades of C. auris after 3 days post-infection are shown here. Absolute numbers of (D) IL-17A+ ILCs, (E) IL-17F+ ILCs, (F) TCRβ+ IL-17A+, (G) CD4+ IL-17A+, and (H) CD4+ IL-17F+ T cells in four clades of C. auris-infected mice skin tissue after 14 days post-infection are shown here. Seven to ten mice were used for each group and data represented were mean ± SEM for each group. Statistical significance was calculated using Kruskal-Wallis test followed by Dunn’s multiple comparisons test. *P ≤ 0.05 and **P ≤ 0.01 were considered as significant.
DISCUSSION
C. auris is an emerging multi-drug-resistant fungal pathogen that can cause fatal infections in humans (1, 19, 20). C. auris colonizes the human skin, which leads to nosocomial transmission and outbreaks of skin infection and candidemia (4, 5). However, determinants of colonization resistance and the local host immune response to C. auris in the skin are still poorly defined. Therefore, we developed an intradermal mouse model of C. auris skin infection and compared the fungal load and skin immune response of C. auris with that of C. albicans. Our findings show a significant increase in fungal load in the skin tissue of C. auris-infected mice compared to C. albicans-infected mice. Persistent fungal colonies were observed in >80% of C. auris-infected mice even after 2 weeks post-infection. Recent findings indicate that mice epicutaneously inoculated with C. auris had significantly increased fungal load in the skin swab and underlying skin tissue compartment compared to C. albicans-infected groups (4). C. auris persisted in mice skin tissue for 4 months even after skin swab was found to be negative for live fungal colonies (4). Furthermore, mice epicutaneously infected with different clades of C. auris had significantly different fungal loads in the skin tissue (4). However, in our intradermal route of infection, four clades of C. auris showed almost similar levels of fungal load in skin tissues. This might be due to difference in route of infection and or difference in invasion potential among different clades of C. auris from skin surface to the dermis, which is an important future area to investigate (21). Previous findings reported the localization of C. auris and C. albicans in the skin tissue (4, 22, 23). Future studies using epicutaneous and intradermal routes of infection with different clades of C. auris and C. albicans might reveal the invasion potential and localization of these fungal species in the skin. Collectively, previous findings (4), along with ours, indicate that persistent fungal colonies were observed in the skin tissues of mice infected with epicutaneous or intradermal routes of infection with C. auris. The higher skin burdens for C. auris also correlate with clinical observations of persistent skin colonization and ex vivo models of C. auris growing on human skin (24).
Using the epicutaneous mouse model of infection, Huang et al. demonstrated that a local IL-17A/IL-17F-dependent response mounted by both innate and adaptive lymphoid cell subsets is associated with protection against C. auris skin infection (4). Using mice deficient in IL-17R-associated adaptor molecule Act1, which lack IL-17 responses, the authors demonstrated that IL-17A/IL-17F produced by αβ T cells, γδ T cells, and ILCs is associated with protection against C. auris (4). Furthermore, previous findings suggest that Th17 cells are required for cutaneous host defense, while Th1 response is critical against systemic C. albicans infection (18, 25). These findings suggest that IL-17-mediated immune responses are critical for protection against C. auris and C. albicans skin infection (4, 18, 25). Recent evidence indicates that C. auris elicits less potent innate immune responses compared to C. albicans in the mouse model of systemic infection (13). In addition, C. auris persisted longer than C. albicans in kidneys of infected mice (26). Although C. auris preferentially colonizes the skin, the innate immune cells involved in the cutaneous host response against C. auris had not been defined to date. Furthermore, the host factors that contribute to persistent colonization of this emerging pathogen are still not clear. Therefore, using this intradermal mouse model of infection, we compared the innate and adaptive cellular immune responses of C. auris with C. albicans.
Surprisingly, our results indicate that significantly decreased numbers of neutrophils were observed in the skin tissue of C. auris-infected mice compared to the C. albicans-infected group. This agrees with previous findings that C. auris elicits less pronounced innate immune responses compared to C. albicans in a mouse model of systemic infection (13). The decreased number of neutrophils observed in the skin tissue of C. auris-infected mice may be an immune evasion strategy employed by this fungus. On the other hand, C. auris infection is associated with an increased number of CD11b+ Ly6 Chi inflammatory monocytes and LCs. Recent evidence indicates that differences in mannan composition between C. auris and C. albicans contribute to decreased innate immune recognition, phagocytic activity, and clearance of C. auris in human neutrophils (13, 27, 28). It is interesting to postulate that these cell wall mannosylation differences may also play a role in the differential innate immune responses observed for C. auris in our skin model. Our findings, along with others, suggest that increased fungal load in the C. auris-infected mice on day 3 post-infection might be in part due to the decreased number of neutrophils observed in the skin tissue. Previous findings suggest that C. albicans morphology, DCs subsets, and LCs elicit different skin immune responses in an epicutaneous and intradermal routes of infection (25). However, future studies are required to better understand the role of C. auris morphology and specific innate immune cells such as neutrophils, monocytes, macrophages, and DC subsets in different anatomical locations of the skin that are critical to control C. auris infection.
Next, we compared the lymphoid cell immune response between C. auris and C. albicans. Surprisingly, we found that C. auris infection elicits significantly less potent adaptive immune response compared to C. albicans. CD4+ IL-17+ T cells and CD4+ IL-22+ T cells that are important for host defense against fungal pathogens were significantly decreased in the skin tissue of C. auris-infected mice. In addition, C. auris infection induces a significantly decreased number of IL-17A+ ILCs and IL-17F+ ILCs in skin tissue relative to C. albicans infection. However, using an intestinal mouse model of infection, we recently found that C. auris and C. albicans elicit similar levels of cellular adaptive immune responses in the intestine (29). Mice orally infected with C. auris but not C. albicans had increased fungal-specific IgA levels in their serum (29). These results suggest that adaptive immune responses are Candida species specific and may differ in different host mucocutaneous niches such as the gut and skin.
Because U.S. isolates are closely related to those of South America and South Asia, we used the genome sequenced South Asian clade CA 0387 (B8441) to compare with C. albicans (4, 30 – 32). In order to rule out any differences in the major innate and adaptive immune cells among different clades of C. auris, we compared skin immune response between four clades of C. auris. Significant differences in the number of LCs and IL-17+ ILCs were observed among different clades of C. auris. However, no significant differences in the absolute number of CD11b+ Ly6G+ neutrophils, CD11b+ Ly6 Chi inflammatory monocytes, and IL-17+ T cells were observed among four clades of C. auris. Taken together, intradermal infection of mice with C. auris had a significantly increased fungal load compared to C. albicans infected skin tissue. Persistent infection of C. auris in mouse skin tissues correlates with less potent innate and adaptive immune response elicited by this emerging pathogen. Our findings for the first time characterized the major innate immune cells involved in host defense against C. auris skin infection and identified quantitative differences relative to C. albicans skin infection. Furthermore, persistent infection of C. auris in the mouse skin tissue appears to be in part due to a less potent innate and adaptive immune response compared to C. albicans. Further studies are required to understand the fungal factors that contribute to differences in skin immune responses to different clades of C. auris. In addition, understanding the genetic and host factors that contribute to the differential immune response to C. auris and C. albicans infections is critical to prevent and treat this emerging skin tropic fungal pathogen (14, 33).
ACKNOWLEDGMENTS
This study was supported by Purdue University start-up funds provided to S.T. This study was supported in part by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (ZIA AI001175 to M.S.L.).
A.D. and D.D. performed the experiments, analyzed the data, prepared the figures, and wrote methods section. S.T. supervised the project, reviewed the data analysis, and wrote the manuscript. J.N., J.V., and M.S.L. reviewed data analysis and edited the manuscript. All authors contributed and approved the final version of the manuscript.
Contributor Information
Shankar Thangamani, Email: sthangam@purdue.edu.
Joshua J. Obar, Geisel School of Medicine at Dartmouth, Lebanon, New Hampshire, USA
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.02215-23.
Fig. S1 to S5.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Ahmad S, Alfouzan W. 2021. Candida auris: epidemiology, diagnosis, pathogenesis, antifungal susceptibility, and infection control measures to combat the spread of infections in healthcare facilities. Microorganisms 9:807. doi: 10.3390/microorganisms9040807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . 2022. WHO fungal priority pathogens list to guide research, development and public health action. World Health Organization, Geneva. Available from: https://www.who.int/publications/i/item/9789240060241 [Google Scholar]
- 3. Kadri SS. 2020. Key takeaways from the U.S. CDC’s 2019 antibiotic resistance threats report for frontline providers. Crit Care Med 48:939–945. doi: 10.1097/CCM.0000000000004371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang X, Hurabielle C, Drummond RA, Bouladoux N, Desai JV, Sim CK, Belkaid Y, Lionakis MS, Segre JA. 2021. Murine model of colonization with fungal pathogen Candida auris to explore skin tropism, host risk factors and therapeutic strategies. Cell Host Microbe 29:210–221. doi: 10.1016/j.chom.2020.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shastri PS, Shankarnarayan SA, Oberoi J, Rudramurthy SM, Wattal C, Chakrabarti A. 2020. Candida auris candidaemia in an intensive care unit - prospective observational study to evaluate epidemiology, risk factors, and outcome. J Crit Care 57:42–48. doi: 10.1016/j.jcrc.2020.01.004 [DOI] [PubMed] [Google Scholar]
- 6. Proctor DM, Drummond RA, Lionakis MS, Segre JA. 2023. One population, multiple lifestyles: commensalism and pathogenesis in the human mycobiome. Cell Host Microbe 31:539–553. doi: 10.1016/j.chom.2023.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sparber F, De Gregorio C, Steckholzer S, Ferreira FM, Dolowschiak T, Ruchti F, Kirchner FR, Mertens S, Prinz I, Joller N, Buch T, Glatz M, Sallusto F, LeibundGut-Landmann S. 2019. The skin commensal yeast malassezia triggers a type 17 response that coordinates anti-fungal immunity and exacerbates skin inflammation. Cell Host Microbe 25:389–403. doi: 10.1016/j.chom.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 8. Sakamoto K, Goel S, Funakoshi A, Honda T, Nagao K. 2022. Flow cytometry analysis of the subpopulations of mouse keratinocytes and skin immune cells. STAR Protocols 3:101052. doi: 10.1016/j.xpro.2021.101052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Naik S, Bouladoux N, Linehan JL, Han S-J, Harrison OJ, Wilhelm C, Conlan S, Himmelfarb S, Byrd AL, Deming C, Quinones M, Brenchley JM, Kong HH, Tussiwand R, Murphy KM, Merad M, Segre JA, Belkaid Y. 2015. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature 520:104–108. doi: 10.1038/nature14052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Netea MG, Joosten LAB, van der Meer JWM, Kullberg B-J, van de Veerdonk FL. 2015. Immune defence against Candida fungal infections. Nat Rev Immunol 15:630–642. doi: 10.1038/nri3897 [DOI] [PubMed] [Google Scholar]
- 11. Leonardi I, Xin L, Alexa S, Dalin L, Itai D, Gregory P, Agnieszka B, Daniel P, Maria R, Dermot PBM, Jesus P, Iliyan DI. 2018. Cx3Cr1(+) mononuclear phagocytes control immunity to intestinal fungi. Science 359:232–236. doi: 10.1126/science.aao1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koh AY, Köhler JR, Coggshall KT, Van Rooijen N, Pier GB. 2008. Mucosal damage and neutropenia are required for Candida albicans dissemination. PLoS Pathog 4:e35. doi: 10.1371/journal.ppat.0040035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Y, Zou Y, Chen X, Li H, Yin Z, Zhang B, Xu Y, Zhang Y, Zhang R, Huang X, Yang W, Xu C, Jiang T, Tang Q, Zhou Z, Ji Y, Liu Y, Hu L, Zhou J, Zhou Y, Zhao J, Liu N, Huang G, Chang H, Fang W, Chen C, Zhou D. 2022. Innate immune responses against the fungal pathogen Candida auris. Nat Commun 13:3553. doi: 10.1038/s41467-022-31201-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lionakis MS, Drummond RA, Hohl TM. 2023. Immune responses to human fungal pathogens and therapeutic prospects. Nat Rev Immunol 23:433–452. doi: 10.1038/s41577-022-00826-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kashem SW, Kaplan DH. 2016. Skin immunity to Candida albicans . Trends Immunol 37:440–450. doi: 10.1016/j.it.2016.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Igyártó BZ, Haley K, Ortner D, Bobr A, Gerami-Nejad M, Edelson BT, Zurawski SM, Malissen B, Zurawski G, Berman J, Kaplan DH. 2011. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity 35:260–272. doi: 10.1016/j.immuni.2011.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gladiator A, LeibundGut-Landmann S. 2013. Innate lymphoid cells: new players in IL-17-mediated antifungal immunity. PLoS Pathog 9:e1003763. doi: 10.1371/journal.ppat.1003763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kagami S, Rizzo HL, Kurtz SE, Miller LS, Blauvelt A. 2010. IL-23 and IL-17A, but not IL-12 and IL-22, are required for optimal skin host defense against Candida albicans . J Immunol 185:5453–5462. doi: 10.4049/jimmunol.1001153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vu CA, Jimenez A, Anjan S, Abbo LM. 2022. Challenges and opportunities in stewardship among solid organ transplant recipients with Candida auris bloodstream infections. Transpl Infect Dis 24:e13919. doi: 10.1111/tid.13919 [DOI] [PubMed] [Google Scholar]
- 20. Ademe M, Girma F. 2020. Candida auris: from multidrug resistance to pan-resistant strains. Infect Drug Resist 13:1287–1294. doi: 10.2147/IDR.S249864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Muñoz JF, Welsh RM, Shea T, Batra D, Gade L, Howard D, Rowe LA, Meis JF, Litvintseva AP, Cuomo CA. 2021. Clade-specific chromosomal rearrangements and loss of subtelomeric adhesins in Candida auris . Genetics 218:iyab029. doi: 10.1093/genetics/iyab029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Santus W, Mingozzi F, Vai M, Granucci F, Zanoni I. 2018. Deep dermal injection as a model of Candida albicans skin infection for histological analyses. J Vis Exp 2018:136. doi: 10.3791/57574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bai W, Wang Q, Deng Z, Li T, Xiao H, Wu Z. 2020. TRAF1 suppresses antifungal immunity through CXCL1-mediated neutrophil recruitment during Candida albicans intradermal infection. Cell Commun Signal 18:30. doi: 10.1186/s12964-020-00532-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eix EF, Johnson CJ, Wartman KM, Kernien JF, Meudt JJ, Shanmuganayagam D, Gibson ALF, Nett JE. 2022. Ex vivo human and porcine skin effectively model Candida auris colonization, differentiating robust and poor fungal colonizers. J Infect Dis 225:1791–1795. doi: 10.1093/infdis/jiac094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kashem SW, Igyarto BZ, Gerami-Nejad M, Kumamoto Y, Mohammed JA, Jarrett E, Drummond RA, Zurawski SM, Zurawski G, Berman J, Iwasaki A, Brown GD, Kaplan DH. 2015. Candida albicans morphology and dendritic cell subsets determine T helper cell differentiation. Immunity 42:356–366. doi: 10.1016/j.immuni.2015.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vila T, Montelongo-Jauregui D, Ahmed H, Puthran T, Sultan AS, Jabra-Rizk MA. 2020. Comparative evaluations of the pathogenesis of Candida auris phenotypes and Candida albicans using clinically relevant murine models of infections. mSphere 5:e00760-20. doi: 10.1128/mSphere.00760-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johnson CJ, Davis JM, Huttenlocher A, Kernien JF, Nett JE. 2018. Emerging fungal pathogen Candida auris evades neutrophil attack. mBio 9:e01403-18. doi: 10.1128/mBio.01403-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Horton MV, Johnson CJ, Zarnowski R, Andes BD, Schoen TJ, Kernien JF, Lowman D, Kruppa MD, Ma Z, Williams DL, Huttenlocher A, Nett JE. 2021. Candida auris cell wall mannosylation contributes to neutrophil evasion through pathways divergent from Candida albicans and Candida glabrata. mSphere 6:e0040621. doi: 10.1128/mSphere.00406-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Das D, HogenEsch H, Thangamani S. 2023. Intestinal colonization with Candida auris and mucosal immune response in mice treated with Cefoperazone oral antibiotic. Front Immunol 14:1123200. doi: 10.3389/fimmu.2023.1123200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rybak JM, Laura AD, Andrew TN, Katherine SB, Glen EP, P David Rogers R. 2019. Abrogation of Triazole resistance upon deletion of Cdr1 in a clinical isolate of Candida Auris. Antimicrob Agents Chemother 63. doi: 10.1128/aac.00057-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Muñoz JF, Gade L, Chow NA, Loparev VN, Juieng P, Berkow EL, Farrer RA, Litvintseva AP, Cuomo CA. 2018. Genomic insights into multidrug-resistance, mating and virulence in Candida auris and related emerging species. Nat Commun 9:5346. doi: 10.1038/s41467-018-07779-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vallabhaneni S, Kallen A, Tsay S, Chow N, Welsh R, Kerins J, Kemble SK, Pacilli M, Black SR, Landon E, Ridgway J, Palmore TN, Zelzany A, Adams EH, Quinn M, Chaturvedi S, Greenko J, Fernandez R, Southwick K, Furuya EY, Calfee DP, Hamula C, Patel G, Barrett P, MSD, Lafaro P, Berkow EL, Moulton-Meissner H, Noble-Wang J, Fagan RP, Jackson BR, Lockhart SR, Litvintseva AP, Chiller TM. 2016. Investigation of the first seven reported cases of Candida auris, a globally emerging invasive, multidrug-resistant fungus - United States, may 2013-August 2016. MMWR Morb Mortal Wkly Rep 65:1234–1237. doi: 10.15585/mmwr.mm6544e1 [DOI] [PubMed] [Google Scholar]
- 33. Tharp B, Zheng R, Bryak G, Litvintseva AP, Hayden MK, Chowdhary A, Thangamani S. 2023. Role of microbiota in the skin colonization of Candida auris. mSphere 8:e0062322. doi: 10.1128/msphere.00623-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S5.